Abstract
The use of fractional exhaled nitric oxide (FeNO) has been suggested as a quantitative marker for pulmonary arterial hypertension (PAH) in humans. To further characterize FeNO in PAH we investigated this marker in a rodent model. Since there is no standardized technique for FeNO measurement in animals, we intended to reduce measuring errors and confounders of an existing published method by mathematical modification and tested its applicability in an NO-regulating therapy concept of PAH. Thirty-three male Sprague-Dawley rats underwent unilateral pneumonectomy and monocrotaline (MCT) injection and were observed for 49 days. A telemetric catheter was introduced into the left pulmonary artery to continuously record mean pulmonary arterial pressure (mPAP), and FeNO was assessed. After 35 days, animals were randomized to receive either oral l-arginine (300 mg/kg) in combination with tetrahydrobiopterin (20 mg/kg) therapy (n = 12) or vehicle (n = 11) daily over a period of 14 days. mPAP at baseline was 17.19 ± 9.62 mmHg, which increased to 53.1 ± 10.63 mmHg 28 days after monocrotaline exposure (P < 0.001). Using the modified technique, we found an inverse correlation between exhaled NO and pulmonary pressures before (r = −0.366, P = 0.043) and after MCT (r = −0.363, P = 0.038) as well as after therapy administration (r = −0.657, P = 0.02). Our modified technique proved robust in a rodent model, since valid and reproducible data were gained and showed an inverse correlation between exhaled NO and mPAP, whereas the existing method did not.
Keywords: fractional exhaled nitric oxide, noninvasive measurement, rat, monocrotaline
pulmonary arterial hypertension (PAH) is a progressive disease, leading to early death if left untreated (9, 19). It is marked by increased vascular resistance in the distal pulmonary arteries as a consequence of vasoconstriction, proliferative remodeling of the pulmonary vasculature, inflammation, and thrombosis. Impaired production of vasodilators such as prostacyclin and nitric oxide (NO) has been proposed to be involved in disease pathogenesis (12). NO plays a major role in vascular homeostasis, smooth muscle cell proliferation and migration, platelet aggregation, and leukocyte adhesion on the endothelium (36). Low expiratory NO concentrations have been found in PAH patients (10, 16, 35) and even an inverse relation between exhaled NO, its biochemical reaction products, and mean pulmonary arterial pressure (mPAP) has been shown (16). Although there are established and uniform measurement techniques for the assessment of exhaled NO in humans (1), noninvasive measurement in animals is challenging, since the expected NO concentration lies close to the quantification threshold. Most groups solved this problem by adding an accumulation process (2, 6, 21, 30, 32), varying in time and setup. At such low concentrations the error by sampling NO for its quantification alters the concentration in the measuring chamber.
The aim of our study was to establish a reliable and reproducible method for fractional exhaled NO (FeNO) measurement in a rat model of PAH, to compare the obtained exhaled NO values to an already-published method, and to test whether the novel technique is able to trace even minimal effects of an NO-regulating therapy with the NO-substrate l-arginine and the cofactor tetrahydrobiopterin (BH4).
MATERIALS AND METHODS
Animal model.
This study was approved by the Austrian Ministry for Science and Research and was performed according to the Helsinki convention for the use and care of animals. A total of 62 male Sprague-Dawley rats [weight: 412.15 ± 46.3 g, age: 98.22 ± 20.74 days (29)] underwent unilateral pneumonectomy and simultaneous implantation of a DSI PA-C40 pressure catheter in the common pulmonary artery trunk (22, 33). For anesthesia the animals received 2% xylazine (4 mg/kg)/ketamine (100 mg/kg) and were intubated. After connection to a small animal ventilator (Biegler, Mauerbach, Austria) the animals received 1.5% isoflurane at an inspired oxygen fraction of 0.8 and a respiratory volume of 1.5 l/min. All animals were given a single shot of enrofloxacine (Baytril, Bayer, Vienna, Austria) as perioperative antibiotic prophylaxis. For placement of the DSI catheter a refined technique was used. In brief, the catheter tip was directly inserted into the pulmonary artery after resection of the left lung via a lateral thoracotomy (27). The excised lung was utilized as nondiseased control tissue for all experiments. Hemodynamic data were received and processed by DSI 4.2 ART software system (Datascience International, Boston, MA). In every rat, mPAP and heart rate were recorded over 5 min per hour, and a mean value for each day was calculated. PAH was defined as mPAP > 25 mmHg.
Rats were provided tap water and common rat chow (ssniff, Soest, Germany) ad libitum. Seven days after the surgical intervention, PAH was induced by subcutaneous administration of 60 mg/kg monocrotaline (MCT; Sigma, Vienna, Austria). From day 35 onward, a daily combination of l-arginine (300 mg/kg) (28) (Sigma) and BH4 (20 mg/kg) (18) (kindly provided by AOP Orphan Pharmaceuticals, Vienna, Austria) was administered in a subset of 12 rats for the following 14 days. A control group of 11 rats received water instead. After sedation with isoflurane, therapy or vehicle was administered by orogastric gavage. To prevent aspiration, animals remained in an upright position until they regained consciousness. FeNO values were determined before the administration of MCT, on day 35 (at which point all included animals had developed PAH), and at the end of observation.
Nitric oxide measurement.
A chemiluminescence analyzer (CLD 66, Eco Physics, Duernten, Switzerland) with a sensitivity of 0.5 ppb and a withdrawal rate of 100 ml/min was used to measure gas phase NO. For the measurement of FeNO, single spontaneously breathing rats were placed in a whole body plethysmography (WBP) chamber (3.9 liters, PLY 3213 Buxco Research Systems, Wilmington, DE). The WBP chamber was connected to the Denox88 system (Eco Medics, Duernten, Switzerland), which produces and carries a continuous flow of NO-free air (300 ml/s) into the WBP chamber and into the chemiluminescence analyzer. Measurements were recorded by excel Macro (cld2xls, Jörg Hoffmann, ECO PHYSICS). Fast elimination of NO from the WBP chamber was achieved by an outlet located at the top of the chamber. Sampling was planned after the setup described by Ahmad et al. (2). The WBP chamber was flushed with NO-free air and was sealed when NO levels were back to baseline. The exhaled breath of the rat was collected for 300 s. This would be the approximate time that the entire gas volume inside the WBP chamber would have been inhaled and exhaled by the rats. Then the outflow stopcock was opened for NO measurement, and the recording continued for another 300 s.
Modified concept of nitric oxide measurement.
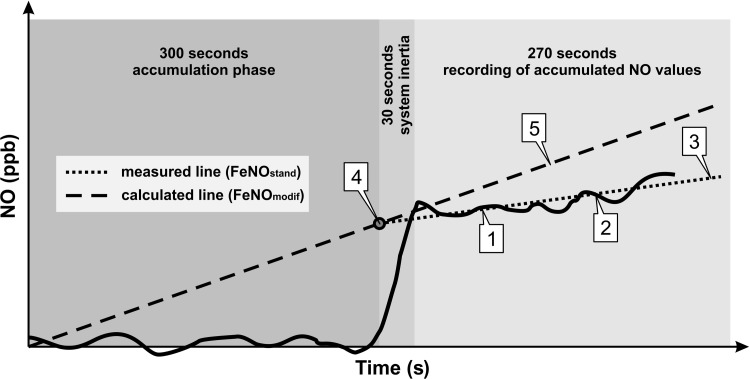
In undisturbed closed systems (like the WBP chamber) concentrations display an exponential curve at steady accumulation. However, because of the instability of NO and time limitations, this approximation value cannot be used in practice. In the period during which NO is allowed to accumulate in the plethysmograph, the measured NO concentration vs. time produces a near-linear relationship. In addition, after opening the closed system, the true NO concentration is underestimated because of its constant removal for sampling. To overcome this technical issue, we first determined the NO values (parts per billion per hour, ppb/h) as described above. The first 30 s of transient condition at the beginning of recording were excluded to reduce the bias caused by the inertia of the measuring system. By averaging the NO levels of the remaining 270 s, two points were generated through which a partial regression line was laid to determine the standard slope (FeNOstand) (Fig. 1, slope 3). For the mathematical modification FeNOstand was used for extrapolation and determination of the calculated initial chamber concentration (cENOi; Fig. 1, point 4). The oscillation of the baseline values was evened out by average determination and a zero point was created. The modified slope (FeNOmodif; Fig. 1, slope 5) was calculated by laying a partial regression line through the zero point and cENOi, receiving a steeper slope than FeNOstand. We presume that this increase represents the successful elimination of bias created by dilution and oscillating values that may be crucial at such a low concentration.
Fig. 1.
Scheme of data acquisition. FeNOstand, slope of NO values generated with the standard method; FeNOmodif: slope of NO values generated with the mathematical modification; cENOi, calculated initial NO chamber concentration. Points 1 and 2: Data from the final 270 s are used to generate 2 points by average determination. Slope 3: a partial regression line is laid through points 1 and 2 resulting in FeNOstand. Point 4: by extrapolation of points 1 and 2 cENOi can be established. Slope 5: finally, a straight line is laid through point 4 and the zero baseline to determine FeNOmodif.
To validate our modified method we applied it to a cohort of six untreated rats (no pneumonectomy, no MCT) in an intervention that predictably affects NO production. l-NG-nitroarginine methyl ester (l-NAME hydrochloride, Bertin Pharma, Montigny-le-Bretonneux, France) nonselectively inhibits NO synthase and therefore reduces NO exhalation in rodent models (4, 17). l-NAME was administered by two intraperitoneal injections (2 × 100 mg/kg) with an interval of 30 min (17, 32). Prior to and 30 min after l-NAME application, FeNO was assessed by both the standard and the modified method.
Immunohistochemistry and morphometry.
At the end of the observation period animals were anesthetized and euthanized by intracardial thiopental bolus injection. The remaining lung was removed for further analysis. Endothelial NO synthase (eNOS) mRNA expression was measured from nitrogen-snap-frozen pulmonary tissue samples by using the RNeasy fibrous tissue kit according to the manufacturer's protocol (Qiagen, Hilden, Germany) and determined by real-time PCR using eNOS primer Rn02132634_S1 (7). eNOS expression was visualized in immunohistochemically stained lung tissue slides (anti-eNOS antibody ab66127, Abcam, Cambridge, UK) and intraindividually compared with the nondiseased sample. We chose third order pulmonary arteries with an outer diameter <350 μm (5), since they were relatively easy to detect and displayed all features of hypertensive pulmonary arteries and compared them to vessels ≥350 μm. Percent medial wall thickness (%MWT) was calculated as described elsewhere (25). In brief, %MWT was calculated by the following equation: %MWT = [(medial thickness × 2)/external diameter] × 100. Six vessels of each section were randomly chosen and analyzed by two independent observers using ImageJ 1.46 (National Institutes of Health, Bethesda, MD).
Statistical analysis.
Results were compared using SPSS19 Statistic software (IBM, Armonk, NY). An unpaired Student's t-test was used for comparison of data between methods. Data generated by both methods were assessed by Spearman correlation analysis and linear regression analysis. A P value of <0.05 was regarded as significant.
RESULTS
Animal model.
Twenty-seven rats had to be excluded from our follow-up (perioperative death in 9 animals, death due to wound infection in 2 animals, death attributed to PAH in 9 rats, temporary malfunction of the NO measuring apparatus in 7 rats). Of the remaining 35 animals, two did not develop PAH and were also excluded from this analysis. The mPAP at baseline (postpneumonectomy) was 17.19 ± 9.62 mmHg, which increased to 53.13 ± 10.63 mmHg after 28 days of MCT exposure (P < 0.001; Table 1).
Table 1.
Animal characteristics
| pre-P/MCT (day 7) | post-P/MCT (day 35) | post-VH (day 49) | post-TH (day 49) | |
|---|---|---|---|---|
| N | 33 | 33 | 11 | 12 |
| TBW, g | 412.15 ± 46.30 | 414.61 ± 59.79 | 422.97 ± 69.13 | 402.00 ± 77.52 |
| HR, beats/min | 343.5 ± 34.30 | 349.02 ± 34.40 | 432.09 ± 60.59 | 399.57 ± 71.60 |
| BR, breaths/min | 104.81 ± 21.82 | 120.73 ± 29.77 | 306.57 ± 16.57 | 332.97 ± 16.50 |
| mPAP, mmHg | 17.19 ± 9.62 | 53.13 ± 10.63 | 51.1 ± 17.6 | 47.2 ± 16.2 |
| FeNOstand, ppb/h | 16.16 ± 7.19 | 14.33 ± 6.50 | 18.81 ± 4.11 | 18.31 ± 11.18 |
| FeNOmodif, ppb/h | 21.21 ± 8.27 | 23.96 ± 7.60 | 27.55 ± 10.72 | 29.31 ± 12.56 |
| eNOS, log10RQ | 0.00 ± 0.57 | n. a. | 0.60 ± 0.86 | −0.84 ± 0.84 |
pre-P/MCT, prior to monocrotaline application; post-P/MCT, after pneumonectomy and monocrotaline application; post-TH, after therapy administration; post-VH, after vehicle administration; N, number of animals; TBW, total body weight; HR, heart rate; BR, breathing rate; mPAP, mean pulmonary arterial pressure; FeNOstand, slope of exhaled nitric oxide values generated with the standard method; FeNOmodif, slope of exhaled nitric oxide values generated with the mathematical modification; eNOS, endothelial nitric oxide synthase results from real-time PCR. n. a., not available. For P values, see text.
Modified FeNO measurement.
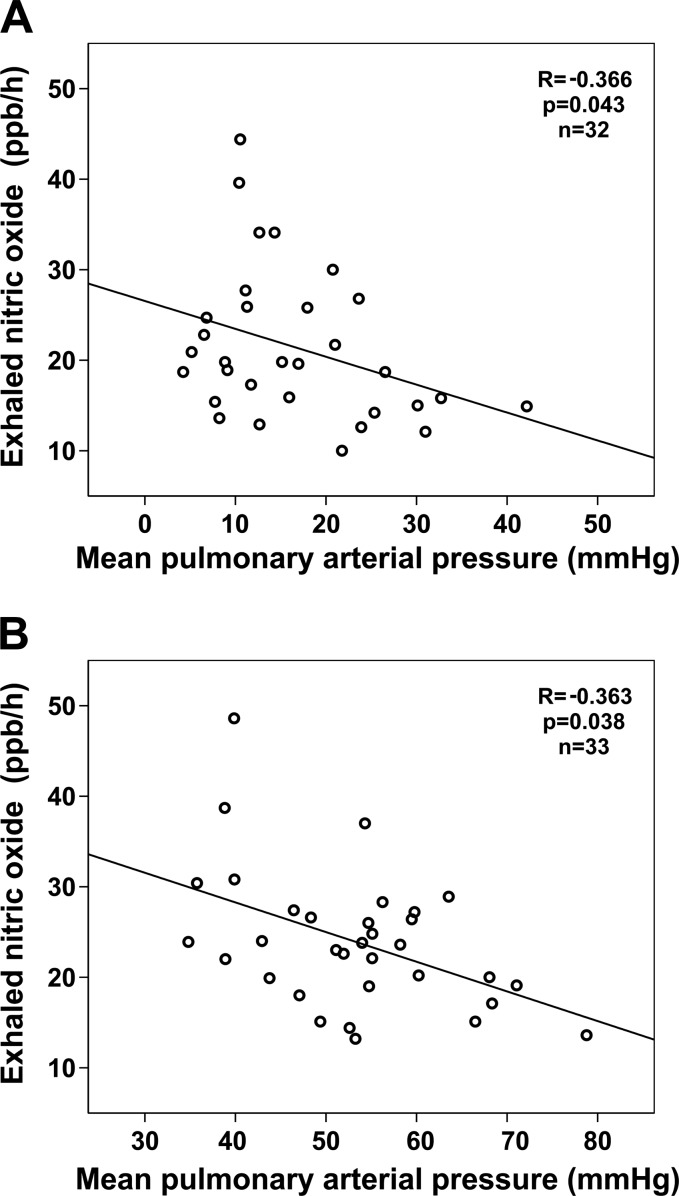
Basal standard NO levels were 16.16 ± 7.19 ppb/h whereas basal modified values were 21.21 ± 8.27 ppb/h. Twenty-eight days after MCT application, modified NO levels increased by 3.17 ± 6.17 ppb/h after establishment of PAH (P = 0.01; Fig. 2) whereas standard NO levels remained unaffected (−1.62 ± 8.35 ppb/h; P = 0.2). Relying on the conventional FeNO assessment (2), neither correlation could be identified between FeNO and pulmonary pressures before (r = −0.282, P = 0.124) nor after (r = −0.258, P = 0.148) development of PAH. After modification of FeNO assessment, we found a moderate inverse correlation between FeNO and pulmonary pressures before (r = −0.366, P = 0.043) and after (r = −0.363, P = 0.038) development of PAH (Fig. 3).
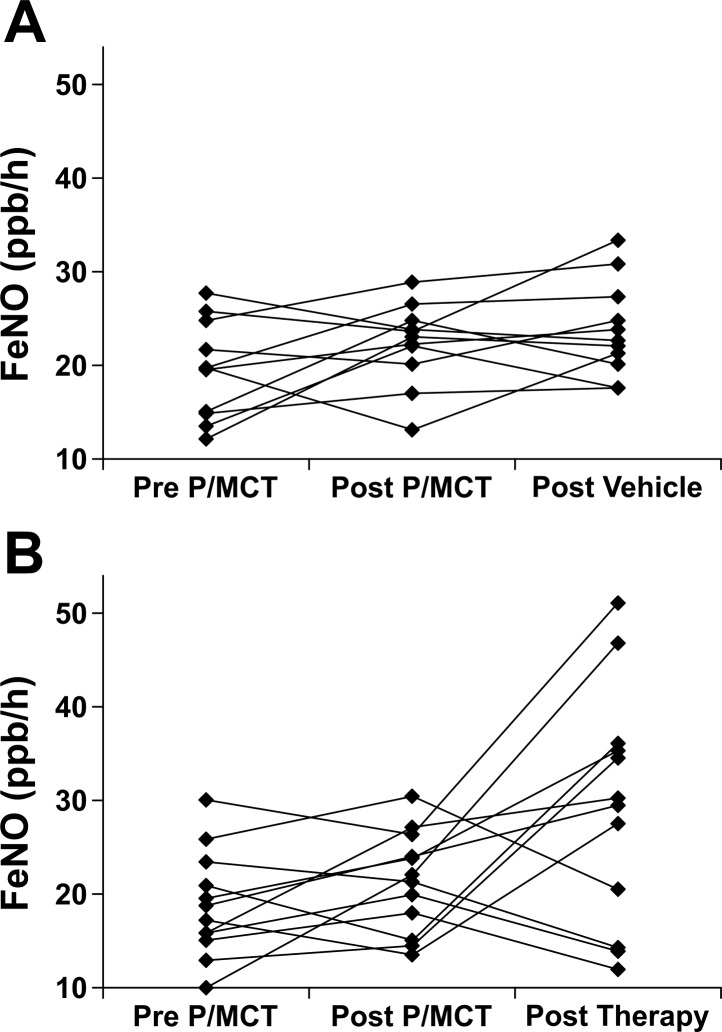
Fig. 2.
Fractional exhaled NO (FeNO) courses of individual rats in response to pneumonectomy plus monocrotaline (MCT) injection (P/MCT). A: vehicle-treated group. B: verum group.
Fig. 3.
Scatterplot illustrating the correlation of mean pulmonary arterial pressure (mPAP) with the exhaled nitric oxide values after pneumonectomy before (A) and after (B) MCT application. The modified NO measurement technique was applied. mPAP values exceeding 25 mmHg occur in some rats at baseline. After unilateral pneumonectomy, an adaptation to the increasing blood flow in the remaining lung is seen (15) that has not been completed yet in animals with mPAP > 25 mmHg.
In a linear regression model, the conventional technical setup failed to predict pulmonary pressure before (R2 = 0.076, P = 0.133) and after (R2 = 0.059, P = 0.175) the application of unilateral pneumonectomy and MCT injection (P/MCT). With the modified setup, there was a moderate relation between FeNO and mPAP before (R2 = 0.118, P = 0.059) and a significant association between FeNO and mPAP (R2 = 0.213, P = 0.007) after P/MCT.
Therapy administration led to a significant decrease of mPAP (−7.8 ± 15.4 mmHg, P = 0.003) and a trend toward increasing FeNO measured by the modified setup (P = 0.068). Furthermore, a significant inverse correlation was shown between mPAP and FeNO (r = −0.657, P = 0.02).
FeNO measurement after l-NAME application.
Intraperitoneal application of l-NAME led to a significant decrease of NO after 30 min. The mean baseline value as measured by the standard method was 9.87 ± 1.87 ppb/h and decreased to 7.17 ± 2.29 ppb/h (P = 0.05). In the modified method baseline NO was 15.82 ± 1.29 ppb/h and decreased to 11.3 ± 2.15 ppb/h (P = 0.001).
eNOS expression.
As supported by prior findings (20, 31) our data showed eNOS mRNA upregulation in diseased vs. healthy contralateral lungs [0.603 log10 relative quantity (RQ) after P/MCT, P = 0.03]. Therapy administration led to a significant eNOS downregulation to −0.839log10 RQ (P = 0.008). Staining showed impaired eNOS expression in the endothelium of P/MCT exposed pulmonary vasculature (Fig. 4). The ratio of eNOS-positive endothelial cells to the total number of cells per vessel was significantly lower in PAH (0.99 ± 2.92%) compared with native lungs (8.32 ± 10.49%, P < 0.001) whereas %MWT was significantly higher (PAH: 23.45 ± 2.6% vs. native: 14.33 ± 1.65%, P < 0.001). These changes were even more prominent in smaller pulmonary arteries (outer diameter <350 μm, mean 166.13 ± 143.67 μm) characterized by a higher %MWT (26.65 ± 4.56%) than larger vessels (20.11 ± 3.72%, P < 0.001) and correlating with less eNOS-positive cells (r = −0.491, P < 0.001). Therapy administration did not significantly improve %MWT or eNOS ratio.
Fig. 4.
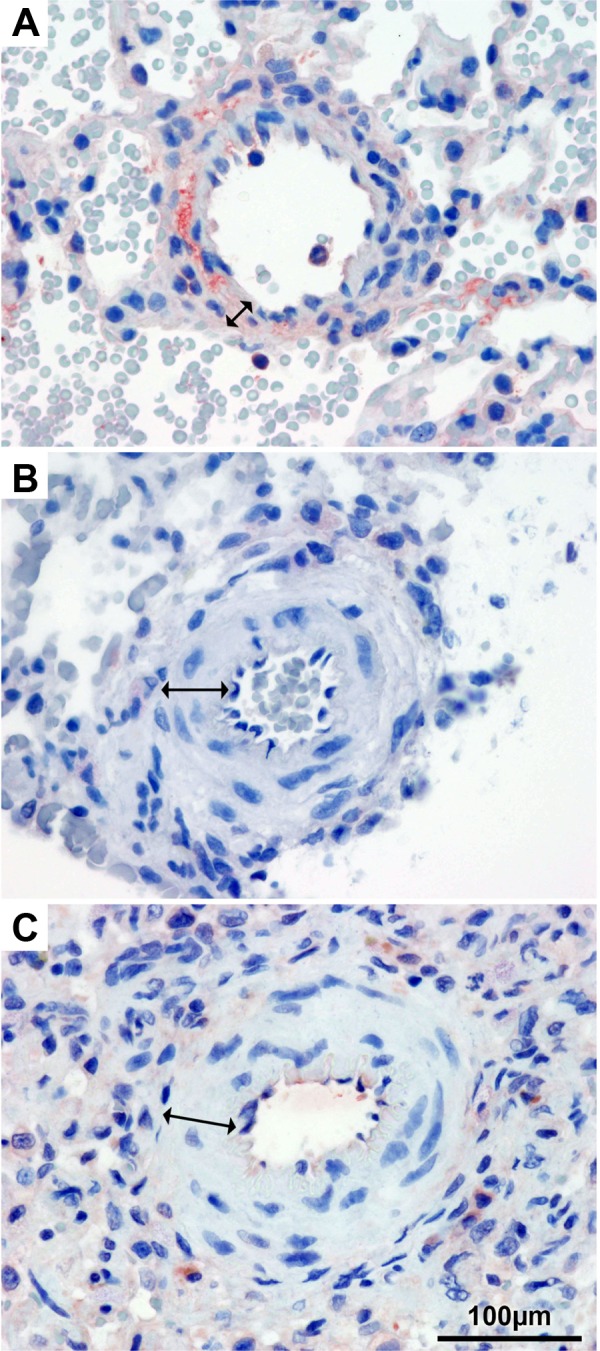
Immunohistochemistry for endothelial NO synthase. Pulmonary arteries of healthy lung tissue (A), after P/MCT (B), and after P/MCT and therapy administration (C). Arrows indicate the medial layer, which shows hypertrophy after P/MCT with and without therapy (B and C).
DISCUSSION
By mathematical modification of a standard measuring technique we developed a novel method of NO measurement in the P/MCT rat model. Because of a better correlation with hemodynamic parameters our concept seems to be promising in terms of a superior noninvasive preclinical evaluation of pathophysiology and therapy in PAH.
Since 2005 the measurement of FeNO in human subjects is defined by the ERS/ATS workshop (1). In animals and especially small rodents these guidelines cannot be adapted, because of the high mortality of invasive ventilation in pulmonary hypertensive rats.
We chose an optimal setup of NO-free air for flushing the animal chamber, followed by a short accumulation time paired with a withdrawal of a small volume of gas and a long acquisition phase. We excluded the first 30 s of recordings to eliminate the bias of dead volume and evened out oscillations in measurement to receive a linear calculated slope predicting the accumulation of NO. This calculation allows a standardization of the different accumulation times and setups used in other publications [e.g., a NO concentration of ∼5 ppb after 20 min of accumulation (6), compared with 5.38 ppb when using our standard measurement in the same setup]. After applying our modification a value of 7.07 ppb would be read out. This result suggests that measurement inaccuracy and error can be compensated by mathematical modification only.
A decreased NO production has been proposed to be an important prognostic factor in the course of PAH (16, 23). In accordance, our study also found an inverse correlation between FeNO and mPAP with the new modified technique. However, the overall NO increased after P/MCT. This might represent an unspecific surrogate marker for the inflammatory response to MCT application as seen in asthma (10, 34). In our rat study low NO values are associated with high pulmonary pressures, so these findings might predict changes in pulmonary hemodynamics also in the P/MCT model. The observed differences in correlation coefficients (r) are explained by a distortion caused by the experimental setting. At comparable correlations, differences in scattering of measured variables may be responsible for this phenomenon. At baseline (before any manipulation), pulmonary pressures in study animals are homogenous with low scattering. After therapy, individual mPAPs display a more heterogeneous distribution. As a reflection of these differences in scattering, correlation coefficients increased from −0.366 at baseline to −0.657 after therapy.
As previously described MCT has deleterious effects on pulmonary vasculature (24), which is reflected by a low number of eNOS-positive cells (11) and by the increasing media thickness of affected arteries (26). In our model, %MWT increased after P/MCT application, proving that PAH could be effectively established. After therapy with the NO substrate l-arginine and the cofactor BH4, NO levels rose and consequently eNOS mRNA was downregulated. No reduction of %MWT was observed, suggesting that the therapeutic effect is predominantly because of NO-induced vasodilation and to a lesser extent to vascular remodeling.
In humans, orally administered l-arginine led to a significant increase of NO concentration in exhaled breath (13). A single dose of BH4 significantly increased FeNO in hypoxic and normoxic rats (14). In line with these observations, a combination of both led to a slightly higher NO concentration in our animal model.
Study limitations.
Animal studies might not fully represent the pathology in humans. However, our data correspond to results found in PAH patients, so that the conclusions seem to be transferable to human disease.
Our modification itself has not been independently verified. Nevertheless our simple and reproducible FeNO measurement data correlated better with mPAP values than the standard measurements. Moreover, we were able to prove this methodological superiority by evaluation in a model of eNOS inhibition by l-NAME. Introduction of a new diagnostic technique requires both positive and negative predictive values. Defining a cutoff value for determination of true positive and negative calls would result in loss of information compared with simple correlations of continuous variables. Therefore a higher case number would be required for valid results. In more extended experiments we subjected 11 additional animals to combined therapy (data not shown). We defined therapeutic success as reduction of mPAP below a cutoff value of 30 mmHg. In this setting, we calculated a positive predictive value of 88.9% [95% confidence interval (CI) 67.2–96.9%] and a negative predictive value of 18.8% (95% CI 6.6–43.0%). This yields a large CI, which can only be narrowed by increasing the number of animals. We calculated that for a clinically acceptable CI of ∼10% an ethically unjustifiably high total number of 200 animals would be necessary.
Another shortcoming is the lack of a treatment group, which demonstrably improved PAH. A number of therapeutic strategies have been used that yield better resolution of MCT-induced PAH (3). By reviewing the literature on the efficacy of drugs licensed for the treatment of human PAH we found that the treatment effects of most substances in the rat MCT model were comparable to the mean reduction of mPAP in our study [e.g., mean reduction of mPAP ∼10 mmHg with either bosentan or sildenafil vs. 6 mmHg in our setting (8)]. Even if a few more rats had reached the cutoff of 30 mmHg, the total number of animals would only be marginally reduced. In addition, our model of PAH rather reflects advanced disease since it combines MCT application with unilateral pneumonectomy. It is unknown whether the substances referenced above (8) would achieve the same effect in this more severe stage of disease.
Conclusion.
We successfully established an NO measurement method that led to valid and reproducible data in a rat model of PAH. This new technique is based on mathematical modification of an established NO measuring procedure and leads to more reliable data by diminishing measuring errors. An inverse correlation between FeNO and mPAP before and after P/MCT application and after a combined l-arginine and BH4 administration was shown. This relation can be used to noninvasively investigate the course of P/MCT-induced PAH and response to therapy in rats.
GRANTS
The study was supported by two grants of the FWF Austrian Science Fund L 513-B11 and KLI246 to D. Bonderman.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S., C.S., and D.B. conception and design of research; M.S., C.S., A.P., M.-P.W., and H.B. performed experiments; M.S. and C.S. analyzed data; M.S., C.S., P.W., and D.B. interpreted results of experiments; M.S. and J.J. prepared figures; M.S. drafted manuscript; M.S., P.W., and D.B. edited and revised manuscript; M.S., C.S., A.P., M.-P.W., H.B., J.J., J.M., I.M.L., P.W., and D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the keepers of the Institute of Biomedical Research, Vienna, for the excellent care of our rats. Furthermore, we wish to thank Herbert Strobl, MEng, for support concerning all technical matters, and Patricia Sabanas, MD, for proofreading the manuscript.
REFERENCES
- 1.American Thoracic Society; European Respiratory Society ATS/ERS recommendations for standardized procedures for the online, and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 171: 912–930, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ahmad T, Mabalirajan U, Joseph DA, Makhija L, Singh VP, Ghosh B, Agrawal A. Exhaled nitric oxide estimation by a simple and efficient noninvasive technique and its utility as a marker of airway inflammation in mice. J Appl Physiol 107: 295–301, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, Zamel N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 138: 682–692, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bernareggi M, Rossoni G, Clini E, Pasini E, Bachetti T, Cremona G, Ambrosino N, Berti F. Detection of nitric oxide in exhaled air of different animal species using a clinical chemiluminescence analyser. Pharmacol Res 39: 221–224, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Billaud M, Dahan D, Marthan R, Savineau JP, Guibert C. Role of the gap junctions in the contractile response to agonists in pulmonary artery from two rat models of pulmonary hypertension. Respir Res 12: 30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birrell MA, McCluskie K, Hardaker E, Knowles R, Belvisi MG. Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model. Eur Respir J 28: 1236–1244, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bonderman D, Jakowitsch J, Redwan B, Bergmeister H, Renner MK, Panzenbock H, Adlbrecht C, Georgopoulos A, Klepetko W, Kneussl M, Lang IM. Role for staphylococci in misguided thrombus resolution of chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol 28: 678–684, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med 231: 967–973, 2006 [PubMed] [Google Scholar]
- 9.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184: 602–615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerard P, Rakotoniaina Z, Goirand F, Rochette L, Dumas M, Lirussi F, Bardou M. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol 373: 401–414, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kharitonov SA, Lubec G, Lubec B, Hjelm M, Barnes PJ. l-Arginine increases exhaled nitric oxide in normal human subjects. Clin Sci (Lond) 88: 135–139, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Koubsky K, Durisova J, Mikova D, Herget J. Chronic hypoxia inhibits tetrahydrobiopterin-induced NO production in rat lungs. Respir Physiol Neurobiol 185: 547–552, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Lan CC, Chang CY, Peng CK, Wu CP, Huang KL, Lee SC, Chang H. Effect of body positions on hemodynamics and gas exchange in anesthetized pigs shortly after pneumonectomy. Shock 34: 482–487, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Machado RF, Londhe Nerkar MV, Dweik RA, Hammel J, Janocha A, Pyle J, Laskowski D, Jennings C, Arroliga AC, Erzurum SC. Nitric oxide and pulmonary arterial pressures in pulmonary hypertension. Free Radic Biol Med 37: 1010–1017, 2004 [DOI] [PubMed] [Google Scholar]
- 17.McCluskie K, Birrell MA, Wong S, Belvisi MG. Nitric oxide as a noninvasive biomarker of lipopolysaccharide-induced airway inflammation: possible role in lung neutrophilia. J Pharmacol Exp Ther 311: 625–633, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Muntau AC, Roschinger W, Habich M, Demmelmair H, Hoffmann B, Sommerhoff CP, Roscher AA. Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N Engl J Med 347: 2122–2132, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi N. 2009 ESC/ERS pulmonary hypertension guidelines and connective tissue disease. Allergol Int 60: 419–424, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Nakazawa H, Hori M, Ozaki H, Karaki H. Mechanisms underlying the impairment of endothelium-dependent relaxation in the pulmonary artery of monocrotaline-induced pulmonary hypertensive rats. Br J Pharmacol 128: 1098–1104, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes H, Lebrec D, Mazmanian M, Capron F, Heller J, Tazi KA, Zerbib E, Dulmet E, Moreau R, Dinh-Xuan AT, Simonneau G, Herve P. Role of nitric oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med 164: 879–885, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 151: 1019–1025, 1997 [PMC free article] [PubMed] [Google Scholar]
- 23.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung 179: 233–243, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Rakotoniaina Z, Guerard P, Lirussi F, Goirand F, Rochette L, Dumas M, Bardou M. The protective effect of HMG-CoA reductase inhibitors against monocrotaline-induced pulmonary hypertension in the rat might not be a class effect: comparison of pravastatin and atorvastatin. Naunyn Schmiedebergs Arch Pharmacol 374: 195–206, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Sahara M, Sata M, Morita T, Hirata Y, Nagai R. Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. PLoS One 7: e33367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation 115: 509–517, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Schreiber C. Modification of an Established Rodent Model for Pulmonary Arterial Hypertension (diploma thesis) Vienna, Austria: Medical University of Vienna, 2012 [Google Scholar]
- 28.Scott GS, Bolton C. l-arginine modifies free radical production and the development of experimental allergic encephalomyelitis. Inflamm Res 49: 720–726, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Sengupta P. A scientific review of age determination for a laboratory rat: how old is it in comparison with human age? Biomed Int 2(2), 2011 [Google Scholar]
- 30.Steudel W, Kirmse M, Weimann J, Ullrich R, Hromi J, Zapol WM. Exhaled nitric oxide production by nitric oxide synthase-deficient mice. Am J Respir Crit Care Med 162: 1262–1267, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 276: L297–L303, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Weicker S, Karachi TA, Scott JA, McCormack DG, Mehta S. Noninvasive measurement of exhaled nitric oxide in a spontaneously breathing mouse. Am J Respir Crit Care Med 163: 1113–1116, 2001 [DOI] [PubMed] [Google Scholar]
- 33.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L583–L590, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Wilson DW, Segall HJ, Pan LC, Dunston SK. Progressive inflammatory and structural changes in the pulmonary vasculature of monocrotaline-treated rats. Microvasc Res 38: 57–80, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Zuckerbraun BS, George P, Gladwin MT. Nitrite in pulmonary arterial hypertension: therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovasc Res 89: 542–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]