Abstract
Quantitative trait locus (QTL) mapping is a powerful method to find modifier loci that influence disease risk and progression without prior knowledge of underlying genetic mechanisms. The aim of this study is to identify gene loci that contribute to individual differences in liver fibrosis following chronic liver damage. For this purpose, we carried out a mapping study across a panel of 21 BXD recombinant inbred strains using primary hepatocytes challenged with transforming growth factor (TGF)-β for 48 h. We identified a 6 Mb interval on chromosome 11 that is a major modifier of TGF-β-induced hepatocyte injury. Corresponding in vivo genetic analysis of fibrosis after chronic hepatotoxic injury by carbon tetrachloride (CCl4 ip for 6 wk) highlighted the same locus. Expression QTL (eQTL) analysis in liver tissues in the BXD family identified six polymorphisms in this region that are associated with strong cis eQTLs and that correlate well with gene expression in liver after both 6 wk CCl4 treatment and acute ethanol damage of the liver. Within this interval we rank two genes containing coding sequence variants as strong candidates that may modulate the severity of liver fibrosis: 1) the extracellular proteinase inhibitor gene Expi (also known as Wdnm1 or Wfdc18) and 2) musashi RNA-binding protein 2 (Msi2). The powerful combination of experimental, genetics, and bioinformatics methods, as well as combined in vitro and in vivo approaches can be used to define QTLs, genes, and even candidate sequence variants linked to hepatotoxicity and fibrosis.
Keywords: hepatocellular damage, fibrogenesis, murine reference population, quantitative trait locus, recombinant inbred line, systems genetics
progressive fibrosis represents the end stage of any chronic liver injury of viral, toxic, or dietary origin. The worldwide incidence of fibrotic liver disease is increasing, mainly due to metabolic overload and alcohol abuse (42). Only a subset of affected patients develop advanced fibrosis (10). Age, sex, and environmental factors are known contributors (29), but genetic predisposition has been shown to play a role in disease pathogenesis and progression (17). The genetics of liver fibrosis is highly complex, because of the variety of hepatic cell types involved and their interaction during the activation, regulation, and termination of hepatic wound healing responses (3, 11, 34). Hepatic Kupffer and stellate cells represent the major source of proinflammatory mediators and extracellular matrix proteins, respectively, which are secreted in response to injury of hepatocytes, the predominant liver parenchymal cells. On the basis of the concept that transforming growth factor (TGF)-β represents one of the key profibrogenic cytokines in liver (7, 13), we aimed to identify genetic loci conveying differential susceptibility of hepatocytes to TGF-β-mediated damage in vitro. We hypothesized that this reduction of complexity would allow us to identify genetic factors that not only contribute to the susceptibility of a specific cellular subpopulation in vitro but are also relevant in vivo. Hepatocytes account for two-thirds of the total number of liver cells and about four-fifths of total liver volume (21), and they are the primary targets in most etiologies of liver damage.
For this study, we exploited a reference panel of recombinant inbred mouse lines derived by sib mating progeny of an intercross between C57BL/6J and DBA/2J parental strains (1). This so-called BXD family of inbred lines, of which there are now nearly 160 members, has been studied for several decades. One of their main experimental advantages is that identical sets of genotypes can be tested under many different conditions by many research groups and used to identify networks of quantitative trait loci (QTLs) and sequence variants that cause differences in phenotypes (31, 35). These strains have already been thoroughly genotyped and are ideal for long-term collaborative research.
In this study we isolated and cultivated primary hepatocytes from a subset of the BXD lines and challenged these cells with TGF-β. The aim of this in vitro component of our study was to limit the complexity of interaction to a single prominent signaling molecule, TGF-β, and a single cell type, hepatocytes. A second goal is to test whether increased susceptibility to TGF-β-induced cell death in vitro represents a suitable model of chronic in vivo liver injury. Dying hepatocytes release various proinflammatory cytokines, and this in turn activates stellate and Kupffer cells, which release cytokines and chemokines. Genetic difference in hepatocyte susceptibility is likely to be a major factor that explains differences in response to chronic liver injury among humans.
To compare in vitro with in vivo data, we exploited two large datasets of fibrosis-associated phenotypes. We also analyzed experimental whole liver transcriptome datasets to assess the impact of different modes of damage on gene expression This has enabled us to systematically screen candidate genes within our in vitro and in vivo QTL (4, 6, 33). We mapped variation in mRNA levels in the fibrosis liver expression data sets by standard QTL mapping methods (5, 9). A strong correlation between differences in mRNA level and differences in alleles at a genetic marker is referred to as an expression QTL (eQTL). We hypothesized that a detectable QTL will be result in considerable changes in the transcriptome caused by genetic differences in the respective area altering expression levels in cis or trans. Therefore we searched liver expression datasets following damage of two different etiologies [ethanol and carbon tetrachloride (CCl4)] for genes that were impacted by sequence variants in the common “in vitro hepatocyte damage” and “in vivo fibrogenesis” candidate region. By combining these different sets of results, we were able to identify genetic loci that modify the liver cells' response to intoxication by virtue of genetic variation.
METHODS
Inbred mouse and murine reference lines.
BXD lines were obtained from The Jackson Laboratory (http://www.jax.org) or from Oak Ridge National Laboratory (lines BXD43, BXD51, BXD61, BXD73) and were bred in the facility of the Neurobsik consortium from the Vrije Universiteit (Amsterdam, the Netherlands). Parental lines C57BL/6J and DBA/2J were purchased from local suppliers (Charles River Germany and Janvier Labs Europe). Mice were maintained in a vivarium under temperature-controlled conditions (22 ± 1°C) and treated in accordance with local guidelines as approved by the Saarland University Committee for care and use of animals and the local county regulatory authority “Landesamt für Verbraucherschutz, Abteilung H Lebensmittel- und Veterinärwesen.”
Isolation of primary mouse hepatocytes and in vitro analysis of hepatocellular damage by TGF-β.
Hepatocytes were isolated by a two-step collagenase perfusion method, following the standard operation procedures of the Virtual Liver Network (12, 18, 24) from animals aged between 12 and 16 wk. We assayed 21 BXD lines and performed a minimum of three preparations per sex and per BXD line. After purification, cells were plated in collagen-coated six-well culture plates (150,000 cells per well) and allowed to adhere for 4 h in Williams E medium (Sigma-Aldrich, St. Louis, MO) containing 10% (vol/vol) fetal bovine serum (PAA, Cölbe, Germany). After 4 h, the medium was changed to Williams E medium without supplementation, and cells were serum-starved for 18 h. Afterwards, the cell culture medium was replaced with Williams E medium containing 5 ng/ml recombinant TGF-β (PreproTech, Hamburg, Germany) for 48 h. This concentration was based on previous dynamic cell culture experiments investigating TGF-β-mediated signal transduction in AML12 hepatocytes (39). Control wells were grown in fresh, serum-free Williams E medium. After 48 h, culture supernatants were collected, and adherent cells were lysed in 2% (vol/vol) Triton X-100 (Sigma-Aldrich).
We assayed hepatocellular damage by measuring lactate dehydrogenase (LDH) in the supernatant and from adherent cells following lysis in 2% Triton X-100 spectrometrically with a colorimetric assay as suggested by the manufacturer (Roche, Basel, Switzerland). We measured total cell damage by calculating the relative amount of LDH released into the cell culture supernatant as a percentage of the total LDH from the adherent, intact cells plus the supernatant:
The difference in LDH release between TGF-β-treated cells and untreated cells, expressed as a percentage of total LDH in untreated cells, was used as trait for QTL mapping:
Trait values were uploaded into the “BXD Published Phenotypes Database” of GeneNetwork, (http://www.genenetwork.org), trait number 16231.
QTL analysis of TGF-β-induced cellular damage in vitro.
Normal distribution of total trait data and within each BXD line was analyzed by Kolmogorov-Smirnov tests in SPSS v21.0 (IBM, Ehningen, Germany). For QTL analyses, median ΔLDH values were used as trait. Within-strain and between-strain variances were calculated with analysis of variance (36). Heritability (h2) was estimated with the intraclass correlation coefficient (27).
QTLs were identified by interval mapping: effects of hypothetical QTLs between tested markers were estimated from the genotypes at the markers that flank the interval (14). This analysis provided information on the most likely chromosomal localization of the QTL relative to the associated marker. For composite interval mapping, a single genetic marker representing an identified QTL with the same strain conferring the susceptible allele or an interacting locus from pairwise interaction scans was included as covariate. This methodology increases the power to identify QTLs in complex phenotypes by removing the effect of the pre-eminent or interacting QTL (25, 26). The significance of each association was expressed as likelihood ratio statistic (LRS) (14). LRS values can be converted to LOD (logarithm of the odds) scores by division by 4.61. To estimate the empirical significance threshold of QTL peaks, we mapped 2,000 or more permutations of each trait.
In vivo analysis of fibrogenesis following CCl4 damage.
Our in vivo model of liver damage was the well-established chronic challenge with CCl4 (15, 38). We induced chronic liver damage in 33 BXD lines by 12 intraperitoneal injections (0.7 mg CCl4/kg body wt twice weekly) over 6 wk and measured fibrosis-related phenotypes. Hepatic collagen contents were quantified with a biochemical assay of hydroxyproline in liver hydrolysates (19). Sirius red-stained collagen areas and semiquantitative fibrosis stages (F0–F4) were determined in paraffin sections from the livers. Trait values were uploaded into the “BXD Published Phenotypes Database” of GeneNetwork, trait number 14383. Furthermore, we generated hepatic expression data in livers from females of all 33 BXD lines, using the Affymetrix Mouse Gene 1.0 expression arrays as described by Hall RA, Weber SN, Lammert F (unpublished observations). The expression dataset was uploaded into GeneNetwork under the description SUH_BXD_Liver_Affy_Mouse_Gene_1.0_ST (Jun11)_RMA.
eQTL analysis of susceptibility genes.
We exploited whole liver expression data generated using tissue from cases (4 per sex and per strain) treated with a single injection of 6 g/kg ip ethanol and allowed to survive for 24 h (data provided courtesy of Dr. Robert Rooney, Genome Explorations). These data were generated with the Affymetrix MOE 430.2 array. We also exploited whole liver expression data generated by our own group. We treated 33 BXD strains with CCl4. To identify eQTLs that aligned with the main TGF-β-associated QTL we used the search term “LRS=(15 999 Chr11 83 95)” in GeneNetwork (37). We included a broader interval to account for a QTL peak in vivo (LRS = 12) around 92 Mb (see Fig. 3). This search retrieves genes from the BXD database that are correlated with genetic markers positioned between 83 and 95 Mb on chromosome 11 at an LRS ≥ 15. The majority of these loci are cis eQTLs with cognate genes that are themselves located within this interval. For all markers showing strong eQTLs we searched for coding variants differing between C57BL/6J and DBA/2J.
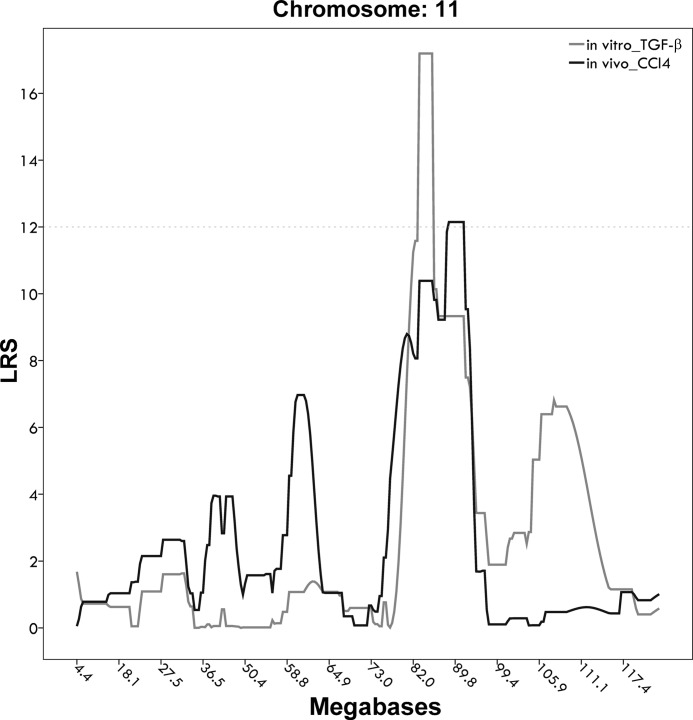
Fig. 3.
Superimposed quantitative trait locus peaks for in vivo fibrosis stages following carbon tetrachloride (CCl4) challenge and in vitro LDH increase following TGF-β treatment. The x-axis shows the megabase position on mouse chromosome 11; the y-axis shows LRS values for the respective traits. Hepatocyte susceptibility to TGF-β-mediated damage is shown in light gray; F-score following CCl4 injection is shown in dark gray.
Gene expression network analysis.
Coexpression between genes was analyzed by Pearson's correlation coefficients. To illustrate networks of genes that share common genetic control, we display results in the form of graphs with correlation strengths represented by edges between transcripts, as implemented in GeneNetwork.
RESULTS
Variation and QTL analysis of TGF-β-induced hepatocellular damage in vitro.
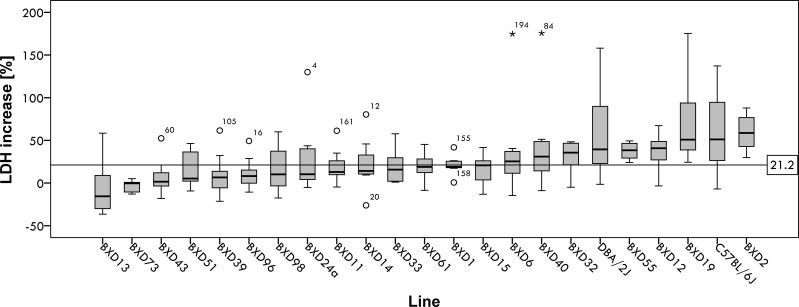
Figure 1 shows hepatocellular damage after 48 h TGF-β challenge in vitro, expressed as relative difference of LDH activity in tissue culture supernatant between TGF-β-treated cells and untreated control cells. The trait shows marked differences between BXD reference lines. Table 1 summarizes the individual trait values, with a trait value of zero defined as no difference in LDH release between hepatocytes treated with 5 ng/ml TGF-β and those maintained in serum-free E medium. The average increase of LDH release by TGF-β is 21.2%, as indicated by the horizontal line in Fig. 1. It is noteworthy that a few lines yielded a negative trait value, indicating decreased cell death in TGF-β-treated samples compared with cells maintained in serum-free Williams medium. For hepatocytes from these BXD lines, TGF-β appears to exert a protective effect in vitro. h2 of the cellular trait in the total population was rather low at 0.22. When separated by sex, h2 in male mice was 0.46 but only 0.16 in female mice, indicating that a substantial amount of within-strain variation is due to sex-specific differences such as hormonal status variation (Table 2).
Fig. 1.
Distribution of hepatocellular damage increase in vitro [percentage increase in lactate dehydrogenase (LDH) release between untreated hepatocytes and transforming growth factor (TGF)-β-treated hepatocytes] in response to TGF-β in all BXD lines assayed (∑ = 199, medians ± 95% confidence interval), in ascending order. The horizontal line at 21.2% shows the mean increase for all samples assayed. Boxes represent the interquartile range (IQR = 25th percentile to the 75th percentile); bold horizontal line represents the median value. Whiskers or inner fences represent 1.5 times the IQR or, if no case has a value in that range, to the minimum or maximum values. If the data are distributed normally, ∼95% of the data are expected to lie between the inner fences. Circular dots represent outliers which differ from the mean by >1.5 times the IQR. *Extreme outliers that differ from the mean by >3 times the IQR.
Table 1.
Hepatocellular damage in vitro by 48 h TGF-β in 21 BXD lines and parental strains
| Group | n | Mean | Median | SD | SE |
|---|---|---|---|---|---|
| BXD1 | 7 | 21.24 | 19.01 | 12.30 | 4.65 |
| BXD2 | 8 | 59.28 | 58.64 | 20.43 | 7.22 |
| BXD6 | 8 | 38.52 | 25.44 | 57.62 | 20.37 |
| BXD11 | 8 | 19.29 | 12.99 | 20.19 | 7.14 |
| BXD12 | 8 | 37.16 | 40.86 | 22.06 | 7.80 |
| BXD13 | 9 | −4.41 | −15.55 | 33.12 | 11.04 |
| BXD14 | 7 | 22.20 | 14.12 | 33.21 | 12.55 |
| BXD15 | 8 | 16.21 | 20.75 | 17.83 | 6.30 |
| BXD19 | 8 | 70.82 | 50.84 | 50.04 | 17.69 |
| BXD24a | 9 | 29.06 | 10.26 | 41.76 | 13.92 |
| BXD32 | 9 | 29.87 | 35.59 | 18.42 | 6.14 |
| BXD33 | 9 | 19.29 | 15.71 | 19.40 | 6.47 |
| BXD39 | 12 | 7.65 | 6.63 | 22.34 | 6.45 |
| BXD40 | 13 | 38.12 | 31.04 | 45.27 | 12.56 |
| BXD43 | 10 | 6.88 | 1.64 | 19.21 | 6.07 |
| BXD51 | 7 | 17.09 | 5.24 | 22.11 | 8.36 |
| BXD55 | 8 | 37.71 | 38.42 | 9.64 | 3.41 |
| BXD61 | 9 | 19.87 | 18.98 | 17.21 | 5.74 |
| BXD73 | 6 | −3.03 | −0.32 | 7.09 | 2.89 |
| BXD96 | 9 | 10.60 | 8.22 | 18.52 | 6.17 |
| BXD98 | 8 | 16.39 | 10.22 | 28.75 | 10.16 |
| C57BL/6J | 12 | 58.70 | 51.19 | 44.91 | 12.97 |
| DBA/2J | 7 | 60.19 | 39.50 | 55.53 | 20.99 |
TGF, transforming growth factor.
Table 2.
Hepatocellular damage in vitro by 48 h TGF-β in 21 BXD lines and parental strains for both sexes
| Group | n Female | n Male | Mean Female | Mean Male | Median Female | Median Male | SD Female | SD Male | SE Female | SE Male |
|---|---|---|---|---|---|---|---|---|---|---|
| BXD1 | 3 | 4 | 28.47 | 15.81 | 24.85 | 18.18 | 11.96 | 10.76 | 6.90 | 5.38 |
| BXD2 | 4 | 4 | 44.88 | 73.68 | 42.87 | 76.70 | 14.11 | 14.90 | 7.06 | 7.45 |
| BXD6 | 4 | 4 | 23.96 | 53.08 | 25.44 | 26.09 | 9.72 | 84.18 | 4.86 | 42.09 |
| BXD11 | 4 | 4 | 17.91 | 20.67 | 13.94 | 12.99 | 11.96 | 28.33 | 5.98 | 14.17 |
| BXD12 | 4 | 4 | 33.98 | 40.34 | 39.30 | 48.74 | 12.24 | 30.96 | 6.12 | 15.48 |
| BXD13 | 4 | 5 | 25.19 | −28.09 | 21.70 | −29.98 | 26.75 | 8.92 | 13.38 | 3.99 |
| BXD14 | 4 | 3 | 3.76 | 46.78 | 10.60 | 45.84 | 20.41 | 33.14 | 10.20 | 19.13 |
| BXD15 | 4 | 4 | 22.33 | 10.09 | 26.10 | 14.68 | 19.40 | 16.29 | 9.70 | 8.14 |
| BXD19 | 4 | 4 | 79.35 | 62.29 | 50.84 | 57.21 | 64.16 | 39.15 | 32.08 | 19.58 |
| BXD24a | 5 | 4 | 36.60 | 19.64 | 10.26 | 19.11 | 55.36 | 18.71 | 24.76 | 9.36 |
| BXD32 | 4 | 5 | 29.06 | 30.52 | 28.48 | 39.64 | 16.37 | 21.83 | 8.18 | 9.76 |
| BXD33 | 5 | 4 | 10.88 | 29.81 | 6.06 | 29.66 | 11.99 | 23.39 | 5.36 | 11.69 |
| BXD39 | 5 | 7 | 17.71 | 0.46 | 9.58 | −3.67 | 25.24 | 18.59 | 11.29 | 7.02 |
| BXD40 | 6 | 7 | 51.93 | 26.28 | 32.61 | 31.04 | 62.78 | 21.45 | 25.63 | 8.11 |
| BXD43 | 5 | 5 | 4.86 | 8.90 | −3.44 | 7.99 | 27.41 | 8.27 | 12.26 | 3.70 |
| BXD51 | 4 | 3 | 9.14 | 27.68 | 2.27 | 31.39 | 22.18 | 20.83 | 11.09 | 12.03 |
| BXD55 | 4 | 4 | 36.56 | 38.87 | 38.42 | 39.56 | 9.24 | 11.31 | 4.62 | 5.65 |
| BXD61 | 3 | 6 | 27.15 | 16.23 | 28.18 | 18.88 | 14.40 | 18.53 | 8.31 | 7.56 |
| BXD73 | 3 | 3 | −7.55 | 1.49 | −10.53 | 0.46 | 7.34 | 3.23 | 4.24 | 1.87 |
| BXD96 | 5 | 4 | 18.69 | 0.48 | 15.24 | 2.08 | 20.86 | 9.42 | 9.33 | 4.71 |
| BXD98 | 3 | 5 | 43.54 | 0.09 | 58.80 | 2.75 | 27.50 | 13.55 | 15.88 | 6.06 |
| C57BL/6J | 6 | 6 | 76.92 | 40.48 | 81.88 | 42.63 | 54.88 | 25.10 | 22.40 | 10.25 |
| DBA/2J | 6 | 1 | 43.88 | 158.03 | 35.73 | 158.03 | 38.29 | 15.63 |
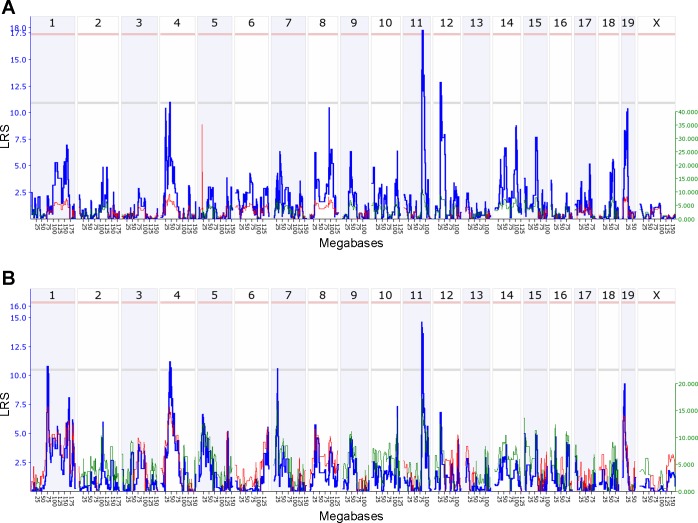
Interval mapping of TGF-β-induced damage using trait values all 21 BXD lines revealed that a 6 Mb locus on mouse chromosome 11 (rs13481128@83.5 Mb–rs3659504@89.5 Mb) was associated with hepatocellular damage, with two further suggestive loci on chromosomes 4 (LRS = 13.0) and 5 (LRS = 12.5) modulating the trait. When we accounted for the effect of the chromosome 5 locus (rs13478133) by composite interval mapping (see methods), chromosome 11 emerged as the most significant driver of the trait. The LRS of 18.0 indicated significant linkage at the genome-wide level (Fig. 2A), as determined by permutation tests. The restriction of QTL analysis to male BXD lines resulted also in the detection of the susceptibility locus on chromosome 11 (LRS = 14.0, Fig. 2B). Interval mapping of trait values from female mice resulted in the detection of a single suggestive linkage peak on chromosome 5 (LRS = 13.0, data not shown).
Fig. 2.
Genome-wide composite interval mapping of hepatocellular damage after TGF-β treatment. A: composite interval mapping of trait values from both sexes. B: mapping of trait values from male mice only. The bold blue line shows the likelihood ratio statistics (LRS) for association between the trait and the markers tested for every interval of the mouse genome assayed. The scale for LRS values is given at left in blue numbers. Empiric significance thresholds determined by 2,000 random permutations are indicated in the genome-wide association plots by a gray horizontal line for the suggestive and a red horizontal line for the significant linkage thresholds. Thin green and red lines indicate effect sizes for every locus of the mouse genome as percentage of the total trait variation explained by the locus assayed. The red line indicates that C57BL/6J alleles increase trait values; the green line indicates that DBA/2J alleles increase trait values. The scale for effect size values is given in green numbers at right.
QTL analysis of experimental fibrogenesis in vivo.
Composite interval mapping of histopathological fibrosis stages in 33 BXD lines during chronic liver injury in vivo resulted in two significant QTLs on chromosomes 7 (rs3703247@48.2–rs8255275@53.7) and chr 17 (rs13483077@64.9–rs13483081@71.1). When we controlled for the effect of gnf06.037.785 at 40.7 Mb on chromosome 6, composite interval mapping revealed involvement of the same region of chromosome 11 as implicated in vitro (Fig. 3). The former had been identified as a strong interacting locus by a genome wide pairwise interaction scan. Here, the LRS of 12.1 (using mean fibrosis stages in males) is suggestive but not significant, which is not unexpected in view of the complex nature of fibrogenesis in vivo. Direct comparison between both phenotypes showed a moderate correlation between hepatocyte damage following 48 h TGF-β treatment in vitro and F-score following 6 wk of CCl4 injections in vivo (r = 0.36, P = 0.11).
eQTL analysis of ethanol- and CCl4-damaged liver tissue.
We searched expression databases for markers inside the candidate region (83–95 Mb) that are significantly correlated with gene expression under conditions of hepatocellular stress. Six markers at 83.5, 87.7, 88.5, 88.9, 90.2, and 90.7 Mb, respectively, were detected that exerted a substantial impact on the expression of other genes. These markers accounted for all eQTLs with LRS > 15.0 in short-term ethanol-treated livers (Table 3). The SNP at 83.5 Mb represents the main eQTL locus within the candidate region associated with the regulation of gene expression, accounting for 15 genes from a total of 43 genes associated at LRS > 15.0. The other five loci at 87.7, 88.5, 88.9, 90.2, and 90.7 Mb were associated with the expression of 11, eight, four, two, and three genes, respectively (Table 3).
Table 3.
eQTLs with LRS > 15.0 from the chromosome 11 QTL at 83–95 Mb in ethanol- and CCl4-damaged livers in vivo ranked by chromosomal location (proximal to distal) and LRS
| eQTL Location | LRS | Gene Symbol | Description | Gene Location |
|---|---|---|---|---|
| Etiology: Ethanol | ||||
| 83.521935 | 57.8 | Dhx40 | DEAH (Asp-Glu-Ala-His) box polypeptide 40; last two exons and 3′-UTR | 86.6 |
| 83.521935 | 47.2 | Ggnbp2 | gametogenetin binding protein 2 | 84.7 |
| 83.521935 | 42.4 | BC022224 | cDNA sequence BC022224 | 84.6 |
| 83.521935 | 38.3 | Acac | acetyl-Coenzyme A acyltransferase 2 (mitochondrial 3-oxoacyl-Coenzyme A thiolase) | 84.1 |
| 83.521935 | 36.8 | Rps6kb1 | ribosomal protein S6 kinase, polypeptide 1; last three exons and proximal 3′-UTR | 86.3 |
| 83.521935 | 32.7 | 2700008B19Rik | RIKEN cDNA 2700008B19 gene; distal end of last exon and proximal 3′-UTR | 83.6 |
| 83.521935 | 30.5 | Acac | acetyl-Coenzyme A acyltransferase 2 (mitochondrial 3-oxoacyl-Coenzyme A thiolase) | 84.1 |
| 83.521935 | 28.5 | Acac | acetyl-Coenzyme A acyltransferase 2 (mitochondrial 3-oxoacyl-Coenzyme A thiolase); distal 3′-UTR | 84.2 |
| 83.521935 | 25.4 | Cltc | clathrin, heavy polypeptide (Hc); mid-3′-UTR | 86.5 |
| 83.521935 | 25.1 | Bcas3 | breast carcinoma amplified sequence 3 | 85.4 |
| 83.521935 | 20.5 | Aatf | apoptosis antagonizing transcription factor; exons 4, 5, 7, 8 and 9 | 84.2 |
| 83.521935 | 16.9 | Pigx | phosphatidylinositol glycan, class X | Chr16: 32.1 |
| 83.521935 | 16.0 | Acat1 | acetyl-Coenzyme A acetyltransferase 1 | Chr9: 53.4 |
| 83.521935 | 15.8 | Ash1l | ash1 (absent, small, or homeotic)-like (Drosophila) | Chr3: 88.8 |
| 83.521935 | 15.8 | 1110054O05Rik | RIKEN cDNA 1110054O05 gene | Chr4: 59.8 |
| 87.668081 | 39.5 | Msi2 | Musashi RNA-binding protein 2; mid-3′-UTR | 88.2 |
| 87.668081 | 18.0 | Usp32 | ubiquitin specific protease 32; distal 3′-UTR (transQTL on Chr 4 in BXD eye data) | 84.8 |
| 87.668081 | 17.6 | Wdr53 | WD repeat domain 53 | Chr16: 32.3 |
| 87.668081 | 17.1 | Ldhd | lactate dehydrogenase D | Chr8: 114.2 |
| 87.668081 | 17.0 | Grm1 | glutamate receptor, metabotropic 1 (group I, PLC-IP3 coupled); last exon and proximal 3′-UTR | Chr10: 10.4 |
| 87.668081 | 16.8 | Sfrs1 | splicing factor, arginine/serine-rich 1 (ASF/SF2); distal 3′-UTR | 87.9 |
| 87.668081 | 16.6 | C130020C13Rik | RIKEN cDNA C130020C13 gene | Chr5: 34.2 |
| 87.668081 | 16.3 | Adam4 | a disintegrin and metalloprotease domain 4 | Chr12: 82.5 |
| 87.668081 | 16.2 | Mapk12 | mitogen-activated protein kinase 12 | Chr15: 89.0 |
| 87.668081 | 15.5 | Epn2 | epsin 2; middistal 3′-UTR | 61.3 |
| 87.668081 | 15.2 | Pmvk | phosphomevalonate kinase | Chr3: 89.3 |
| 88.522933 | 29.3 | 2210409E12Rik | RIKEN cDNA 2210409E12 gene | 88.8 |
| 88.522933 | 24.1 | 2700008B19Rik | RIKEN cDNA 2700008B19 gene | 83.6 |
| 88.522933 | 18.0 | Appbp2 | amyloid beta precursor protein (cytoplasmic tail) binding protein 2; far 3′-UTR | 85.1 |
| 88.522933 | 17.9 | 5730526G10Rik | RIKEN cDNA 5730526G10 gene | Chr9: 15.2 |
| 88.522933 | 17.0 | CR517518 | CR517518 EST in olfactory receptor cluster (EST associated with similarity to erythroid differentiation regulator); unknown, perhaps intron of Or1 g1 | Chr19: 11.9 |
| 88.937291 | 18.2 | Rae1 | RAE1 RNA export 1 homolog (S. pombe) | N/A |
| 88.937291 | 16.2 | Nfkbib | nuclear factor of kappa light chain gene enhancer in B-cells inhibitor, beta | Chr7: 29.5 |
| 88.937291 | 15.8 | Pnma1 | paraneoplastic antigen MA1 | Chr12: 85.5 |
| 89.612736 | 23.2 | Trygn16 | trypsinogen 16 | Chr6: 41.4 |
| 89.612736 | 17.8 | Nrbf1 | nuclear receptor binding factor 1 | Chr4: 131.4 |
| 89.612736 | 15.1 | Rabgef1 | RAB guanine nucleotide exchange factor (GEF) 1; last exon and proximal 3′-UTR | Chr5: 130.7 |
| 89.938911 | 16.0 | Bcl11a | B-cell CLL/lymphoma 11A (zinc finger protein) | 24.0 |
| 90.203179 | 23.2 | Pctp | phosphatidylcholine transfer protein; distal half of 3′-UTR | 89.8 |
| 90.203179 | 15.5 | Pctp | phosphatidylcholine transfer protein; last 3 exons and 3′-UTR | 89.9 |
| 90.664416 | 25.6 | Stxbp4 | syntaxin binding protein 4; 3′-UTR | 90.3 |
| 90.664416 | 20.1 | Gdpd1 | glycerophosphodiester phosphodiesterase domain containing 1 | 86.5 |
| 90.664416 | 17.3 | Foxo1 | forkhead box O1; last intron | Chr3: 52.1 |
| Etiology: CCl4 | ||||
| 83.521935 | 37.1 | Dhrs11 | dehydrogenase/reductase (SDR family) member 11 | 84.6 |
| 83.521935 | 26.2 | Myo19 | myosin XIX | 84.7 |
| 83.521935 | 23.9 | Affy_10358428 | Affymetrix Mouse Gene 1.0 ST probe set 10358428 | Chr1: 146.8 |
| 83.521935 | 22.2 | Affy_10502482 | Affymetrix Mouse Gene 1.0 ST probe set 10502482 | Chr3: 142.4 |
| 83.521935 | 22.0 | Appbp2 | amyloid beta precursor protein (cytoplasmic tail) binding protein 2 | 85.0 |
| 83.521935 | 21.7 | Cuedc1 | CUE domain containing 1 | 87.9 |
| 83.521935 | 18.1 | Affy_10339384 | Affymetrix Mouse Gene 1.0 ST probe set 10339384 | N/A |
| 83.521935 | 17.9 | Serpinh1 | serine (or cysteine) peptidase inhibitor, clade H, member 1 | Chr7: 106.5 |
| 83.521935 | 16.4 | Ipp | IAP promoted placental gene | Chr4: 116.2 |
| 83.521935 | 16.2 | Affy_10491484 | Affymetrix Mouse Gene 1.0 ST probe set 10491484 | Chr3: 35.6 |
| 83.521935 | 15.3 | Zfp616 | zinc finger protein 616 | 73.9 |
| 83.521935 | 15.3 | Txlng | taxilin gamma | Chr5: 105.2 |
| 88.522933 | 31.1 | Dhx40 | DEAH (Asp-Glu-Ala-His) box polypeptide 40 | 86.6 |
| 88.522933 | 17.2 | mir-465a | Affymetrix Mouse Gene 1.0 ST probe set 10604877 | ChrX: 64.1 |
| 88.522933 | 16.9 | Xlr3a | X-linked lymphocyte-regulated 3A | ChrX: 70.3 |
| 88.522933 | 16.6 | Gm15698 | predicted gene 15698 | 88.8 |
| 88.522933 | 15.7 | Affy_10466841 | Affymetrix Mouse Gene 1.0 ST probe set 10466841 | Chr19: 25.5 |
| 88.522933 | 15.3 | Saa3 | serum amyloid A 3 | Chr7: 54.0 |
| 88.522933 | 15.2 | Bsdc1 | BSD domain containing 1 | Chr4: 129.1 |
| 89.612736 | 21.3 | Pctp | phosphatidylcholine transfer protein | 89.8 |
| 89.612736 | 19.5 | Fuz | fuzzy homolog (Drosophila) | Chr7: 52.2 |
| 89.612736 | 16.5 | Gm5712 | predicted gene 5712 | Chr3: 129.1 |
eQTL, expression quantitative trait locus; LRS, likelhood ratio statistic; CCl4, carbon tetrachloride.
In CCl4-treated livers, three genomic loci at 83.5, 88.5, and 89.6 Mb accounted for the entire set of 22 eQTLs within the candidate region that were associated with the expression of genes following toxic liver damage for 6 wk at LRS > 15.0 (Table 3). The genomic marker at 83.5 Mb was associated with the expression of 12 out of 22 genes associated with loci within the candidate region at LRS > 15.0. The genomic locus around 88.5 Mb accounted for the seven associations, and a single nucleotide polymorphism (SNP) at 89.6 Mb was associated with the remaining three genes (Table 3).
These high-impact eQTLs were associated with the following markers used to test for association with expression values in BXD lines: rs13481128 at 83.5 Mb, rs3720112 at 87.7 Mb, rs3659504 at 88.5 Mb, D11Mit41 (rs6370920) at 88.9 Mb, rs13481150 at 89.6 Mb, rs13481154 at 90.2 Mb, and rs13481156 at 90.7 Mb. On the basis of our initial assumption that TGF-β is a common mediator of liver damage and fibrogenesis in the etiologies investigated, the two highest-impact eQTL loci at 83.5 and 88.5 Mb represent creedal candidate loci for causing the TGF-β-induced differential effects. The proximal locus is associated with the expression of 27 out of a total of 65 genes, and the distal SNP shows association with the expression levels of 18 out of 65 genes with LRS > 15.0 (Table 3).
Identification of susceptibility genes.
Following the delineation of two genomic loci within our candidate region that show substantial association with gene expression following liver damage, the next step was the identification of nearby amino-acid alterations that differentiate between the two parental mouse strains C57BL/6J and DBA/2J. A variant search of the 83–95 Mb region of chromosome 11 at the Jax phenome database (Sanger1 dataset) for coding/nonsynonymous and splice site variants retrieved 111 nonsynonymous amino acid differences between these strains. Focused search near expression-associated SNPs revealed a coding variant in close proximity of rs13481128 at 83.5219 Mb, the SNP in the region that showed correlation with the highest number of transcripts in ethanol- and CCl4 liver damage (Table 3). rs13462283 at 83.5234 Mb results in the amino acid substitution p.R46Q in the WAP four-disulfide core domain 18 gene Wfdc18, a.k.a. extracellular proteinase inhibitor Expi or Kalman syndrome domain gene Kal1 (http://www.informatics.jax.org/marker/MGI:107506), which is localized in 1,481 bp distance from the expression-associated SNP.
Two other coding variants are located in the wider proximity of rs3659504 at 88.5234 Mb: rs29388491 at 88.4036 Mb, resulting in the variant p.R15Q in the musashi RNA-binding protein 2 (Msi2), and rs29454667 at 88.1803 Mb, resulting in p.Q142R in Msi. The Msi2 gene, which is sometimes also called Msi2h (http://www.informatics.jax.org/marker/MGI:1923876), stretches from 88.339382 to 88.718513 Mb across almost 400 kb of genomic sequence and is oriented from the telomeric (distal) 5′-promoter area near rs3659504 toward the centromeric (proximal) 3′-end near rs13481145. Expression of the phosphatidylcholine transfer protein (Pctp) gene is correlated with the marker rs13481154 at 90.2032 Mb (LRS = 23.2) in ethanol-treated livers and with rs13481150 at 89.6127 Mb (LRS = 21.3) in CCl4-treated livers. The coding variant rs27067571 is localized between both expression associated SNPs at position 89.9911 and results in an alanine-to-threonine replacement at position 69 of the translated protein product (p.A69T).
Identification of gene expression networks.
We speculate that the associations between SNPs and gene transcription levels revealed by eQTL analysis are not an effect of the variants themselves, but more likely to be mediated by a nearby coding variant in linkage disequilibrium. If the coding variant alters a protein, expression of the allelic variants may have a knock-on effect on the transcription of various other genes affected by the resulting structural changes. In fact, the downstream affected genes can be identified and grouped into networks of co-regulation by statistical analysis of data from all BXD lines investigated for coexpression.
The resulting network graphs can serve as identifiers of biological interaction pathways (32). Of note, the coexpression analysis of genes that were associated with markers in the candidate region with LRS > 15.0 in the two models of liver damage available for eQTL analysis, 6 wk of CCl4 injections or 6 g/kg of ethanol for 24 h, revealed the DEAH (Asp-Glu-Ala-His) box polypeptide 40 (Dhx40) and the Pctp as the only common genes. All other transcripts associated with loci on chromosome 11 (83–95 Mb) in either ethanol- or CCl4-induced liver damage are different, suggesting largely divergent genetic responses to both etiologies of liver damage.
DISCUSSION
The aim of our study was to identify susceptibility genes for liver fibrogenesis by cellular phenotyping of isolated murine liver cells after challenge with the central profibrogenic stimulus TGF-β. The study design was based on the hypothesis that increased susceptibility to TGF-β-induced cell death in vitro may be a suitable model for the physiological processes underlying individual differences in response to chronic liver injury in vivo.
Here, we employed QTL mapping of TGF-β-induced cell death to identify a locus at position 83–89 Mb on mouse chromosome 11 that is significantly associated with in vitro susceptibility of hepatocytes as well as two suggestive loci on chromosomes 4 and 5. The observation that the trait “fibrosis stage in vivo” colocalizes with this QTL on chromosome 11 supports our working hypothesis and indicates that mouse strains with hepatocytes that are highly susceptible to TGF-β-mediated damage in vitro appear to develop more progressive fibrosis in vivo. While there are obviously multiple differences between both phenotypes measured and the damage models, the impact of TGF-β on hepatocytes appears sufficient to result in a genotype-phenotype correlation peak consistent across experimental designs.
In the past, the identification of genes underlying major QTL was frequently hampered by the size of the region and the number of genes of unknown function contained within. However, we now have access to complete variation maps for numerous mouse strains and lines, as well as to liver-specific gene expression data for tens of thousands transcripts per mice under various conditions of stress or damage (8, 28). Correlation analyses in our datasets allowed us to identify gene variants within the critical QTL that are associated with the transcriptional response to environmental challenges. When we analyzed the expression data from livers that were damaged in vivo with hepatotoxins for correlation with genetic variation in the susceptibility locus, we were able to identify four critical single nucleotide variants. These were defined by most significant linkage with hepatic expression levels under stress in vivo. In particular, rs13481128 at 83.5 Mb showed a particularly strong regulatory impact in both models (Table 3).
Expi, the nearest gene to rs13481128 bearing a nonconservative polymorphism, belongs to a group of three adjacent, structurally similar “WFDC” (whey acid-four cytosine domain) proteins (22, 23, 30, 41). Of these three transcripts (GM11048/Wfdc17/AMWAP, Expi/WDNM1/Wfdc18, WDNM1-like/1100001G20Rik), only Expi shows a sequence difference resulting in an amino acid substitution between the parental strains C57BL/6J and DBA/2J. The WFDC structural element is indicative of potential inflammatory and antimicrobial properties, similar to defensins (2, 40). Previous studies showed that Expi plays a role in epithelial cell apoptosis, involving the TNF-α/NF-κB signaling pathway (20, 23). These studies indicate potential mechanisms how the amino acid alteration in this short peptide might cause differential priming of hepatocytes for proapoptotic signals, including TGF-β, although further studies are required.
At the distal end of the QTL, two coding variants (rs29454667 at 88.2 Mb and rs29388941 at 88.4 Mb) in the hematopoietic stem cell marker Msi2 point to another candidate gene. It is entirely unknown at present how Msi2 might play a role in the sensitivity of hepatocytes to TGF-β. Since TGF-β is involved in angiogenesis (16) and might render hepatocytes more apoptosis susceptible, a potential role of a transcriptional regulator involved in hematopoiesis does not seem entirely impossible. In both models of liver damage analyzed, Dhx40 appears as common denominator. Coincidentally, the physical position of Dhx40 is also central to the QTL candidate region. However, no nonsynonymous variants between the parental strains (C57BL/6J and DBA/2J) were identified in this gene. The phosphatidylcholine transfer protein Pctp is located just outside the candidate region for TGF-β-induced hepatocyte damage in vitro, but well within the candidate area for CCl4-induced F-score in vivo (Fig. 3). It is also one of only two genes associated with markers in the candidate region in both etiologies of liver damage amenable to eQTL analysis. In contrast to Dhx40, the Pctp gene bears an amino-acid exchanging polymorphism (rs27067571) that differentiates between both parental mouse strains.
Only genes bearing variants between the parental strains or loci exerting differential regulatory or biochemical activity in the tissues analyzed are able to cause an inheritable phenotype that can be mapped by QTL analysis. Regions that are identical-by-descent in both strains do not generate any difference in the trait measured, thereby excluding parts of the genome from analysis. This exclusion may conceal some factors influencing the complex response to liver damage from detection in our experimental setting. At the same time, it removes sources of variation, and this reduction may facilitate QTL mapping in a limited murine reference panel such as the BXD strains (1) used in this study.
In summary, we have identified a genomic locus that has a significant impact on hepatocyte sensitivity to TGF-β signaling in vitro and plays a potential role in experimental fibrogenesis in vivo. Within this locus we identified sequence variants and amino acid differences in Expi that were associated with differential transcriptional activities in response to hepatotoxins. Coexpression of Dhx40 and Pctp in both etiologies of liver damage might suggest a role for these genes in mediating the damage-associated response. These associations require closer functional studies to dissect the role in genetic predisposition to liver damage and fibrogenesis.
GRANTS
The experimental work was supported by Deutsche Forschungsgemeinschaft (DFG Grants LA997/5-1 to R. Liebe, S. Dooley, and F. Lammert and LA997/6-1 to F. Lammert), the Helmholtz Virtual Institute GENESYS (German Network of Systems Genetics, VH-VI-242), and COST Action BM0901 (SYGENET).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L., R.W.W., S.D., and F.L. conception and design of research; R.L. and R.A.H. performed experiments; R.L., R.A.H., R.W.W., and F.L. analyzed data; R.L., R.A.H., and F.L. interpreted results of experiments; R.L., R.A.H., and F.L. prepared figures; R.L. and R.W.W. drafted manuscript; R.L., R.W.W., S.D., and F.L. edited and revised manuscript; R.L., R.A.H., R.W.W., S.D., and F.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Expert technical assistance by Annika Bohner is gratefully acknowledged.
This paper was presented in part at the 35th Annual Research Society on Alcoholism scientific meeting in San Francisco, CA, in May 2012 and the 63rd American Association for the Study of Liver Diseases annual meeting in Boston, MA, November 2012, and published in abstract form in Hepatology 56, Suppl 1: 764A, 2012.
The ethanol-treated dataset is from the GenExQTL database, developed by Genome Explorations with SBIR support (R44AA016225, HHSN267200700050C, HHSN275200900017C) from the National Institute of Alcohol Abuse and Alcoholism.
REFERENCES
- 1.Andreux PA, Williams EG, Koutnikova H, Houtkooper RH, Champy MF, Henry H, Schoonjans K, Williams RW, Auwerx J. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell 150: 1287–1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol 29: 444–453, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Breitkopf K, Nagy LE, Beier JI, Mueller S, Weng H, Dooley S. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res 33: 1647–1655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW. Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics 1: 343–357, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chesler EJ, Williams RW. Brain gene expression: genomics and genetics. Int Rev Neurobiol 60: 59–95, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Damerval C, Maurice A, Josse JM, de Vienne D. Quantitative trait loci underlying gene product variation: a novel perspective for analyzing regulation of genome expression. Genetics 137: 289–301, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res 347: 245–256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durrant C, Swertz MA, Alberts R, Arends D, Moller S, Mott R, Prins P, van der Velde KJ, Jansen RC, Schughart K. Bioinformatics tools and database resources for systems genetics analysis in mice–a short review and an evaluation of future needs. Brief Bioinform 13: 135–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara CT, Wang P, Neto EC, Stevens RD, Bain JR, Wenner BR, Ilkayeva OR, Keller MP, Blasiole DA, Kendziorski C, Yandell BS, Newgard CB, Attie AD. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet 4: e1000034, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol 38, Suppl 1: S38–S53, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Muller A, Tuschl G, Mueller SO, Dooley S. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology 49: 2031–2043, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 10: 76–99, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity (Edinb) 69: 315–324, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Hillebrandt S, Goos C, Matern S, Lammert F. Genome-wide analysis of hepatic fibrosis in inbred mice identifies the susceptibility locus Hfib1 on chromosome 15. Gastroenterology 123: 2041–2051, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res 102: 637–652, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, Layden TJ, Wright TL, White T, Cheung RC. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology 46: 297–306, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Huard J, Mueller S, Gilles ED, Klingmuller U, Klamt S. An integrative model links multiple inputs and signaling pathways to the onset of DNA synthesis in hepatocytes. FEBS J 279: 3290–3313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem 112: 70–75, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Jung DJ, Bong JJ, Baik M. Extracellular proteinase inhibitor-accelerated apoptosis is associated with B cell activating factor in mammary epithelial cells. Exp Cell Res 292: 115–122, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kanel GC. Liver: anatomy, microscopic structure, and cell types. In: Atlas of Gastroenterology, edited by Yamada T. Oxford, UK: Wiley-Blackwell, 2009, p. 598–606 [Google Scholar]
- 22.Karlstetter M, Walczak Y, Weigelt K, Ebert S, Van den Brulle J, Schwer H, Fuchshofer R, Langmann T. The novel activated microglia/macrophage WAP domain protein, AMWAP, acts as a counter-regulator of proinflammatory response. J Immunol 185: 3379–3390, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Kho Y, Kim S, Yoon BS, Moon JH, Kwak S, Park G, Woo J, Oh S, Hong K, Kim H, You S, Choi Y. WDNM1 is associated with differentiation and apoptosis of mammary epithelial cells. Anim Biotechnol 19: 89–103, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Klingmuller U, Bauer A, Bohl S, Nickel PJ, Breitkopf K, Dooley S, Zellmer S, Kern C, Merfort I, Sparna T, Donauer J, Walz G, Geyer M, Kreutz C, Hermes M, Gotschel F, Hecht A, Walter D, Egger L, Neubert K, Borner C, Brulport M, Schormann W, Sauer C, Baumann F, Preiss R, MacNelly S, Godoy P, Wiercinska E, Ciuclan L, Edelmann J, Zeilinger K, Heinrich M, Zanger UM, Gebhardt R, Maiwald T, Heinrich R, Timmer J, von Weizsacker F, Hengstler JG. Primary mouse hepatocytes for systems biology approaches: a standardized in vitro system for modelling of signal transduction pathways. Syst Biol (Stevenage) 153: 433–447, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lammert F, Wang DQ, Wittenburg H, Bouchard G, Hillebrandt S, Taenzler B, Carey MC, Paigen B. Lith genes control mucin accumulation, cholesterol crystallization, and gallstone formation in A/J and AKR/J inbred mice. Hepatology 36: 1145–1154, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Manly KF, Olson JM. Overview of QTL mapping software and introduction to map manager QT. Mamm Genome 10: 327–334, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Mhyre TR, Chesler EJ, Thiruchelvam M, Lungu C, Cory-Slechta DA, Fry JD, Richfield EK. Heritability, correlations and in silico mapping of locomotor behavior and neurochemistry in inbred strains of mice. Genes Brain Behav 4: 209–228, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet 8: 58–69, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 349: 825–832, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, Groden J. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res 65: 166–176, 2005 [PubMed] [Google Scholar]
- 31.Robertson A. Linkage between marker loci and those affecting a quantitative trait. Behav Genet 3: 389–391, 1973 [DOI] [PubMed] [Google Scholar]
- 32.Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, Guhathakurta D, Sieberts SK, Monks S, Reitman M, Zhang C, Lum PY, Leonardson A, Thieringer R, Metzger JM, Yang L, Castle J, Zhu H, Kash SF, Drake TA, Sachs A, Lusis AJ. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet 37: 710–717, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, Colinayo V, Ruff TG, Milligan SB, Lamb JR, Cavet G, Linsley PS, Mao M, Stoughton RB, Friend SH. Genetics of gene expression surveyed in maize, mouse and man. Nature 422: 297–302, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Siegmund SV, Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res 29: 102S–109S, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Taylor BA, Heiniger HJ, Meier H. Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc Soc Exp Biol Med 143: 629–633, 1973 [DOI] [PubMed] [Google Scholar]
- 36.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era–concepts and misconceptions. Nat Rev Genet 9: 255–266, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Williams RW, Manly KF. WebQTL: web-based complex trait analysis. Neuroinformatics 1: 299–308, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33: 105–136, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Wegner K, Bachmann A, Schad JU, Lucarelli P, Sahle S, Nickel P, Meyer C, Klingmuller U, Dooley S, Kummer U. Dynamics and feedback loops in the transforming growth factor beta signaling pathway. Biophys Chem 162: 22–34, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson TS, Roghanian A, Simpson AJ, Sallenave JM. WAP domain proteins as modulators of mucosal immunity. Biochem Soc Trans 39: 1409–1415, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Smas CM. Wdnm1-like, a new adipokine with a role in MMP-2 activation. Am J Physiol Endocrinol Metab 295: E205–E215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 9: 524–530, 2011 [DOI] [PubMed] [Google Scholar]