Abstract
Aging is associated with attenuated thermoregulatory function that varies regionally over the body. Decrements in vasodilation and sweating are well documented with age, yet limited data are available concerning the regional relation between these responses. We aimed to examine age-related alterations in the relation between regional sweating (RSR) and skin blood flow (SkBF) to thermal and pharmacological stimuli. Four microdialysis fibers were inserted in the ventral forearm, abdomen, thigh, and lower back of eight healthy aged subjects (64 ± 7 yr) and nine young (23 ± 3 yr) during 1) ACh dose response (1 × 10−7 to 0.1 M, mean skin temperature 34°C) and 2) passive whole body heating to Δ1°C rise in oral temperature (Tor). RSR and SkBF were measured over each microdialysis membrane using ventilated capsules and laser-Doppler flowmetry. Maximal SkBF was measured at the end of both protocols (50 mM SNP). Regional sweating thresholds and RSR were attenuated in aged vs. young at all sites (P < 0.0001) during whole body heating. Vasodilation thresholds were similar between groups (P > 0.05). Attenuated SkBF were observed at the arm and back in the aged, representing 56 and 82% of those in the young at these sites, respectively (0.5 ΔTor). During ACh perfusion, SkBF (P = 0.137) and RSR were similar between groups (P = 0.326). Together these findings suggest regional age-related decrements in heat-activated sweat gland function but not cholinergic sensitivity. Functional consequences of such thermoregulatory impairment include the compromised ability of older individuals to defend core temperature during heat exposure and a subsequently greater susceptibility to heat-related illness and injury.
Keywords: sweating, aging, laser-Doppler flowmetry, skin blood flow, sweating
human aging is associated with altered thermoregulatory function during both passive and exercise-induced hyperthermia, including attenuated reflex cutaneous vasodilation (26) and eccrine sweating responses (3, 18, 24). A functional loss of active cholinergic vasodilation cotransmitter function and dysfunction of second-messenger pathways (i.e., nitric oxide bioavailability) (8, 11, 12, 31) contribute to attenuated cutaneous vasodilatory responses to elevations in core temperature (Tcore), hindering convective heat loss. An age-related decline in thermoregulatory sweating further compromises heat loss and increases thermal strain in warm ambient conditions and during exercise. Reduced evaporative heat loss from the skin surface results from a decline in thermoregulatory sweating, due to a decreased thermal sensitivity (32) and atrophy of the sweat glands (17, 19), producing a lower sweat output per gland (23, 24, 39).
Regional variation in sweating and skin blood flow (SkBF) responses to passive heating are well documented (29); however, limited data are available concerning the relation between these responses and how they may vary with aging (19, 20). It has been suggested that an age-related decline in reflex cutaneous vasodilation precedes decrements in sweat gland function (15, 19), with the latter resulting from an initial reduction in sweat gland output followed by a subsequent decline in the number of heat-activated sweat glands (HASG) (15). Physiological mechanisms contributing to the disproportionate temporal progression of sudomotor and vasomotor dysfunction with age remain unclear. Furthermore, despite attempts to identify a putative signaling pathway or cotransmitter relating the two responses, including speculation over VIP (40), bradykinin (4), and nitric oxide (33), a single mechanism has not yet been elucidated. The regional progression of age-related vasomotor and sudomotor dysfunction is not well characterized, with limited vasomotor data available and conflicting results of regional sudomotor adjustments. Several studies have indicated a peripheral-to-core (torso) progression in sweat gland dysfunction with aging (20), whereas others have reported a sequential progression from lower to upper body regions (19). In contrast to these data, a gap in knowledge exists regarding regional variation in SkBF in an aged population. Although age-related decrements in sweating and SkBF are widely recognized, a thorough examination of regional differences in an aged population is largely lacking, with changes in distribution subsequently less well understood. Furthermore, most studies investigating sudomotor and vasomotor adjustments with age have not matched subject groups for anthropometric parameters and/or aerobic capacity (15), confounding separation of these influences from aging effects per se. Understanding such age-related thermoregulatory dysfunction is particularly important in the current aging population, who are particularly susceptible to heat-related illness and injury both during passive heat stress and during physical activity in the heat.
The present study aimed to examine age-related decrements in the relation between regional sweating and SkBF during whole body passive heating using subject groups matched for anthropometric characteristics and V̇o2max. Based on previous regional sweating rate (RSR) data and the putative relation between RSR and SkBF, we hypothesized that regional, age-related decrements in sweating and SkBF would occur in a nonuniform manner over the body, indicating a peripheral-to-central (torso) progression. Considering decrements in SkBF have been observed in advance of RSR, we further hypothesized that attenuated SkBF responses would be greater than decrements in RSR.
METHODS
Subjects
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent were voluntarily obtained from all subjects before participation. Nine healthy aged men and women (64 ± 2 yr, 4 men and 5 women) participated in the study and were compared with nine young men and women (23 ± 2 yr, 5 men and 4 women) from a previous study following the same experimental protocol (37). Subjects underwent a complete medical screening, including blood chemistry, complete lipid, renal, and liver enzyme profile evaluation (Quest Diagnostics Nichol Institute, Chantilly, VA), resting electrocardiogram, and physical examination. All subjects were screened for the presence of cardiovascular, dermatological, and neurological disease. Subjects were nonsmokers, nondiabetic, normally active (neither sedentary nor highly exercise trained), and were not taking medications, including antihypertensives or other drugs that may affect the cardiovascular system, including antioxidants, hormone replacement therapy, or oral contraceptives. All young women were normally menstruating and were tested during the early follicular phase (days 1–7) of their menstrual cycle. All subjects completed a graded exercise test (Bruce protocol) on a semirecumbent bike to determine V̇o2 peak in a room maintained at 23°C, 40% relative humidity. Subjects underwent a dual x-ray absorptiometry scan for assessment of regional body composition.
Instrumentation
All subjects completed the following two experimental protocols: 1) whole body heating and 2) ACh dose response, of which the order was randomized between subjects. Experiments were conducted on different days separated by a minimum of 1 wk to allow full healing of microdialysis insertion sites and ensure recovery from whole body heating. Protocols were performed in a thermoneutral laboratory with the subjects in a supine position. Four intradermal microdialysis fibers (MD 2000; Bioanalytical Systems) (10 mm, 20-kDa cutoff membrane) were inserted as previously described (9) in the ventral forearm, abdomen, thigh, and lower back. Foam was placed underneath the subject to prevent pressure being placed on the equipment and the measurement site at the lower back. Fibers were inserted following temporary anesthesia of each site using ethyl chloride spray (Gebauer, Cleveland, OH) (14). Ethyl chloride spray was selected because of its short period of anesthesia, ease of application to the MD fiber insertion sites, and showing no effect on SkBF during or after hyperemia, compared with using ice (pilot data from our laboratory, unpublished observation). Sites were selected based on high and low sweat regions from whole body sweat maps and using similar anatomical measurements to determine MD fiber placement (35, 36). Briefly, the abdomen site was identified on the left side of the body as midway between the anterior superior iliac spine and the navel; the thigh site was calculated as 0.6× the upper leg length (distance from anterior superior iliac spine to the proximal edge of the patella); the lower back site was identified on the left side of the body at the height of the anterior superior iliac spine, 5–10 cm lateral to the vertebrae. Initial insertion trauma was allowed to subside for 60–90 min, during which time lactated Ringer solution was perfused through all fibers at a rate of 2 μl/min (Bioanylitical Systems Bee hive and Baby Bee microinfusion pumps, West Lafayette, IN).
Whole body heating was achieved using a water-perfused suit that covered the entire body with exception to the head, hands, and feet. Local skin temperature (Tsk) was continuously measured at six sites using copper-constantan thermocouples (calf, thigh, abdomen, chest, upper arm, and upper back), and an unweighted mean skin temperature (Tsk mean) was calculated. Oral temperature (Tor) was measured as an index of body Tcore using a thermistor placed in the sublingual sulcus throughout baseline and whole body heating. Following verification of appropriate placement against temperature readings, the thermistor was taped in place, and subjects were instructed to maintain a closed mouth for the duration of the protocol. Tor was closely monitored throughout heating. Mean body temperature (Tbody) was calculated as Tbody = (0.9 × Tor) + (0.1 × mean Tsk) (5). An index of SkBF was measured using laser-Doppler flowmetry probes (MoorLAB; Moor Instruments) placed over each microdialysis site measuring cutaneous red blood cell flux, which was recorded continuously during the experiment. Arterial blood pressure was measured via brachial auscultation every 5 min following resolution of hyperemia induced by MD fiber insertion. Mean arterial pressure (MAP) was calculated as 1/3 systolic blood pressure + 2/3 diastolic blood pressure. SkBF was expressed as cutaneous vascular conductance (CVC; red blood cell flux/MAP) and normalized as percent of maximal CVC values (%CVCmax).
Total body sweating rate was determined from the change in body mass during whole body hearting using a scale accurate to ±10 g (SECA. model 770 1321143). Values were not corrected for metabolic and respiratory losses. Local sweating rates were measured using ventilated capsules with compressed medical-grade nitrogen used as the perfusion gas (28, 34), specifically manufactured so that sweating rate and laser-Doppler flux could be measured simultaneously. Sweat capsules (4.46 cm2) were positioned over the center of the membrane portion of each microdialysis fiber. The temperature and humidity of the air flowing out of the capsules were measured using capacitance hygrometers that were calibrated by the manufacturer and regularly calibrated using reference solutions of known temperature and humidity (model HMT330; Vaisala, Helsinki, Finland). The sensitivity of the system was assessed by injecting set volumes of distilled water (5–20 μl) in the capsules, and the area under the curve was calculated (unpublished pilot data). Sweating rate (SR) was calculated based on the change in relative humidity of the air as it passed through the capsule (Δrh) at a determined air flow (AF), the density of saturated steam at the given temperature (D), and the capsules surface area (SA), using the following equation (28):
Experimental Protocol
Whole body heating.
Upon arrival to the laboratory, subjects provided a urine sample for assessment of hydration status via urine specific gravity and osmolality, and body mass was recorded. Following MD fiber insertion and resolution of insertion trauma, baseline data were collected for 20 min with mean Tsk maintained at thermoneutral using a water-perfused suit (34°C). After collection of baseline data, 50°C water was perfused through the suit to elevate Tor by 1°C, after which Tor was clamped for 5 min by reducing the temperature of the water perfusing the suit. After 5 min of steady-state laser-Doppler flux values, water perfusing the suit was returned to 34°C and 50 mM SNP (Nitropress; Abott Laboratories, Chicago, IL) was perfused for 20 min through each MD site at a rate of 4 μl/min to obtain maximal CVC values.
ACh dose response.
Following MD fiber insertion and resolution of insertion trauma, baseline data were collected for 20 min. Immediately after baseline measurement, Tsk was clamped at 34°C (water-perfused suit) during perfusion of seven ascending concentrations of ACh at a rate of 2 μl/min for 10 min each: 1 × 10−7 to 1 × 10−1 M dissolved in Ringer solution in 10-fold increments. This amount of time allowed for a plateau in SkBF at each concentration of ACh. After completion of the ACh dose response, 50 mM SNP was perfused through all sites at a rate of 4 μl/min to induce maximal cutaneous vasodilation (LDF) (13, 22).
Data and Statistical Analysis
Data were digitalized at 40 Hz, recorded, and stored for offline analysis using Windaq software and the Dataq data acquisition system (Windaq; Dataq Instruments, Akron, OH). Baseline values were determined as the last 5 min before commencing whole body heating. Local sweating and SkBF data are presented as mean values over 60 s at each 0.1°C rise in Tor throughout the duration of the heating protocol. Maximal SkBF values were averaged over a stable 60-s plateau during perfusion of 50 mM SNP. Sweating and cutaneous vasodilation thresholds were defined as the values that exceeded the nonsweating transepidermal water loss and baseline CVC values, respectively, determined by two independent investigators who were blinded to the subject group during analysis (1). Student's unpaired t-tests were used to compare physical characteristics between subject groups. %CVCmax and sweating data were analyzed using the three-way, mixed-model, repeated-measures ANOVA protocol [group × site × heating phase (ΔTor) or ACh dose; proc mix SAS 9.2]. Specific planned comparisons were performed when appropriate to determine where differences between groups and sites occurred with appropriate Bonferroni correction. To assess the regional relation between RSR and SkBF within each age group, Pearson's r correlations were performed for each subject, and a group mean (±SE) was calculated due to differences in absolute values between subjects. The level of significance was set at α = 0.05. Values are presented as means ± SE.
RESULTS
Subject characteristics are presented in Table 1. Subjects were matched for anthropometric parameters, lipid profile, blood pressure, and V̇o2max. Aged subjects showed a greater serum low-density lipoprotein (LDL) concentration compared with young subjects (P = 0.008); however, values were still within clinically defined normal limits.
Table 1.
Participant characteristics
| Young | Primary Aged | |
|---|---|---|
| Subjects (male, female) | (5, 4) | (4, 5) |
| Age, yr | 23 ± 1 | 64 ± 2*** |
| BMI, kg/m2 | 25 ± 1 | 24 ± 1 |
| Body surface area, m2 | 1.95 ± 0 | 1.81 ± 0 |
| Total body fat, % | 27 ± 2 | 27 ± 4 |
| Systolic BP, mmHg | 108 ± 3 | 116 ± 2 |
| Diastolic BP, mmHg | 69 ± 3 | 70 ± 2 |
| MAP, mmHg | 82 ± 3 | 86 ± 2 |
| HbA1c, mmol/mol | 35 ± 0 | 37 ± 0 |
| HDL, mg/dl | 66 ± 7 | 65 ± 7 |
| LDL, mg/dl | 81 ± 9 | 118 ± 8** |
| V̇o2max, ml·kg−1·min−1 | 35 ± 3 | 28 ± 3 |
Values are means ± SE. BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; V̇o2max, maximal O2 uptake. Values significantly different from young group:
P < 0.01 and
P < 0.001.
Whole Body Heating
Core temperature, total sweat loss, and body composition.
The aged subjects achieved a 1°C ΔTor significantly faster compared with the young group (58 ± 5 vs. 88 ± 9 min, P < 0.05). Heating time did not correlate significantly with total lean mass, total body fat, body surface area, or V̇o2max (P > 0.05). Total sweat loss during heating was similar between groups (young 767 ± 84 g·m−2·h−1 vs. aged 799 ± 101 g·m−2·h−1), and no differences were present in absolute Tor, mean Tsk or calculated Tbody throughout the protocol (Table 2). Within-subject correlations of SkBF, RSR, and body composition data (Table 3) at 0.5 and 1.0°C ΔTor showed considerable variation, with mean group values showing a strong correlation between regional lean mass and RSR only within the young group (0.5°C ΔTor r = 0.79, 1.0°C ΔTor r = 0.64). No correlation was present within the aged group.
Table 2.
Regional skin temperature during baseline, 0.5°C, and 1°C rise in Tor during the whole body heating protocol
| Regional Skin Temperature, °C |
||||||
|---|---|---|---|---|---|---|
| Young |
Aged |
|||||
| Site | Baseline | 0.5°C ΔTor | 1.0°C ΔTor | Baseline | 0.5°C ΔTor | 1.0°C ΔTor |
| Calf | 34.4 ± 0.4 | 37.8 ± 0.9 | 38.9 ± 0.7 | 34.3 ± 0.1***†§ | 39.0 ± 1.1 | 39.0 ± 0.4** |
| Thigh | 33.8 ± 0.4 | 38.6 ± 0.6 | 38.3 ± 0.6* | 33.9 ± 0.2***†§ | 39.1 ± 0.5 | 39.4 ± 0.5†§ |
| Abdomen | 35.3 ± 0.4 | 37.5 ± 0.5 | 38.0 ± 0.5* | 35.7 ± 0.3# | 38.3 ± 0.5 | 38.2 ± 0.6** |
| Chest | 35.9 ± 0.3 | 38.6 ± 0.3 | 38.9 ± 0.3* | 35.9 ± 0.3 | 38.2 ± 0.3 | 38.2 ± 0.5** |
| Upper arm | 36.2 ± 0.3 | 38.4 ± 0.2 | 38.9 ± 0.3*† | 35.8 ± 0.2#‡ | 38.1 ± 0.4 | 38.8 ± 0.4** |
| Shoulder | 36.2 ± 0.3 | 39.3 ± 0.5 | 39.8 ± 0.5 | 35.9±.02 | 40.1 ± 0.3 | 40.5 ± 0.25 |
Regional skin temperature (mean ± SE) during baseline, 0.5°C, and 1°C rise in oral temperature (Tor) during the whole body heating protocol. Significant vs. shoulder:
P < 0.05,
P < 0.01, and
P < 0.001; significant vs. abdomen:
P < 0.05; significant vs. chest:
P < 0.05; significant vs. thigh:
P < 0.05; and significant vs. calf:
P < 0.001.
Table 3.
Regional body composition corresponding to skin blood flow and sweating rate measurement regions in young and primary aged subjects
| Regional Body Composition |
||||||
|---|---|---|---|---|---|---|
| Young |
Primary Aged |
|||||
| Site | Fat, kg | Lean, kg | Fat, % | Fat, kg | Lean, kg | Fat, % |
| Left arm | 0.99 ± 0.11 | 2.63 ± 0.30 | 27 ± 4 | 0.97 ± 0.17 | 2.40 ± 0.32 | 28 ± 5 |
| Trunk | 8.71 ± 1.20 | 26.09 ± 1.44 | 24 ± 2 | 9.48 ± 1.46 | 2.48 ± 1.96 | 27 ± 3 |
| Right leg | 4.46 ± 0.46 | 8.58 ± 0.60 | 33 ± 3 | 3.47 ± 0.57 | 7.34 ± 0.74 | 31 ± 5 |
| Total body | 20.52 ± 2.24 | 52.51 ± 3.36 | 27 ± 2 | 19.10 ± 2.63 | 48.34 ± 4.30 | 27 ± 4 |
Values are means ± SE.
Regional sweating rates.
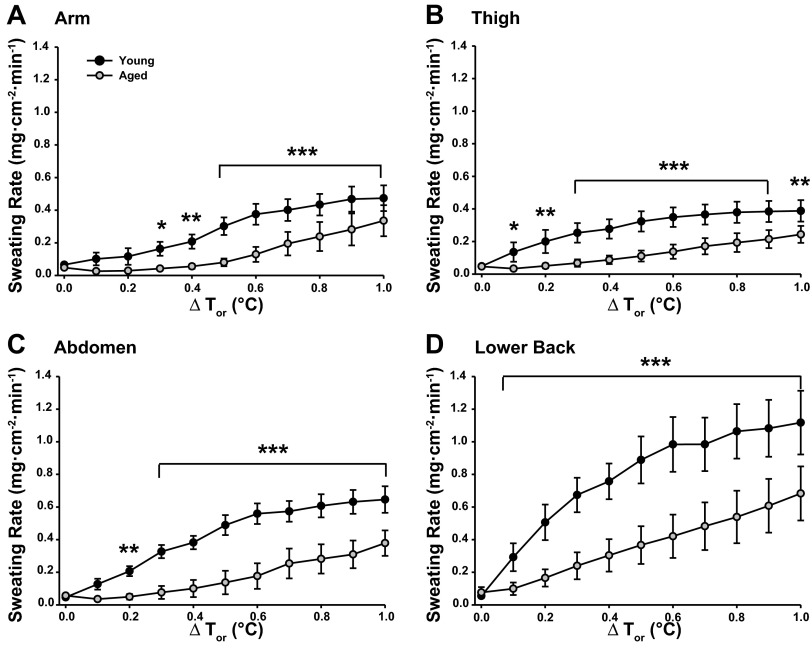
Figure 1 illustrates RSR data in young and aged subjects during passive whole body heating. RSR varied between sites in both groups (main effect P < 0.0001) throughout the heating protocol. No differences in RSR were observed at baseline between sites in either group. Young subjects showed a greater RSR on the lower back compared with the other sites throughout heating (all sites P < 0.05). Differences in RSR between the remaining sites varied with increasing Tor. Less variation was present between sites in the aged group with higher RSRs on the back compared with both the arm and abdomen from ≥0.2°C ΔTor (P < 0.05), and the thigh vs. abdomen at 1.0°C ΔTor (P < 0.05). Aged subjects had significantly attenuated RSRs at all sites compared with the young group with the exception of the arm (0.1–0.2°C ΔTor) and abdomen (0.1°C ΔTor) during whole body heating. Despite differences in absolute RSRs between groups, changes in sweating rates within each region were similar between young and aged subjects with increasing Tor (group × site × phase interaction, P = 0.517). Sweating onset progressed in a similar manner between groups, beginning at the lower back and occurring last on the arm. Sweating thresholds were significantly higher in aged compared with young subjects at all sites (Table 4).
Fig. 1.
Regional sweating rates (mg·cm−2·min−1) during whole body heating to 1°C rise in oral temperature (Tor) at the ventral forearm (A), thigh (B), abdomen (C), and lower back (D) in young and primary aged subjects. Values significantly different from primary aged group: *P < 0.05, **P < 0.01, and ***P < 0.001 corrected for multiple comparisons.
Table 4.
Relative thresholds (ΔTor from baseline, °C) for the onset of cutaneous vasodilation and sweating at four sites in young and primary aged subjects
| Relative Thresholds (°C above baseline Tor) During Heating Protocol |
LDF EC50 Values (log-molar concentration) ACh Dose Response |
Maximal LDF Values (50 mM SNP) |
||||
|---|---|---|---|---|---|---|
| Group | Site | Vasodilation | Sweating | Heating protocol | ACh dose response | |
| Young | Arm (ventral forearm) | 0.2 ± 0.1 | 0.3 ± 0.1 | −4.39 ± 0.31 | 112 ± 10† | 104 ± 8 |
| Abdomen | 0.2 ± 0.1 | 0.2 ± 0.0 | −3.47 ± 0.22‡ | 139 ± 9††† | 106 ± 9 | |
| Thigh | 0.2 ± 0.1 | 0.2 ± 0.0§ | −4.11 ± 0.21§ | 113 ± 15 | 107 ± 6 | |
| Lower back | 0.1 ± 0.0 | 0.1 ± 0.0§ | −3.38 ± 0.32§ | 79 ± 7 | 112 ± 14 | |
| Primary aged | Arm (ventral forearm) | 0.4 ± 0.1 | 0.6 ± 0.1* | −3.97 ± 0.39 | 134 ± 10 | 125 ± 17 |
| Abdomen | 0.2 ± 0.1 | 0.5 ± 0.1** | −4.33 ± 0.29 | 119 ± 10 | 132 ± 38 | |
| Thigh | 0.4 ± 0.1 | 0.4 ± 0.1*§§ | −3.46 ± 0.30 | 149 ± 21 | 111 ± 19 | |
| Lower back | 0.3 ± 0.1* | 0.4 ± 0.1*§ | −4.19 ± 0.25 | 90 ± 19 | 92 ± 18 | |
Values are means ± SE. SNP, sodium nitroprusside. Values significantly different from young group:
P < 0.05 and
P < 0.01; values significantly different from arm site within each group:
P < 0.05 and
P < 0.01; significantly different from thigh within each group:
P < 0.05; significantly different from back within each group:
P < 0.05 and
P < 0.001.
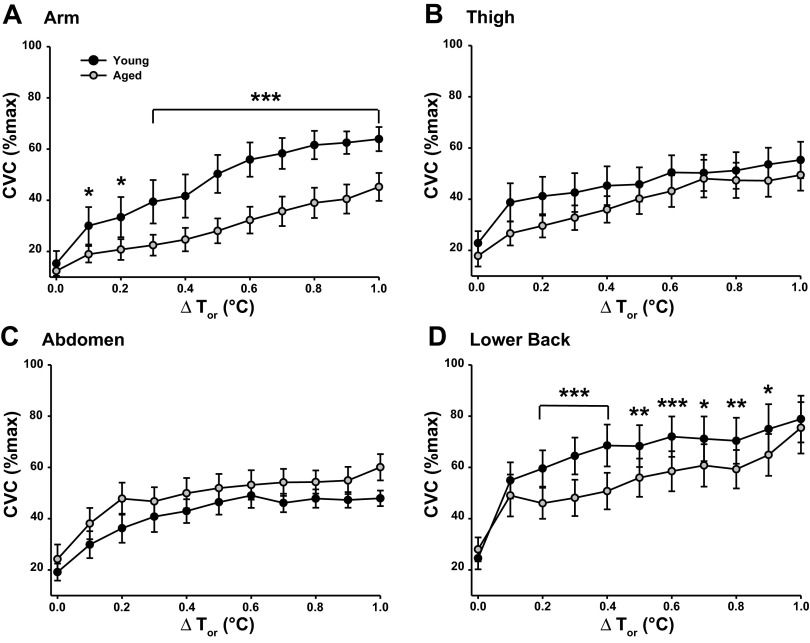
Regional skin blood flow.
Figure 2 illustrates regional SkBF normalized to maximal vasodilation (%CVCmax) during whole body heating. There were no differences in SkBF between groups at any site during baseline when Tsk was clamped at 34°C (unweighted mean Tsk: young 35.2 ± 0.3°C, aged 35.2 ± 0.1°C). SkBF was similar between groups at the thigh for the duration of heating, but was significantly attenuated at the arm in the aged compared with young group throughout heating and at the lower back from 0.2 to 0.9°C ΔTor. Maximal vasodilation (50 mM SNP) varied between sites within the young group (Table 3), but no differences were present between sites in the aged subjects (P = 0.218). Maximal vasodilation was similar between groups at all sites.
Fig. 2.
Cutaneous vascular conductance (%CVCmax) during whole body heating to 1°C rise in Tor at ventral forearm (A), thigh (B), abdomen (C), and lower back (D) in young and primary aged subjects. Values significantly different from primary aged group: *P < 0.05, **P < 0.01, and ***P < 0.001 corrected for multiple comparisons.
When considering alterations in cutaneous vasodilation, it is important to consider regulation in terms of absolute flow rather than values normalized as a percentage of a maximal value. To consider the regional relation between absolute SkBF (LDF) and RSR, within-subject analysis showed strong correlation coefficients (Pearson's r > 0.7) during the early stages (BL-0.5°C ΔTor) of heating in the young (group mean: arm r = 0.77 ± 0.09, thigh r = 0.81 ± 0.08, abdomen r = 0.89 ± 0.04, lower back r = 0.86 ± 0.04) but not the aged (all Pearson's r < 0.3) subjects. Conversely, during the latter stages of heating (0.6–1°C ΔTor), the young subjects illustrated a weak (Pearson's r < 0.4) SkBF-RSR correlation compared with strong (Pearson's r > 0.7) correlations observed in the aged group at most sites (aged group mean: arm 0.73 ± 0.26, thigh 0.74 ± 0.28, abdomen 0.56 ± 0.15, back 0.69 ± 0.15).
ACh Dose Response
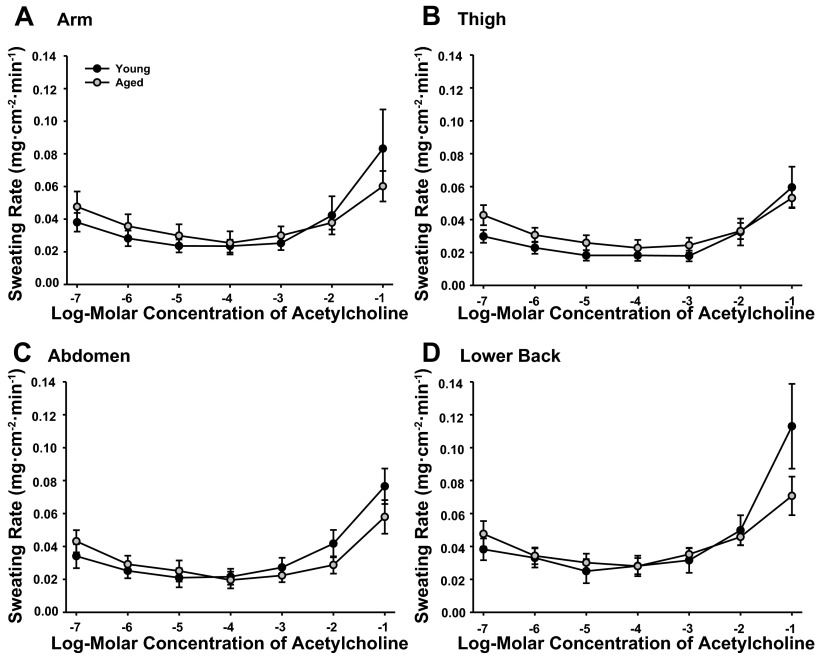
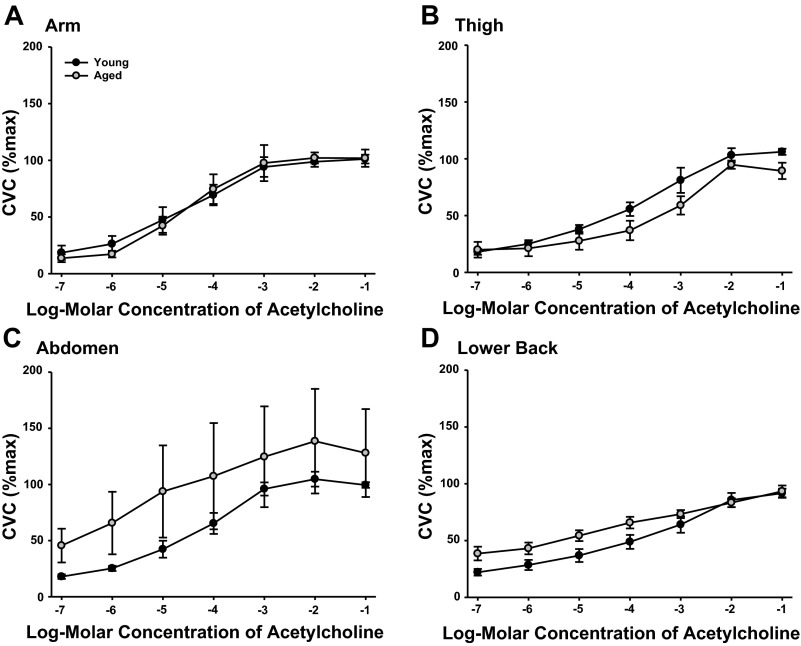
RSR were similar between sites in both the young and aged subjects throughout the ACh dose-response protocol (Fig. 3). No differences in RSR were present between groups (main effect P = 0.326). To examine the relation between SkBF and RSR, both absolute (LDF) and normalized SkBF values were assessed. No differences in the pattern of SkBF were observed between absolute and normalized values, therefore CVC (%CVCmax) are presented in Fig. 4. SkBF was similar between groups (main effect P = 0.137) and showed no difference between sites (main effect: young P = 0.203, aged P = 0.098).
Fig. 3.
Regional sweating rates (mg·cm−2·min−1) during local administration of 10−7 to 10−1 M ACh at ventral forearm (A), thigh (B), abdomen (C), and lower back (D) of young and primary aged subjects.
Fig. 4.
%CVCmax during local administration of 10−7 to 10−1 M ACh at ventral forearm (A), thigh (B), abdomen (C), and lower back (D) of young and primary aged subjects.
The EC50 values for SkBF (Table 3) differed significantly between sites in the young subjects only (main effect: young P = 0.027, aged P = 0.341). No differences in SkBF EC50 values were observed between groups (main effect P = 0.472). RSR EC50 values were not calculated due to a maximal sweating rate not being elicited. No significant differences in maximal SkBF were present between sites in the young (P = 0.623) or aged (P = 0.218) subjects or between groups during perfusion of SNP (P = 0.475; Table 3).
DISCUSSION
The major new findings of the present study were that, when matched for anthropometric parameters and aerobic fitness, aged subjects showed 1) greater age-related decrements in regional sweating compared with cutaneous vasodilation during whole body heating, with both responses occurring in a nonuniform manner over the body, and 2) these differences were not attributable to cholinergic sensitivity. Together these findings suggest regional age-related decrements in HASG function but not cholinergic sensitivity, which exhibit greater age-related decrements compared with the attenuation in cutaneous vasodilation.
Regional variations in sweating and cutaneous vasodilation in response to thermal stimuli are well documented in young healthy subjects. The present data support the regional patterns observed in young subjects (2, 30, 35, 36) and confirm the widely reported decrements with aging (19, 20). In the present study, the magnitude of age-related decrements in RSR were dependent upon the phase of heating. First, the significantly delayed Tor thresholds for sweating in aged subjects were evident at all sites compared with the young group. At 0.5°C ΔTor the largest reductions in sweating in the aged occurred at the arm and abdomen, representing 26 and 29% of the sweat produced in the young subjects, respectively. At 1°C ΔTor sweating rates on the abdomen in the aged group represented 58% of that in the young, followed by the lower back (61%), thigh (62%), and the arm (72%). The difference in RSR observed between groups conflicts with the sequential progression of RSR decline from the lower limbs to the upper body and head reported in a longitudinal study of young and older men (19) and an observed peripheral-to-central pattern of age-related reductions in sweat gland function (2). Moreover, the age-related decrements in RSR in the present study could not be explained by alterations in cholinergic sensitivity, with no differences observed between sites or groups during ACh perfusion. These data are in disagreement with similar studies using methacholine-stimulated sweating in which sweat gland output was significantly lower in older compared with younger subjects (25). Because we did not measure regional HASG density, we are unable to determine if these decrements were due to a decline in the number of active glands or output per gland.
We did observe a delay in the sweating thresholds in the older subjects, which may explain the opposing relations observed for SkBf and RSR between groups. The poor correlations present at all sites in the aged subjects during the early stages of heating may reflect the mismatch between initial vasodilation and a delayed sweating response compared with the young group. The slower initial regional sweating responses to passive heating in the aged subjects have similarly been observed by Inoue and colleagues to differing severities of heat stress (19, 21), which they attribute to potential age-related modifications of thermoreceptor sensitivity, reduced cholinergic sensitivity of the sweat glands, or delayed vasodilation. In the present study, age-related alterations in cutaneous vasodilation thresholds and limited differences in SkBF were not observed during the initial stages of heating between groups, suggesting altered thermoreceptor and/or cholinergic sensitivity (24) to be a more likely cause. The present ACh dose-response data showed no age-related alteration in SkBF, suggesting altered thermoreceptor function or attenuation of putative cotransmission may play a greater role in the reduced vasodilation as opposed to cholinergic sensitivity in older subjects upon exposure to heat.
The present data indicate a greater age-related decline of RSR compared with cutaneous vasodilation responses to passive heating. This is in disagreement with studies that observe reductions in cutaneous vasodilation preceding decrements in sweat gland function with aging (15, 19). Regional SkBF varied significantly between sites, with the greatest decrements observed on the arm, representing 56 and 70% of blood flow observed in the young subjects at 0.5 and 1.0°C ΔTor, respectively. Values were significantly lower on the back in the aged subjects throughout most of the heating protocol, achieving 82 and 85% of SkBF observed in young at 0.5 and 1.0°C ΔTor, respectively. A number of possible explanations for the discrepancy in these findings with some of the literature may be the specific regional measurement site, the method of stimulation (pharmacological, exercise, passive heating), and the matching of groups for physical characteristic and aerobic capacity. The influences of aerobic fitness and anthropometric characteristics on vasodilation and sweating responses are well documented (7, 16). Despite some subject variation, the present groups were relatively well matched for sex, anthropometric characteristics, blood pressure, and blood chemistry. The observed decrements in vasomotor and sudomotor responses to passive heating likely indicate changes due to aging per se, rather than the reduced aerobic capacity, increased adiposity, or reductions in muscle mass commonly associated with aging. Few studies have matched young and older subjects for physical characteristics and V̇o2max, thus discerning between such factors and true age effects is often difficult. One study reported declines in sweat gland function in 60- to 70-yr-old men despite similarities in anthropometric parameters and aerobic capacity during a 5-yr longitudinal study (15), supporting the attenuation of thermal responses due to age itself. By matching subject groups in the present study, one group may not be truly representative of the general population; however, the decrements in vascular and thermal function may potentially become greater when using unmatched groups. Regardless of absolute changes in thermoregulatory dysfunction, we would expect a similar pattern of regional vasomotor and sudomotor dysfunction for “average” groups of younger and older subjects.
The attenuated vasomotor responses in aged subjects at two sites during the present whole body heating protocol, yet the similarity in ACh-induced vasodilation, support the theory of a loss of cotransmitter function (8). Our laboratory has previously found similarities in cutaneous vasodilation to the local perfusion of ACh in young and aged subjects. Using microdialysis in the ventral forearm, we observed no age-related difference in vasodilation to perfusion of ascending doses of ACh. Together, this may explain the blunted SkBF responses at the arm and abdomen in the present data but does not account for the significantly lower sweating responses observed in the aged subjects. Grassi and colleagues (6) have identified an age-related decline in skin sympathetic nerve activity (SSNA) in response to thermal stimuli. This supports our findings of age-related decrements in sweating and SkBF in the absence of altered cholinergic sensitivity. Notably, however, the multinode approach used by these authors to measure SSNA prevents discrimination between sympathetic activity to blood vessels and sweat glands, preventing us from determining the relative decrements in vasomotor and sudomotor function with age. Furthermore, SSNA was recorded at only a single site and may not be generalizable to the entire skin surface. It is unclear as to the potential heterogeneity of SSNA to sudomotor and vasomotor function both in a young, healthy population and possible region-dependent alterations in SSNA with aging. Putative heterogeneity in SSNA responses to thermal stimuli may help explain regional sweating and SkBF observed over the body; however, we are unable to provide any definite conclusions as to its role in the nonuniform, age-related attenuation in vasomotor and sudomotor responses observed in the present data.
Finally, considering the age-related rightward shift in sweating thresholds, and the vasomotor and sudomotor dysfunction observed in response to whole body heating, it is not surprising that older individuals reached a 1°C ΔTor rise significantly faster than the younger group. Despite similarities in anthropometric characteristics and V̇o2max between groups, this relatively healthy group of aged individuals achieved the target Tcore 30 min faster than their younger counterparts. This highlights the important issue of increased susceptibility to heat-related illness and injury in older individuals, and particularly those with pathological conditions that may further compromise cardiovascular and thermoregulatory responses in the heat.
Limitations
Utilizing microdialysis in conjunction with sweating rate measurement may have potentially blocked sweat glands at the skin sites being measured, which we are unfortunately unable to detect. The present RSR data are consistent with literature values measured at similar sites, suggesting the effects of the microdialysis fibers to be minimal, or certainly consistent, across sites. During the ACh perfusion, RSR values were slightly low, which may be explained by the skin surface area of the capsules (4.46 cm2) compared with the 0.5-cm2 capsules used over microdialysis fibers by other authors (27). Moreover, maximal sweating rates may not have been elicited using a 100 mM ACh concentration during the dose-response protocol, with regional differences in sensitivity being missed at the higher concentrations. Other authors have obtained maximal sweating rates on the ventral forearm with a 1 M concentration of ACh (27). It does, however, seem unlikely that such large variation in RSR would emerge between the present data and perfusion of 1 M ACh concentration to explain the observed variation between sites during exposure to thermal stimuli. Regional HASG density was not measured in the present study, preventing the identification of the source of age-related decrements in sweating from reduced output per gland and/or HASG density. A further point to note during ACh perfusion is the possibility of eliciting sweating via both a direct stimulation of muscarinic receptors on the sweat glands and indirectly via stimulation of an axon reflex. We are unable to discern between the two pathways using the present sweat capsule technique; however, there were no differences in RSR between age groups in response to ACh perfusion, and any further discussion is beyond the scope of this study.
The aged subjects in the present study showed significantly higher LDL values compared with the young subjects, which our laboratory has recently demonstrated to cause attenuated cutaneous nitric oxide-dependent vasodilation (10). The present LDL values of the aged subjects were still within normal limits set forth by the American Heart Association. Although it remains possible that this may cause small changes in SkBF, recently published data from our laboratory suggest any potential changes to be minimal within these LDL limits (38). Finally, the present study included similar numbers of males and females within each age group; however, assessing sex differences was not a focus of this study and was underpowered to do so. Future studies examining regional age- and sex-related alterations in vasomotor and sudomotor function may be a logical extension of the present data.
Perspectives and Significance
Few studies have used sweat capsules and laser-Doppler flowmetry to simultaneously measure regional sweating and SkBF responses in conjunction with cutaneous drug delivery via microdialysis. Nonuniform decrements in cutaneous vasodilation and sweating responses to passive heating occurred in primary aged compared with young individuals, resulting in less regional variation with age. This is of particular importance in understanding the age-related progression of vascular and thermoregulatory dysfunction, in addition to highlighting the importance of the measurement site. An age-related right shift in sweating thresholds and decrements in sweating rates were more pronounced than the observed attenuation in cutaneous vasodilation, occurring independently of anthropometric variables and aerobic fitness. Considering sweating is the greatest avenue of heat loss from the body in hot environments and during exercise, the disproportionately high attenuation of sweating vs. SkBF responses with aging per se highlights the susceptibility of older individuals to heat-related illness and injury. Mechanistically, no differences in cholinergic sensitivity were observed between groups for RSR or SkBF, suggesting potential alterations in SSNA, thermoreceptor function, and/or attenuation of a putative cotransmitter as playing a role in these age-related declines. Such findings have important implications for understanding thermal and vascular dysfunction in disease populations, by first understanding regional variation in healthy populations.
GRANTS
This work was supported by National Institute on Aging Grant R01-AG-007004-22 (W. L. Kenney) and American College of Sports Medicine Foundation Research Endowment 2012 (C. J. Smith).
DISCLOSURES
The authors have nothing to disclose or conflicts of interest to report.
AUTHOR CONTRIBUTIONS
Author contributions: C.J.S., L.M.A., and W.L.K. conception and design of research; C.J.S. performed experiments; C.J.S. analyzed data; C.J.S., L.M.A., and W.L.K. interpreted results of experiments; C.J.S. prepared figures; C.J.S. drafted manuscript; C.J.S., L.M.A., and W.L.K. edited and revised manuscript; C.J.S., L.M.A., and W.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sue Slimak (Pennsylvania State University) for assistance with data collection and Jane Pierzga for Institutional Review Board application preparation.
REFERENCES
- 1.Cheuvront SN, Bearden SE, Kenefick RW, Ely BR, DeGroot DW, Sawka MN, Montain SJ. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol 107: 69–75, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Cotter JD, Patterson MJ, Taylor NA. The topography of eccrine sweating in humans during exercise. Eur J Appl Physiol Occup Physiol 71: 549–554, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ellis FP, Exton-Smith AN, Foster KG, Weiner JS. Eccrine sweating and mortality during heat waves in very young and very old persons. Isr J Med Sci 12: 815–817, 1976 [PubMed] [Google Scholar]
- 4.Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol 142: 219–232, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagge AP, Nishi Y. Heat exchange between human skin surface and thermal environment agents. In: Handbook of Physiology Reactions to Environmental Agents. Bethesda, MD: Am Physiol Soc, 1977, p. 69–72 [Google Scholar]
- 6.Grassi G, Seravalle G, Turri C, Bertinieri G, Dell'Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation 108: 729–735, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Havenith G, Inoue Y, Luttikholt V, Kenney WL. Age predicts cardiovascular, but not thermoregulatory, responses to humid heat stress. Eur J Appl Physiol Occup Physiol 70: 88–96, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293: H1090–H1096, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Holowatz LA, Kenney WL. Oral atorvastatin therapy increases nitric oxide-dependent cutaneous vasodilation in humans by decreasing ascorbate-sensitive oxidants. Am J Physiol Regul Integr Comp Physiol 301: R763–R768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Holowatz LA, Thompson CS, Kenney WL. l-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 574: 573–581, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe Iii HR, Heidal K, Choi MD, Kraus RM, Boyle K, Hickner RC. Increased adipose tissue lipolysis after a 2-week high-fat diet in sedentary overweight/obese men. Metabolism 60: 976–981, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue Y. Longitudinal effects of age on heat-activated sweat gland density and output in healthy active older men. Eur J Appl Physiol Occup Physiol 74: 72–77, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Inoue Y, Havenith G, Kenney WL, Loomis JL, Buskirk ER. Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int J Biometeorol 42: 210–216, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Inoue Y, Nakao M, Araki T, Murakami H. Regional differences in the sweating responses of older and younger men. J Appl Physiol 71: 2453–2459, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Nakao M, Okudaira S, Ueda H, Araki T. Seasonal variation in sweating responses of older and younger men. Eur J Appl Physiol Occup Physiol 70: 6–12, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Inoue Y, Shibasaki M. Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating (Abstract). Eur J Physiol 74: 84, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Inoue Y, Shibasaki M, Hirata K, Araki T. Relationship between skin blood flow and sweating rate, and age related regional differences. Eur J Appl Physiol 79: 17–23, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Shibasaki M, Ueda H, Ishizashi H. Mechanisms underlying the age-related decrement in the human sweating response. Eur J Appl Physiol Occup Physiol 79: 121–126, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Kenney WL, Anderson RK. Responses of older and younger women to exercise in dry and humid heat without fluid replacement. Med Sci Sports Exerc 20: 155–160, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol 65: 1082–1086, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol 65: 1082–1086, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol 272: H1609–H1614, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kimura K, Low DA, Keller DM, Davis SL, Crandall CG. Cutaneous blood flow and sweat rate responses to exogenous administration of acetylcholine and methacholine. J Appl Physiol 102: 1856–1861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Love AHG, Shanks RG. The relationship between the onset of sweating and vasodilation in the forearm during body heating. J Physiol (Lond) 162: 121–128, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado-Moreira CA, Smith FM, van den Heuvel AM, Mekjavic IB, Taylor NA. Sweat secretion from the torso during passively-induced and exercise-related hyperthermia. Eur J Appl Physiol 104: 265–270, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Natsume K, Ogawa T, Sugenoya J, Ohnishi N, Imai K. Preferred ambient-temperature for old and young men in summer and winter. Int J Biometeorol 36: 1–4, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol 90: 757–762, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Smith CJ, Havenith G. Body mapping of sweating patterns in athletes: a sex comparison. Med Sci Sports Exerc 44: 2350–2361, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Smith CJ, Havenith G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur J Appl Physiol 111: 1391–1404, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Smith CJ, Kenney WL, Alexander LM. Regional relation between skin blood flow and sweating to passive heating and local administration of acetylcholine in young, healthy humans. Am J Physiol Regul Integr Comp Physiol 304: R566–R573, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol 112: 791–797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner JA, Robinson S, Tzankoff SP, Marino RP. Heat tolerance and acclimatization to work in the heat in relation to age. J Appl Physiol 33: 616–622, 1972 [DOI] [PubMed] [Google Scholar]
- 40.Yamashita Y, Ogawa T, Ohnishi N, Imamura R, Sugenoya J. Local effect of vasoactive intestinal polypeptide on human sweat-gland function. Jpn J Physiol 37: 929–936, 1987 [DOI] [PubMed] [Google Scholar]