Abstract
When freshwater turtles acclimatize to winter hibernation, there is a gradual transition from aerobic to anaerobic metabolism, which may require adjustments of blood O2 transport before turtles become anoxic. Here, we report the effects of protons, anionic cofactors, and temperature on the O2-binding properties of isolated hemoglobin (Hb) isoforms, HbA and HbD, in the turtle Trachemys scripta. We determined the primary structures of the constituent subunits of the two Hb isoforms, and we related the measured functional properties to differences in O2 affinity between untreated hemolysates from turtles that were acclimated to normoxia and anoxia. Our data show that HbD has a consistently higher O2 affinity compared with HbA, whereas Bohr and temperature effects, as well as thiol reactivity, are similar. Although sequence data show amino acid substitutions at two known β-chain ATP-binding site positions, we find high ATP affinities for both Hb isoforms, suggesting an alternative and stronger binding site for ATP. The high ATP affinities indicate that, although ATP levels decrease in red blood cells of turtles acclimating to anoxia, the O2 affinity would remain largely unchanged, as confirmed by O2-binding measurements of untreated hemolysates from normoxic and anoxic turtles. Thus, the increase in blood-O2 affinity that accompanies winter acclimation is mainly attributable to a decrease in temperature rather than in concentrations of organic phosphates. This is the first extensive study on freshwater turtle Hb isoforms, providing molecular evidence for adaptive changes in O2 transport associated with acclimation to severe hypoxia.
Keywords: globin, adaptation, hypoxia, allostery
freshwater turtles are among the vertebrates that are most tolerant of severe hypoxia or even anoxia, and they are known to stay submerged for minutes to hours when diving during summer or for even months during winter hibernation (46). This outstanding ability to tolerate prolonged O2 deprivation is made possible by the abilities of freshwater turtles to drastically decrease metabolic rate and to tolerate oxidative stress following reoxygenation, as well as to tolerate increased levels of metabolic end-products (4), in particular, lactate and protons from glycolysis (45), but also free Ca2+ (23). Before turtles become anoxic during winter submergence, there is a gradual transition from aerobic to anaerobic metabolism (24), as ambient temperature and metabolic rate decrease, which may, in principle, require adjustments of blood O2 transport, while animals undergo prolonged diving but still have access to air. In the case of the painted turtle (Chrysemys picta), depression of metabolism results in a gradual decrease in the erythrocytic ATP concentration (28). Given that ATP is the major organic phosphate in turtle red blood cells (3, 28) and that it serves as the major allosteric regulator of O2 affinity in the hemoglobins (Hbs) of turtles and most other ectothermic vertebrates (48), reductions in ATP levels might be expected to gradually induce a left shift of the O2-binding curve and decrease O2 delivery to tissues during the progressive decrease in the O2 consumption rate (28). At the same time, the decrease in temperature during winter will have a similar effect on the Hb-O2 affinity (10, 28). In principle, erythrocytic responses to hypoxic or anoxic conditions could also involve regulatory adjustments in the relative concentrations of alternative Hb isoforms (isoHbs) that have different O2-binding properties.
During postnatal life, turtles coexpress two functionally distinct isoHb tetramers, HbA (αA2β2) and HbD (αD2β2) (33, 41), as do the majority of other sauropsid taxa that have been examined (8, 9, 11, 14, 17, 21, 22, 40). The HbA isoform incorporates products of the αA-globin gene, and HbD incorporates products of the αD-globin gene. Among birds and reptiles that have been investigated to date, HbD has a consistently higher O2 affinity than HbA. Given that the αD-globin gene is the duplicated product of an embryonic α-like globin gene, the elevated O2 affinity of HbD may reflect a retained ancestral feature that is characteristic of embryonic Hb (19).
To understand the coupling between metabolic adjustments and O2 transport in the freshwater turtle, Trachemys scripta (red-eared slider), we have investigated the O2-binding properties of HbA and HbD, their allosteric regulation by protons and anions, and the effect of temperature. We also sequenced the full complement of adult-expressed globin genes in T. scripta to gain insights into the structural basis of the observed functional properties of the HbA and HbD isoforms and the differences between them. The main objectives were 1) to identify factors that may regulate the oxygenation in red blood cells during acclimatization to anoxia, and 2) to characterize the nature of isoHb differentiation.
MATERIALS AND METHODS
Animals and blood samples.
Adult red-eared sliders Trachemys scripta with a body mass of 396 ± 68 g (means ± SD) were kept at 21°C in aquaria at the animal care facility at Zoophysiology, Aarhus University, with free access to dry platforms for thermoregulation (25). To obtain maximal changes in red blood cell allosteric cofactors, we exposed turtles to anoxia using a controlled protocol. In the acclimatization protocol, turtles were exposed to 12°C for 1 wk, and then to 5°C for 2 days followed by 10 days with (normoxia, n = 5) or without (anoxia n = 5) access to air at 5°C. Access to air was denied by keeping the turtles submerged, as described in detail previously (25). At the conclusion of the acclimation treatment, turtles were euthanized with 1 ml 20% pentobarbital sodium, the plastron was excised with a bone saw, and blood was collected by cardiac (sinus) puncture using a heparinized syringe. Blood was centrifuged at 10,000 g for 2 min, plasma was removed, and packed red blood cells were immediately frozen in liquid nitrogen and stored at −80°C. Procedures were in accordance with the laws of animal care and experimentation in Denmark, and protocols were approved by the Animal Experimentation Board.
Preparation and analysis of hemolysate by isoelectrofocusing.
An approximately five-fold volume of ice-cold 10 mM HEPES buffer, at pH 7.8, was added to the frozen red blood cells and left on ice for 20 min. Hemolysates from normoxic and anoxic turtles were centrifuged at 9,000 g for 10 min at 15°C to remove membranes and cellular debris and stored in aliquots at −80°C. Hb multiplicity in individual hemolysates from normoxic and anoxic turtles was analyzed by isoelectric focusing (IEF) at a pH range of 3–9 on thin polyacrylamide gels using a PhastSystem (GE Healthcare, Uppsala, Sweden). Relative content of HbA and HbD was obtained by densitometric analysis of IEF gels using ImageJ (http://rsb.info.nih.gov/nih-image/).
Hemoglobin purification.
Separation of the hemolysate into HbA and HbD components and stripping of organic phosphates was achieved by FPLC-ion exchange chromatography using a HiTrap Q 5-ml column (GE Healthcare) equilibrated with 20 mM Tris·HCl, at pH 7.6, and eluted with a linear gradient of 0–400 mM NaCl at a flow rate of 1 ml/min. Individual peaks containing HbA and HbD were collected and concentrated by ultrafiltration (2,800 g, 4°C) in Amicon 4-ml Ultra centrifugal tubes fitted with a 10-kDa cutoff filter (Millipore, Tullagreen, Ireland). Purity of separated Hbs was subsequently verified by IEF. Purified Hbs were then dialyzed against three changes of a 200-fold volume of 10 mM HEPES buffer pH 7.6 at 4°C to eliminate NaCl, concentrated to a final heme concentration of 1.3 mM, and stored in aliquots at −80°C.
O2 equilibria.
Solutions of HbA and HbD were freshly prepared in 0.1 M HEPES at 0.2 mM heme in the absence and presence of 0.1 M KCl and 0.15 mM ATP (ATP/Hb4=3). O2 equilibria were then measured using a modified diffusion chamber connected to two serially coupled Wösthoff gas mixing pumps (Wösthoff, Bochum, Germany) to create humidified gas mixtures at varying O2 tensions by mixing pure N2 (>99.998%) and atmospheric air, as previously described (51, 52). Using 3–6-μl samples, we monitored absorbance (A) at 436 nm after equilibration with pure N2 and pure O2 to obtain absorbance at zero (A0) and full O2 saturation (A100), respectively, and fractional saturations (S) were then obtained by stepwise equilibration with known Po2 values (Eq. 1):
| (1) |
O2 equilibrium curves were generated from the fractional saturations as a function of Po2. The O2 tension and cooperativity coefficient at half saturation, P50 and n50, respectively, were calculated from the zero intercept and the slope, respectively, of linear regression (r2 ≥ 0.99) of Hill plots, log[S/(1− S)] vs. logPo2, based on ≥4 equilibrium saturation steps in the 20–80% saturation range. The apparent heat of oxygenation (ΔHapp) was calculated from the van't Hoff equation:
| (2) |
where R is the gas constant and T is absolute temperature (Kelvin).
The pH of Hb and hemolysate solutions used in the determination of O2 equilibrium curves was measured at the same temperature of the experiment using a Mettler Toledo thermostatted pH/ion meter S220 (Schwerzenbach, Switzerland). The Bohr factor (φ=ΔlogP50/ΔpH), equivalent to the additional number of protons bound per O2 molecule (i.e., the number of additional protons released upon oxygenation when the Bohr factor is negative) was calculated in the 6.9–8.0 pH range, with logP50 values at the exact pH values interpolated from the logP50 vs. pH plot. The apparent affinity constant of oxygenation-linked ATP binding to HbA and HbD (KATP) was measured at 0.2 mM heme in 0.1 M HEPES in the absence and presence of 0.1 M KCl at pH 6.9 and 20°C. By fitting a hyperbolic function to the data, the apparent affinity constant was calculated as the ATP concentration at which the increase in logP50 was half the maximum (20). From these data, the apparent O2-linked affinity constant for Cl− binding to the same ATP site of each Hb was estimated from the relationship (7):
| (3) |
To establish whether acclimation to anoxia had generated changes in red blood cell composition of organic phosphates, O2 equilibria of individual hemolysates from normoxic and anoxic samples (unstripped, i.e., still containing endogenous ATP) were measured in 0.1 M HEPES, pH 7.5, at 1.0 mM heme concentration at 10 and 20°C. Calculations of P50 and ΔHapp were made as described for the purified Hb samples.
Sulhydryl reactivity.
Cysteine reactivity was measured by monitoring the stoichiometric thiol-mediated conversion of 4-PDS (4,4′-dithiodipyridine) into 4-TP (4-thiopyridone) (6). A 5-molar excess 4-PDS over heme (∼6–8 μM) was added to purified HbA and HbD samples in 50 mM Tris buffer, pH 7.0 at 20°C. Concentration of 4-TP (equivalent to that of protein reacting thiols) was calculated using the extinction coefficient at 324 nm of 19.8 mM−1/cm (13, 15). Purified HbA and HbD had been treated with a 5-molar excess of DTT for 30 min at room temperature and desalted on a PD-10 column (GE Helathcare) before reaction with 4-PDS to eliminate possible S-S bonds.
Statistical analysis.
Values are presented as means ± SE. Effect of temperature and anoxia exposure on P50 was tested using a 2-way ANOVA. The effect of anoxia exposure on the relative densities of HbA and HbD and on hemolysate ΔHapp was tested using a t-test. Comparisons were considered to be significant when P ≤ 0.05.
Globin sequencing.
Nucleotide sequences of the αD-, αA-, and β-globin genes were obtained by sequencing a total mRNA library generated from red blood cells of an adult T. scripta. Briefly, total RNA was isolated with TRIzol (Life Technologies, Carlsbad, CA), and mRNA libraries were constructed using the Illumina TruSeq RNA sample prep kit (Illumina, San Diego, CA) and were sequenced as 100-bp paired-end reads in an Illumina HiSeq. The resulting reads were assembled with Trinity (12). We then used BLAST (2) to identify contigs containing α-globin and β-globin coding sequence, and the transcript identities were verified by comparison against available EST databases and the Chrysemys picta genome assembly (1). Sequence alignments and comparisons were conducted using MEGA version 5 (44). Reported amino acid sequences were based on conceptual translations of nucleotide sequences. All DNA sequences were deposited in GenBank under the accession nos. KF660537–KF660539.
RESULTS
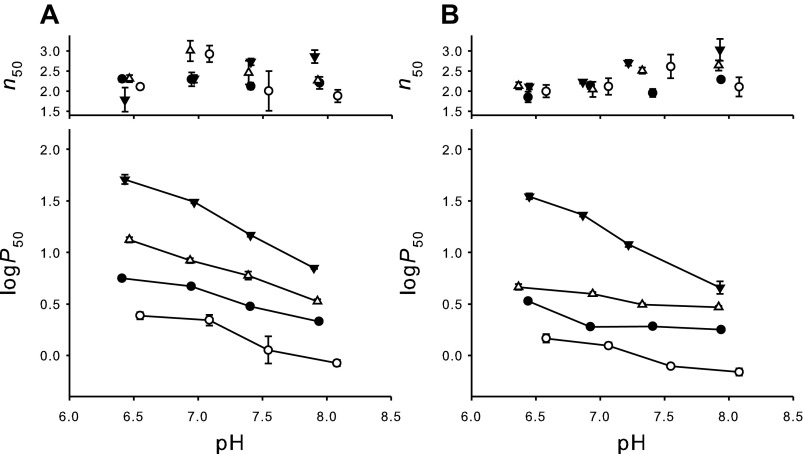
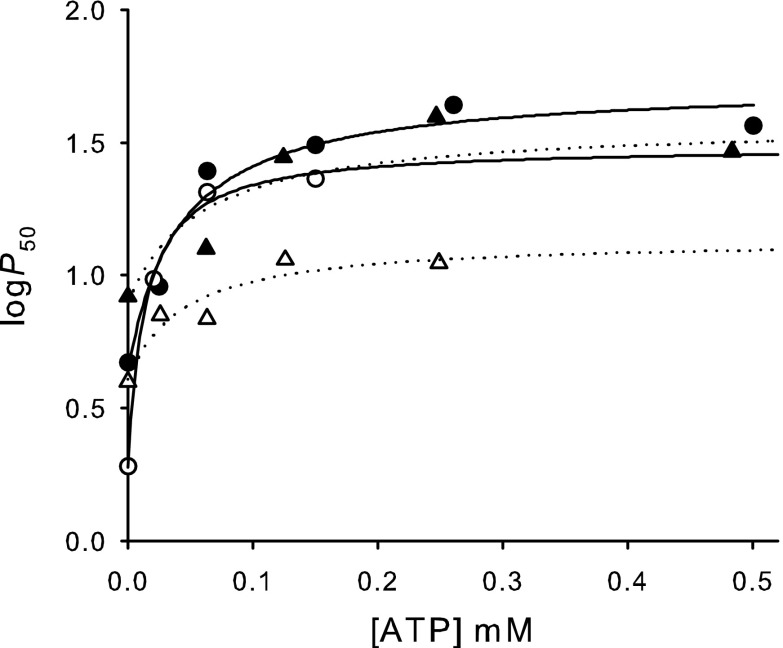
O2 equilibrium measurements of purified isoHbs showed that HbD exhibits a consistently higher O2 affinity compared with HbA under all conditions tested (e.g., P50=3.00 ± 0.07 and 1.92 ± 0.04 mmHg at pH 7.4 for stripped HbA and HbD, respectively) (Table 1, Fig. 1). Both isoHbs were highly cooperative (n50 > 1.95; Fig. 1) and showed a normal Bohr-effect, i.e., an increase in proton activity decreased Hb-O2 affinity. Bohr factors in the pH range 6.9–8.0 under physiological conditions (i.e., with ATP present) were similar in HbA and HbD (φ = −0.68 and −0.65, respectively; Table 1), whereas the Bohr effect was more pronounced in HbA than HbD in the absence of ATP at 20°C (φ = −0.34 and −0.03, respectively) (Fig. 1). The addition of ATP markedly decreased O2 affinity of both isoHbs across the full range of pH, whereas Cl− had less effect (Fig. 1). HbD had a higher affinity for ATP than HbA, with estimated apparent binding constants of 14 μM and 41 μM for HbD and HbA, respectively (Fig. 2). The apparent ATP binding constants increased (i.e., the ATP affinities decreased) in the presence of 0.1 M Cl− to 43 μM and 87 μM for HbD and HbA, respectively (Fig. 2), indicating that these anions compete, at least in part, for binding at the same site. However, the finding that binding constants are in the micromolar range indicates that the affinity for ATP for both Hbs remains high, even in the presence of 0.1 M Cl−. From these values, the apparent affinity for Cl− binding to the same ATP site was roughly estimated as ∼90 and ∼50 mM, for HbA and HbD, respectively. In HbD, logP50 at saturating concentrations of ATP increased by addition of Cl− (Fig. 2), indicating an additional O2-linked Cl− binding to a different site. HbA and HbD exhibited similar and pronounced temperature sensitivities, with overall heats of oxygenation (ΔHapp) of −12.9 and −11.8 kcal/mol at pH 7.4 for stripped HbA and HbD, respectively (1 kcal = 4.18 kJ).
Table 1.
Oxygenation properties (0.1 M HEPES, 20°C, pH 7.4) of T. scripta HbA and HbD, including O2 affinity, cooperativity coefficient, and Bohr factor
|
P50 (mmHg) |
n50 |
φ |
||||
|---|---|---|---|---|---|---|
| HbA | HbD | HbA | HbD | HbA | HbD | |
| Stripped | 3.00 ± 0.07 | 1.92 ± 0.04 | 2.13 ± 0.08 | 1.95 ± 0.10 | −0.34 ± 0.02 | −0.03 ± 0.02 |
| 0.15 mM ATP | 14.90 ± 0.56 | 9.36 ± 0.60 | 2.73 ± 0.08 | 2.70 ± 0.07 | −0.68 ± 0.02 | −0.65 ± 0.06 |
| 0.1 M Cl− | 5.87 ± 0.51 | 3.09 ± 0.10 | 2.46 ± 0.29 | 2.51 ± 0.07 | −0.40 ± 0.05 | −0.12 ± 0.03 |
Values are expressed as means ± SE. HbA, hemoglobin isoform A; HbD, hemoglobin isoform D; P50, O2 affinity; n50, cooperativity coefficient; φ, Bohr factor. φ = ΔlogP50/ΔpH, pH range 6.9–8.0.
Fig. 1.
Effect of pH, temperature, ATP, and KCl on cooperativity (n50; top) and Hb-O2 affinity (P50; bottom) for T. scripta HbA (A) and HbD (B). Conditions: stripped Hb (0.2 mM heme) in the absence (●) and the presence of 0.15 mM ATP (▲) or 0.1 M KCl (△) at 20°C and stripped at 10°C (○). All samples were run in triplicate in 0.1 M HEPES buffer. Data points with error bars indicate means ± SE.
Fig. 2.
Effect of ATP on the O2 affinity (P50) for HbA (solid symbols) and HbD (open) in the absence (circles) and presence (triangles) of 0.1 M KCl. Measurements were made at 0.2 mM heme, 0.1 M HEPES buffer, pH 6.9, and 20°C. Hyperbolic fittings to the data for HbA (solid lines) and HbD (dotted lines) are shown.
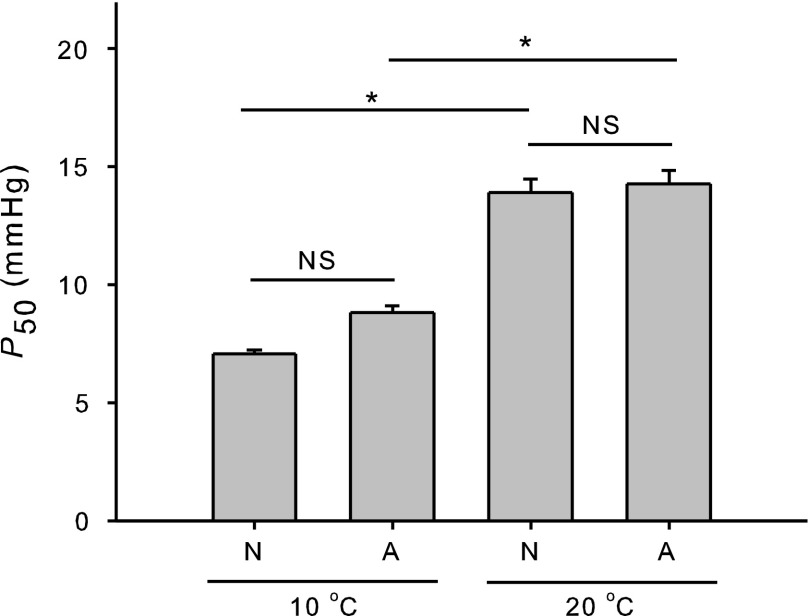
When analyzing the composite hemolysate, we found no difference in Hb-O2 affinity between hemolysates from anoxic and normoxic turtles (n = 5) when measured at the same temperature (e.g., P50=13.9 ± 1.3 mmHg and 14.3 ± 1.3 mmHg at 20°C, respectively; P = 0.468, two-way ANOVA) (Fig. 3), but we found a significant effect of temperature on P50 (P < 0.001, two-way ANOVA) (Fig. 3). Untreated hemolysates from normoxic and anoxic turtles showed large and similar (P = 0.174, t-test) heats of oxygenation, with ΔHapp values at pH 7.5 of −11.2 ± 0.8 and −7.4 ± 0.6 kcal/mol, respectively. Furthermore, isoelectric focusing of hemolysates from normoxic and anoxic turtles showed similar HbA:HbD ratios (73:26 ± 3.7 and 66:34 ± 2.5, respectively), indicating no significant change in the relative expression of isoHbs during acclimatization to anoxia (P = 0.226, t-test).
Fig. 3.
O2 affinity (P50, means ± SE) of unstripped hemolysates (i.e., containing endogenous levels of organic phosphates) from normoxic (N; n = 5) and anoxic (A; n = 5) T. scripta specimens at 10°C and 20°C in 0.1 M HEPES buffer, pH 7.5, at 1 mM heme. Asterisk and NS denote significant (P ≤ 0.05) and no significant difference, respectively, in P50 (two-way ANOVA).
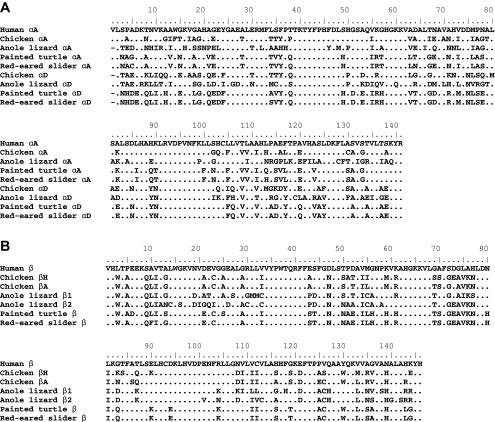
Sulhydryl reactivity measurements revealed a similar number of reactive Cys residues in HbA and in HbD (0.8 and 1.2 per heme, respectively). Given that the primary structure contained 3 Cys residues in the common β-chain (positions 23, 93, and 126), two Cys in the αA chain (positions 5 and 104), and one Cys in the αD chain (position 104) (Fig. 4), these data suggest that only a few of these Cys residues are able to react with 4-PDS. Sequencing of the adult-expressed globin mRNAs from T. scripta and comparisons with orthologous genes from other tetrapod vertebrates revealed clear homology with the turtle αA-, αD-, and β-globins (Fig. 4).
Fig. 4.
Alignment of amino acid sequences representing the complete repertoire of postnatally expressed α- and β-like globin genes from red-eared slider (T. scripta) and four outgroup taxa: human (Homo sapiens), chicken (Gallus gallus), anole lizard (Anolis carolinensis), and painted turtle (Chrysemys picta). Translated sequences of α-like (A) and β-like (B) globin genes are shown.
Sequence comparisons between T. scripta and the painted turtle (Chrysemys picta) yielded estimates of amino acid sequence divergence of 2.1, 0.7, and 4.1% for the αA-, αD-, and β-globins, respectively. Given the observed differences in O2 affinity and ATP affinity between HbA and HbD, we examined amino acid sequence differences between the αA- and αD-globin genes to gain insights into the structural basis of the observed functional differences. Amino acid sequence divergence between the αA- and αD-globins of T. scripta was 35%, corresponding to 50 amino acid replacements.
DISCUSSION
Turtles express two major isoHbs (HbA and HbD) that differ in their O2, ATP, and Cl− affinities, but the two isoHbs exhibit similar responses to temperature and pH (Fig. 1, Table 1) that are comparable to those of other vertebrate Hbs. Both isoforms show O2 affinities within the range reported previously for stripped Hbs of Emydidae (P50 = 1.9–10.7 mmHg at pH 8 and 20°C) (42), and they appear to be similar to those of reptilian Hbs investigated so far (32). The higher O2 affinity of HbD relative to HbA is consistent with the reported functional differences between the same isoHbs in birds (5, 14). A previous study (8) also suggested coexpression of isoHbs in isolated intact red blood cells of T. scripta.
In turtles and birds, HbA and HbD each consist of two α- and two β-chains, where the β-chains are identical in the two tetramers and the α-chains are encoded by the two distinct αA- and αD-globin genes (14, 38). Thus, the different functional properties of the two isoHbs must be attributable to amino acid substitutions between the two α-chains.
The O2 affinities of both isoHbs are reduced by the binding of ATP and Cl− (Fig. 1). The allosteric effect of both anionic cofactors is most pronounced at low pH, indicating cofactor-induced activation of the basic groups involved in proton binding. ATP had a much stronger inhibitory effect on Hb-O2 affinity compared with Cl− (as apparent binding constants for the two anions differ by three orders of magnitude), indicating that ATP binding results in better stabilization of the low-affinity T state. In contrast to bird Hbs, where HbA shows a stronger response to the allosteric cofactors ATP, IHP, and DPG (5, 14, 27, 47), turtle HbD shows a higher affinity for ATP compared with HbA (Fig. 2).
The αA-globin gene and the embryonic αE-globin gene originated via tandem duplication of a proto α-globin after the ancestral lineage of tetrapod vertebrates split from the lobe-finned fishes >400 million years ago in the Devonian, and the αD-globin gene originated subsequently via tandem duplication of the ancestral, single-copy αE-globin gene prior to the diversification of tetrapods (18, 19). Although the duplicative origins of the αA- and αD-globin genes vastly predate the divergence of extant sauropsid lineages, surprisingly consistent differences in the O2-binding properties of HbA and HbD have been maintained in birds and turtles (5, 14, 35, 53). Interestingly, this consistent difference in O2 affinity between the two isoHbs has persisted in spite of evolutionary changes in the relative sensitivity of HbA and HbD to different allosteric effectors.
The ATP binding site described in fish Hbs (31) consists of β2Glu for binding the NH2 group of adenosine and of β1Val-NH2, β82Lys, and β143Arg for binding the three phosphate groups. Although β-globin of red-eared slider and other turtles contains His at β2 and Leu at β143 [Fig. 4; (38)], our data indicate that HbA and HbD have both retained a high responsiveness to ATP. Furthermore, the higher ATP affinity found in HbD compared with HbA is noteworthy, as amino acid residues responsible for ATP binding in the Hbs of other vertebrates are found in the shared β-chain. Because the two isoHbs only differ in their α-chains, the differential ATP-binding affinities suggest that amino acid substitutions in the αA- and/or αD-globin chains may indirectly perturb ATP binding site in the β-β interface, as found recently for the DPG sensitivity of deer mouse Hb (29). Alternatively, the different ATP-binding affinities of HbA and HbD could be due to changes at possible α-chain ATP binding sites (14, 37, 43).
Experiments with 4-PDS show that HbA and HbD have the same content of fast reactive cysteines (∼1 per heme), likely including β93Cys, which is highly conserved in mammals, birds, and reptiles (34), on the common β-chain. These reactive Hb thiols along with glutathione will then contribute to the high total thiol levels found previously in the red blood cells of this turtle species (25). Hb thiols (including perhaps other Cys residues that are not sufficiently exposed to the surface to react with 4-PDS) may then take part in the overall antioxidant defenses against reactive oxygen species, which are generated after hypoxia and reoxygenation, thereby contributing to the ability of this turtle species to survive extreme hypoxia (34).
During winter, turtles face changing O2 demands, which require acclimatization responses of the convective O2 transport system from the lungs to the respiring tissues before turtles become anoxic. In addition, turtles submerged in aerated water at low temperatures appear able to sustain low levels of aerobic energy metabolism by extrapulmonary O2 uptake (45). In principle, a change in the relative expression of Hb isoforms with distinct functional properties could potentially provide a mechanism for regulating the O2 affinity of blood. However, the HbA-to-HbD ratio was not affected by exposure to anoxia, as also found previously (28).
The concentration of red blood cell ATP in the normoxic and anoxic turtles used in this study was 3.5 and 1.2 mM, respectively (F. B. Jensen, personal communication). These values for red blood cell ATP concentrations are in good agreement with those of 5.9 and 1.3 mM for summer and winter painted turtles, respectively, measured in a previous study (28). As also proposed earlier (28), this decrease in ATP concentration would be expected to increase the Hb-O2 affinity. Turtle erythrocytes possess mitochondria, which permits potential regulation of blood O2 transport by the energy state of the red blood cell (49). However, our study shows that the O2 affinities of hemolysates remained unchanged after anoxia when measured at the same pH and temperature (Fig. 3), revealing that the changes in red blood cell ATP content occurring during anoxia acclimation did not affect Hb oxygenation. This can be explained by the high affinity (with equilibrium dissociation constants in the micromolar range) of the O2-linked ATP binding site of both HbA and HbD, whereby even when erythrocytic ATP concentrations decreases down to ∼1 mM, both Hbs would still be largely saturated with ATP (Fig. 2). For comparison, the apparent equilibrium dissociation constant for DPG in human Hb is ∼0.45 mM (0.1 M NaCl, 37°C) (20), i.e., ∼5- and 10-fold higher than the constants for ATP to turtle HbA and HbD, respectively. Together with a previous study (28) that also reported identical P50 values (measured at the same temperature) of whole blood from summer and winter submerged painted turtles, our data indicate that changes in the ionic composition of red blood cells do not have significant effects on the Hb-O2 affinity. This also implies that other potential allosteric cofactors of turtle Hb such as lactate and Ca2+ that are known to massively increase during severe hypoxia and anoxia (23, 45) should have limited effects on the Hb-O2 affinity, although this prediction remains to be confirmed. Furthermore, the effect of a blood pH decrease on the Hb-O2 affinity is less pronounced in vivo for winter-acclimated turtles than for summer turtles, partly because of the rise in pH induced by low temperatures during winter acclimation (28).
Conversely, the temperature drop during acclimation to anoxia in winter appears to be a major factor determining the position of the O2 equilibrium curve in vivo (Fig. 3), with changes in logP50 of ∼0.025/°C similar to that of ∼0.032 earlier found (28). According to the exothermic nature of Hb-O2 binding, a marked left shift of the O2-binding curve will occur during natural winter acclimation to hypoxia or even anoxia (10, 28). Here, we found values for the heat of oxygenation (ΔHapp) of the purified Hbs (−12.9 and −11.8 kcal/mol for HbA and HbD, respectively) and of the untreated hemolysates (−11.2 and −7.4 kcal/mol for normoxic and anoxic hemolysates, respectively) that are remarkably similar to those of −12.4 and −12.5 kcal/mol reported earlier for blood from winter turtles and summer animals, respectively (28), and that indicate a pronounced temperature sensitivity of turtle Hb oxygenation. The resulting higher Hb-O2 affinity at lower acclimation temperatures would then function to counterbalance O2 delivery to acidotic tissues, where O2 consumption is progressively depressed. This effect would prevent a harmful mismatch between O2 delivery and O2 consumption. Interestingly, hibernation in bears that is a nonhypoxic metabolic depression is also associated with a left-shifted O2 binding curve of the Hb, although the mechanism involves a combined decrease in temperature, as well as in the level of red blood cell organic phosphates (36). Furthermore, the observation that the heat of oxygenation remains constant in either hemolysate (this study) or whole blood (28), despite the large change in the red blood cell allosteric cofactor ATP, further supports the conclusion that in turtles Hb is fully saturated with ATP under normoxia as well as during anoxia.
Perspectives and Significance
This study shows that the main mechanism for regulating convective O2 transport in turtles during the progressive winter acclimation to O2 deprivation seems to be a temperature-dependent and ATP-independent increase in Hb-O2 affinity along with extensive reduction of heart rate (16, 24, 30) and peripheral vasoconstriction (39). Therefore, metabolic downregulation of red blood cell organic phosphate allosteric effectors found by us and others in turtles (28) and other hibernating (36, 50) or estivating (26) vertebrates appears to be a consequence of an as yet poorly understood general reorganization of metabolism, and it is not necessarily a cause of increased Hb-O2 affinity. Perhaps most interestingly, results of functional experiments yielded two findings with important implications for structure-function relationships of vertebrate Hbs, particularly of turtles and birds that express distinct HbA and HbD isoforms. First, HbA and HbD both retained a high sensitivity to ATP in spite of β-chain substitutions that eliminate positively charged residues that are known phosphate-binding sites in the Hbs of other vertebrates. Second, differences in ATP sensitivity between HbA and HbD must be attributable to α-chain substitutions, even though the main phosphate-binding site of vertebrate Hb comprises multiple, positively charged β-chain residues. These findings suggest that future structural investigations of turtle Hbs may reveal novel molecular mechanisms of allosteric regulation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.D. and F.G.H. performed experiments; C.D., J.F.S., and F.G.H. analyzed data; C.D., J.F.S., F.G.H., and A.F. interpreted results of experiments; C.D. and F.G.H. prepared figures; C.D. and A.F. drafted manuscript; C.D., J.F.S., F.G.H., and A.F. edited and revised manuscript; C.D., J.F.S., F.G.H., and A.F. approved final version of manuscript; A.F. conception and design of research.
ACKNOWLEDGMENTS
We thank Anny Bang, Signe Helbo, Tobias Wang and Heidi Meldgaard Jensen (Aarhus, Denmark) and Mike W. Vandewege (Mississippi State University) for assistance and an anonymous reviewer for useful comments on the manuscript. The research was funded by grants from the Danish Council for Independent Research, Natural Sciences (10-084565 to AF), the National Institutes of Health/National Heart, Lung, and Blood Institute (R01 HL087216 and HL087216-S1 to JFS), and the National Science Foundation (IOS-0949931 to JFS and EPS-0903787 to FGH).
REFERENCES
- 1.Abramyan J, Badenhorst D, Biggar KK, Borchert GM, Botka CW, Bowden RM, Braun EL, Bronikowski AM, Bruneau BG, Buck LT, Capel B, Castoe TA, Czerwinski M, Delehaunty KD, Edwards SV, Fronick CC, Fujita MK, Fulton L, Graves TA, Green RE, Haerty W, Hariharan R, Hillier LH, Holloway AK, Janes D, Janzen FJ, Kandoth C, Kong L, de Koning J, Li Y, Literman R, Mardis ER, McGaugh SE, Minx P, Mork L, 8217 Laughlin M O, Paitz RT, Pollock DD, Ponting CP, Radhakrishnan S, Raney BJ, Richman JM, St John J, Schwartz T, Sethuraman A, Shaffer B, Shedlock AM, Spinks PQ, Storey KB, Thane N, Thomson RC, Valenzuela N, Vinar T, Warren DE, Warren WC, Wilson RK, Zimmerman LM, Hernandez O, Amemiya CT. The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol 14: R28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 215: 403–410, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Bartlett GR. Phosphate compounds in reptilian and avian red blood cells; developmental changes. Comp Biochem Physiol A Physiol 61: 191–202, 1978 [Google Scholar]
- 4.Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu Rev Physiol 69: 145–170, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Borgese TA, Nagel RL. Differential effects of 2,3-DPG, ATP and inositol pentaphosphate (IP5) on the oxygen equilibria of duck embryonic, fetal and adult hemoglobins. Comp Biochem Physiol A Physiol 56: 539–543, 1977 [Google Scholar]
- 6.Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber RE. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J Biol Chem 279: 44417–44426, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Fersht A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. New York: W. H. Freeman, 1999 [Google Scholar]
- 8.Frische S, Bruno S, Fago A, Weber RE, Mozzarelli A. Oxygen binding by single red blood cells from the red-eared turtle Trachemys scripta. J Appl Physiol 90: 1679–1684, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Fushitani K, Higashiyama K, Moriyama EN, Imai K, Hosokawa K. The amino acid sequences of two alpha chains of hemoglobins from Komodo dragon Varanus komodoensis and phylogenetic relationships of amniotes. Mol Biol Evol 13: 1039–1043, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Glass ML, Boutilier RG, Heisler N. Ventilatory control of arterial Po2 in the turtle Chrysemys picta bellii: Effects of temperature and hypoxia. J Comp Physiol B 151: 145–153, 1983 [Google Scholar]
- 11.Gorr TA, Mable BK, Kleinschmidt T. Phylogenetic analysis of reptilian hemoglobins: trees, rates, and divergences. J Mol Evol 47: 471–485, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassetti DR, Murray JF., Jr Determination of sulfhydryl groups with 2,2′- or 4,4′-dithiodipyridine. Arch Biochem Biophys 119: 41–49, 1967 [DOI] [PubMed] [Google Scholar]
- 14.Grispo MT, Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Storz JF. Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J Biol Chem 287: 37647–37658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helbo S, Fago A. Allosteric modulation by S-nitrosation in the low-O2 affinity myoglobin from rainbow trout. Am J Physiol Regul Integr Comp Physiol 300: R101–R108, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Hicks JW, Wang T. Cardiovascular regulation during anoxia in the turtle: an in vivo study. Physiol Biochem Zool 71: 1–14, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hiebl I, Weber RE, Schneeganss D, Braunitzer G. High-altitude respiration of Falconiformes. The primary structures and functional properties of the major and minor hemoglobin components of the adult White-headed vulture (Trigonoceps occipitalis, Aegypiinae). Biol Chem Hoppe Seyler 370: 699–706, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann FG, Storz JF, Gorr TA, Opazo JC. Lineage-specific patterns of functional diversification in the α-and β-globin gene families of tetrapod vertebrates. Mol Biol Evol 27: 1126–1138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann FG, Storz JF. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol Biol Evol 24: 1982–1990, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hofmann O, Mould R, Brittain T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem J 306: 367, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaacks RE, Harkness DR, Adler JL, Goldman PH. Studies on avian erythrocyte metabolism: Effect of organic phosphates on oxygen affinity of embryonic and adult-type hemoglobins of the chick embryo. Arch Biochem Biophys 173: 114–120, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Isaacks RE, Harkness DR. Erythrocyte organic phosphates and hemoglobin function in birds, reptiles, and fishes. Am Zool 20: 115–129, 1980 [Google Scholar]
- 23.Jackson DC, Ultsch GR. Long-term submergence at 3°C of the turtle, Chrysemys picta bellii, in normoxic and severely hypoxic water. II. Extracellular ionic responses to extreme lactic acidosis. J Exp Biol 96: 29–43, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Jackson DC. Metabolic depression and oxygen depletion in the diving turtle. J Appl Physiol 24: 503–509, 1968 [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen SB, Hansen MN, Jensen FB, Skovgaard N, Wang T, Fago A. Circulating nitric oxide metabolites and cardiovascular changes in the turtle Trachemys scripta during normoxia, anoxia and reoxygenation. J Exp Biol 215: 2560–2566, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Johansen K, Lykkeboe G, Weber RE, Maloiy GMO. Respiratory properties of blood in awake and estivating lungfish, Protopterus amphibius. Respir Physiol 27: 335–345, 1976 [DOI] [PubMed] [Google Scholar]
- 27.Lutz PL. On the oxygen affinity of bird blood. Am Zool 20: 187–198, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Maginniss LA, Tapper SS, Miller LS. Effect of chronic cold and submergence on blood oxygen transport in the turtle, Chrysemys picta. Respir Physiol 53: 15–29, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340: 1324–1327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overgaard J, Gesser H, Wang T. Tribute to P. L. Lutz: cardiac performance and cardiovascular regulation during anoxia/hypoxia in freshwater turtles. J Exp Biol 210: 1687–1699, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Perutz MF, Brunori M. Stereochemistry of cooperative effects in fish and amphibian haemoglobins. Nature 299: 421–426, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Pough FH. Blood oxygen transport and delivery in reptiles. Am Zool 20: 173–185, 1980 [Google Scholar]
- 33.Ramirez JR, Dessauer HC. Isolation and characterization of two hemoglobins found in the turtle, Pseudemys scripta elegans. Proc Soc Exp Biol Med 96: 690–694, 1957 [DOI] [PubMed] [Google Scholar]
- 34.Reischl E, Dafre AL, Franco JL, Wilhelm Filho D. Distribution, adaptation and physiological meaning of thiols from vertebrate hemoglobins. Comp Biochem Physiol C 146: 22–53, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Reischl E, Höhn M, Jaenicke R, Bauer C. Bohr effect, electron spin resonance spectroscopy and subunit dissociation of the hemoglobin components from the turtle Phrynops hilarii. Comp Biochem Physiol Part B Comp Biochem 78: 251–257, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Revsbech IG, Malte H, Fröbert O, Evans A, Blanc S, Josefsson J, Fago A. Decrease in the red cell cofactor 2,3-diphosphoglycerate increases hemoglobin oxygen affinity in the hibernating brown bear Ursus arctos. Am J Physiol Regul Integr Comp Physiol 304: R43–R49, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Riccio A, Tamburrini M, Giardina B, di Prisco G. Molecular dynamics analysis of a second phosphate site in the hemoglobins of the seabird, south polar skua. Is there a site-site migratory mechanism along the central cavity? Biophys J 81: 1938–1946, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rücknagel KP, Braunitzer G. Hemoglobins of reptiles. The primary structure of the major and minor hemoglobin component of adult western painted turtle (Chrysemys picta bellii). Biol Chem Hoppe Seyler 369: 123–132, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Stecyk JAW, Overgaard J, Farrell AP, Wang T. α-Adrenergic regulation of systemic peripheral resistance and blood flow distribution in the turtle Trachemys scripta during anoxic submergence at 5°C and 21°C. J Exp Biol 207: 269–283, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Storz JF, Hoffmann FG, Opazo JC, Sanger TJ, Moriyama H. Developmental regulation of hemoglobin synthesis in the green anole lizard Anolis carolinensis. J Exp Biol 214: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan B, Riggs A. Structure, function and evolution of turtle hemoglobins-II. Electrophoretic studies. Comp Biochem Physiol 23: 449–458, 1967 [DOI] [PubMed] [Google Scholar]
- 42.Sullivan B, Riggs A. Structure, function and evolution of turtle hemoglobins-III. Oxygenation properties. Comp Biochem Physiol 23: 459–474, 1967 [DOI] [PubMed] [Google Scholar]
- 43.Tamburrini M, Riccio A, Romano M, Giardina B, di Prisco G. Structural and functional analysis of the two haemoglobins of the Antarctic seabird Catharacta maccormicki. Eur J Biochem 267: 6089–6098, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ultsch GR, Jackson DC. Long-term submergence at 3°C of the turtle, Chrysemys picta bellii, in normoxic and severely hypoxic water. I. Survival, gas exchange, and acid-base status. J Exp Biol 96: 11–28, 1982 [DOI] [PubMed] [Google Scholar]
- 46.Ultsch GR. Ecology and physiology of hibernation and overwintering among freshwater fishes, turtles and snakes. Biol Rev 64: 435–515, 1989 [Google Scholar]
- 47.Vandecasserie C, Paul C, Schnek A, Léonis J. Oxygen affinity of avian hemoglobins. Comp Biochem Physiol A Physiol 44: 711–718, 1973 [DOI] [PubMed] [Google Scholar]
- 48.Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir Physiol Neurobiol 144: 141–159, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Weber RE, Jensen FB. Functional adaptations in hemoglobins from ectothermic vertebrates. Annu Rev Physiol 50: 161–179, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Weber RE, Lykkeboe G. Respiratory adaptations in carp blood influences of hypoxia, red cell organic phosphates, divalent cations and CO2 on hemoglobin-oxygen affinity. J Comp Physiol 128: 127–137, 1978 [Google Scholar]
- 51.Weber RE. Cationic control of O2 affinity in lugworm erythrocruorin. Nature 292: 386–387, 1981 [Google Scholar]
- 52.Weber RE. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J Appl Physiol 72: 1611–1615, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Wood SC, Lykkeboe G, Johansen K, Weber RE, Maloiy GM. Temperature acclimation in the pancake tortoise, Malacochersus tornieri: Metabolic rate, blood pH, oxygen affinity and red cell organic phosphates. Comp Biochem Physiol A 59: 155–160, 1978 [Google Scholar]