Abstract
Previously, we investigated the role of neuropeptide Y and leptin-sensitive networks in the mediobasal hypothalamus in sleep and feeding and found profound homeostatic and circadian deficits with an intact suprachiasmatic nucleus. We propose that the arcuate nuclei (Arc) are required for the integration of homeostatic circadian systems, including temperature and activity. We tested this hypothesis using saporin toxin conjugated to leptin (Lep-SAP) injected into Arc in rats. Lep-SAP rats became obese and hyperphagic and progressed through a dynamic phase to a static phase of growth. Circadian rhythms were examined over 49 days during the static phase. Rats were maintained on a 12:12-h light-dark (LD) schedule for 13 days and, thereafter, maintained in continuous dark (DD). After the first 13 days of DD, food was restricted to 4 h/day for 10 days. We found that the activity of Lep-SAP rats was arrhythmic in DD, but that food anticipatory activity was, nevertheless, entrainable to the restricted feeding schedule, and the entrained rhythm persisted during the subsequent 3-day fast in DD. Thus, for activity, the circuitry for the light-entrainable oscillator, but not for the food-entrainable oscillator, was disabled by the Arc lesion. In contrast, temperature remained rhythmic in DD in the Lep-SAP rats and did not entrain to restricted feeding. We conclude that the leptin-sensitive network that includes the Arc is required for entrainment of activity by photic cues and entrainment of temperature by food, but is not required for entrainment of activity by food or temperature by photic cues.
Keywords: feeding, obesity, hypothalamus, saporin, BMAL-1, glucose
the arcuate nucleus (arc) of the hypothalamus is a key site for the regulation of feeding, adiposity, and metabolism, which is dependent on the adipose tissue-derived hormone leptin (19, 56). Receptors for leptin occur densely in the Arc and are often coexpressed with neuropeptide Y (NPY) (3, 52). The mediobasal hypothalamus, including the Arc, contributes to the hypothalamic control of circadian rhythms, including feeding and rest/activity (22, 49, 59, 28). Obese Zucker rats lacking leptin receptors maintain modified light-entrained temperature and locomotor rhythms and enhanced food anticipatory activity (41, 37). Diet-induced obesity decreases food anticipatory activity (43), suggesting that the Arc leptin/NPY circuits are critical to the anticipation of scheduled food availability, inhibiting activity when leptin levels are enhanced by obesity and facilitating activity when leptin signaling is absent (38). Similar observations in obese lesioned rats for core body temperature (Tb), activity, and food anticipation have been reported for rats with electrolytic lesions of the ventromedial hypothalamus (11) and for monosodium glutamate-treated rats (36), both of which have compromised Arc function. Moreover, the Arc contains oscillators with intrinsic rhythms lasting for days (1), including Per2 oscillators (23). Interestingly, Per2 mutant mice do not exhibit food anticipatory rhythms (21). Because the Arc is critical for the integration of signals important to feeding and metabolism, the Arc may also be critical for food anticipatory elevation of activity and temperature, which are integral to these functions.
Lesions targeted to the Arc block the feeding and anorexic effects of exogenous leptin, induce severe hyperphagia and obesification (7, 28), produce rest-activity disturbances (59), and result in circadian arrhythmia for ad libitum feeding (28). We hypothesized that leptin-receptive neurons in the Arc area are important in locomotor and temperature rhythms. To test this hypothesis, we targeted leptin receptor-expressing neurons within the Arc with the toxin saporin conjugated to leptin (Lep-SAP). This conjugate binds to the leptin receptor, is internalized, and ultimately causes cell death by disrupting ribosomal function (60). We examined Lep-SAP effects on locomotor and temperature rhythms under light-dark (LD) conditions and in constant darkness (DD). We restricted feeding to 4 h per day over 10 days to evaluate food anticipatory activity and temperature changes and followed with a 3-day fast to examine persistence of rhythm. The results demonstrate a required role for the leptin network of the arcuate nucleus in light-entrained activity rhythms and food-entrained temperature rhythms.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats, purchased from Simonsen Laboratories (Gilroy, CA), were housed individually in plastic cages in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were maintained on a 12-h light (0700–1900) and 12-h dark cycle with ad libitum access to standard pelleted rodent chow (F6 Rodent diet; Harlan Teklad, Madison, WI) and tap water, except as noted. Room temperature was set at 20°C. All experimental procedures were approved by Washington State University Institutional Animal Care and Use Committee, which conforms to National Institutes of Health guidelines.
Surgical instrumentation and injection of saporin conjugates.
Rats were anesthetized using 1.0 ml/kg body wt of a ketamine-xylazine-acepromazine cocktail [5 ml ketamine HCl, 100 mg/ml (Fort Dodge Animal Health, Fort Dodge, IA); 2.5 ml xylazine, 20 mg/ml, (Vedco, St. Joseph, MO); 1 ml acepromazine, 10 mg/ml (Vedco), and 1.5 ml 0.9% saline solution]. Stereotaxic microinjection parameters, modified from previous work using NPY-SAP and Lep-SAP to lesion Arc neurons (59, 28), were used to deliver Lep-SAP and control solution. Lep-SAP (24 ng/50 nl; Advanced Targeting Systems, Carlsbad, CA) was dissolved in 0.1 M PBS (pH 7.4) and injected bilaterally into two sites per side into the Arc. Controls were injected at the same sites with an equivalent amount of blank-sap (B-SAP), saporin conjugated to a “blank peptide” with no known function or receptor. Rostral injection sites were 2.5 mm caudal, ± 0.4 mm lateral to bregma, and 8.3 mm ventral to dura mater. Caudal sites were 3.5 mm caudal, ± 0.4 mm lateral to bregma, and 8.5 mm ventral to dura mater. Solutions were delivered through a pulled glass capillary pipette (30-μm tip diameter) using a Picospritzer (Parker; Cleveland, OH). Solutions were injected slowly over a 5-min period, and their movement through the injection pipette was monitored microscopically. Rats injected with Lep-SAP and B-SAP were each implanted with a PDT-4000 Emitter that was sewn into the inner surface of the abdominal muscles covering the peritoneal cavity for monitoring activity and temperature (Mini Mitter, Philips Respironics, Bend, OR).
Experimental schedules.
Twenty-two rats with Arc-targeted injections (Lep-SAP = 12, B-SAP = 10) were placed in standard plastic cages in an isolated room. Lep-SAP rats were in the static phase of obesification, during which they were not hyperphagic, as shown previously (28). Activity and temperature data were collected and evaluated with Vital View Data Acquisition Software (Mini Mitter, Philips Respironics), and the circadian rhythms were analyzed in 10-min bins using ClockLab (ActiMetrics). Double-raster plotted actograms (for activity) and tempograms (for temperature; see Ref. 50) were generated as percentiles, and Lomb-Scargle periodograms (44) were analyzed for significant circadian rhythmicity (i.e., possessing a tau of 20–28 h). The batch function, where all data for each group are averaged into a single plot, was used to examine rhythmic tendencies in eatograms and periodograms. Batched analyses are valuable in showing and quantifying strong synchronous rhythms within a group. However, batch analyses also can be misleading because they may obscure individual rhythms that are asynchronous across the group. Therefore, we analyzed and report both batch and individual data from our experiments. Group average waveforms for the last 4 days of restricted feeding were generated in 10-min bins to evaluate enhanced anticipation, as has been observed in obese Zucker rats (38). Additional group average waveforms for the last 4 days of ad libitum feeding in DD were generated in 10-min bins for comparison with the waveforms of restricted feeding. The rats were monitored over 49 days in light-dark (LD, 13 days) and dark-dark (DD, 36 days) conditions. During LD, lights came on at 7 AM. Rats were allowed ad libitum access to food for 13 days in DD followed by 10 days of food restriction (RF) to the 4-h period from 9 AM until 1 PM, while remaining under DD conditions. Rats were fasted for 3 days, immediately after restricted feeding, also in DD, for analysis of the persistence of activity and temperature rhythms, and were then returned to ad libitum feeding. Both anticipation and persistence are required for confirmation of entrainment. Entry to the testing room was restricted, and required maintenance was conducted at irregular times. After rats were removed from circadian test cages (5 mo after surgery), measures were taken for 24-h food intake. In addition, a glucose tolerance test was conducted in 3-h fasted rats challenged with 2 ml/kg ip of 50% glucose. Glucose was measured at intervals prior to and following glucose challenge using a hand-held glucose meter (Abbott Diabetes Care, Alameda, CA).
Real-time PCR.
The Lep-SAP lesion was analyzed using RT-PCR to detect Agrp and Pomc, genes expressed in leptin-sensitive neurons in the Arc. Rats were anesthetized deeply with isoflurane (Halocarbon Products) at the end of experimentation and killed by decapitation. After total RNA isolation from hypothalamic homogenates and reverse transcription, real-time PCR reaction was performed in triplicate using Platinum Taq DNA polymerase (Invitrogen), SYBR Green I, with 5 μl of diluted cDNA in a final reaction volume of 25 μl, as reported previously (28). The amplification was performed with a CFX96 real-time system (Bio-Rad Laboratories; Hercules, CA), by 40 cycles of denaturation at 94°C for 15 s, annealing at 58°C for 10–15 s and extension at 72°C for 15–20 s. Finally, a melting curve was generated by stepwise increases in temperature (0.5°C increase every 10 s) for 80 cycles starting at 55°C. The threshold cycle (Ct) was determined with CFX manager software (Bio-Rad Laboratories). Gene expression was evaluated by means of a comparative Ct method and normalized to β-actin expression. The primers used in the experiments were for agouti gene-related peptide (Agrp; GenBank accession no. AF206017), 5′-gca gac cga gca gaa gat gt-3′ and 5′-gac tcg tgc agc ctt aca ca-3′; for Pomc (no. BC058443), 5′-ctc ctg ctt cag acc tcc at-3′ and 5′-ttt cag tca agg gct gtt ca-3′; and for β-actin (no. BC063166), 5′-aga tta ctg ccc tgg ctc ct-3′ and 5′-aca tct gct gga agg tgg ac-3′. The dissociation curves of each primer pair used in the present study showed a single peak, and the samples tested after the PCR reactions had a single expected DNA band on agarose gels.
Immunohistochemistry for lesion analysis.
On the basis of recent reports of robust levels of BMAL-1 in the Arc (33, 40, 61), as well as in the dorsomedial nucleus (DMN) and suprachiasmatic nucleus (SCN), an additional subset of six rats (n = 3 controls and 3 Lep-SAP) was used to evaluate the lesion using BMAL-1 as the immunohistochemical marker. These rats were a subset of those prepared for rhythm analysis, but which were not needed for these tests. When killed, they were late in the static stage and comparable in weight to the rats used in rhythm analysis. At time point ZT8 (3 PM), rats were euthanized by deep isoflurane (Webster Veterinary Supply, Devens, MA) anesthesia and perfused transcardially with PBS (pH 7.4) and then with fresh 4% formalin solution prepared with phosphate buffer (PB) (pH 7.4). Brains were removed, postfixed at room temperature for 4 h in 4% formalin, and cryoprotected overnight in 25% sucrose solution. Coronal cryostat sections (30 μm) through the length of the Arc and SCN were directly mounted. Sections were processed using previously described immunohistochemical techniques (28). After pretreatment with 50% ethanol for 20 min, slides were washed (3 × 5 min) in 0.1 M PB and incubated for 1 h in 10% normal horse serum. The blocking solution was removed, and the tissue was incubated in the primary antibody, goat polyclonal anti-BMAL-1 (Santa Cruz SC-8550; lot no. D0209, 1:1,000), made up in 10% normal horse serum-TPBS. After 72 h, the primary antibody was removed, the sections washed (3 × 10 min) in TPBS, and then incubated in a secondary antibody (biotinylated donkey anti-goat 1:500; Jackson ImmunoResearch Laboratories, West Grove, PA) made in 1% normal horse serum-TPBS. After 24 h, the slide was washed (3 × 10 min) in TPBS, incubated with extravidin-peroxidase (1:1,500 in TPBS; Sigma-Aldrich) for 3 h, washed again (3 × 10 min), and reacted for visualization of BMAL-1 immunoreactivity using nickel-intensified diaminobenzidine in the peroxidase reaction to produce a black reaction product. Slides were then cover-slipped for microscopic evaluation.
Data analysis.
Data were statistically analyzed with SigmaStat or ClockLab (Actimetrics) software. Body weight, mean values, anticipation ratios, and table measures were analyzed by t-test. Anticipation ratios were calculated as the ratio of the mean values for 3 h immediately before RF divided by the mean 24-h values for each group over the last 4 days of restricted feeding (compare Ref. 38). Two-way repeated-measures or three-way ANOVA analyses were performed as appropriate. P < 0.05 was considered significant. Results are presented as means ± SE.
RESULTS
Lep-SAP reduces cellularity and Agrp and Pomc expression in Arc without damaging the SCN.
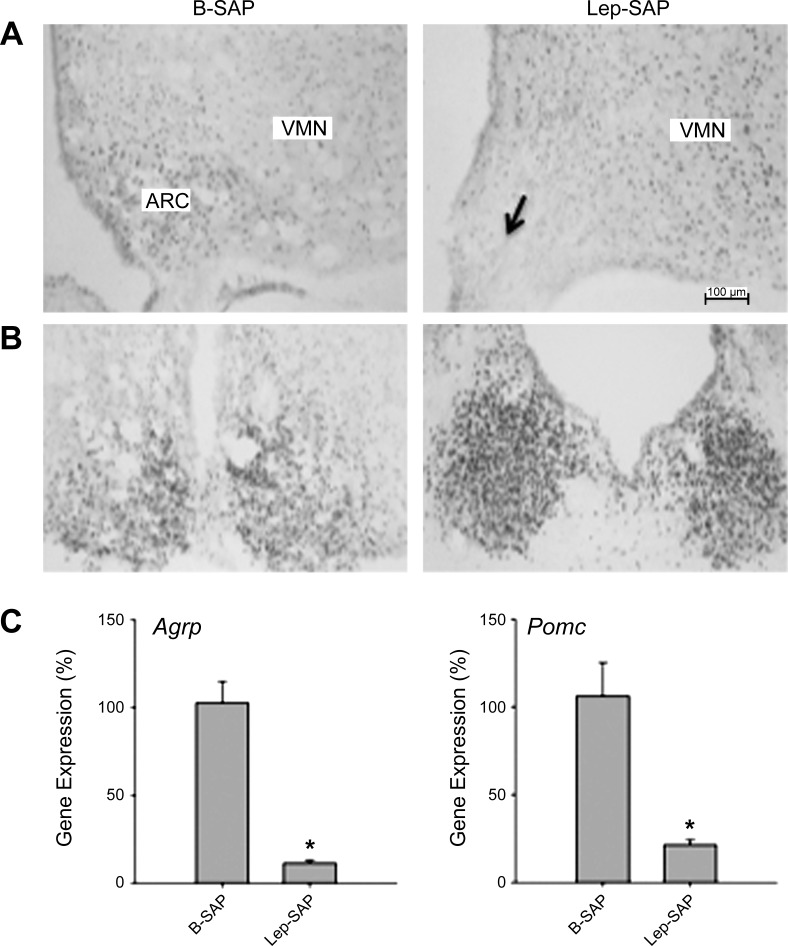
Photomicrographs of coronal sections showing BMAL-1 immunoreactivity in the Arc injection site and SCN are shown in Fig. 1, A and B. The Arc injection site in Lep-SAP rats was diminished in overall size and in the density of BMAL-1-immunoreactive nuclei. However, the SCN did not appear to be damaged by Arc-directed Lep-SAP. PCR analysis (Fig. 1C) revealed that expression of Agrp and Pomc were reduced to 11% and 21% of control, indicating that Lep-SAP was effective in lesioning Arc leptin-sensitive neurons.
Fig. 1.
Photomicrographs of coronal sections showing mediobasal hypothalamus showing the Arc (A) and the suprachiasmatic nucleus (SCN; B) in controls (left column) and Lep-SAP (right column)-injected rats. Lep-SAP injection site is indicated by the arrow in the Arc (arrow). The immunoreactive product (dark nuclear stain) reveals BMAL-1. The Lep-SAP lesion caused a loss of cellularity in the Arc, where many leptin receptor-expressing neurons are located. Calibration bar = 100 μm. C: results of RT-PCR analysis of hypothalamic Pomc and Agrp in B-SAP controls (n = 5) and Lep-SAP-lesioned rats (n = 6). These genes, expressed by leptin-sensitive neurons that heavily populate the Arc, were significantly reduced by Lep-SAP (*P < 0.001 vs. B-SAP control).
Arc Lep-SAP injections increased body weight, food intake, and glucose tolerance.
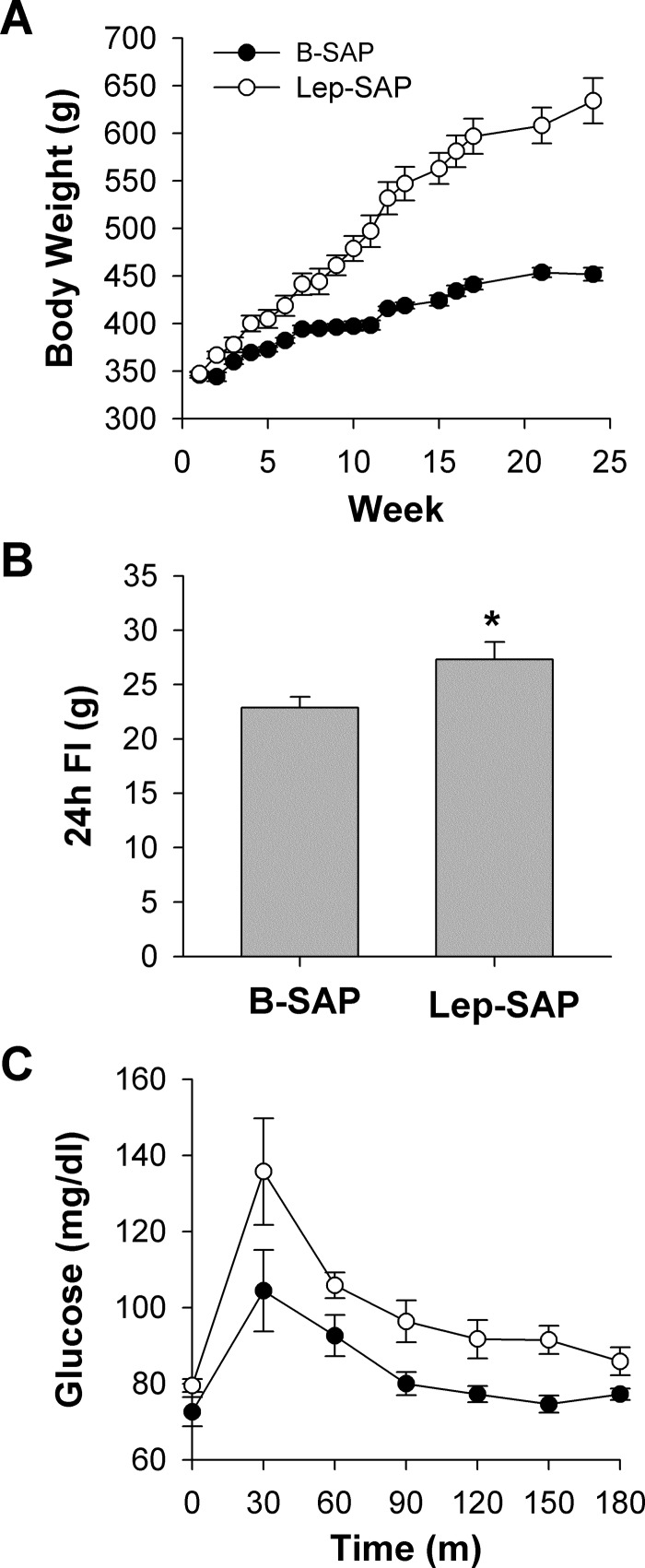
As we reported previously (28), Lep-SAP lesion of Arc neurons caused hyperphagia and rapid weight gain, referred to historically as the dynamic phase of an obesifying ventral hypothalamic lesion, followed by a phase of normophagia with near-normal rates of weight gain but sustained obesity, referred to as the static phase of the lesion. At surgery, body weights were not different between B-SAP (346.0 ± 3.0 g) and Lep-SAP rats (347.0 ± 2.3 g). Body weight, which was measured weekly throughout the experiment except during testing for circadian rhythms, is shown in Fig. 2A. Terminal body weights for Lep-SAP rats (634.0 ± 23.9 g) were significantly elevated compared with B-SAP rats (452.0 ± 6.8 g) with a maximum individual weight of 824 g. After circadian testing, 24-h food intake was measured daily for 3 days and adjusted for spillage. Lep-SAP rats displayed a continued mild, but significant, hyperphagia in the static phase (Fig. 2B).
Fig. 2.
Body weight (A), daily food intake (B), and glucose tolerance (C) in rats injected into the Arc with B-SAP or Lep-SAP. Body weight (A) was significantly elevated in Lep-SAP rats by the end of the first week after surgery (*P < 0.05). B and C: testing was conducted in the light period of the LD cycle 5 mo after surgery when circadian analysis was complete. Daily food intake at the end of treatment remained mildly elevated (B). Glucose tolerance tests (2 ml/kg of 50% glucose ip) in 3-h fasted rats resulted in significant main effects for group (P = 0.003) and time (P < 0.001), but the interaction was not significant (P = 0.491), and baseline glucose levels were within the normal range in Lep-SAP rats (C).
Although severely obese, the Lep-SAP rats did not develop severe diabetes, a condition that may be linked to circadian disruption (17). Their 3-h fasted blood glucose baseline levels 2 mo after surgery were in the normal range (79.5 ± 1.7 mg/dl) and not significantly different from B-SAP controls (72.6 ± 3.8 mg/dl; Fig. 2C). However, glucose tolerance curves of the Lep-SAP group were mildly elevated with significant main effects for time (P < 0.001) and group (P = 0.003) but not for their interaction (P = 0.491).
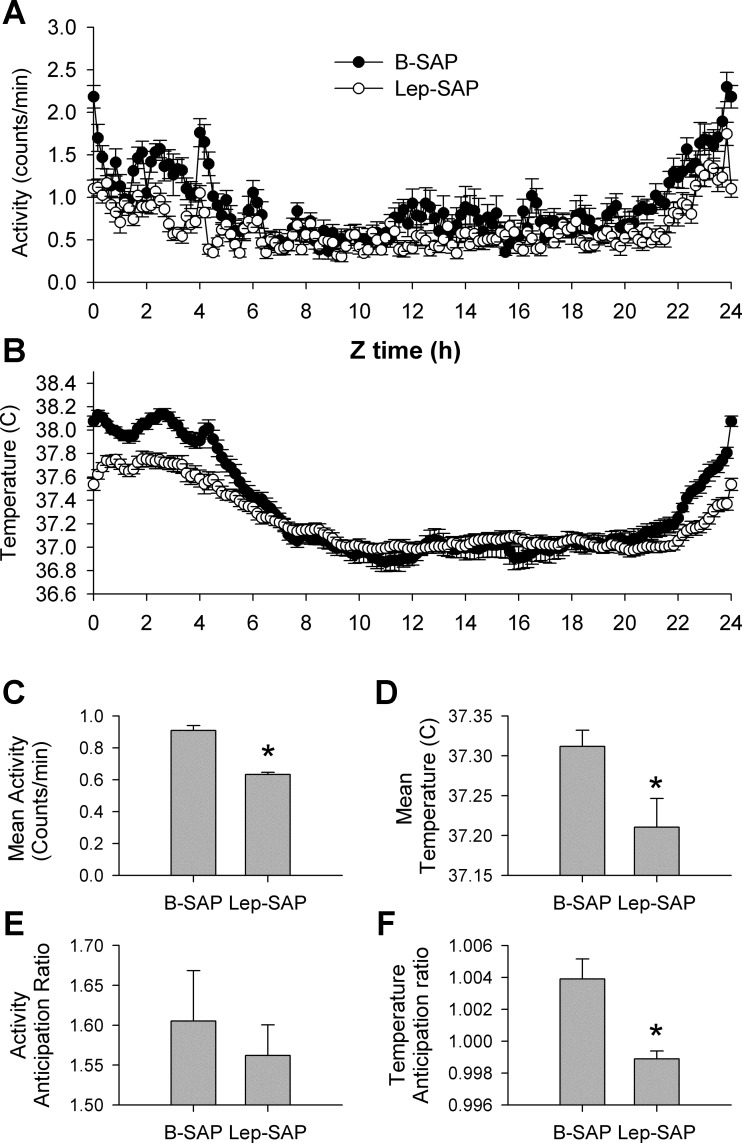
Activity, but not core temperature, was altered during the static phase of the Arc Lep-SAP lesion.
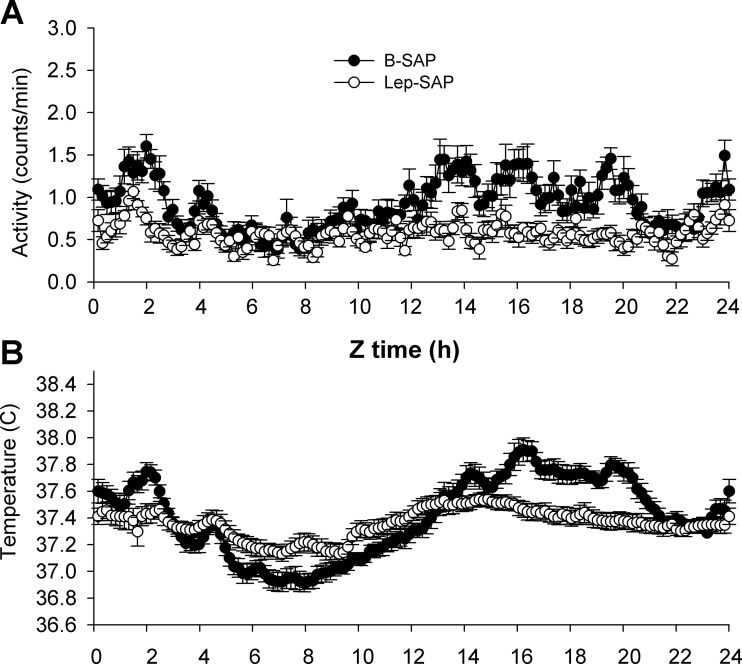
Table 1 provides mean 24-h core Tb and activity values for each of the five stages of the experiment for each group. Mean body temperature did not differ significantly between groups during any stage of the experiment. However, the range of temperature fluctuation was significantly less in Lep-SAP rats than in B-SAP rats for both maximum and minimum values. Lep-SAP rats were significantly less active than B-SAP rats during all stages of the experiment, as reflected in mean, maximum, and minimum values. The robustness of the activity rhythm was less than the robustness of the temperature rhythm, but waveform analysis (Fig. 8) clearly shows enough range and pattern of activity to provide rhythm analysis. There is sufficient robustness in activity levels to find a significant rhythm in Lep-SAP rats during LD cycling (Table 1). The loss of rhythm in Lep-SAP rats during DD is unlikely due to either decreased activity levels or lowered levels of robustness. Indeed, mean activity levels were not changed in the transition from LD to DD (Table 1).
Table 1.
Mean 24-h core body temperature and activity values for each of the five stages of the experiment
| LD | DD |
||||
|---|---|---|---|---|---|
| 13 days | AdLib1 13 days | FR 10 days | FAST 3 days | AdLib2 10 days | |
| Mean Tb, °C | |||||
| B-SAP | 37.52 ± 0.03 | 37.46 ± 0.02a | 37.33 ± 0.02a | 37.19 ± 0.03a | 37.42 ± 0.02a |
| Lep-SAP | 37.45 ± 0.04 | 37.40 ± 0.05 | 37.26 ± 0.04a | 37.16 ± 0.03a | 37.34 ± 0.04a |
| Max Tb, °C | |||||
| B-SAP | 38.43 ± 0.05 | 38.42 ± 0.07 | 38.43 ± 0.02 | 38.48 ± 0.05 | 38.31 ± 0.07 |
| Lep-SAP | 38.11 ± 0.03* | 38.08 ± 0.05* | 38.07 ± 0.05* | 38.06 ± 0.07* | 37.95 ± 0.05*a |
| Min Tb, °C | |||||
| B-SAP | 36.66 ± 0.08 | 36.65 ± 0.07 | 36.29 ± 0.02a | 35.92 ± 0.11a | 36.44 ± 0.08a |
| Lep-SAP | 36.83 ± 0.05* | 36.80 ± 0.09 | 36.68 ± 0.05*a | 36.59 ± 0.05*a | 36.63 ± 0.06*a |
| Activity | |||||
| Mean activity | |||||
| B-SAP | 1.05 ± 0.03 | 0.97 ± 0.10 | 0.94 ± 0.03a | 0.90 ± 0.03a | 0.74 ± 0.03a |
| Lep-SAP | 0.64 ± 0.03* | 0.60 ± 0.03* | 0.65 ± 0.03* | 0.69 ± 0.03* | 0.57 ± 0.03*a |
| Max activity | |||||
| B-SAP | 3.05 ± 0.13 | 3.17 ± 0.13 | 2.87 ± 0.16 | 2.62 ± 0.16a | 2.60 ± 0.15a |
| Lep-SAP | 1.83 ± 0.10* | 1.87 ± 0.09* | 2.13 ± 0.10*a | 1.94 ± 0.09* | 1.96 ± 0.08* |
For the first 13 days, the rats are in 12:12-h light-dark (LD) and, thereafter, in dark:dark (DD). For the first 13 days of DD, all rats were ad libitum fed (AdLib1). For the next 10 days, food availability was restricted to food availability for 4 h per day from 9 AM until 1 PM (FR). This was followed by a 3-day fast (Fast). Then all rats were returned to ad libitum feeding (AdLib2). Tb, body temperature.
Significant difference between groups (P < 0.05; “a” is significant difference within groups compared to LD (P < 0.05).
Fig. 8.
Group average wave forms (A and B), mean responses (C and D), and anticipation ratios (E and F) for locomotor (A, C, E) and temperature (B, D, F) rhythms in 10-min bins across the last 4 days of restricted feeding beginning at time 0 (Z0), the time of food availability. Restricted feeding occurs from Z0 through Z3; anticipation occurs at the end of the cycle. For activity, waveform patterns were similar between groups, with mean responses depressed in Lep-SAP rats (C), but anticipation ratios not significantly different. For temperature, waveform patterns were distinctly different (B), with mean responses depressed in Lep-SAP rats (D), and anticipation ratios elevated above baseline only in B-SAP rats. During the last 4 days of restricted feeding, the mean temperature of Lep-SAP rats was significantly reduced for the only time during the experiment (see Table 1). *Significant difference between groups by t-test (P < 0.05).
Periodicity and/or robustness of activity and temperature rhythms during ad libitum feeding were altered by Lep-SAP.
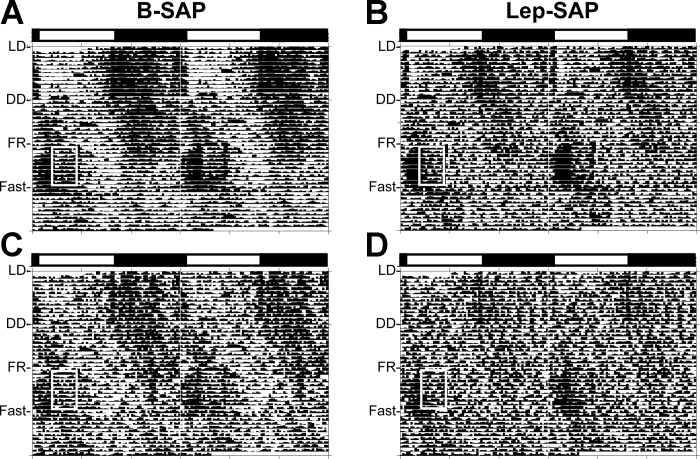
Table 2 provides quantitative data showing circadian period lengths and robustness of temperature and activity rhythms under the different photic and feeding conditions for each group. Actograms (Fig. 3) of these data are presented as double-raster plots over 49 days in B-SAP (n = 10) and Lep-SAP (n = 12) rats with both a batched group analysis and an individual example. Lomb-Scargle periodograms are presented for activity (Fig. 4). Tempograms showing robustness of temperature are presented in Fig. 5, while Lomb-Scargle plots also present data for temperature (Fig. 6) during each stage of the circadian experiment.
Table 2.
Mean 24-h rhythm analysis for core body temperature and activity
| LD | DD |
|||
|---|---|---|---|---|
| 13d | AdLib1 13 days | FR 10 days | AdLib2 10 days | |
| Tb °C | ||||
| Period, h | ||||
| B-SAP | 24.0 ± 0.0 | 24.4 ± 0.0* | 24.4 ± 0.2 | 24.8 ± 0.2a |
| Lep-SAP | 24.1 ± 0.0 | 24.3 ± 0.3 | 24.3 ± 0.3a | 24.6 ± 0.3 |
| Amplitude (robustness) | ||||
| B-SAP | 487.0 ± 34.5 | 305.9 ± 34.8a | 219.4 ± 41.7a | 181.7 ± 24.3a |
| Lep-SAP | 248.7 ± 34.4* | 118.6 ± 23.5*a | 207.4 ± 21.3 | 62.9 ± 9.5*a |
| % Rhythmic | ||||
| B-SAP | 100 | 100 | 100 | 100 |
| Lep-SAP | 100 | 100 | 100 | 67 |
| Activity | ||||
| Period, h | ||||
| B-SAP | 24.0 ± 0.0 | 24.3 ± 0.0a | 24.4 ± 0.1a | 24.1 ± 0.1 |
| Lep-SAP | 24.0 ± 0.1 | No rhythm | 24.0 ± 0.1* | 23.9 |
| Amplitude (robustness) | ||||
| B-SAP | 117.3 ± 13.6 | 40.3 ± 6.0a | 25.6 ± 8.1a | 32.3 ± 7.5a |
| Lep-SAP | 22.2 ± 2.8* | No rhythm | 24.7 ± 2.2 | 15* |
| % Rhythmic | ||||
| B-SAP | 100 | 80 | 100 | 30 |
| Lep-SAP | 50 | 0 | 100 | 8 |
Amplitude (robustness) refers to the consistency of the rhythm across days. For the first 13 days the rats are in 12:12-h light:dark (LD) and, thereafter, in dark:dark (DD). For the first 13 days of DD, all rats were ad libitum fed (AdLib1). For the next 10 days, food availability was restricted to food availability for 4 hours per day from 9 AM until 1 PM (FR). This was followed by a 3-day fast (insufficient time for rhythm analysis). Then all rats were returned to ad libitum feeding (AdLib2).
Asterisk is significant difference between groups (P < 0.05; “a” is significant difference within groups compared to LD (P < 0.05).
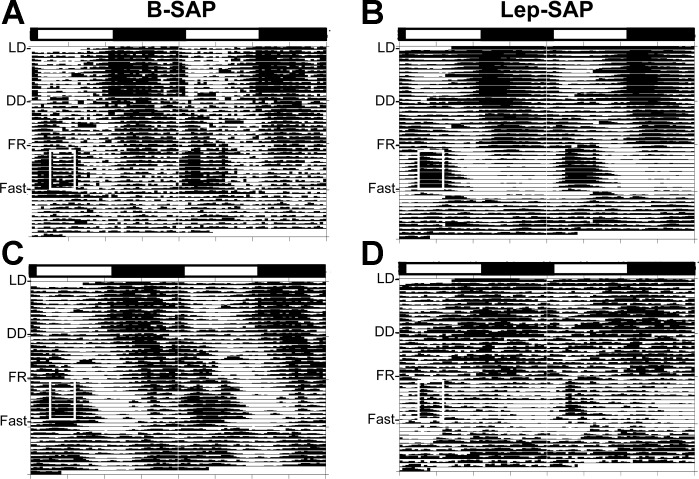
Fig. 3.
Double-raster plots for activity rhythms (actograms) over 49 days in B-SAP (n = 10) and Lep-SAP (n = 12) rats. Rats were maintained in 12:12-h light-dark (LD) for 13 days and, thereafter, maintained in dark:dark (DD). During DD rats had ad libitum access to food for 13 days to assess photically driven feeding rhythms, then access to food was restricted to food 4 h per day from 9 AM until 1 PM (white box) for 10 days to measure entrainment, followed immediately by a 3-day fast to evaluate persistence of rhythms. After the fast, the rats were returned to ad libitum feeding. The light bar marks the light/dark status of the rats during LD. A and B: collective batch actograms for B-SAP and Lep-SAP rats, respectively. C and D: actograms for an individual B-SAP or Lep-SAP rat, respectively.
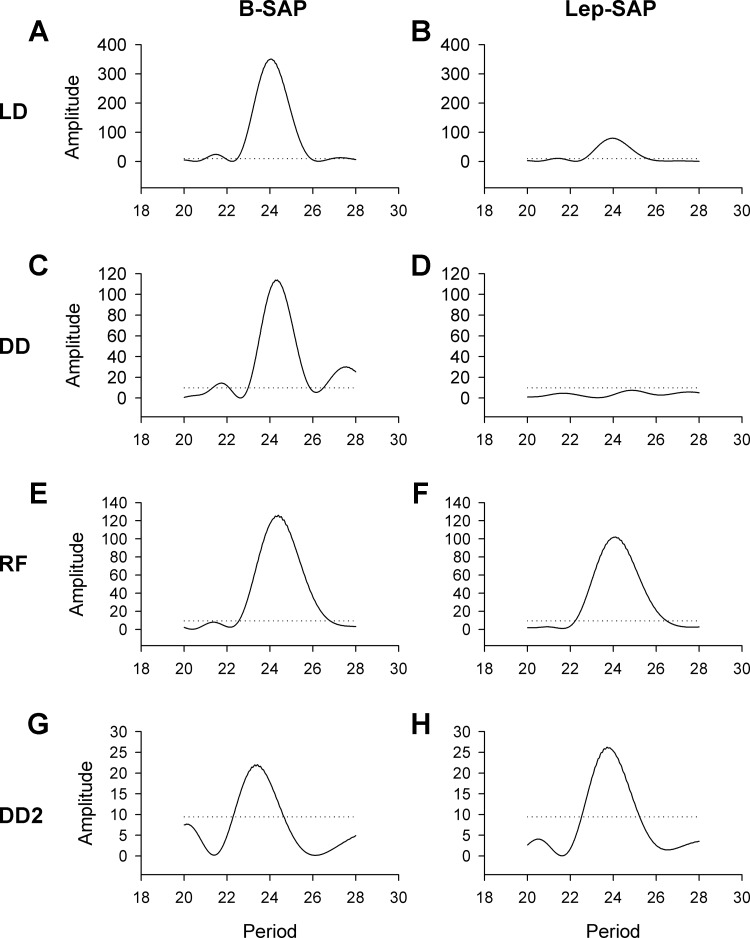
Fig. 4.
Activity Lomb-Scargle periodograms for B-SAP rats (A, C, E, G) and Lep-SAP rats (B, D, F, H) during light:dark (LD; A and B), dark:dark with ad libitum food (DD: C and D), and DD with restricted feeding (FR; E and F), and DD2 with ad libitum food (G and H). The dotted line represents statistical significance (P < 0.05). The robustness (or consistency) of the rhythm across time is indicated by the amplitude of the periodogram.
Fig. 5.
Double raster plots for temperature rhythms (tempograms) over 49 days as in Fig 4. A and B: collective batch actograms for B-SAP and Lep-SAP rats, respectively. C and D: actograms for an individual B-SAP or Lep-SAP rat. Anticipation of feeding elevated temperature before food was available in B-SAP rats (A, area immediately before white box) but not in Lep-SAP rats (B). Persistence of the elevated rhythm during the 3 days of fast occurs in B-SAP rats (A) but not Lep-SAP rats (B).
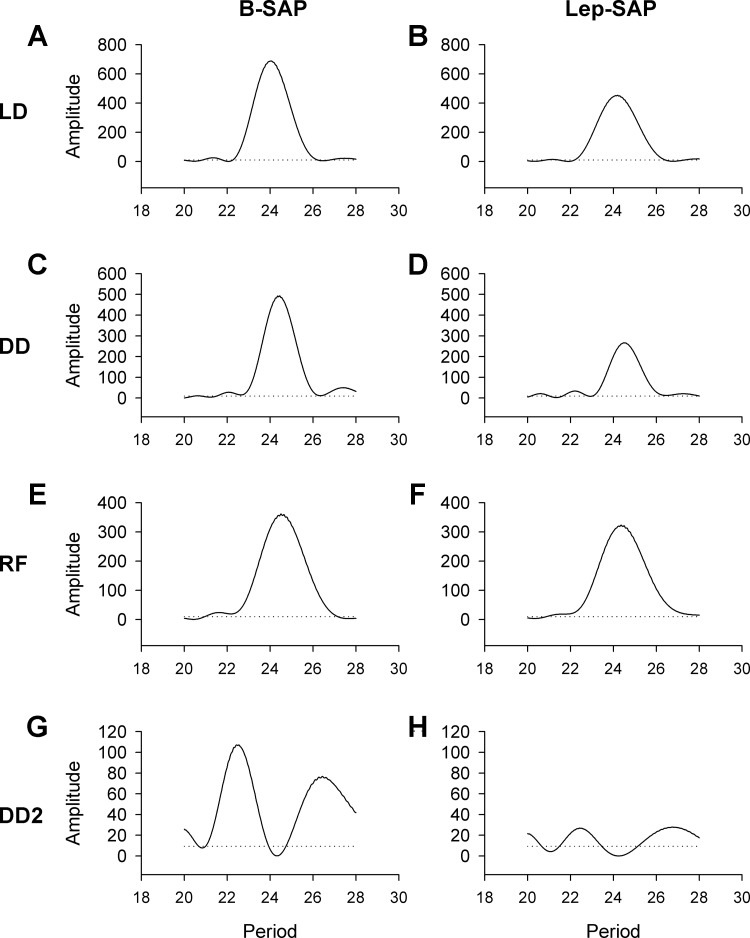
Fig. 6.
Temperature Lomb-Scargle periodograms for B-SAP rats (A, C, E, G) and Lep-SAP rats (B, D, F) during light:dark (LD; A and B), dark:dark with ad libitum food (DD: C and D), DD restricted feeding (FR; E and F), and with ad libitum food (DD2; G and H). The dotted line represents statistical significance (P < 0.05). The robustness (or consistency) of the rhythm across time is indicated by the amplitude of the periodogram.
The robustness (or consistency) of the rhythm across time is indicated by the amplitude of the periodogram. For activity, Lep-SAP rats were weakly rhythmic during LD and arrhythmic during DD compared with B-SAP rats. For activity, amplitude was always significantly higher in B-SAP rats than in Lep-SAP rats. Lep-SAP rats were arrhythmic in DD during ad libitum feeding. In contrast, temperature rhythms were always significant for both groups with taus in the circadian range. However, periodograms show that amplitude of the temperature rhythm was significantly higher in B-SAP rats than Lep-SAP rats under ad libitum conditions.
Activity but not temperature rhythm was entrained by restricted feeding in DD.
Grouped anticipatory and persistence rhythm analysis are presented for activity and temperature during the 2 h before food availability and the first hour of food availability (Fig. 7). Waveform analysis showing circadian patterns for activity and temperature during the last 4 days of restricted feeding are shown in Fig. 8, A and B, and anticipation ratios for locomotor activity and core temperature during this period are shown in Fig. 8, C–F.
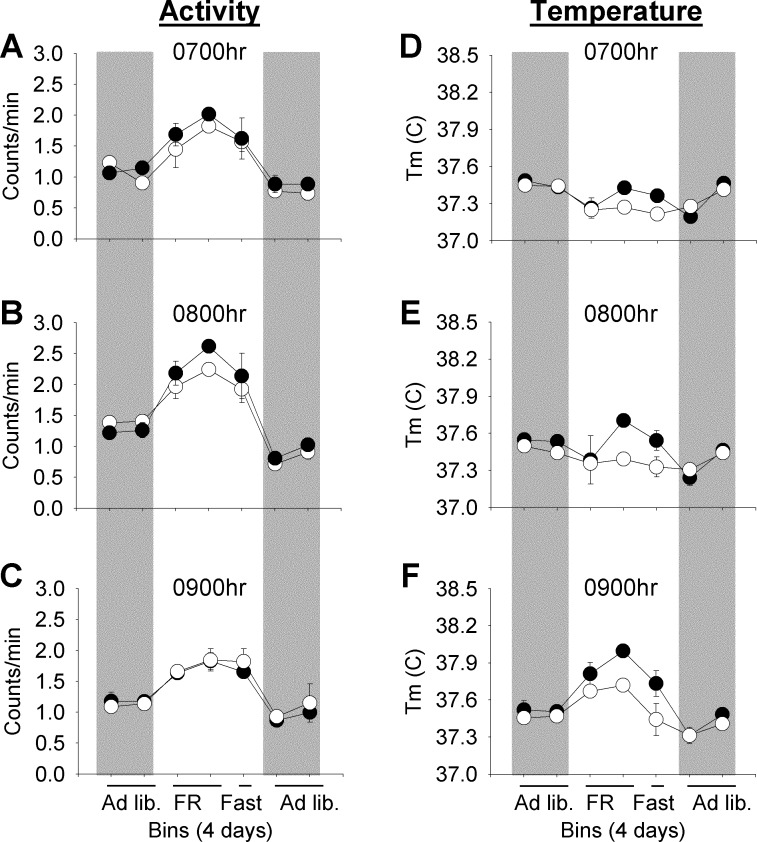
Fig. 7.
Anticipatory and persistence rhythms in response to 4-h restricted feeding (FR) during DD in Lep-SAP (○) rats compared with B-SAP (●) rats. One-hour activity counts/min or mean core body temperature are binned over 4 days during ad libitum feeding, restricted feeding, and fasting (a 3-day bin), followed by a second ad libitum feeding period. A–C: binned hourly activity measures taken 2 h (0700) and 1 h (0800) before FR and during the first hour of FR (0900), respectively. D–F: equivalent measures for body temperature. Ad libitum feeding is indicated by gray rectangles. There are two bins during ad libitum feeding, two bins during FR, one bin during fasting, and two bins during the final ad libitum feeding period. Activity did not differ significantly between groups during the first ad libitum feeding period but was significantly elevated (P < 0.05) during each of the 3 h during the FR period and during the same hours in the subsequent 3-day fast. Body temperature (Tb) was not different between B-SAP and Lep-SAP rats during ad libitum feeding before or after FR. Tb was sharply elevated during the 0800 (E) in B-SAP rats but not in Lep-SAP rats. Tb increased in Lep-SAP rats only during feeding (0900; F). In B-SAP rats, but not in Lep-SAP rats, Tb was significantly elevated (P <0.05) during the fast compared with subsequent ad libitum feeding during both 0800 and 0900.
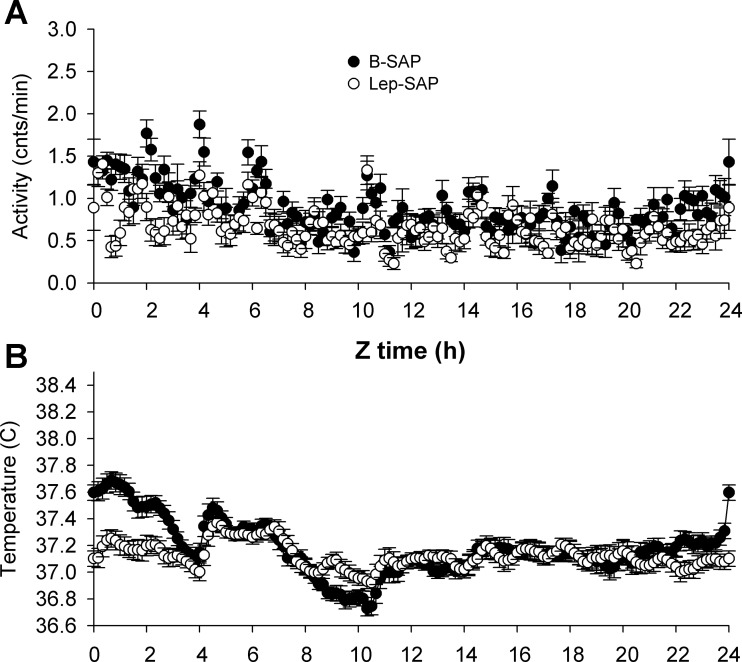
For activity and core body temperature, the anticipation and rhythm persistence were dissociated in Lep-SAP rats when food was restricted, with temperature significantly lower in fasted Lep-SAP rats (P = 0.04). In both B-SAP rats and Lep-SAP rats, activity during the last 4 days of restricted feeding was significantly elevated during the 2 h immediately prior to food presentation and during the first hour of feeding and did not differ between groups during these hours. Waveform analysis of the last 4 days of restricted feeding (Fig. 8, A and B) confirms the difference in circadian patterns for activity and temperature. Twenty-four hour mean activity levels remained significantly lower in Lep-SAP rats than in B-SAP rats (P < 0.05, Fig. 8C), but the anticipation ratio for activity was not different between groups (Fig. 8E). For Lep-SAP rats, core temperature was suppressed during the last 4 days of restricted feeding (P < 0.05; Fig. 8D). But in contrast to activity, the anticipation ratios for temperature were significantly different between groups (P < 0.05) and the ratios for the Lep-SAP group were >1, indicating that there was no anticipation for temperature in Lep-SAP rats (Fig. 8F). Wave-form analysis of temperature (Fig. 8B) suggests that anticipation was not completely lost in Lep-SAP rats; however, this absolute rise from nadir was in our view due to masking by the intact activity rhythm. Indeed, upon commencement of feeding temperature in Lep-SAP increased to control levels, as expected, due to the thermal effects of food and postprandial thermogenesis. The arrhythmia of locomotor activity and the blunted but intact rhythm of core body temperature are observed with waveform analysis during the last 4 days of ad libitum feeding (Fig. 9, A and B). Fasting rhythms of anticipation and meal time elevation are persistent for activity in both groups but are not persistent for temperature in Lep-SAP rats (Fig. 10, A and B). Without persistence, the evidence for entrainment of temperature is insufficient.
Fig. 9.
Group average waveforms for locomotor (A) and temperature (B) rhythms in 10-min bins across the last 4 days of ad libitum feeding in DD beginning at time 0 (Z0), the time of food availability during subsequent food restriction (compare Fig 8, A and B). Activity was arrhythmic; temperature was rhythmic, but the temperature range was compressed (see Table 1).
Fig. 10.
Group average wave forms for locomotor (A) and temperature (B) rhythms in 10-min bins across the last 2 days of fasting in DD beginning at time 0 (Z0), the time of food availability during previous food restriction (compare Fig. 8, A and B). Entrainment by restricted feeding requires persistence of rhythms before and during the food restriction window. For activity anticipation occurs in both groups, but for temperature, only B-SAP rats demonstrate anticipation or meal time elevation.
DISCUSSION
Richter was the first to describe anticipation of bodily rhythms, including both activity and temperature rhythms, to a restricted feeding schedule (46). Bolles and de Lorge (5) were the first to demonstrate endogenous circadian control of food anticipation (reviewed in Ref. 10). The oscillators linking such rhythms to feeding schedule are referred to as food-entrainable oscillators (FEO; Ref. 53). The contribution of an FEO to an observable rhythm is identified both by the presence of a food anticipatory component of the rhythm and by the persistence of the rhythm when food is subsequently withheld (34). In the present experiment, we used Lep-SAP, a ribosomal toxin targeted to the leptin receptor, to selectively destroy leptin receptor-expressing neurons in the Arc region and examined the effect of this lesion on rhythms of activity and core temperature and on the entrainability of these rhythms to a restricted feeding schedule. Our major findings are that this lesion disrupted the circadian rhythm of activity, but not temperature, during DD conditions and, in addition, dissociated the control of temperature and activity by food. Although core body temperature remained rhythmic in DD in lesioned rats, their temperature did not entrain to a restricted feeding schedule. In contrast, activity was arrhythmic during DD in the lesioned rats, but was, nevertheless, entrainable to the restricted feeding schedule and demonstrated persistence of the entrained rhythm during subsequent fasting. Results were not caused by lesion of the SCN. It has been shown previously that entrainment of both activity and temperature rhythms to a restricted feeding schedule persists in SCN-lesioned rats (54, 6, 57, 34). In addition, examination of the SCN histology in this experiment indicates that the SCN was not damaged by the Arc Lep-SAP injections, as also shown previously in experiments using the same Lep-SAP injection parameters (28). These results reveal an anatomical dissociation of the photic and FEOs governing activity, suggest that the circuitry of FEOs for temperature and activity differ in anatomical location, and indicate that the FEO-controlling activity is not dependent on leptin receptor-expressing neurons in the Arc.
Activity and temperature rhythms generated by FEOs are usually coupled (34), even when arrhythmic (21). Indeed, the dissociation of these two rhythms by food restriction has been reported only after hypophysectomy or infralimbic cortical lesions with rats that have lowered mean temperature and no postprandial temperature elevation (15, 45), suggesting that thermoregulation in these cases is downstream of an intact FEO or controlled by a different mechanism altogether. There is currently no agreement on the location of any FEO critical for generating food anticipatory activity rhythms, and a distributed mechanism has been suggested (57, 14, 20), possibly under hormonal (9), metabolic, and/or reward controls (11). One distributed clock gene that may be critical for anticipatory FEO responses is Per2 (21), which is densely expressed in the Arc, but not in the adjacent ventromedial nucleus (VMN) (23). Indeed, FEOs may not be subject to known clock genes (55). Another approach to identification of components of distributed FEOs has been the evaluation of brain areas sensitive to food restriction as measured by diminished local glucose utilization. The latter approach has identified the Arc, but not the VMN, as a site of interest (16). Collectively, these data are consistent with our present results indicating a role for the Arc as an FEO governing core temperature.
Positive masking by rest/activity cycles accounts for a highly variable expression of core body temperature rhythm. This did not appear to contribute to the significant difference in anticipatory temperature in the present experiment. Although obese Lep-SAP rats were less active than B-SAP controls, body temperatures between the two groups did not differ under basal conditions. Just prior to scheduled food presentation, body temperature rose in the B-SAP group, but significantly less in Lep-SAPs (Fig. 7), despite the fact that anticipatory locomotor ratios did not differ between groups. In addition, the anticipatory ratio for temperature in Lep-SAP rats was <1, indicating no difference between mean anticipatory temperature and mean 24-h temperature. There was an absolute rise in temperature from nadir before food availability observed in the waveform (Fig. 8B), indicative of masking by the intact activity entrainment to food. However, only temperature in B-SAP rats remained elevated during persistence testing. These factors suggest that differences in activity under these experimental conditions are not accountable for between-group differences in the food-anticipatory temperature. Rather, our results suggest that leptin-sensitive networks of the Arc are necessary for the entrainment of body temperature rhythms to restricted feeding.
Lesion results, however, do not rule out the contribution of additional integrative sites to circadian rhythm generation. Multiple structures and their interactions are likely to be required for generation of circadian rhythms of a complex behavioral function, such as locomotor activity. For example, food-anticipatory wakefulness and locomotor activity are lost in orexin neuron-ablated transgenic mice (32) and compromised in mice lacking Npas2 (18). In the present study, our Lep-SAP injections reduced Arc BMAL-1, but BMAL-1 in the DMN appeared unchanged. Thus, a possible role for Arc BMAL-1 in the circadian changes reported here is supported.
The altered rhythms produced by lesion of leptin receptor expressing Arc neurons do not necessarily indicate that leptin itself entrains the altered rhythms, as these neurons express other receptors, and experiments using exogenous leptin have yet to support such a role (29, 9, 35). Leptin rhythms appear to follow feeding: increased circulating levels of leptin follow feeding (51, 25, 48) and decreased circulating levels follow fasting (3). Indeed, restricted feeding abolishes the diurnal rhythm of circulating leptin along with rhythmic expression of mRNA for leptin in adipocytes and mRNA for hypothalamic NPY, POMC, and galanin, while maintaining the anticipatory rise in leptin before scheduled food availability (63). However, loss of leptin in the Lepob/ob mouse does produce hypoactivity that is reversed when exogenous leptin is provided (42). Hypoactivity occurs after loss of leptin signaling in FLPe-reactivatable leptin receptor-null allele (Leprneo/neo) mice. When leptin signaling for these mice is reactivated only in the Arc, activity levels are elevated specifically in the dark period, such that overall activity levels are normalized (13), suggesting that leptin itself is important to the control of day and night activity levels.
For both temperature and activity, circadian period length was comparable between groups in LD, but robustness was reduced by half in Lep-SAP rats for temperature and almost eliminated for activity. Indeed, half of the Lep-SAP rats were arrhythmic for activity in LD. Constant darkness further reduced the robustness of temperature rhythms for both groups, and all Lep-SAP rats were arrhythmic for activity. These data support a required role for the Arc in the control of light-entrainable oscillator activity rhythms. This view is consistent with reports that disrupted activity rhythms resulting from genetic disruption of leptin receptors are restored when these receptors are reactivated selectively in the Arc (13). A retinorecipient pathway flowing through the intergeniculate leaflet to the Arc dopamine cells independent of the SCN has been reported (24). However, both the SCN and Arc may be required for photically driven activity rhythms. Electrophysiological analyses and anatomical studies have revealed the presence of neural connections between the Arc and the SCN that might contribute to these rhythms (58, 39, 27). More than 80% of both SCN and peri-SCN cells are responsive to Arc stimulation, and ∼13% of Arc cells contribute to the SCN/peri-SCN projection (47). Arcuate neurons contributing to this projection, some of which are sensitive to leptin, include the POMC (26), galanin (2), and ghrelin (64) populations. Thus, on the basis of what is known about the anatomical connections of the SCN and Arc, the present results are consistent with the hypothesis that light-entrained activity rhythms, like feeding rhythms (28), are generated by an interconnected neural circuitry coupling these two sites.
In previous work (28), we found that injection of Lep-SAP into the Arc of S/D rats eliminated ∼80% of Arc NPY/AGRP and α-MSH neurons, both known to express LepB-R (31). In S/D rats, Lep-SAP also impaired feeding and body weight responses typically produced by leptin administration. Moreover, Lep-SAP toxicity was without effect in obese Zucker rats, which lack functional leptin receptors. These results demonstrate that Lep-SAP is both a selective and effective lesioning agent: its toxicity is primarily dependent on internalization of the LepR-B receptor; it destroys neuronal phenotypes known to express leptin receptors, and it impairs physiological responses mediated by leptin-sensitive neurons at the injection site. Similarly, in the present experiment, Lep-SAP reduced expression of Arc Agrp and Pomc to 18% and 21% of control, respectively. Importantly, the Arc Lep-SAP injections did not appear to lesion either adjacent dorsomedial or ventromedial nuclei or SCN (28).
In general, there are two required tests of entrainment for an FEO: 1) elevation of the measure before food availability (anticipation); and 2) continued elevation for more than 1 day without any food (persistence). We provided both tests. Elevation without persistence is usually due to masking. For Lep-SAP rats, temperature was not elevated above the daily mean before food availability, which is the most common test of anticipation. In addition, there was a significant difference in the anticipatory curves between the B-SAP control rats and the Lep-SAP rats. However, the slope of the temperature curve is upward. Thus, the anticipation evaluation strongly suggests a loss of entrainment by food restriction for Lep-SAP rats. But there is some doubt due to the upward slope of the curve. For the second test, persistence, Figs. 7D and 10B clearly show no persistence for Lep-SAP rats. Because some effect of activity on temperature was expected, some masking by increased activity is likely. To us, it is remarkable that activity does not have more of an impact on temperature in Lep-SAP rats. Our conclusion is that the circuitry for the temperature FEO requires leptin sensitivity within the Arc.
The Arc contains multiple clocks (1) that respond to meals (30) or fasting (23), and the SCN contains clocks that respond to photic cues. Our work suggests that both are required for generation and maintenance of endogenous activity rhythms entrained to the LD cycle, while also revealing an essential role for the Arc in maintaining food anticipatory temperature rhythms by FEOs. Moreover, the dissociation of temperature and activity responses to both light and food restriction suggests a complex circuitry for circadian control, potentially leading to a state of internal desynchrony. Chronic desynchrony has been hypothesized to play a role in the development of obesity and metabolic disorders in humans and has been repeatedly demonstrated to cause obesity in rodents (reviewed in Ref. 62). Thus, the absence of a global synchronizing signal, such as temperature (8) may represent a mechanism, whereby the loss of leptin-sensitive neurons in the Arc contributes to the massive weight gains observed.
Perspectives and Significance
Both discrete and dissociable elements are required to entrain activity, core body temperature, and sleep and feeding rhythms by photic and nonphotic cues. The present results reveal that intrahypothalamic circuitry between the Arc and SCN represents a key component of this process. Because the Arc is a primary site for metabolic integration and the coordination of metabolic, activity, and endocrine rhythms is essential for this function, it is likely that the Arc plays an important role in the disruptive effects of desynchrony. The use of Lep-SAP represents a highly selective method by which the interaction among these multiple clock-containing systems can be studied.
GRANTS
This work was supported by Public Health Service Grants DK-40498 and DK-81546 to S. Ritter and DA-023202 to H. T. Jansen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.F.W., A.-J.L., H.T.J., and S.R. conception and design of research; M.F.W., A.-J.L., and T.T.D. performed experiments; M.F.W. and A.-J.L. analyzed data; M.F.W., A.-J.L., H.T.J., and S.R. interpreted results of experiments; M.F.W., A.-J.L., and S.R. prepared figures; M.F.W., A.-J.L., and S.R. drafted manuscript; M.F.W., A.-J.L., H.T.J., and S.R. edited and revised manuscript; M.F.W., A.-J.L., T.T.D., H.T.J., and S.R. approved final version of manuscript.
REFERENCES
- 1.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 22: 350–356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamson EE, Moore RY. Lesions of suprachiasmatic nucleus efferents selectively affect rest-activity rhythm. Mol Cell Endocrinol 252: 46–56, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Baskin DG, Breininger JF, Bonigut S, Miller MA. Leptin binding in the arcuate nucleus is increased during fasting. Brain Res 828: 154–158, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem 47: 353–362, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bolles RC, de Lorge J. The rat's adjustment to a-diurnal feeding cycles. J Comp Physiol Psychol 55: 760–762, 1962 [DOI] [PubMed] [Google Scholar]
- 6.Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res 1: 39–65, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146: 1179–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330: 379–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carneiro BT, Araujo JF. The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals? Chronobiol Int 26: 1273–1289, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Carneiro BT, Araujo JF. Food entrainment: major and recent findings. Front Behav Neurosci 6: 83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Challet E, Mendoza J. Metabolic and reward feeding synchronises the rhythmic brain. Cell Tissue Res 341: 1–11, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Challet E, Pévet P, Lakhdar-Ghazal N, Malan A. Ventromedial nuclei of the hypothalamus are involved in the phase advance of temperature and activity rhythms in food-restricted rats fed during daytime. Brain Res Bull 43: 209–218, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Davidson AJ. Lesion studies targeting food-anticipatory activity. Eur J Neurosci 30: 1658–1664, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Davidson AJ, Stephan FK. Feeding-entrained circadian rhythms in hypophysectomized rats with suprachiasmatic nucleus lesions. Am J Physiol Regul Integr Comp Physiol 277: R1376–R1384, 1999 [DOI] [PubMed] [Google Scholar]
- 16.de Vasconcelos AP, Bartol-Munier I, Feillet CA, Gourmelen S, Pevet P, Challet E. Modifications of local cerebral glucose utilization during circadian food-anticipatory activity. Neuroscience 139: 741–748, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Delezie J, Challet E. Interactions between metabolism and circadian clocks: reciprocal disturbances. Ann NY Acad Sci 1243: 30–46, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301: 379–383, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav 74: 703–708, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: the driving force for food-anticipatory activity. Eur J Neurosci 30: 1665–1675, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Curr Biol 16: 2016–2022, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Gerkema MP, Groos GA, Daan S. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J Biol Rhythms 5: 81–95, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Guilding C, Hughes AT, Brown TM, Namvar S, Piggins HD. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain 2: 28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath TL. An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. J Neurosci 18: 1546–1558, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalra SP, Bagnasco M, Otukonyong EE, Dube MG, Kalra PS. Rhythmic, reciprocal ghrelin and leptin signaling: new insight in the development of obesity. Regul Pept 111: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kineman RD, Kraeling RR, Crim JW, Leshin LS, Barb CR, Rampacek GB. Localization of proopiomelanocortin (POMC) immunoreactive neurons in the forebrain of the pig. Biol Reprod 40: 1119–1126, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience 110: 73–92, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Li AJ, Wiater MF, Oostrom MT, Smith BR, Wang Q, Dinh TT, Roberts BL, Jansen HT, Ritter S. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. Am J Physiol Regul Integr Comp Physiol 302: R1313–R1326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Merlos MT, Angeles-Castellanos M, Díaz-Muñoz M, Aguilar-Roblero R, Mendoza J, Escobar C. Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Endocrinol 181: 53–63, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Mendoza J, Pévet P, Felder-Schmittbuhl MP, Bailly Y, Challet E. The cerebellum harbors a circadian oscillator involved in food anticipation. J Neurosci 30: 1894–1904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett 387: 113–116, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci 24: 10493–10501, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minana-Solis MC, Angeles-Castellanos M, Feillet C, Pevet P, Challet E, Escobar C. Differential effects of a restricted feeding schedule on clock-gene expression in the hypothalamus of the rat. Chronobiol Int 26: 808–820, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci 30: 1718–1729, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav 104: 535–545, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Mistlberger RE, Antle MC. Neonatal monosodium glutamate alters circadian organization of feeding, food anticipatory activity and photic masking in the rat. Brain Res 842: 73–83, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Mistlberger RE, Lukman H, Nadeau BG. Circadian rhythms in the Zucker obese rat: assessment and intervention. Appetite 30: 255–267, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Mistlberger RE, Marchant EG. Enhanced food-anticipatory circadian rhythms in the genetically obese Zucker rat. Physiol Behav 66: 329–335, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol 389: 508–534, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci 29: 1447–1460, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol 269: R1038–R1043, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Persons JE, Stephan FK, Bays ME. Diet-induced obesity attenuates anticipation of food access in rats. Physiol Behav 54: 55–64, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Piccione G, Caola G, Refinetti R. Feeble weekly rhythmicity in hematological, cardiovascular, and thermal parameters in the horse. Chronobiol Int 21: 571–589, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Recabarren MP, Valdés JL, Farías P, Serón-Ferré M, Torrealba F. Differential effects of infralimbic cortical lesions on temperature and locomotor activity responses to feeding in rats. Neuroscience 134: 1413–1422, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Richter CP. A behavioristic study of the rat. Comp Psychol Monogr 1: 1–55, 1922 [Google Scholar]
- 47.Saeb-Parsy K, Lombardelli S, Khan FZ, McDowall K, Au-Yong IT, Dyball RE. Neural connections of hypothalamic neuroendocrine nuclei in the rat. J Neuroendocrinol 12: 635–648, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Sánchez J, Oliver P, Picó C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflügers Arch 448: 500–506, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res 153: 243–252, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Satinoff E. Patterns of circadian body temperature rhythms in aged rats. Clin Exp Pharmacol Physiol 25: 135–140, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest 100: 1882–1887, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms 17: 284–292, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol 25: 545–554, 1979 [DOI] [PubMed] [Google Scholar]
- 55.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA 106: 6808–68013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149: 1773–1785, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verwey M, Amir S. Food-entrainable circadian oscillators in the brain. Eur J Neurosci 30: 1650–1657, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol 258: 204–229, 1987 [DOI] [PubMed] [Google Scholar]
- 59.Wiater MF, Mukherjee S, Li AJ, Dinh TT, Rooney EM, Simasko SM, Ritter S. Circadian integration of sleep-wake and feeding requires NPY receptor-expressing neurons in the mediobasal hypothalamus. Am J Physiol Regul Integr Comp Physiol 301: R1569–R1583, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiley RG, Kline IR. Neuronal lesioning with axonally transported toxins. J Neurosci Methods 103: 73–82, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res 1337: 21–31, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Wyse CA, Selman C, Page MM, Coogan AN, Hazlerigg DG. Circadian desynchrony and metabolic dysfunction; did light pollution make us fat? Med Hypotheses 77: 1139–1144, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Xu B, Kalra PS, Farmerie WG, Kalra SP. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology 140: 2868–2875, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147: 283–294, 2006 [DOI] [PubMed] [Google Scholar]