Abstract
The reduction in nitric oxide (NO)-mediated vascular function with age has largely been determined by flow-mediated dilation (FMD). However, in light of recent uncertainty surrounding the NO dependency of FMD and the recognition that brachial artery (BA) vasodilation during handgrip exercise is predominantly NO-mediated in the young, we sought to determine the contribution of NO to BA vasodilation in the elderly using the handgrip paradigm. BA vasodilation during progressive dynamic (1 Hz) handgrip exercise performed at 3, 6, 9, and 12 kg was assessed with and without NO synthase (NOS) inhibition [intra-arterial NG-monomethyl-l-arginine (l-NMMA)] in seven healthy older subjects (69 ± 2 yr). Handgrip exercise in the control condition evoked significant BA vasodilation at 6 (4.7 ± 1.4%), 9 (6.5 ± 2.2%), and 12 kg (9.5 ± 2.7%). NOS inhibition attenuated BA vasodilation, as the first measurable increase in BA diameter did not occur until 9 kg (4.0 ± 1.8%), and the change in BA diameter at 12 kg was reduced by ∼30% (5.1 ± 2.2%), with unaltered shear rate (Control: 407 ± 57, l-NMMA: 427 ± 67 s−1). Although shifted downward, the slope of the relationship between BA diameter and shear rate during handgrip exercise was unchanged (Control: 0.0013 ± 0.0004, l-NMMA: 0.0011 ± 0.007, P = 0.6) as a consequence of NOS inhibition. Thus, progressive handgrip exercise in the elderly evokes a robust BA vasodilation, the magnitude of which was only minimally attenuated following NOS inhibition. This modest contribution of NO to BA vasodilation in the elderly supports the use of the handgrip exercise paradigm to assess NO-dependent vasodilation across the life span.
Keywords: vascular function, endothelium, nitric oxide
an age-associated reduction in brachial artery (BA) nitric oxide (NO)-mediated vascular function has been documented noninvasively by attenuated postischemic flow-mediated vasodilation (FMD) (5, 17, 54). However, recent findings have questioned the NO dependency of the traditional postischemic FMD technique (36, 39, 52, 57), leaving doubt as to whether FMD is truly a functional bioassay of endothelium-derived NO (14, 24). These recent challenges and the uncertainty pertaining to conventional FMD technique highlight the need to develop alternative approaches capable of noninvasively assessing vascular function and NO bioavailability across the life span.
One approach to noninvasively assess vascular function is to induce BA vasodilation with progressively more intense handgrip exercise. Compared with conventional FMD, which relies upon a single vasodilatory response to a somewhat complex and dynamic change in shear rate, progressive handgrip exercise evokes multiple stepwise increases in shear rate, resulting in a linear BA vasodilatory response (47), providing a robust method with which to assess vascular function (56). Unlike hyperemia, during handgrip exercise, which is largely dictated by downstream resistance vessels and is only minimally NO-dependent in the young (10–20%) (4, 16, 19–21, 44–46, 56) and even less so in the old (0–10%) (4, 44), the contribution of NO to exercise-induced vasodilation of the conduit vessels has not been extensively investigated. We recently reported that BA vasodilation during handgrip exercise is predominantly (∼70%) NO-mediated in young adults (56); however, the contribution of NO to BA vasodilation in the elderly has not been examined. Given the recognized age-associated reductions in NO bioavailability and endothelium-dependent vasodilation (5, 9, 17, 18, 48, 49), the contribution of NO to BA vasodilation during progressive handgrip exercise might be expected to be less in the elderly than previously reported in the young (56). However, currently, the usefulness of this paradigm to assess vascular function across the human life span remains limited as the contribution of NO to BA vasodilation in the elderly has yet to be determined.
Therefore, this study was designed to determine the degree to which exercise-induced vasodilation in the elderly is mediated through a NO-dependent mechanism. With the understanding that NO bioavailability is likely reduced with aging, we hypothesized that inhibition of NOS during progressive handgrip exercise would only minimally alter BA vasodilation, revealing a negligible reliance on NO-mediated vasodilation in the elderly. To test this hypothesis, progressive handgrip exercise was performed, and BA vasodilation was assessed under control and NOS-inhibited conditions.
METHODS
Subjects.
Seven older (3 men and 4 women, 69 ± 2 yr) healthy subjects were enrolled in this study. All subjects were nonsmokers and were not participating in any regular exercise program. Subjects were not taking any prescription medication and were free from overt cardiovascular disease. Protocol approval and written informed consent were obtained, according to the University of Utah and Salt Lake City Veterans Affairs Medical Center (VAMC) Institutional Review Board, in accordance with the principles outlined in the Declaration of Helsinki. All data collection took place in the Utah Vascular Research Laboratory at the Salt Lake City VAMC Geriatric Research, Education, and Clinical Center. It should be noted that a similar investigation has been published in healthy young adults (56) and because of the complexity of placing the BA catheter high in the upper arm near the brachial plexus, we have, for ethical reasons, chosen not to repeat this protocol in a second group of young subjects. Thus, although highly relevant to the current investigation, comparisons to our previous study in young adults are limited to the discussion of this study.
Protocols.
Subjects performed a minimum of two familiarization trials ∼1 wk prior to the experimental trials. Handgrip maximal voluntary contraction (MVC) was determined, and the progressive handgrip exercise protocol to be used during the experimental trials was performed during these familiarization visits. On the experimental day, subjects reported to the laboratory between 0700 and 0800 after an overnight fast. Using a sterile technique, we placed arterial and venous catheters (18 gauge, 20 cm; Arrow, Reading, PA) in the BA and an antecubital vein of the experimental arm, after local anesthesia (2% lidocaine). The BA catheter was placed ∼10 cm distal to the axilla and advanced 6–8 cm in the retrograde direction. The BA catheter was placed in the upper arm to ensure that l-NMMA entered the artery upstream to the ultrasound Doppler sample volume, allowing the assessment of local drug effects on BA diameter and blood velocity.
After 30 min of recovery following the catheter placement, resting measurements were made. Subjects then performed dynamic handgrip exercise (1 Hz) using a commercially available handgrip dynamometer (TSD121C; Biopac Systems, Goleta, CA), interfaced with an analog-to-digital conversion box. Cadence was guided by a metronome, accompanied by real-time visual feedback of dynamometer force. Subjects were encouraged to perform rapid contractions with the goal of limiting contraction time to <25% of the duty cycle. Subjects exercised at 3, 6, 9, and 12 kg (1 Hz), corresponding to ∼15, 25, 40, and 50% of MVC. Each exercise stage was performed for 2.5 min with a 1-min break allotted between each workload to limit fatigue. Immediately following the Doppler ultrasound assessment of BA velocity and diameter, arterial and venous blood samples were collected and later analyzed for plasma nitrite. It should be noted that blood samples were only obtained at baseline, during infusion, and at the 6- and 12-kg workloads in the control and l-NMMA conditions to limit the total volume of blood withdrawn from subjects. Following a recovery period of 30 min to allow BA blood flow and metabolism to return to resting levels, the same handgrip protocol was repeated with intra-arterial l-NMMA infusion.
l-NMMA infusion.
Lower and upper arm volumes were determined anthropometrically and then used for the calculation of l-NMMA (Bachem, Bubendorf, Switzerland) dosing. Total arm volume receiving l-NMMA infusate was calculated as follows: total volume (dl) = forearm volume + (upper arm × 0.5). A portion of the upper arm was included in this calculation due to the proximal location of the arterial catheter.
l-NMMA was diluted from 250-mg lyophilized powder in normal saline to a concentration of 2.5 mg/ml. l-NMMA was infused at a loading dose of 0.48 mg/dl arm volume/min for 5 min prior to exercise. During handgrip exercise, l-NMMA was infused at a maintenance dose 0.24 mg·dl arm volume−1·min−1. Handgrip exercise commenced 3 min after switching to the maintenance dose. Previously, an l-NMMA dose-response performed in young subjects revealed no further reduction in BA blood flow from 0.24 to 0.48 mg·dl arm volume−1·min−1 (56).
Brachial artery diameter and blood velocity measurements.
Simultaneous measurements of BA blood velocity and vessel diameter of the infused arm were performed using a Logiq 7 ultrasound Doppler system (GE Medical Systems, Milwaukee, WI). At rest, BA blood velocity and diameter of the contralateral limb were performed using a Logic e ultrasound Doppler system (GE Medical Systems) in five of the seven subjects. Both ultrasound systems were equipped with linear array transducers operating at an imaging frequency of 12–14 MHz. Blood velocity was obtained using the same transducer with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel on the basis of real-time duplex ultrasound visualization. Mean velocity values (Vmean, angle-corrected, and intensity-weighted area under the curve) were calculated using commercially available software (Logic 7 and Logic e). End-diastolic, ECG R-wave-gated images were collected via video output from the Logic 7 for off-line analysis of BA vasodilation using automated edge detection software (Medical Imaging Applications, Coralville, IA). With the use of arterial diameter and Vmean, BA blood flow [Vmean π (vessel diameter/2)2·60], and shear rate (8Vmean/BA diameter) were calculated.
Heart rate, mean arterial pressure, stroke volume, and cardiac output.
Heart rate (HR) was monitored from a standard three-lead ECG. Arterial blood pressure was collected continuously from within the BA, with the pressure transducer placed at the level of the catheter (Transpac IV; Abbott Laboratories, Abbott Park, IL). Mean arterial pressure (MAP) was calculated using the time integral of the directly measured arterial waveform. BA vascular conductance was then calculated as BA blood flow/MAP. Stroke volume (SV) and cardiac output (CO) were determined with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). SV was calculated using the Modelflow method, a validated model (50) that uses an algorithm to compute the aortic flow waveform from an arterial blood pressure pulsation by simulating a nonlinear, self-adaptive (three-element Windkessel) model of the aortic input impedance (Beatscope, version 1.1; Finapres Medical Systems). CO was then calculated as the product of HR and SV.
Assays.
A lipid panel and complete blood count were assessed by standard clinical techniques. Plasma nitrite levels were measured using a standard fluorometric assay kit (Caymen Chemical, Ann Arbor, MI).
Data and statistical analysis.
Ultrasound images and Doppler velocity spectra were recorded continuously at rest and during each exercise stage. During the last 60 s of each ultrasound Doppler segment, Vmean was averaged across five 12-s intervals, which were matched with intima-to-intima BA diameter measurements evaluated during diastole. Linear regression analysis was performed on individual data across all handgrip exercise stages for BA vasodilation and shear rate, with r values and slope determined to evaluate BA vasodilation in response to shear rate before and after l-NMMA infusion. Statistics were performed with the use of commercially available software (SigmaPlot 11.0, Systat Software, Point Richmond, CA). A two-way repeated-measures ANOVA was used to evaluated difference between trials, and a least significant difference test identified means that were significantly different with P ≤ 0.05. A paired t-test was used to compare the effect of drug on slope and y-intercept values from linear regression analysis. All group data are expressed as means ± SE.
RESULTS
Subject characteristics are presented in Table 1.
Table 1.
Subject and blood characteristics
| Characteristics | Value |
|---|---|
| Age, yr | 69 ± 2 |
| Height, cm | 172 ± 4 |
| Weight, kg | 76 ± 6 |
| Body mass index, kg/m2 | 26 ± 1 |
| Arm Volume, dl | 13 ± 1 |
| Glucose, mg/dl | 76 ± 3 |
| Sodium, mmol/l | 142 ± 1 |
| Potassium, mmol/l | 4.0 ± 0.1 |
| Chloride, mmol/l | 105 ± 1 |
| Creatine, mg/dl | 0.9 ± 0.07 |
| Cholesterol, mg/dl | 185 ± 11 |
| Triglycerides, mg/dl | 72 ± 13 |
| HDL, mg/dl | 60 ± 4 |
| LDL, mg/dl | 115 ± 11 |
Values are expressed as means ± SE.
Impact of l-NMMA at rest.
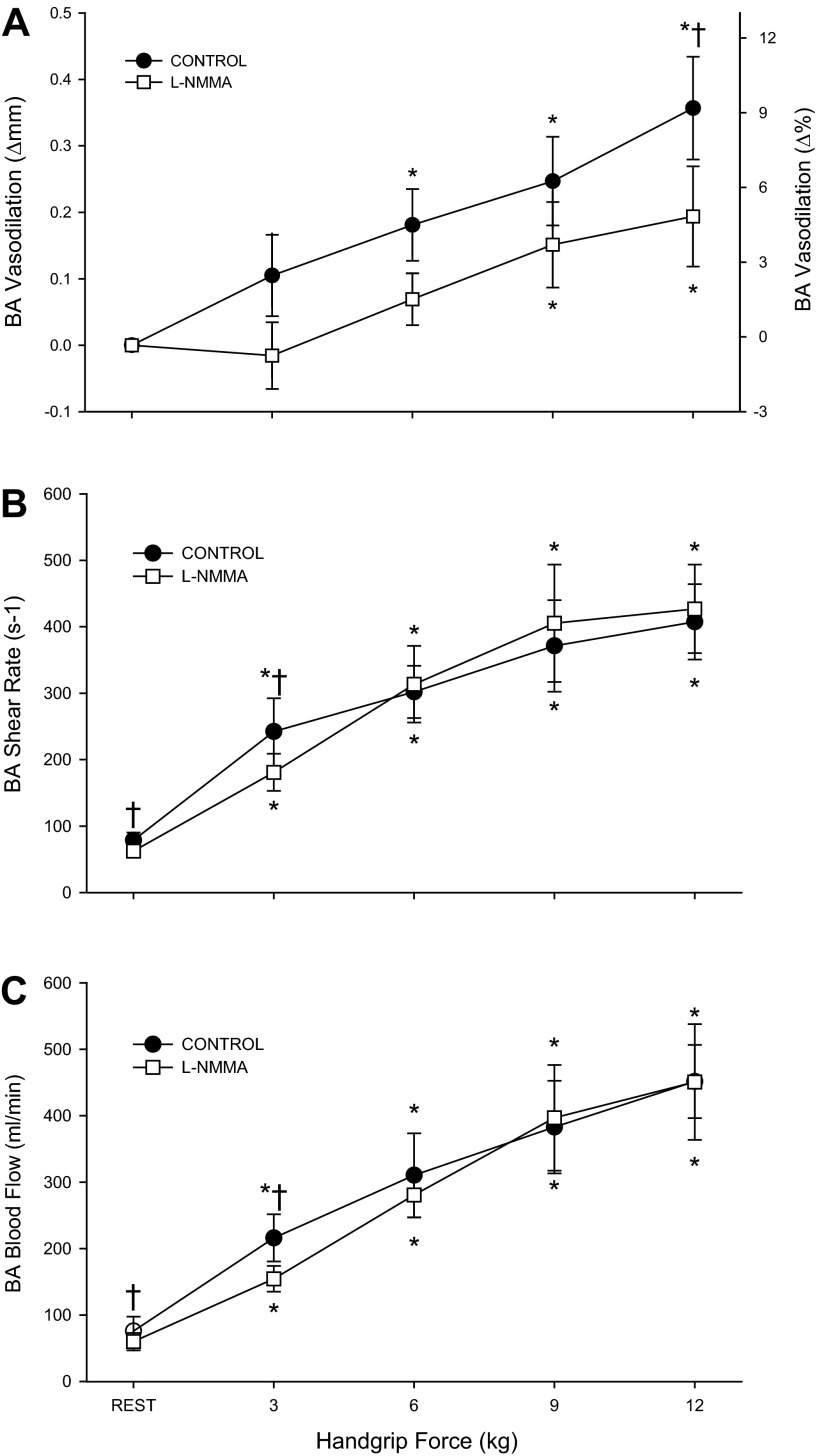
Vascular measures at rest in the experimental arm, prior to and during l-NMMA infusion, are presented in Table 2. At rest, l-NMMA reduced BA mean blood velocity, blood flow, shear rate, and vascular conductance by 35–40% (P < 0.05) (Fig. 1 and Table 2). Resting BA diameter did not change in response to l-NMMA infusion (P = 0.18) (Table 2). HR and MAP were not altered by l-NMMA, indicating that the drug remained localized to the vasculature of the infused arm (Table 2). Additionally, during l-NMMA infusion, BA diameter, mean blood velocity, blood flow, shear rate, and vascular conductance of the contralateral limb remained unchanged, further confirming the regional effect of l-NMMA with no measureable systemic impact of the drug on peripheral hemodynamics (Table 3).
Table 2.
Cardiovascular variables at rest and during progressive handgrip exercise
| Exercise Intensity |
|||||
|---|---|---|---|---|---|
| Absolute, kg | Rest | 3 | 6 | 9 | 12 |
| Relative, % MVC | 13 ± 1 | 26 ± 1 | 39 ± 2 | 52 ± 3 | |
| Control | |||||
| HR, beats/min | 57 ± 3 | 64 ± 4 | 74 ± 7* | 74 ± 7* | 74 ± 7* |
| MAP, mmHg | 102 ± 3 | 110 ± 4* | 112 ± 4* | 117 ± 5* | 124 ± 6* |
| BA diameter, mm | 4.3 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.3* | 4.5 ± 0.3* | 4.6 ± 0.3* |
| BA Vasodilation, %Δ | 2.7 ± 1.5 | 4.7 ± 1.4* | 6.5 ± 2.2* | 9.5 ± 2.7* | |
| BA Velocity, cm/s | 10 ± 1 | 24 ± 3* | 32 ± 3* | 40 ± 5* | 45 ± 4* |
| BA VC, ml/min/mmHg | 0.7 ± 0.2 | 2.0 ± 0.3* | 2.7 ± 0.5* | 3.2 ± 0.5* | 3.6 ± 0.4* |
| l-NMMA | |||||
| HR, beats/min | 59 ± 3 | 66 ± 4 | 73 ± 7* | 73 ± 7* | 73 ± 7* |
| MAP, mmHg | 102 ± 4 | 108 ± 4* | 113 ± 5* | 118 ± 6* | 127 ± 6* |
| BA diameter, mm | 4.3 ± 0.3 | 4.3 ± 0.4 | 4.4 ± 0.4 | 4.5 ± 0.3* | 4.5 ± 0.3* |
| BA Vasodilation, %Δ | −0.6 ± 1.0 | 1.6 ± 0.9 | 4.0 ± 1.8* | 5.1 ± 2.2*† | |
| BA Velocity, cm/s | 6 ± 1† | 18 ± 1*† | 32 ± 3* | 42 ± 6* | 46 ± 5* |
| BA VC, ml/min/mmHg | 0.6 ± 0.1† | 1.4 ± 0.2*† | 2.5 ± 0.3* | 3.3 ± 0.6* | 3.6 ± 0.7* |
Values are expressed as means ± SE.
HR, heart rate; MAP, mean arterial pressure; BA, brachial artery; VC, vascular conductance.
Significantly different from rest.
†Significantly different from control, P < 0.05.
Fig. 1.
Brachial artery (BA) vasodilation (A), shear rate (B), and blood flow (C) during progressive handgrip exercise in the control (●) and l-NMMA (□) conditions. *P < 0.05, significant difference from rest. †P < 0.05, significant difference from control.
Table 3.
Resting peripheral vascular measures in the contralateral arm prior to and during l-NMMA infusion.
| Control | l-NMMA | |
|---|---|---|
| Vmean, cm/s | 8 ± 2 | 8 ± 1 |
| BA diameter, mm | 4.4 ± 0.4 | 4.4 ± 0.4 |
| Shear rate, s−1 | 74 ± 14 | 77 ± 12 |
| Vascular conductance, ml·min−1·mmHg−1 | 0.8 ± 0.2 | 0.8 ± 0.3 |
| Forearm blood flow, ml/min | 80 ± 27 | 83 ± 27 |
Values are expressed as means ± SE; n = 5.
Impact of l-NMMA during exercise.
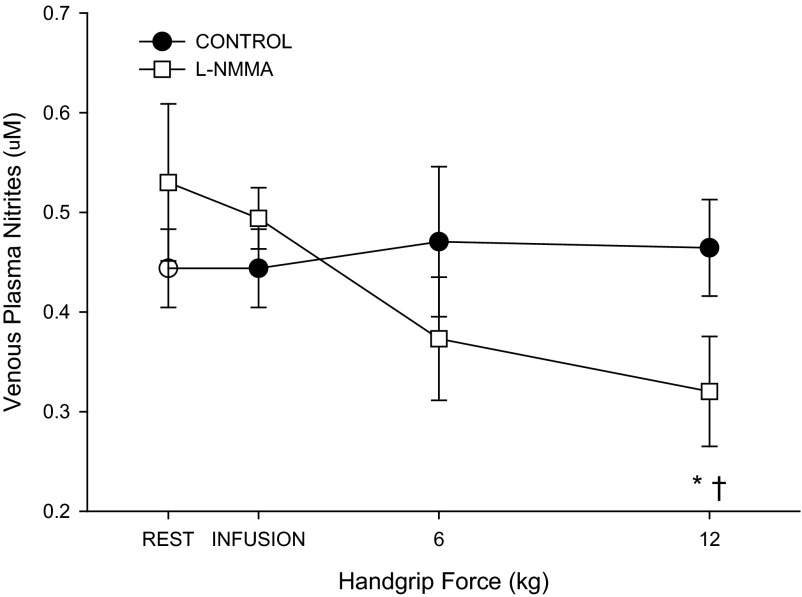
In the control condition, handgrip exercise resulted in immediate and intensity-dependent increases in BA velocity (Table 2), shear rate (Fig. 1B), blood flow (Fig. 1C), and vascular conductance (Table 2). Likewise, HR and MAP displayed intensity-dependent increases during handgrip exercise (Table 2). The first significant increase in BA diameter occurred at 6 kg (Fig. 1A). Venous plasma nitrite levels remained unchanged throughout handgrip exercise in the control condition (Fig. 3).
Fig. 3.
Venous plasma nitrite level during progressive handgrip exercise (n = 6). *P < 0.05, significantly different from rest. †P < 0.05, significant difference from control.
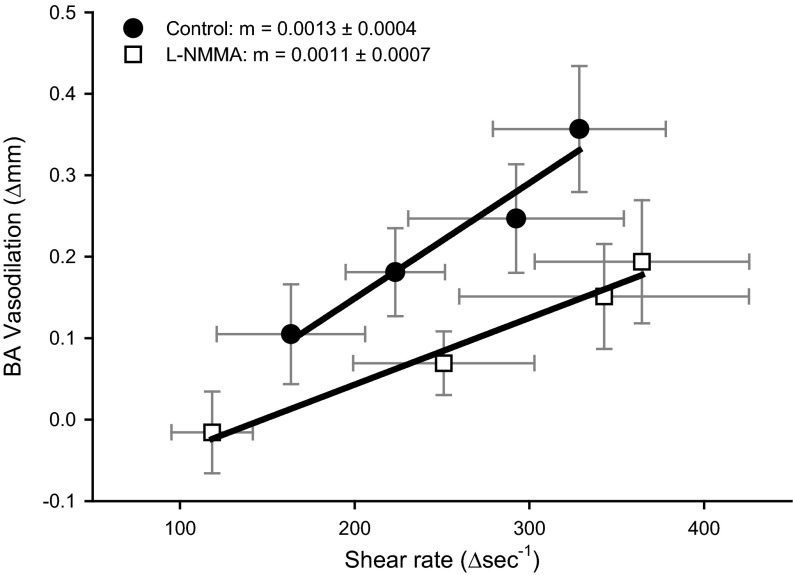
After l-NMMA, BA velocity (Table 2), shear rate (Fig. 1B), blood flow (Fig. 1C), and vascular conductance (Table 2) increased in an intensity-dependent manner; however, each of these variables was reduced at 3 kg compared with the control trial (P < 0.05). At higher exercise intensities (i.e., ≥ 6 kg), there were no measurable differences between conditions for BA velocity, shear rate, blood flow, or vascular conductance. Increases in HR and MAP were not different from the control trial (Table 2). Compared with the control trial l-NMMA delayed the onset of measurable BA vasodilation as the first significant increase in BA diameter occurred at 9 kg. Additionally, l-NMMA attenuated the increase in BA diameter at 12 kg by ∼30%. (P = 0.02) (Fig. 1A). The relationship between BA diameter and shear rate exhibited a downward shift. However, the slope of this relationship, determined after the initiation of BA vasodilation, was not altered by l-NMMA (Control: 0.0013 ± 0.0004; l-NMMA; 0.0011 ± 0.007, P = 0.6, Fig. 2). Venous plasma nitrite levels were reduced by 34 ± 12% at 12 kg during handgrip exercise following l-NMMA infusion compared with the control condition (Fig. 3).
Fig. 2.
Relationship between changes in brachial artery shear rate and the associated change in brachial artery vasodilation during progressive handgrip exercise in control (●) and l-NMMA (□) conditions. There were no differences in the slopes between conditions despite a downward and parallel shift in the relationship between BA vasodilation and BA shear rate.
DISCUSSION
This study sought to examine the contribution of NO to BA vasodilation during progressive handgrip exercise in healthy older subjects. The primary novel finding of this study is that progressive handgrip exercise induced a robust BA vasodilation, the magnitude of which, was modestly, but significantly (∼30%) attenuated following NOS inhibition. Additionally, NOS inhibition altered the relationship between BA vasodilation and shear rate, such that the BA vasodilation evoked by a given shear rate was reduced following administration of l-NMMA. Interestingly, the slope of the relationship between BA vasodilation and shear rate during handgrip exercise was not altered. This modest contribution of NO to BA vasodilation in the elderly supports the use of the handgrip exercise paradigm to assess NO-dependent vasodilation across the lifespan.
Age, NO, and vascular function.
The main purpose of this study was to determine the contribution of NO to BA vasodilation during progressive handgrip exercise in healthy older subjects. On the basis of the present findings, NO contributes to ∼30% of the BA vasodilation that occurs during handgrip exercise in healthy older subjects. In contrast, nearly 70% of this response is NO-dependent in healthy young subjects (56), documenting a predominant shear rate-mediated vasodilatory role for NO in the young but not the old. This finding in combination with the robust vasodilation during handgrip exercise exhibited by the elderly reveals, by the simple process of elimination, that NO-independent mechanisms are primarily responsible for the vasodilatory response to handgrip-induced increases in shear stress in this population. Interestingly, the older subjects exhibited a similar capacity for BA vasodilation (∼10% increase in BA diameter) compared with our previously published data from young subjects (56). Without further examination of the mechanisms involved in BA vasodilation, this finding suggests preserved BA vascular function with aging. However, normalizing BA vasodilation for shear rate (6, 22, 51) reveals impaired BA vascular function, as a 40% greater increase in shear rate was required to elicit the same BA vasodilation in the older subjects compared with the young. Interestingly, the slope of the relationship under control conditions in the old closely resembled the NOS-inhibited state in the young (56), further supporting the notion of reduced NO bioavailability and impaired vascular function with age (4, 44). On the basis of our current and previous findings, the slope of the relationship between BA vasodilation and shear rate may prove to be a useful index of NO-mediated vascular function in health and disease.
The exact mechanisms contributing to this preserved BA vasodilation during progressive handgrip exercise in the old are not entirely clear. Utilizing a combination of NOS and prostaglandin inhibition, Parker et al. (36) reported substantial heterogeneity in the pathways underlying conduit artery vasodilation in response to FMD. Similarly, vasodilatory mechanisms regulating coronary microvascular function appear to shift with advancing age and disease from predominantly prostaglandins and NO to endothelial derived hyperpolarizing factors (EDHF) (2). Additionally, altered ATP-induced vasodilation (26) and/or an augmented contribution of hydrogen peroxide to FMD (31) may account for the shift in vasodilatory regulation. Further investigation employing multiple blockades is warranted to elucidate the mechanisms accounting for ∼70% of the exercise-induced BA vasodilation that occurs with advancing age that is currently unexplained.
Using the same approach as our previous investigation (56), we placed the arterial catheter above the site of Doppler ultrasound interrogation, allowing the direct assessment of BA diameter during administration of l-NMMA. NOS inhibitors are typically infused distal to the site of arterial diameter measurements (11, 16, 19, 21, 45, 46). Such an experimental design focuses the impact of the drug on downstream resistance vessels leaving the conduit vessel unaffected by NOS inhibition. The proximal arterial catheterization and subsequent l-NMMA infusion revealed, for the first time, that the inhibition of NOS reduced BA vasodilation in the elderly (Fig. 1A). Of note, l-NMMA reduced plasma nitrite levels by ∼30% (Fig. 4), indicating a direct reduction in local NOS activity and NO bioavailability, likely resulting in the observed reduction in shear-induced vasodilation (28). These data indicate that NO appears to be a significant, albeit reduced, contributor to exercise-induced BA vasodilation in the elderly.
Handgrip-induced vasodilation to assess NO bioavailability.
FMD following arterial occlusion, as is conventionally used to evaluate vascular function, provides only a single transient bolus of vascular shear stress that gradually decreases over time, leading to a peak dilation that is delayed in relation to the maximal shear stimulus (40). This method provides a somewhat complex and time-dependent assessment of vascular function. Aging is typically associated with a reduction in vascular function, as assessed using this FMD approach (3, 5, 17). However, this is not always true, as two reports from our laboratory report a lack of age-associated reductions in BA vascular function following normalization for shear rate (35, 55). Moreover, we recently reported that, in healthy young subjects, only 30% of the increase in BA diameter during arterial occlusion FMD testing is NO-dependent (57). Although we have yet to perform a similar FMD test with NOS inhibition in older subjects, we would expect even less of a contribution from NO in healthy older subjects due to age-associated reductions in NO bioavailability (48, 49). Therefore, it is proposed that the sustained and stepwise increase in shear rate during handgrip exercise not only provides a powerful stimulus for BA vasodilation (12, 47, 56) but may provide a more robust measure of vascular function than traditional arterial occlusion-induced FMD.
Age, NO, and skeletal muscle blood flow.
At rest, the magnitude of l-NMMA-induced reductions in resting blood velocity, shear rate, forearm vascular conductance, and forearm blood flow were similar to our previous data in young subjects (56) and are in agreement with the reductions reported by Taddei et al. (49). This suggests that NO contributes equally to the regulation of resting skeletal muscle blood flow across the life span. However, this comparable contribution of NO across ages is not preserved during exercise, as NOS inhibition did not alter skeletal muscle blood flow in the current elderly subjects, while previous studies reported attenuated blood flow in young subjects (4, 44, 56). In the present study, the reduction in BA blood flow at 3 kg of handgrip exercise likely represents a carry-over effect of reduced blood flow at rest because normalizing the change in BA blood flow for the l-NMMA-induced reduction at rest eliminated the difference between the control and l-NMMA conditions. These findings are in agreement with the notion that distinct mechanisms regulate resting and exercising skeletal muscle blood flow (41, 42) and further identifies age and exercise intensity as important contributors to the differential regulation of muscle blood flow by NO.
Age-associated reductions in exercise-induced hyperemia have been thoroughly examined, and differences between young and old subjects appear to be limb-specific, as attenuated blood flow is often evident during leg, but not arm, exercise (1, 13, 23, 30, 32, 34, 35, 37, 38). In the current study, BA blood flow was similar to our previously reported data in the young (56), supporting prior reports from our laboratory (12) and other laboratories (4, 23) of preserved exercise-induced blood flow in the arm of the elderly. Again, in agreement with Schrage et al. (44), l-NMMA did not alter exercise-induced increases in blood flow in the old. This lack of change in exercise-induced hyperemia in the old provides strong support for a diminished role for NO in regulating blood flow during arm exercise with aging. In light of our previous findings (56), NO appears to act as an important regulator of vasodilation during exercise ensuring appropriate matching of tissue perfusion and metabolism in the young (25); however, a redundancy of mechanisms governing exercise hyperemia appears to be altered, such that NO no longer contributes to BA blood flow regulation in the old (29, 44).
Age and the exercise pressor reflex.
The current finding of a preserved BA vasodilation despite impaired vascular function may appear paradoxical. However, examination of the mechanisms responsible for this preserved vasodilation emphasizes the importance of integrating multiple factors involved in peripheral hemodynamics and vascular function during exercise. Specifically, the impact of the exercise pressor response and resultant increase in mean blood velocity, BA blood flow, and shear rate must be considered when assessing exercise-induced vascular function. The current elderly subjects exhibited an exaggerated exercise pressor response during handgrip exercise (+ Δ22 mmHg) compared with our previously studied group of young subjects (+ Δ9mmHg) (56). The underlying cause of this exaggerated increase in blood pressure is not entirely clear but may be related to an elevated muscle metaboreflex (8). The resultant increase in blood pressure increased blood velocity in the BA of the exercising forearm, elevating shear rate and resulting in a greater vasodilatory stimulus (27, 33, 40). During handgrip exercise, the exaggerated exercise pressor response and the associated increase in shear rate in the elderly appear to partially compensate for the reduction in NO bioavailability associated with aging and may make teleological sense. The exact vasodilatory pathways contributing to the observed preservation of BA vasodilation during handgrip exercise are not entirely clear but may involve augmented prostaglandin and/or EDHF-induced mechanisms.
l-NMMA-specific experimental considerations.
The dosages of l-NMMA used in the current study (loading of 6.4 ± 0.6 mg/min and maintenance of 3.2 ± 0.3 mg/min) are among the highest reported (1–5 mg/min), all of which established effective NOS inhibition during handgrip exercise (11, 15, 43). This l-NMMA dosing regimen proved effective at altering the response of the peripheral vasculature in the exercising forearm, while central hemodynamics were unchanged. Additionally, in the contralateral arm, there were no measurable changes during resting l-NMMA infusion, confirming the localized effect of the drug (Table 3). Because of lasting effects of l-NMMA (10), the control trial was always performed prior to the l-NMMA trial. However, our group and others have reported high reproducibility in the hyperemic response when multiple exercise bouts are performed sequentially with adequate rest between trials (7, 53, 56).
Summary.
Progressive handgrip exercise elicits stepwise increases in shear rate, evoking a linear and robust BA vasodilation in the elderly. Inhibition of NOS attenuated this BA vasodilation by ∼30%, indicating a significant, albeit modest, contribution of NO to exercise-induced BA vasodilation. Additionally, the relationship between BA vasodilation and shear rate was altered during NOS inhibition, such that the change in BA diameter for a given change in shear was reduced, but not abolished. Compared with our previous investigation in the young (56), the older subjects required a 40% greater shear rate to evoke a similar BA vasodilation, indicating impaired NO-mediated vascular function and augmented NO-independent vasodilation. Overall, these findings lend credence to the use of progressive handgrip exercise as a novel method for the noninvasive assessment NO-dependent vascular function across the life span.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.T., D.W.W., M.A.H.W., Z.B.-O., S.J.I., J.D.C., and R.S.R. conception and design of research; J.D.T., D.W.W., M.A.H.W., G.L., Z.B.-O., S.J.I., J.D.C., V.R., and R.S.R. performed experiments; J.D.T., M.A.H.W., G.L., Z.B.-O., S.J.I., and V.R. analyzed data; J.D.T., D.W.W., M.A.H.W., G.L., Z.B.-O., S.J.I., V.R., and R.S.R. interpreted results of experiments; J.D.T. prepared figures; J.D.T. and R.S.R. drafted manuscript; J.D.T., D.W.W., M.A.H.W., G.L., Z.B.-O., S.J.I., J.D.C., V.R., and R.S.R. edited and revised manuscript; J.D.T., D.W.W., M.A.H.W., G.L., Z.B.-O., S.J.I., J.D.C., V.R., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
J. D. Trinity, M. A. Witman, and S. J. Ives were supported by the Advanced Fellowship in Geriatrics awarded by the Veterans Affairs Medical Center. This work was funded by National Institutes of Health PO1 HL-091830 (to R. S. Richardson), VA Merit Award E6910R (to R. S. Richardson), and AHA Scientist Development Grant 0835209N (D. W. Wray).
REFERENCES
- 1.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Beyer AM, Gutterman DD. Regulation of the human coronary microcirculation. J Mol Cell Cardiol 52: 814–821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black MA, Cable NT, Thijssen DHJ, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato AJ, Uberoi A, Bailey DM, Walter Wray D, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298: H671–H678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001 [PubMed] [Google Scholar]
- 15.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JLD, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol 92: 2019–2025, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation 90: 2886–2890, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation 90: 2853–2858, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med 7: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol 474: 353–360, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J 14: 1685–1696, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res 72: 1276–1284, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laughlin MH, Korzick DH. Vascular smooth muscle: integrator of vasoactive signals during exercise hyperemia. Med Sci Sports Exerc 33: 81–91, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res 23: 741–747, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol 301: H1118–H1126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rådegran G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 168: 575–591, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol 98: 1251–1257, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovasc Res 35: 125–131, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci (Lond) 106: 371–376, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MAH, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wray DW, Witman MAH, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wray DW, Witman MAH, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 62: 345–351, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]