Abstract
Nitric oxide (NO) regulates lung development through incompletely understood mechanisms. NO controls pulmonary vascular smooth muscle cell (SMC) differentiation largely through stimulating soluble guanylate cyclase (sGC) to produce cGMP and increase cGMP-mediated signaling. To examine the role of sGC in regulating pulmonary development, we tested whether decreased sGC activity reduces alveolarization in the normal and injured newborn lung. For these studies, mouse pups with gene-targeted sGC-α1 subunit truncation were used because we determined that they have decreased pulmonary sGC enzyme activity. sGC-α1 knockout (KO) mouse pups were observed to have decreased numbers of small airway structures and lung volume compared with wild-type (WT) mice although lung septation and body weights were not different. However, following mild lung injury caused by breathing 70% O2, the sGC-α1 KO mouse pups had pronounced inhibition of alveolarization, as evidenced by an increase in airway mean linear intercept, reduction in terminal airway units, and decrease in lung septation and alveolar openings, as well as reduced somatic growth. Because cGMP regulates SMC phenotype, we also tested whether decreased sGC activity reduces lung myofibroblast differentiation. Cellular markers revealed that vascular SMC differentiation decreased, whereas myofibroblast activation increased in the hyperoxic sGC-α1 KO pup lung. These results indicate that lung development, particularly during hyperoxic injury, is impaired in mouse pups with diminished sGC activity. These studies support the investigation of sGC-targeting agents as therapies directed at improving development in the newborn lung exposed to injury.
Keywords: soluble guanylate cyclase, cGMP, lung development, alveolarization
nitric oxide (NO) and cGMP have an important role in regulating pulmonary vascular function and development. NO produced by nitric oxide synthase (NOS) in endothelial cells diffuses into subjacent vascular smooth muscle cells (SMCs), where it stimulates soluble guanylate cyclase (sGC) to increase cGMP production. Functional sGC is a heterodimeric protein that consists of an α- and β-subunit. Although each subunit can exist as two isoforms, the subunit isoforms exhibit differential expression in tissues. sGC-α1β1 and sGC-α2β1 are expressed in the lung although the former dimer is more abundant (40). cGMP regulates pulmonary vascular tone (27) and SMC differentiation (21, 80) primarily through interacting with cGMP-dependent protein kinase (PKGI), stimulating its proteolysis and nuclear localization (29, 60, 70).
Several studies suggest that NO regulates the maturation of the normal and injured lung. The role of NO in modulating pulmonary development is supported by studies in which NO donors were observed to increase branching morphogenesis of cultured fetal rat lungs (78) and in which mouse pup lungs deficient in the constitutive endothelial isoform of NOS (NOSIII) were reported to have decreased microvascular development and expression of angiogenic factors, such as vascular endothelial growth factor-A, Flk-1, and TIE2 (32). Emerging evidence indicates that NO also regulates the maturation of the injured newborn lung. For example, the expression of several NOS isoforms was reported to be decreased in a prematurely born baboon model of lung injury (1), and the level of NOSIII was noted to be decreased in the small pulmonary arteries of both fetal lambs exposed to endotoxins and chronically ventilated preterm lambs (36, 44). Moreover, although NOSIII-deficient mouse pups have normal alveolar development in infancy, when reared during exposure to mild levels of hypoxia, they had decreased alveolarization and pulmonary microvascular development (10). Furthermore, exposure to exogenous NO was reported to improve alveolarization in the injured developing lung. For example, inhaled NO gas decreased abnormal pulmonary SMC proliferation in rat pups with lung vascular injury (63, 64) and increased alveolar development following hyperoxic lung injury in rat pups (42) or following hypoxic lung injury in NOSIII-deficient mouse pups (7). In addition, inhaled NO improved alveolarization in prematurely born baboons and lambs with oxygen- and ventilator-induced lung injury (12, 45). When taken together, these studies indicate that NO regulates lung development, particularly during pulmonary injury.
The mechanisms through which NO regulates lung maturation are incompletely understood. Indirect evidence suggests that sGC might play a role in protecting the development of the injured lung. For example, sGC expression and activity have been observed to be downregulated in newborn lung injury models (6, 13, 72), and increasing cGMP levels with phosphodiesterase inhibitors has been reported to improve the maturation of the injured lung (77). However, several studies indicate that NO also mediates protein nitrosylation (41), and it is thus possible that NO could regulate lung development through mechanisms that are independent of sGC and cGMP (5, 67). Moreover, cGMP is also produced from natriuretic peptide-stimulated particulate guanylate cylases in the lung, and such NO-independent cGMP sources could regulate lung development through mechanisms that do not require sGC.
Here we tested the hypothesis that sGC regulates lung development and its response to injury. We observed that, in the lungs of mouse pups deficient in one sGC isoform, sGC-α1, the levels of sGC-β1 protein expression, sGC enzyme activity, and cGMP are diminished. This result confirmed the utility of the sGC-α1 KO mouse pup to test our hypothesis. We then observed that the decreased sGC activity level was associated with diminished lung volumes in air-breathing knockout mouse pups although airway septation and somatic growth were unchanged. However, the decreased sGC activity combined with mild O2-induced pulmonary injury produced striking reductions in alveolarization, expression of pulmonary artery SMC differentiation marker proteins, and activation of peripheral lung fibroblasts. The results of these studies suggest that sGC plays an important role in modulating newborn pulmonary injury and help identify sGC as a potential target for therapies directed at mitigating alveolarization in the injured newborn lung.
MATERIALS AND METHODS
Experimental design.
The Subcommittee for Research Animal Studies at the Massachusetts General Hospital approved the animal study protocols used in this investigation. Generation of sGC-α1 KO mice with a targeted deletion of exon 6 of sGC-α1 on the C57BL/6 background was described previously (18). These sGC-α1 KO mice were previously shown to express a catalytically inactive, mutant sGC-α1 isoform (18, 19, 73). C57BL/6 mice were studied because previous studies indicated that this strain of mouse pups exhibits decreased alveolarization when exposed to oxygen-rich environments (53, 58).
Three days after full-term delivery, paired litters of newborn mouse pups and their mothers were moved to a quiet, special purpose room, where they remained in their cages and breathed either 70% O2 while in a 15-l plastic exposure chamber or air while residing on a laboratory bench. The oxygen concentration in the chamber was measured using an electrochemical analyzer (Maxtec OM-25RME). The flows of oxygen and nitrogen were carefully regulated using flow meters (Cole-Palmer) to maintain a fresh O2 gas flow rate 8–10 times the calculated oxygen consumption of the litter and mother (59). Both mothers and pups tolerated this exposure well, as also reported by others (8, 24). The mice had continuous access to food and water, and, every 4 days, fresh bedding was placed in the cages. The room was illuminated for 12 h per day. The effect of sGC-α1 deficiency and air or 70% O2 exposure on lung structure was determined after 10 days, on postnatal day 13, and lung myofibroblast differentiation was assessed after 9 days of exposure to the gases because it is at approximately this postnatal age that sGC subunit expression and activity and alveolar and microvascular development peak in wild-type (WT) rodents (14, 17, 48, 52). Moreover, the effect of sGCα1 deficiency on lung structure in air-breathing pups at postnatal day 3 was determined because this is before the age when secondary septation is observed in the mouse pup lung (2).
Detection of cGMP signaling enzymes using immunoblotting.
sGC isoform and PKGI protein expression levels were determined in soluble fractions of whole lungs using protein blot hybridization and commercially available antibodies. To obtain the protein for analysis, sGC-α1 KO and WT 10-day-old mouse pups were killed using an intraperitoneal injection of 200 mg/kg pentobarbital sodium, and then lung tissues were obtained by dissection, blotted to remove excess blood, quickly frozen using liquid N2, and stored at −80°C. Subsequently, the frozen lungs were pulverized, and proteins were solubilized using ice-cold lysis buffer containing 50 mM Tris·HCl, pH 7.4, 1 mM EDTA, 1 mM dithiothreitol, and supplemented with 1:100 volume protease inhibitors (Sigma P8340). After determining the protein in soluble fractions, using BCA protein assay reagent (Pierce), we resolved 50 μg of protein using SDS-PAGE, electroblotted onto polyvinylidene difluoride membranes, and blocked using 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20. The membranes were then reacted with the following antibodies: anti-sGC-α1 (Cayman Chemical 160895), anti-sGC-α2 (Lifespan Biosciences LS-C98331), anti-sGC-β1 (Cayman Chemical 160897), and anti-PKGI (Cell Signaling clone C8A4). This anti-PKGI antibody detects the COOH-terminal catalytic domain, which is conserved in both PKGI-α and PKGI-β, the prominent isoforms of PKGI expressed in the lung. The PKGI isoforms migrate similarly in the SDS-PAGE gel employed in this study. The immunocomplexes were detected using peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. Equal protein transfer was confirmed by reprobing the membranes with an antibody that detects α-tubulin (Sigma clone DM1A).
sGC enzyme activity in mouse pup lungs.
sGC enzyme activity was determined in soluble lysates of whole lungs by measuring cGMP production using previously described methods (19). The lungs of 10-day-old mouse pups were collected and rapidly frozen, and soluble protein extracts were obtained from pulverized frozen lungs using lysis buffer defined above. sGC activity was measured by assessing cGMP levels in reaction buffers containing 75 μg of soluble lung protein, 1 mM GTP, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 0.1 mg/ml creatine phosphokinase, 7.5 mM creatine phosphate, 4 mM MgCl2, and 50 mM Tris·HCl, pH 7.4, with or without 1 mM sodium nitroprusside (SNP), an NO donor. This amount of SNP has been observed to maximally stimulate sGC enzyme activity (14). IBMX is an inhibitor of cGMP hydrolysis by phosphodiesterases. After incubating the solutions at 37°C for 10 min, the reaction was terminated by the addition of 0.2 ml ice-cold 0.05 N HCl. The cGMP levels were measured after 10 min of SNP stimulation because, in pilot studies, we observed that the sGC activity in the reaction mixture at this time point was not limited by substrate availability. Following lyophilization and acetylation, the cGMP concentration in the samples was measured by ELISA using a commercially available kit (Biomedical Technologies BT-740).
Analysis of lung structure.
The mouse pups were weighed and then killed as above. The lungs were fixed in a distended state by instilling 4% paraformaldehyde in the airways at 22 cmH2O water pressure, as previously described (53). Subsequently, the lungs were removed from the thorax, and the total lung volume was measured using the Archimedes principle. Subsequently, a 2–3-mm-thick transverse section was obtained from the middle of the left lung, dehydrated using graded ethanol baths and Clear Rite 3 (Richard Allen Scientific), and embedded in paraffin. After obtaining 6-μm-thick sections, we removed the paraffin, and we stained the lung tissue with toluidine blue. Lung images used for the structural analysis were captured using an integrated microscope and digital camera system (Nikon CoolScope) and an unbiased systematic sampling approach. Blood cells and debris in the alveoli and large vascular structures in the lung images were masked using an image-processing program (Adobe Photoshop). During the image capture and masking, the investigators were unaware of the genotype and oxygen exposure level of the lung specimen.
The structure of lungs obtained from 13-day-old (P13) mouse pups was analyzed objectively using the following methods. To analyze mean linear intercept length (Lm), the lung slices were divided into four regions, and two 0.08 mm × 0.06 mm images that excluded large airway and vascular structures were obtained from within each region. The Lm was determined using a dissector containing horizontal lines that were 12.6 μm apart, ImageJ (61), and methods described previously (53). Such grid-line spacing provides an appropriate sampling of chords in the mouse lung (51). Moreover, we determined that this horizontal line spacing permitted assessment of chord lengths in each airspace of the peripheral pup lung (data not shown). As previously described, chord lengths <8 and >500 μm long were discarded to reduce data associated with artifacts and large conducting airways (53). At least 38,000 chords from lungs of 12 pups per genotype and oxygen-treatment level were analyzed using this method.
The novel log-area and circularity (LArC) analysis of lung airway structures takes advantage of the notion that gas exchange units formed during alveolarization and affected by injury in the newborn tend to be smaller and rounder (have a circularity closer to 1), whereas conducting airways that are relatively unchanged during lung maturation or injury in the newborn are larger and more oblong (have a circularity <1). Although others have examined airway circularity itself as a means to evaluate alveolar expansion by pulmonary surfactant (31) or alveolar heterogeneity in lung injury (23), to our knowledge, this report contains the first attempt to assess lung development by analyzing both airspace area and circularity and comparing their distribution by applying standard statistical testing.
The peripheral lung LArC data were obtained using the same images that were used for the Lm analysis. After the images were thresholded using a Renyi entropy method (37) and segmented using binary morphological processing, the regions representing airspace were enumerated using connected component labeling (33). Airspaces touching the image boundary were removed. Area and perimeter length for the remaining airspaces were determined using pixel counting. The circularity of each airspace was determined using the following equation: (4 π × area)/perimeter2. This isoperimetric factor defines circularity as the ratio of the area of the airspace to the area of a circle with the same perimeter. Defined this way, circularity is bounded between a value of 0 and 1, with a value of 1 for circular airspaces, and lower values for less round ones. Of note, in previous reports, airway circularity was examined by using the Heywood circularity factor (HCF). However, we chose to improve the comparability of the airspace shapes in the genotype and O2-exposure groups in this study by using 1/√HCF to estimate circularity, because 1 and 0 would bound the resulting metric, whereas the HCF is unbounded. The LArC data were analyzed using an automated method and a custom script (Mathworks MATLAB). Airspaces with areas ≤64 μm2 and ≥2,500 μm2 were discarded to reduce the analysis of artifacts and large conducting airways. Using this method, over 3,000 airspace structures were analyzed in each treatment group consisting of 12 pups.
To facilitate visual comparisons between the airspace area and circularity in the different groups, we introduced three additional aspects to the analysis. First, we performed a log-transformation of the airspace area, thereby generating data with a Gaussian distribution (data not shown) to visualize disparate values more easily. Second, we divided the log area-circularity values into data bins, and the number of assignments to each bin was tabulated to create a discrete estimate, or frequency map, of the underlying joint distribution. Third, a color scale was applied to distinguish the number of airspaces in each bin, and the data were assembled into graphical heat maps. The bin widths in the LArC data heat maps were determined analytically from the data from the air-breathing WT mouse pup lungs using an algorithm defined by Wand (74) employing the following formula: 3.49 × [min(s, IQR/1.349)] × n−1/3. The selection of bin width is important for computing frequency maps that faithfully represent the underlying distributions, allowing distinguishing differences to be noted while still classifying similar data into common bins. The importance of using appropriate bin numbers to represent quantized LArC data was verified by comparing the output of LArC data analyses performed with varying numbers of bins (data not shown). With too few bins, the differences between the LArC data distributions of the groups are difficult to appreciate. Finally, we note that the LArC data analysis permits unbiased comparisons of lung structure between groups through the use of Chi-square analysis of independence and the determination of distance maps.
The septal density and alveolar ring estimator (ARE) data were obtained using 0.85 mm × 0.65 mm images from sequential sections of the lungs, using a modification of a method described by others (57). To facilitate the analysis, images from adjacent lung sections were placed in a stack and then simultaneously registered using an unwarping method based on elastic deformations represented by B-splines and the bUnwarpJ ImageJ macro (3). Subsequently, three nonoverlapping 200 × 200 μm dissectors were applied to the registered figures, in areas that did not include large airways or vessels, and the resulting images were unstacked. The total number of septae was determined in each dissector; the median number of septae was used in determining the septal density. For determining the numbers of alveolar openings and closings, the bridges and islands within the dissectors were counted. As defined by others (35, 57), a bridge (B) was counted when two free edges of an airspace structure in one section united into a single structure in the adjacent section, and an island (I) was counted when an isolated portion of an airspace wall appeared in one section but not in the adjacent lung section. Bridges and islands that originated outside of the dissector window were ignored. The identification of structures occurred in both directions, i.e., using each serial section as a reference for comparison with the other. The ARE was calculated using the formula: ARE = −Δχ = −(I − B)/2. Δχ represents the contribution from the two adjacent dissector fields to the total Euler number of the lung. Because I < B, −Δχ was analyzed to convert the estimator to a positive integer. The dissectors employed in this study allowed the sampling of the alveolar rings in at least twice the relative lung area used by others (57). During the identification of airspace septal structures, bridges, and islands, the investigator was unaware of the genotype or oxygen exposure level of the lung section.
Detection of lung smooth muscle myosin heavy chain protein expression.
Smooth muscle myosin heavy chain (SMMHC) immunoreactivity was detected in P13 mouse pup lung sections, obtained as described above. The lung sections were treated with antigen retrieval solution (BD Biosciences Retrievagen A), blocked with 1% horse serum, and then incubated with an anti-SMMHC antibody (Biomedical Technologies BT-562) or preimmune rabbit serum overnight at 4°C. Subsequently, the lungs were treated with a biotinylated anti-rabbit antibody, avidin-biotin complexed alkaline phosphatase, and then a colorimetric substrate (Vector Laboratories Vector Red). After the lung sections were counterstained using hematoxylin, images were captured using a wide-field microscope with an attached CCD camera system. Nonspecific immunoreactivity was not observed in the lung sections treated with the preimmune rabbit serum (data not shown).
Evaluation of pulmonary fibroblast activation.
Fibroblasts were isolated from peripheral segments of mouse pup lung tissue using previously described methods (6). In brief, after the mouse pups were killed with pentobarbital sodium, an ∼1-mm-thick layer of the peripheral lung that excluded large airways and vessels was obtained with the aid of a dissecting microscope. The tissue was then minced and treated with 2 mg/ml collagenase (Worthington CLS-2) in Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine (complete media, CM) for 2–4 h at 37°C with intermittent agitation. Cells released from the digested lung tissue were then collected by centrifugation at 300 g for 5 min and resuspended in CM. The fibroblasts from four pup lungs in each group were pooled and then were allowed to adhere to tissue culture dishes or chamber slides. After 1 h, the media was removed, and the attached cells were maintained in CM.
α-Smooth muscle actin (α-SMA) immunoreactivity in isolated peripheral pup lung fibroblasts was quantified by flow cytometry, using a modification of the method described by Kimani and coworkers (39). After maintenance in CM for 4 days, the isolated cells were removed from tissue culture dishes using EDTA-trypsin, washed with CM and then PBS, and then fixed with 2% paraformaldehyde in PBS (vol/vol) at 4°C for 20 min. After being permeabilized with 0.1% saponin in PBS (wt/vol) at 4°C for 10 min, the cells were blocked with 5% (wt/vol) nonfat dry milk at 4°C. Subsequently, ∼2 × 105 cells were reacted for 30 min at room temperature with either Alexa Fluor 488-conjugated anti-α-SMA 1A4 monoclonal mouse antibody (R&D Systems IC1420G) or isotype control IgG2A antibody (R&D Systems IC003G) diluted in 0.1% saponin in PBS. After being washed with 0.1% saponin in PBS, the cells were resuspended in PBS. Flow cytometry was performed using at least 5,000 gated events captured with an analytic B-D LSRII flow cytometer employing a 488-nm laser.
Analytical methods.
The lungs used for structural analysis were obtained from at least three independent litters and exposure experiments. Previous experiments indicated that 12 pup lungs per group would be necessary to detect at least a 10% change in Lm with a Type I error of 0.05 and a power of 0.90 (53). Therefore, lung images of this number of pups were used for the Lm analysis. In agreement with reports by others (43), kernel density plots revealed that the Lm data from the pup lungs had a positive skew. Accordingly, Lm medians and 95% confidence intervals (CI) were determined after performing a log-transformation, which normalized the distribution as demonstrated by quantile-quantile plots. Subsequently, an exponential transformation of the Lm values was employed to convert the data back to a linear scale. The ARE data appeared to have a bimodal distribution; consequently, the median and quartile ARE data were represented using a box plot. The group Lm and ARE data were first compared using a Kruskal-Wallis rank sum test, and then post hoc analysis was performed using a Mann-Whitney U-test with a Bonferroni adjustment. The LArC analysis data distributions from each group were compared using χ2 test of independence. Because several LArC analysis data bins contained no members, this χ2 analysis was performed using the original data set as well as one in which the data was rebinned in a 4 × 9 array, a resolution that resulted in fewer zero bins. A Bonferroni correction for multiple comparisons indicated that the significance P value for χ2 test of independence should be 8 × 10−3. χ2 Distance (D) was calculated using the numbers of elements within corresponding LArC analysis bins (i) of the compared genotypes and exposure gas groups (x, y) and the following equation (20): D(x,y) = ½Σi[(xi − yi)2/(xi + yi)].
The α-SMA immunoreactivity of the fibroblasts isolated from the peripheral mouse pup lung was analyzed using the Bioconductor package of R (28). The background fluorescence of the cells detected with the nonimmune IgG control antibody was minimal and equal in cells derived from WT and sGC-α1 KO mouse pups treated with air or 70% O2. After applying identical rectangular laser beam side- and forward-scatter gates to compensate for fragmented and dead cells and a boundary filter to remove oversaturated data, we performed a sine hyperbolic function transformation for the α-SMA immunoreactivity/Alexa 488.A channel of the data. An unsupervised model-based clustering approach described by Naumann and coworkers [curvHDR, (54, 55)] was then employed to group the peripheral lung fibroblasts based on similarity in the α-SMA immunoreactivity levels. This method classes cell characteristics by first identifying data group boundaries that have statistically significant high negative curvatures and then refining the region limits by examining local density functions. This approach for gating cells is especially suitable for data that does not have a parametric distribution, such as was observed in our flow cytometry study. In our studies, optimization of the data bandwidth used for the Hessian matrix estimation at a P < 0.05 statistical threshold revealed that the cells are best divided into four clusters based on their size and α-SMA immunoreactivity. A similar number of groupings was suggested by inspection of the frequency distribution of the α-SMA reactivity data. In a second stage of analysis, polygonal gates representing the group boundaries were applied to the data, and the number of cells within the groups was determined.
The frequency distributions of the α-SMA immunoreactivity data that were obtained using flow cytometry were compared using probability binning (65) and a custom R script. We report T(X) from this analysis because this statistic indicates the number of standard deviations that the normalized χ2, which is calculated from the binned distributions, is above the one in which there is no distribution difference. The data obtained from the peripheral lung fibroblasts of the WT mouse pups that breathed air were used to construct the control frequency distribution. The number of bins used in this analysis was determined using the method described by Wand (74). To adjust for multiple comparisons of the fluorescence distributions using a Bonferroni correction, a T(X) ≥ 4, which corresponds to P < 0.01 (65), was considered significant.
The effect of sGC-α1 deficiency and oxygen-induced lung injury on body weights and lung volume was analyzed using a factorial model of ANOVA. When significant differences were detected, a Tukey's HSD test was used post hoc. Unless otherwise indicated, the data are presented as means ± SD. With each test, significance was determined at P < 0.05, except as noted above.
RESULTS
sGC-β1 protein expression and sGC activity are decreased in sGC-α1 KO mouse pup lungs.
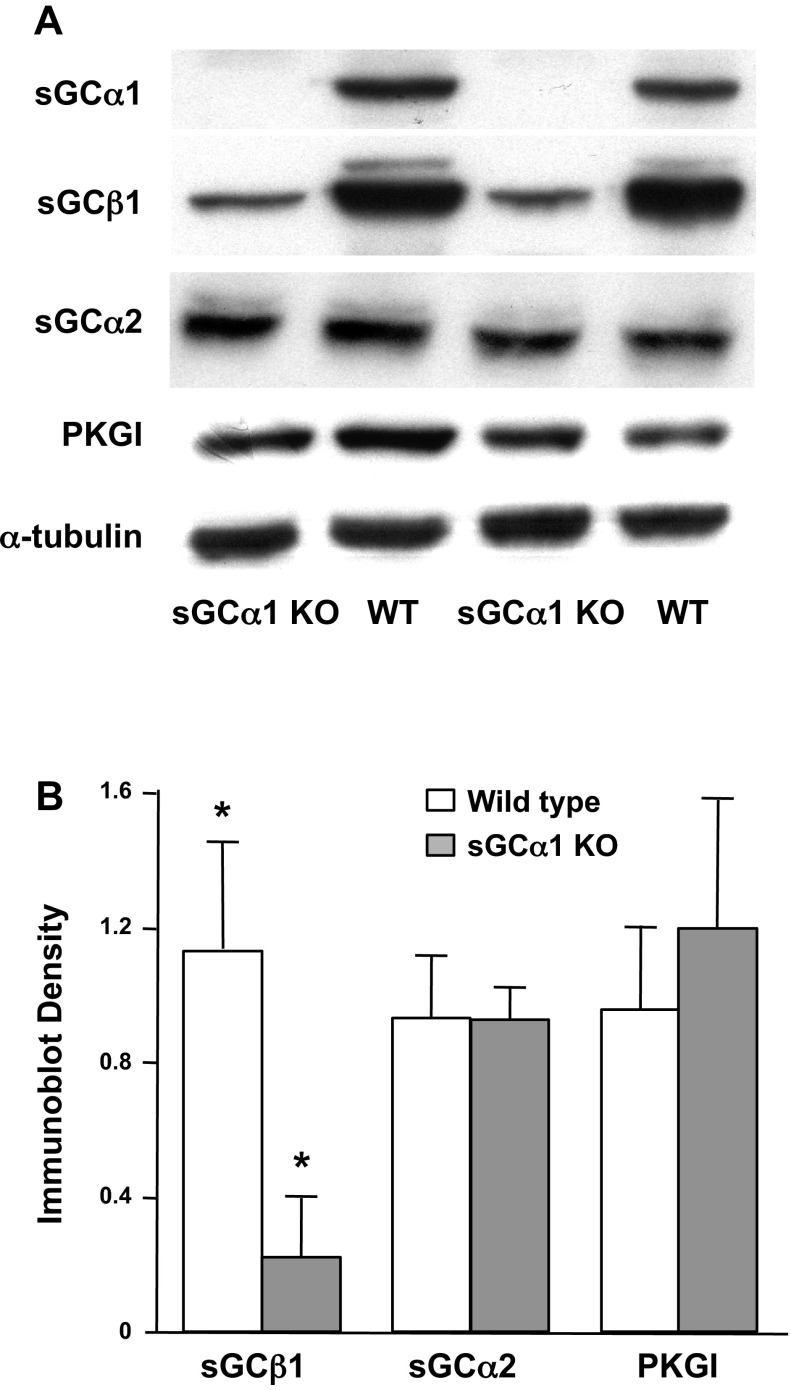
Although sGC subunit and activity levels have been reported to be decreased in the adult sGC-α1 KO mouse lung (19, 47), it was unknown whether the upregulation of sGC isoform expression observed in the developing lung (14), particularly of the sGC-α2 subunit, would preserve sGC activity in the sGC-α1 KO mouse pup lung. Therefore, we examined sGC subunit expression and enzyme activity in the sGC-α1 KO mouse pup lung. The protein levels of sGC-α1, sGC-α2, and sGC-β1 subunit isoforms were examined because they are the most abundant ones expressed in the mouse pup lung. Moreover, PKGI protein expression was determined because cGMP activation of this kinase is critical for the vasodilatory (27) and cell phenotype regulation activities of sGC (21), and sGC can regulate PKGI gene expression in cultured vascular SMC (15, 16). As shown in Fig. 1, deficiency of sGC-α1 expression in the KO mouse pup lung was associated with a marked decrease in sGC-β1 but preservation of sGC-α2 and PKGI protein expression levels compared with those observed in the WT pup lungs. Quantitation of the protein immunoreactivity revealed that the sGC-β1 protein levels are decreased by >75% in the sGC-α1 KO mouse pup lung although the levels of the other cGMP signaling proteins that we measured are unchanged.
Fig. 1.
Soluble guanylate cyclase (sGC) isoform protein expression is decreased in sGC-α1 knockout (KO) mouse pup lung. cGMP signaling enzyme protein expression in lungs of 10-day-old sGC-α1 KO and wild-type (WT) mouse pups was determined using immunoblotting. A: representative protein blots of 50 μg of soluble lung proteins from 2 mouse pups in each group that were reacted with indicated antibodies. α-Tubulin immunoreactivity was assessed to demonstrate that equal amounts of lung proteins were analyzed. PKGI, cGMP-dependent protein kinase. B: band densities of indicated protein immunoreactivity normalized to the band density of α-tubulin immunoreactivity. N = 5–6 mice per group. *P < 0.05 for difference between genotypes.
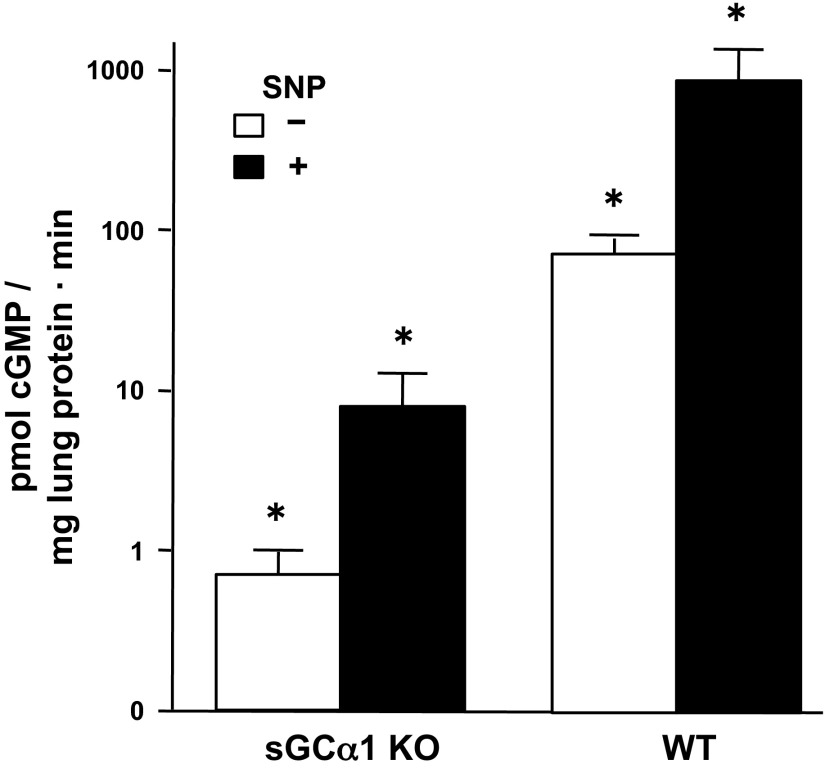
Because sGC subunit isoform expression levels do not always correlate with sGC enzyme activity (30), we next examined whether the decreased sGC-α1 and sGC-β1 protein levels observed in the sGC-α1 KO mouse pup lung are associated with diminished sGC activity. Both basal and stimulated sGC enzyme activity were measured by assessing the cGMP levels in soluble protein fractions from whole lung lysates of 10-day-old pups in the presence of a cGMP hydrolysis inhibitor with or without SNP. As shown in Fig. 2, the basal and SNP-stimulated sGC activity levels were decreased by about 100-fold in sGC-α1 KO pup pulmonary lysates compared with the basal and NO-stimulated sGC activity levels observed in WT pup lung lysates. Also of note, SNP treatment was found to cause nearly a 10-fold increase in cGMP production in both the sGC-α1 KO and WT lung lysates. This similar sGC activation by SNP suggests that, although sGC enzyme activity is decreased in the sGC-α1 KO lung, it responds normally to NO stimulation. The low levels of cGMP in the lungs of the sGC-α1 KO pups are likely generated by residual sGC-α2β1 activity.
Fig. 2.
sGC enzyme activity level is decreased in the sGC-α1 KO mouse pup lung. sGC activity was determined by measuring cGMP levels in soluble fractions of the indicated mouse pup lungs treated with or without 1 mM sodium nitroprusside (SNP; a nitric oxide donor) for 10 min; cGMP levels were referenced to the lung protein quantity in the sample. sGC enzyme activity level in sGC-α1 KO pup lung lysates was 100-fold less than in WT. Nevertheless, SNP stimulated a 10-fold increase in sGC enzyme activity in lung lysates from sGC-α1 KO and WT pups, suggesting that residual sGC-β1 formed functional dimers with sGC-α2 in the sGC-α1 KO pup lung. N = 12 per group. *P < 0.05 for the difference between SNP-stimulated and unstimulated lung lysates, and for the difference between WT and sGC-α1 KO lung samples.
sGC deficiency exacerbates alveolar growth inhibition caused by hyperoxic injury.
Because decreased sGC activity was detected in the newborn sGC-α1 KO mouse lung, we next examined whether these mouse pups had decreased alveolarization with and without lung injury. The mouse pup lung structure was examined in 3-day-old (P3) sGC-α1 KO pups because this would provide a comparison of baseline pulmonary structure before secondary lung septation, a period when sGC expression peaks in the WT lung (14). Moreover, the lung structure was examined in P13 mouse pups because alveolarization is nearly complete in mice by that age (17, 48, 52). The pups were exposed to 70% oxygen because pilot studies determined that this oxygen level produced only minimal changes in alveolarization in WT mice.
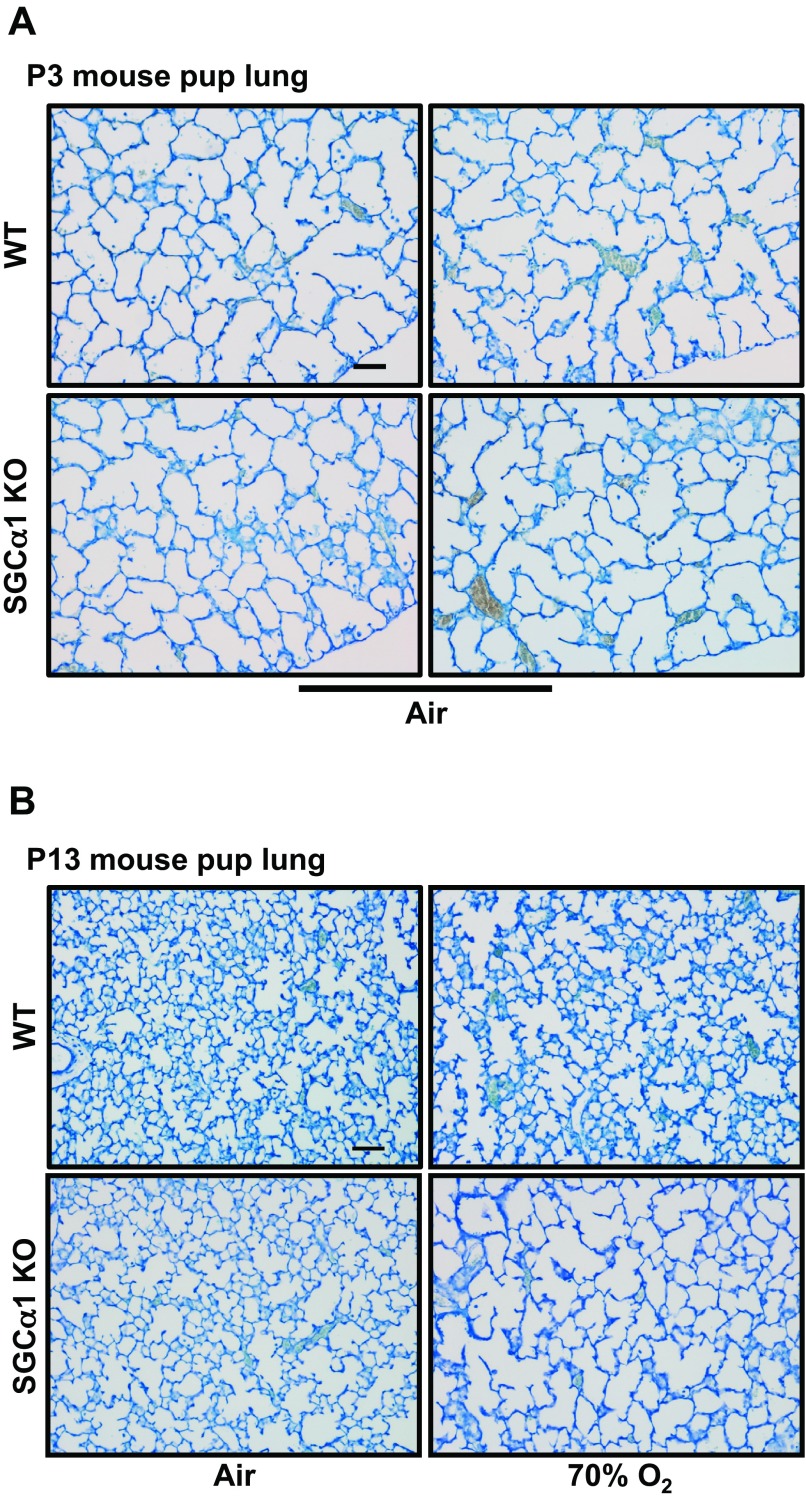
As shown in Fig. 3, there appeared to be no difference in the peripheral lung structure of P3 sGC-α1 KO mouse pups compared with WT controls. This was expected because the low sGC subunit expression observed in the WT fetal lung was thought to have little influence on the embryonic and fetal stages of lung development. However, the P13 hyperoxic WT and air-breathing sGC-α1 KO pup lung appeared to have similar mild changes in acinar structure, and a decrease in alveolarization was most pronounced in the sGC-α1 KO pup lungs that were exposed to 70% O2.
Fig. 3.
Alveolarization is decreased in the sGC-α1 KO mouse pup lung. Representative images of toluidine blue-stained sections of mouse pup lungs that were inflation fixed at postnatal day 3 (P3) and 13 (P13). A: peripheral lung structure of P3 sGC-α1 KO pup lungs appeared to be similar to that of WT ones. B: in contrast, P13 sGC-α1 KO pup lungs had fewer and larger airspace structures than WT ones at the indicated oxygen exposure levels. The most severe lung phenotype was observed in the sGC-α1 KO mouse pups exposed to 70% O2. Scale bar = 20 μm.
For an unbiased examination of the effects of decreased sGC activity and injury on lung development in the P13 pups, morphometric analyses were performed. The Lm was analyzed because it has been a standard metric that is often used to examine newborn lung development. The Lm measures intra-airspace distances (including alveoli and conducting airways), inversely correlates with lung internal surface area, and decreases during alveolarization. Although we observed that the Lms of the air-breathing sGC-α1 KO and WT pup lungs were not different (Lm in μm, median ± 95% CI, WT-air 24 ± 1, sGC-α1 KO 24 ± 1), the Lms were larger after hyperoxic lung injury and greatest in the sGC-α1 KO pup lung exposed to 70% O2 (WT-70% O2 25 ± 1, sGC-α1 KO-70% O2 28 ± 1, P < 0.01 air vs. 70% O2 and sGC-α1 KO vs. WT). Although similar Lms were observed in the air-breathing sGC-α1 KO and WT mouse pups, the reduction in sGC-α1 KO mouse pup lung volumes that we detail below suggests that sGC deficiency likely decreases lung internal surface area (34, 71).
Inspection of the P13 mouse pup lung images suggested that small peripheral airspaces were fewer in number in the air-breathing sGC-deficient mouse compared with the air-exposed WT mouse. Thus we next employed morphometric tools that detail the structure of small airspaces. Previously described approaches that measure airway area have relied on arbitrary size cut-offs, limiting their success in distinguishing alveoli from conducting airways (43, 46, 75). Therefore, to focus on small gas-exchanging structures that might be modified in the injured lung, we employed a novel area-circularity analysis method, which we report here for the first time, and an alveolar ring estimator, which is a minor modification of the connectivity method of alveolar counting that was developed by others (35, 57).
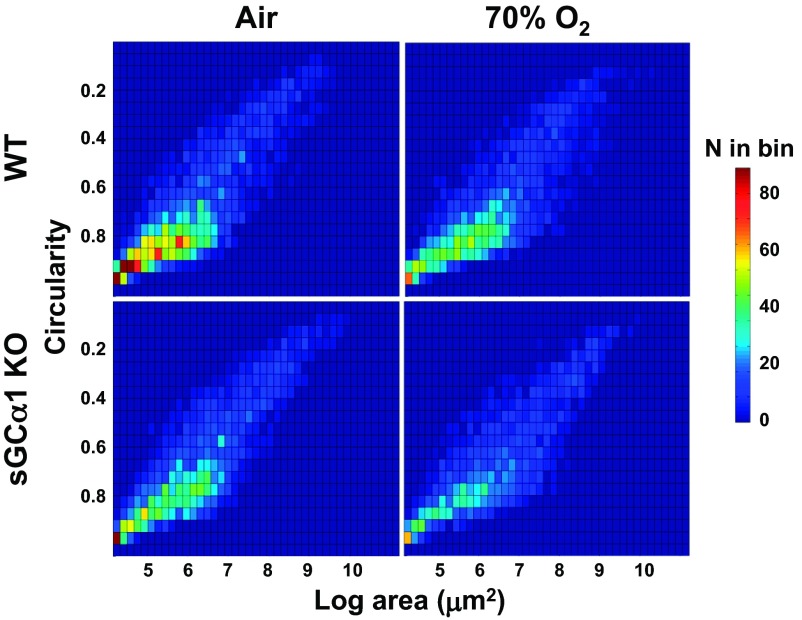
The LArC data analysis stems from our postulate that the alveoli can be distinguished from other types of airspace structures (e.g., bronchioles) by their relatively small size and round shape in cross-section. As described in materials and methods, such data can be collected effectively from peripheral lung images using algorithms designed for object pattern recognition, quantized in a manner that preserves airway size and shape information, visualized using a heat map, and compared using standard statistical methods. The LArC analysis permitted the observation of important differences in small airway development in the sGC-α1 KO mouse pups. As shown in Fig. 4, and in agreement with inspection of lung images, the greatest difference in the number of small circular peripheral airspace structures was observed between air-breathing WT pup lungs and 70% O2-exposed sGC-α1 KO mouse pup lungs. However, when compared with air-breathing WT pups, both air-exposed sGC-α1 KO and 70% O2-treated WT mouse pups had similarly decreased numbers of small round peripheral lung airspace structures. The differences between the LArC distributions of the groups, illustrated by the heat maps (Fig. 4), were supported by χ2 analysis; the χ2 distances and P values for the data reduced to 5 × 9 bins are shown in Table 1. These data suggest that sGC deficiency importantly decreases alveolar development, although to a lesser extent in air-exposed mouse pup lungs.
Fig. 4.
70% Oxygen exposure decreases the abundance of small-circular airspaces in the periphery of sGC-α1 KO lungs. The log area and circularity (LArC) of peripheral airspace structures of toluidine blue-stained inflation-fixed P13 mouse pup lung images was measured, binned, and arranged in a heat map. Circularity ranges from 1 (round) to 0 (oblong); log area corresponds to structures with areas between 64 and 2,500 μm2. The number of airspaces classed per bin ranges from low (dark blue) to high (dark red) according to the scale on the right. The lungs from 12 pups per group were analyzed. The distributions of airspace structures were different in the groups by χ2 analysis.
Table 1.
Analysis of sGC-α1 KO and WT mouse lung LArC data
| WT |
sGC-α1 KO |
|||
|---|---|---|---|---|
| Air | 70% O2 | Air | 70% O2 | |
| WT | ||||
| Air | 8 × 10−13 | 2 × 10−13 | 3 × 10−10 | |
| 70% O2 | 294 | 8 × 10−9 | 2 × 10−7 | |
| sGC-α1 KO | ||||
| Air | 265 | 242 | 1 × 10−10 | |
| 70% O2 | 464 | 263 | 293 | |
Standard font, χ2 test of independence P value; boldface, χ2 distance; sGC, soluble guanylate cyclase; KO, knockout; WT, wild-type; LArC, log area and circularity.
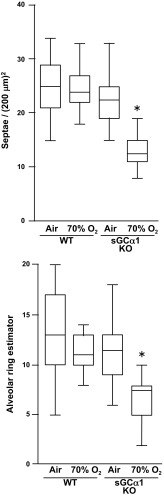
To further test whether decreased sGC activity alters mouse pup alveolar development, we determined the septal densities in the mouse pup lungs. As shown in Fig. 5, the injured sGC-α1 KO mouse pup lung had decreased septation. To further detail pulmonary development, we also applied a recently described morphometric method, originally employed to quantify the total number of alveoli in the lung, to examine the effect of decreased sGC activity on alveolar structures in peripheral sections of mouse pup lungs. The method estimates the number of alveoli in the whole lung by performing a topological analysis of the connectivity of the lung (35, 56, 57). This approach is specific for alveolar structures because it estimates the number of connections in the two-dimensional network (alveolar rings or openings) located within a three-dimensional space (the lung). Another strength of this approach is that it makes no assumptions regarding shape, size, or orientation of the alveoli. Because we were interested in examining alveolar development, we focused our analysis of lung connectivity to the peripheral lung and designated our metric as the ARE (see materials and methods). In agreement with the most marked change in Lm, septal density, and LArC values, the ARE was significantly decreased in the 70% O2-exposed sGC-α1 KO pup lungs compared with the others (Fig. 5), suggesting that sGC deficiency most impairs alveolar formation in the injured newborn lung.
Fig. 5.
70% Oxygen exposure decreases alveolarization in sGC-α1 KO pup lung. Alveolarization was assessed in the indicated pup lungs by determining the septal density and using an alveolar ring estimator (ARE), a derivative of the Euler number of the network of alveolar openings as described in the text, using 6 mouse pup lungs per group. *P < 0.05 for 70% oxygen-exposed sGC-α1 KO lung compared with each other group.
sGC-α1 deficiency inhibits lung and somatic growth in mouse pups.
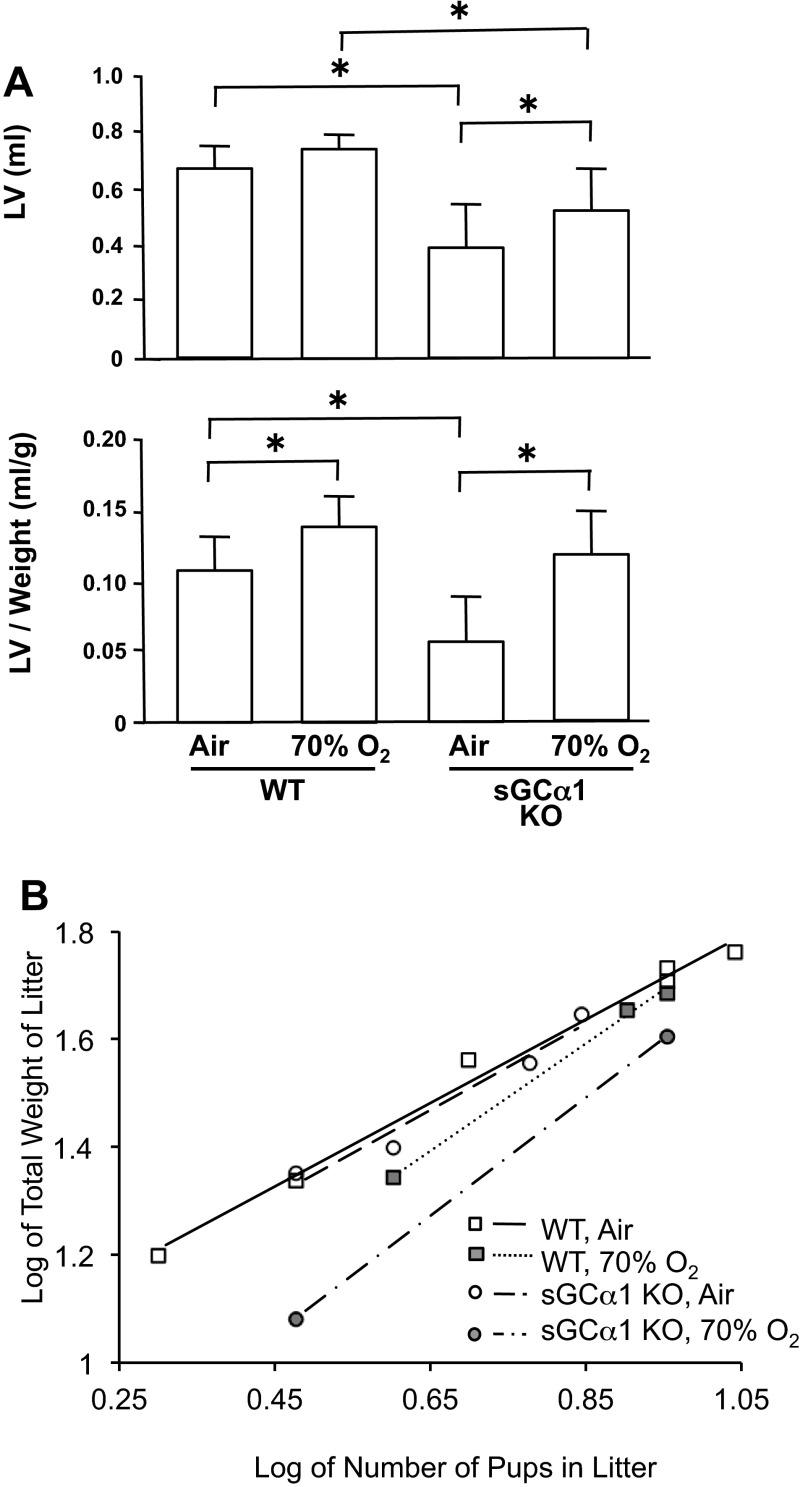
As shown in Fig. 6A, air-exposed sGC-α1 KO pups had smaller lung volumes, with and without normalizing to body weight, than air-exposed WT pups. Lung volume normalized for body weight was significantly greater in both WT and sGC-α1 KO pups exposed to 70% oxygen for 10 days, compared with air-exposed pups. These data suggest that decreased sGC activity diminishes lung growth but does not affect the mild increase in lung volume caused by oxygen-induced lung injury. The effect of decreased sGC activity on somatic growth was assessed by weighing pups on postnatal day 13. Although there was no difference in body weights between sGC-α1 KO pups breathing air and WT pups breathing air or 70% O2, sGC-α1 KO pups breathing 70% O2 had decreased somatic growth (body weight in g, WT-air 5.89 ± 0.88, WT-70% O2 5.51 ± 0.65, sGC-α1 KO-air 6.39 ± 0.64, sGC-α1 KO-70% O2 4.36 ± 0.30; 12–34 per group, sGC-α1 KO-70% O2, P < 0.05 vs. others). To control for differences in pup weights due to litter size, the relationship between the number of pups in a litter and the combined pup weights in the litter were also compared, as described (22). As shown in Fig. 6B, this relationship confirmed that somatic growth was inhibited primarily in the sGC-α1 KO mouse pups exposed to 70% O2. Of note, both WT and sGC-α1 KO pups conform to the previously reported weight-litter size relationship (22) and have similar slopes, suggesting that pup growth varies similarly with litter size regardless of genotype or hyperoxia exposure. However, the significantly lower y-intercept of the line based on the 70% O2-exposed sGC-α1 KO litters confirms that the overall average lower weight of these pups is not due to skewing by a preponderance of large litters (with smaller pups) in this group. Furthermore, because more severe impairments in lung development have been correlated with greater reductions in somatic growth (10, 53, 58, 66), this result supports our observations showing that hyperoxia impairs lung development to a greater extent in sGC-α1 KO than in WT newborns.
Fig. 6.
Lung volume (LV) is decreased in sGC-α1 KO mouse pups, and 70% oxygen exposure decreased sGC-α1 KO mouse pup body weight. A: LV and LV-to-body weight ratio of 13-day-old mice. N = 12–14 mice per group. *P < 0.05. B: relationship between total pup weights and pup numbers in a litter. Each point represents data from 1 13-day-old litter; lines are linear regression models. All groups exhibit an effect of litter size on weight. Air-exposed WT and sGC-α1 KO litters and 70% oxygen-exposed WT litters showed similar growth, whereas sGC-α1 KO litters exposed to 70% oxygen had decreased growth (P < 0.05).
Smooth muscle myosin heavy chain expression is decreased in the injured sGC-α1 KO mouse pup lung.
cGMP plays an important role in regulating vascular SMC differentiation and proliferation (reviewed in Ref. 60). Therefore, we examined whether the sGC-α1 KO mouse pup lung has decreased expression of the SMC marker SMMHC in the pulmonary microvasculature. SMMHC is a subunit of myosin that is expressed in SMCs residing in pulmonary arteries, but not veins, and in the acinar wall commencing in the saccular phase of lung development (49, 76). As shown in Fig. 7, and in agreement with observations by others (50, 76), SMMHC immunoreactivity was detected in the mouse pup pulmonary arteries in the peripheral lung and in cells in the alveolar septae. However, in the O2-injured sGC-α1 KO mouse pup lung, the SMMHC expression levels were decreased in these cells. A similar reduction of α-SMA immunoreactivity was observed in the injured sGC-α1 KO pup lung (data not shown) although α-SMA is not exclusively expressed in SMCs in the lung.
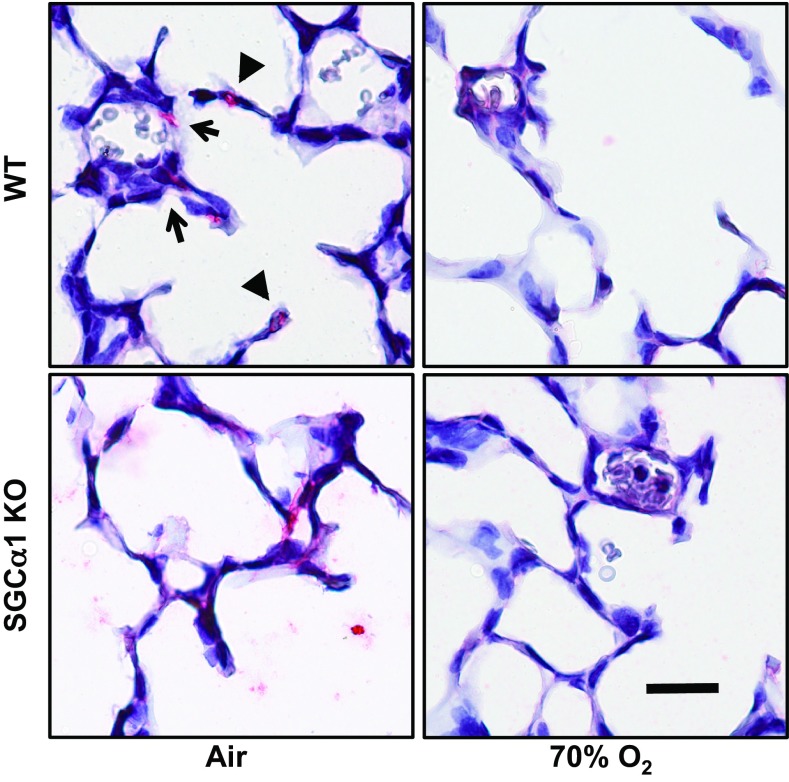
Fig. 7.
Diminished sGC activity decreases smooth muscle myosin heavy chain (SMMHC) expression in the 70% oxygen-injured peripheral mouse pup lung. SMMHC immunoreactivity was determined using mouse pup lung sections using a specific polyclonal antibody and immunohistochemistry (depicted in red); cells were counterstained with hematoxylin. Whereas the peripheral lungs from P13 air- and 70% O2-exposed WT and air-treated sGC-α1 KO pups exhibited SMMHC immunoreactivity in cells in the wall of peripheral lung arteries (arrow) and in alveolar septae (arrow head), SMMHC expression was decreased in 70% O2-treated sGC-α1 KO pups. Representative images of 3 separate experiments are shown. Scale bar = 20 μm.
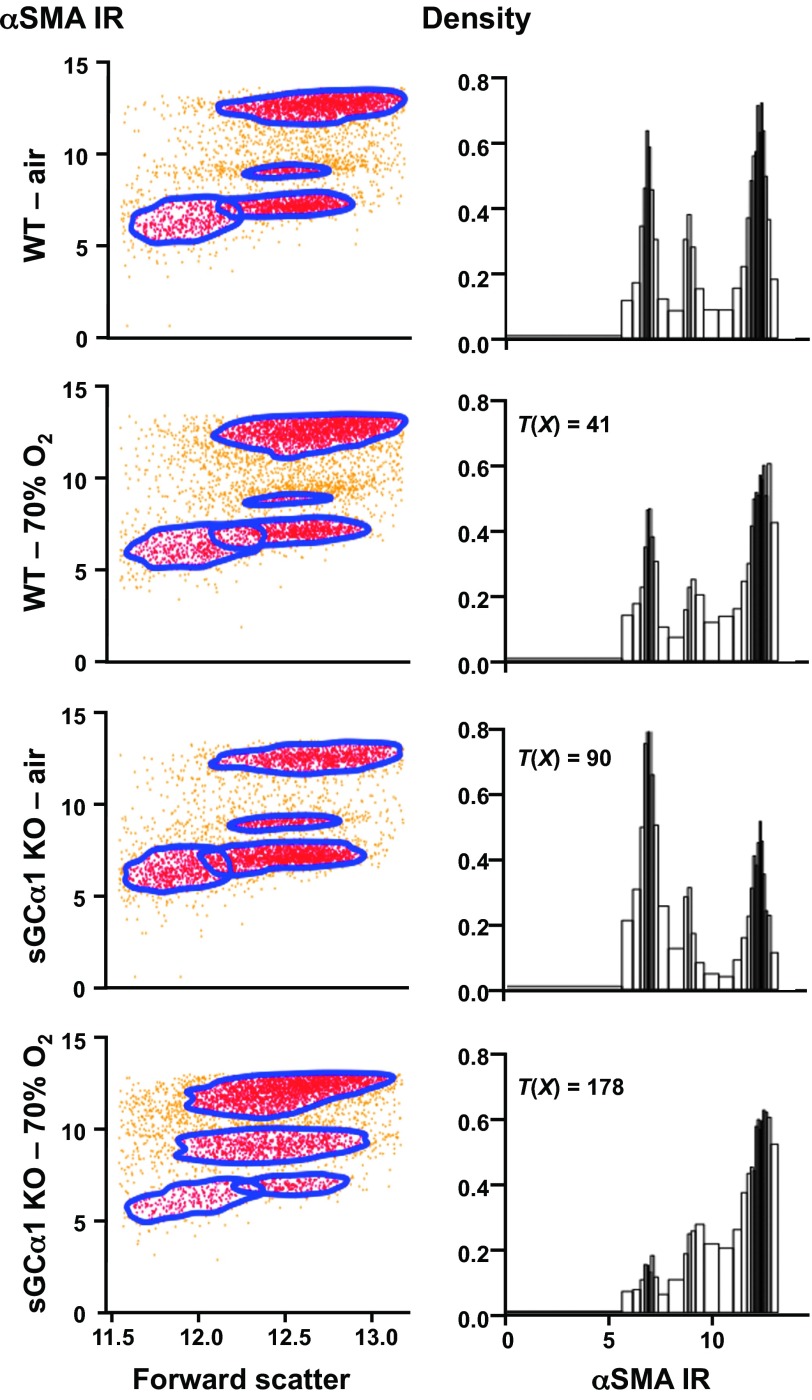
To examine whether decreased sGC activity is associated with increased fibroblast activation in the O2-injured sGC-α1 KO pup lung, we analyzed α-SMA immunoreactivity in fibroblasts isolated from the peripheral pup lung using an anti-α-SMA antibody and flow cytometry. Studies by others show that fibroblast activation is associated with an increase in α-SMA expression in the lung (68). As shown in Fig. 8, four populations of peripheral pup lung fibroblasts were identified based on cell size characteristics (forward scatter) and α-SMA immunoreactivity. The cells were automatically parsed into these groups using data-driven gating algorithms (55), and the differences in the α-SMA immunoreactivity were shown using probability binning methods (65). The T(X) values indicate that each distribution of α-SMA immunoreactivity in the peripheral lung fibroblasts is different from that observed in cells derived from the other mouse pups (P < 0.01). The normalized χ2 distances associated with the distributions are shown in Table 2. The two cohorts of cells with the lowest α-SMA immunoreactivity essentially did not express this SMC marker because they had the same fluorescence signal as cells reacted with an isotype-control antibody conjugated with the fluoroprobe (data not shown). Of the peripheral lung myofibroblasts interacting with the α-SMA antibody, two staining levels were observed. This suggested that the peripheral lung contains myofibroblasts with two levels of differentiation indicated by α-SMA expression and is consistent with the varying level of α-SMA immunoreactivity that we observed in peripheral lung myofibroblasts isolated from the WT mouse pup lung using epifluorescence microscopy (data not shown).
Fig. 8.
Reduced sGC activity levels and 70% oxygen exposure decrease α-smooth muscle actin (SMA) expression in peripheral lung myofibroblasts. α-SMA expression was determined in peripheral lung myofibroblasts isolated from 9-day-old WT and sGC-α1 KO mouse pups exposed to air or 70% O2, as indicated, using a fluorescently labeled α-SMA-reactive antibody and flow cytometry. The cell data were automatically gated based on α-SMA staining level; representative α-SMA immunoreactivity (IR) scatter grams and associated probability-binned histograms and T(X) are depicted. α-SMA expression in each genotype was differently modulated by 70% oxygen exposure. A minimum number of 5,000 cells was analyzed, which were obtained from 4 mouse pups in each group.
Table 2.
Analysis of sGC-α1 KO and WT peripheral lung fibroblast α-SMA flow cytometry data
| Normalized χ2 Distance Values |
||||
|---|---|---|---|---|
| WT |
sGC-α1 KO |
|||
| Air | 70% O2 | Air | 70% O2 | |
| WT—Air | 0.052 | 0.113 | 0.207 | |
SMA, smooth muscle actin.
Decreased sGC activity in the sGC-α1 KO mouse pup lung was associated with diminished α-SMA immunoreactivity in isolated lung myofibroblasts. The proportion of isolated cells exhibiting the highest level of α-SMA immunoreactivity was smaller for sGC-α1 KO compared with WT mouse pup lungs (Fig. 8, highest cluster or right-most peak of the histograms). Cell counts within the polygonal gates illustrated in the scatter grams in Fig. 8 revealed that 52% of the cells isolated from the WT pup lungs exhibited the highest α-SMA immunoreactivity, but only 28% of the cells isolated from the air-exposed sGC-α1 KO pup lungs had a similarly high level of α-SMA expression. The numbers of cells with intermediate α-SMA immunoreactivity were nearly 10%, and similar in both sGC-α1 KO and WT lungs. Of interest, although exposure to 70% O2 did not change the distribution of cells with and without α-SMA immunoreactivity in cells isolated from WT lungs, it diminished the number of non-α-SMA-reacting cells and increased the number of α-SMA-expressing cells isolated from the sGC-α1 KO mouse pup lungs. The differential effects of hyperoxia on α-SMA expression in peripheral pup lung fibroblasts depending on the mouse genotype suggest that sGC function may regulate myofibroblast differentiation in the injured mouse pup lung periphery.
DISCUSSION
Previously, we observed that hyperoxic lung injury decreases sGC expression as well as alveolarization and peripheral lung myofibroblast differentiation in the developing mouse lung (6, 53). However, a direct link between sGC activity and lung development had not been defined. We now report that sGC has a role in regulating alveolarization in the injured newborn lung. We observed that, in contrast with WT animals, mouse pups deficient in sGC-α1 have decreased pulmonary sGC enzyme activity, mild reductions in small airspaces, and reduced lung volumes and peripheral lung myofibroblast differentiation. However, acinar septation and somatic growth remained intact. Importantly, mild hyperoxic injury caused a severely disrupted alveolarization phenotype in the mouse pup lungs with decreased cGMP levels. In sGC-α1 KO pup lungs, chronic inhalation of 70% O2 decreased lung septation and somatic growth, decreased SMC contractile protein expression in peripheral pulmonary arteries and alveolar septae, and caused activation of peripheral lung fibroblasts compared with oxygen-injured WT or air-treated sGC-α1 pups. These studies indicate for the first time the importance of sGC in promoting alveolarization in the injured lung.
sGC is a heterodimeric protein that produces cGMP upon stimulation by NO. However, the importance of the expression of each enzyme subunit isoform in forming functional sGC in the newborn lung is not fully understood. In the sGC-α1 KO mouse pup model used for these studies, we observed that deficiency in the pulmonary expression of only one isoform, sGC-α1, was sufficient to greatly decrease sGC enzyme activity levels in lung lysates. Although previously it was understood that sGC subunit expression increases in the peripheral lungs of newborn rodents (14), we observed a >75% decrease in sGC-β1 expression and no change in sGC-α2 expression in the sGC-α1 KO mouse pup lung compared with WT. Interestingly, the reduction of sGC-β1 expression observed in lungs of our sGC-α1-deficient pup model has not been observed in all tissues in sGC-α1 KO mice. For example, the level of sGC-β1 protein expression was reported to be comparable in the aorta of adult sGC-α1 KO and WT mice. However, a reduction of sGC-β1 protein expression, similar to that which we observed in sGC-α1 KO pup lungs, was measured in adult lungs with the same genotype (19, 47). It is likely that diminished sGC activity in the lungs of sGC-α1 KO mice is not only due to this reduction of sGC-β1 expression. It is also possible that truncated sGC-α1 expressed in the sGC-α1 KO mouse binds to residual sGC-β1 and thereby inhibits sGC activity through a transdominant-negative mutation mechanism (19). Furthermore, although it is possible that sGC-β1 might homodimerize and thereby reconstitute cGMP-producing activity, for homodimerization has been observed in sGC isoforms overexpressed in cultured cells, generally such sGC homodimers are catalytically inactive (79). Therefore, it is likely that residual sGC activity observed in sGC-α1 KO lungs results from sGC-α2β1 (25). Our observation that sGC enzyme activity is decreased by about 100-fold in the sGC-α1 KO mouse pup compared with the WT lung confirms the reports of others that sGC-α2β1 plays less of a role in producing cGMP than sGC-α1β1 in the lung (19, 47).
The observation that pulmonary development is decreased in sGC-α1 KO mouse pups, especially those exposed to hyperoxic lung injury, supports the notion that sGC plays a direct role in regulating alveolarization. On postnatal day 3, no difference in peripheral lung development was observed in sGC-α1 KO mouse pups compared with age-matched WT controls. This observation was expected because very little sGC is expressed during fetal rodent lung development (14). Therefore, the similar low level of fetal WT and sGC-α1 KO sGC expression has no apparent role in modulating lung embryogenesis or branching and primary septation. In contrast, at nearly 2 wk of age, the air-breathing sGC-α1 KO mouse pups had mild structural changes in the distal airways compared with WT pups. Inspection of the airspace structure in lung sections suggested that sGC-α1 KO mouse pups had larger and more simplified peripheral airway units than the control pups. To objectively quantify these observations, we developed a novel approach that simultaneously quantifies the size and shape of the distal airways, the LArC analysis. We believe that using the LArC analysis in conjunction with other indicators of internal surface area (Lm and lung volume) and of alveolar formation (alveolar ring estimator and septal density) can provide a comprehensive survey of the anatomical changes that occur during septation of the developing lung. Moreover, we believe that such a survey might permit determination of the way septation of the saccular airways in the developing lung is modified by injury or therapeutic interventions. Although these indices have some overlap in the structural features that they assess, they consider different aspects of alveolarization. Through the LArC analysis, one can focus on changes in the abundance of small and round airways, which are most likely gas-exchanging units that form during alveolarization. Although the ARE can be used to assess the increase in distal airway connectivity that occurs during alveolarization, the Euler characteristic from which ARE is derived does not examine the size of the newly formed peripheral lung units, which might be influenced by lung injury. Moreover, although the Lm does provide an index of airway size, the Lm samples both terminal and conducting airway dimensions and is therefore not optimized to parse and then permit examination of newly forming alveoli. Although other investigators have used a curve-fitting approach to help resolve the Lm data obtained from adult gerbils into chord-length distributions attributed by alveoli and conducting airways (43), this approach does not work in the mouse, and it does not take advantage of the use of airway geometry to help distinguish differences in these lung compartments. Moreover, although the LArC analysis samples alveoli and conducting airways, given the abundance of the former in the mouse lung, we believe that the LArC analysis can give an estimate of alveolar formation. The LArC analysis in these pups suggested that the air-breathing sGC-α1 KO pup had an abnormality in the development of small-round airspaces, the characteristic size and shape of alveoli. This finding is consistent with the decrease in lung volume of these pups. A lack of decrease in the ARE suggested that decreased sGC activity, alone, did not inhibit the formation of alveolar rings. These data suggest that alveolar formation might be driven by other signaling systems in the lung.
In this investigation, we also examined the effect of decreased sGC activity on SMMHC expression in the peripheral mouse pup lung. We chose to examine the expression of SMMHC, which is a marker of SMC differentiation, because studies suggest that NO and cGMP signaling play an important role in regulating vascular SMC phenotype and proliferation. For example, previously, we reported that NO and cGMP regulate pulmonary artery SMC differentiation through stimulating PKGI (21). Nuclear cGMP signaling in vascular SMC appears to require PKGI proteolysis, in part mediated by proprotein convertases, and the nuclear translocation of an active kinase fragment that transactivates gene expression (38, 70). Moreover, others have observed decreased pulmonary vascular development in newborn (10) and increased muscularization of peripheral lung arteries in adult mice with NOS deficiency (69). We previously observed that inhaled NO decreased abnormal SMC proliferation in the small pulmonary arteries of rat pups with lung injury (64) in a manner that did not require vasodilatation (63). Furthermore, we previously observed that hyperoxia decreases sGC-α1 expression and α-SMA protein levels in the peripheral lungs of mouse pups (6, 53). In the present study, we report that the decreased sGC enzyme activity in the sGC-α1 KO mouse pup is associated with decreased SMMHC expression in SMCs located in peripheral lung pulmonary arteries and alveolar septae in the oxygen-injured lung. We also tested whether the decreased sGC level in the sGC-α1 KO pup lung was associated with fibroblast activation in the O2-induced injury. Although the number of α-SMA-expressing fibroblasts was decreased in the sGC-α1 KO pup lung, we observed that 70% oxygen-induced lung injury increased fibroblast activation in these lungs, as shown by increased numbers of α-SMA-expressing fibroblasts. These studies suggest that sGC enzyme activity modulates the differentiation of cells in the peripheral lung that contribute to formation of microvasculature and alveoli, particularly in the setting of lung injury.
The results of these studies support the role of sGC in regulating the development of the injured mouse pup lung, and further investigation is needed. For example, because sGC-α1 was not selectively knocked out in the lung in our mouse model, we do not know whether impaired lung development was due exclusively to abnormal pulmonary cGMP signaling. However, a suitable model of animals with genes selectively knocked out in pulmonary vascular SMC is not currently available (62). Recent studies have pointed to the importance of circulating mesenchymal or bone marrow-derived stem cells in the promotion of lung development (4, 9). Although it is unknown whether these cells express NO-signaling enzymes, it is possible that sGC deficiency in such cells in sGC-α1 KO mice inhibited newborn lung development. Moreover, our model does not exhibit complete deficiency of pulmonary sGC. Although the cGMP levels were low in the sGC-α1 KO mouse pup lung lysates, they contained some residual sGC activity as demonstrated by the induction of cGMP production following SNP treatment. Nevertheless, our results are likely to be relevant to in vivo processes contributing to inhibited alveolarization in the newborn because other newborn lung injury models demonstrate reduced, but not absent, sGC isoform expression (11, 13, 72). Interestingly, some studies have demonstrated that inhaled NO improves lung development in premature animals (12, 45). Our work reported here suggests that decreased sGC expression may play a role in preventing inhaled NO therapy from ameliorating lung development in the injured newborn lung. Future studies should investigate whether cGMP replacement in the sGC-α1 KO mouse pup lung rescues the effects of hyperoxia on diminished lung development. Furthermore, because our results suggest that sGC regulates SMC/myofibroblast phenotype in the developing lung, future studies should investigate the role of sGC modulation of these cells in lung development with and without injury.
Although the mechanisms through which sGC regulates or protects lung alveolarization are unknown, several studies suggest that cGMP has a role in regulating the differentiation and proliferation of cells that program lung development. For example, cGMP-stimulated PKGI has been shown, not only to regulate pulmonary vascular cytoskeletal kinetics, but also to modulate cell phenotype and proliferation (reviewed in Refs. 26 and 60). Although the precise mechanisms through which cGMP regulates cell phenotype are incompletely understood, recently, cGMP-stimulated PKGI has been observed to undergo proteolysis, in part through the activity of proprotein convertases (38), and a constitutively active PKGI kinase fragment has been observed to localize in the nucleus of cells (70). This nuclear PKGI has been observed in some studies to be required for the transactivation of gene expression by cGMP (29, 70). The studies detailed here suggest that sGC plays an important role in regulating lung development and support the importance of future work to determine whether the protective activity of cGMP occurs through modulation of pulmonary PKGI.
In conclusion, the studies detailed here indicate that sGC is important for normal and injured lung development in the newborn. They suggest that decreased sGC activity directly contributes to diminished alveolarization in the injured developing lung and therefore that further understanding the role of sGC in this context will yield insights into potential new therapies to prevent important newborn lung diseases.
GRANTS
This work was supported by the National Institutes of Health (5T32GM0759 to P. Bachiller and 1R01HL096779 to J. Roberts), the Foundation for Anesthesia Education and Research (MRTG to P. Bachiller), the American Heart Association (Scientist Development Grant 10SDG2610313 to E. Buys), Ikaria/INO Therapeutics, the Harvard Medical School (Shore Fellowship to P. Bachiller), and the MGH Department of Anesthesia, Critical Care, and Pain Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.R.B. and J.D.R. conception and design of research; P.R.B., K.H.C., R.K., E.S.B., and J.D.R. performed experiments; P.R.B., K.H.C., R.K., E.S.B., and J.D.R. analyzed data; P.R.B., K.H.C., R.K., E.S.B., and J.D.R. interpreted results of experiments; P.R.B., K.H.C., R.K., and J.D.R. prepared figures; P.R.B. and J.D.R. drafted manuscript; P.R.B., K.H.C., E.S.B., and J.D.R. edited and revised manuscript; P.R.B., K.H.C., R.K., E.S.B., and J.D.R. approved final version of manuscript.
REFERENCES
- 1.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 284: L749–L758, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 124: 131–151, 1977 [PMC free article] [PubMed] [Google Scholar]
- 3.Arganda-Carreras I, Sorzano COS, Marabini R, Carazo JM, de Solorzano CO, Kybic J. Consistent and elastic registration of histological sections using vector-spline regularization. Com Vis Approach Med Image Anal 4241: 85–95, 2006 [Google Scholar]
- 4.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180: 1122–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 176: 291–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachiller PR, Nakanishi H, Roberts JD., Jr Transforming growth factor-β modulates the expression of nitric oxide signaling enzymes in the injured developing lung and in vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 298: L324–L334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L1073–L1084, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol 298: L315–L328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 284: L964–L971, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med 172: 899–906, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bland RD, Ling CY, Albertine KH, Carlton DP, MacRitchie AJ, Day RW, Dahl MJ. Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 285: L76–L85, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bloch KD, Filippov G, Sanchez LS, Nakane M, de la Monte SM. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol Lung Cell Mol Physiol 272: L400–L406, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Browner NC, Dey NB, Bloch KD, Lincoln TM. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem 279: 46631–46636, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C88–C96, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung. I. Morphometry. Anat Rec 178: 711–730, 1974 [DOI] [PubMed] [Google Scholar]
- 18.Buys ES, Cauwels A, Raher MJ, Passeri JJ, Hobai I, Cawley SM, Rauwerdink KM, Thibault H, Sips PY, Thoonen R, Scherrer-Crosbie M, Ichinose F, Brouckaert P, Bloch KD. sGCα1β1 attenuates cardiac dysfunction and mortality in murine inflammatory shock models. Am J Physiol Heart Circ Physiol 297: H654–H663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res 79: 179–186, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Chardy P, Glemarec A, Laurec A. Applications of inertial methods to benthic marine ecology: Practical implications of the basic options. Est Cost Marine Sci 4: 179–205, 1976 [Google Scholar]
- 21.Chiche JD, Schlutsmeyer SM, Bloch DB, de la Monte SM, Roberts JD, Jr, Filippov G, Janssens SP, Rosenzweig A, Bloch KD. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem 273: 34263–34271, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Crozier WJ, Enzmann EV. On the relation between litter size, birth weight, and rate of growth, in mice. J Gen Physiol 19: 249–263, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czaplik M, Biener I, Dembinski R, Pelosi P, Soodt T, Schroeder W, Leonhardt S, Marx G, Rossaint R, Bickenbach J. Analysis of regional compliance in a porcine model of acute lung injury. Respir Physiol Neurobiol 184: 16–26, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest 123: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533–559, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 90: 1291–1335, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudi T, Lohmann SM, Pilz RB. Regulation of gene expression by cyclic GMP-dependent protein kinase requires nuclear translocation of the kinase: identification of a nuclear localization signal. Mol Cell Biol 17: 5244–5254, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haase N, Haase T, Seeanner M, Behrends S. Nitric oxide sensitive guanylyl cyclase activity decreases during cerebral postnatal development because of a reduction in heterodimerization. J Neurochem 112: 542–551, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Halliday H, Robertson B, Nilsson R, Rigaut JP, Grossmann G. Automated image analysis of alveolar expansion patterns in immature newborn rabbits treated with natural or artificial surfactant. Br J Exp Pathol 68: 727–732, 1987 [PMC free article] [PubMed] [Google Scholar]
- 32.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res 94: 1115–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Haralick RM, Shapiro LG. Computer and Robot Vision. Boston: Addison-Wesley, 1991 [Google Scholar]
- 34.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyde DM, Tyler NK, Putney LF, Singh P, Gundersen HJ. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings. Anat Rec A Discov Mol Cell Evol Biol 277: 216–226, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 287: L1178–L1185, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Kapur JN, Sahoo PK, Wong AKC. A new method for gray-level picture thresholding using the entropy of the histogram. Comp Vis Graphics Image Proc 29: 273–285, 1985 [Google Scholar]
- 38.Kato S, Zhang R, Roberts JD., Jr Proprotein convertases play an important role in regulating PKGI endoproteolytic cleavage and nuclear transport. Am J Physiol Lung Cell Mol Physiol 305: L130–L140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimani PW, Holmes AJ, Grossmann RE, McGowan SE. PDGF-Ralpha gene expression predicts proliferation, but PDGF-A suppresses transdifferentiation of neonatal mouse lung myofibroblasts. Respir Res 10: 119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int 45: 813–819, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22–29, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Lum H, Huang I, Mitzner W. Morphological evidence for alveolar recruitment during inflation at high transpulmonary pressure. J Appl Physiol 68: 2280–2286, 1990 [DOI] [PubMed] [Google Scholar]
- 44.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, Clair PM, Dahl MJ, Godfrey EA, Carlton DP, Bland RD. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol 281: L1011–L1020, 2001 [DOI] [PubMed] [Google Scholar]
- 45.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 288: L450–L459, 2005 [DOI] [PubMed] [Google Scholar]
- 46.McGrath-Morrow SA, Cho C, Soutiere S, Mitzner W, Tuder R. The effect of neonatal hyperoxia on the lung of p21Waf1/Cip1/Sdi1-deficient mice. Am J Respir Cell Mol Biol 30: 635–640, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest 116: 1731–1737, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyrick B, Reid L. Pulmonary arterial and alveolar development in normal postnatal rat lung. Am Rev Respir Dis 125: 468–473, 1982 [DOI] [PubMed] [Google Scholar]
- 49.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res 75: 803–812, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Mitchell JJ, Reynolds SE, Leslie KO, Low RB, Woodcock-Mitchell J. Smooth muscle cell markers in developing rat lung. Am J Respir Cell Mol Biol 3: 515–523, 1990 [DOI] [PubMed] [Google Scholar]
- 51.Mitzner W, Fallica J, Bishai J. Anisotropic nature of mouse lung parenchyma. Ann Biomed Eng 36: 2111–2120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mund SI, Stampanoni M, Schittny JC. Developmental alveolarization of the mouse lung. Dev Dyn 237: 2108–2116, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD., Jr TGF-β-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Naumann U, Luta G, Wand MP. The curvHDR method for gating flow cytometry samples. BMC Bioinformatics 11: 44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naumann U, Wand MP. Automation in high-content flow cytometry screening. Cytometry A 75: 789–797, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Ochs M. A brief update on lung stereology. J Microsc 222: 188–200, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, Richter J, Gundersen HJ. The number of alveoli in the human lung. Am J Respir Crit Care Med 169: 120–124, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Pappas CT, Obara H, Bensch KG, Northway WH., Jr Effect of prolonged exposure to 80% oxygen on the lung of the newborn mouse. Lab Invest 48: 735–748, 1983 [PubMed] [Google Scholar]
- 59.Penatti E, Barina A, Schram K, Li A, Nattie E. Serotonin transporter null male mouse pups have lower ventilation in air and 5% CO2 at postnatal ages P15 and P25. Respir Physiol Neurobiol 177: 61–65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pilz RB, Broderick KE. Role of cyclic GMP in gene regulation. Front Biosci 10: 1239–1268, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Rasband WS. ImageJ. NIH: Bethesda, MD, 1997–2007 [Google Scholar]
- 62.Rawlins EL, Perl AK. The a“MAZE”ing world of lung-specific transgenic mice. Am J Respir Cell Mol Biol 46: 269–282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts JD, Jr, Chiche JD, Weimann J, Steudel W, Zapol WM, Bloch KD. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ Res 87: 140–145, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Roberts JD, Jr, Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circ Res 76: 215–222, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Roederer M, Treister A, Moore W, Herzenberg LA. Probability binning comparison: a metric for quantitating univariate distribution differences. Cytometry 45: 37–46, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Schmalisch G, Wilitzki S, Roehr CC, Proquitte H, Buhrer C. Development of lung function in very low birth weight infants with or without bronchopulmonary dysplasia: longitudinal assessment during the first 15 months of corrected age. BMC Pediatr 12: 37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stenger MR, Rose MJ, Joshi MS, Rogers LK, Chicoine LG, Bauer JA, Nelin LD. Inhaled nitric oxide prevents 3-nitrotyrosine formation in the lungs of neonatal mice exposed to >95% oxygen. Lung 188: 217–227, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75: 23–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugiura T, Nakanishi H, Roberts JD., Jr Proteolytic processing of cGMP-dependent protein kinase I mediates nuclear cGMP signaling in vascular smooth muscle cells. Circ Res 103: 53–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomkeieff I. Linear intercepts, areas, volumes. Nature 155: 24; 107, 1945 [Google Scholar]
- 72.Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol 31: 97–105, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, Pellens M, Gillijns H, Swinnen M, Graveline A, Collen D, Dewerchin M, Brouckaert P, Bloch KD, Janssens S. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation 116: 936–943, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Wand MP. Data-based choice of histogram bin width. Am Stat 51: 59–64, 1997 [Google Scholar]
- 75.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998 [DOI] [PubMed] [Google Scholar]
- 76.Woodcock-Mitchell J, White S, Stirewalt W, Periasamy M, Mitchell J, Low RB. Myosin isoform expression in developing and remodeling rat lung. Am J Respir Cell Mol Biol 8: 617–625, 1993 [DOI] [PubMed] [Google Scholar]
- 77.Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Ruhlmann A, Weissmann N, Ghofrani HA, Gunther A, Seeger W, Grimminger F, Morty RE, Schermuly RT. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J 33: 861–870, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol 282: L379–L385, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Zabel U, Hausler C, Weeger M, Schmidt HH. Homodimerization of soluble guanylyl cyclase subunits. Dimerization analysis using a glutathione S-transferase affinity tag. J Biol Chem 274: 18149–18152, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L1459–L1466, 2007 [DOI] [PubMed] [Google Scholar]