Abstract
Osterix (Osx) is essential for both intramembranous or endochondral bone formation. Osteoblast-specific ablation of Osx using Col1α1-Cre resulted in osteopenia, because of impaired osteoblast differentiation in adult mice. Since Osx is also known to be expressed in chondrocytes, we evaluated the role of Osx expressed in chondrocytes by examining the skeletal phenotype of mice with conditional disruption of Osx in Col2α1-expressing chondrocytes. Surprisingly, Cre-positive mice that were homozygous for Osx floxed alleles died after birth. Alcian blue and alizarin red staining revealed that the lengths of skeleton, femur, and vertebrae were reduced by 21, 26, and 14% (P < 0.01), respectively, in the knockout (KO) compared with wild-type mice. To determine if haploid insufficiency of Osx in chondrocytes influenced postnatal skeletal growth, we compared skeletal phenotype of floxed heterozygous mice that were Cre-positive or Cre-negative. Body length was reduced by 8% (P < 0.001), and areal BMD of total body, femur, and tibia was reduced by 5, 7, and 8% (P < 0.05), respectively, in mice with conditional disruption of one allele of Osx in chondrocytes. Micro-CT showed reduced cortical volumetric bone mineral density and trabecular bone volume to total volume in the femurs of Osxflox/+;col2α1-Cre mice. Histological analysis revealed that the impairment of longitudinal growth was associated with disrupted growth plates in the Osxflox/+;col2α1-Cre mice. Primary chondrocytes isolated from KO embryos showed reduced expression of chondral ossification markers but elevated expression of chondrogenesis markers. Our findings indicate that Osx expressed in chondrocytes regulates bone growth in part by regulating chondrocyte hypertrophy.
Keywords: osterix, chondrocyte, endochondral bone formation, col2α1-Cre, haploinsufficiency
endochondral bone formation is a process that involves tightly controlled proliferation and differentiation of chondrocytes at the growth plates of long bones. During long bone development, the cartilage anlagen surrounded by the perichondrium derived from mesenchymal cells are first laid down and gradually replaced by bone (17, 28). The cartilage anlagen elongate and expand in width by proliferation of chondrocytes as well as by deposition of collagen II (Col2) rich cartilage matrix. Subsequently, chondrocytes in the central region of the cartilage undergo further differentiation to hypertrophic chondrocytes and synthesize collagen X (Col10)-rich extracellular matrix, which is subsequently mineralized by calcium deposition. The calcified cartilage is then resorbed by chondroclasts and replaced by bone matrix via osteoblasts that are brought in by invading blood vessels. Thus, in the growth plate, a coordinated sequence of chondrocyte proliferation, hypertrophy, and apoptosis results in longitudinal growth of long bones (16, 17, 28). During endochondral bone formation, chondrocyte differentiation plays a critical role in extracellular matrix formation, mineralization, and the stimulation of osteoblast differentiation. Changes in proliferation and differentiation of chondrocytes will dramatically alter the amount of cartilage and bone tissue. Therefore, identification of the regulatory factors that modulate the process of chondrocyte proliferation and differentiation is essential to develop therapies to treat skeletal defects related to the endochondral ossification process.
Endochondral ossification is tightly regulated by several transcriptional factors including Runx2 and Osterix (Osx), which are considered master transcription factors for osteoblast development (11, 19, 23, 28). Runx2 is expressed in the prehypertrophic and hypertrophic chondrocytes of growth plates (5, 7) and is required for both osteoblast and chondrocyte development (12, 23, 26). Osx, a downstream target of Runx2, was originally identified as an osteoblast-specific transcription factor that is required for osteoblast maturation and bone formation (19, 20). While mice with total knockout of Osx failed to form intramembranous as well as endochondral bone and died at birth (19), mice with osteoblast-specific disruption of Osx using col1α1-Cre were viable with no bone phenotype at birth (2). However, these mice developed osteopenia as they reached adulthood (2). These data suggest that Osx expressed in other cell types besides osteoblasts contribute to early embryonic skeletal development in mice. Furthermore, human genetic studies have shown that mutations in Osx are associated with skeletal diseases, thus implying a significant role for Osx in human skeletal development (3, 15). Besides osteoblasts, pre- and hypertrophic chondrocytes have also been shown to express Osx albeit at a lower level (19). An in vitro study showed that knocking down Osx expression in ATDC5 cells also inhibited the expression of hypertrophic chondrocyte markers, suggesting Osx is a positive regulator of chondrocyte differentiation (22). While the majority of these studies have been focused on the role of Osx in osteoblast development and functions, the role of Osx expressed in chondrocytes on skeletal development remains unclear. In this study, we evaluated the function of chondrocyte produced Osx by generating a chondrocyte-specific conditional knockout of Osx in type II collagen-producing chondrocytes. We used the Col2α1-Cre driver mice as we and others have used this line of Cre mice to specifically disrupt genes of interest in chondrocytes (9, 24, 30). We found that Cre-positive mice that are homozygous for Osx floxed alleles died immediately after birth, a finding consistent with a recent report by Oh and colleagues (21). We also found that mice with an Osx haploinsufficiency in chondrocytes exhibited delayed growth of both trabecular and cortical bone that was caused in part by impaired chondrocyte hypertrophy. Our findings demonstrate that both alleles of Osx in chondrocytes are required for postnatal skeletal growth.
MATERIALS AND METHODS
Animals.
Chondrocyte-specific Osx knockout mice were generated by crossing Osx floxed mice (Osxflox/flox) with a Cre transgenic mouse line driven by the col2α1 promoter (col2α1-Cre) (1, 24). Cre-positive mice with homozygous loxp alleles were referred to as KO (Osxflox/flox;col2α1-Cre) and were compared with the corresponding Cre-negative wild-type (WT) mice with homozygous loxp alleles (Osxflox/flox). Cre-positive mice with heterozygous loxp alleles were referred to as Het (Osxflox/+;col2α1-Cre) and were compared with mice with Cre-negative WT mice with heterozygous loxp alleles (Osxflox/+). All animal rearing and experimental procedures were performed according to approved standards by the Institutional Animal Care and Use Committees of the Jerry L. Pettis Memorial VA Medical Center. Mice were anesthetized with approved anesthetics, isoflurane. For euthanasia, mice were exposed to CO2 followed by cervical dislocation.
Skeletal staining and measurement.
Pregnant mothers were euthanized at 18 days of pregnancy (E18). E18 embryos were deskinned, fixed, and stained with alizarin red and alcian blue according to established procedures published previously (18). Briefly, embryos were fixed in 95% ethanol for 5 days and acetone for an additional 4 days. The fixed carcasses were then stained with a solution containing 0.1% alizarin red, 0.3% alcian blue, acetic acid, and 70% ethanol at a volumetric ratio of 1:1:1:17. The embryos were then cleared with 1% KOH in 20% glycerol and stored in glycerol. Whole bone lengths and mineralized bone lengths were measured with a caliper.
Evaluation of bone phenotypes.
Bone area, bone mineral content (BMC), and bone mineral density (BMD) of WT and Het mice were evaluated by dual-energy X-ray absorptiometry (DEXA) with the PIXImus instrument (LunarCorp, Madison, WI) as previously described (10). Femur cortical and trabecular bone microarchitecture from 3 wk old WT and Het mice were assessed using the microcomputed tomography (μCT) (VIVA CT40; SCANO Medical, Bruttisellen, Switzerland) as previously reported (10). The femurs were scanned by X-ray at 55 and 75 kVp for the distal metaphysis and middiaphysis, respectively, with a voxel size of 10.5 μm. Reconstruction analysis were performed with SCANCO software (SCANO Medical). Sections of ∼1 mm (adjusted to lengths of femurs) at the middiaphysis were analyzed for cortical bone parameters, and ∼0.63 mm (adjust to lengths of femurs) starting from 0.36 mm proximal to the growth plate were analyzed to obtain trabecular bone parameters using the SCANO software.
Histomorphometric analysis.
Tibias of 3 wk old mice were isolated and processed for paraffin sections as previously described (25), followed by Safranin-O staining to visualize cartilage at the growth plates. Columnar and hypertrophic chondrocytes were identified by Safranin-O staining as well as cellular morphology. Heights of the columnar and hypertrophic zones were measured using the OsteoMeasure software (Ostometrics, Atlanta, GA). Tartrate-resistant acid phosphatase (TRAP) staining of osteoclasts were performed as previously described (4). TRAP staining of two fields from primary or secondary spongiosa were measured using the OsteoMeasure software (Ostometrics).
Immunohistochemistry.
Immunohistochemistry was carried out according to a previously published procedure (4, 10). Osx antibody (ab22552, Abcam) was diluted at 1:500 in blocking solution and incubated at 4°C overnight. The secondary antibody was detected using the VECTASTAIN ABC-AP kit (AK-5000, Vector Laboratories) followed by color development with the Vector Blue AP substrate (SK-5300, Vector Laboratories).
Primary cell culture.
Pregnant mothers were euthanized at E18. E18 embryos were isolated and genotyped. Isolation of primary chondrocytes was performed according to a previously established procedure (8), and the chondrocyte phenotype was confirmed by cell morphology and expression of chondrocyte markers. Briefly, rib cages were deskinned and digested with collagenase type D (3 mg/ml in 1× PBS) at 37°C with shaking (210 rpm) for 1 h. The solution was removed and the ribs were washed with 1× PBS three times followed by additional 3 h of digestion with collagenase type D. Chondrocytes were collected and grown in α-MEM containing 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). The cells were grown until 70% confluent and collected for RNA extraction.
Quantitative real-time RT-PCR.
RNA was extracted from primary cells with Trizol reagent (Invitrogen) according to manufacturer's instruction. An aliquot of RNA (400 ng) was reverse-transcribed into cDNA with the oligo(dT)12–18 primer in a 20 μl reaction volume. Quantitative real-time PCR was performed as previously described. (4). Ppia was used as reference gene. Primer sequences used for real-time PCR are listed in Table 1.
Table 1.
Primer sequences used for real-time RT-PCR
| Gene | Primer | Sequences |
|---|---|---|
| Osx | forward | 5′-AGAGGTTCACTCGCTCTGACGA-3′ |
| reverse | 5′-TTGCTCAAGTGGTCGCTTCTG-3′ | |
| Runx2 | forward | 5′-AAAGCCAGAGTGGACCCTTCCA-3′ |
| reverse | 5′-ATAGCGTGCTGCCATTCGAGGT-3′ | |
| Sox9 | forward | 5′-CGGAGGAAGTCGGTGAAGA-3′ |
| reverse | 5′-GTCGGTTTTGGGAGTGGTG-3′ | |
| Hes1 | forward | 5′-CTGAGCACAGAAAGTCATCAAAGCC-3′ |
| reverse | 5′-GGTATTTCCCCAACACGCTCG-3′ | |
| Tgfbr2 | forward | 5′-CGCTTCTCCCAAGTGTGTCATG-3′ |
| reverse | 5′-GGCTGACACCCGTCACTTGGA-3′ | |
| Fgfr3 | forward | 5′-GAGAAGGCTGCTTTGGACAGGTG-3′ |
| reverse | 5′-CAGGTGAGCTGTTCCTCTGGCA-3′ | |
| Col2 | forward | 5′-TGGCTTCCACTTCAGCTATG-3′ |
| reverse | 5′-AGGTAGGCGATGCTGTTCTT-3′ | |
| Col10 | forward | 5′-ACGGCACGCCTACGATGT-3′ |
| reverse | 5′-CCATGATTGCACTCCCTGAA-3′ | |
| Mmp13 | forward | 5′-CATCCATCCCGTGACCTTAT-3′ |
| reverse | 5′-TCATAACCATTCAGAGCCCA-3′ | |
| Ppia | forward | 5′-CCATGGCAAATGCTGGACCA-3′ |
| reverse | 5′-TCCTGGACCCAAAACGCTCC-3′ |
Statistics.
Student's t-test or ANOVA were used for statistical as appropriate. Two-way ANOVA was performed using STATISTICA software (Statsoft, Tulsa, OK).
RESULTS
Chondrocyte-specific ablation of Osx impairs skeletal development and mineralization in mice.
To disrupt Osx specifically in chondrocytes, we crossed mice with floxed Osx alleles to mice where Cre expression was driven by the chondrocyte-specific col2α1 promoter (Fig. 1A). After two generations of breeding, four genotypes (25% of each) were obtained: Osxflox/flox;col2α1-Cre (homozygous conditional knockout, KO), Osxflox/+;col2α1-Cre (heterozygous conditional knockout, Het), and their wild-type littermates (Osxflox/flox and Osxflox/+, WT). The conditional KO mice died shortly after birth from a difficulty in breathing. No surviving pups were found to be Cre positive, consistent with a recent report (21). To examine whether or not the expression of Osx is ablated in KO chondrocytes, we performed immunohistochemistry with sections of E18 embryos. Osx protein is detected in the pre- and hypertrophic zones of the growth plate in E18 WT embryos. By contrast, Osx expression was sparse in the Cre-positive conditional KO chondrocytes (Fig. 1B, arrows). The expression of Osx was also reduced in the hypertrophic chondrocytes of the Het mice compared with WT littermates (Fig. 1C, arrows).
Fig. 1.
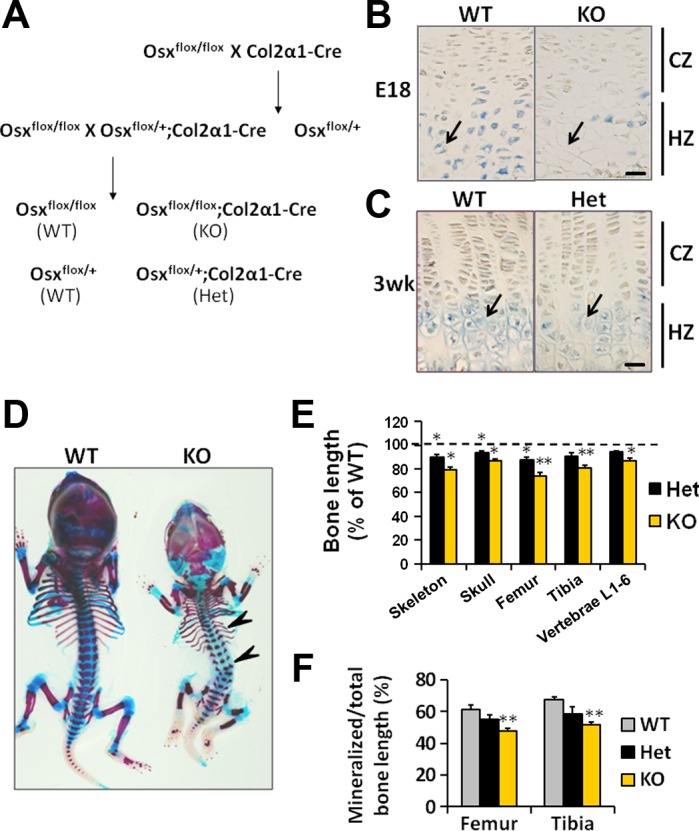
Chondrocyte-specific knockout of osterix (Osx) alleles resulted in impaired skeletal growth. A: breeding theme to generate wild-type mice (WT), homozygous (KO), and heterozygous (Het) conditional Osx knockout mice in chondrocytes. B: Osx protein expression was abundant in the hypertrophic zone (HZ), but not in the columnar zone (CZ) of the growth plate in WT mice. Expression of Osx was sparse in the KO mice. E18, 18 days of pregnancy. C: Osx protein expression was reduced in the Het mice compared with WT mice. Arrows indicate hypertrophic chondrocytes. D: E18 conditional KO of Osx in chondrocytes showed severe skeletal deficits. Arrowheads show the bent ribs and vertebral column. E: bone lengths of the skeleton, skull, femur, tibia, and lumbar vertebrae (L1–L6) in the Het and KO mice normalized to WT mice. F: the ratio of mineralized to total bone length in WT, Het, and KO mice. *P < 0.05, **P < 0.001; n = 4/group. Data are presented as means ± SE. Bar = 50 μm.
To examine skeletal development in conditional KO embryos, we stained E18 embryos with alizarin red and alcian blue. By comparison to the WT embryos, the skeleton of the conditional KO embryos were noticeably smaller with bent ribs and a bent vertebral column (Fig. 1D, arrowheads). The lengths of the skeleton, skull, femur, tibia, and lumbar vertebrae (L1–L6) were reduced by 21, 13, 26, 19, and 14% (P < 0.05), respectively, in the conditional KO embryos compared with the WT embryos. Bone lengths of skeleton, skull, and femur were also significantly reduced (by 7–13%, P < 0.05) in the Het mice compared with WT mice (Fig. 1E). In addition, mineralized portions of the long bones were significantly reduced in the conditional KO embryos, compared with WT embryos, while the reduction in the Het mice did not reach statistical significance (Fig. 1F).
Haploinsufficiency of Osx in chondrocytes results in delayed postnatal skeletal growth.
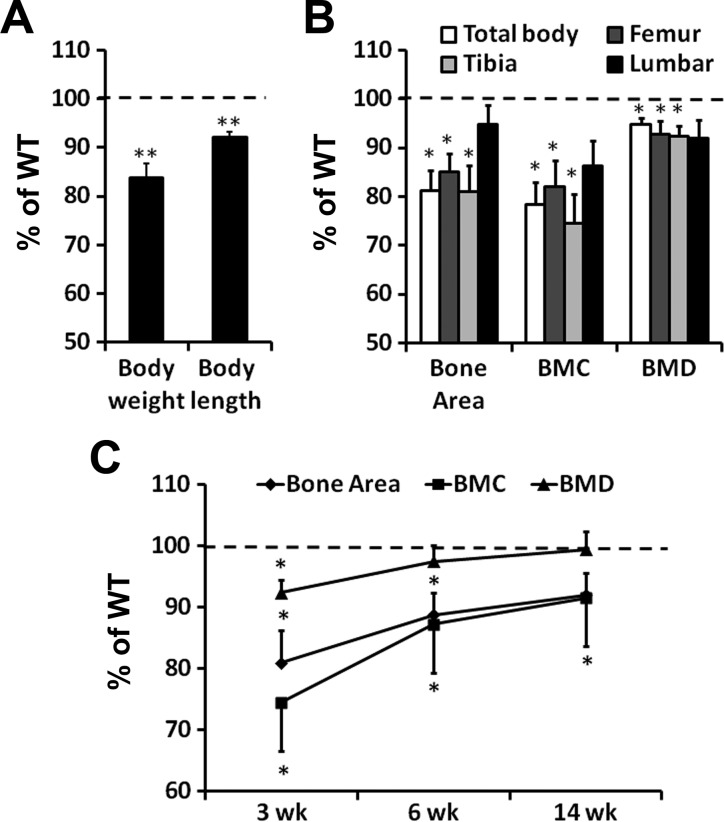
Because conditional KO of Osx in chondrocytes caused neonatal lethality and because Osx expression was significantly reduced in chondrocytes of heterozygous mice, we next investigated the consequence of loss of one copy of the Osx gene in chondrocytes on skeletal growth. At 3 wk of age, the Het mice of Osx are slightly smaller than WT mice. The Het mice exhibited a 16% decrease in body weight (Fig. 2A, P < 0.001) and 8% decrease in body length (P < 0.001). Bone area, BMC, and BMD were measured at multiple sites by DEXA (Fig. 2B). Bone area of the total body, femur, and tibia was decreased by 19, 15, and 19% (P < 0.05), respectively, in the Het mice compared with WT mice. BMC of total body, femur, and tibia were similarly reduced by 22, 18, and 25% (P < 0.05), respectively, in the Het mice. BMD of total body, femur, and tibia were also decreased by 5, 7, and 8%, respectively, in the Het mice compared with WT mice. By contrast, lumbar vertebrae showed no statistically significant difference in the bone area, BMC, or BMD between Het and WT mice (Fig. 2B). By 6 wk of age, the bone parameter deficits in the tibia were reduced in the Het mice compared with the WT mice (Fig. 2C). Bone area and BMC of tibia were 11 and 13% (P < 0.05) less in the Het mice compared with the WT mice, while BMD showed no difference between the two genotypes. By 14 wk of age, only BMC was significantly less (P < 0.05) in Het mice when compared with WT mice. Thus, skeletal growth parameters in Het mice appeared to catch up with WT mice with age.
Fig. 2.
Dual-energy X-ray absorptiometric analysis of bone parameters in WT and Het mice. A: 3 wk old Het mice exhibited reduced body weight and length. B: 3 wk old Het mice showed reduced bone area, bone mineral content (BMC), and bone mineral density (BMD) in the total body, femur, and tibia but not in the lumbar vertebrae. C: the bone deficits in the Het tibia diminished with mouse age. *P < 0.05, **P < 0.001; n = 14/group. Data are presented as means ± SE.
Changes in cortical and trabecular bone parameters in mice with chondrocyte-specific Osx haploinsufficiency.
Next we performed μCT to investigate whether or not the reduced area BMD in Het mice was due to changes of properties in the cortical or trabecular bones. μCT measurements revealed that the volumetric BMD (vBMD) at the femur middiaphysis was reduced by 8% (Fig. 3, A and B) in Het mice compared with WT mice at age of 3 wk. Accordingly, cortical thickness at the middiaphysis was not significantly altered (Fig. 3C). Furthermore, loss of a single copy of Osx in chondrocytes did not alter bone size (Fig. 3D), as measured by femur cross-sectional area. μCT at the femur distal metaphysis revealed less developed trabecular bone in mice with chondrocytic Osx haploinsufficiency compared with WT mice (Fig. 3E). Total volume (TV) of trabecular bone was comparable between Het and WT mice (data not shown), but bone volume to total volume (BV/TV) was significantly reduced by 31% (Fig. 3F, P < 0.05). Trabecular number (Tb. N) in the Het mice was reduced by 24% (Fig. 3G, P < 0.05) while trabecular separation was increased by 27% (Fig. 3I, P < 0.05) in the Het mice compared with WT mice. However, trabecular thickness was not altered in the Het mice compared with WT mice (Fig. 3H, P = 0.06).
Fig. 3.
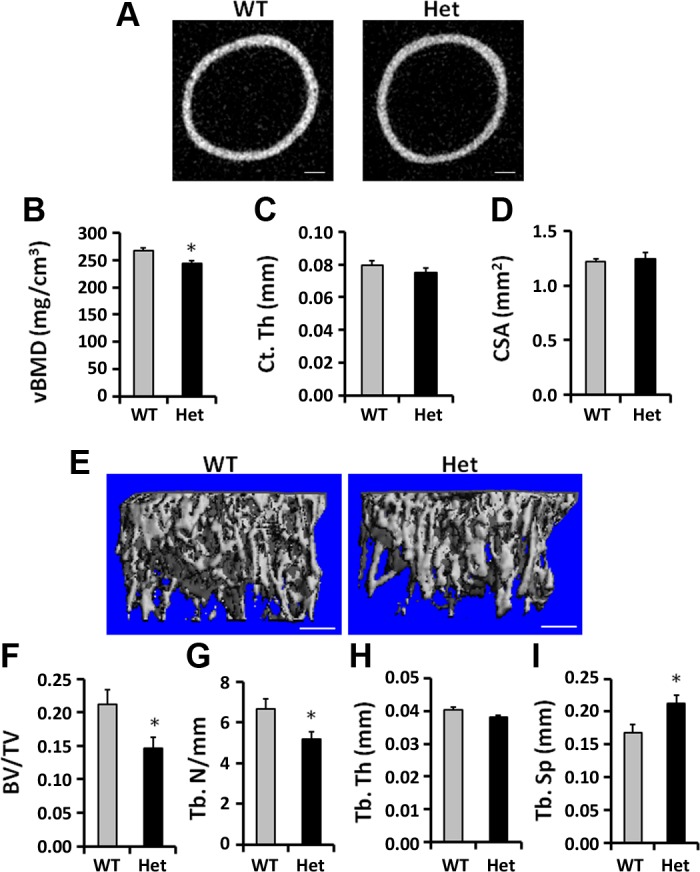
Femur cortical and trabecular bone microarchitecture in 3 wk old WT and Het mice. A: cross-sectional images of the femurs at the middiaphysis in the WT and Het mice. B: microcomputed tomography (μCT) of the femur middiaphysis showed reduced volumetric (v) BMD. C, D: cortical thickness (Ct. Th) and cross-sectional area (CSA) of the femur middiaphysis were not significantly altered in the Het mice compared with WT mice. E: the 3D rendering of the trabecular bone at the distal femur of WT and Het mice exhibited reduced trabecular bone architecture in the Het mice compared with WT mice. F–I: bone volume to total volume (BV/TV) and trabecular number (Tb. N) were reduced, while trabecular separation (Tb. Sp) was increased in the Het mice compared with WT mice. Tb. Th, trabecular thickness. *P < 0.05, n = 10/group. Data are presented as means ± SE. Bar = 200 μm.
Haploinsufficiency of Osx in chondrocytes impairs chondrocyte hypertrophy in the growth plate.
Since Osx is expressed in growth plate chondrocytes and contributes to trabecular bone parameters via endochondral bone formation, we next investigated if lack of single copy of Osx in chondrocytes influenced growth plate morphology. Longitudinal sections of tibia from 3 wk old heterozygous Osx conditional KO and WT mice were stained with Safranin-O. We found that the growth plates in the Het mice were thinner than in the WT mice (Fig. 4, A and B). The heights of columnar and hypertrophic zones were decreased by 13% (P < 0.01) and 15% (P < 0.05), respectively, in the Het mice compared with WT mice (Fig. 4, C and D). In addition, many of the chondrocytes in the hypertrophic zone of the Het mice appeared smaller with atypical hypertrophic morphology, compared with the hypertrophic chondrocytes in the WT mice (Fig. 4, A and B, arrows).
Fig. 4.
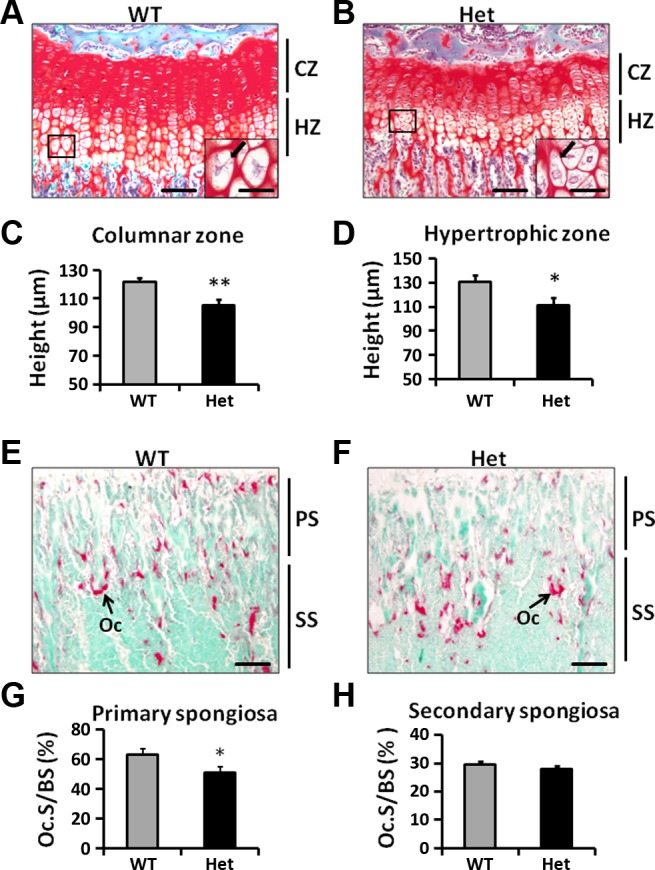
Reduced heights of CZ and HZ in Het mice. A, B: Safranin-O staining of growth plates in WT and Het mice. The growth plate in Het mice appeared to be thinner compared with WT mice. Insets: hypertrophic chondrocytes under high magnification (×400). Hypertrophic chondrocytes in the Het mice appeared to be smaller compared with WT chondrocytes (arrows). C, D: heights of the CZ and HZ were reduced in Het mice compared with WT mice. E, F: Tartrate-resistant acid phosphatase staining of the WT and Het tibia metaphysic. G: osteoclast surface to bone surface (Oc.S/BS) was reduced in the Het primary spongiosa (PS) in the Het tibia. H: osteoclast Oc.S/BS was unaltered in the secondary spongiosa (SS) in the Het tibia. *P < 0.05, **P < 0.01; n = 8/group. Data are presented as means ± SE. Bar = 100 μm. Inset bar = 25 μm.
Hypertrophic chondrocytes also produce RANKL and OPG and influence trabecular bone resorption by regulating osteoclastogenesis. Deletion of RANKL expression in mesenchymal lineage abolished osteoclastogenesis in the femur metaphysic. (13, 27, 29). We next analyzed TRAP activity in the WT and Het mice. We found that the TRAP-positive surface to bone surface (Oc.S/BS) is reduced by 23% (P < 0.05) in the primary spongiosa adjacent to the growth plate in the Het tibia (Fig. 4, E–G). However, osteoclast activity in the secondary spongiosa was not altered in the Het mice. The ratios of TRAP-positive Oc.S/BS were similar between WT and Het tibia secondary spongiosa (Fig. 4, E, F, and H).
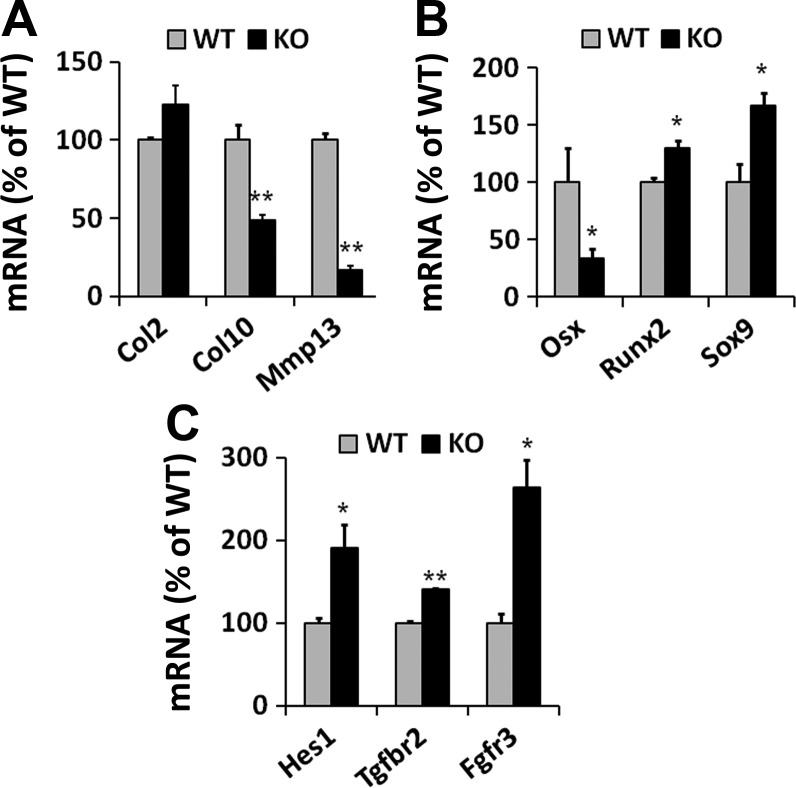
Since Osx is expressed in the differentiating chondrocytes and Osx haploinsufficiency caused atypical chondrocyte hypertrophy in the growth plate, we next examined gene expression of chondrogenesis and focused on chondral ossification markers in the primary chondrocytes isolated from conditional KO and WT embryos. While the expression of Col2 was not altered in the Het chondrocytes, terminal differentiation markers Col10 and Mmp13 were markedly downregulated in KO chondrocytes compared with WT chondrocytes (Fig. 5A). In contrast, the expression of chondrogenic transcription factor Sox9 and early differentiation marker Runx2 was significantly upregulated in KO chondrocytes (Fig. 5B). As expected, Osx transcript was significantly reduced by 67% in the KO chondrocytes compared with WT chondrocytes (Fig. 5B). Markers for chondrogenic signaling Hes1, Tgfbr2, and Fgfr3 were also significantly upregulated in KO chondrocytes (Fig. 5C).
Fig. 5.
Expression of chondrocyte markers detected by real-time RT-PCR in cultured primary chondrocytes. A: markers of chondrocyte terminal differentiation, Col10 and Mmp13, were reduced in KO chondrocytes compared with WT mice, while Col2 expression was not altered. B: Osx expression was reduced by 67% in the KO chondrocytes. Expression of chondrogenic transcription factor Sox9 and early differentiation marker Runx2 were increased in the KO chondrocytes. C: markers of chondrogenic signaling, Hes1, Tgfbr2, and Fgfr3, were increased in KO chondrocytes compared with WT chondrocytes. *P < 0.05, **P < 0.005; n = 3/group. Data are presented as means ± SE.
DISCUSSION
In this study, we demonstrate that the expression of Osx in chondrocytes is essential for skeletal growth in mice. Chondrocyte-specific disruption of Osx resulted in severe skeletal defects including a small body, reduced bone length, bent long bones, and reduced bone mineralization in embryos, a phenotype comparable to that of a total Osx gene knockout in mice (19). Though previous studies have been focused on the role of Osx in osteoblast function, the osteoblast-specific conditional knockout of Osx did not yield significant skeletal defects in embryonic mice. These studies strongly suggest that the bone phenotype in mice with a complete Osx knockout is also likely due to missing Osx functions in chondrocytes, as well as its well-studied function in osteoblasts. Chondrocyte-based endochondral bone formation is essential for long bone growth especially during embryonic stages, while osteoblast-mediated ossification becomes critical at later stages in long bone ossification. Our findings suggest that Osx is critically important in both of these processes.
As a master transcriptional factor for endochondral and intramembranous bone formation, the importance of Osx is also evident from clinical genetic studies involving mutations in the Osx gene (3, 15). It is now known that genetic variations influencing gene expression contribute to differences in peak bone mass in humans (6, 14). In our mouse model, we found that haploid insufficiency of Osx in chondrocytes caused delayed skeletal growth reflected by reduced total body length and weight, as well as reduced BMC and BMD in long bones of young mice. Though lumbar BMD is reduced to the same extent as femur and tibia BMD in Het mice compared with WT mice, the P value does not reach statistical significance (P = 0.21) because of larger variations in lumbar BMD measurements caused by smaller size of lumbar vertebra. The length of long bone is mainly gained through endochondral bone formation at the growth plates, and delayed skeletal growth in Het mice suggests impairment of endochondral bone formation when missing one allele of Osx. However, the growth deficits in the mice with chondrocyte-specific Osx haploinsufficiency diminished with age. Thus we demonstrated that variations in Osx expression may influence the rate of skeletal growth, particularly during the prepubertal growth period.
Besides influencing longitudinal growth, chondrocytic Osx haploinsufficiency also reduced vBMD and cortical thickness in the middiaphysis of the femur. By contrast, neither the bone size at the middiaphysis nor at the femur metaphysis is affected by a reduction in the Osx gene copy number. These results support the hypothesis that chondrocytic Osx haploinsufficiency primarily affects endochondral bone formation in mice, as bone size is known to be mainly controlled by periosteal cells. Consistent with this notion, we found poorly developed trabecular bone in the mice with chondrocytic Osx haploinsufficiency compared with WT mice, suggesting impairment of endochondral ossification in mice with chondrocytic Osx haploinsufficiency.
The process of endochondral bone formation begins with chondrocyte hypertrophy. Impairment of endochondral ossification may be related to abnormalities in chondrocyte hypertrophy. Indeed, we observed reduced height of the hypertrophic zone with abnormal chondrocytic morphology in the Het mice, suggesting Osx expression in chondrocytes is required for chondrocyte hypertrophy. During the process of endochondral bone formation, resorption of ossified cartilage by osteoclasts is crucial for the remodeling of primary spongiosa in the metaphysis. Indeed, we found reduced osteoclast activity in the primary spongiosa immediately below the growth plate in the Het tibia. The abnormal osteoclast activity impairs remodeling of the ossified cartilage.
Gene expression data further revealed a marked reduction in the expression of hypertrophic chondrocyte markers Col10 and Mmp13. We speculate that the hindrance of chondrocyte hypertrophy could in part contribute to the elevated expression of chondrogenic markers Sox9, Hes1, Tgfbr2, and Fgfr3 in the absence of Osx in chondrocytes. In addition to the differentiating chondrocytes at the growth plates, the expression of Osx has also been found in the chondrogenic cell line, ATDC5 (20, 22). In line with our findings, Omoteyama and Takagi (22) found that knocking down Osx using shRNA impaired differentiation of ATDC5 cells. These data strongly suggest that Osx is important for chondrocyte differentiation. Based on our findings and other recently published findings, we conclude that Osx expressed in chondrocytes plays a much more important role than previously thought in regulating chondrocyte hypertrophy and thereby endochondral bone formation. Future studies will address the molecular mechanism by which Osx exerts its important role on chondral ossification.
Conclusions
By generating heterozygous conditional knockout of Osx in collagen II-expressing chondrocytes, we found that both alleles of Osx are required for skeletal growth in mice. Haploinsufficiency of Osx in chondrocytes resulted in reduced cortical vBMD, trabecular BV/TV, and trabecular number. Disrupted bone growth in Osx haploinsufficient mice was associated reduced heights of columnar and hypertrophic zones of growth plates. The abnormal chondrocyte hypertrophy in Osx haploinsufficient mice was associated with reduced expression of chondrocyte differentiation markers. Our study demonstrates that both alleles of Osx are required for the chondrocyte hypertrophy and endochondral bone formation.
GRANTS
This study was supported by funding from the National Institutes of Arthritis and Musculoskeletal Diseases R01 Grant AR-048139 to S. Mohan and Veterans Administration Biomedical Laboratory Research and Development Merit Review Grant 10826917 to S. Mohan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.C. and S.M. conception and design of research; S.C., W.X., and X.Z. performed experiments; S.C. and S.M. analyzed data; S.C., W.X., and S.M. interpreted results of experiments; S.C. and S.M. prepared figures; S.C., W.X., and S.M. drafted manuscript; S.C., W.X., X.Z., and S.M. edited and revised manuscript; S.C., W.X., X.Z., and S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
All work was performed at facilities provided by the Veterans' Administration. The authors thank Catrina Alarcon, Sheila Pourteymour, and Nancy Lowen for technical assistance.
REFERENCES
- 1.Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA 102: 14665–14670, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B, Kim JE. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J Bone Miner Res 24: 1055–1065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao T, Xiao Y, de Crombrugghe B, Somerman MJ, Feng JQ. Genetic evidence for the vital function of Osterix in cementogenesis. J Bone Miner Res 27: 1080–1092, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S, Zhao SL, Nelson B, Kesavan C, Qin X, Wergedal J, Mohan S, Xing W. Targeted disruption of ephrin B1 in cells of myeloid lineage increases osteoclast differentiation and bone resorption in mice. PLoS One 7: e32887, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi KY, Lee SW, Park MH, Bae YC, Shin HI, Nam S, Kim YJ, Kim HJ, Ryoo HM. Spatio-temporal expression patterns of Runx2 isoforms in early skeletogenesis. Exp Mol Med 34: 426–433, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Edderkaoui B, Baylink DJ, Beamer WG, Wergedal JE, Porte R, Chaudhuri A, Mohan S. Identification of mouse Duffy antigen receptor for chemokines (Darc) as a BMD QTL gene. Genome Res 17: 577–585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem 275: 8695–8702, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc 3: 1253–1260, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIαI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics 30: 354–362, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 148: 5706–5715, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hojo H, Ohba S, Yano F, Chung UI. Coordination of chondrogenesis and osteogenesis by hypertrophic chondrocytes in endochondral bone development. J Bone Miner Metab 28: 489–502, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev 80: 159–170, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto K, Kitazawa R, Kurosaka M, Maeda S, Kitazawa S. Expression profile of genes related to osteoclastogenesis in mouse growth plate and articular cartilage. Histochem Cell Biol 125: 593–602, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science 303: 229–232, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Lapunzina P, Aglan M, Temtamy S, Caparros-Martin JA, Valencia M, Leton R, Martinez-Glez V, Elhossini R, Amr K, Vilaboa N, Ruiz-Perez VL. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet 87: 110–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol 5: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40: 46–62, 2008 [DOI] [PubMed] [Google Scholar]
- 18.McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22: 299–301, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Nishio Y, Dong Y, Paris M, O'Keefe RJ, Schwarz EM, Drissi H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 372: 62–70, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Oh JH, Park SY, de Crombrugghe B, Kim JE. Chondrocyte-specific ablation of Osterix leads to impaired endochondral ossification. Biochem Biophys Res Commun 418: 634–640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omoteyama K, Takagi M. The effects of Sp7/Osterix gene silencing in the chondroprogenitor cell line, ATDC5. Biochem Biophys Res Commun 403: 242–246, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89: 765–771, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26: 145–146, 2000 [PubMed] [Google Scholar]
- 25.Rundle CH, Wang X, Sheng MH, Wergedal JE, Lau KH, Mohan S. Bax deficiency in mice increases cartilage production during fracture repair through a mechanism involving increased chondrocyte proliferation without changes in apoptosis. Bone 43: 880–888, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev 15: 467–481, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usui M, Xing L, Drissi H, Zuscik M, O'Keefe R, Chen D, Boyce BF. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res 23: 314–325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuelling M, Vortkamp A. Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification. Pediatr Nephrol 25: 625–631, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 17: 1235–1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development 131: 2161–2171, 2004 [DOI] [PubMed] [Google Scholar]