Abstract
In rats with ligated femoral arteries, the exercise pressor reflex is exaggerated, an effect that is attenuated by stimulation of peripheral μ-opioid receptors on group IV metabosensitive afferents. In contrast, δ-opioid receptors are expressed mostly on group III mechanosensitive afferents, a finding that prompted us to determine whether stimulation of these opioid receptors could also attenuate the exaggerated exercise pressor reflex in “ligated” rats. We found femoral arterial injection of [D-Pen2,D-Pen5]enkephalin (DPDPE; 1.0 μg), a δ-opioid agonist, significantly attenuated the pressor and cardioaccelerator components of the exercise pressor reflex evoked by hindlimb muscle contraction in both rats with ligated and patent femoral arteries. DPDPE significantly decreased the pressor responses to muscle mechanoreflex activation, evoked by tendon stretch, in ligated rats only. DPDPE (1.0 μg) had no effect in either group on the pressor and cardioaccelerator responses to capsaicin (0.2 μg), which primarily stimulates group IV afferents. DPDPE (1.0 μg) had no effect on the pressor and cardioaccelerator responses to lactic acid (24 mM), which stimulates group III and IV afferents, in rats with patent femoral arteries but significantly decreased the pressor response in ligated rats. Western blots revealed the amount of protein comprising the δ-opioid receptor was greater in dorsal root ganglia innervating hindlimbs with ligated femoral arteries than in dorsal root ganglia innervating hindlimbs with patent femoral arteries. Our findings support the hypothesis that stimulation of δ-opioid receptors on group III afferents attenuated the exercise pressor reflex.
Keywords: neural control of the circulation, decerebrate rats, thin-fiber muscle afferents, peripheral artery disease
activation of the exercise pressor reflex by muscle contraction increases arterial pressure, heart rate, and ventilation, effects that function to increase the delivery of blood and oxygen to the working muscles (1, 3, 9, 27, 35, 42). Group III and group IV fibers with endings in skeletal muscle comprise the afferent arm of the reflex (27). Thinly myelinated group III afferent fibers are predominantly activated by mechanical stimuli arising in contracting muscle, whereas unmyelinated group IV afferents are predominantly activated by metabolic stimuli produced by contracting muscle (12, 23, 24). Both group III and IV afferents synapse onto dorsal horn cells of the spinal cord, which, in turn, project to neurons in the ventrolateral medulla (18, 19) and the nucleus tractus solitarius (10, 49). These medullary interneurons eventually synapse onto autonomic preganglionic neurons to increase sympathetic outflow to the heart and vasculature as well as decrease parasympathetic outflow to the heart (10, 21). Suprapontine pathways appear to play little, if any, role in the exercise pressor reflex arc (20).
We have recently shown that the exercise pressor reflex is exaggerated in a rat preparation that simulates the blood flow patterns to a hindlimb with peripheral artery disease (44). In this preparation, one of the rat's femoral arteries was tightly ligated distal to the inguinal ligament 72 h before an experiment. Using radiolabeled microspheres, Terjung and colleagues showed that femoral artery ligation reduced blood flow reserve capacity during exercise to ∼10–20% of normal; ligation, however, did not impair resting blood flow in cage-restrained rats (37, 52). Using this preparation, which simulates blood flow patterns to both resting and exercising muscles afflicted with peripheral artery disease, we found that the pressor responses to static contraction of the hindlimb muscles were significantly greater in rats whose femoral artery was ligated 72 h before the start of the experiment than were the pressor responses to contraction in rats whose femoral arteries were freely perfused (44). We also found that femoral arterial injection of [D-Ala, MePhe4, Gly(ol)5]enkephalin (DAMGO), a μ-opioid agonist, attenuated the exercise pressor reflex in rats with ligated femoral arteries but had no effect on the reflex in rats with patent femoral arteries (45). Likewise, DAMGO had no effect on the pressor and cardio-accelerator responses to tendon stretch (45) in either ligated or freely perfused rats (45). Tendon stretch is a pure mechanical stimulus that selectively activates group III afferents (23). The pressor and cardio-accelerator responses to tendon stretch have been termed the muscle mechanoreflex (43, 50).
Recently, Scherrer et al. reported that μ-opioid receptors in mice are expressed on unmyelinated somatic afferent fibers, whereas δ-opioid receptors are expressed on thinly myelinated somatic afferent fibers (40). This report prompted us to test the hypothesis that a peripheral δ-opioid receptor agonist inhibited both the muscle mechanoreflex, which is evoked by tendon stretch (43), as well as the exercise pressor reflex (9, 27, 30), which is evoked by contraction. We tested this hypothesis in rats whose femoral arteries were patent and in rats whose femoral arteries were ligated 72 h before the start of the experiment.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Hershey Medical Center Pennsylvania State University. Adult male Sprague-Dawley rats (n = 92; average weight was 435 ± 5 g) were used in these experiments. All rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle and fed a standard diet and tap water ad libitum. In 48 rats, we ligated the left femoral artery 72 h before the experiment. Briefly, rats were anesthetized with isoflurane (2–3%) in 100% oxygen. Under sterile procedure, the left femoral artery was surgically exposed and ligated with suture (5-0 silk) just distal to the inguinal ligament. Rats were then allowed to recover for 72 h. This technique has been shown to reduce blood flow capacity to ∼10–20% of normal while having little effect on resting blood flow (37, 52).
Surgical preparation.
On the day of the experiment, rats were anesthetized with isoflurane gas (2–3%) in oxygen. The trachea was cannulated, and their lungs were mechanically ventilated with the gas anesthetic until decerebration was performed. Both carotid arteries and a jugular vein were cannulated (PE-50) to measure arterial blood pressure and to administer drugs and fluids, respectively. Arterial blood gases and pH were measured using an automated blood gas analyzer (ABL 700 Series, Radiometer). Pco2 and arterial pH were maintained within normal ranges by adjusting ventilation and oxygen or through an intravenous administration of sodium bicarbonate (8.5%). Body temperature was maintained between 36.5 and 38.0°C by an isothermal heating pad and lamp.
In rats in which the left femoral artery was ligated, on the day of the experiment, we inserted a catheter (PE-10) into this artery distal to the suture. In rats in which the femoral artery was patent, on the day of the experiment, we inserted in a retrograde manner a catheter (PE-10) into the right femoral artery. The tip of this catheter was located at the bifurcation of the abdominal aorta. In all rats, including both those whose femoral arteries were ligated and those whose femoral arteries were patent, we placed a reversible snare around the abdominal aorta and inferior vena cava just above where the aorta bifurcates into the iliac arteries. When tightened, the snare directed and maintained the injectate into the circulation of the left hindlimb.
A laminectomy was performed to expose the spinal cord and the lower lumbar roots (L2–L6). The rats were then secured in a Kopf customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the back skin, we formed a pool, which was filled with warm (37.0°C) mineral oil. The dura was then cut and reflected so that L4 and L5 ventral roots, which innervate the muscles of the hindlimb, could be identified and sectioned. The left calcaneal bone was sectioned, and the triceps surae muscles were isolated.
A precollicular decerebration was performed, and the forebrain was aspirated using the method described previously (42, 44, 45). To minimize bleeding, small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with gauze. Immediately after precollicular transection, gas anesthesia was discontinued. After decerebration, the rats were allowed to stabilize for at least 1 h before any experimental protocol was initiated.
Experimental protocols.
The cut peripheral ends of ventral roots L4 and L5 were placed on a shielded stimulating electrode. The cut end of the calcaneal tendon was connected to a force transducer (Grass Instruments, FT10), which in turn was attached to a rack-and-pinion. The tendon was stretched so that baseline tension was set between 100 and 200 g. Static contraction of the hindlimb muscles was evoked by electrical stimulation of the cut peripheral ends of the left L4 and L5 ventral roots (one to three times motor threshold, 0.1-ms pulse duration, 40 Hz). In a subset of 17 animals, the muscle mechanoreflex was activated by stretching the triceps surae muscles by manually turning the rack-and-pinion attached to the calcaneal tendon. Both muscle contraction and tendon stretch lasted for 30 s.
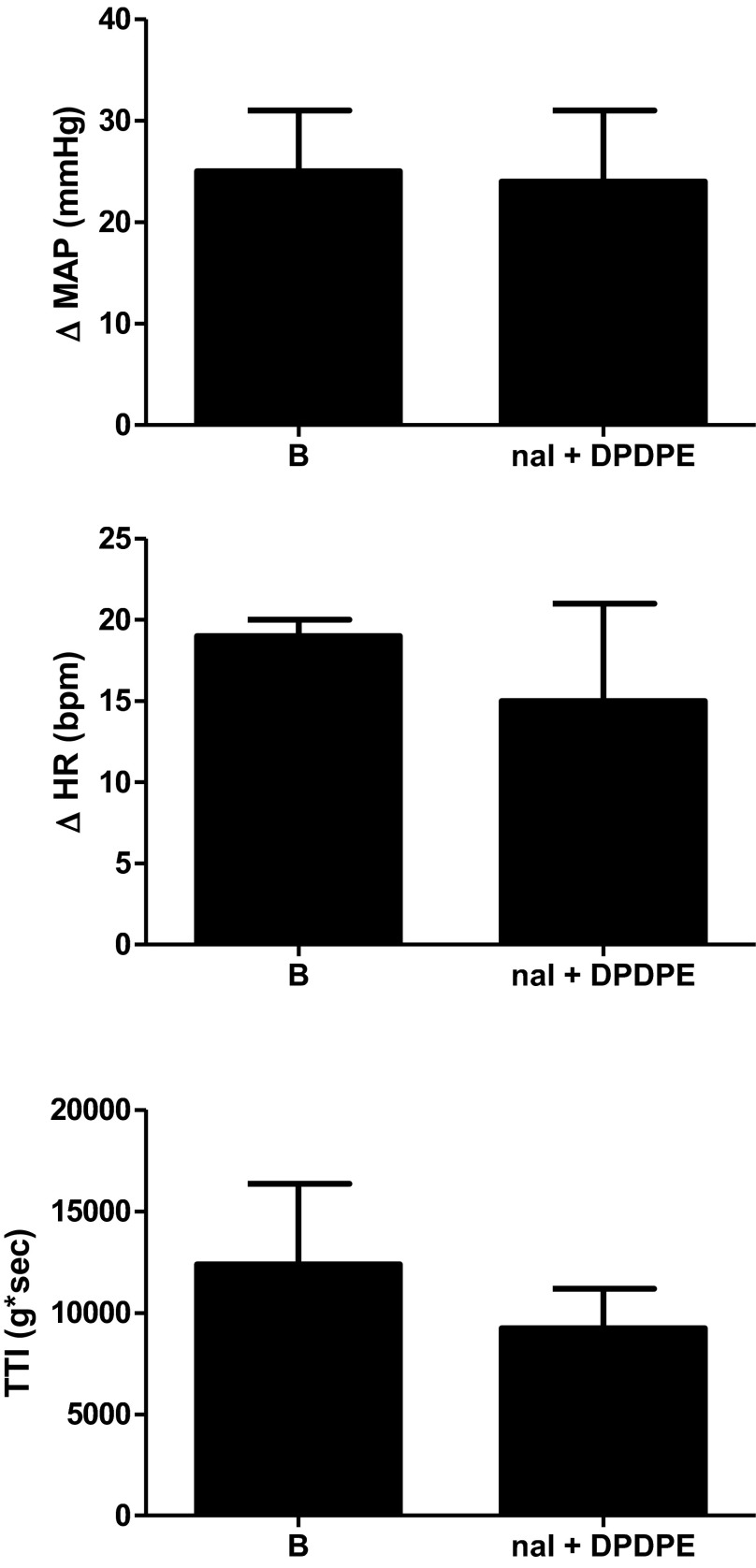
We stimulated δ-opioid receptors by injecting [D-Pen2,5]enkephalin (DPDPE; Tocris, 1.0 μg in 0.1 ml of saline) into the arterial supply of the left hindlimb via the arterial catheter (see above). Before injection, the snare was tightened to trap the injectate within the circulation of the left hindlimb. After DPDPE had been trapped for 5 min, the snare was released, and the muscles were freely perfused for 10 min before we again initiated contraction or calcaneal tendon stretch. In four rats whose left femoral artery had been ligated for 72 h, the highly selective δ-opioid receptor antagonist naltrindole (100 μg, Tocris) was injected with DPDPE (1.0 μg) into the arterial supply of the left hindlimb via the femoral arterial catheter. In these rats, the snare was tightened to trap the drugs in the circulation of the contracted limb for 5 min, after which the hindlimb was allowed to reperfuse for 10 min before static contraction was performed. In these experiments, we found that the combined injection of naltrindole and DPDPE appeared to reduce the ability of the hindlimb muscles to develop tension when the ventral roots were stimulated (see Fig. 6).
Fig. 6.
Combined femoral arterial injection of DPDPE (1.0 μg) and naltrindole (100 μg) had no effect on the peak pressor and cardioaccelerator responses to contraction in four ligated rats. The TTI did not differ before (B) and after combined naltrindole + DPDPE injection.
This finding caused us to change our approach when attempting to determine whether blockade of δ-opioid receptors with naltrindole had an effect on the exercise pressor reflex. Consequently, to determine the effect of δ-opioid receptor blockade on the exercise pressor reflex, we injected naltrindole (200 μg) into a carotid arterial catheter whose tip was placed in the abdominal aorta 1 mm above its bifurcation into the iliac arteries. Just before injection, we clamped the right iliac artery and vein so that the injectate was directed to the left hindlimb. This experiment was performed in five rats with ligated left femoral arteries and in four rats with patent femoral arteries. For unknown reasons, injection of 200 μg of naltrindole into the abdominal aorta had no effect on the ability of the hindlimb muscles to develop tension when the ventral roots were stimulated.
In five freely perfused and five ligated rats, 1.0 μg of DPDPE was injected into the jugular vein to test whether our data with the femoral arterial injection of DPDPE could be explained by its circulation to the spinal cord. Static hindlimb contraction was evoked 15 min after the intravenous injection of DPDPE.
In 21 rats not used in the above experiments, we measured the cardiovascular responses to hindlimb intra-arterial injections of lactic acid (24 mM; 0.4 ml; Sigma-Aldrich) and capsaicin (0.2 μg in 0.1 ml of saline; Sigma-Aldrich) both before and after intra-arterial injection of DPDPE (1.0 μg). Lactic acid stimulates both group III and IV afferents (39, 41), whereas capsaicin stimulates mostly group IV afferents (23).
At the end of the experiment, in 10 rats whose femoral arteries were ligated and in 8 whose femoral arteries were patent, we injected Evan's blue dye into the femoral circulation of the left hindlimb. The rats were randomly selected, and the injection catheter was the same one that was used to inject DPDPE. In three randomly selected rats with ligated femoral arteries, we also injected Evan's blue dye into the carotid catheter whose tip was advanced to the abdominal aorta near its bifurcation into the iliac arteries. In every case, regardless of whether the dye was injected into a femoral artery or into the abdominal aorta, the triceps surae muscles were stained blue, verifying that DPDPE or naltrindole had access to these muscles.
Western blots.
In 11 rats, we ligated the femoral artery as described above. The rats were then killed 72 h afterward, and the L4 and L5 dorsal root ganglia (DRG) from the freely perfused and ligated sides were dissected in ice-cold Hanks' balanced salt solution. A total of 22 samples were collected (11 freely perfused and 11 ligated, each containing the L4 and L5 DRG). After removal, the DRG tissue was quickly frozen and stored at −80°C until protein samples were processed with the Nucleospin RNA/protein kit (Macherey-Nagel) according to the manufacturer's instructions. Protein concentration for each sample was determined with the Qubit 2.0 Fluorometer (Life Technologies, Grand Island, NY). Electrophoresis of the protein samples (25 μg/lane) was performed on NuPAGE 10% Bis-Tris precast gels (Life Technologies), employing 90 V for 110 min at 4°C. The protein was next transferred onto PVDF membranes (0.2-μm pore) for 95 min with 30 V at room temperature. The membranes were blocked for 60 min at room temperature in Tris-buffered saline-Tween 20 (TBS-T) supplemented with 7% milk. After the blocking period, the membranes were incubated with the anti-δ-opioid receptor (1:500) rabbit polyclonal antibody (catalog no. AB1560, EMD Millipore) for 60 min at room temperature. The membranes were rinsed and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:5,000; GE Healthcare) for 60 min at room temperature. Following the rinse of the membranes, the proteins were visualized with the enhanced chemiluminescent reagent, SuperSignal West Femto (Thermo Scientific). The images were acquired with the ChemiDoc-It imaging system (UVP) equipped with a charge-coupled device camera. To normalize for protein loading, the membranes were stripped with the Restore Western Blot Stripping Buffer (Thermo Scientific) for 5 min at room temperature and retested with anti-actin (1:2,500) mouse monoclonal antibody (Abcam). The δ-opioid receptor and actin bands were quantified with the VisionWorksLS software (UVP).
Data analysis.
In all experiments, baseline as well as reflex changes in mean arterial pressure (MAP), heart rate (HR), and developed tension were recorded continuously with a Spike 2 data acquisition system (CED, Cambridge) and stored on a computer hard drive (Dell). The initial 30 s before stimuli were taken as baseline to compare peak responses to experimental maneuvers. MAP is expressed in millimeters mercury (mmHg) and HR is in beats per minute (beats/min). The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level and is expressed in gram seconds (g·s).
All values are expressed as means ± SE. Statistical comparisons were performed with two-way repeated-measures ANOVA. If the overall F value was significant, post hoc tests were performed with the Scheffe's test between individual means. The criterion for statistical significance was set as P < 0.05.
RESULTS
Exercise pressor reflex and δ-opioid receptors.
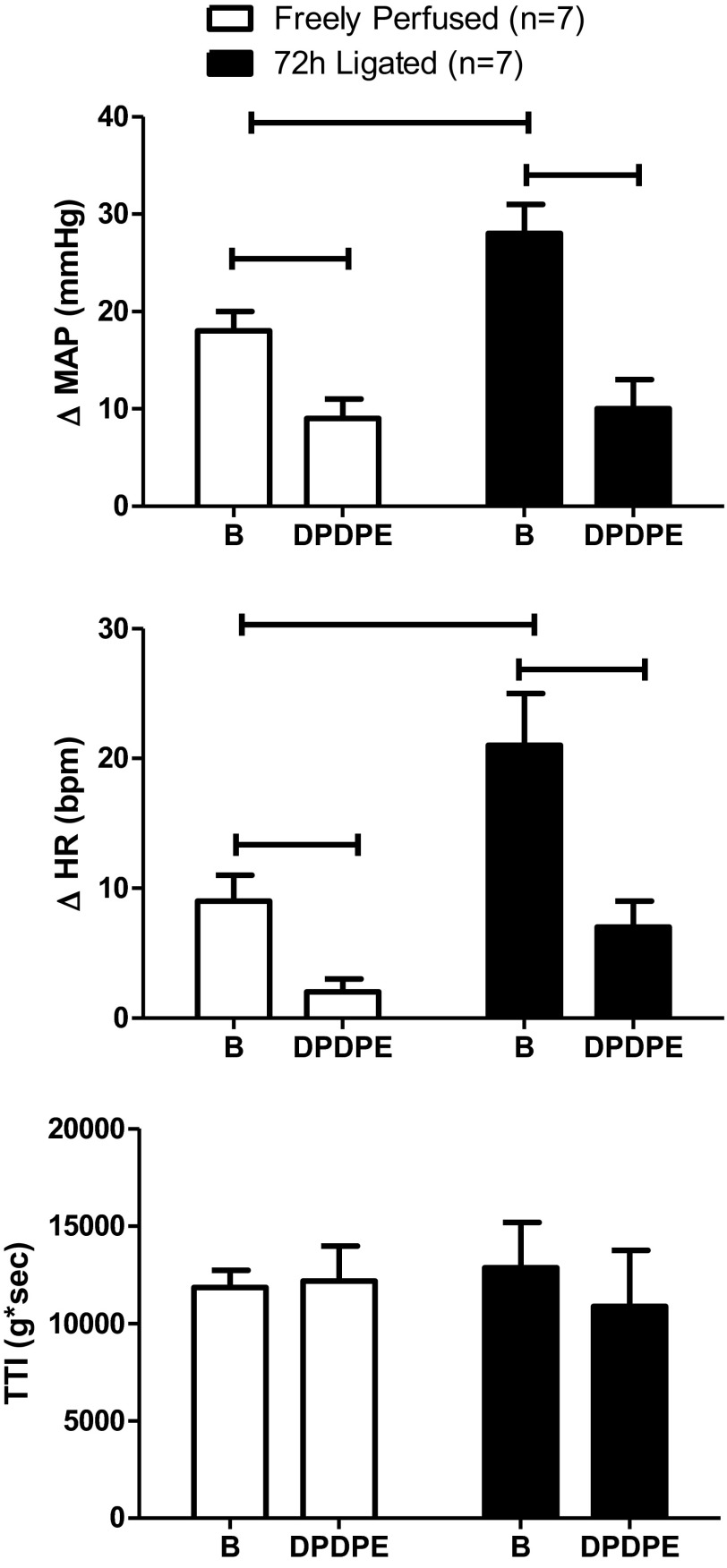
As previously reported (44, 45), the pressor and cardioaccelerator responses to static contraction of the hindlimb muscles were significantly greater in rats whose femoral arteries were ligated 72 h before the experiment than in rats whose femoral arteries were patent (P = 0.03; Fig. 1). In most instances (n = 12), DPDPE (1.0 μg), injected into the femoral artery, had no significant effect on either baseline MAP or HR in either group of rats (Table 1). In the four instances where DPDPE did decrease either baseline, the magnitude of the effect was not large and the baseline value remained well within the normal range (Table 1).
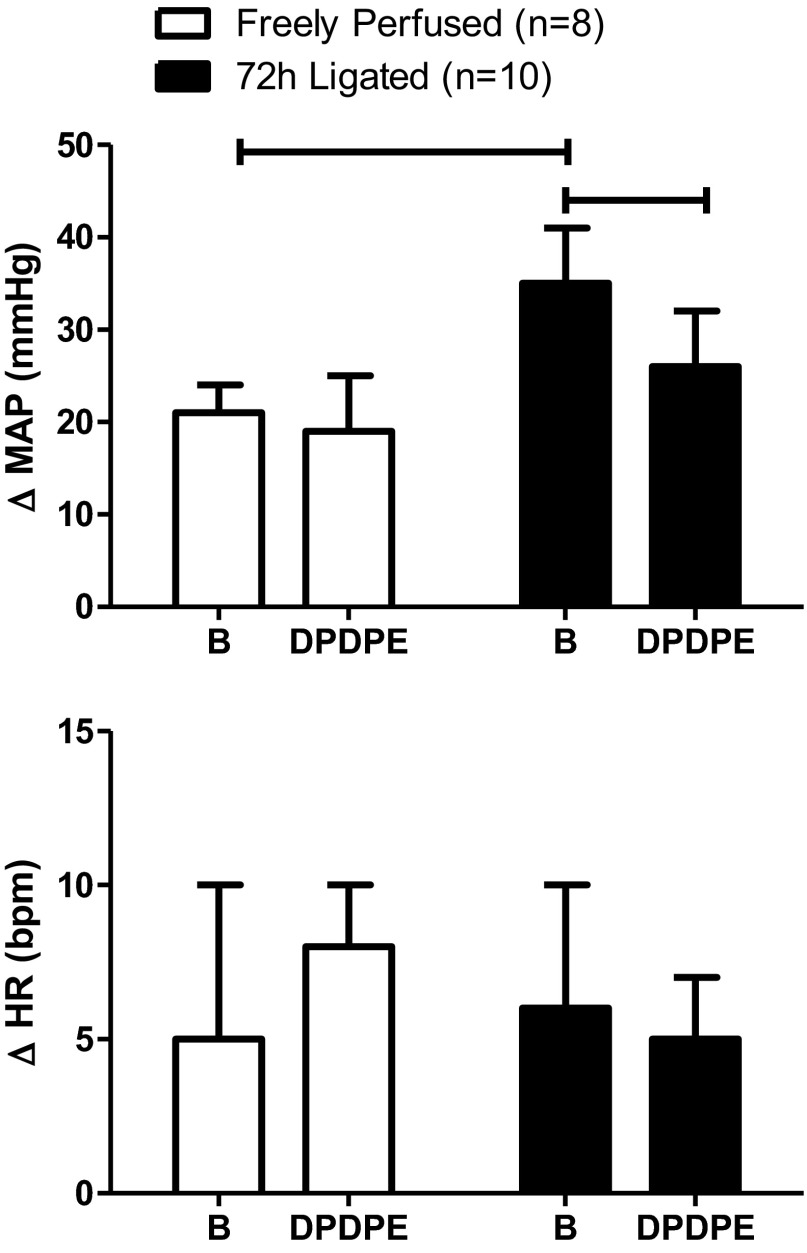
Fig. 1.
Effect of static contraction on the peak increases in mean arterial blood pressure (MAP) and heart rate (HR) before (B) and after femoral arterial injection of [D-Pen2,D-Pen5]enkephalin (DPDPE; 1.0 μg). The tension-time index (TTI) did not differ before and after DPDPE injection in either freely perfused or ligated rats. In this and subsequent figures, vertical bars represent means, and vertical brackets represent standard errors. The horizontal brackets connect means that were significantly different from each other (P < 0.05).
Table 1.
Baseline values for MAP and HR before and after femoral arterial injection of 1 μg of DPDPE
| Condition | MAP, mmHg | HR, beats/min |
|---|---|---|
| Static Contraction (n = 7) | ||
| Before | 103 ± 9 | 412 ± 24 |
| After DPDPE | 101 ± 9 | 406 ± 19 |
| Freely perfused | ||
| Tendon stretch (n = 11) | ||
| Before | 98 ± 7 | 417 ± 21 |
| After DPDPE | 87 ± 7* | 419 ± 21 |
| Lactic acid injection (n = 8) | ||
| Before | 94 ± 9 | 452 ± 18 |
| After DPDPE | 95 ± 10 | 454 ± 17 |
| Capsaicin injection (n = 8) | ||
| After | 82 ± 7 | 467 ± 18 |
| After DPDPE | 96 ± 10 | 446 ± 16* |
| Static contraction (n = 7) | ||
| Before | 108 ± 9 | 466 ± 33 |
| After DPDPE | 109 ± 12 | 487 ± 34 |
| 72-h ligated | ||
| Tendon stretch (n = 11) | ||
| Before | 102 ± 8 | 475 ± 18 |
| After DPDPE | 90 ± 7* | 487 ± 20 |
| Lactic acid injection (n = 10) | ||
| Before | 86 ± 10 | 496 ± 14 |
| After DPDPE | 88 ± 11 | 465 ± 20* |
| Capsaicin injection (n = 11) | ||
| Before | 79 ± 9 | 461 ± 17 |
| After DPDPE | 79 ± 10 | 460 ± 19 |
Values are means ± SE. MAP, mean arterial blood pressure; HR, heart rate.
Significant difference (P < 0.05) between corresponding values before and after injection of DPDPE.
In rats with patent femoral arteries as well as in rats with ligated femoral arteries, DPDPE (1.0 μg) significantly decreased the pressor and cardio-accelerator responses to static contraction. Specifically, in rats with patent arteries, DPDPE significantly decreased the pressor response to static contraction from 18 ± 2 to 9 ± 2 mmHg (P < 0.05; n = 7). Likewise, DPDPE significantly decreased the cardio-accelerator response to contraction from 9 ± 2 to 2 ± 1 beats/min (P < 0.05; n = 7). In the rats with ligated femoral arteries, DPDPE significantly decreased the pressor response to contraction from 27 ± 4 to 10 ± 3 mmHg (P < 0.05; n = 7). Likewise, DPDPE significantly decreased the cardio-accelerator response from 20 ± 4 to 6 ± 3 beats/min (P < 0.05; n = 7). Peak tension development and TTIs during static contraction did not differ significantly between groups both before and after the δ-opioid receptor agonist was injected (Fig. 1).
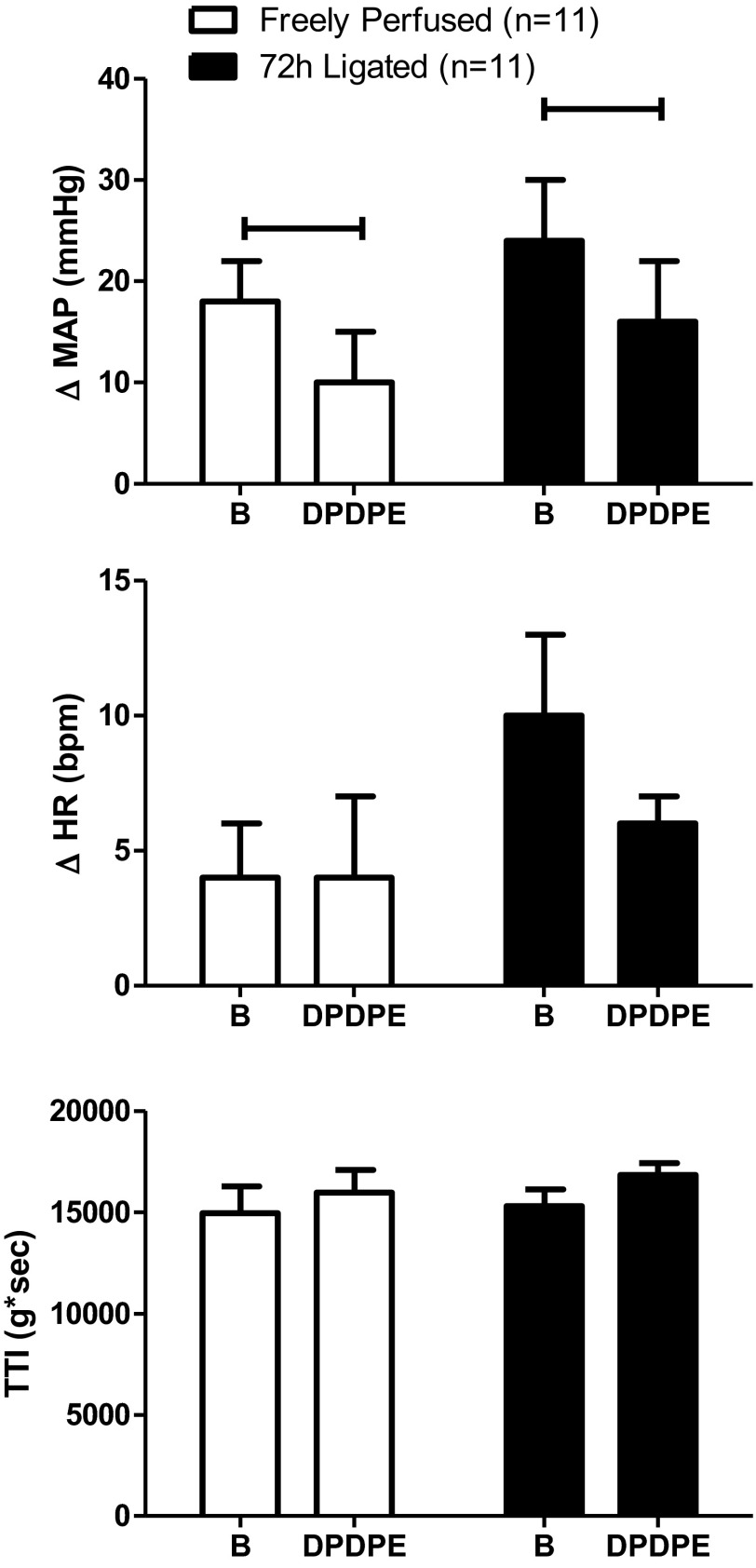
Muscle mechanoreceptor reflex and δ-opioid receptors.
DPDPE (1.0 μg), injected into the femoral artery, decreased the pressor response to calcaneal tendon stretch in rats with patent arteries from 18 ± 4 to 10 ± 5 mmHg (P < 0.05; n = 11); likewise in rats with ligated arteries, DPDPE significantly decreased the pressor response to tendon stretch from 24 ± 6 to 16 ± 6 mmHg (P < 0.05; n = 11). DPDPE did not attenuate the cardio-accelerator responses to tendon stretch in either group of rats. Although the pressor response to tendon stretch in rats with ligated femoral arteries tended to be larger than the pressor response to stretch in rats with patent arteries, the difference was not significant (P = 0.19). TTIs during tendon stretch were not significantly different before and after injection of DPDPE (Fig. 2).
Fig. 2.
Effect of stretch on peak increases in MAP and HR before (B) and after femoral arterial injection of DPDPE (1.0 μg). The TTI did not differ before and after DPDPE injection in either freely perfused or ligated rats. Values are means ± SE. Horizontal brackets signify that the responses were significantly different from each other (P < 0.05).
Muscle chemoreflex and δ-opioid receptors.
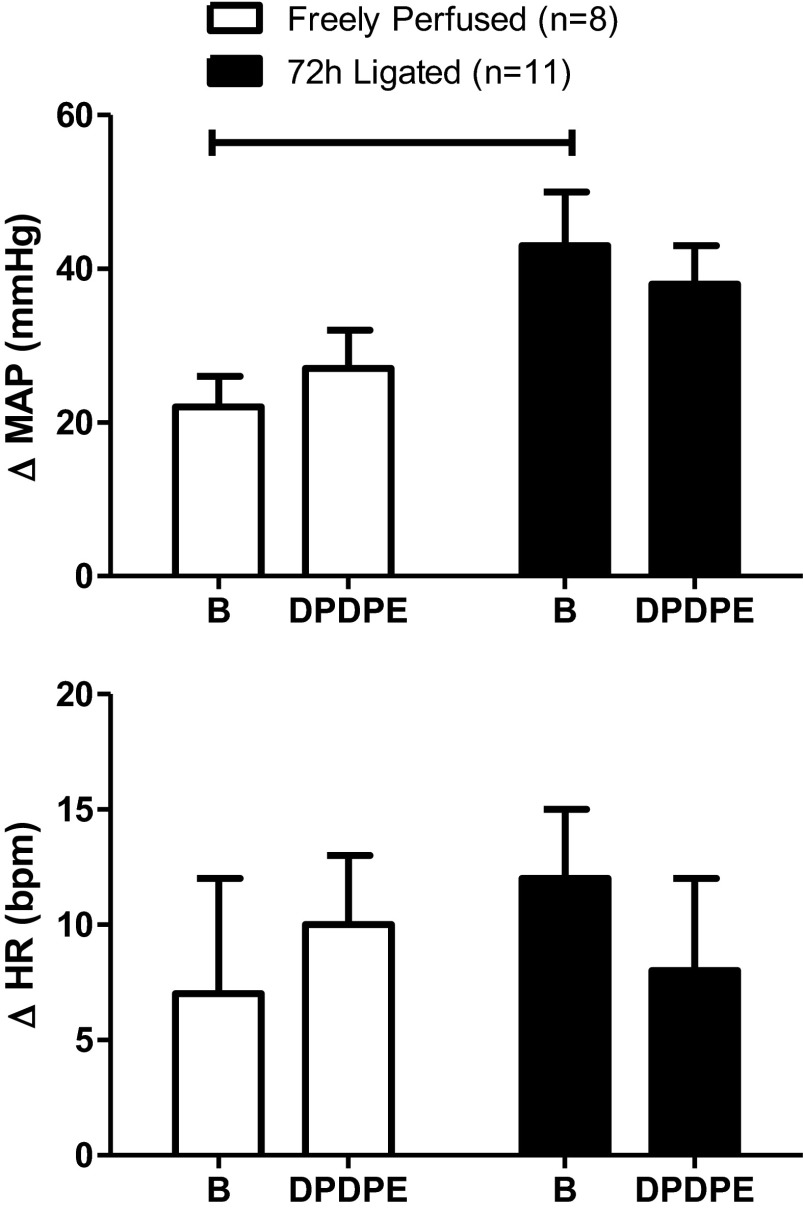
In rats not used in the experiments described above, we measured the pressor and cardioaccelerator responses to femoral artery injection of lactic acid (24 mM; 0.4 ml) and capsaicin (0.2 μg in 0.1 ml) both before and after femoral artery injection of DPDPE (1.0 μg). In both rats with patent femoral arteries (n = 8) and in rats with ligated femoral arteries (n = 11), DPDPE had no effect on the pressor and cardioaccelerator responses to capsaicin injections (Fig. 3). DPDPE also had no effect on the pressor and cardioaccelerator responses to lactic acid injection in rats with patent arteries (Fig. 4; n = 8). However, DPDPE significantly decreased the pressor responses to lactic acid injections in the rats with ligated femoral arteries (from 35 ± 6 to 26 ± 6 mmHg; P < 0.05; n = 10; Fig. 4).
Fig. 3.
DPDPE (1.0 μg) had no effect on the peak pressor and cardioaccelerator responses to femoral arterial injection of capsaicin (0.2 μg). Horizontal brackets connect means that were significantly different from each other (P < 0.05). B, response before DPDPE injection.
Fig. 4.
DPDPE (1.0 μg) attenuated the peak pressor response to femoral arterial injection of lactic acid (24 mM; 0.4 ml) in ligated rats but not in freely perfused rats. Horizontal brackets signify that the responses were significantly different from each other (P < 0.05). B signifies the response before DPDPE injection.
Control experiments.
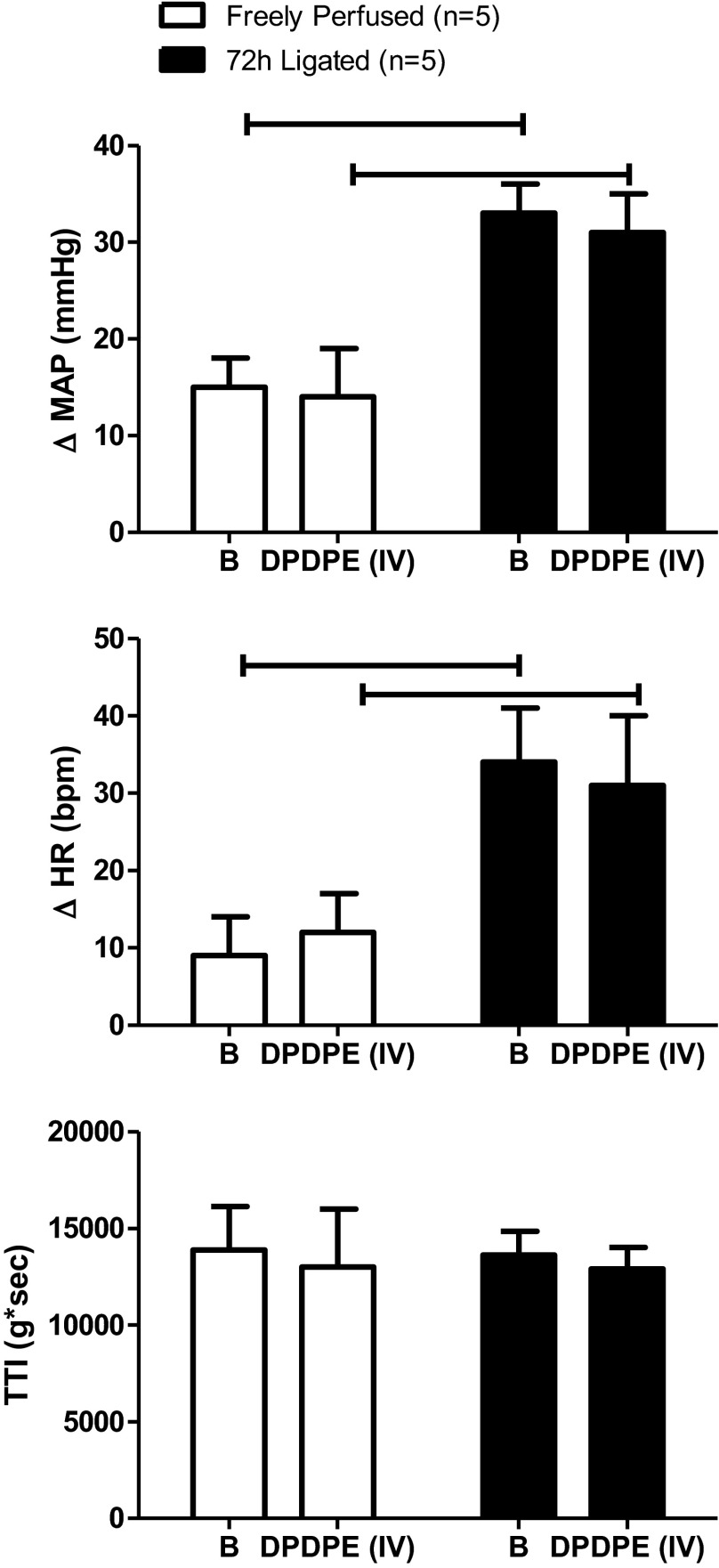
Intravenous injection of DPDPE (1.0 ug) in five rats with patent and in five rats with ligated femoral arteries had no effect on either the pressor or the cardio-accelerator responses to static contraction (P > 0.05; Fig. 5). TTIs were not affected by intravenous injection of DPDPE. Finally, co-administration of the selective δ-opioid receptor antagonist naltrindole (100 μg) with DPDPE (1.0 ug) into the femoral artery prevented the attenuating effect of DPDPE on the pressor and cardioaccelerator responses to static contraction in rats with ligated femoral arteries (P < 0.05; n = 4; Fig. 6). Naltrindole alone (200 μg), injected into the abdominal aorta, had no effect on the pressor responses to contraction in either rats with ligated femoral arteries (n = 5) or rats with patent femoral arteries (n = 4; Fig. 7). Likewise, naltrindole (200 μg), injected into the abdominal aorta, had no effect on the pressor response to tendon stretch in either rats with ligated femoral arteries (n = 4) or rats with patent femoral arteries (n = 4; Fig. 7).
Fig. 5.
Intravenous injection of DPDPE (1.0 μg) had no effect on the peak pressor and cardioaccelerator responses to static contraction in either freely perfused or ligated rats. The TTI did not differ before and after DPDPE injection in either freely perfused or ligated rats. Horizontal brackets signify that the responses were significantly different from each other (P < 0.05). B signifies the response before DPDPE injection.
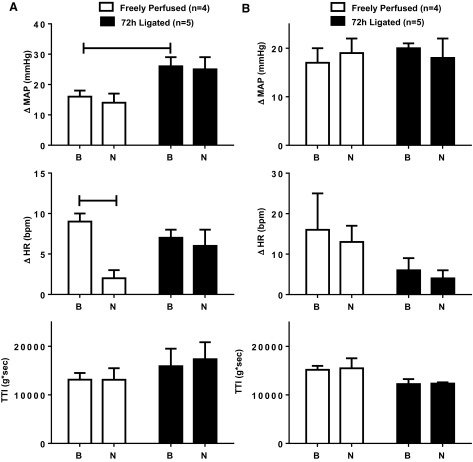
Fig. 7.
Naltrindole (200 μg), injected into the abdominal aorta, had no effect on the peak pressor responses to both static contraction (A) and tendon stretch (B) in either freely perfused or ligated rats. Naltrindole slightly but significantly (P < 0.05) attenuated the cardioaccelerator response to contraction in the freely perfused but not in the ligated rats (A). TTI for both contraction and tendon stretch were not altered by naltrindole. Horizontal brackets connect means that were significantly different from each other (P < 0.05). B signifies the response before naltrindole injection; N signifies the response after naltrindole injection.
Western blots.
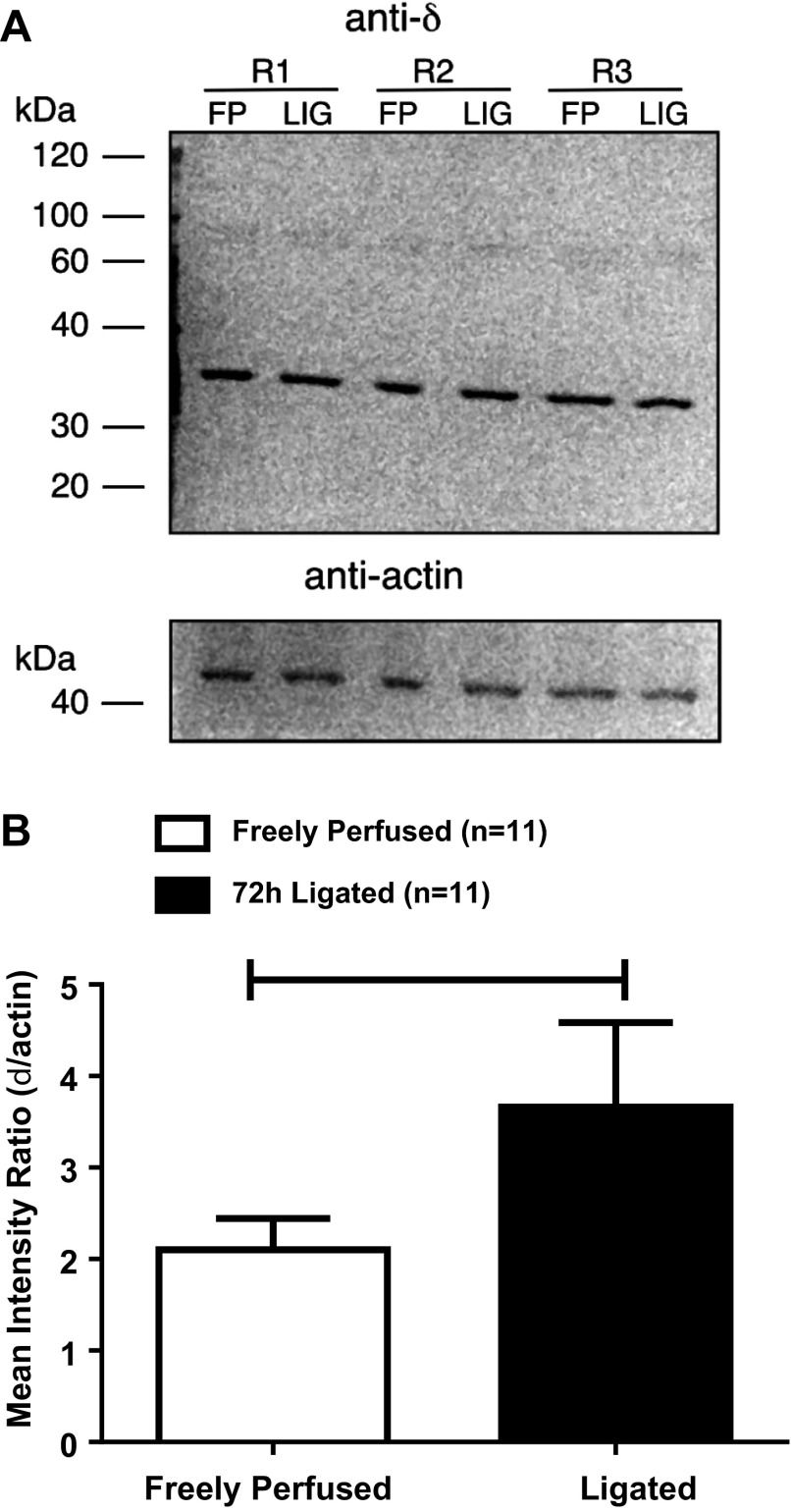
Binding of the respective antibodies to the δ-opioid receptor and actin were measured as pixel densities and then calculated as a ratio with the former as the numerator and the latter as the denominator. In protein extracted from the L4 and L5 DRGs innervating the hindlimbs whose femoral arteries were ligated, the ratio averaged 3.66 ± 0.92, whereas, in protein extracted from the dorsal root ganglia innervating the hindlimbs whose femoral arteries were patent, the ratio averaged 2.10 ± 0.34 (P = 0.04; n = 11; Fig. 8).
Fig. 8.
A: Western blot illustrating results with anti-δ-opioid receptor and anti-actin in DRG tissue isolated from three rats with freely perfused (FP) and ligated (LIG) femoral arteries. Each lane was loaded with ∼25 μg of protein corresponding to each rat's DRG tissue. Lines indicate approximate molecular weights (kDa). B: densitometric analysis of Western blots for δ-opioid receptor and actin quantified by measuring the area density (i.e., intensity). The plot illustrates the mean ratio of each receptor value normalized to its respective actin value. Horizontal brackets signify that the intensities were significantly different from each other (P < 0.05).
DISCUSSION
The primary purpose of our experiments was to determine whether stimulation of δ-opioid receptors on the peripheral endings of thin-fiber muscle afferents attenuated the exercise pressor reflex in decerebrated, unanesthetized rats. We found that stimulating these receptors with DPDPE, a δ-opioid agonist, attenuated the pressor and cardioaccelerator components of the exercise pressor reflex in both rats with patent femoral arteries as well as in rats with ligated femoral arteries. The attenuation of the reflex by DPDPE with the dose used in our experiments was not caused by its circulation to the central endings of thin-fiber muscle afferents synapsing in the dorsal horn of the spinal cord (5) because intravenous injection of DPDPE had no effect on the exercise pressor reflex. In addition, the attenuating effect of DPDPE on the pressor and cardio-accelerator components of the exercise pressor reflex was prevented by naltrindole, a highly selective δ-opioid receptor antagonist (36).
The secondary purpose of our experiments was to provide clues about which afferent population was responsible for the DPDPE-induced attenuation of the exercise pressor reflex. We used three stimuli known to activate specific populations of thin-fiber afferents. The first stimulus was tendon stretch, which is known to stimulate group III mechanoreceptors but not group IV metaboreceptors (23, 24). DPDPE, in our experiments, attenuated the pressor responses to stretch in rats with ligated femoral arteries but had no effect on the pressor responses to stretch in rats with freely perfused arteries. The second stimulus was lactic acid, an ASIC3 agonist that stimulates both group III and group IV afferents (17, 39, 41). DPDPE attenuated the pressor responses to injection of lactic acid in rats with ligated femoral arteries but had no effect on the pressor responses to lactic acid in rats with patent femoral arteries. The third stimulus was capsaicin, a TRPV1 agonist (8) that stimulates mostly group IV afferents (22, 23). DPDPE had no effect on the pressor responses to injection of capsaicin in either rats with ligated femoral arteries or rats with patent femoral arteries. Our findings, when considered together, suggest that DPDPE decreased the excitability of group III afferents to attenuate the exercise pressor reflex in the rats with ligated femoral arteries and perhaps in the rats with patent femoral arteries.
The information obtained from our Western blots allows us to speculate as to why DPDPE did not significantly attenuate the pressor and cardioaccelerator responses to tendon stretch in the rats with patent arteries. We found that ligating the femoral artery for 72 h significantly increased the amount of protein comprising the δ-opioid receptor in the dorsal root ganglia innervating the hindlimbs. This increase in δ-opioid receptors in the thin-fiber afferents innervating the hindlimb muscles of rats with ligated femoral arteries might have been necessary to reach the threshold level required to decrease afferent excitability to tendon stretch, a stimulus that excites an overlapping but different population of group III mechanoreceptors than does contraction (15). This speculation might also apply to the inability of DPDPE to attenuate the pressor and cardioaccelerator responses to lactic acid injection in the rats with freely perfused hindlimbs.
Previously, we found that stimulation of μ-opioid receptors on group III and IV muscle afferents attenuated the exercise pressor reflex in rats with ligated femoral arteries. We also found that stimulating μ-opioid receptors had no effect on the muscle mechanoreceptor reflex, which is evoked by tendon stretch. These findings led us to speculate that the μ-opioid receptor agonist attenuated the exercise pressor reflex in ligated rats by decreasing the excitability of group IV afferents (45). Our speculation was consistent with the findings of Scherrer et al. (40), who reported that murine μ-opioid receptors were concentrated on the peptide containing cell bodies of dorsal root ganglia neurons with unmyelinated axons. Scherrer et al. also found that δ-opioid receptors were distributed exclusively on cell bodies of dorsal root ganglia neurons with myelinated axons. In addition, Scherrer et al. found that stimulation of δ-opioid receptors increased the mechanical pain threshold in mice but had no effect on their response to noxious heat (40), a stimulus that is transduced at least in part by TRPV1 receptors that, in turn, are found on group IV afferents. Based on these findings and our present data, we hypothesize that DPDPE stimulated δ-opioid receptors located predominantly on group III mechanically sensitive muscle afferents (23). This hypothesis is supported by our findings showing that DPDPE had no effect on the pressor and cardio-accelerator responses to the TRPV1 agonist capsaicin in either group of rats. We conclude, therefore, that the δ-opioid agonist did not exert its effect on the exercise pressor reflex by decreasing the excitability of group IV muscle afferents.
Our finding that DPDPE, a δ-opioid agonist, attenuated the muscle mechanoreflex in rats with ligated femoral arteries but had no effect on the capsaicin-induced muscle chemoreflex is consistent with the conclusion by Scherrer et al. (40) that μ-opioid receptors are found on unmyelinated somatic afferents, whereas δ-opioid receptors are found on thinly myelinated somatic afferents. Likewise, our finding that DPDPE attenuated the exercise pressor reflex in rats with freely perfused hindlimb muscles is consistent with the finding by Scherrer et al. (40) that δ-opioid receptors are present in the plasma membrane of the neuron and do not have to be transported to the plasma membrane by μ-opioid receptors. Nevertheless, both concepts are controversial. For example, previous reports indicated that the two opioid receptors were coexpressed on the same nociceptive neuron (4, 47). In addition, μ-opioid receptors are hypothesized to regulate the surface density of the δ-opioid receptor by transporting them to the plasma membrane where they can be accessed by δ-opioid receptor agonists (14, 25, 29, 46). Support for the latter hypothesis comes from studies showing that δ-opioid receptor agonists were not effective analgesics in μ-opioid receptor knockout mice (13, 32, 33). Furthermore, morphine pretreatment is thought to cause the recruitment of δ-opioid receptors to neuronal plasma membranes in the dorsal horn of the rodent spinal cord (7, 31).
The primary source of opioid peptides in the periphery is leukocytes, which migrate toward inflamed or damaged tissue. Leukocytes can be stimulated to release opioid peptides by norepinephrine, interleukin-1β, and corticotrophin releasing factor (26). Opioid peptides, in turn, bind to inhibitory G-protein-coupled receptors on the peripheral endings of thin-fiber afferents, thereby attenuating their excitability. We found in both rats whose femoral arteries were freely perfused and rats whose femoral arteries were ligated that neither the exercise pressor reflex nor the muscle mechanoreceptor reflex was increased by naltrindole, a δ-opioid receptor antagonist. The simplest interpretation of our findings is that neither contraction nor tendon stretch caused leukocytes to release peptides that stimulated δ-opioid receptors on thin-fiber muscle afferents. Nevertheless, this conclusion must be tempered by the specific circumstances of our experiments. For example, we do not know whether a ligation period of more than 3 days would have resulted in the migration of leukocytes to the hindlimb muscles undergoing contraction.
Femoral artery ligation in our experiments increased the amount of protein comprising the δ-opioid receptor in the L4 and L5 dorsal root ganglia. One possible stimulus for this increase is inflammation, which when induced by hindpaw injection of Complete Freund Adjuvant increased δ-opioid receptor mRNA and protein levels in the dorsal root ganglia of rats (6, 38, 48). In our experiments, the rats were restrained in cages and, as a consequence, had limited opportunity to exercise. At rest, hindlimb blood flow to the limb with a ligated femoral artery meets metabolic demand, although during hindlimb contraction blood flow capacity is reduced to ∼10–20% of normal (37). Consequently, a mismatch between blood supply and demand in cage-restrained rats whose capacity to exercise was limited would probably not be a major stimulus to increase the protein comprising the δ-opioid receptor in dorsal root ganglia. Despite this reservation, we cannot exclude the possibility that some inflammatory process induced by femoral artery ligation increased the protein comprising the δ-opioid receptor in our experiments. Nevertheless, the nature of this stimulus remains to be discovered; it should be noted that femoral artery ligation also increases the protein levels comprising the TRPV1 and ASIC3 receptor in the dorsal root ganglia of rats (11, 51).
Our study has three limitations, each of which need to be considered. The first limitation concerns our use of stretch to selectively stimulate group III afferents, which are well known to be mechanically sensitive (23). In cats, only about half of the group III mechanoreceptors that responded to stretch also responded to static contraction (15). Consequently, tendon stretch, although an effective mechanical stimulus to some group III muscle afferents, cannot be used to recruit the same population of group III afferents as does static contraction. Despite this limitation, our finding showing that DPDPE attenuated the cardiovascular responses to passive stretch lends support to our conclusion that DPDPE's site of action was on δ-opioid receptors located on group III afferent fibers. The second limitation concerns the selectivity of DPDPE, which can activate μ-opioid receptors at high doses (34). We minimized this concern by showing that the attenuating effect of the dose of DPDPE (1.0 μg) used in our experiments was prevented by its co-injection with naltrindole, a highly selective δ-opioid receptor antagonist (36). The third and final limitation is that the distribution of δ-opioid receptors in both the dorsal root ganglion and the dorsal horn of the spinal cord differ between rodents and primates (28), findings that establish a need for further experiments before the applicability of our findings in rats can be translated to humans.
In conclusion, opioids are widely believed to exert their attenuating effects on the cardiovascular responses to exercise on receptors in the spinal cord and brain stem (2, 16). Although there is no doubt that this is the case, opioids are also capable of exerting their attenuating effects on the peripheral endings of group IV muscle afferents (45). In this study, we have shown that stimulation of the peripheral endings of thin-fiber muscle afferents with DPDPE, a δ-opioid receptor agonist, attenuated the exercise pressor reflex in rats whose femoral arteries were patent and in rats whose femoral arteries were ligated 72 h before the start of the experiment. DPDPE attenuated the cardiovascular responses to passive stretch and to femoral artery injection of lactic acid, maneuvers that stimulate group III afferent fibers, in rats whose femoral arteries were ligated. Our findings are consistent with the hypothesis that δ-opioid receptors are located on group III afferents (40) and that ligation of the femoral artery increases δ-opioid receptors in the dorsal root ganglia, innervating the hindlimb with the compromised blood supply.
GRANTS
This work was supported by National Institutes of Health Grants PO1 HL-096570, RO1 AR-059397, and F32 HL-108406.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.K.L. and M.P.K. conception and design of research; A.K.L., K.Y., J.K., and V.R.-V. performed experiments; A.K.L., J.K., and V.R.-V. analyzed data; A.K.L., V.R.-V., and M.P.K. interpreted results of experiments; A.K.L. and V.R.-V. prepared figures; A.K.L. and M.P.K. drafted manuscript; A.K.L. and M.P.K. edited and revised manuscript; A.K.L., V.R.-V., and M.P.K. approved final version of manuscript.
REFERENCES
- 1.Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci 31: 13068–13077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol 440: 65–84, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain 101: 199–208, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci 21: 7598–7607, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Craig AD, Heppelmann B, Schaible HG. The projection of the medial and posterior articular nerves of the cat's knee to the spinal cord. J Comp Neurol 276: 279–288, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Craig AD, Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat as revealed by the transport of horseradish peroxidase. Neurosci Lett 41: 233–238, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience 150: 807–817, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA 101: 5135–5139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68: 2466–2472, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto GA, Parnaveles JG, Kaufman MP, Botterman BR, Mitchell JH. Activation of caudal brainstem cell groups during the exercise pressor reflex as elucidated by 2-[14C]deoxyglucose. Brain Res 304: 178–182, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto GA, Waldrop TG, Bauer RM, Mitchell JH. Pressor responses to muscular contraction in the cat: contributions by caudal and rostral ventrolateral medulla. Prog Brain Res 81: 253–263, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. J Appl Physiol 59: 459–467, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. II. Control of Respiratory and Cardiovascular Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, p. 381–447 [Google Scholar]
- 22.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 24.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem 280: 11152–11164, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Machelska H, Stein C. Leukocyte-derived opioid peptides and inhibition of pain. J Neuroimmune Pharmacol 1: 90–97, 2006 [DOI] [PubMed] [Google Scholar]
- 27.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O'Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol 465: 349–360, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of mu-, delta- and kappa-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Brain Res Mol Brain Res 30: 203–210, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O'Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci 24: 5549–5559, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci 23: 4888–4898, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain 109: 266–273, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci USA 80: 5871–5874, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol 146: 185–186, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience 129: 473–479, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137: 1148–1159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Smith SA, Mitchell GS, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchimochi H, McCord JL, Kaufman MP. Peripheral mu-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol 10: 73–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA 107: 13117–13122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Adelta-nociceptors. Pain 153: 900–914, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Wilson LB, Andrew D, Craig AD. Activation of spinobulbar lamina I neuron by static contraction. J Neurophysiol 87: 1641–1645, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Wilson LB, Wall PT, Pawelczyk JA, Matsukawa K. Cardiorespiratory and phrenic nerve responses to graded muscle stretch in anesthetized cats. Respir Physiol 98: 251–266, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Xing J, Lu J, Li J. Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol 590: 1261–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]