Abstract
Strong epidemiological and experimental evidence indicate that hypertension in the elderly predisposes to the development of Alzheimer's disease (AD), but the underlying mechanisms remain elusive. The present study was designed to characterize the additive/synergistic effects of hypertension and aging on the expression of genes involved in β-amyloid generation and AD in the hippocampus, an area of brain contributing to higher cognitive function, which is significantly affected by AD both in humans and in mouse models of the disease. To achieve that goal, we induced hypertension in young (3 mo) and aged (24 mo) C57BL/6 mice by chronic (4 wk) infusion of angiotensin II and assessed changes in hippocampal mRNA expression of genes involved in amyloid precursor protein (APP)-dependent signaling, APP cleavage, Aβ processing and Aβ-degradation, synaptic function, dysregulation of microtubule-associated τ protein, and apolipoprotein-E signaling. Aged hypertensive mice exhibited spatial memory impairments in the Y-maze and impaired performance in the novel object recognition assay. Surprisingly, hypertension in aging did not increase the expression of APP, β- and γ-secretases, or genes involved in tauopathy. These genes are all involved in the early onset form of AD. Yet, hypertension in aging was associated with changes in hippocampal expression of APP binding proteins, e.g., [Mint3/amyloid β A4 precursor protein-binding family A member 3 (APBA3), Fe65/amyloid β A4 precursor protein-binding family B member 1 (APBB1)], amyloid β (A4) precursor-like protein 1 (APLP1), muscarinic M1 receptor, and serum amyloid P component, all of which may have a role in the pathogenesis of late-onset AD. The hippocampal gene expression signature observed in aged hypertensive mice in the present study provides important clues for subsequent studies to elucidate the mechanisms by which hypertension may contribute to the pathogenesis and clinical manifestation of AD.
Keywords: hypertension, Alzheimer's disease, vascular cognitive impairment, senescence, vascular aging, β-amyloid, tauopathy, dementia
there is growing epidemiological evidence that hypertension increases the risk (risk ratio = ∼1.5) for sporadic Alzheimer's disease (AD), the most common cause of dementia in the elderly (23, 27). The amyloid cascade hypothesis remains the most frequently invoked hypothesis to explain the cause of AD. It posits that altered production and/or processing of amyloid-β peptides, derived from amyloid precursor protein (APP), play a central role in the development of AD, a concept that is supported by substantial genetic and biochemical data. Yet, despite the old concept that the pathomechanisms of AD and vascular cognitive impairment are inherently different, recent clinical and experimental studies strongly suggest that cerebromicrovascular impairment plays a key role in the pathogenesis of AD as well (18, 21, 26, 47). As predicted by the vascular hypothesis of AD (60), many vascular risk factors, including hypertension, increase the probability to develop AD in the elderly. Although there are studies extant investigating the possible vascular mechanisms underlying the cognitive decline induced by hypertension (9, 20, 30), the pathophysiological mechanistic links among hypertension, Aβ peptides, and the development of AD still remain elusive.
Recent experimental studies give further support for both the amyloid cascade hypothesis and the vascular hypothesis of AD by demonstrating that hypertension in mice (induced by transverse aortic coarctation) promotes Aβ-deposition, which associates with cognitive decline (10–12). Importantly, in mice brain Aβ deposits are detectable as early as 4 wk after induction of hypertension (10–12), suggesting that hypertension-induced cerebromicrovascular impairment is sufficient to trigger molecular processes leading to AD. The aforementioned studies implicate oxidative stress and neuroinflammation in the development of AD-like pathologies in hypertensive mice (10–12). Despite these advances, the cellular and molecular mechanisms by which hypertension affect neuronal function and phenotype in the brain are not well understood. Importantly, there are no studies extant characterizing the effect of hypertension on the neuronal gene expression.
Aging is the single most important risk factor for AD, but the interaction between aging and hypertension in the genesis of AD has not been understood. Our recent studies provide important evidence that aging exacerbates hypertension-induced cognitive decline and neuroinflammation (50), likely because of the increased vulnerability of the aged cerebral microcirculation to the deleterious effects of hypertension. On the basis of these findings and recent data showing that neuroinflammation contribute to cognitive decline in hypertension (11), we predict that aging may exacerbate hypertension-induced alterations in hippocampal expression of AD-related genes.
The present study was designed to characterize the additive/synergistic effects of hypertension and aging on the expression of genes involved in β-amyloid generation and AD in the hippocampus, an area of brain contributing to higher cognitive function, which is significantly affected by AD both in humans and in mouse models of the disease. To achieve that goal, we induced hypertension in young and aged C57BL/6 mice by chronic infusion of angiotensin II (ANG II) followed by tests for learning and spatial memory and assessment of changes in hippocampal expression of relevant genes.
METHODS
Animals.
Young (3 mo, n = 20) and aged (24 mo, n = 20) male C57/BL6 mice were purchased from the National Institute on Aging and were housed 3–5/cage in the rodent barrier facility at University of Oklahoma Health Sciences Center. All mice were maintained under specific pathogen-free barrier conditions. Water and normal laboratory diet were available ad libitum. All procedures were approved by the Institutional Animal Use and Care Committees of the participating institutions.
Infusion of ANG II.
To induce hypertension, Alzet mini-osmotic pumps (model 2006, 0.15 μl/h, 42 days; Durect, Cupertino, CA) were implanted into young and aged mice. Pumps were filled either with saline vehicle or solutions of ANG II (Sigma Chemical, St. Louis, Mo) that subcutaneously delivered 1,000 ng·min−1·kg−1 of ANG II for 28 days (55). Pumps were placed into the subcutaneous space of ketamine-xylazine anesthetized mice through a small incision in the back of the neck that was closed with surgical sutures. All incision sites rapidly healed without the need for any medication.
Blood pressure measurements.
Systolic blood pressure of mice in each experimental group was measured by the tail-cuff method (CODA Non-Invasive Blood Pressure System, Kent Scientific, Torrington, CT) before and 4 wk after the minipump implantation.
Spatial memory testing of mice in Y-maze.
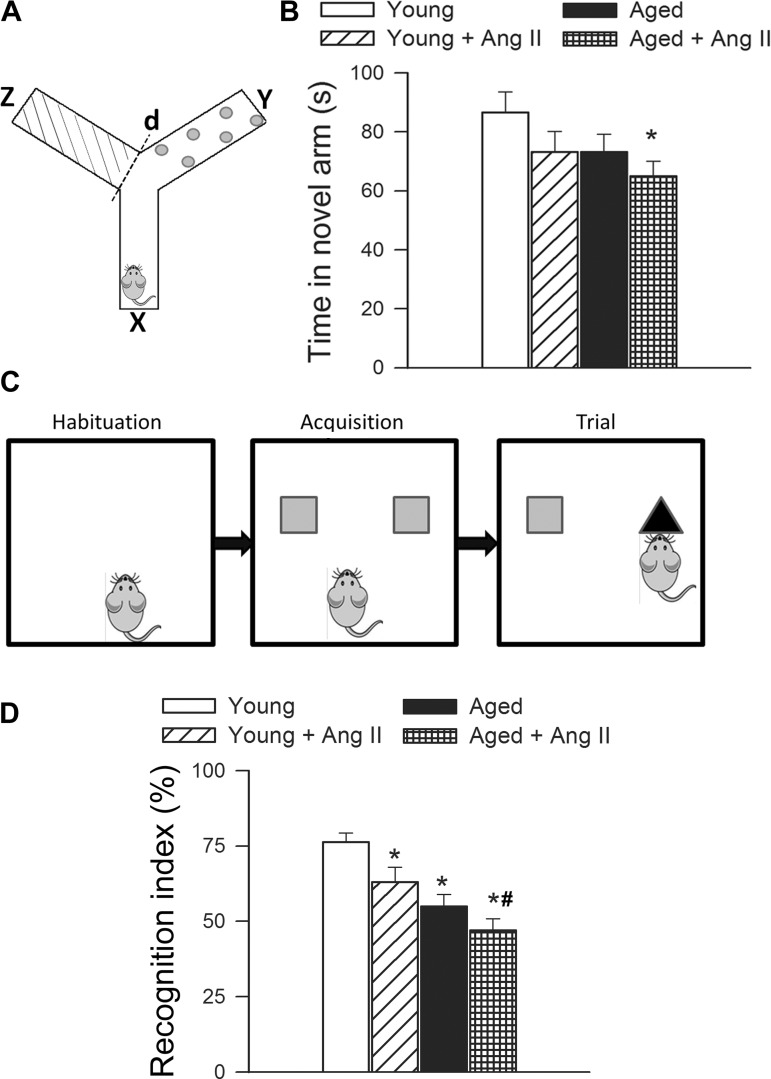
Animals were tested for spatial working memory in Y-maze. The Y-maze apparatus, made up of three enclosed transparent Plexiglas arms (40 cm length × 9 cm width × 16 cm height) with extra-maze visual cues around the maze (Fig. 2A) was used to assess hippocampal-dependent spatial recognition memory. The test consisted of two trials separated by an intertrial interval (4 h). All mice were transported to the behavioral testing room in their home cages at least 1 h before testing. In the first training (acquisition) trial, mice were placed in the maze facing the end of a pseudorandomly chosen start arm and allowed to explore the maze for 5 min with one of the arms closed (novel arm). Mice were returned to their home cage until the second (retrieval) trial, during which they could explore freely all three arms of the maze. The time spent in each arm was measured and analyzed from video recordings. Mice were required to enter an arm with all four paws in order for it to be counted as an entry. The time spent in the novel arm was calculated as a percentage of the total time in all three arms during the 2-min retrieval trial. The maze was cleaned with 70% ethanol between the trials.
Fig. 2.
A: spatial memory testing of mice in Y-maze. B: exploratory time spent by young control, young hypertensive (young + ANG II) and aged control and aged hypertensive (aged + ANG II) mice in the novel arm of the Y-maze during retrieval trial. Data are means ± SE (n = 10–15 in each group). C: novel object recognition task test used to evaluate recognition memory in mice. During the habituation phase, the animals explored for 5 min the empty open-field arena. In the acquisition phase the mice then explored two identical objects during 2 min. After a 4-h delay, a trial phase occurred. D: recognition index (representing the time spent investigating the novel object relative to the total object investigation) was used as the main index of retention. Data are means ± SE (n = 10–15 in each group). *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II.
Novel object recognition test.
The novel object recognition task was used to evaluate recognition memory (4). The results of the novel object recognition test are influenced by both hippocampal and cortical impairment (2). Previous studies demonstrate that the novel object recognition task is a sensitive indicator of cognitive decline in mouse models of AD. The novel object recognition task test consists of the habituation phase, the acquisition (familiarization) phase, and the trial phase (Fig. 2C). During the habituation phase the animals explored for 5 min the empty open-field arena. In the acquisition phase, the mice then explored two identical objects during 2 min. After a 4-h delay, a trial phase occurred. During the trial period, animals explored the familiar object and a novel object for 2 min. Exploration of the objects was defined as directing the nose at a distance ≤2 cm to the object and/or touching it with the nose, whereas running around the object or sitting or climbing on it was not considered as an exploration. All objects used in this study were made of washable odorless plastic and were different in shapes and colors but identical in size. A percentage of time spent exploring the novel object relative to the total time spent exploring both objects was used as a measure of novel object recognition. The recognition index [RI, representing the time spent investigating the novel object (Tnovel) relative to the total object investigation] was used as the main index of retention, which was calculated according to the following formula: RI = Tnovel/(Tnovel + Tfamiliar) (2). The arena and the objects were cleaned with 70% ethanol between the trials to prevent the existence of olfactory cues.
Quantitative real-time RT-PCR.
At the end of the experimental period, mice were decapitated, the brains were removed, and the hippocampi were isolated. Total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as previously described (3). A quantitative real-time RT-PCR technique was used to analyze hippocampal mRNA expression of genes known to be involved in β-amyloid generation and AD using validated TaqMan Gene Expression Assays (Applied Biosystems) and a Strategen MX3000 platform, as previously reported (3). Quantification was performed using the ΔΔCq method. The relative quantities of the reference genes Actb, Hprt1, and Gapdh were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Statistical analysis.
Data are expressed as means ± SE and were analyzed using a one-way ANOVA, followed by Tukey's post hoc test. The time in the novel arm comparison with the other arms of the Y-maze within a group was performed by Student's paired t-test. Significance was set at P < 0.05.
RESULTS
Hypertension in aging elicits significant decline in hippocampal cognitive function.
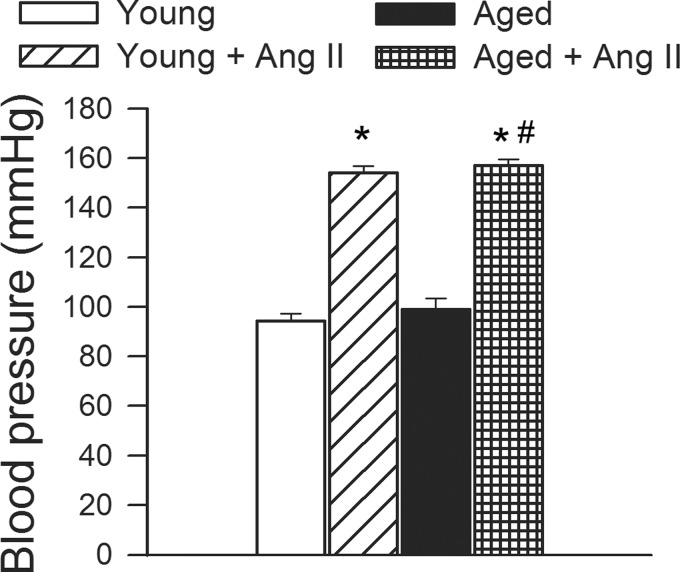
Blood pressure was significantly increased in both young and aged mice receiving ANG II infusion, as shown in Fig. 1.
Fig. 1.
Chronic infusion of angiotensin II (ANG II) on systolic blood pressure in young and aged mice. Data are means ± SE. *P < 0.05 vs. young; #P < 0.05 vs. aged.
For the hippocampus-dependent spatial memory test, young normotensive mice spent significantly (P < 0.05) more time in the novel arm than the previously visited arms following the intertrial interval. The time spent in the novel arm in both young hypertensive mice (by ∼−15%) and aged mice (by ∼−15%) tended to decrease, compared with that in young control mice, although the differences did not reach statistical significance. For old hypertensive mice, time in the novel arm was significantly decreased (by ∼−25%), indicating that these mice had significant hippocampal spatial memory impairments.
Subsequently, we also tested the performance of the mice in the novel object recognition assay. We found no significant difference in the time that mice from each group spent exploring the two identical objects placed at the opposite ends of the arena during the acquisition phase, confirming that the location of the objects does not affect the exploration behavior of mice. In the trial phase with two different objects (one novel, the other familiar), young normotensive mice explored the novel object for a significantly longer time period, with a calculated RI of ∼76%, indicating their memory for the familiar object (Fig. 2D). In contrast, both young hypertensive mice and aged normotensive mice had a significantly lower RI at ∼63 and 55%, respectively, which is consistent with their impaired cognitive function (Fig. 2D). When hypertension was induced in the aged mice, it further decreased the RI in aged mice to ∼47% (Fig. 2D), reflecting the additive effect of aging and hypertension to cause cognitive decline.
Effects of hypertension and aging on the hippocampal expression of APP and genes involved in APP-dependent signaling.
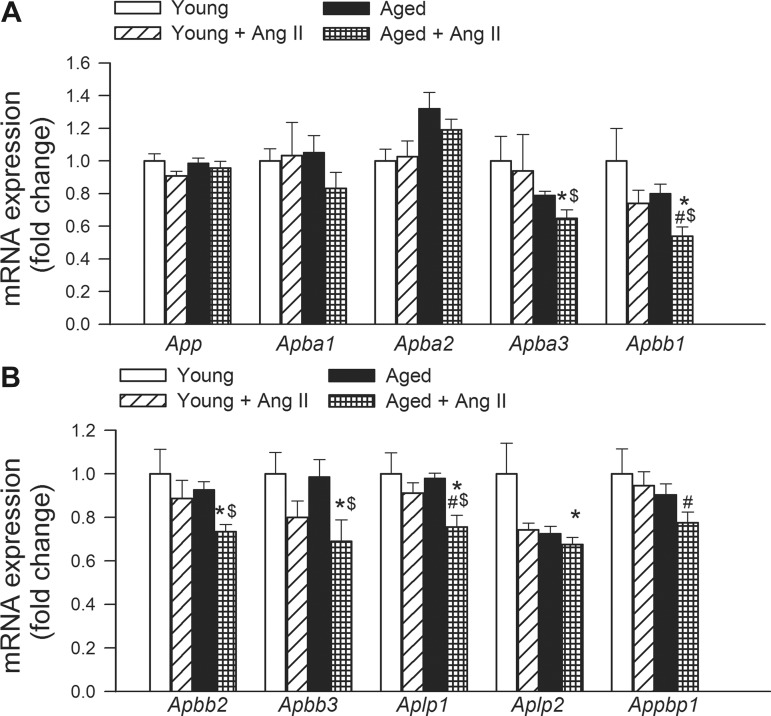
Neither hypertension nor aging altered the expression of App (amyloid β A4 precursor protein), Apba1 [amyloid β (A4) precursor protein-binding, family A, member 1; MUNC18-interacting protein 1 (MINT-1)] and Apba2 [amyloid β (A4) precursor protein-binding, family A, member 2; MINT-2] in the mouse hippocampus (Fig. 3A). Importantly, hypertension in aged mice significantly decreased the expression of Apba3 [amyloid β (A4) precursor protein-binding, family A, member 3; MINT-3], Apbb1 [amyloid β (A4) precursor protein-binding, family B, member 1; FE65], Apbb2 [amyloid β (A4) precursor protein-binding, family B, member 2; FE65L1], Apbb3 [amyloid β (A4) precursor protein-binding, family B, member 3; FE65L2], Aplp1 [amyloid β (A4) precursor-like protein 1], Aplp2 [amyloid β (A4) precursor-like protein 2], and Appbp1 (NEDD8-activating enzyme E1 regulatory subunit), whereas hypertension in young mice was without effect (Fig. 3).
Fig. 3.
Effects of aging and hypertension on the hippocampal expression of amyloid precursor protein (APP) and genes involved in APP-dependent signaling. Quantitative RT (qRT)-PCR data showing mRNA expression of App, Apba1, Apba2, Apba3, Apbb1, Apbb2, Apbb3, Aplp1, Aplp2, and Appbp1. See results for definitions of gene abbreviations. Data are means ± SE (n = 6 in each group) *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II; $P < 0.05 vs. aged.
Effects of hypertension and aging on the hippocampal expression of α-, β-, and γ-secretases.
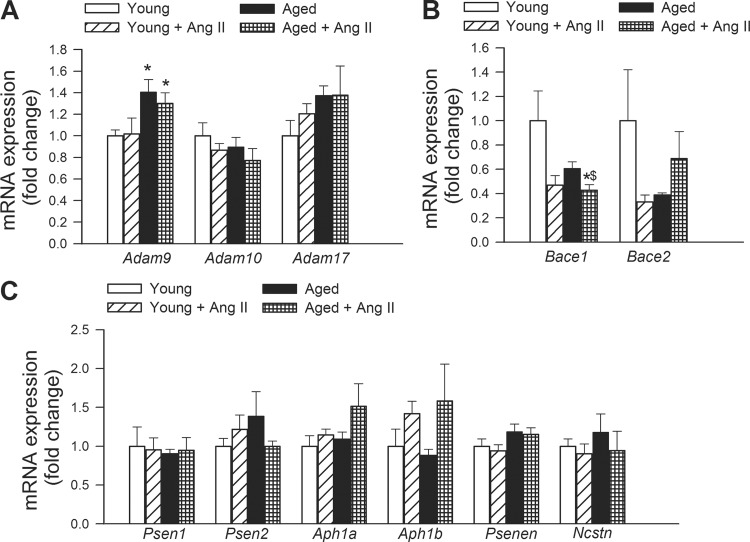
The effects of hypertension and aging on the expression of α- (Fig. 4A), β- (Fig. 4B) and γ-secretases (Fig. 4C) were assessed in the mouse hippocampus. We found that aging tended to increase the expression of Adam9 (ADAM metallopeptidase domain 9). Aging and hypertension did not alter the expression of Adam10 (ADAM metallopeptidase domain 10), and Adam17 (ADAM metallopeptidase domain 17; TNF-α converting enzyme/TACE) (Fig. 4A). Hypertension tended to downregulate Bace1 (β-site APP-cleaving enzyme 1; β-secretase) both in aged mice, whereas expression of Bace2 (β-site APP-cleaving enzyme 2) did not show the same pattern (Fig. 4B). We found that aging and hypertension did not alter the expression of Psen1 (presenilin 1, PS-1), Psen2 (presenilin 2), Aph1a [anterior pharynx defective 1 homolog A (Caenorhabditis elegans); γ-secretase subunit APH-1A; Presenilin-stabilization factor] and Aph1b [anterior pharynx defective 1 homolog B (C. elegans); γ-secretase subunit APH-1B; Presenilin-stabilization factor-like], Psenen [presenilin enhancer 2 homolog (C. elegans)], and Ncstn (nicastrin) (Fig. 4C).
Fig. 4.
Effects of aging and hypertension on the hippocampal expression of α-, β-, and γ-secretases. qRT-PCR data showing mRNA expression of the α-secretases Adam9, Adam10, and Adam17 (A), the β-secretases Bace1 and Bace2 (B), and the γ-secretase components Psen1, Psen2, Aph1a and Aph1b, and Psenen and Ncstn (C). See results for definitions of gene abbreviations. Data are means ± SE (n = 6 in each group) *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II; $P < 0.05 vs. aged.
Effects of hypertension and aging on the hippocampal expression of genes involved in aβ processing and aβ degradation.
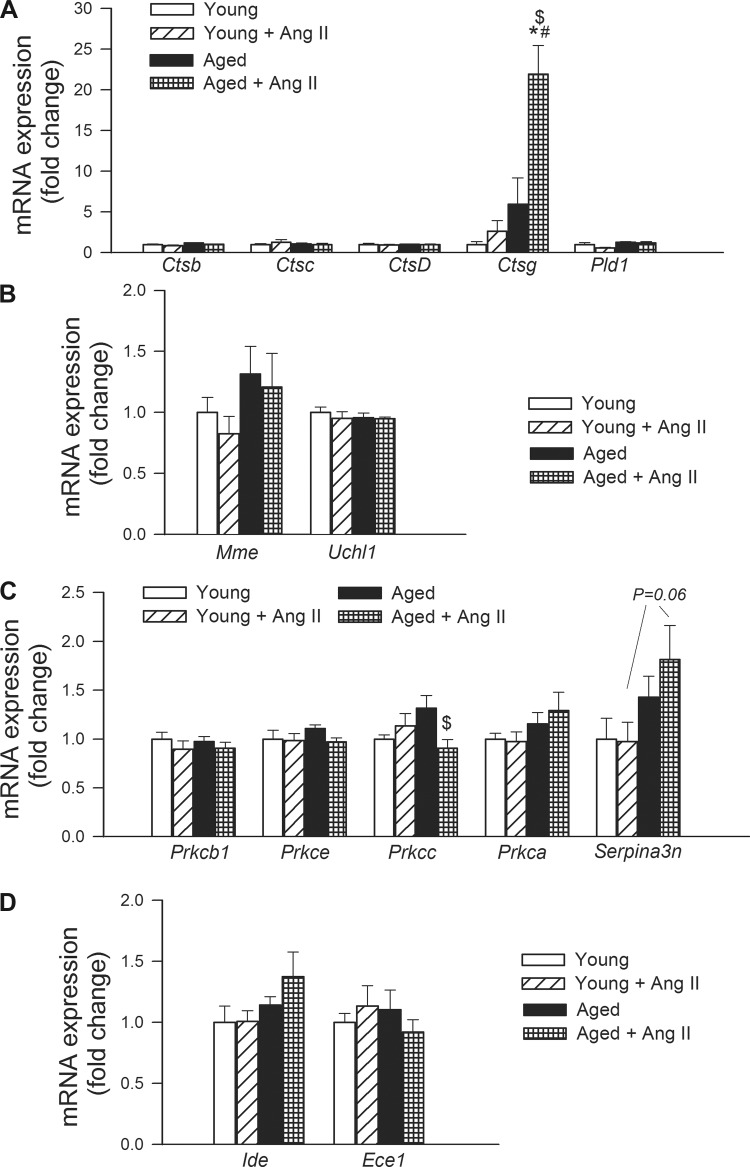
We also assessed the effects of hypertension and aging on the expression of genes involved in Aβ processing (Fig. 5A) and Aβ-degradation (Fig. 5B) in the mouse hippocampus. We found that neither aging nor hypertension affected the expression of cathepsins Ctsb (cathepsin B), Ctsc (cathepsin C), and Ctsd (cathepsin D) and Pld1 (phospholipase D1; Fig. 5A). In contrast, hypertension in aging was associated with a marked upregulation of expression of Ctsg (cathepsin G; Fig. 5A). Aging and hypertension did not alter the expression of Mme (membrane metallo-endopeptidase) and Uchl1 (ubiquitin carboxyl-terminal esterase L1; Fig. 5B) PKC isoforms are also involved in the processing of the APP and PKC activators exert therapeutic effects of in mouse models of AD. Among the protein kinase C-related genes investigated, neither aging nor hypertension altered the expression of Prkcb1 (protein kinase C, β), Prkce (protein kinase C, ε), and Prkca (protein kinase C, α). Hypertension decreased expression of Prkcc (protein kinase C, γ) in aged mice, but it was without effect in young mice (Fig. 5C). The expression of Serpina3n [serpin peptidase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 3] tended to be upregulated in hypertensive aged animals (P = 0.06 vs. young + ANG II), whereas hypertension in young animals was without effect (Fig. 5C). Neither aging nor hypertension affected the expression of insulin-degrading enzyme (Ide) and endothelin-converting enzymes (Ece1), both of which are capable of degrading Aβ (Fig. 5D).
Fig. 5.
Effects of aging and hypertension on the hippocampal expression of genes involved in Aβ processing and Aβ-degradation. qRT-PCR data showing mRNA expression of Ctsb, Ctsc, Ctsd, Ctsg, and Pld1 (A), Mme and Uchl1 (B), and Prkcb1, Prkce, Prkcc, Prkca, and Serpina3n (C). See results for definitions of gene abbreviations. Data are means ± SE (n = 6 in each group). *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II; $P < 0.05 vs. aged.
Effects of hypertension and aging on the hippocampal expression of postsynaptic neurotransmitter receptors.
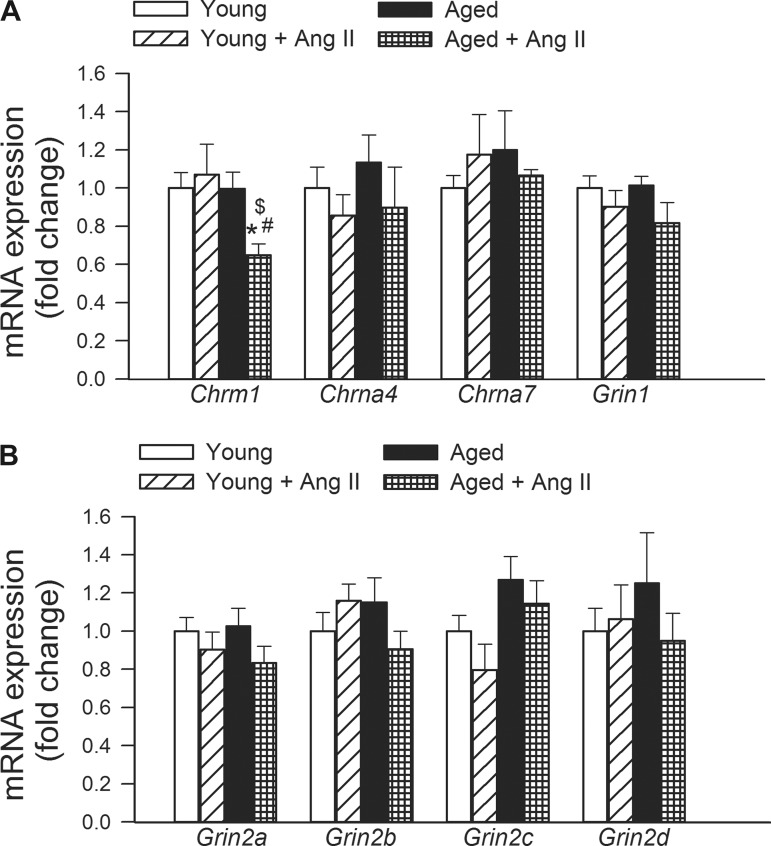
In the present study hypertension in aged mice, but not in young mice, was associated with downregulation of Chrm1 (muscarinic M1 acetylcholine receptor; Fig. 6). Expression of Chrna4 (neuronal acetylcholine receptor subunit α-4), Chrna7 (cholinergic receptor, nicotinic, α 7), Grin1a (NMDAR1), Grin2a (glutamate receptor, ionotropic, N-methyl-d-aspartate 2A; NMDAR2A), Grin2b (NMDAR2b), Grin2c (NMDAR2C), and Grin2d (NMDAR2D) was unaffected by aging and hypertension (Fig. 6).
Fig. 6.
Effects of aging and hypertension on the expression of postsynaptic neurotransmitter receptors. A: qRT-PCR data showing mRNA expression of Chrm1 (muscarinic M1 receptor), Chrna4 (neuronal acetylcholine receptor subunit α-4), Chrna7 (cholinergic receptor, nicotinic, α 7), and Grin1a (NMDAR1). B: mRNA expression of Grin2a (glutamate receptor, ionotropic, N-methyl d-aspartate 2A; NMDAR2A), Grin2b (NMDAR2b), Grin2c (NMDAR2C), and Grin2d (NMDAR2D). Data are means ± SE (n = 6 in each group). *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II; $P < 0.05 vs. aged.
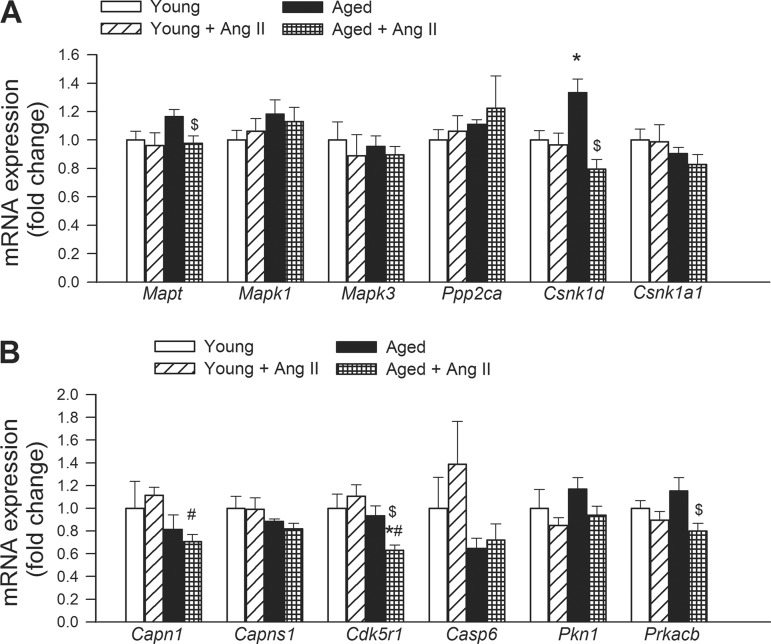
Effects of hypertension and aging on the hippocampal expression of genes involved in taupathies.
Hypertension in aged mice significantly decreased hippocampal expression of Mapt (microtubule-associated protein τ) and Csnk1d (casein kinase 1, δ), which tended to be upregulated in aged normotensive mice, whereas hypertension was without effect in young mice (Fig. 7). Neither aging or hypertension significantly alters the expression of Mapk1 (mitogen-activated protein kinase 1), Mapk3 (mitogen-activated protein kinase 3), Ppp2ca (protein phosphatase 2, catalytic subunit, α-isozyme), Csnk1a1 (casein kinase 1, α1), Capns1 (calpain, small subunit 1) and Casp6 (caspase 6) compared with young controls (Fig. 7). Expression of Capn1 (calpain 1, μ/I large subunit) was decreased in aged hypertensive mice compared with young hypertensive mice. Hypertension in aged mice decreased the expression of Cdk5r1 (cyclin-dependent kinase 5, regulatory subunit 1; p35), Pkn1 (protein kinase N1) and Prkacb (protein kinase, cAMP-dependent, catalytic, β), whereas it was without effect in young mice (Fig. 7).
Fig. 7.
Effects of aging and hypertension on the expression of genes involved in abnormalities of microtubule-associated tau protein. qRT-PCR data showing mRNA expression of Mapt, Mapk1, Mapk3, Ppp2ca, Csnk1d, Csnk1a1, Capn1, Capns1, Cdk5r1, Casp6, Pkn1, and Prkacb. See results for definitions of gene abbreviations. Data are means ± SE (n = 6 in each group). *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II; $P < 0.05 vs. aged.
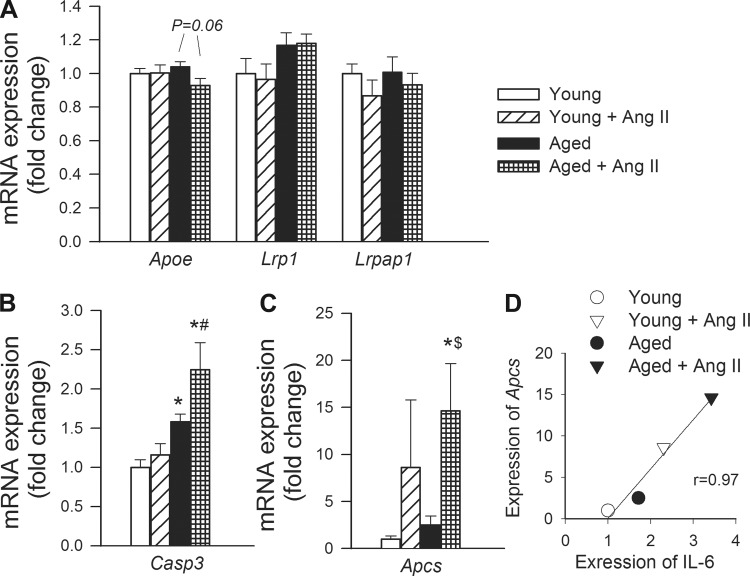
Effects of hypertension and aging on the hippocampal expression of caspase 3, serum amyloid P (SAP), and genes involved in apolipoprotein-E signaling.
We found that hypertension did not affect the expression of Apoe (apolipoprotein E, which modulates APP proteolytic processing and localization), the ApoE receptor Lrp1 [low-density lipoprotein receptor-related protein 1, which is a major Aβ clearance receptor in cerebral vascular smooth muscle cell acting to prevent Aβ accumulation (29)], and Lrpap1 (low-density lipoprotein receptor-related protein-associated protein 1, which is involved with trafficking of LRP1) in the mouse hippocampus (Fig. 8A). Aging was associated with an increased expression of Casp3 (caspase 3) in the mouse hippocampus (Fig. 8B). Hypertension tended to increase hippocampal expression of Casp3 in aged mice, whereas it was without effect in young mice (Fig. 8B). Hypertension significantly increased hippocampal expression of Apcs (SAP) in aged mice, whereas its effect did not reach statistical significance in young mice (Fig. 8C).
Fig. 8.
Effects of aging and hypertension on the hippocampal expression of genes involved in apolipoprotein-E (ApoE) signaling and caspase 3, serum amyloid P. qRT-PCR data showing mRNA expression of Apoe, Lrp1, and Lrpap1 (A), Casp3 (B), and Apcs (C). Data are means ± SE (n = 6 in each group). *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II; $P < 0.05 vs. aged. See results for definitions of gene abbreviations. D: relationship between hippocampal expression of IL-6 and Apcs.
DISCUSSION
The results of this study suggest that increased vulnerability of the aged cerebral microcirculation to hypertension is associated with dysregulation of hippocampal expression of distinct genes involved in AD.
It is commonly accepted that AD is associated with a progressive decline in several forms of declarative memory. Here we report that hypertension in aging (Fig. 1) results in significant spatial memory deficits in mice, as shown by the Y-maze and the novel object recognition tests (Fig. 2). In the novel object recognition memory formation, dorsal hippocampus plays an important role, and if there are lesions or functional impairment in this structure, moderate and reliable anterograde memory impairment will occur (2). The synergistic negative effect of aging and hypertension on hippocampally encoded functions of learning and memory have been recently confirmed also using the elevated plus maze learning protocol (50). The aforementioned findings provide evidence that the presence of hypertension in aging is sufficient to trigger significant impairment of hippocampal function, mimicking aspects of mild cognitive impairment.
Previous studies provide evidence that in addition to the established association of high blood pressure with cerebrovascular lesions and vascular cognitive impairment, there is a direct relationship between high blood pressure and pathogenesis of AD. Morphological analysis of brains from deceased members of the Honolulu Heart Program/Honolulu-Asia Aging Study (HHP/HAAS) cohort demonstrate that elevated systolic blood pressure, (≥160 mmHg) in midlife is associated with an increased presence of neuritic plaques in both the neocortex and hippocampus (40). In addition, increased diastolic pressure (≥95 mmHg) was also associated with increased presence of neurofibrillary tangles in the hippocampi (40). Similar conclusions were reached by other studies as well (49). Importantly, the use of antihypertensive medication in elderly patients is associated with lower AD neuropathology (25). Epidemiological studies suggest that in addition to the increased prevalence of hypertension in older individuals, the deleterious cerebrovascular effects of hypertension are also exacerbated in elderly patients (6, 33, 43). As predicted on the basis of the vascular hypothesis of AD, treatment of hypertension resulted in a 19% and 50% reduction in dementia incidence in the elderly in the Perindopril Protection Against Recurrent Stroke Study (PROGRESS) (42) and Systolic Hypertension in Europe (Syst-Eur) (17) studies, respectively. Among elderly patients with hypertension with mild cognitive impairment, the Study on Cognition and Prognosis in the Elderly (SCOPE) also found evidence suggesting that treatment with candesartan (an ANG II type 1 receptor antagonist) may prevent cognitive decline (51). There are data available suggesting that treatment of hypertension with diuretics may also be associated with reduced incidence of AD (31), although more robust evidence is evidently needed (37). In contrast, in a recent small-scale study treatment of patients at risk for AD with ramipril (an angiotensin-converting enzyme inhibitor) did not affect Aβ(1–42) levels in the cerebrospinal fluid and did not improve cognition (58). There are studies extant showing that angiotensin-receptor blockers also confer protection against AD in mouse models (52). In addition, propranolol, a β-receptor antagonist antihypertensive drug, was also reported to ameliorate the cognitive impairments and inhibit AD-related processes in the senescence-accelerated (SAMP8) mice (16).
Multiple lines of experimental evidence suggest that hypertension-induced cerebromicrovascular impairment precedes/triggers cognitive decline and AD neuropathology. First, experimental hypertension was shown to induce early cerebromicrovascular oxidative stress and vasomotor dysfunction in mice (8, 9, 30), which are associated with impaired cognitive performance and accumulation of amyloid-β (10–12). Second, induction of hypertension in APP(swe) Tg2576 mice is associated with a significant increase of the accumulation of amyloid-β in the brain (15). Our recent studies (50) demonstrate that aging impairs functional adaptation of cerebral vessels to high blood pressure, which exacerbates hypertension-induced microvascular damage. Downstream consequences of cerebrovascular autoregulatory dysfunction in aged hypertensive mice include exacerbated microvascular injury, disruption of the blood-brain barrier, microglia activation and neuroinflammation (50). To determine whether hypertension-induced microvascular alterations and chronic low-grade neuroinflammation in aged mice were sufficient to trigger early processes involved in the development of AD, we studied hippocampal expression of genes involved in regulation of the cellular APP-dependent signaling pathways, β-amyloid generation and processing, postsynaptic neurotransmitter receptor expression, the pathogenesis of tauopathy and ApoE signaling.
APP is an integral membrane protein abundantly expressed in the neuronal synapses. It has important signaling roles, regulating the formation of synapses, long-term potentiation and neural plasticity. In AD its proteolysis generates β amyloid (Aβ), the main component of amyloid plaques. Interestingly, neither hypertension nor aging affect cerebral expression of App (Fig. 3). In contrast, aging exacerbated hypertension-induced downregulation of mRNA expression of multiple APP-binding proteins, including APBA3, APBB1, APBB2, APBB3, APLP1, APLP2, and APPBP1 (Fig. 3). APBA1, -2 and -3 are synaptic adapter proteins best known for their interaction through the PTB domain with the C-terminal part of APP (5) and binding to synaptic proteins involved in exocytosis. Among those the best known interaction happens with MUNC18-1, therefore this protein group is also called as MINTs. Overexpression of APBAs (MINTs) in HEK293 cells were reported to reduce β-amyloid production (46) by regulating the traffic of APP from the trans-Golgi network. Haploefficiency of APBA1 in vivo had an enhanced AD pathology in heterozygous knockout mice (44), whereas effect of reduced APBA1 and 2 levels in double knockout mice decreased amyloid secretion (24).
APBB1 (FE65) is a nuclear adaptor protein, and its genetic depletion was shown to impair performance in learning and memory tasks, with a striking deficit in reversal spatial learning (56). APBB1 and APBB2 (FE65L1) interact with the cytoplasmic tail of APP and were demonstrated to potently stimulate transcription (7). Interestingly, overexpression of APP-cytoplasmic tail and Fe65 in a transgenic mouse strain induces neurofibrillary tangle formation and marked memory deficits by 8 mo of age, followed by neurodegeneration (19). Genetic depletion of both APBB1 and APBB2 results in cortical dysplasia (22). APBB3 is expressed in the cytoplasm and binds to the intracellular domain of the APP and may modulate its internalization. APLP1 is a membrane-associated glycoprotein that is cleaved by secretases, but this cleavage does not produce β-amyloid. The intracellular cytoplasmic fragment of APLP1 is believed to act also as a transcriptional activator, which plays a role in synaptic maturation. APLP2 is essential for normal synaptic transmission, spatial learning, and long-term potentiation (57). APPBP1 is thought to contribute to the regulation of neurogenesis. In addition, APPBP1 may inhibit Aβ42 production by interacting with presenilin-1 (14).
It has been well established that generation of the amyloid-β peptides that form aggregates in the brain of AD patients requires two sequential cleavages of APP. Extracellular cleavage of APP by β-secretases results in the release of a soluble extracellular fragment and a cell membrane-bound fragment. Cleavage of the later fragment within its transmembrane domain by γ-secretase produces amyloid-β. α-Secretases are a family of proteolytic enzymes that cleave APP in its transmembrane region closer to the cell membrane than β-secretases. Initial cleavage of APP by α-secretases rather than β-secretases prevents the generation of amyloid-β, and therefore it is considered to be part of the non-amyloidogenic pathway in APP processing. Because hypertension in mice was shown to increase amyloid-β deposition (12), in the present study we assessed the effects of hypertension and aging on the expression of α- (Fig. 4A), β- (Fig. 4B) and γ-secretases (Fig. 4C) in the mouse hippocampus. We found that hypertension does not increase expression of β-secretases, yet the possibility cannot be excluded that it alters β-secretase activity. The γ-secretase complex consists of presenilin (which regulates proteolysis of APP and affect its intracellular trafficking), nicastrin (which promotes the maturation of the other proteins in the γ-secretase complex), APH-1 (which is required for proteolytic activity), and presenilin enhancer 2 (which facilitates the γ cleavage of APP into β-amyloid). We found that aging and hypertension did not alter the expression of Psen1, Psen2, Aph1a and Aph1b, Psenen, and Ncstn (Fig. 4C). Aging, but not hypertension, tended to increase the expression of Adam9, which has α-secretase activity. In contrast, aging and hypertension did not alter the expression of the α-secretases Adam10 and Adam17 (Fig. 4).
Among the factors involved in Aβ processing and Aβ-degradation (Fig. 5), hypertension in aging was associated with upregulation of cathepsin G, a chymotrypsin-like protease that is capable of cleaving APP to generate several breakdown products (45). Yet, previous reports suggest that cathepsin G in the AD brain is predominantly localized to leukocytes (45), and presently it is unclear whether cathepsin G contributes to amyloid deposition in the brain in AD. Further studies are warranted to elucidate the cellular localization and pathophysiological role of cathepsin G in aged hypertensive mice. Interestingly, hippocampal expression of the gene Serpina3n encoding the acute phase protein α-1 antichymotrypsin (serine protease inhibitor A3) also tended to increase in aged hypertensive mice. Serine protease inhibitor A3 is an inhibitor of several proteases including cathepsin G and its expression in the vascular wall is known to be upregulated in atherosclerosis (54). The function of serine protease inhibitor A3 in the brain is not completely understood. It is believed to associate with ApoE and protein amyloid β and is an integral component of the plaques in AD (41).
In previous studies the hippocampal levels of postsynaptic neurotransmitter receptors have been directly correlated with cognitive performance, particularly on spatial learning and memory tasks. Among the postsynaptic receptors tested in this study, hypertensive aged mice exhibited the greatest decline in the expression of M1 muscarinic acetylcholine receptor gene (Fig. 6). The M1 muscarinic acetylcholine receptor is the most densely distributed muscarinic receptor in the hippocampus contributing to learning and memory (1). Mice with genetic depletion of this receptor exhibit reduced long-term potentiation in the hippocampus and cognitive impairment (1). Thus the potential role of M1 receptor dysregulation in hypertension induced cognitive decline in aged mice should be considered in future studies. Recent studies show that in P301L-tauTg mice hypertension exacerbates the progression of motor dysfunction associated with tauopathy (15). Yet, in the present study hypertension did not affect significantly the expression of genes, which are involved in abnormalities of microtubule-associated protein τ (Fig. 7).
Recent studies demonstrate that patients with AD exhibit significant increases both in synaptic procaspase-3 and active caspase-3 expression levels, suggesting that caspase 3 may play an important role in synapse degeneration during progression of AD (35). In that regard it is significant that aging and hypertension exert synergistic effect, upregulating hippocampal expression of caspase 3 (Fig. 8). Here we report for the first time that hypertension in aging is associated with upregulation of SAP component in the brain (Fig. 8). SAP, a pentraxin like C-reactive protein (51% sequence homology), is involved in innate immunity. SAP is always present in the cerebral and cerebrovascular amyloid lesions in patients with AD and is believed to stabilize aggregates by preventing proteolytic cleavage (32). Increased level of SAP may also contribute to neuronal death (32) as SAP is toxic and induces apoptosis of neurons at the nanomolar concentration (53). Importantly, scintigraphy with circulating 123I-SAP component in AD revealed no detectable localization of tracer within the brain (36), suggesting that increased expression of SAP in the brain of patient with AD (38) may significantly contribute to the development of plaques. Important for the present discussion, recent studies demonstrate that SAP is produced by vascular smooth muscle cells in the brain (39); that in AD prominent perivascular SAP, component staining can be observed in the affected gray matter (39); and that expression is also upregulated in animal models of vascular disease (48). Furthermore, data from the Cardiovascular Health Study show that SAP associates with vascular disease in humans (28). Previous studies showed that expression of SAP in cultured hepatocytes is regulated by cytokines, including IL-6 (34, 59). Importantly, hypertension in aging is associated with exacerbation of neuroinflammation, including upregulation of inflammatory cytokines (e.g., IL-6) in the hippocampus (50). There appears to be a positive correlation between expression of IL-6 and expression of SAP in the hippocampus (Fig. 8), supporting the hypothesis that increased SAP expression may be secondary to the heightened inflammatory status of the hippocampi in aged hypertensive mice.
Limitations of the study.
A number of important limitations of the present study need to be considered. First, we do not have data on protein expression of the investigated target genes. Second, as many proteins are known to be modulated at the posttranslational level in their contributions to Alzheimer pathology, further studies are needed to investigate the interaction of aging and hypertension both at the translational and at the posttranslational levels as well. Third, previous studies demonstrate that in addition to the effects of hypertension-mediated cerebromicrovascular injury, ANG II can directly affect neuronal function. For example, a recent study provides evidence that ANG II significantly increases production of reactive oxygen species via a mitochondrial-localized NADPH oxidase 4-dependent manner (13). Thus further studies should elucidate the mechanistic roles of hypertension per se and ANG II in neuronal dysfunction and cognitive decline in aged mice with ANG II-induced hypertension.
Conclusion.
In conclusion, the hippocampal gene expression signature observed in aged hypertensive mice in the present study provides important clues for subsequent studies to elucidate the mechanisms by which hypertension may contribute to the pathogenesis and clinical manifestation of AD. Further studies are evidently needed to determine whether pharmacological treatments that confer microvascular protection or anti-inflammatory effects will attenuate hypertension-induced alterations in neuronal gene expression preventing/delaying cognitive decline.
GRANTS
This work was supported by an American Heart Association grant (to P. Toth, A. Csiszar, and Z. Ungvari), National Center for Complementary and Alternative Medicine Grant R01-AT006526 (to Z. Ungvari), National Institute on Aging Grants AG031085 (to A. Csiszar) and AG038747 (to W. E. Sonntag), American Federation for Aging Research grant (to A. Csiszar), Oklahoma Center for the Advancement of Science and Technology grant (to A. Csiszar, Z. Ungvari, and W. E. Sonntag), Hungarian Scientific Research Fund (OTKA; K 108444), Nemzeti Fejlesztési ügynökség SROP-4.2.2.a-11/1/KONV-2012-0024 and -0017 (to Z. Ungvari and A. Koller), and the Ellison Medical Foundation (to W. E. Sonntag).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.C., P.T., and Z.I.U. conception and design of research; A.C., Z.T., D.S., and T.G. performed experiments; A.C., Z.T., and Z.I.U. analyzed data; A.C., Z.T., P.T., D.S., T.G., A.K., F.D., W.E.S., and Z.I.U. interpreted results of experiments; A.C., Z.T., P.T., D.S., T.G., A.K., F.D., W.E.S., and Z.I.U. edited and revised manuscript; A.C., Z.T., P.T., D.S., T.G., A.K., F.D., W.E.S., and Z.I.U. approved final version of manuscript; Z.I.U. prepared figures; Z.I.U. drafted manuscript.
REFERENCES
- 1.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6: 51–58, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93–110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci 67: 313–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1: 1306–1311, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Borg JP, Yang Y, De Taddeo-Borg M, Margolis B, Turner RS. The X11alpha protein slows cellular amyloid precursor protein processing and reduces Abeta40 and Abeta42 secretion. J Biol Chem 273: 14761–14766, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 67: 564–569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao X, Südhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293: 115–120, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol 300: H397–H407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 32: 4878–4886, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnevale D, Lembo G. ‘Alzheimer-like’ pathology in a murine model of arterial hypertension. Biochem Soc Trans 39: 939–944, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Carnevale D, Mascio G, Ajmone-Cat MA, D'Andrea I, Cifelli G, Madonna M, Cocozza G, Frati A, Carullo P, Carnevale L, Alleva E, Branchi I, Lembo G, Minghetti L. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging 33: 205 e219–e229, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60: 188–197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Case AJ, Li S, Basu U, Tian J, Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Bodles AM, McPhie DL, Neve RL, Mrak RE, Griffin WS. APP-BP1 inhibits Abeta42 levels by interacting with Presenilin-1. Mol Neurodegener 2: 3, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Ruiz C, Wang J, Ksiezak-Reding H, Ho L, Qian X, Humala N, Thomas S, Martinez-Martin P, Pasinetti GM. Role of hypertension in aggravating Abeta neuropathology of AD type and tau-mediated motor impairment. Cardiovasc Psychiatry Neurol 2009: 107286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobarro M, Orejana L, Aguirre N, Ramirez MJ. Propranolol restores cognitive deficits and improves amyloid and Tau pathologies in a senescence-accelerated mouse model. Neuropharmacology 64: 137–144, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 352: 1347–1351, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol 5: 649–658, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci USA 106: 18367–18372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol 26: 826–832, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenette S, Chang Y, Hiesberger T, Richardson JA, Eckman CB, Eckman EA, Hammer RE, Herz J. Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J 25: 420–431, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Z, Qiu C, Viitanen M, Fastbom J, Winblad B, Fratiglioni L. Blood pressure and dementia in persons 75+ years old: 3-year follow-up results from the Kungsholmen Project. J Alzheimers Dis 3: 585–591, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Ho A, Liu X, Sudhof TC. Deletion of Mint proteins decreases amyloid production in transgenic mouse models of Alzheimer's disease. J Neurosci 28: 14392–14400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, Haroutunian V. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 72: 1720–1726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke 40: S40–S44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Israeli-Korn SD, Masarwa M, Schechtman E, Abuful A, Strugatsky R, Avni S, Farrer LA, Friedland RP, Inzelberg R. Hypertension increases the probability of Alzheimer's disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology 34: 99–105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 29: 594–599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer's amyloid-beta. J Neurosci 32: 16458–16465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol 63: 686–692, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Kolstoe SE, Ridha BH, Bellotti V, Wang N, Robinson CV, Crutch SJ, Keir G, Kukkastenvehmas R, Gallimore JR, Hutchinson WL, Hawkins PN, Wood SP, Rossor MN, Pepys MB. Molecular dissection of Alzheimer's disease neuropathology by depletion of serum amyloid P component. Proc Natl Acad Sci USA 106: 7619–7623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, Kawas C, Breitner JC, Fitzpatrick A, Dulberg C. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64: 1548–1552, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin BF, Ku NO, Zahedi K, Whitehead AS, Mortensen RF. IL-1 and IL-6 mediate increased production and synthesis by hepatocytes of acute-phase reactant mouse serum amyloid P-component (SAP). Inflammation 14: 297–313, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, Trojanowski JQ, Arnold SE. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am J Pathol 173: 1488–1495, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovat LB, O'Brien AA, Armstrong SF, Madhoo S, Bulpitt CJ, Rossor MN, Pepys MB, Hawkins PN. Scintigraphy with 123I-serum amyloid P component in Alzheimer disease. Alzheimer Dis Assoc Disord 12: 208–210, 1998 [DOI] [PubMed] [Google Scholar]
- 37.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev: CD004034, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulder SD, Hack CE, van der Flier WM, Scheltens P, Blankenstein MA, Veerhuis R. Evaluation of intrathecal serum amyloid P (SAP) and C-reactive protein (CRP) synthesis in Alzheimer's disease with the use of index values. J Alzheimers Dis 22: 1073–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Perlmutter LS, Barron E, Myers M, Saperia D, Chui HC. Localization of amyloid P component in human brain: vascular staining patterns and association with Alzheimer's disease lesions. J Comp Neurol 352: 92–105, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging 21: 57–62, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Potter H, Wefes IM, Nilsson LN. The inflammation-induced pathological chaperones ACT and apo-E are necessary catalysts of Alzheimer amyloid formation. Neurobiol Aging 22: 923–930, 2001 [DOI] [PubMed] [Google Scholar]
- 42.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358: 1033–1041, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 64: 1734–1740, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saluja I, Paulson H, Gupta A, Turner RS. X11alpha haploinsufficiency enhances Abeta amyloid deposition in Alzheimer's disease transgenic mice. Neurobiol Dis 36: 162–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savage MJ, Iqbal M, Loh T, Trusko SP, Scott R, Siman R. Cathepsin G: localization in human cerebral cortex and generation of amyloidogenic fragments from the beta-amyloid precursor protein. Neuroscience 60: 607–619, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Shrivastava-Ranjan P, Faundez V, Fang G, Rees H, Lah JJ, Levey AI, Kahn RA. Mint3/X11gamma is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer's Precursor protein from the trans-Golgi network. Mol Biol Cell 19: 51–64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res 28: 605–611, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Song Z, Cai L, Guo L, Tsukamoto Y, Yutani C, Li XA. Accumulation and expression of serum amyloid P component in human atherosclerotic lesions. Atherosclerosis 211: 90–95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC., 3rd Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci 131: 162–169, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregutory dysfunction exacerbates cerebromicrovascular injury in mice with angiotensin II induced hypertension. J Cereb Blood Flow Metab. 2013. August 14. 10.1038/jcbfm.2013.143 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trenkwalder P. The Study on COgnition and Prognosis in the Elderly (SCOPE)—recent analyses. J Hypertens Suppl 24: S107–S114, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Tsukuda K, Mogi M, Iwanami J, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension 54: 782–787, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Urbanyi Z, Lakics V, Erdo SL. Serum amyloid P component-induced cell death in primary cultures of rat cerebral cortex. Eur J Pharmacol 270: 375–378, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Wagsater D, Johansson D, Fontaine V, Vorkapic E, Backlund A, Razuvaev A, Mayranpaa MI, Hjerpe C, Caidahl K, Hamsten A, Franco-Cereceda A, Wilbertz J, Swedenborg J, Zhou X, Eriksson P. Serine protease inhibitor A3 in atherosclerosis and aneurysm disease. Int J Mol Med 30: 288–294, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab 30: 56–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Hu Q, Hearn MG, Shimizu K, Ware CB, Liggitt DH, Jin LW, Cool BH, Storm DR, Martin GM. Isoform-specific knockout of FE65 leads to impaired learning and memory. J Neurosci Res 75: 12–24, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Weyer SW, Klevanski M, Delekate A, Voikar V, Aydin D, Hick M, Filippov M, Drost N, Schaller KL, Saar M, Vogt MA, Gass P, Samanta A, Jaschke A, Korte M, Wolfer DP, Caldwell JH, Muller UC. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J 30: 2266–2280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wharton W, Stein JH, Korcarz C, Sachs J, Olson SR, Zetterberg H, Dowling M, Ye S, Gleason CE, Underbakke G, Jacobson LE, Johnson SC, Sager MA, Asthana S, Carlsson CM. The effects of ramipril in individuals at risk for Alzheimer's disease: results of a pilot clinical trial. J Alzheimers Dis 32: 147–156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zahedi K, Whitehead AS. Regulation of mouse serum amyloid P gene expression by cytokines in vitro. Biochim Biophys Acta 1176: 162–168, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12: 723–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]