Abstract
The effect of hypercapnia on outwardly rectifying currents was examined in locus coeruleus (LC) neurons in slices from neonatal rats [postnatal day 3 (P3)–P15]. Two outwardly rectifying currents [4-aminopyridine (4-AP)-sensitive transient current and tetraethyl ammonium (TEA)-sensitive sustained current] were found in LC neurons. 4-AP induced a membrane depolarization of 3.6 ± 0.6 mV (n = 4), while TEA induced a smaller membrane depolarization of 1.2 ± 0.3 mV (n = 4). Hypercapnic acidosis (HA) inhibited both currents. The maximal amplitude of the TEA-sensitive current was reduced by 52.1 ± 4.5% (n = 5) in 15% CO2 [extracellular pH (pHo) 7.00, intracellular pH (pHi) 6.96]. The maximal amplitude of the 4-AP-sensitive current was reduced by 34.5 ± 3.0% (n = 6) in 15% CO2 (pHo 7.00, pHi 6.96), by 29.4 ± 6.8% (n = 6) in 10% CO2 (pHo 7.15, pHi 7.14), and increased by 29.0 ± 6.4% (n = 6) in 2.5% CO2 (pHo 7.75, pHi 7.35). 4-AP completely blocked hypercapnia-induced increased firing rate, but TEA did not affect it. When LC neurons were exposed to HA with either pHo or pHi constant, the 4-AP-sensitive current was inhibited. The data show that the 4-AP-sensitive current (likely an A current) is inhibited by decreases in either pHo or pHi. The change of the A current by various levels of CO2 is correlated with the change in firing rate induced by CO2, implicating the 4-AP-sensitive current in chemosensitive signaling in LC neurons.
Keywords: A current, carbon dioxide, central control of breathing, neuronal acid sensing, potassium channels
there are a number of neurons within various brain regions, called chemosensitive neurons, that respond to altered CO2 and/or pH. One such region that contains a large percentage of chemosensitive neurons is the locus coeruleus (LC). The LC is an important noradrenergic region in the pons and has been implicated to play a role in many pathological conditions, including panic disorder, Rett syndrome, and posttraumatic stress disorder (1, 55, 65, 66). In addition, chemosensitive LC neurons are believed to be a part of a distributed network of neurons that are involved in the control of breathing (18, 35). In addition to the LC (13, 15, 16, 20, 44), this network includes neurons from the ventral medullary surface (32, 54), the medullary raphé (3, 49, 50), the pre-Bötzinger complex (56), the retrotrapezoid nucleus (14, 19, 52), and the nucleus tractus solitarius (NTS) (8, 11). There has, thus, been great interest in the properties of chemosensitive neurons within the brain.
The cellular and molecular mechanisms underlying the response of these chemosensitive neurons are not well elucidated. Increased CO2 (hypercapnia) results in decreased outside pH (pHo) and intracellular pH (pHi). It is believed that decreased pH alters ion channel activity, resulting in decreased neuronal membrane potential (Vm) and increased neuronal firing rate (47). However, it is also clear that the pH response of a neuron does not define it as a chemosensitive neuron (4, 46). It is likely that the properties and types of channels on the membrane of a neuron determine whether it is chemosensitive or not (46).
A variety of channels can potentially serve as chemosensitive channels, including K+ channels (44), acid-sensing ion channels (22), transient receptor potential (TRP) channels (10), a Ca2+-activated nonselective ion channel (9), Cl− currents (44), and L-type Ca2+ channels (16, 23). We focus here on the K+ channel targets of pH changes, since these channels, if active at or near resting Vm, can regulate Vm and determine neuronal excitability (41). Several such K+ channel targets have been identified. Decreased pH has been shown to inhibit inwardly rectifying K+ (Kir) channels, Ca-activated K+ (KCa) channels, and TASK channels (2, 43, 63, 64). In LC neurons, Kir channels have been shown to respond to hypercapnic acidosis (HA) (45, 65). Filosa and Putnam (16) reported that HA inhibited a tetraethyl ammonium (TEA)-sensitive K+ channel and probably a TASK channel in LC neurons. No report has been made of a potential role for a 4-aminopyridine (4-AP)-sensitive transient A current in the chemosensitive response of LC neurons, even though these channels have been shown to be present in LC neurons (17).
In the current study, whole cell patch-clamp was used to study the role of outwardly rectifying K+ channels in the chemosensitive response to HA in LC neurons in brain stem slices from neonatal rats. Our main goals in this study were 1) to determine which outward currents are present in LC neurons from neonatal rat, to demonstrate which, if any, of these outward currents are involved in the firing rate response of LC neurons to hypercapnia; and 2) to see whether these currents are inhibited by decreases in either pHi or pHo. We found that a transient A current is involved in the firing rate response to hypercapnia of LC neurons from neonatal rats.
Preliminary reports of this work have previously been published (31, 48).
MATERIALS AND METHODS
Slice Preparation
All procedures involving animals were reviewed and approved by the Wright State University Institutional Animal Care and Use Committee and are in agreement with standards set out in the National Institutes of Health Guide for Care and Use of Laboratory Animals. Wright State University is accredited by Association for the Assessment and Accreditation of Laboratory Animal Care and is covered by National Institutes of Health assurance (no. A3632–01).
Pontine LC slices were prepared from neonatal Sprague-Dawley rats (postnatal; P, aged P3–P15), as previously described (15, 28, 51). Briefly, rats were anesthetized either by hypothermia (<P10) or by 100% CO2 (≥P10) and rapidly decapitated. The brain stem was subsequently removed and placed onto a vibratome (Pelco Vibratome 1000). Coronal brain stem slices (300 μm) were cut in ice-cold artificial cerebral spinal fluid (aCSF) and then incubated at room temperature in aCSF until used.
Solutions
aCSF contained (in mM): 3 KCl, 124 NaCl, 1.3 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, and 2.4 CaCl2 and was equilibrated with 5% CO2/95% O2 (pHo ∼ 7.45). Various hypercapnic acidotic (HA) solutions were identical in composition but were equilibrated with either 10% CO2/90% O2 (pHo ∼ 7.15) or 15% CO2/85% O2 (pHo ∼ 7.0). We also employed a solution that was hypercapnic but with a normal pHo, isohydric hypercapnia (IH). This solution was made by modifying aCSF by elevating external NaHCO3 to 77 mM (isosmotically substituting for an equal amount of NaCl) and equilibrating with 15% CO2/85% O2 so that pHo remained constant at 7.45 (15). A solution that was hypocapnic and alkalotic was also used and consisted of aCSF equilibrated with 2.5% CO2/97.5% O2 (pHo ∼ 7.75). Synaptic blockade medium (SNB) contained (in mM): 3 KCl, 121 NaCl, 11.4 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, and 0.2 CaCl2, plus carbenoxolone (CAR; 100 μM) to block gap junctions (8, 37). In many experiments, tetrodotoxin (TTX; 1 μM) and CoCl2 (2 mM) were added to aCSF to block sodium channels and voltage-gated calcium channels, respectively (16). Whole cell patch pipettes were filled with an internal solution designed to reduce washout of the chemosensitive response (16) that contained (in mM): 130 K-gluconate, 0.4 EGTA, 1 MgCl2, 0.3 Na2GTP, 2 Na2ATP, 10 HEPES; pHo 7.45. When pHi was to be measured, 200 μM pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid) was added to the pipette filling solution (51). K+ channel inhibitors tetraethyl ammonium chloride (TEA-Cl; 20 mM) or 4-AP (5 mM) were added to aCSF isosmotically replacing NaCl. All chemicals were from Fisher Scientific except for K-gluconate, carbenoxolone, Na2ATP, Na2GTP, EGTA, HEPES, TEA-Cl, and 4-AP (which were from Sigma-Aldrich), glucose (VWR Scientific), pyranine (Molecular Probes), TTX (Tocris Bioscience), and Na-acetate (EMD Scientific).
Recording Chamber
Slices were placed on the floor of a perfusion chamber and held with a nylon grid (16, 51). Brain slices were continuously superfused through stainless-steel tubes via a gravity-fed system at a rate of 3–5 ml/min. Cells were visualized with an upright microscope (Nikon Optiphot-2) using near-infrared illumination and a 40× water-immersion objective (Hoffmann modulated contrast, N.A. 0.55, 3.0-mm working distance). All experiments were done at a temperature of about 35°C (16), achieved by maintaining solution reservoirs in a water bath at around 40°C and adjusted with an in-line thermoelectric Peltier assembly (27).
Electrophysiological Recordings
Patch pipettes (3–6 MΩ) were pulled from thin-walled (1.5-mm outer diameter, 1.12-mm inner diameter) borosilicate glass capillaries (TW150–3; World Precision Instruments) on a vertical electrode puller (Narishige PP-830). Electrical signals from individual neuronal soma were obtained in whole cell patch clamp configuration with an Axopatch 200B amplifier, a Digidata 1440A A/D converter and pCLAMP 10.2 software (all from Molecular Devices). An Ag-AgCl electrode connected to the bath solution via a KCl-agar bridge served as the reference electrode. Data were filtered at 2 kHz, sampled at 10 kHz, and saved on a computer to be analyzed offline. Data were discarded if the series resistance (10.1 ± 0.4 MΩ) changed >20% over the duration of the recording.
Current-clamp recordings.
Membrane potential (Vm) and firing frequency were measured from whole cell patched LC neuronal soma in current clamp mode. A recording was used if the neuron exhibited a stable Vm and a steady firing frequency in the presence of SNB and CAR. Occasionally, a small depolarizing DC current was injected into the cell to maintain an appropriate firing frequency, but this was not necessary in most neurons. We employed episode mode to inject 30-pA hyperpolarizing current for 300 ms every 50 s to calculate the input resistance (Rin) of the neuron. Rin was calculated from Ohm's law by dividing the hyperpolarizing change in Vm by the injected hyperpolarizing current (30 pA). To record Vm in the absence of action potentials, we added TTX (1 μM) and CoCl2 (2 mM) to the aCSF to block fast sodium channels and voltage-gated calcium channels, respectively (16). Membrane potential was corrected for the liquid junction potential of 16-mV (liquid junction potentials were calculated from the Henderson equation using Clampex).
Voltage-clamp recordings.
To determine the presence of specific K+ channels, we studied individual LC neurons within the slice using voltage clamp. In the presence of TTX (1 μM) and CoCl2 (2 mM), the whole cell outwardly rectifying current was elicited by command pulses from −116 to +34 mV with 10-mV increments (300 ms) from an initial holding potential (Vh) of −96 mV. The records were not compensated for series resistance because even attempting modest compensation often resulted in electrode ringing and the loss of the patch. Membrane potential was corrected for the liquid junction potential of 16–18 mV. The membrane steady-state leak current was subtracted with Clampfit software.
We recognize the inherent issues of space clamping in intact neurons within slices. As others have done, we chose to employ slices to obtain at least a qualitative description of the ionic currents (5, 25, 60). We opted not to use isolated or cultured neurons to avoid the potential that they could have altered properties compared with in vivo neurons and to maintain as much of the neuropil intact as possible.
Various techniques were used to divide out individual currents from the whole cell outwardly rectifying current. A TEA-sensitive portion of this whole cell current was isolated by subtracting current in the presence of TEA (20 mM) from that in the absence of TEA. The plateau amplitude at the end of the TEA-sensitive current was measured and was plotted as a function of the conditioning step potentials (I-V plot). Similarly, a 4-AP-sensitive portion of the current was isolated by subtracting current in the presence of 4-AP (5 mM) from that in the absence of 4-AP. Maximum 4-AP-sensitive current was measured as the peak current. A second protocol was used to study the 4-AP-sensitive current, based on the protocol of Sonner et al. (57). Initially, a neuron, in the presence of 1 mM TTX, 2 mM CoCl2, and 20 mM TEA, was held at a holding Vm of −98 mV (to eliminate any K+ channel inactivation), and then currents were measured in response to depolarizing command pulses (from −98 to +42 mV, 10-mV increments, 300 ms). A second set of currents was collected, which included an initial depolarized (−40 mV) conditioning pulse (200 ms), whose purpose was to inactivate any transient K+ currents, before the depolarizing command pulses (from −98 to +42 mV, 10-mV increments, 300 ms). These two sets of currents were digitally subtracted offline using Clampfit 10.2 (Axon Instruments), and the maximum current was plotted as a function of the conditioning step pulse.
Measurement of Intracellular pH
Intracellular pH (pHi) was measured as described previously (20, 51). All pHi measurements were recorded from the cell soma. Briefly, the pH-sensitive dye pyranine (100 μM) was loaded into individual LC neurons with whole cell pipettes. Pyranine-loaded LC neurons were excited (with light from a xenon arc lamp) alternately at 450 ± 10 nm (pH-sensitive excitation wavelength) and 415 ± 10 nm (pH-insensitive wavelength) using a Sutter Lambda 10–2 filter wheel. Image acquisition was achieved within 2 s and was repeated at 60-s intervals. There was no excitation light between acquisitions to minimize photobleaching. Emitted fluorescence at 515 ± 10 nm (all filters were obtained from Omega Optical) was directed to the Nikon multi-image port module and was then directed to a GenIISys image intensifier and a CCD 100 camera (both obtained from Dage-MTI). The subsequent fluorescence images were acquired using a Gateway 2000 E-3100 computer and were collected and processed using Metafluor 4.6r5 software (Universal Imaging), and the 450/415 fluorescence ratios (Rfl) were determined. To normalize Rfl values, we calibrated pyranine using the high-K+/nigericin technique (59) to obtain Rfl values at pH 7.4. Rfl values were normalized to the Rfl value at pH 7.4, yielding normalized Rfl (Nfl). Nfl values were converted to pHi values using the following equation (51): pHi = 7.5561 + log[(Nfl − 0.1459)/(2.0798 − Nfl)].
Intracellular pH was clamped using the technique of Hartzler et al. (20). Briefly, a patched neuron was loaded with a weak acid (acetic acid) by adding K-acetate (50 mM, isosmotically replacing K-gluconate) to the pipette-filling solution. A stable pHi of typical value was obtained by superfusing the neuron with aCSF containing 45 mM Na-acetate (isosmotically replacing NaCl). When the neuron was exposed to hypercapnic acidotic solution (CO2 15%, pHo 7.0), pHi acidified normally when the solution contained 45 mM Na-acetate, but pHi did not change when the aCSF contained only 15 mM Na-acetate due to a counterbalancing efflux of H+ in the form of acetic acid (20).
Data Analysis and Statistical Treatment
The spontaneous firing rates over a 1-min period before and during exposure to acidic or alkalotic stimuli were calculated to get the mean value for every neuron. Three successive input resistances in control and during exposure to acidic or alkalotic stimuli were averaged for every neuron. All values are expressed as means ± SE. Changes in Vm, Rin, or firing rate were tested for being different from zero using a t-test. All comparisons of two means were done using either a two-tailed unpaired or paired Student's t-test, and comparisons of three or more means were done using a one-way ANOVA with multiple paired comparisons done using Tukey's method. The effects of TEA, 4-AP, and the two together on Vm were tested for differences using a one-way repeated-measures ANOVA with Tukey's studentized range for paired comparisons. Differences were assumed to be statistically significant at a level of P ≤ 0.05.
RESULTS
Firing Rate Response of LC Neurons to Hypercapnia or Hypocapnia
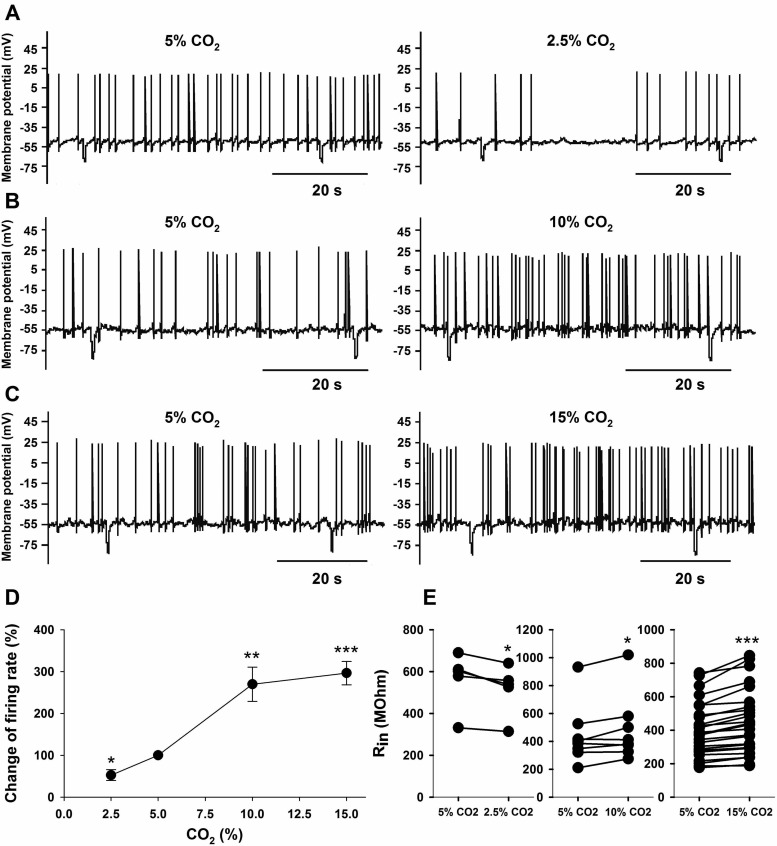
The vast majority (>80%) of LC neurons that we patched responded to hypercapnia with an increased firing rate, as previously observed (15, 40, 45). We studied only LC neurons that showed an intrinsic, reversible increase in firing rate in response to HA. All neurons were tested for their intrinsic firing rate response to various levels of CO2 in aCSF that had elevated Mg2+ and reduced Ca2+ for blockade of chemical synapses and contained 100 μM CAR to block gap junctions (e.g., Ref. 8). As shown in Fig. 1, the firing rate response of LC neurons to CO2 was dependent on the level of CO2. Compared with firing in normocapnic solutions (5% CO2), hypocapnic solutions (2.5% CO2) resulted in decreased firing (from 1.0 ± 0.3 to 0.6 ± 0.2 Hz; n = 6, P = 0.04) (Fig. 1A), while firing rate was increased in 10% CO2 (from 1.1 ± 0.4 to 2.6 ± 0.6 Hz; n = 9, P = 0.002) (Fig. 1B) and in 15% CO2 (from 0.5 ± 0.1 to 1.2 ± 0.2 Hz; n = 33, P < 0.001) (Fig. 1C). Note that the % firing rate increase had nearly reached a plateau by 10% CO2, not significantly increasing further at 15% CO2 (Fig. 1D). Changes of CO2 not only affected firing rate but they significantly altered the Rin of LC neurons as well. The resting Rin of LC neurons under normocapnic (5% CO2) conditions was 446.6 ± 33.2 MΩ (n = 40). Hypocapnia (2.5% CO2) significantly (n = 5; P = 0.01) reduced Rin by 8.1 ± 1.8% (Fig. 1E), while hypercapnia significantly increased Rin by 10.2 ± 4.2% (n = 8; P = 0.04) and by 10.7 ± 1.2% (n = 27; P = 0.001) at 10 and 15% CO2, respectively (Fig. 1E).
Fig. 1.
The effects of hypocapnia and hypercapnia on the spontaneous firing rate and input resistance in locus coeruleus (LC) neurons. A: traces of membrane potential (Vm) vs. time showing action potential frequency. Compared with control (5% CO2), firing rate went down in 2.5% CO2. B: compared with control, firing rate increased in 10% CO2. C: compared with control, firing rate increased in 15% CO2. D: relationship between the CO2 and the change in firing rate induced by different levels of CO2 in LC neurons. The response is presented as the percentage of change of firing rate in altered CO2 vs. control (5% CO2). Symbols represent the mean changes in firing rate ± SE. Note the large effect of CO2 on firing rate between 2.5 and 10% but the plateau of firing rate above 10% CO2. The % change in firing rates at various levels of CO2 were significant at *P < 0.05, **P < 0.01, or ***P < 0.001. E: effect of CO2 on input resistance (Rin). The response is plotted as paired data for Rin at 5% CO2 vs. 2.5%, 10%, or 15% CO2. Each pair shows the response of Rin for a given neuron in response to the indicated change of CO2. Rin is significantly different from the values at 5% CO2 at either *P < 0.05 or ***P < 0.001 based on paired t-tests.
Effects of TEA and 4-AP on Membrane Potential, Input Resistance, and Firing Rate
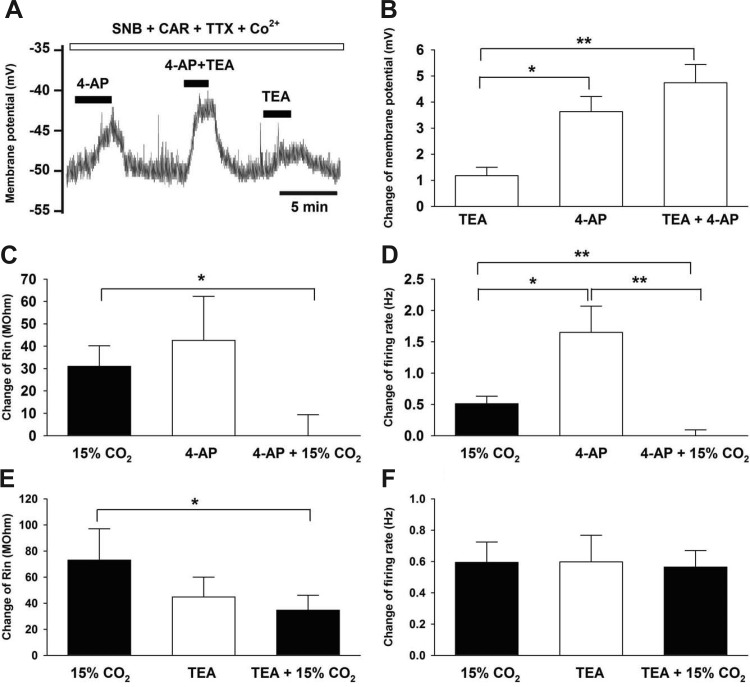
To determine whether voltage-gated K+ channels are active at resting membrane potentials in LC neurons from neonates, we studied the effects of the Kv channel blockers TEA (20 mM) and 4-AP (5 mM) on resting Vm (control Vm was −53.5 ± 0.7 mV; n = 58). In the presence of SNB and CAR, we first showed that an LC neuron was chemosensitive (increased firing rate in response to 15% CO2) (data not shown). TTX and Co2+ were then added to eliminate fast Na+ channels and voltage-gated Ca2+ channels, respectively, which yielded a Vm trace free of action potentials (Fig. 2A). Under these conditions, exposure to 4-AP induced a large and significant (P = 0.0001) depolarization (∼3.6 mV), TEA induced a smaller but significant (P = 0.026) depolarization (∼1.2 mV), and exposure to both 4-AP and TEA induced a depolarization (P < 0.0001) that was equal to the sum of the effects of the two drugs (∼4.8 mV) (Fig. 2, A and B). 4-AP and the combination of 4-AP+TEA caused a significantly larger depolarization than TEA alone (Fig. 2B). The depolarization induced by 4-AP was not significantly different from that induced by 4-AP+TEA (P = 0.17, based on one-way repeated-measures ANOVA with Tukey's studentized range for paired comparisons), but these values differed on the basis of a two-tailed paired Student's t-test (P < 0.05). These results suggest that both 4-AP-sensitive and TEA-sensitive K+ channels are active at resting Vm in chemosensitive LC neurons.
Fig. 2.
The effects of TEA, 4-AP, and CO2 on the membrane potential, input resistance, and firing rate in LC neurons. A: LC neuron in the presence of synaptic blockade medium (SNB), carbenoxolone (CAR), tetrodotoxin (TTX), and Co2+, which shows no action potentials. TEA induced a small, and 4-AP induced a larger, reversible depolarization. The combination of TEA and 4-AP resulted in the largest reversible depolarization, which was the sum of the effects of each drug alone. B: summary of the effects of TEA and 4-AP on Vm. The depolarization induced by 4-AP alone and in combination with TEA was significantly larger than the depolarization induced by TEA alone. The average effects of TEA, 4-AP, and the two together on Vm are shown for 4 LC neurons (all in the presence of 5% CO2). C: effect of 15% CO2, 4-AP, and the combination of the two on the change of Rin. Rin was significantly increased by increasing CO2 from 5% to 15% and was also significantly increased by the addition of 4-AP (in the presence of 5% CO2). However, in the maintained presence of 4-AP, raising CO2 from 5% to 15% resulted in no additional increase of Rin. Thus, the increase in Rin induced by increasing CO2 from 5% to 15% in the presence of 4-AP was significantly smaller than when CO2 was increased in the absence of 4-AP. The average effects for 8 LC neurons is shown. D: effect of 15% CO2, 4-AP, and the combination of the two on the change of firing rate. The firing rate of LC neurons was significantly increased by changing CO2 from 5% to 15% and was also significantly increased by the addition of 4-AP (in the presence of 5% CO2). The addition of 4-AP alone caused a significantly greater increase in firing rate than increasing CO2 to 15%. However, in the maintained presence of 4-AP, increasing CO2 from 5% to 15% resulted in no additional increase in firing rate. Thus, the increase in firing rate induced by increasing CO2 from 5% to 15% in the presence of 4-AP was significantly smaller than when CO2 was increased in the absence of 4-AP. The average effects for 8 LC neurons is shown. E: effect of 15% CO2, TEA, and the combination of the two on the change of Rin. Rin was significantly increased by increasing CO2 from 5% to 15% and was also significantly increased by the addition of TEA (in the presence of 5% CO2). In the maintained presence of TEA, increasing CO2 from 5% to 15% resulted in a significant increase of Rin. The increase in Rin induced by increasing CO2 from 5% to 15% in the presence of TEA was significantly smaller than when CO2 was increased in the absence of TEA. The average effects for five LC neurons are shown. F: effect of 15% CO2, TEA, and the combination of the two on the change of firing rate. The firing rate was significantly increased by increasing CO2 from 5% to 15% and was also significantly increased by the addition of TEA (in the presence of 5% CO2). In the maintained presence of TEA, with the firing rate adjusted back to its initial value by injecting negative current, increasing CO2 from 5% to 15% resulted in a significant increase of firing rate. Both increasing CO2 from 5% to 15% and the addition of TEA (in 5% CO2) resulted in a significant and nearly identical increase in firing rate. Further, in the maintained presence of TEA, increasing CO2 from 5% to 15% caused a nearly identical increase in firing rate to that caused by raising CO2 in the absence of TEA. The average effects for five LC neurons is shown. In all figures, bars show means ± SE. Differences were significant at either *P < 0.05 or at **P < 0.01.
Because hypercapnia can induce an increase in Rin and firing rate in LC neurons (Fig. 1), we next examined the effects of 4-AP and TEA on these parameters. The initial Rin for all neurons in 5% CO2 was 393 ± 44.8 MΩ. Exposure of LC neurons that were in 5% CO2 to 15% CO2 caused a significant (8.8%) increase in Rin that was similar to the significant increase of Rin of 12.2% caused by exposing LC neurons to 4-AP (in 5% CO2) (Fig. 2C). However, in the maintained presence of 4-AP, exposure of LC neurons that were in 5% CO2 to 15% CO2 caused no additional increase in Rin (Fig. 2C). Thus, increasing CO2 from 5% to 15% caused a significantly larger increase in Rin in the absence of 4-AP than in the presence of 4-AP. In parallel with these findings, LC neuron firing rate was increased significantly by increasing CO2 from 5% to 15% or by the addition of 4-AP in 5% CO2, with the increase induced by 4-AP being significantly larger than the increase induced by hypercapnia (Fig. 2D). In the maintained presence of 4-AP at 5% CO2, we injected negative current to return firing rate to its initial value. When these neurons, in the presence of 4-AP and 5% CO2, were exposed to 15% CO2, firing rate showed no increase (Fig. 2D). Thus, the firing rate increase normally seen upon raising CO2 from 5% to 15% CO2 was completely inhibited in the presence of 4-AP (Fig. 2D). These findings show that 4-AP can recapitulate the effects of 15% CO2 on Rin and firing rate and that the effects of 4-AP and 15% CO2 on firing rate appear to be mediated by a similar process since the firing rate response to CO2 is eliminated in the presence of 4-AP.
In a different set of LC neurons, exposure of neurons in 5% CO2 to either TEA (also in 5% CO2) or to a hypercapnic solution (15% CO2) resulted in similar increases in Rin (10.9 and 16.9%, respectively) (Fig. 2E). In the maintained presence of TEA (in 5% CO2), exposure to 15% CO2 resulted in a significant change in Rin, but this change was significantly less than in the absence of TEA (Fig. 2E). Exposure of LC neurons in 5% CO2 to either TEA (still in 5% CO2) or to 15% CO2 resulted in a similar increase in firing rate (Fig. 2F). In the maintained presence of TEA (in 5% CO2), we injected negative current to bring firing rate back down to a value similar to firing rate before exposure to TEA. When we then exposed these neurons to solutions of 15% CO2 (still containing TEA), we observed a similar increase in firing rate to that caused by 15% CO2 in the absence of TEA (Fig. 2F). Thus, the firing rate increase seen upon raising CO2 from 5% to 15% CO2 was the same in the presence and in the absence of TEA (Fig. 2F). These results show that TEA can increase firing rate to the same extent as 15% CO2 but that TEA-inhibited channels do not appear to be significantly involved in the firing rate response of LC neurons to hypercapnia.
Effect of Ba2+ on Firing Rate and Input Resistance
It has been suggested that Kir channels play a role in the CO2 response of LC neurons (45, 65). To test whether Kir channels are also involved in the response of our LC neurons, we studied the response of firing rate and Rin to hypercapnia in the presence of 200 μm Ba2+, which has been shown to inhibit Kir channels (45, 65). Firing rate increased in response to 15% CO2 by 0.54 ± 0.12 Hz (from 0.35 ± 0.07 to 0.89 ± 0.11 Hz; n = 7). Exposure to Ba2+ also increased firing rate in 5% CO2 by 0.65 ± 0.10 Hz (from 0.36 ± 0.09 Hz to 1.01 ± 0.12 Hz; n = 7), indicating that Kir channels are present at rest in LC neurons. We injected negative current to bring control firing rate down to 0.50 ± 0.08 Hz. In the presence of Ba2+, 15% CO2 once again increased firing rate by 0.67 ± 0.17 Hz (to 1.17 ± 0.18 Hz; n = 7). The hypercapnia-induced increased firing rate was not significantly different (P = 0.52) in the presence vs. the absence of Ba2+.
Similarly, Ba2+ had no effect on the hypercapnia-induced increase in Rin. Hypercapnia (15% CO2) increased Rin by 43.5 ± 14.7 MΩ (from 345.8 ± 70.8 to 389.2 ± 85.2 MΩ; n = 7) in the absence of Ba2+ and by 43.4 ± 10.7 MΩ (from 399.4 ± 84.4 to 442.7 ± 90.1 MΩ; n = 7) in the presence of Ba2+. These data show that while LC neurons from neonatal rats contain Kir channels, these channels do not appear to play a role in the response of our LC neurons to hypercapnia.
Characterization of and the Effect of Hypercapnia on the TEA-Sensitive K+ Current Using Voltage Clamp
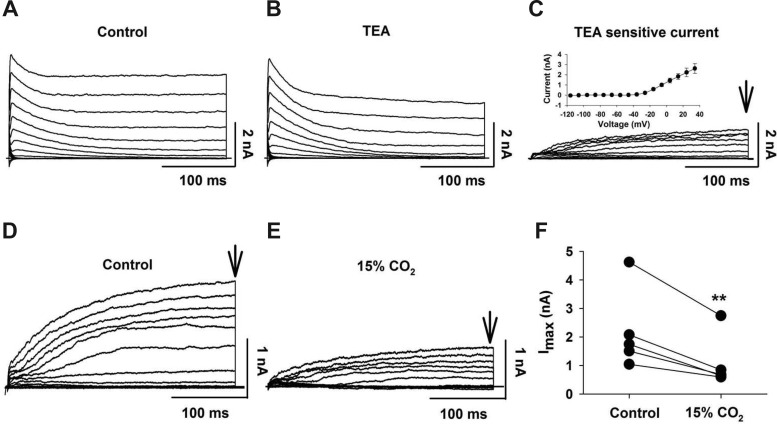
We used whole cell patch clamp of individual LC neurons within a slice to characterize TEA-sensitive K+ current. In the presence of TTX (1 μM) and Co2+ (2 mM), currents were elicited by command pulses in 10-mV increments (300 ms) from a holding potential of −96 mV. The resulting currents had a transient peak component and a sustained component (Fig. 3A). TEA (20 mM) partially inhibited the sustained current but did not affect the transient peak current (Fig. 3B). The difference current, obtained by subtracting the currents in the presence of TEA from control currents in the absence of TEA, is the TEA-sensitive current (Fig. 3C). This current is slowly activating without apparent inactivation, similar to a delayed-rectifying K+ current. The I-V plot for this current is consistent with an outwardly rectifying K+ current (Fig. 3C, inset).
Fig. 3.
TEA-sensitive outwardly rectifying K+ current in LC neurons. A: representative family of outwardly rectifying K+ currents elicited by command pulses (300-ms duration) from −116 to +34 mV with 10-mV increments; Vh = −96 mV. B: representative family of outwardly rectifying K+ currents elicited by the same command pulses in the presence of TEA (20 mM). C: representative family of TEA-sensitive outwardly rectifying K+ currents was obtained by subtracting the currents in B from those in A. Arrow shows time at which the current amplitude was quantified. Inset: activation current-voltage (I-V) plot for TEA-sensitive outwardly rectifying K+ current (n = 4). Symbols represent means ± SE. D: representative family of TEA-sensitive outwardly rectifying K+ currents. E: family of TEA-sensitive outwardly rectifying K+ currents from the same neuron as in D but in the presence of 15% CO2. Note the markedly smaller currents in the hypercapnic solution. F: LC neurons showing paired currents for each neuron in control (5% CO2) and in 15% CO2. Currents were quantified at the points indicated by the vertical arrows in D and F. Note that 15% CO2 significantly (**P < 0.01) reduced the TEA-sensitive outwardly rectifying K+ current.
We studied the effect of HA (15% CO2, pHo 7.0) on the TEA-sensitive K+ current. This TEA-sensitive current was significantly (P = 0.010) inhibited by 15% CO2 (Fig. 3, D and E), decreasing the maximal current by 52.1 ± 4.5% (Fig. 3F). These results show that the TEA-sensitive outwardly rectifying current can be inhibited by hypercapnia, but because our previous results suggest that inhibition of this current does not play a role in the chemosensitive response of LC neurons (Fig. 2F), we did not study this current further at more physiological values of CO2.
Characterization of and the Effect of Hypercapnia on the 4-AP-Sensitive K+ Current Using Voltage Clamp
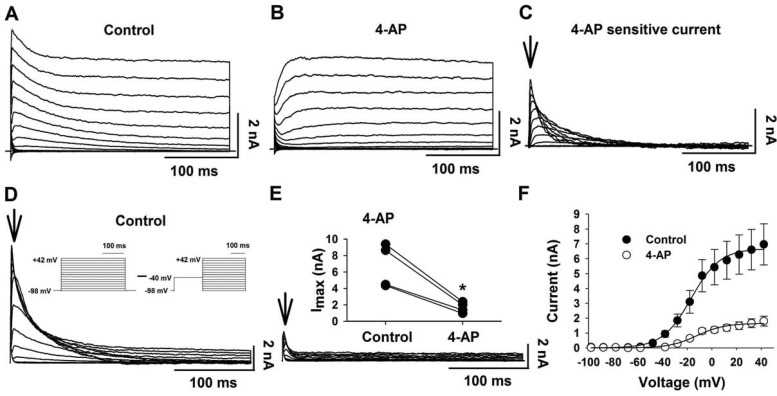
For the rest of our study, we focused on the transient K+ current due to the large effect of 4-AP on Vm (Fig. 2B) and the ability of 4-AP to block the hypercapnia-induced increased firing rate (Fig. 2D). We did experiments similar to the TEA experiments but using 4-AP (5 mM). The control current had a transient and sustained component (Fig. 4A). 4-AP resulted in the loss of the transient component but not the sustained current (Fig. 4B). The difference current (current in the absence minus current in the presence of 4-AP) shows the 4-AP-sensitive K+ current (Fig. 4C), which has a rapid activation and a rapid inactivation, like an A current (IA).
Fig. 4.
4-AP-sensitive outwardly rectifying K+ current in LC neurons. A: representative family of outwardly rectifying K+ currents elicited by command pulses (300-ms duration) from −116 to +34 mV with 10-mV increments; Vh = −96 mV. B: representative family of outwardly rectifying K+ currents elicited by the same command pulses in the presence of 4-AP (5 mM). C: representative family of 4-AP-sensitive outwardly rectifying K+ currents were obtained by subtracting the currents in B from those in A. Arrow shows time at which the current amplitude was quantified. D: representative family of A currents elicited by a different voltage-clamp protocol (inset) designed to identify the activation characteristics of the A current. TTX, Co2+, and TEA were added to the perfusate to eliminate Na+, Ca2+, and TEA-sensitive K+ currents. E: A currents in the presence of 4-AP (5 mM). Arrows show the times at which the current amplitudes were quantified. Inset: paired plot of A current amplitude in the absence (control) and the presence (4-AP) of 4-AP for 4 LC neurons. Note that 4-AP significantly inhibited Imax of the transient A current (*P < 0.05). F: activation I-V relationship for 4-AP-sensitive outwardly rectifying K+ current (n = 4) in the absence (●) and the presence (○) of 4-AP. The I–V plots were fit by Boltzmann curves. Symbols represent means ± SE.
We used a different electrophysiological protocol combined with pharmacological methods (TTX, Co2+, and TEA) to study the transient K+ current (Fig. 4D). In this case, activation was measured as the difference in the currents using the standard protocol and the currents using a conditioning pulse of −40 mV for 200 ms before repeating the activation protocol (Fig. 4D, inset) (54). The difference current showed a rapidly activating and inactivating current (Fig. 4D), similar to the 4-AP-sensitive current shown previously (Fig. 4C). The maximal amplitude of the difference current was significantly (P = 0.02) reduced (Fig. 4E) by 74.7 ± 1.9% by 4-AP (Fig. 4E, inset). The I-V plot for this curve is consistent with an outward current and was fit by a Boltzmann curve (Fig. 4F). The I-V plot showed that the A current is activated at around −50 mV, has a maximal current of 6.7 ± 1.3 nA with a voltage for half-activation of −10.4 ± 0.9 mV (n = 7).
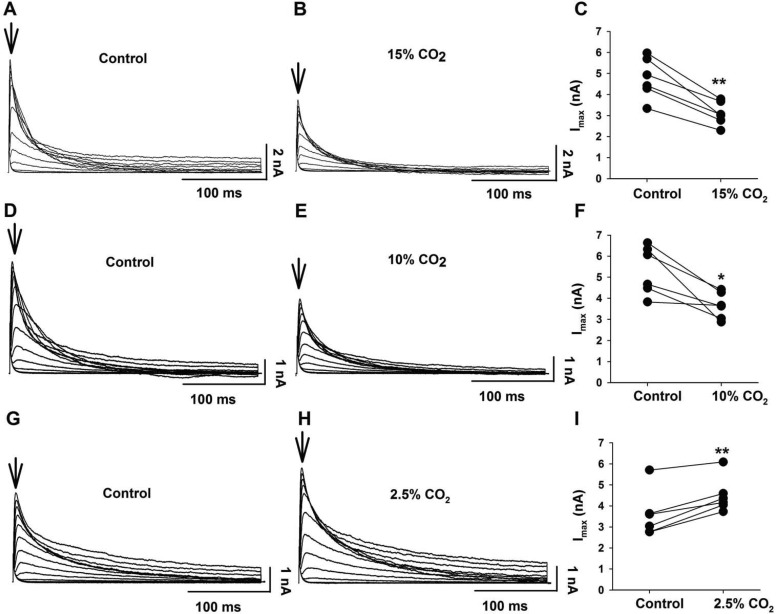
We studied the effect of HA (15% CO2, pHo 7.0) on the 4-AP-sensitive K+ current. The maximum transient K+ current was significantly (P = 0.01) inhibited by hypercapnia (Fig. 5, A and B) by 34.5 ± 3.0% (n = 5) (Fig. 5C). However, a stimulus of 15% CO2 is a very large hypercapnic stimulus. We repeated the measurements of inhibition of transient K+ currents using a milder HA (10% CO2, pHo 7.15). This milder HA also significantly (P = 0.01) decreased the maximal amplitude of the transient K+ current (Fig. 5, D and E) by 29.4 ± 6.8% (n = 6) (Fig. 5F). We also determined the effect of hypocapnic alkalotic solutions (2.5% CO2, pHo 7.75) on the transient K+ current. Hypocapnia significantly (P = 0.002) increased the maximal amplitude of this current (Fig. 5, G and H) by 29.0 ± 6.4% (Fig. 5I). Taken together, these results suggest that the transient K+ current can be modulated by different levels of CO2.
Fig. 5.
The effect of 15%, 10%, and 2.5% CO2 on the transient A current in LC neurons. A and B: representative family of transient A currents in control (5% CO2) (A) and hypercapnia (15% CO2) (B) aCSF. 15% CO2 decreased the transient A current. Arrows show times at which the current amplitudes were determined. C: paired plot of A current amplitude in control (5% CO2) and in 15% CO2 for 6 LC neurons. Note that 15% CO2 significantly inhibited Imax of the transient A current (**P < 0.001). D and E: representative family of transient A currents in control (5% CO2) (D) and hypercapnia (10% CO2) (E) aCSF. 10% CO2 decreased the transient A current. Arrows show times at which the current amplitudes were determined. F: paired plot of A current amplitude in control (5% CO2) and in 10% CO2 for 6 LC neurons. Note that 10% CO2 significantly inhibited Imax of the transient A current (*P < 0.05). G and H: representative family of transient A currents in control (5% CO2) (G) and hypocapnia (2.5% CO2) (H) aCSF. 2.5% CO2 increased the transient A current. Arrows show times at which the current amplitudes were determined. I: paired plot of A current amplitude in control (5% CO2) and in 2.5% CO2 for 6 LC neurons. Note that 2.5% CO2 significantly increased Imax of the transient A current (**P < 0.001).
Effect of Changes of CO2 on pHi and Maximum Transient K+ Current
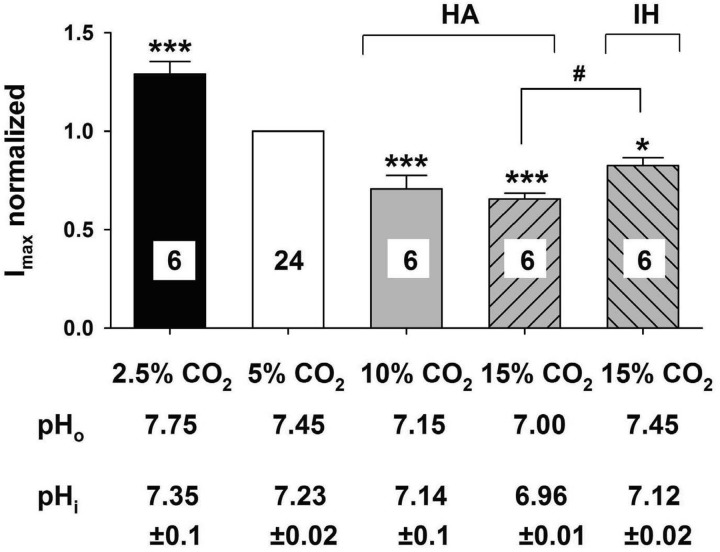
We next measured pHi in LC neurons in response to the various hypercapnic and hypocapnic challenges that we used. pHi varied from 6.96 in the presence of 15% CO2 (HA) to 7.35 in the presence of 2.5% CO2 (Fig. 6). The normalized maximum transient K+ current varied sharply from 2.5% to 10% CO2 but appeared to reach a minimum plateau above 10% CO2 (HA) (Fig. 6). This pattern of the effects of CO2 on transient K+ current is similar to the effects of CO2 on firing rate (Fig. 1). This relationship suggests that the inhibition of the transient K+ current by hypercapnia plays a role in the increased firing rate induced by hypercapnia in LC neurons, which supports the findings of the effect of 4-AP on the hypercapnia-induced increased firing rate (Fig. 2D).
Fig. 6.
Summary of the effects of hypercapnia and hypocapnia on the transient A current in LC neurons. The transient A currents in various levels of CO2 were expressed as a function of the maximum current at 5% CO2. Each bar represents the mean ± SE for the maximum transient A current in 2.5% (black bar), 5% (white bar), 10% (gray bar), 15% HA (upward hatched bar) CO2 and 15% isohydric hypercapnia (IH) solution (downward hatched bar). The number of neurons is given in each bar. For each level of CO2, the maximum transient A current was significantly different than in 5% CO2 (***P < 0.001) and 15% CO2-IH was also significantly different than in 5% CO2 (*P < 0.05). Further, the maximum transient A current was significantly smaller in 15% CO2-HA vs. 15% CO2-IH (#P < 0.05). Below each bar is the value of pHo measured for aCSF equilibrated with the corresponding value of CO2 and of the mean ± SE of the pHi measured using pyranine fluorescence in LC neurons exposed to aCSF equilibrated with the corresponding level of CO2.
Inhibition of Transient K+ Currents by pHi or pHo?
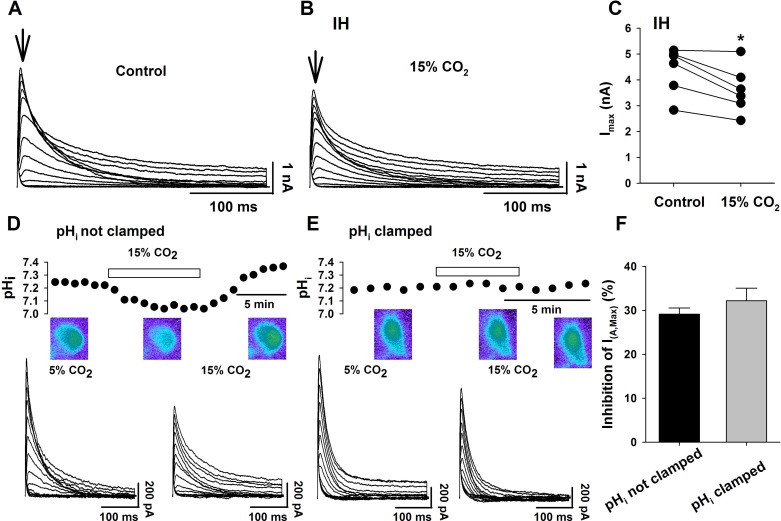
The decrease in the maximal transient K+ current in response to HA is accompanied by decreases in both pHo and pHi (Fig. 6). To initially determine whether the transient K+ current is inhibited by changes of pHo or by changes of pHi, we employed IH solutions, whereby a neuron is exposed to hypercapnia (15% CO2) but with no change of pHo. We directly measured the effect of IH solutions on LC neuron pHi and found that pHi acidified from a value of about 7.23 in 5% CO2 to 7.12 in IH (Fig. 6). This acidification is smaller than the intracellular acidification seen in HA solutions equilibrated with 15% CO2 (Fig. 6), as expected (15), and in fact is nearly identical to the fall in pHi seen with HA solutions equilibrated with only 10% CO2 (Fig. 6). IH solutions significantly (P = 0.01) reduced the transient K+ current by 17.4 ± 3.9% (Fig. 7, A–C). Interestingly, the reduction of the transient K+ current caused by IH solutions was significantly less than the reduction caused by HA solutions, even though both solutions were equilibrated with 15% CO2 (Fig. 6). This smaller inhibition of transient K+ current by IH vs. HA solutions is correlated with the smaller pHi change induced by IH vs. HA solutions (Fig. 6). However, both the degree of inhibition of the transient K+ current and the fall of pHi are similar in LC neurons exposed to IH solutions (equilibrated with 15% CO2) vs. those exposed to HA solutions (equilibrated with 10% CO2) (Fig. 6). These data clearly indicate that a fall of pHi is sufficient to inhibit the transient K+ current in LC neurons even without a change of pHo.
Fig. 7.
The effects of 15% CO2 on the transient A current with either pHo or pHi held constant. A and B: representative family of transient A currents in control (5% CO2) (A) and isohydric hypercapnia (IH) (15% CO2) (B) aCSF. IH solution decreased the transient A current. Arrows show times at which the current amplitudes were determined. C: paired plot of A current amplitude in control (5% CO2) and in IH (15% CO2) solutions for six LC neurons. Note that 15% CO2 IH solutions significantly inhibited Imax of the transient A current (*P < 0.05) despite the fact that pHo did not change. D: trace of the effect of HA (15% CO2) on the pHi of an LC neuron. pHi was measured by fluorescence imaging microscopy of the pH-sensitive dye pyranine, injected into the neuron from the patch pipette. Note the reversible hypercapnia-induced acidification of the neuron when pHi was not clamped. The color images show a pyranine-loaded LC neuron soma. A shift from green to blue fluorescence indicates acidification. Bottom: associated transient A current traces in control (5% CO2; left traces) and in 15% CO2 (right traces). Note that when hypercapnia was allowed to acidify the neuron, the transient A current was decreased. E: trace of the effect of HA (15% CO2) on the pHi of an LC neuron when pHi was clamped. Note the lack of an effect of hypercapnia on pHi. The color images show a pyranine-loaded LC neuron soma whose pHi was clamped during hypercapnic exposure. Bottom: associated transient A current traces in control (5% CO2; left traces) and in 15% CO2 (right traces). Note that when the LC neuron was exposed to hypercapnia while its pHi was maintained constant (i.e., clamped), the transient A current was still decreased by hypercapnia. F: inhibition of the maximum transient A current upon exposure to hypercapnic aCSF (15% CO2) with pHi clamped (gray bar) or not clamped (black bar). The bars represent the mean % inhibition of the maximum transient A current ± SE; n = 3. Note that hypercapnia inhibited the maximum transient A current to the same extent regardless of whether pHi was allowed to acidify or not.
We tested the opposite possibility, that a change of pHo, in the absence of any change of pHi, could also inhibit the transient K+ current in LC neurons. We used a technique that we previously developed (20) to clamp pHi at a constant value in the face of changes of CO2 and pHo. When pHi was not clamped, HA resulted in a reversible fall of pHi (from 7.24 ± 0.008 to 7.02 ± 0.014; n = 3; P = 0.007) (Fig. 7D, top). Under these conditions, both pHi and pHo were acidified during hypercapnia and the magnitude of the transient K+ current was decreased (Fig. 7D, bottom). When pHi was clamped, hypercapnia did not result in any change of pHi (from 7.21 ± 0.003 to 7.21 ± 0.009; n = 3, P = 0.784) (Fig. 7E, top). Under these conditions, hypercapnia resulted in a fall of pHo, but no change of pHi, and yet the magnitude of the transient K+ current still was decreased by hypercapnia (Fig. 7E, bottom). The degree of inhibition of the maximum value of the transient K+ current was about 30% whether pHi was clamped or not (Fig. 7F). These data clearly indicate that the transient K+ current can also be inhibited by a decrease of pHo, even in the absence of a decrease in pHi. These findings, combined with the IH data above, show that the transient K+ current in LC neurons can be inhibited by either intracellular or extracellular acidosis.
DISCUSSION
The main conclusions of this study are that 1) the firing rate response of chemosensitive LC neurons to altered CO2 from neonatal rats is very similar to the response seen in LC neurons from adult rats; 2) there are at least two outwardly rectifying currents in LC neurons from neonatal rats: a 4-AP-sensitive transient current (the A current) and a sustained TEA-sensitive delayed-rectifying K+ current; 3) both the 4-AP and the TEA-sensitive currents are active at resting Vm and both outwardly rectifying currents are inhibited by hypercapnic acidosis in LC neurons; 4) altered activity of the A current in response to altered levels of CO2 is largely responsible for the CO2-induced changes of firing rate in LC neurons; and 5) hypercapnic acidosis inhibits the A current by either decreased pHo or decreased pHi in LC neurons (Fig. 7).
LC Firing Rate Response to Changes of CO2 in LC Neurons
The pattern of our firing rate response to altered levels of CO2 (Fig. 1D) is remarkably similar in our LC neurons from neonatal rats compared with the pattern previously shown in LC neurons from adult rats (Fig. 1C in Ref. 45). The data are reported as % change in firing rate induced by various levels of CO2 vs. the initial firing rate in 5% CO2, which was the same in both studies (0.7 Hz in 45 vs. 0.5–1.1 Hz in the present study). However, the maximal firing rate in response to hypercapnia was smaller (∼150%) in LC neurons from adults vs. the response in LC neurons from neonates (∼300%). This finding is consistent with previous reports of a smaller chemosensitive response in LC neurons from older vs. younger rats (18, 36). The similarity in the pattern of response in neurons from neonates vs. adults suggests that the general pattern of the chemosensitive response of LC neurons is established early in development and maintained into adulthood.
Ion Channels in LC Neurons
The chemosensitive response of a neuron is most likely determined by its complement of ion channels that are responsive to changes of CO2 and/or pH (14). A number of different ion channels have been described in LC neurons. These include a 4-AP-inhibitable A current (17, 63), a TTX-sensitive Na+ channel (16), an inwardly rectifying K+ channel (45, 65), a Ca2+-activated K+ (KCa) channel (29, 34, 53), an L-type Ca2+ channel (16, 21, 23), TASK channels (2, 61), a TRP channel (10), and a delayed-rectifying K+ channel (17). Any or all of these channels could be affected by hypercapnia and ultimately contribute to the chemosensitive firing response of LC neurons. It is not clear that all of these channels coexist in single LC neurons. For instance, TRPC5 channels are expressed in only 35% of LC neurons (10). Further, some of the K+ channels have only been shown to be expressed in adult rodents, such as the A current (63), Kir (45, 65), and TASK 1 channels (61), although A currents have been demonstrated from neonatal LC neurons in culture for up to 6 mo. Thus, we did not assume that the LC neurons from our preparation expressed a given channel without evidence for it.
In the current study, we found clear evidence for a 4-AP-sensitive transient A current (Figs. 2, A and B, and 4) and a TEA-sensitive sustained K current (Figs. 2, A and B and 3) in LC neurons from neonatal rats. Further, these channels were expressed in the same neurons (Fig. 2A). Given space clamp issues with studying neurons in slices, we were concerned about our characterization of these channels using voltage clamp. Thus, we compared our values with those of Forsythe et al. (17), who studied these currents in cultured LC neurons, a preparation that has better space clamping than our slice preparation. They found a sustained current that we estimate had a magnitude of 3.5 nA at 0 mV and a slope conductance of 140 nS (Fig. 1 from 17), which compares with our values of 1.5 nA and 40 nS (Fig. 3C, inset). Our measured properties for the A current from LC neurons in neonatal slices also compare favorably to those measured in cultured LC neurons (17). Our maximum whole cell A current was 6.7 nA (Fig. 4F) compared with 5–10 nA in cultured neurons, and our voltage for half maximum activation was −10 mV (Fig. 4F) compared with about −20 mV for cultured neurons. Further, Forsythe et al. (17) found that with 2.5 mM 4-AP the A current was inhibited by 36%, whereas we noted a 75% inhibition with 5 mM 4-AP (Fig. 4E, inset). The similarity in the properties of these two currents in these two very different preparations suggest that the channel properties are robust and imply that we may have adequate space clamping to study them. One way that this could occur in intact neurons within a brain slice is if the channels that constitute these currents are largely localized to the soma. Somal localization would be consistent with the findings, using localized acidification, that the chemosensitive response of LC neurons is largely restricted to the soma (51), which would require that the chemosensitive channels be largely restricted to the soma.
Our I-V plots suggest that the TEA-sensitive sustained current is not active at resting Vm (Fig. 3C, inset), but the 4-AP-sensitive current should have some activity (Fig. 4F). In fact, on the basis of our inhibitor studies, both TEA-sensitive and 4-AP-sensitive currents have activity at resting Vm, since both drugs resulted in membrane depolarization (Fig. 2B), an increase in Rin (Fig. 2, C and E), and an increase in firing rate (Fig. 2, D and F). 4-AP resulted in a three-fold higher membrane depolarization and increased firing rate compared with TEA (Fig. 2, B, D, and F). These findings suggest that the 4-AP-sensitive current is considerably larger under resting conditions than the TEA-sensitive current in LC neurons from neonates.
CO2 and pH Sensitivity of Outwardly Rectifying Channels in LC Neurons
In the current study, both outwardly rectified currents observed were inhibited by a large HA challenge (15% CO2, pHo 7.0) (Fig. 5), the TEA-sensitive sustained current by ∼50% (Fig. 3, D and E) and the 4-AP-sensitive A current by about ∼35%. Despite both channels being inhibited by 15% CO2, our data with inhibitors also clearly show that inhibition of the TEA-sensitive current does not appear to play a role in hypercapnia-induced increased firing rate (Fig. 2F), while the 4-AP-sensitive A current appears to account for all of the increased firing rate induced by 15% CO2 (Fig. 2D). This lack of a contribution from the TEA-sensitive current is consistent with the much smaller membrane depolarization induced by TEA vs. 4-AP. Thus, while a high dose of TEA can inhibit the sustained channels sufficiently to increase firing rate, we believe that the effect of hypercapnia on normal TEA-sensitive channel activity is sufficiently small as to contribute very little, if at all, to the increased firing rate induced by high CO2. For this reason, we focused on the effect of altered CO2 on the A current.
The maximum A current was inhibited by 35%, 30%, and increased by 25–30% by HA solutions equilibrated with 15% CO2 (Fig. 5, A and B), 10% CO2 (Fig. 5, D and E) and 2.5% CO2 (Fig. 5, G and H), respectively. Thus, the A current is affected by both increases and decreases in CO2 over the physiological range (Fig. 6). This range of sensitivity of the maximum A current matches quite well the sensitivity of LC neuron firing rate at CO2 values between 2.5 and 15% (Fig. 1D). As expected, the relationship is inverse (Fig. 6). At CO2 values where A current is high, firing rate is low and firing rate increases as A current decreases. The relationship between the two is further strengthened by the fact that between 10 and 15% CO2, the firing rate saturates, increasing only slightly, and, in parallel, inhibition of the A current flattens out (Fig. 1D and 6). These data indicate that inhibition of A current by HA seems to play an important role in determining the firing rate of LC neurons, which is consistent with our findings that the effect of hypercapnia on LC neuron firing rate is eliminated in the presence of 4-AP (Fig. 2D). These are the first data to indicate an important role for the A current in chemosensitive signaling in mammalian neurons, although the A current has been shown to play a major role in the response of chemosensitive neurons from the snail, Helix aspersa (12).
It may seem strange that a common K+ current like the A current, which is present in nearly all neurons, should be involved in the chemosensitive response of a neuron. However, common channels, like Kir channels (45), TASK channels (2), and KCa channels (62) have been implicated in central chemosensitive signaling in mammalian neurons. Further, the involvement of an A current and a delayed-rectifying K+ current in chemosensitivity in snail neurons has been shown (12). The fact that these channels are widely distributed but contribute to central chemosensitivity in select neurons suggests that these channels come in a variety of forms involving different subunits or splice variants with differing sensitivities to elevated CO2/H+. For instance, the Kir current from chemosensitive LC neurons is believed to derive from a highly pH-sensitive hybrid channel formed from the Kir4.1-Kir5.1 subunits (64, 65). Thus, in many chemosensitive neurons, their pH responsiveness may indeed derive from altered forms of channels commonly found in many neurons. It may also seem odd that we find no evidence here for a role of Kir channels in the chemosensitive response of our LC neurons given that they appear to be present (based on the Ba2+ experiments) and given previous reports of a role for this channel in LC neuron chemosensitivity (45, 65). These previous studies were done in adult rats and mice, while we are working with neonates, so it is possible that the basis of the chemosensitive response in LC neurons shifts from one based largely on the A current in neurons from neonates to one involving Kir currents in neurons from adults. This would also suggest a shift in the molecular basis for Kir channels in LC neurons from non-pH-sensitive subunits to more highly pH-sensitive subunits during development.
The degree of inhibition of the A current by HA seems rather small, with at most about a 35% inhibition of the maximum current. This small inhibition of the A current may account for the small increase of Rin seen in response to hypercapnia in LC neurons (Figs. 1E and 2, C and E). However, a mathematical model of chemosensitive neurons from the snail showed that firing rate could be increased nearly 4-fold with about a 10% inhibition of K+ conductance (see Fig. 3 in Ref. 6). In LC neurons from neonatal rats, the Rin is rather high (400–500 MΩ) under normocapnic conditions, and thus, small changes in Rin induced by hypercapnia may result in substantial changes in firing rate. This high value for Rin is about twice previously measured values of around 200–250 MΩ, but all of these values were measured in neurons from adult rats using sharp-tipped electrodes (7, 26, 33, 38), so it may be that LC neurons from neonates have higher Rin values. Thus, it appears that hypercapnia does not need to inhibit K+ conductance greatly to have a substantial impact on neuronal firing rate.
Finally, we addressed whether it is the change of pHi or pHo associated with HA that is responsible for the inhibition of the A current (15, 46, 47). We have previously suggested that a decrease in either pHi or pHo is sufficient to increase LC firing rate with hypercapnia (20). A currents have commonly been observed with channels made from the voltage-gated K+ channel subunit Kv1.4 (24, 39, 42, 58). Ishii et al. (24) have shown that the maximum A current from channels made from Kv1.4 can be inhibited by decreases in pHo. In contrast, Padanilam et al. (42) showed an inhibition of the maximum A current from channels made from Kv1.4 by decreases in pHi, with a pK for inhibition in the physiological range (pK= 7.5). We used IH solutions and a technique that we had previously developed to clamp pHi at a constant value in neurons exposed to HA solutions (20) to determine whether the A current in LC neurons is inhibited by decreased pHi or pHo. Our results show that the A current in LC neurons can be inhibited by a fall of either pHo or pHi (Fig. 7). This is consistent with our previous suggestion that either decreased pHo or pHi can induce increased firing in LC neurons (18). We do not know which Kv subunit(s) are responsible for the A current in LC neurons nor do we know whether the A current derives from a single channel type that is sensitive to changes of both pHo and pHi or whether multiple channel types lead to the A current in LC neurons, some of which are inhibited by decreased pHo and others by decreased pHi. A similar pattern of sensitivity to both decreased intracellular and extracellular pH has been shown recently for TRP channels in LC neurons (10).
Perspectives and Significance
Our findings are the first direct evidence that the A current is involved in chemosensitive signaling in mammalian neurons. In LC neurons, we find not only that the A current is affected by HA, but that the sensitivity of the current to various levels of CO2 is inversely correlated to the firing rate of LC neurons at those same levels of CO2 (Figs. 1D and 6), suggesting an important role for the A current in the chemosensitive response of LC neurons. Thus, in mammalian central chemosensitive neurons, the A current plays a role in chemosensitive signaling in LC neurons and likely in NTS neurons as well (31). Combined with previous work that showed a role for an A current in the response of chemosensitive neurons from the invertebrate Helix aspersa (12), our data suggest that the A current may play a widespread role in chemosensitive neurons from many different animals from various taxa.
The presence of multiple hypercapnia-sensitive channels, as well as multiple signals (e.g., changes of pHo and pHi) is consistent with a polyphyletic origin for pH-sensing in neurons, arising many different times and based on different channels. It is curious that numerous CO2 and acid-sensitive neurons are present in LC neurons. Although our work suggests that A currents play a predominant role in LC neurons from neonates, other work suggests that Kir channels may be important in LC neurons from adults (45, 65). There is precedence for a developmental change in the channel involved in the chemosensitive response of neurons. In medullary raphé neurons, it has been shown that TASK 1 and TASK 3 channels mediate the small firing rate response to hypercapnia in young neonates (<P10) but that a Ca-activated nonspecific cation current mediates the much larger firing rate response to hypercapnia in raphé neurons from older rats (9). The ion-channel basis for the development of chemosensitivity in LC neurons merits further study since there appears to be a change in the channel basis with age, but the overall firing rate pattern in response to different levels of CO2 is very similar in LC neurons from neonates vs. adults.
Our understanding of the cellular basis of chemosensitive signaling has presented a large number of potential targets for drug therapy aimed at altering central chemosensitive gain and anxiety/panic disorders. Further, the fact that the cellular basis for the chemosensitive response seems to differ among neurons from different chemosensitive regions offers an approach to help answer the question of why there are so many different chemosensitive regions and what are the roles of these various regions.
In summary, the ability to sense acid involves different channels in different neurons, and our work suggests that the A current plays a major role in LC neurons from neonates. Since LC neurons have been implicated both in the control of breathing and in mediating anxiety, modulation of acid-sensitive A currents could potentially alter either or both of these important neural functions.
GRANTS
This work was supported by National Heart, Lung and Blood Institute Grant R01 HL-56683 and a Research Challenge Augmentation Grant from Wright State University to R. W. Putnam.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.-Y.L. and R.W.P. conception and design of research; K.-Y.L. performed experiments; K.-Y.L. analyzed data; K.-Y.L. and R.W.P. interpreted results of experiments; K.-Y.L. prepared figures; K.-Y.L. drafted manuscript; K.-Y.L. and R.W.P. edited and revised manuscript; K.-Y.L. and R.W.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ann Imber for reading an earlier version of the manuscript and providing useful comments.
REFERENCES
- 1.Bailey CR, Cordell E, Sobin SM, Neumeister A. Recent progress in understanding the pathophysiology of post-traumatic stress disorder: implications for targeted pharmacological treatment. CNS Drugs 27: 221–232, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Respir Physiol 129: 159–174, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphé. J Appl Physiol 80: 108–115, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Bouyer P, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J Physiol 559: 85–101, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro PA, Cooper EC, Lowenstein DH, Baraban SC. Hippocampal heterotopia lack functional Kv4.2 potassium channels in the methylazoxymethanol model of cortical malformations and epilepsy. J Neurosci 21: 6626–6634, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernov MM, Daubenspeck JA, Denton JS, Pfeiffer JR, Putnam RW, Leiter JC. A computational analysis of central CO2 chemosensitivity in Helix aspersa. Am J Physiol Cell Physiol 292: C278–C291, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chiu TH, Chen TY, Ho CL, Chiang ST. Electrophysiological effects of dermorphin on locus coeruleus neurons of rat. Neuropharmacology 29: 747–755, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respir Physiol Neurobiol 166: 4–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson Corcoran GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui N, Zhang X, Tadepalli JS, Yu L, Gai H, Petit J, Pamulapati RT, Jin X, Jiang C. Involvement of TRP channels in the CO2 chemosensitivity of locus coeruleus neurons. J Neurophysiol 105: 2791–2801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Denton JS, McCann FV, Leiter JC. CO2 chemosensitivity in Helix aspersa: three potassium currents mediate pH-sensitive neuronal spike timing. Am J Physiol Cell Physiol 292: C292–C304, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Elam M, Yao T, Thorén P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res 222: 373–381, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Erlichman JS, Putnam RW, Leiter JC. Glial modulation of CO2 chemosensory excitability in the retrotrapezoid nucleus of rodents. Adv Exp Med Biol 605: 317–321, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol 541: 493–509, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 284: C145–C155, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Forsythe ID, Linsdell P, Stanfield PR. Unitary A-currents of rat locus coeruleus neurones grown in cell culture: rectification caused by internal Mg2+ and Na+. J Physiol 451: 553–583, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central. chemosensitivity. Resp Physiol Neurobiol 173: 264–273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyenet PG. The 2008 Carl Ludwig Lecture. Retrotrapezoid nucleus, CO2, homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Med Biol 605: 333–337, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. Brain activation pattern induced by stimulation of L-type Ca2+-channels: contribution of Ca(V)1.3 and Ca(V)12 isoforms. Neuroscience 139: 1005–1015, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Huda R, Pollema-Mays SL, Chang Z, Alheid GF, McCrimmon DR, Martina M. Acid-sensing ion channels contribute to chemosensitivity of breathing-related neurons of the nucleus of the solitary tract. J Physiol 590: 4761–4775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imber AN, Putnam RW. Postnatal development, and activation of L-type Ca2+ currents in locus coeruleus neurons: implications for a role for Ca2+ in central chemosensitivity. J Appl Physiol 112: 1715–1726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii K, Nunoki K, Yamagishi T, Okada H, Taira N. Differential sensitivity of Kv1.4, Kv12, and their tandem channel to acidic pH: involvement of a histidine residue in high sensitivity to acidic pH. J Pharmacol Exp Ther 296: 405–411, 2001 [PubMed] [Google Scholar]
- 25.Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci 8: 650–656, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov A, Aston-Jones G. Local opiate withdrawal in locus coeruleus neurons in vitro. J Neurophysiol 85: 2388–2397, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Kelso SR, Nelson DO, Silva NL, Boulant JA. A slice chamber for intracellular and extracellular recording during continuous perfusion. Brain Res Bull 10: 853–857, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Kersh AE, Hartzler LK, Havlin K, Belcastro B, Nanagas V, Kalra A, Chua J, Whitesell R, Ritucci NA, Dean JB, Putnam RW. pH regulating transporters in neurons from various chemosensitive brainstem regions in neonatal rats. Am J Physiol Regul Integr Comp Physiol 297: R1409–R1420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama S, Jin YH, Akaike N. ATP-sensitive and Ca2+-activated K+ channel activities in the rat locus coeruleus neurons during metabolic inhibition. Brain Res 828: 189–192, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Li K, Putnam RW. Hypercapnia inhibits both transient and sustained potassium currents in chemosensitive neurons from neonatal rat locus coeruleus (LC). FASEB J: Program No. 621.5, 2009 [Google Scholar]
- 31.Li K, Putnam RW. Hypercapnia inhibits a transient K+ current in chemosensitive neurons from the nucleus tractus solitarius (NTS) of neonatal rats. Soc Neurosci Abstr 36: Program No. 188.20, 2010 [Google Scholar]
- 32.Loeschcke HH, De Lattre J, Schläfke ME, Trouth CO. Effects on respiration and circulation of electrically stimulating the ventral surface of the medulla oblongata. Respir Physiol 10: 184–197, 1970 [DOI] [PubMed] [Google Scholar]
- 33.McFadzean I, Lacey MG, Hill RG, Henderson G. Kappa opiod receptor activation depresses excitatory synaptic input to rat locus coeruleus neurons in vitro. Neuroscience 20: 231–239, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Murai Y, Ishibashi H, Koyama S, Akaike N. Ca2+-activated K+ currents in rat locus coeruleus neurons induced by experimental ischemia, anoxia, and hypoglycemia. J Neurophysiol 78: 2674–2681, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex neurons from adult rats. Am J Physiol Regul Integr Comp Physiol 296: R763–R773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieber K, Sevcik J, Illes P. Hypoxia changes in rat locus coeruleus neurons in vitro. J Physiol 486: 33–46, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris AJ, Nerbonne JM. Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv43, and Kv14 alpha-subunits. J Neurosci 30: 5092–5101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyamada Y, Ballantyne D, Mückenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol 513: 381–398, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyamada Y, Yamaguchi K, Murai M, Ishizaka A, Okada Y. Potassium channels in the central control of breathing. Adv Exp Med Biol 580: 339–344, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Padanilam BJ, Lu T, Hoshi T, Padanilam BA, Shibata EF, Lee HC. Molecular determinants of intracellular pH modulation of human Kv1.4 N-type inactivation. Mol Pharmacol 62: 127–134, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Pedersen KA, Jørgensen NK, Jensen BS, Olesen SP. Inhibition of the human intermediate-conductance, Ca2+-activated K+ channel by intracellular acidification. Pflügers Arch 440: 153–156, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Petheö GL, Molnár Z, Róka A, Makara JK, Spät A. A pH-sensitive chloride current in the chemoreceptor cell of rat carotid body. J Physiol 535: 95–106, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Putnam RW. CO2 chemoreception in cardiorespiratory control. J Appl Physiol 108: 1796–1802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Putnam RW, Li K. Transient and sustained potassium currents are inhibited by hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr 35: Program No. 89.16, 2009 [Google Scholar]
- 49.Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol 289: C1094–C1104, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289: R851–R861, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sausbier U, Sausbier M, Sailer CA, Arntz C, Knaus HG, Neuhuber W, Ruth P. Ca2+ -activated K+ channels of the BK-type in the mouse brain. Histochem Cell Biol 125: 725–741, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Schläfke ME, See WR, Loeschcke HH. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respir Physiol 10: 198–212, 1970 [DOI] [PubMed] [Google Scholar]
- 55.Singewald N, Philipu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol 56: 237–267, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Solomon IC, Edelman NH, Neubauer JA. Pre-Bötzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol 83: 2854–2868, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol 586: 1605–1622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol 582: 1219–1238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Erhlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979 [DOI] [PubMed] [Google Scholar]
- 60.Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol 509: 183–194, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Zhang C, Li N, Su L, Wang G. Expression of TASK-1 in brainstem and the occurrence of central sleep apnea in rats. Respir Physiol Neurobiol 161: 23–28, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Wellner-Kienitz MC, Shams H, Scheid P. Contribution of Ca2+-activated K+ channels to central chemosensitivity in cultivated neurons of fetal rat medulla. J Neurophysiol 79: 2885–2894, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurons. Neuroscience 13: 137–156, 1984 [DOI] [PubMed] [Google Scholar]
- 64.Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1, Kir5.1, by hypercapnia and intracellular acidosis. J Physiol 524: 725–735, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol Cell Physiol 301: C729–C738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139: 1012–1021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]