Abstract
Maintenance of body water homeostasis is critical for preventing hyperthermia, because evaporative cooling is the most efficient means of dissipating excess body heat. Water homeostasis is achieved by regulation of water intake and water loss by the kidneys. The former is achieved by sensations of thirst that motivate water acquisition, whereas the latter is regulated by the antidiuretic action of vasopressin. Vasopressin secretion and thirst are stimulated by increases in the osmolality of the extracellular fluid as well as decreases in blood pressure and/or blood volume, signals that are precipitated by water depletion associated with the excess evaporative water loss required to prevent hyperthermia. In addition, they are stimulated by increases in body temperature. The sites and molecular mechanisms involved in integrating thermal and osmotic regulation of thirst and vasopressin secretion are reviewed here with a focus on the role of the thermal and mechanosensitive transient receptor potential-vanilloid (TRPV) family of ion channels.
Keywords: thirst, TRPV, vasopressin, hypothalamus
maintenance of a constant body temperature by homeotherms is critically dependent on body water homeostasis, because evaporative cooling is the most efficient means that homeotherms have for dissipating excess body heat. Even at rest, heat is generated as a by-product of basal cellular metabolism and the ongoing muscle activity of the heart beating, respiration, and gastrointestinal motility. For a typical 70-kg man the resting (basal) rate of metabolic heat production is about 80 kcal/h, but intense exercise can increase heat production to 400–600 kcal/h. Thus the body must have efficient mechanisms for dissipating heat to prevent hyperthermia. In humans, body heat is dissipated by passive transfer of heat from the skin to the air. The heat generated by muscles and internal organs is transferred to the skin by the blood flowing through epidermal capillaries. Thus heat loss can be increased by increasing skin blood flow and by increasing the movement of air next to the skin by removing clothing and standing near a fan or in a breeze. However, evaporative cooling significantly augments heat loss from the skin, because evaporation of 1 gram of water from the skin dissipates 0.6 kcal. Thus a sweat rate of 1 l/h could theoretically remove 600 kcal/h from the body. Fur-covered mammals also rely heavily on evaporative cooling for maintaining body temperature with increased body temperature promoting panting, increased salivation, and saliva spreading in dogs, cats, and rats. However, water loss of the magnitude required in many instances to adequately dissipate excess body heat must be compensated to prevent electrolyte imbalance and preserve blood volume for adequate cardiovascular function. Thus mechanisms integrating thermal and fluid homeostasis are critical for homeotherms.
Regulation of Water Balance
Water balance is maintained by matching intake to loss. In addition to the water loss from sweating, salivation, and panting mentioned above, water is lost from the respiratory and gastrointestinal tract (insensible water loss) and in the urine. Water loss in the urine is regulated by vasopressin (VP; aka antidiuretic hormone). VP increases the permeability of the distal tubule and collecting ducts to water allowing increased water reabsorption from the renal filtrate and thereby decreasing water loss in the urine. The perception of thirst motivates water intake. Thirst and VP secretion are regulated in concert.
Water loss or a deficit in body water results in a decrease in the volume of the extracellular fluid (ECF) resulting in contraction of the blood volume and an increase in concentration of solutes in the ECF (increased osmolality). Both thirst and VP secretion are stimulated by increases in ECF osmolality and decreases in blood volume and/or pressure (75). ECF osmolality is monitored by “osmoreceptive” neurons in the hypothalamus. An increase in osmolality simultaneously drives increased water intake and stimulates VP secretion from the posterior pituitary. Osmotic regulation of VP secretion has been studied extensively, and VP secretion is exquisitely sensitive to small changes in osmolality of the extracellular fluid with a 3- to 5-mosmol/kg H2O increase in osmolality being sufficient to raise plasma VP secretion to a level that induces maximal urinary concentration (24). As reviewed previously (12, 13), osmoreceptive neurons located in the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO) activate the VP neurons of the supraoptic (SON) and paraventricular nuclei (PVN) of the hypothalamus that synthesize and release VP into the peripheral circulation from the posterior pituitary. The VP and oxytocin (OT) neurons are also intrinsically osmosensitive (45, 59, 60). Osmoreceptive neurons in OVLT and SFO also induce water intake (48) and activate a thalamocortical pathway including the insular and cingulate cortex components of the motivated behavior circuit. The latter is thought to represent the neuroanatomical substrate for generation of thirst (34).
Regulation of Thermal Homeostasis
The hypothalamus plays a central role in monitoring core body temperature and in activating responses aimed at either increasing or decreasing body temperature. The hypothalamus regulates endocrine and autonomic as well as voluntary and involuntary somatic motor mechanisms to modulate heat loss and gain. Specifically, hypothalamic regulation of thyroxin production modulates the basal metabolic rate and thus basal heat production (85). Skin blood flow, thermogenesis by brown adipose tissue (BAT), and sweating are regulated by the sympathetic nervous system and can be modulated by the hypothalamus (52). Shivering is an involuntary somatic motor system mechanism for heat gain that can be initiated by hypothalamic cooling, and heating or cooling of the hypothalamus induces voluntary motivated behaviors aimed at either increasing or decreasing heat loss by altering skin exposure (clothing) and/or environmental temperature (53, 54).
Classic studies of central thermoreception focused on the medial preoptic (MPO)/anterior hypothalamic region (10, 11). Early ablation studies identified the anterior hypothalamus as important for maintenance of body temperature, and local warming or cooling of the preoptic area elicited appropriate heat loss or heat gain responses. Nakayama and colleagues (55) were the first to demonstrate in vivo that neurons in the MPO behaved as “thermosensors” by increasing or decreasing their firing rate in response to local warming or cooling respectively of the MPO. About 40% of MPO neurons are thermosensitive. The majority function as “warm sensors,” increasing their firing rate as local temperature increases (11). A smaller population of “cold sensors” also exist in MPO. The firing rate of these neurons is inversely related to changes in the local temperature (11). This review will focus predominantly on the “warm sensors.” In addition to monitoring local temperature, the MPO “warm-sensor” neurons receive afferent projections carrying information from cutaneous thermoreceptors. Thus they integrate information about local temperature with information about environmental temperature (52). The information from cutaneous warm and cold sensors is relayed to the MPO via glutamatergic spinal afferents projecting to the lateral parabrachial nucleus (LPB), glutamatergic LPB afferents to the median preoptic nucleus (MnPO), and finally glutamatergic (from cutaneous warm sensors) or GABAergic (from cutaneous cold sensors) MnPO neurons innervate the “warm sensors” in the MPO (51, 52). The warm sensors are predominantly GABAergic and tonically inhibit regions in the hypothalamus, brain stem, and spinal cord responsible for initiating involuntary heat gain mechanisms [vasoconstriction, thermogenesis in BAT, and shivering (51, 52)].
As eloquently delineated and described by Morrison et al. (51–53), multiple and distinct populations of warm-sensitive neurons in MPO independently regulate the diverse thermoeffector pathways. This is evident from the fact that the various heat-loss and heat-gain mechanisms (e.g., thermoeffectors) have different thermal thresholds for activation. Specifically, as MPO temperature decreases, skin vasoconstriction is activated before BAT thermogenesis, and an even greater decrease in MPO temperature is required for initiation of shivering (51). Furthermore, the efferent projections from these thermoreceptors diverge. Thermosensitive efferents that activate BAT thermogenesis and shivering synapse in the dorsomedial nucleus (DMN) of the hypothalamus before innervating autonomic premotor neurons in the rostral raphe pallidus (rRP). In contrast, initiation of vasoconstriction by MPO cooling bypasses the DMN and directly innervates the rRP. The involvement of discrete populations of thermoreceptors and divergent effector pathways for autonomic, involuntary, and voluntary heat-gain mechanisms supports the possibility that the mechanisms and pathways for integrating thermal and fluid homeostasis are distinct and independent from these well-characterized pathways for maintaining body temperature homeostasis.
The existence of thermoreceptive mechanisms in addition to the “warm- and cold-sensors” in MPO is illustrated by the evidence that in contrast to the involvement of the warm-sensitive MPO neurons in regulating autonomic heat-gain mechanisms, the MPO does not seem to play a major role in recruiting voluntary behaviors aimed at conserving body heat (52). Whereas recruitment of thermoregulatory behaviors in response to environmental changes may primarily reflect responses to cutaneous thermoreceptors, thermoregulatory behaviors are also recruited during fever without ambient temperature changes.
Integration of Temperature and Fluid Homeostasis
The importance of evaporative cooling for preventing hyperthermia discussed above suggests the existence of complex mechanisms integrating thermal and fluid balance. In fact, an effect of body temperature on renal water retention was recognized in the 1880s with the description of “cold diuresis” (see Ref. 8 for review). The subsequent elucidation of a hormonal mechanism underlying cold diuresis (6), and the discovery of VP as an antidiuretic agent linked the phenomenon of cold diuresis to inhibition of VP secretion. The more recent observations that dehydration reduces evaporative cooling responses resulting in hyperthermia (7, 57) further supports the importance of mechanisms integrating thermal and fluid homeostasis.
In addition to hyperosmolality and hypovolemia and/or hypotension, an increase in hypothalamic temperature also stimulates VP secretion and thirst (27, 28, 77). Classic studies from the laboratory of Andersson (3, 4) using thermodes to locally heat and cool the preoptic-anterior hypothalamic area in the goat demonstrated that increasing and decreasing the temperature enhanced and inhibited drinking, respectively. Similar effects have been reported in pigs (35) and baboons (76). Although similar studies in dogs and rats reported inhibition rather than stimulation of drinking in response to heating of the hypothalamus (30, 77), this may have reflected compensatory responses to changes in whole body temperature rather than direct effects of temperature changes in the preoptic area.
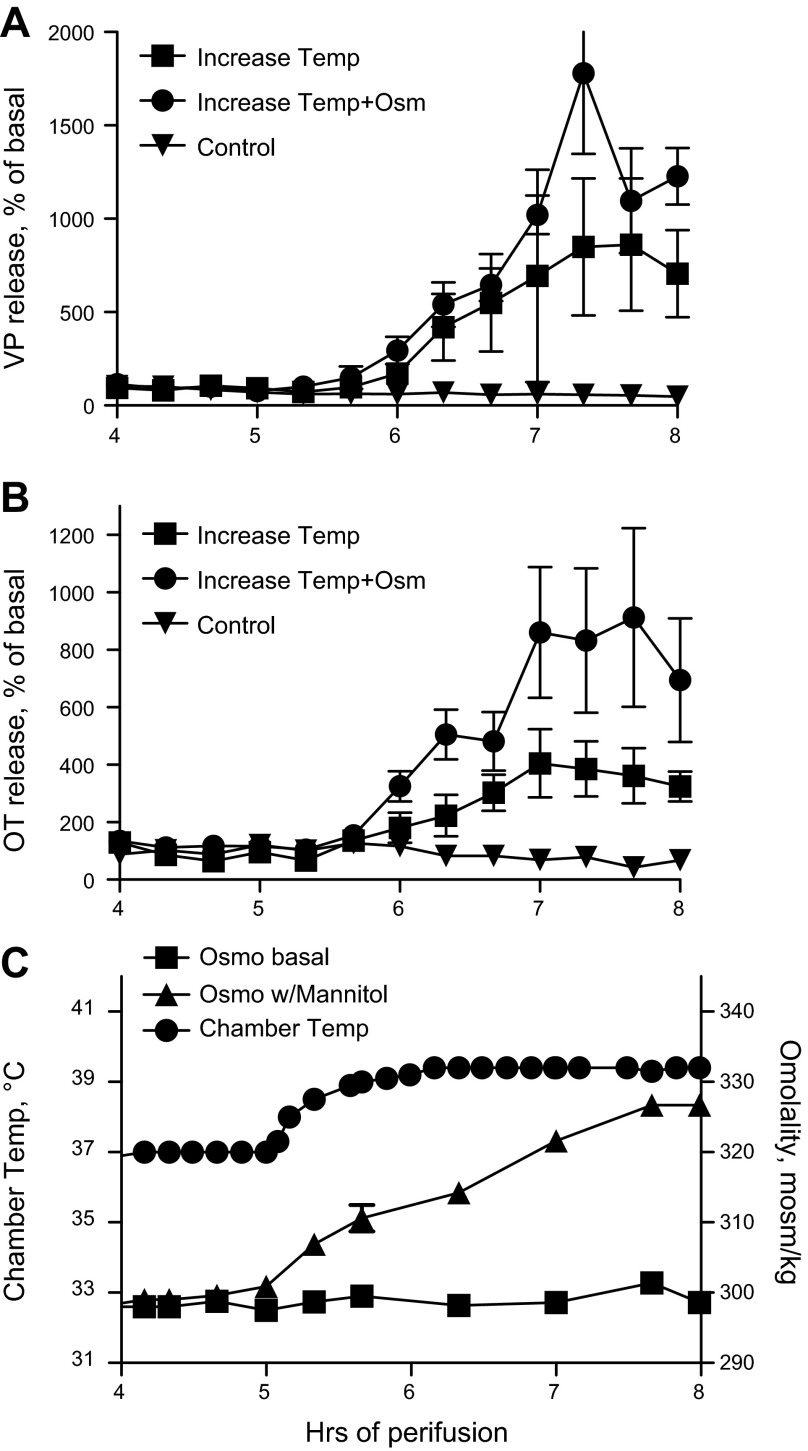
In the case of VP secretion, the problem of compensatory changes in whole body temperature was obviated by evaluating the effect of an increase in temperature on VP release from an in vitro preparation. As shown in Fig. 1, VP and OT release from perifused explants of the hypothalamo-neurohypophyseal system (HNS) is dramatically increased when the temperature is increased from 37–39.5°C over 1 h. This increase in temperature, which is well within the range experienced in fever, results in a 800% increase in VP secretion from HNS explants. A simultaneous increase in osmolality results in further stimulation of VP and OT release (Fig. 1).
Fig. 1.
Effect of temperature (Temp) on basal and osmotically (Osm) stimulated vasopressin (VP) and oxytocin (OT) release from explants of the hypothalamo-neurohypophyseal system. Increasing Temp (C) resulted in an 800 and 400% increase in VP (A) and OT (B) release (P < 0.0001). Simultaneously increasing temperature and osmolality (Temp+Osm; C) further increased release, although the effect on VP release just missed statistical significance (B: OT, P = 0.0012; A: VP, P = 0.06). Control, no change in Temp or Osm. n = 5/group. In these studies, hormone release in response to an increase in temperature or osmolality is expressed as percentage of the basal release for each explant. Mean basal release for these explants was the following: Control, 119 ± 20 pg/ml (VP), 164 ± 52 pg/ml (OT); Increase in Temp, 140 ± 61 pg/ml (VP), 207 ± 43 pg/ml (OT); Increase in Temp and Osm, 63 ± 15 pg/ml (VP), 151 ± 58 pg/ml (OT).
Sites for Integration of Temperature and Fluid Homeostasis
In searching for the mechanisms integrating temperature and water homeostasis, it might be expected that integration would occur in regions identified as monitoring body temperature and/or osmolality. Thus studies addressing this issue have focused on the regions identified as critical for osmoreception [e.g., the circumventricular organs (CVOs), OVLT, and SFO] and the regions involved in monitoring body temperature [e.g., the MPO/anterior hypothalamus (AH)].
In synchrony with this, the periventricular tissue surrounding the anteroventral part of the ventral third ventricle (AV3V), which includes the OVLT, has been identified as being essential for maintenance of both fluid and thermal homeostasis and a likely site for integration of thermal and osmotic reception in rats and humans (25, 88). Rats with lesions in this region are initially adipsic but can be weaned through this period with sweet, palatable liquids. However, they retain deficiencies in osmotic and angiotensin II-induced drinking (15). These lesioned rats also fail to concentrate their urine in response to the adipsia (36), and HNS explants obtained from AV3V-lesioned rats fail to increase VP release in response to an increase in osmolality (72). Also, AV3V-lesioned rats have a markedly reduced thermal tolerance showing attenuated salivation and impaired cardiovascular responses in response to heat stress (88). In addition to the OVLT, the “AV3V” region includes the ventral portion of the MnPO, the preoptic periventricular nucleus, and medial aspects of the MPO (15, 88). As described above, the MnPO innervates the “warm-sensitive” neurons in the MPO and transmits information from cutaneous thermal receptors to the MPO warm sensors. Efferent projections to the MnPO from the SFO have been implicated in osmoreception, and although AV3V lesions do not destroy SFO, many efferents from the SFO are disrupted. For example, following AV3V lesions, anterograde degeneration was observed in SON and PVN, and retrograde degeneration was observed in SFO (16, 17). AV3V lesions destroy connections between the MnPO and MPO and therefore indirect SFO projections to MPO.
Integration of thermal and osmosensitivity by neurons in the MPO and MnPO has been specifically evaluated in studies using electrophysiology on hypothalamic slices. Subsets of thermosensitive neurons in MPO and MnPO responded to changes in osmolality as well as temperature (69, 81). However, these studies were not performed under conditions of synaptic blockade, and thus, did not establish whether the MnPO and/or MPO neurons were inherently osmo- or temperature sensitive. Nevertheless, since both areas have been shown to influence drinking and VP secretion, and MnPO innervates MPO, these areas clearly represent important way stations for integrating osmotic and thermal regulation of efferents even if the primary osmo- and thermoreceptors are located elsewhere and the neurons in MnPO and MPO are efferent to osmoreceptive neurons in SFO and OVLT.
Although the primary monitoring and integration of thermal and osmotic information may occur in OVLT and SFO, integrative sensing of thermal and osmotic information also occurs in at least one effector system: the magnocellular VP secreting neurons in SON and presumably PVN. Studies of the cellular mechanisms underlying osmotic and thermal sensing in SON neurons has provided significant insights into the cellular and molecular mechanisms available for integration of thermal and osmotic information (see Cellular/Molecular Mechanisms of Osmoreception). However, since this represents only one arm of fluid homeostasis, e.g., water conservation, additional mechanisms may be important in the “intake/replenshing” arm of fluid homeostatsis that is critical for evaporative heat loss and thus, prevention of hyperthermia.
Cellular/Molecular Mechanisms of Osmoreception
Early in vivo studies by Verney et al. (84) suggested that “osmoreceptors” responded to changes in their cell volume, because antidiuretic activity was enhanced by agents that could not cross the cell membrane (e.g., sodium or sucrose), but not by agents that were membrane permeant (urea or glucose). Verney localized “osmoreception” to the anterior hypothalamus. However, observations in goats suggested that cerebral receptors monitored sodium rather than osmolality, and it was suggested that the effect of the non-sodium osmolytes was explained by the blood-brain barrier limiting their access to the brain and, in turn, altering the CSF sodium concentration (2). This led to the “osmoreceptor versus sodium sensor” controversy. However, a role for the blood-brain barrier was minimized by findings that membrane nonpermeant agents, but not membrane permeant agents, stimulated VP release from HNS explants, a preparation that eliminated potential contributions of the blood-brain barrier (73). Ultimately, definitive demonstrations that “osmoreceptive” elements were located in regions of the hypothalamus that lacked a blood-brain barrier, e.g., the OVLT and SFO (14, 49, 80), put the controversy to rest. Current evidence supports roles for both osmoreceptors and sodium receptors (33) in regulating thirst and VP secretion.
In addition to the osmoreceptive neurons in OVLT and SFO, neurons in the SON that synthesize VP and OT and project to posterior pituitary are also “osmoreceptive.” Intrinsic osmosensitivity of SON neurons was first demonstrated by Mason (45) with intracellular recordings of SON neurons in coronal slices. He demonstrated that increasing the osmolality by 15 mosmol/kg H2O with membrane impermeant ions induced depolarization in SON neurons. Subsequent studies on acutely dissociated SON neurons confirmed that these neurons can operate as cell autonomous osmosensors (59, 60, 89). Mason's work also demonstrated that the SON neurons function as part of an “osmoreceptive complex,” because in addition to a hypertonic stimulus inducing depolarization of the SON neuron, it also increased the frequency of excitatory postsynaptic potentials (EPSP) recorded in SON neurons. The latter were required for firing of the SON neurons in response to an increase in osmolality. Much has been learned about the mechanisms of osmoreception by studying the easily identified SON neurons. For example, Bourque and his colleagues (13) confirmed the role of cell volume and membrane mechanosensitivity (e.g., membrane stretch and contraction) in altering the activity of SON neurons. Their work demonstrated the involvement of stretch-inactivated nonselective cation (SIC) channels in osmoreception. Their subsequent search for the molecular underpinnings of this channel led to the hypothesis that modified TRPV1 (mTRPV1) channels are responsible for osmoreception in SON neurons.
Mechanisms of Temperature Reception
Multiple neurophysiological mechanisms may underlie the endogenous thermosensitivity of MPO warm sensors. One possibility is that a heat-induced membrane depolarization determines the threshold for firing. Select MPO neurons recorded under conditions of synaptic blockade demonstrated a thermal-sensitive depolarization, and upon reaching threshold, fired repetitively. The heat-sensitive conductance showed nonselective cation properties (39). Although this is consistent with characteristics of TRPV channels, the participation of TRPV channels in “warm-sensitive” neurons has not been definitely demonstrated: Systemic administration of TRPV1 channel agonists activates MPO warm sensors, but this may be indirect reflecting actions on efferents to MPO warm sensors (46, 63). On the other hand, other TRPV channels may be expressed in MPO (56) and TRPV expression may be altered in fever (26). Thus TRPV channels may play a role in MPO thermosensitivity.
Several types of K+ channels have been postulated to contribute to thermosensitivity in MPO. A voltage-sensitive A-type potassium current that contributes to afterhyperpolarization has been studied extensively in MPO. In this case, heating increases the inactivation rate of the K+ current. This reduces the hyperpolarization period following an action potential and results in an increase in firing rate of the neuron (90). K+ leak channels such as TASK and TRAAK or hyperpolarization activated cyclic nucleotide-gated (HCN) channels are also possible candidates for the temperature sensitive K+ channel (87) as they are expressed in MPO. However, these channels are broadly expressed throughout the central nervous system including temperature-insensitive neurons. Thus their expression is not unique to temperature-sensitive neurons.
TRPV Channels: Sensors Underlying Thermal and Mechanosensitivity of Neurons
The transient receptor potential (TRP) channel super family includes a diverse group of proteins that form nonselective cation channels and are involved in sensory transduction. Several members of the vanilloid subfamily of TRP channels (TRPV) are molecular candidates for integrators of thermal and osmotic sensitivity, because in addition to functioning in multimeric complexes to form gated calcium-permeable nonselective cation channels, they are polymodal sensors that respond to temperature, membrane stretch, protons, and chemical ligands.
TRPV1.
TRPV1 is the best characterized of the TRPV channels due to its involvement in mediating pain perception. It is responsible for the burning sensation induced by capsaicin, and because it has a temperature threshold >42°C, TRPV1 has been implicated in transducing information about noxious heat (29). Since SON neurons are not capsaicin sensitive and are responsive to temperature changes in the physiological range, TRPV1 seems a poor candidate for mediating thermal and osmosensitivity in these neurons. Nevertheless, TRPV1 is expressed in SON (34), and Bourque and colleagues (22, 65) have provided extensive evidence that the SIC conductance that underlies osmosensitivity of SON is, in fact, mediated by a mTRPV1 channel. The mTRPV1 expressed in SON is a truncated form of TRPV1 that includes the COOH-terminal portion of the molecule but not the NH2-terminal region (65). The molecular evidence from the Bourque lab for this mTRPV1 channel is compelling, because it is supported by findings at both the mRNA and protein level (22, 65). They showed, by using RT-PCR, that primer sets to the NH2-terminal region did not detect TRPV1 mRNA in SON, but primer sets to the COOH-terminal region detected a shorter mRNA in SON. Furthermore, SON was robustly labeled with an antibody to the COOH-terminal region, but not the NH2-terminal region, and the COOH-terminal labeling was absent in TRPV1 knockout (KO) mice (65). This demonstrates that the mTRPV1 expressed in SON is a trpv1 gene product. The osmoreceptive role of the mTRPV1 was demonstrated by the presence of a hypertonic-induced cation current in wild-type mice that was sensitive to ruthenium red (RR; a broad-spectrum blocker of TRP channels) and SB366791 (a selective TRPV1 antagonist) (65). Furthermore, the increase in cationic conductance evoked by hyperosmolality was absent in mice lacking TRPV1 expression (65). Expression of an NH2-terminal truncated form of TRPV1 is consistent with the absence of capsaicin responses in SON neurons, because the NH2-terminal region is required for capsaicin sensitivity (37).
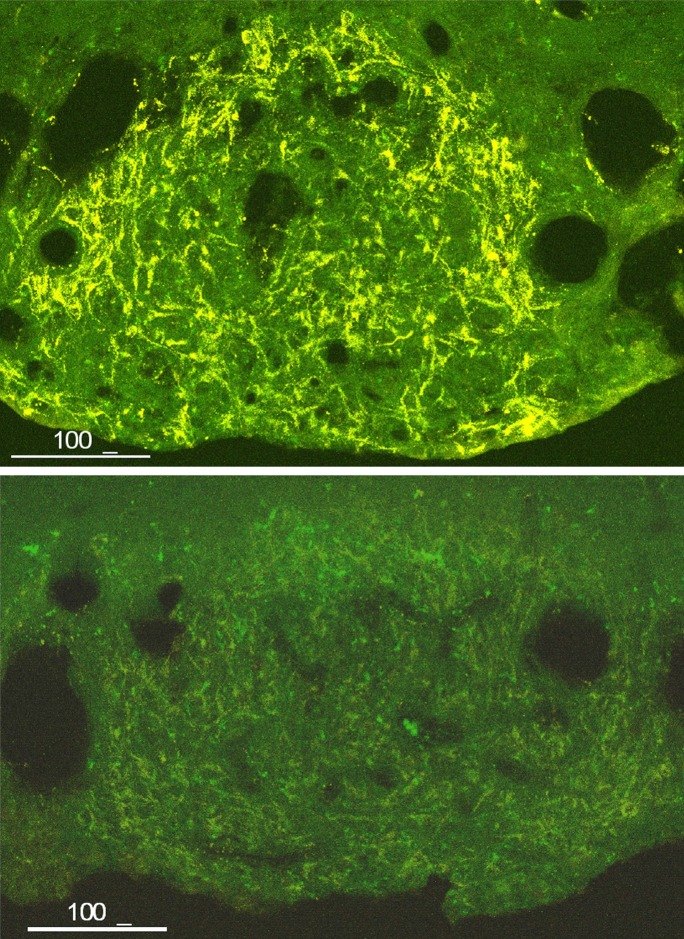
Osmoreception in OVLT neurons also involves SIC channels (58) and is TRPV1 dependent (22). However, the nature of the TRPV1 gene product expressed in OVLT is still controversial (22, 44). TRPV1 is robustly expressed in OVLT and SFO (Fig. 2), but in the CVOs, it is most prominent in thick astrocytic processes (Fig. 2 and Ref. 44). It is also observed in neuronal dendrites in OVLT and SFO (44), but it was also evident in neuronal somata in OVLT in one study (34). Unlike SON, TRPV1 expressed in OVLT and SFO includes the NH2-terminal portion and is the expected size for full-length TRPV1 (44). However, this contrasts to the finding by Ciura and Bourque (22) that OVLT neurons were insensitive to capsaicin, and raises the possibility that the form of TRPV1 expressed in neurons and glia may be different.
Fig. 2.
Transient receptor potential vanilloid (TRPV1) expression in subfornical organ (SFO). Top: immunohistochemical demonstration of TRPV1 expression in SFO. Note the prominent expression in thick astrocytic processes and the lack of neuronal perikaryal staining. Bottom: significant reduction in TRPV1 staining in SFO 28 days postintracerebroventricular injection of AAV1/2-shRNA-TRPV1 (GCG CAU CUU CUA CUU CAA C) demonstrating antibody specificity. A COOH-terminal directed antibody (Cat. no. GP14100; Neuromics, Edina, MN) was used for the immunohistochemistry.
Expression of TRPV1 in OVLT and SFO suggests a role for TRPV1 in hyperosmotic drinking. This was supported by a report of reduced drinking to a peripheral hyperosmotic challenge in TRPV1 KO mice (22). However, this was not observed by another group (78). These discrepant results may reflect slight differences in the osmotic challenge that prevented detection of a partial deficit as other mechanisms compensated for the life-time absence of TRPV1 (23, 78). As discussed below, the latter possibility is supported by evidence for expression of other TRPV channels in OVLT and SFO.
The Bourque group also provided evidence based on both pharmacological studies and TRPV1 KO mice that TRPV1 channels are involved in thermosensitivity of SON neurons (66). They showed that isolated SON VP neurons increased their firing rate in response to increasing the perifusate temperature from 36–38°C. In contrast to the warm-sensitive neurons of the MPO, the increase in firing rate was accompanied by membrane depolarization and an increase in membrane conductance mediated by calcium-permeable nonselective cation channels (66). Thermal sensitivity in the 35–39°C range was reduced in isolated VP neurons obtained from TRPV1 KO mice, and the temperature-sensitive current was reduced by the specific TRPV1 antagonist SB366791 in VP neurons from wild-type but not TRPV1 KO mice (66). Thus the Bourque group has provided compelling evidence supporting the involvement of TRPV1 channels in thermal sensitivity of isolated VP neurons, but important questions remain: 1) Since different domains of the TRPV1 molecule are thought to mediate its polymodal sensitivity (e.g., activation by heat and protons, and inactivation by stretch), can the NH2-truncated mTRPV1 expressed in SON monitor temperature? While the NH2-terminal domain required for capsaicin sensitivity is absent in mTRPV1, it is the COOH terminal domain that is thought to mediate osmoreceptivity and thermosensitivity, and this is intact in mTRPV1. 2) What mechanisms are responsible for reducing the high heat threshold of TRPV1? Evidence exists in other cells, for PKC, PKA, and PIP3-dependent lowering of the TRPV1 heat threshold (21, 62, 83). Ligands of several G protein-coupled receptors including bradykinin and ATP also sensitize TRPV1 to temperature (29). Peptide ligands of PKC-linked G protein-coupled receptors have been shown to modulate osmoreceptivity of the SIC channel. Specifically, angiotensin II and neurotensin increased the nonselective cation currents in SON neurons (20, 38). Another possibility is that thermosensitivity of mTRPV1 channels is reduced if they exist in heteromeric complexes with other TRPV family members that have lower heat thresholds (e.g., TRPV4) (19, 64). Heteromeric complexes of TRPV2 and/or TRPV4 with mTRPV1 have been postulated to subserve hyper- and hypotonicity sensitivity (67).
TRPV2.
TRPV2 is also a heat-gated calcium-permeable nonselective cation channel. As shown in Fig. 3, it is prominently and preferentially expressed in the SON, PVN, and neural lobe in primates with coexpression in VP and OT neurons (86). It is also prominently expressed in rat SON, PVN, and neural lobe (31). TRPV2 mRNA is present in VP neurons (56), and water deprivation increases TRPV2 mRNA in SON (31). Prominent expression is also present in the OVLT, MPO, and SFO in the rat brain (56). Although, like TRPV1, the temperature threshold for TRPV2 exceeds the physiological range, it also can be sensitized to lower temperatures by regulatory agents. Insulin and insulin-like growth factor-1 (IGF-1) induce translocation of TRPV2 to the membrane resulting in the presence of constitutively active channels (29). Insulin induced TRPV2-dependent calcium entry in pancreatic β-cells (32). Since receptors for both IGF-1 and insulin are expressed in SON (1, 9, 82), TRPV2 channels are viable candidates for mediating thermosensitivity. TRPV2 has been shown to form heteromeric complexes with TRPV1 (40, 64). Thus formation of heteromeric channels with coexpressed osmosensitive TRPV1 and possibly TRPV4 channels in SON, OVLT, and SFO provides the potential for TRPV2 channels to participate in integration of osmotic and thermal signals for drinking as well as VP secretion.
Fig. 3.
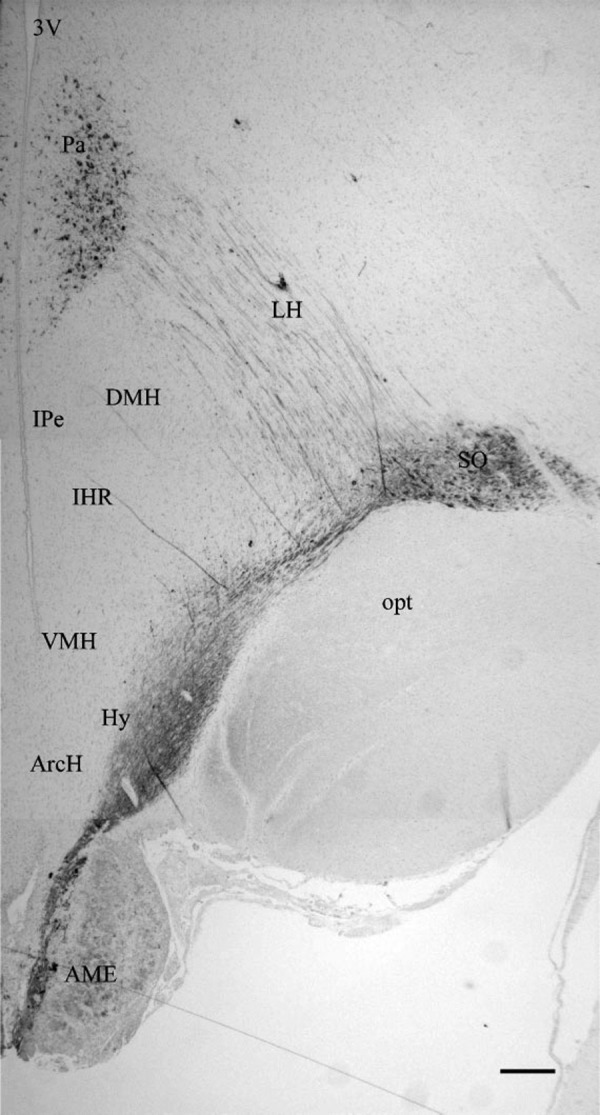
Discrete distribution of TRPV2 in the hypothalamo-neurohypophyseal system (HNS) in the primate brain. 3V, third ventricle; Pa, paraventricular nucleus; LH, lateral hypothalamus; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; SO, supraoptic nucleus; opt, optic tract; ARH, arcuate hypothalamus; AMe, anterior median eminence. Reproduced with permission from Wiley (86).
TRPV4.
TRPV4 was one of the first recognized osmosensitive proteins (41). In transfected cells, it was shown to be gated by exposure to hypotonic challenges within the physiological range, and its osmosensitivity is increased by warming to 37°C. In the rat, it is expressed in SON, PVN, OVLT, and SFO (18, 41). TRPV4 KO mice show deficits in osmoregulation. When compared with wild-type mice, they drink less water either spontaneously or in response to a hypertonic challenge, become hyperosmolar, and have a smaller increase in plasma VP when challenged with hypertonic saline (42). They also became hypoosmolar when treated with dDAVP (42). Thus they show deficits in responses to both hypertonic and hypotonic challenges. cFos expression in OVLT induced by a hypertonic challenge is reduced in TRPV4 KO mice. Although taken together these observations suggest a role for central osmosensing, the exact nature of that role remains unclear due to conflicting reports in the literature: In contrast to the finding that VP release was reduced to a hypertonic challenge in TRPV4 KO mice, Mizuno et al. (50) reported that VP secretion in response to water deprivation was increased in TRPV4 KO mice, and Ciura et al. (23) found that osmotic depolarization of OVLT neurons was dependent on TRPV1 but not TRPV4 expression. However, since Ciura et al. (23) used acutely dissociated OVLT neurons, a role for TRPV4 in glial cells was not excluded in that study. It is likely that these discrepencies reflect diverse contributions of the individual TRPV channels to the osmoreceptor complex such that the type of osmotic perturbation, the location of the channels, and the end point evaluated all impact the perceived involvement or importance of TRPV4.
Summary/Overview
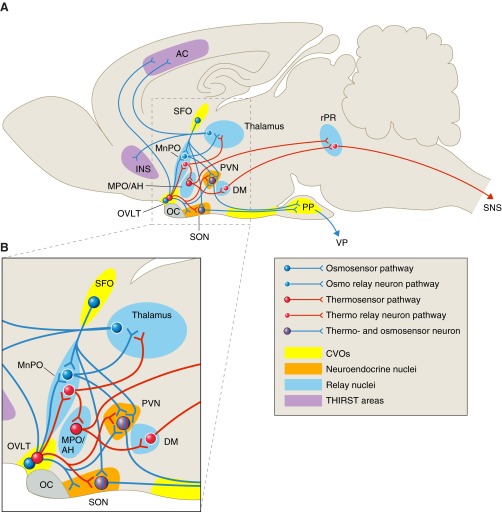
As described above and diagramed in Fig. 4, homeostasis in both body temperature and osmolality is achieved by complexes of temperature and osmotic sensors. In the case of osmotic control of VP secretion, the “osmosensor complex” confers greater sensitivity to osmotic perterbations than is achieved with the inherent osmosensitivity of the VP neurons alone. As mentioned, it is well recognized that with the osmosensor complex intact, approximately a 1% increase in osmolality induces secretion of VP sufficient to generate a maximally concentrated urine. In contrast, a 5–10% increase in osmolality is required to elicit demonstrable electrophysiological responses in dissociated or synaptically blocked SON neurons (45, 59, 60). Similarly, the temperature sensors in MPO are not sufficient to protect animals against lethal hyperthermia in the absence of efferent information coming from the CVOs (88). Thus similar to the hypothalamic-anterior pituitary axis, these osmotic and thermal complexes function to achieve the sensitivity required for the the output systems to meet physiological requirements.
Fig. 4.
Osmo- and thermoregulatory circuits in the mammalian brain. A: sagittal illustration of the rat brain in which the relative positions of relevant structures and nuclei have been compressed onto a single plane. Structures that have been implicated in thermal and osmotic sensing and their connections to relay neurons and effectors for maintenance of body temperature and fluid homeostasis are shown. Neurons and pathways are color coded to distinguish osmosensors (blue) and thermosensors (red) and their relay neurons and effector projections. In regions that house both osmo- and thermoreceptive neurons (OVLT, SON, PVN), these functions may occur in the same cell and may be transduced by the same molecular entity (e.g., TRPV channels). B: enlargement of region in A delineated by dashed box. OVLT contains both osmo- and thermosensitive neurons that project to MnPO, SON, PVN, thalamus, and anterior cinguate cortex; MnPO is an integrating center that receives input from OVLT and SFO as well as other brain areas relaying information from peripheral baro-, thermo-, and osmoreceptors. It projects to SON, PVN, MPO/AH, and thalamus. SON and PVN contain magnocellular VP and OT neurons that project to PP. They function as both osmo- and thermosensors as well as receiving information from the osmo- and thermosensors in OVLT and SFO. The thalamus, insula, and anterior cingulate cortex are components of the motivated behavior circuit involved in thirst and water acquisition. AC, anterior cingulate cortex; DM, dorsomedial nuclei; INS, insula; MnPO, nucleus medianus; MPO/AH, Medial preoptic/anterior hypothalamus; OC, optic chiasma; OVLT, organum vasculosum of lamina terminalis; PP, posterior pituitary; PVN, paraventricular nuclei; rRP, rostral Raphe Palidus; SON, supraoptic nuclei; SFO, subfornical organ; SNS, sympathetic nervous system for regulation of BAT and vascular constriction; THAL, thalamus; VP, vasopressin.
In addition to increasing sensitivity, the osmotic and thermal complexes also provide the substrate for integrating crucial physiological information in addition to temperature and osmolality, and the components of the complex can confer selectivity to output responses. For example, the SFO expresses receptors for angiotensin II, a peptide that conveys information about blood pressure and volume, and the SFO is responsible for eliciting drinking in response to angiotensin II (43, 70, 71). The SFO and OVLT also express estrogen receptors (74) and thus have the potential to modulate osmotic and thermal homeostasis in concert with the reproductive cycle.
TRPV channels expressed in SFO, OVLT, and magnocellular SON and PVN neurons are strong candidates for the molecular mechanism integrating thermal and fluid balance homeostasis. The thermal and osmosensitive cells in OVLT and SFO express TRPV channels and innervate appropriate effectors including: 1) the MnPO, which in turn innervates the various “warm-sensitive” neurons in MPO that independently regulate autonomic control of the heat conservation/dissipation thermoeffector pathways; 2) the VP secreting neurons in SON and PVN, which are themselves thermal and osmosensitive; and 3) the thalamocortical pathways involved in generating thirst (the sensation that motivates behaviors to increase water intake). While the exact nature and composition of the TRPV channels is not yet fully understood, it is clear that endogenously expressed products of the trpv1, trpv2, and trpv4 genes participate. Additionally, the possibility that plasticity exists in expression and/or composition of the TRPV channels (e.g., TRPV subtype and homomeric/heteromeric characteristics) and can be driven by physiological (gender, age, hydration status, etc.) or pathophysiological conditions (e.g., fever, stress, hypertension, etc.) remains to be explored. Thus it is likely that the composition of TRPV heteromeric channels differs between cell types (neurons and glia) and with different physiological states. It also follows that such plasticity in channel composition would result in variable responses to temperature and osmotic stimuli. As such, the predominant parameter (temperature or osmolality) could fluctuate depending on the physiological context.
While this review has focused on the TRPV channels, other channels may also have a role in integrating thermal and osmotic stimuli. For example, the sodium-sensitive, NaX channel is expressed in SFO and MnPO, regions that contain Na+-sensitive neurons (33, 68). Also, all subunits of the epithelial Na channels (ENaC) are expressed in VP neurons (79). Temperature sensitivity of the NaX channels remains to be evaluated, but at least in expression systems ENaC has been shown to be temperature sensitive (5). Involvement of Na+-sensing mechanisms in coordinating thermal and fluid homeostasis is reasonable since sodium loss during sweating can compromise fluid retention and cardiovascular function. Thus future studies may elucidate molecular mechanisms in addition to TRPV channels that contribute to integration of body temperature and water homeostasis.
Because of the focus of this review on the importance of evaporative cooling and thus maintenance of water balance for preventing hyperthermia, we have not discussed another interesting and important aspect of thermal and osmotic integration: The involvement of the “cold-sensing” mechanisms. The early reports of “cold diuresis” mentioned earlier (8), supports a role for cold as well as heat in regulation of VP secretion. Another member of the TRP channel family has been implicated in sensing cold, TRPM8 (47, 61). Its role in regulation of water balance remains to be examined, but it represents yet another potential channel contributing to integration of temperature and water homeostasis.
GRANTS
This work was supported by National Institute of Health Grants NS-27975 and HD-072428 (to C. D. Sladek) and HL-14388, HL-98207, MH-80241 (to A. K. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.D.S. and A.K.J. conception and design of research; C.D.S. and A.K.J. performed experiments; C.D.S. and A.K.J. analyzed data; C.D.S. and A.K.J. interpreted results of experiments; C.D.S. prepared figures; C.D.S. and A.K.J. drafted manuscript; C.D.S. and A.K.J. edited and revised manuscript; C.D.S. and A.K.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Wanida R. Stevens (University of Colorado School of Medicine) for contribution to the work in Fig. 1, and Dr. Daniel Badauê-Passos Jr, (University of Iowa; current address: Departamento de Fisiologia, Universidade Federal de Sergipe, Aracaju, SE, Brazil) for contributions to Fig. 2.
REFERENCES
- 1.Adem A, Jossan SS, D'Argy R, Gillberg PG, Nordberg A, Winblad B, Sara V. Insulin-like growth factor 1 (IGF-1) receptors in the human brain: quantitative autoradiographic localization. Brain Res 503: 299–303, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Andersson B. Regulation of water intake. Physiol Rev 58: 582, 1978 [DOI] [PubMed] [Google Scholar]
- 3.Andersson B, Gale CC, Sundsten JW. Effects of chronic central cooling on alimentation and thermoregulation. Acta Physiol Scand 55: 177–188, 1962 [DOI] [PubMed] [Google Scholar]
- 4.Andersson B, Larsson B. Influence of local temperature changes in the preoptic area and rostral hypothalamus on the regulation of food and water intake. Acta Physiol Scand 52: 75–89, 1961 [DOI] [PubMed] [Google Scholar]
- 5.Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader RA, Eliot JW, Bass DE. Hormonal and renal mechanisms of cold diuresis. J Appl Physiol 4: 649–658, 1952 [DOI] [PubMed] [Google Scholar]
- 7.Baker MA, Doris PA. Control of evaporative heat loss during changes in plasma osmolality in the cat. J Physiol 328: 535–545, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berl T, Kleeman CR. Temperature and fluid balance. Isr J Med Sci 12: 1004–1009, 1976 [PubMed] [Google Scholar]
- 9.Bohannon NJ, Corp ES, Wilcox BJ, Figlewicz DP, Dorsa DM, Baskin DG. Localization of binding sites for insulin-like growth factor-1 (IGF-1) in the rat brain by quantitative radiography. Brain Res 444: 205–213, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Boulant JA. Hypothalamic neurons regulating body temperature. Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. II, chapt. 6, p. 105–126, 1996 [Google Scholar]
- 11.Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol 48: 639–654, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nature Rev Neurosci 9: 519–531, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bourque CW, Oliet SHR, Richard D. Osmoreceptors osmoreception, osmoregulation. Front Neuroendocrinol 15: 231–274, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Buggy J, Hoffman WE, Phillips MI, Fisher AE, Johnson AK. Osmosensitivity of rat third ventricle and interactions with angiotensin. Am J Physiol Regul Integr Comp Physiol 236: R75–R82, 1979 [DOI] [PubMed] [Google Scholar]
- 15.Buggy J, Johnson AK. Anteroventral third ventricle periventricular ablation: Temporary adipsia and persisting thirst deficits. Neurosci Lett 5: 177–182, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Carithers J, Bealer SL, Brody MJ, Johnson AK. Fine structural evidence of degeneration in supraoptic nucleus and subfornical organ of rats with lesions in the anteroventral third ventricle. Brain Res 201: 1–12, 1980 [DOI] [PubMed] [Google Scholar]
- 17.Carithers J, Johnson AK. Lesions of the tissue surrounding the preoptic recess (AV3V region) affect neurosecretory cells in the paraventricular nuclei in the rat. Brain Res 337: 233–243, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Carreno FR, Ji LL, Cunningham JT. Altered central TRPV4 expression and lipid raft association related to inappropriate vasopressin secretion in cirrhotic rats. Am J Physiol Regul Integr Comp Physiol 296: R454–R466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 292: R64–R76, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Chakfe Y, Bourque CW. Peptidergic excitation of supraoptic nucleus neurons: Involvement of stretch-inactivated cation channels. Exp Neurol 171: 210–218, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciura S, Liedtke W, Bourque CW. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci 31: 14669–14676, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn FL, Brennan TJ, Nelson AE, Robertson GL. The role of blood osmolarity and volume in regulating vasopressin secretion in the rat. J Clin Invest 52: 3212–3219, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M, Farrell M, Lancaster J, Jackson G, Fox P, Denton D. Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci USA 100: 15241–15246, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan-xin M, Li-mei S, Bei S, Xin Q, Yu Y, Yu C. Heat shock factor 1 regulates the expression of the TRPV1 gene in the rat preoptic-anterior hypothalamus area during lipopolysaccharide-induced fever. Exp Physiol 97: 730–740, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Forsling ML, Ingram DL, Stanier MW. Effect of changes in hypothalamic temperature on ADH secretion in pigs. J Physiol 247: 49P–50P, 1975 [PubMed] [Google Scholar]
- 28.Forsling ML, Ingram DL, Stanier MW. Effects of various ambient temperatures and of heating and cooling the hypothalamus and cervical spinal cord on antidiuretic hormone secretion and urinary osmolality in pigs. J Physiol 257: 673–686, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 23: 183–191, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hamilton CL, Ciaccia PJ. Hypothalamus, temperature regulation, and feeding in the rat. Am J Physiol 221: 800–807, 1971 [DOI] [PubMed] [Google Scholar]
- 31.Hindmarch C, Yao S, Beighton G, Paton J, Murphy D. A comprehensive description of the transcriptome of the hypothalamoneurohypophyseal system in euhydrated and dehydrated rats. Proc Natl Acad Sci USA 103: 1609–1614, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes 58: 174–184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiyama TY, Watanabe E, Ono K, Inenaga K, Tamkun MM, Yoshida S, Noda M. NaX channel involved in CNS sodium-level sensing. Nature Neurosci 5: 511–512, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Hollis JH, McKinley MJ, D'Souza M, Kampe J, Oldfield BJ. The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: a neuroanatomical framework for the generation of thirst. Am J Physiol Regul Integr Comp Physiol 294: R1390–R1401, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Ingram DL, Stephens DB. The relative importance of thermal, osmotic and hypovolaemic factors in the control of drinking in the pig. J Physiol 293: 501–512, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AK, Buggy J. Periventricular preoptic-hypothalamus is vital for thirst and normal water economy. Am J Physiol Regul Integr Comp Physiol 234: R122–R129, 1978 [DOI] [PubMed] [Google Scholar]
- 37.Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem 277: 44448–44454, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Kirkpatrick K, Bourque CW. Effects of neurotensin on rat supraoptic neurons in vitro. J Physiol 482: 373–381, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi S, Hori A, Matsumura K, Hosokawa H. Point: Heat-induced membrane depolarization of hypothalamic neurons: a putative mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol 290: R1479–R1480, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci 22: 825–834, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA 100: 13698–13703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lind RW, Johnson AK. Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci 2: 1043–1051, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannari T, Morita S, Furube E, Tominaga M, Miyata S. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 61: 957–971, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Mason WT. Supraoptic neurons of rat hypothalamus are osmosensitive. Nature 287: 154–157, 1980 [DOI] [PubMed] [Google Scholar]
- 46.McGaraughty S, Segreti JA, Fryer RM, Brown BS, Faltynek CR, Kym PR. Antagonism of TRPV1 receptors indirectly modulates activity of thermoregulatory neurons in the medial preoptic area of rats. Brain Res 1268: 58–67, 2009 [DOI] [PubMed] [Google Scholar]
- 47.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 48.McKinley MJ, Cairns MJ, Denton DA, Egan G, Mathai ML, Uschakov A, Wade JD, Weisinger RS, Oldfield BJ. Physiological and pathophysiological influences on thirst. Physiol Behav 81: 795–803, 2004 [DOI] [PubMed] [Google Scholar]
- 49.McKinley MJ, Denton DA, Weisinger RS. Sensors for antidiuresis and thirst–osmoreceptors or CSF sodium detectors? Brain Res 141: 89–103, 1978 [DOI] [PubMed] [Google Scholar]
- 50.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 285: C96–C101, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol 110: 1137–1149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 16: 74–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 93: 773–797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science 134: 560–561, 1961 [DOI] [PubMed] [Google Scholar]
- 56.Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, Cunningham JT. Expression and distribution of TRPV2 in rat brain. Exp Neurol 237: 223–237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen B. Effects of changes in plasma volume and osmolarity on thermoregulation during exercise. Acta Physiol Scand 90: 725–730, 1974 [DOI] [PubMed] [Google Scholar]
- 58.Nissen R, Bourque CW, Renaud LP. Membrane properties of organum vasculosum lamina terminalis neurons recorded in vitro. Am J Physiol Regul Integr Comp Physiol 264: R811–R815, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Oliet SHR, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature 364: 341–343, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Oliet SHR, Bourque CW. Steady-state osmotic modulation of cationic conductance in neurons of rat supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol 265: R1475–R1479, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300: 1284–1288, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61: 228–261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutter AR, Ma QP, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport 16: 1735–1739, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nature Neurosci 9: 93–98, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Sharif-Naeini R, Ciura S, Bourque CW. TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron 58: 179–185, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Sharif-Naeini R, Ciura S, Zhang Z, Bourque CW. Contribution of TRPV channels to osmosensory transduction, thirst, and vasopressin release. Kidney Int 73: 811–815, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 54: 59–72, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Silva NL, Boulant JA. Effects of osmotic pressure, glucose, and temperature on neurons in preoptic tissue slices. Am J Physiol Regul Integr Comp Physiol 247: R335–R345, 1984 [DOI] [PubMed] [Google Scholar]
- 70.Simpson JB, Routtenberg A. Subfornical organ: a dipsogenic site of action of angiotensin II. Science 201: 379–381, 1978 [DOI] [PubMed] [Google Scholar]
- 71.Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science 181: 1172–1175, 1973 [DOI] [PubMed] [Google Scholar]
- 72.Sladek CD, Johnson AK. The effect of anteroventral third ventricle lesions on vasopressin release by organ cultured hypothalamo-neurohypophyseal explants. Neuroendocrinology 37: 78–84, 1983 [DOI] [PubMed] [Google Scholar]
- 73.Sladek CD, Knigge KM. Osmotic control of vasopressin release by organ cultured hypothalamo-neurohypophyseal explants from the rat. Endocrinology 101: 1834–1838, 1977 [DOI] [PubMed] [Google Scholar]
- 74.Somponpun SJ, Johnson AK, Beltz T, Sladek CD. Estrogen receptor-alpha expression in osmosensitive elements of the lamina terminalis: regulation by hypertonicity. Am J Physiol Regul Integr Comp Physiol 287: R661–R669, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Stricker EM, Sved AF. Controls of vasopressin secretion and thirst: similarities and dissimilarities in signals. Physiol Behav 77: 731–736, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Sundsten JW. Alterations in water intake and core temperature in baboons during hypothalamic thermal stimulation. Ann NY Acad Sci 157: 1018–1029, 1969 [DOI] [PubMed] [Google Scholar]
- 77.Szczepanska-Sadowska E. Plasma ADH increase and thirst suppression elicited by preoptic heating in the dog. Am J Physiol 226: 155–161, 1974 [DOI] [PubMed] [Google Scholar]
- 78.Taylor AC, McCarthy JJ, Stocker SD. Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol 294: R1285–R1293, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Teruyama R, Sakuraba M, Wilson LL, Wandrey NE, Armstrong WE. Epithelial Na+ sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. Am J Physiol Endocrinol Metab 302: E273–E285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thrasher TN, Keil LC, Ramsay DJ. Lesions of the organum vasculosm of the lamina terminals (OVLT) attenuate osmotically induced drinking and vasopressin secretion in the dog. Endocrinology 110: 1837–1839, 1982 [DOI] [PubMed] [Google Scholar]
- 81.Travis KA, Johnson AK. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. Am J Physiol Regul Integr Comp Physiol 264: R1200–R1205, 1993 [DOI] [PubMed] [Google Scholar]
- 82.Unger J, McNeill TH, Moxley IIIRT, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31: 143–157, 1989 [DOI] [PubMed] [Google Scholar]
- 83.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534: 813–825, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verney EB. The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond 135: 25–106, 1947 [PubMed] [Google Scholar]
- 85.Videla LA, Fernandez V, Tapia G, Varela P. Thyroid hormone calorigenesis and mitochondrial redox signaling: upregulation of gene expression. Front Biosci 12: 1220–1228, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Wainwright A, Rutter AR, Seabrook GR, Reilly K, Oliver KR. Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J Comp Neurol 474: 24–42, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Wechselberger M, Wright CL, Bishop GA, Boulant JA. Ionic channels and conductance-based models for hypothalamic neuronal thermosensitivity. Am J Physiol Regul Integr Comp Physiol 291: R518–R529, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Whyte DG, Johnson AK. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (AV3V) during acute heat stress in the rat. Clin Exp Pharmacol Physiol 32: 457–461, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Bourque CW. Osmometry in osmosensory neurons. Nature Neurosci 6: 1021–1022, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y, Boulant JA. Temperature effects on neuronal membrane potentials and inward currents in rat hypothalamic tissue slices. J Physiol 564: 245–257, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]