Abstract
Systemic lupus erythematosus (SLE) is a chronic inflammatory disorder with prevalent hypertension and renal injury. In this study, we tested whether the renal nerves contribute to the development of hypertension in an established mouse model of SLE (NZBWF1). Female SLE and control (NZW/LacJ) mice were subjected to either bilateral renal denervation or a sham procedure at 32 wk of age. Two weeks later, blood pressure was assessed in conscious mice using carotid artery catheters. Blood pressure was higher in SLE mice compared with controls, as previously reported; however, blood pressure was not altered in the denervated SLE or control mice. The development of albuminuria was markedly blunted in denervated SLE mice; however, glomerulosclerosis was increased. Renal denervation reduced renal cortical expression of monocyte-chemoattractant protein in SLE mice but did not significantly alter renal monocyte/macrophage infiltration. Renal cortical TNF-α expression was also increased in sham SLE mice, but this was not impacted by denervation. This study suggests that the renal nerves do not have a significant role in the pathogenesis of hypertension, but have a complex effect on the associated renal inflammation and renal injury.
Keywords: blood pressure, albuminuria, renal injury, immune, inflammation
systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that primarily affects women during child-bearing years. The prevalence of hypertension, a major cardiovascular risk factor, is markedly increased in women with SLE, and cardiovascular disease is the leading cause of mortality in these women (38). Given the central role of the kidneys in long-term blood pressure control and that nephritis is evident in greater than 50% of patients with SLE (with nearly all patients exhibiting signs of renal injury on biopsy) (4, 13), it is likely that altered renal excretory function during SLE contributes to the prevalent hypertension in this patient population. Our previously published work in a hypertensive mouse model of SLE showing an impaired chronic pressure natriuresis relationship (26) and attenuated renal hemodynamic function (43) supports this concept; however, the mechanisms that promote impaired renal function and hypertension during SLE remain unclear.
The contributions of the renal sympathetic nerves to excretory function and experimental hypertension have been well documented (8, 12, 24). In addition, recent data showing that high-frequency radioablation of the renal arteries is a potentially effective treatment for resistant hypertension suggests an important role for renal nerves in human hypertension (39). Although autonomic dysfunction is common in SLE patients (11), the potential contribution of the renal nerves to the prevalent hypertension associated with SLE has not been tested. To determine whether the renal nerves directly contribute to SLE-associated hypertension, we tested the hypothesis that bilateral renal denervation in an established hypertensive mouse model of SLE prevents the development of hypertension.
METHODS
Animals.
Female NZBWF1 (SLE) and NZW/LacJ (control) mice were obtained from Jackson Laboratories (Bar Harbor, ME). At 32 wk of age, mice were randomly divided into four groups (n = 9–11 animals per group): sham-operated control mice (control/sham), bilaterally renal denervated control mice (control/Dnx), sham-operated SLE mice (SLE/sham), and bilaterally renal denervated SLE mice (SLE/Dnx). Animals with no evidence of albuminuria, measured by Albustix (26, 42, 44) at the time of the procedure, were included in the study. Bilateral renal denervation (Dnx) was performed on animals maintained under gas anesthesia (isoflurane), as previously described by others (1). Briefly, the right and left renal artery and vein were approached retroperitoneally, cleared of connective tissue, and separated from the renal vein. The renal artery was then carefully coated with a 10% phenol solution in ethanol and rinsed with normal saline to remove residual phenol. Sham surgery was performed without dissection of the artery and vein in controls. Mice were maintained on a 12:12-h light-dark cycle in temperature-controlled rooms with access to food and water ad libitum. All studies were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Tissue catecholamines.
To confirm bilateral renal denervation, renal norepinephrine, epinephrine, and dopamine were measured in both kidneys (whole kidney homogenates) using high-performance liquid chromatography as previously reported (32). Denervation was considered successful if the renal norepinephrine level of both kidneys were reduced by greater than 80% compared with the average of the norepinephrine content in sham mice (controls and SLE). One SLE/sham mouse was removed from the study because the animal had very low renal norepinephrine content (<20% of average renal norepinephrine of all sham mice), suggesting that the renal nerves may have been damaged during the sham procedure.
Anti-double-stranded DNA.
Plasma anti-double-stranded DNA (dsDNA) antibodies, a clinical hallmark of SLE, were measured by ELISA (Alpha Diagnostics, San Antonio, TX), as previously described by our laboratory (26). Animals that were positive for anti-dsDNA auto-antibodies were determined to have SLE, as we previously described (10, 42).
Blood pressure.
Two weeks after the denervation or sham procedure, when mice were 34 wk of age, mean arterial blood pressure was measured in conscious, freely moving mice using in-dwelling carotid artery catheters after a 24-h recovery period, as previously described by our laboratory (25, 26, 42) At the conclusion of the study, anesthetized animals were perfused and tissues were harvested and stored for histological and biochemical analysis. The 2-wk time course of the study was chosen to avoid potential reinnervation, which can occur rapidly in rodents (28).
Renal injury.
Weekly urinary albumin was evaluated using dipstick assay, as previously described by our laboratory (25). Albumin levels were confirmed via commercial ELISA (Alpha Diagnostics, San Antonio, TX), according to manufacturer's instructions, in urine samples collected overnight, as previously published by our laboratory (26, 42, 44). Data were normalized to urine creatinine levels.
Kidneys were fixed in formalin and stored in 70% ethanol for paraffin embedding and slicing. Sections were stained with periodic acid Schiff to assess glomerulosclerosis, as defined by mesangial expansion, extracellular matrix deposition, and capillary morphology. A minimum of 30 glomeruli was analyzed for each animal using a scale to define the area (%) of each glomerulus that exhibits injury. The scale is as follows: 0 (no injury), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%) (44).
Renal cortical expression of neutrophil gelatinase-associated lipocalin (NGAL) was measured as an index of renal tubular injury using a goat polyclonal anti-mouse lipocalin-2/NGAL antibody (1:500; R&D Systems, Minneapolis, MN).
Renal inflammation.
Renal cortical and medullary TNF-α and monocyte chemoattractant protein (MCP-1) protein expression, as indices of inflammation, were measured by Western blot analysis. TNF-α was measured as previously described (42), and MCP-1 was detected using a goat polyclonal anti-mouse MCP-1 (Santa Cruz-18 kDa or Abcam-12 kDa). All proteins were visualized on the Odyssey infrared scanner (LI-COR Biosciences, Lincoln, NE) using an appropriate IR700-conjugated antibody (Rockland Immunologicals, Gilbertsville, PA). Data are presented as a ratio of densitometry units of protein (based on band optical density) and normalized to β-actin.
Renal monocyte and macrophage infiltration was assessed in sections (4 μm) incubated with an F4/80 antibody, as previously described by our laboratory (44).
Statistical analysis.
Data are presented as means ± SE. Statistical analyses were performed using either SigmaStat 3.0 software (Systat, Richmond, CA) or GraphPad Prism 5 (La Jolla, CA). Two-way ANOVA was used followed by the Holm-Sidak method for testing comparisons between multiple groups. Because of the large variability in urinary albumin, glomerulosclerosis, and immune cell infiltration common to this model of SLE, the data are presented as geometric means, and the two-way ANOVA was conducted using natural log-transformed raw data. A Student's t-test was used when comparing only two groups. Values were considered statistically different at P < 0.05.
RESULTS
Body weight.
Over the 2-wk course of the study, body weight decreased in all groups to a similar degree (control/sham: 7 ± 3%; control/denervation: 13 ± 2%; SLE/sham: 15 ± 4%; and SLE/denervation: 16 ± 4%). At the time of blood pressure measurement, body weight was higher in SLE mice compared with controls (43 ± 2 vs. 33 ± 1 g; P < 0.001), consistent with our previously published work (37). Body weight was not different between control (31 ± 1 g) or SLE (41 ± 2 g)-denervated mice and their sham controls.
Renal catecholamines.
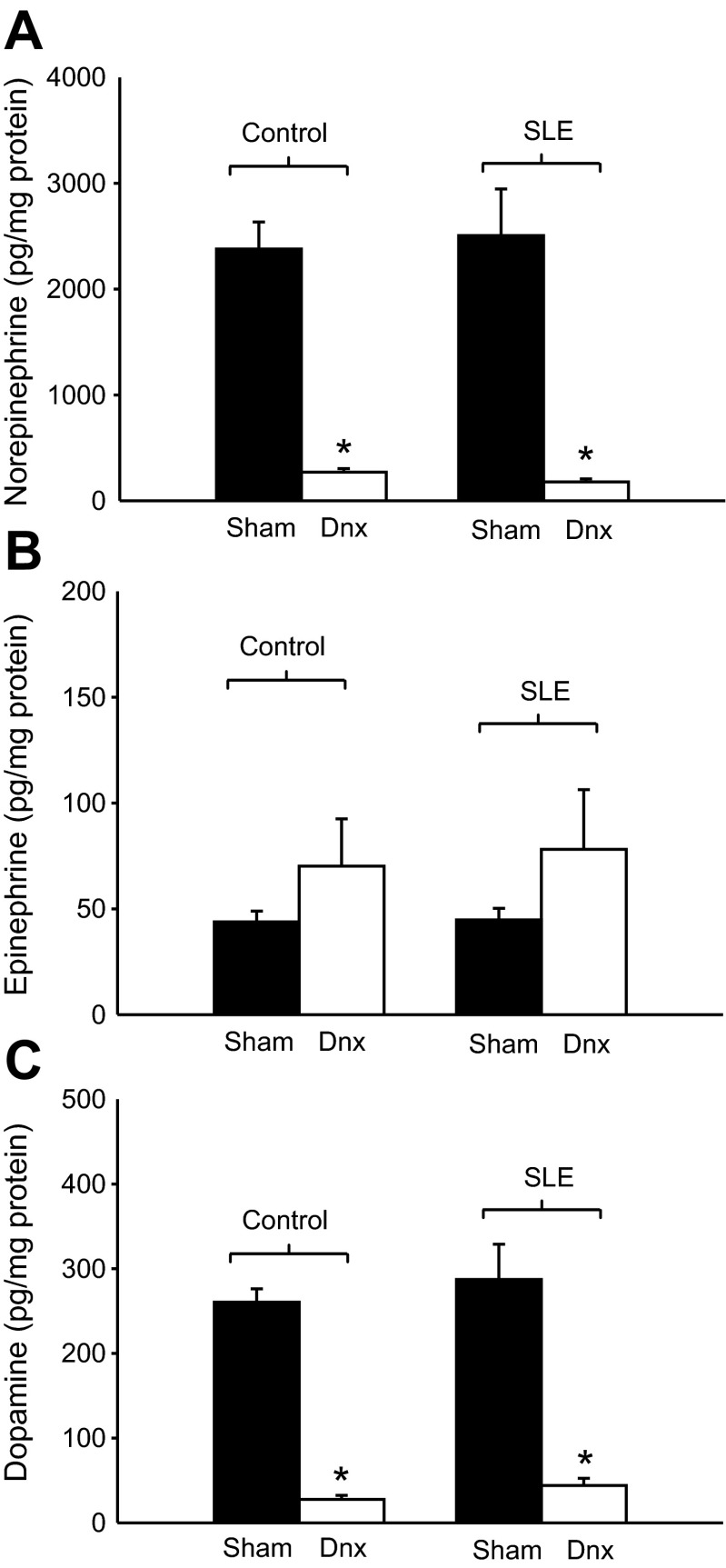
Total renal content of norepinephrine, epinephrine, and dopamine were similar in sham-operated control and SLE mice (Fig. 1, A–C). Renal norepinephrine and dopamine content was markedly reduced following denervation in both control and SLE mice (P < 0.001), whereas renal epinephrine content was not significantly altered in either group. These data confirm successful denervation.
Fig. 1.
Renal denervation reduces renal catecholamine content. Norepinephrine and dopamine (pg/mg protein) were significantly reduced in control and systemic lupus erythematosus (SLE)-denervated animals (n = 6–12). Epinephrine levels (pg/mg protein) were unchanged by denervation (Dnx) in control and SLE mice. *P < 0.05 vs. corresponding sham group.
Plasma anti-dsDNA.
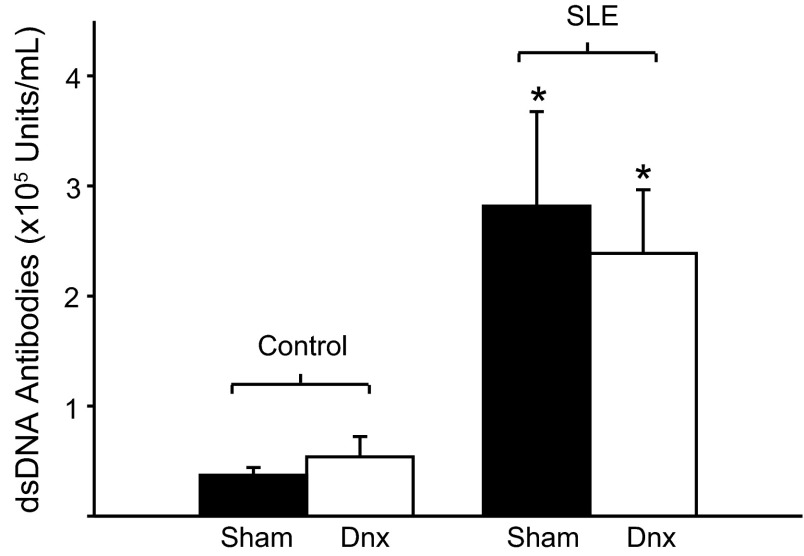
Plasma levels of anti-dsDNA auto-antibodies, a clinical marker of SLE, were significantly elevated in SLE mice (2.8 × 105 ± 8.6 × 104 units/ml; P < 0.05) compared with controls (3.7 × 104 ± 7.1 × 103 units/ml; P = 0.003), as expected (25, 42, 44). Renal denervation did not alter levels of dsDNA autoantibody in SLE (2.4 × 105 ± 5.8 × 104 units/ml) or control (5.6 × 104 ± 2.1 × 104 units/ml) mice (Fig. 2).
Fig. 2.
Renal denervation does not alter dsDNA in SLE mice. Autoantibody levels were significantly elevated in SLE mice compared with control mice (n = 6–9). Renal denervation (Dnx) had no effect in either group. *P < 0.05 vs. corresponding control group.
Blood pressure.
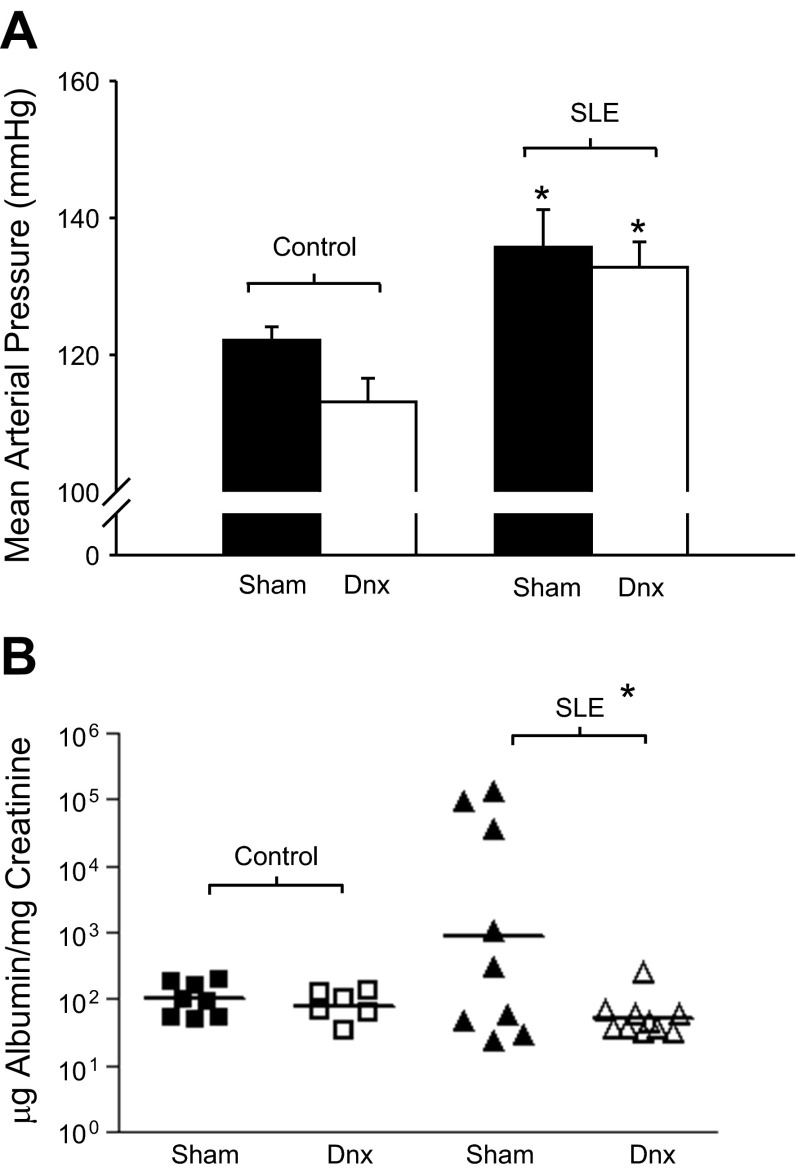
As previously reported, mean arterial pressure was significantly higher in SLE mice compared with controls (136 ± 5 vs. 122 ± 2 mmHg; P = 0.024; Fig. 3A, P < 0.05); however, renal denervation did not affect blood pressure in SLE (133 ± 4 mmHg) or control (113 ± 3 mmHg) mice.
Fig. 3.
Renal denervation reduces albuminuria independently of blood pressure in SLE mice. A: mean arterial blood pressure (mmHg) was significantly elevated in SLE mice compared with controls (n = 4–8). Renal denervation (Dnx) had no effect in either group. *P < 0.05 vs. corresponding Control. B: urinary albumin (μg/mg creatinine) was increased in SLE mice compared with controls (n = 6–9). Renal denervated SLE mice had urinary albumin levels comparable to control sham and control Dnx mice. The horizontal line represents the geometric mean for each group. The impact of Dnx on albuminuria was statistically significant (*P < 0.04).
Renal injury.
Consistent with our previous work, we observed the expected progression of albuminuria in this model with a prevalence of 44% of SLE/sham mice by 34 wk of age (25). In contrast, none of the SLE/Dnx or control (sham or Dnx) mice developed albuminuria over the course of the study (Albustix). A quantitative assessment of urinary albumin levels was performed with samples from 34-wk-old animals using ELISA. Consistent with the Albustix data, albuminuria (geometric mean) was elevated in SLE/sham (886 μg albumin/mg creatinine) compared with control/sham (101 μg albumin/mg creatinine) mice (Fig. 3B) at 34 wk of age. This was markedly attenuated in SLE/Dnx mice (52 μg albumin/mg creatinine) making the urinary albumin comparable to control/sham and control/Dnx mice (81 μg albumin/mg creatinine). The impact of renal Dnx on albuminuria was statistically significant (P < 0.04).
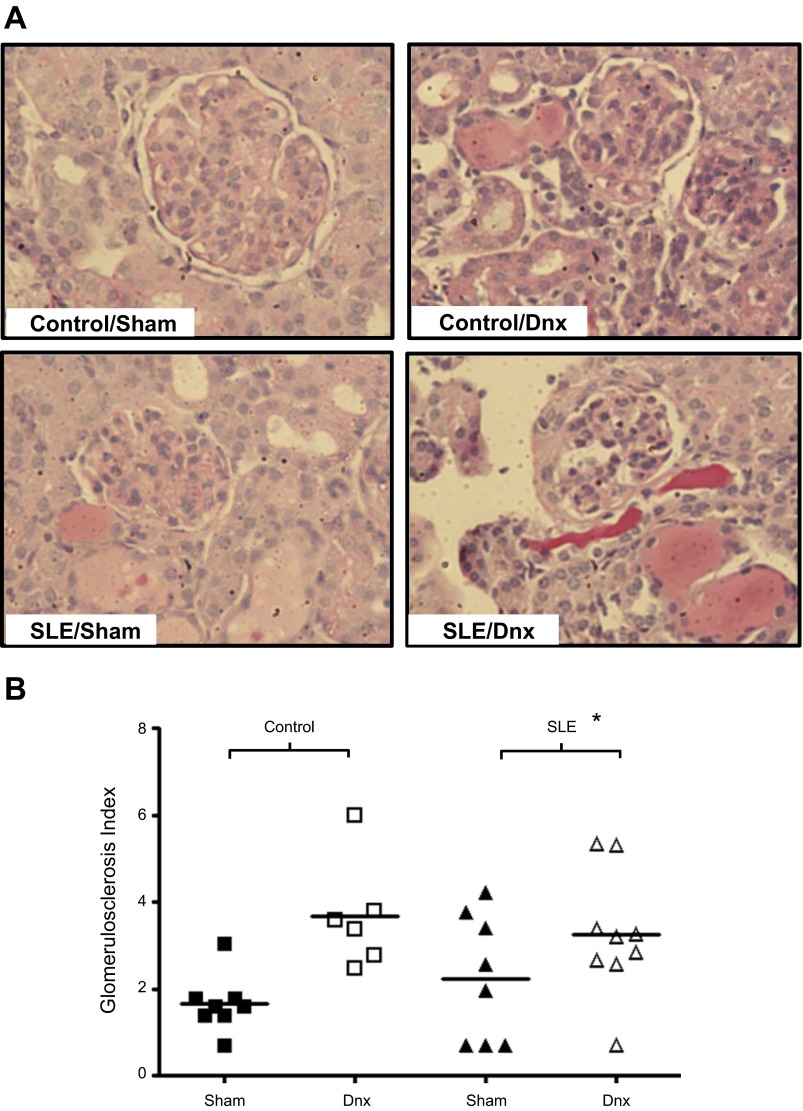
We previously reported the presence of glomerulosclerosis in SLE mice using a semiquantitative analysis (42, 44). Glomerulosclerosis index (GSI) was increased in sham SLE mice compared with sham controls (P < 0.01). On the basis of the albuminuria data, it was anticipated that GSI would be lower in the denervated animals. However, this was not the case, as GSI was increased in both SLE and control mice after denervation (Fig. 4).
Fig. 4.
Renal denervation increased glomerulosclerosis. Representative pictures (×20), indicating that the glomerulosclerosis index (A) and the glomerulosclerosis index (B) was increased following denervation in control and SLE mice. *P < 0.01 vs. control sham.
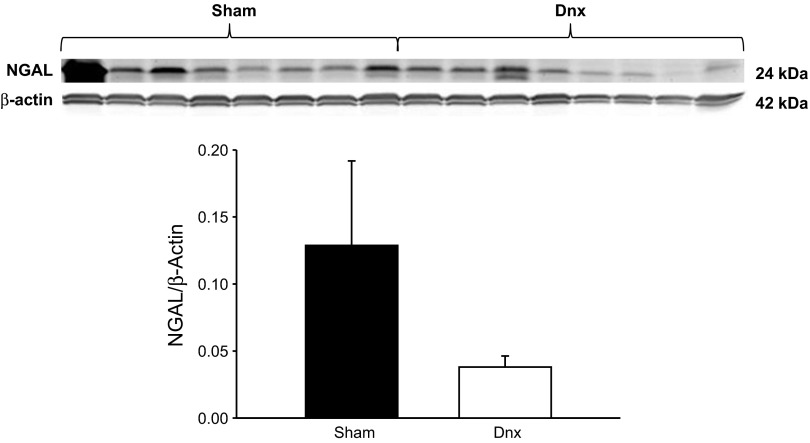
Because the proximal tubule is an important site of albumin reabsorption and is highly innervated (6, 40), we measured renal cortical expression of NGAL, a marker of proximal tubular injury. Although there was a tendency for reduced renal cortical expression of NGAL (Fig. 5) in denervated SLE mice, this did not reach a statistical significance (P = 0.176), making the role of the proximal tubule unclear.
Fig. 5.
Marker of proximal tubule injury after denervation. Renal cortical expression of neutrophil gelatinase-associated lipocalin (NGAL) in sham and denervated SLE mice (P = 0.176).
Renal inflammation.
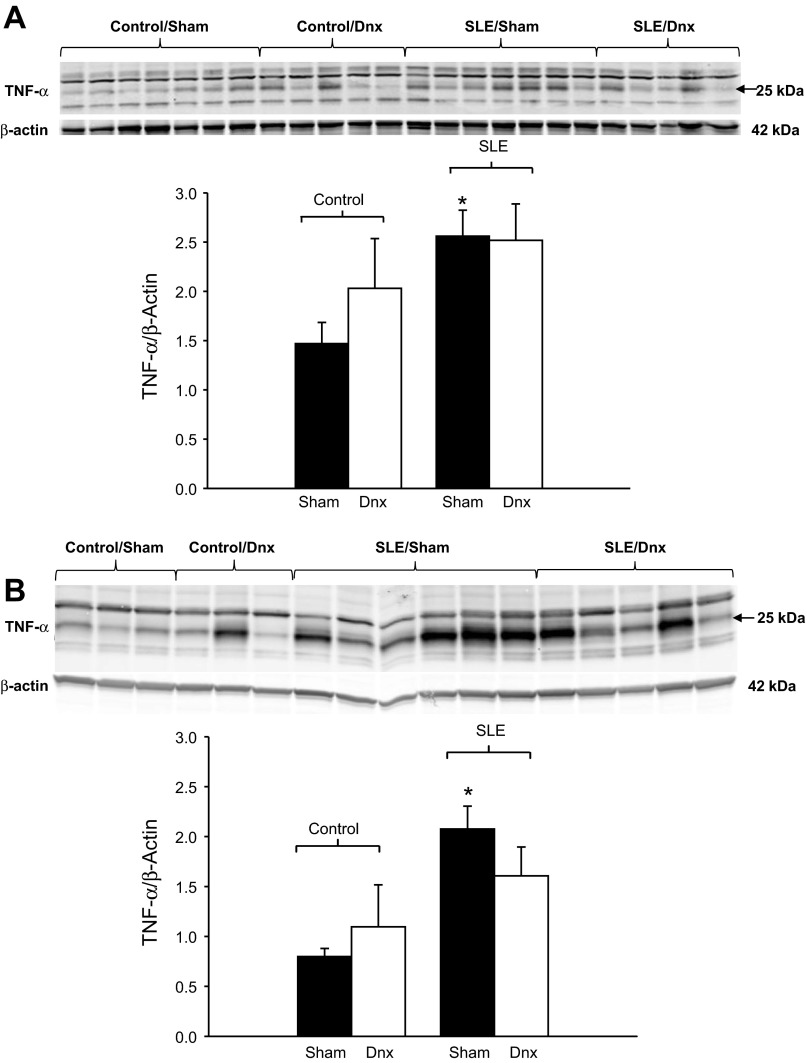
To determine whether denervation altered renal inflammation, we assessed renal cortical and medullary protein expression of TNF-α and MCP-1. Renal cortical expression of TNF-α was significantly increased in SLE mice compared with control mice (Fig. 6A; P = 0.013), as we have previously reported (42). Similarly, renal medullary expression of TNF-α was increased in SLE mice (Fig. 6B; P = 0.008). TNF-α expression was not altered by denervation in either the cortex or medulla.
Fig. 6.
Renal denervation did not alter renal TNF-α expression. Renal cortical expression (A) and renal medullary expression (B) of tumor necrosis factor-α (TNF-α) were both increased in SLE mice and unchanged by renal denervation. *P < 0.05 vs. corresponding sham group.
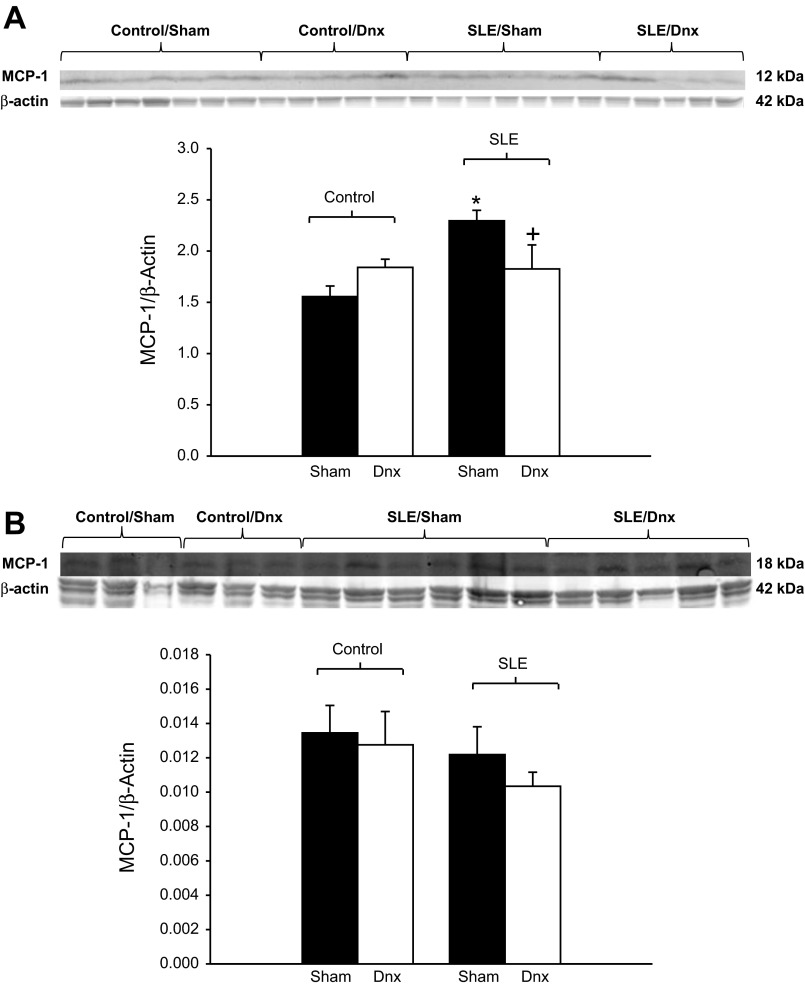
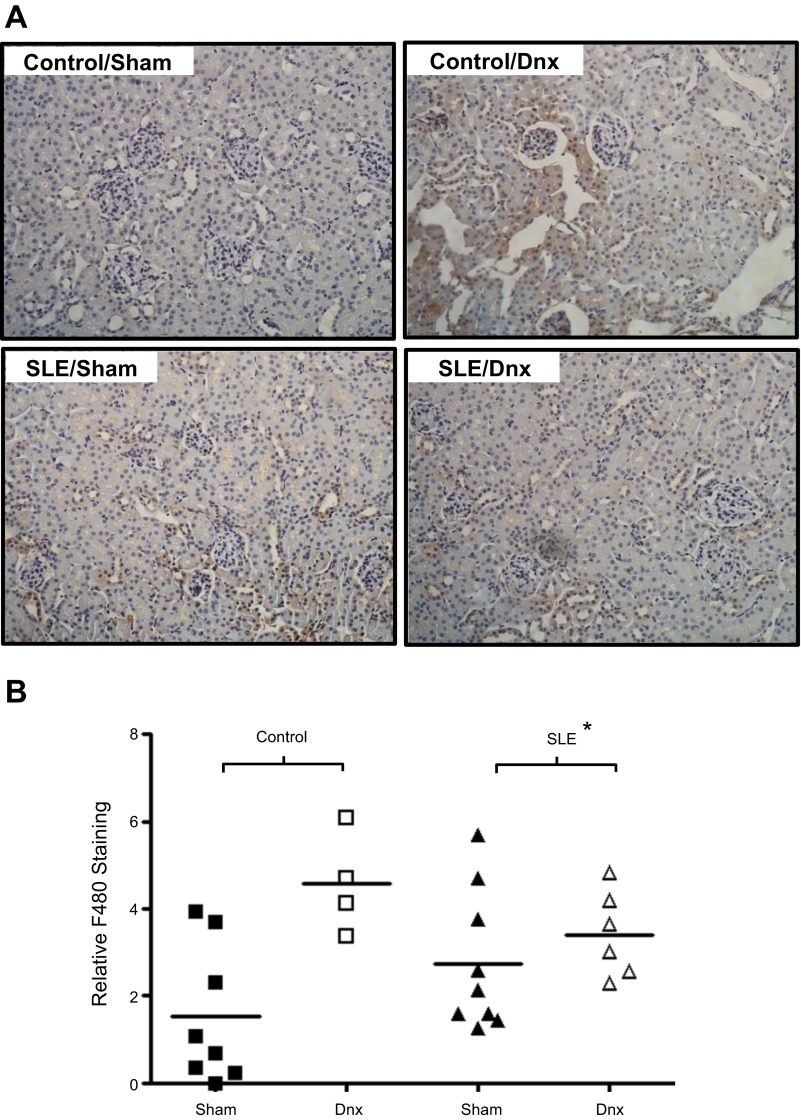
Renal cortical protein expression of MCP-1 was increased in SLE mice (Fig. 7A; P < 0.001) and was significantly lower in denervated SLE mice (P = 0.025). In contrast, renal medullary MCP-1 was similar among all groups (Fig. 7B). Although F4/80 staining was increased in SLE mice compared with sham controls, the reduced renal cortical MCP-1 expression was not associated with lower F4/80 staining in denervated SLE mice (Fig. 8).
Fig. 7.
Renal denervation reduced renal inflammation in SLE mice. A: renal cortical expression of monocyte chemoattractant protein (MCP)-1 was increased in SLE mice compared with controls. MCP-1 expression was lower in denervated SLE mice compared with sham SLE mice. *P < 0.05 vs. corresponding control. +P < 0.05 vs. SLE/Sham. B: renal medullary expression of MCP-1 was similar in all groups (P = 0.727).
Fig. 8.
Renal denervation does not reduce renal cortical F4/80 staining. A: representative pictures (×20) with brown staining indicating monocyte/macrophage infiltration. B: renal cortical staining for F4/80 was increased in mice with SLE. The staining was unchanged in denervated SLE mice and was increased in denervated control mice. *P < 0.05 vs. corresponding control group.
DISCUSSION
In the present study, we investigated whether renal nerves contribute to the development of hypertension during SLE. The rationale was based on the well-known contribution of renal nerves to the pathogenesis of hypertension in humans and experimental animal models, the potential for catecholamines to promote inflammation, and evidence supporting a prominent role for autonomic dysfunction in patients with SLE, a disease with prevalent hypertension and renal disease. The major new findings of this study are 1) renal denervation does not impact blood pressure in female mice with SLE; 2) bilateral renal denervation ameliorates urinary albumin in a female mouse model of SLE; 3) some markers of renal inflammation are lower in denervated mice (i.e., MCP-1), while others are unchanged or even increased (TNF-α, monocyte/macrophage); and 4) glomerulosclerosis is increased after denervation in both control and SLE mice. Taken together, these data suggest that while the renal nerves do not contribute to the hypertension during SLE, at least over the time course studied, there is a complex role for the renal nerves to regulate renal inflammation and injury.
SLE is a chronic autoimmune disorder that predominantly affects women and is associated with prevalent hypertension for reasons that remain unclear. Female NZBWF1 mice represent a widely utilized and established model of lupus nephritis. Our published work has established this as a model of hypertension with a number of factors likely contributing to the hypertension, including endothelial dysfunction (36), impaired renal hemodynamics (26, 43), renal inflammation (42), and oxidative stress (25). Other systems important for blood pressure control may also contribute to the hypertension in this model, including endothelin and the renin-angiotensin system. The previous work of others showed that blockade of the endothelin receptor A ameliorates renal injury in NZBWF1 mice (31). In addition, despite being a low-renin model (35), renin-angiotensin system blockade effectively protects the kidneys in these mice (16). Because the kidneys are highly innervated and sympathetic nerve activity can regulate many of these systems, the present study was designed to test whether renal nerves contribute to the hypertension during SLE.
The sympathetic nervous system has been implicated in both human and experimental models with hypertension and is known to regulate renal hemodynamics. Increased sympathetic activity has been reported in patients with essential hypertension (39) and in obesity-associated hypertension (14). The important role of the renal nerves in blood pressure control is supported by data showing that denervation lowers blood pressure in both humans (22) and in animal models, including obesity-induced hypertension and spontaneous hypertension in rats (17, 19, 20). In addition to the role of renal nerves in regulating tubular sodium and water reabsorption (2, 3), one mechanism by which the renal sympathetic nerves can contribute to the development of hypertension is by promoting increased renal vascular resistance and a rightward shift in the pressure natriuresis relationship (2, 7, 8, 23, 46). These renal hemodynamic changes are consistent with what we recently reported in the NZBWF1 mouse model of SLE (26, 43).
In humans with SLE, autonomic dysfunction is a common occurrence, and data suggest that sympathetic activity may be increased in certain patient populations (11, 15, 47). This is partially based on measurements showing increased serum neuropeptide Y in patients with SLE (15), considered by some to be a marker of sympathetic activity. Consistent with human data, neuropeptide Y is reportedly increased in the kidneys from mice with SLE (5); however, renal sympathetic activity has not been assessed. The current experimental design does not directly assess renal sympathetic nerve activity, and tissue catecholamines are not a good indicator of nerve activity. However, the data showing that renal norepinephrine content is similar between SLE and control mice may indicate that innervation of the kidney is similar.
The finding that blood pressure was not altered by renal denervation in this model of SLE can potentially be explained in the following way. First, it is well known that renal denervation decreases tubular sodium reabsorption (2, 3), an effect that is predicted to lower blood pressure. However, renal injury can reduce filtration fraction, and the data from this study show that glomerulosclerosis is increased in renal denervated mice. In addition, norepinephrine increases filtration fraction (29), suggesting that loss of catecholamines would have the opposite effect. Therefore, it is plausible that the increased glomerular injury and reduced renal catecholamines offset the tubular effects of denervation on sodium reabsorption. Careful assessment of the relationship between chronic renal hemodynamic function and renal injury will ultimately be required to test this hypothesis. Irrespective of the reason behind the blood pressure data, this study suggests that the renal nerves do not have a prominent role in the pathogenesis of hypertension during SLE, at least over the 2-wk time course. This relatively short course of the study was chosen on the basis of evidence that reinnervation occurs quickly in rodents (28) and limits our ability to make conclusions on the long-term impact of denervation on blood pressure during SLE. Several others have also reported that the renal nerves do not figure prominently in the pathogenesis of hypertension. For example, hypertension in the Dahl salt-sensitive rat develops in the same way and to the same degree regardless of whether or not the renal nerves are intact (30). In another example, hypertension resulting from aortic constriction is not altered by renal denervation (9). Finally, renal denervation temporarily delays, but does not prevent, hypertension in a DOCA salt rat model treated with cyclosporine A to induce nephrotoxicity (34).
A somewhat surprising observation made during this study was the marked reduction in urinary albumin from the denervated SLE mice, independent of changes in blood pressure. Albuminuria is an important clinical predictor of future cardiovascular events (33), an important consideration for patients with SLE, in which cardiovascular disease is the leading cause of mortality. The impact of renal denervation to reduce albuminuria independently of blood pressure has also been previously reported in a rat model of anti-Thy-1.1 model of nephritis (41). On the basis of the finding that albuminuria was reduced in denervated SLE mice, it was anticipated that glomerular injury would also be attenuated; however, this was not the case. The increased glomerulosclerosis following denervation in SLE mice may reflect hemodynamic changes in the preglomerular vasculature, given that renal sympathetic activation can increase preglomerular constriction and that denervation can increase renal cortical blood flow (21, 45). It is interesting to speculate that, if filtration is reduced because of the increased glomerulosclerosis or low renal catecholamines, this may also contribute to the reduced albuminuria in the denervated mice. The extent to which this occurs would be difficult to test experimentally. A second factor that could contribute to the reduced levels of albuminuria is related to tubular reabsorption of albumin. The proximal tubule is a major site of albumin reabsorption (40). Therefore, if renal denervation provided protection against tubular injury, the overall ability of the kidney to reabsorb albumin would be predicted to improve. The data showing that NGAL, a marker of proximal tubular injury (27), is reduced in denervated SLE kidneys is consistent with this hypothesis.
The possibility that renal denervation could reduce renal inflammation was also examined in this study. This was based in part on data showing that chronic infusion of catecholamines (phenylephrine) in rats led to increased renal inflammation (osteopontin) and urinary albumin excretion (18). To assess this, renal cortical and medullary TNF-α and MCP-1 protein expression and renal macrophage/monocyte infiltration were examined. As we previously reported, both MCP-1 and TNF-α were increased in the kidneys of SLE mice (42, 44). Bilateral renal denervation significantly reduced cortical MCP-1 expression but did not alter cortical or medullary TNF-α, and did not appear to impact the staining for monocytes and macrophage. These data demonstrate a potentially complex role for the renal nerves in regulating tissue inflammation. The fact that neither TNF-α nor blood pressure was reduced is consistent with our earlier work showing that TNF-α contributes to the pathogenesis of hypertension in this model (42).
Perspectives and Significance
Hypertension is a major risk factor for cardiovascular disease, and chronic inflammation is proposed to contribute to the pathogenesis of hypertension. SLE is a chronic autoimmune inflammatory disorder with prevalent hypertension. It is estimated to affect over 1 million people in the United States, with a majority of the cases occurring in women at an age when the prevalence of hypertension is otherwise very low. The mechanisms for SLE hypertension have not been elucidated. Given the prominent role for the kidney in long-term blood pressure control, the impact of SLE on the kidneys, and the fact that clinical data show that autonomic dysfunction is common in young women with SLE, we hypothesized that the renal nerves play a role in SLE hypertension. However, the studies presented here demonstrate the renal nerves do not play a key role in the development of hypertension during SLE and suggest that there are complex interactions between the renal nerves, tissue injury, and inflammation.
GRANTS
This work was supported by the National Institutes of Health (5T32HL-105324 to K. W. Mathis; HL-085907, HL-085907S3, HL-092284 to M. J. Ryan; and HL-051971 to UMMC-Physiology), the American Heart Association (4350019 to K. W. Mathis, 2260874 to M. Venagas-Pont, and 12060203 to M. J. Ryan), and the University of Mississippi Medical Center-Intramural Research Support Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.W.M., M.R.V.-P., E.R.F., J.M.W., T.M.D., and M.J.R. performed experiments; K.W.M., M.R.V.-P., E.R.F., J.M.W., C.M.-B., T.M.D., and M.J.R. analyzed data; K.W.M., C.M.-B., T.M.D., and M.J.R. interpreted results of experiments; K.W.M. and M.J.R. prepared figures; K.W.M. and M.J.R. drafted manuscript; K.W.M., M.R.V.-P., J.M.W., C.M.-B., T.M.D., and M.J.R. edited and revised manuscript; K.W.M., M.R.V.-P., E.R.F., J.M.W., C.M.-B., T.M.D., and M.J.R. approved final version of manuscript; M.J.R. conception and design of research.
ACKNOWLEDGMENTS
The authors would like to thank Katie W. Corkern, Stephanie P. Evans, C. Warren Masterson, W. Heston Ray, and Haiyan Zhang for their excellent technical assistance with this study.
REFERENCES
- 1.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bell-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest 57: 1104–1107, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bello-Reuss E, Colindres RE, Pastoriza-Munoz E, Mueller RA, Gottschalk CW. Effects of acute unilateral renal denervation in the rat. J Clin Invest 56: 208–217, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boumpas DT, Austin HA, III, Fessler BJ, Balow JE, Klippel JH, Lockshin MD. Systemic lupus erythematosus: emerging concepts. Part 1. Renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med 122: 940–950, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bracci-Laudiero L, Aloe L, Stenfors C, Theodorsson E, Lundeberg T. Development of systemic lupus erythematosus in mice is associated with alteration of neuropeptide concentrations in inflamed kidneys and immunoregulatory organs. Neurosci Lett 248: 97–100, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Dibona GF. Neurogenic regulation of renal tubular sodium reabsorption. Am J Physiol Renal Fluid Electrolyte Physiol 233: F73–F81, 1977 [DOI] [PubMed] [Google Scholar]
- 7.Dibona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens 11: 197–200, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Dibona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Eklof AC, Hokflet T, Aperia A. Renal nerve activity does not contribute to the development of renovascular hypertension in rats with abdominal aortic constriction. Acta Physiol Scand 141: 71–77, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Gilbert EL, Ryan MJ. High dietary fat promotes visceral obesity and impaired endothelial function in female mice with systemic lupus erythematosus. Gend Med 8: 150–155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluck T, Oertel M, Reber T, Zietz B, Scholmerich J, Straub RH. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. I. The hypothalamus-autonomic nervous system axis. J Rheumatol 27: 903–910, 2000 [PubMed] [Google Scholar]
- 12.Granger J, Novak J, Schnackenberg C, Williams S, Reinhart GA. Role of renal nerves in mediating the hypertensive effects of nitric oxide synthesis inhibition. Hypertension 27: 613–618, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Lu X, Miao L, Wu M, Lu S, Luo P. Analysis of clinical manifestations and pathology of lupus nephritis: a retrospective review of 82 cases. Clin Rheumatol 29: 1175–1180, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens 14: 103S–115S, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Harle P, Straub RH, Wiest R, Mayer A, Scholmerich J, Atzeni F, Carrabba M, Cutolo M, Sarzi-Puttini P. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann Rheum Dis 65: 51–56, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herlitz H, Svalander C, Tarkowski A, Westberg G. Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl 6: S684–S686, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol 290: R341–R344, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Gordon KL, Suga S, Duijvestijn AM, Griffin K, Bidani A. Renal injury and salt-sensitive hypertension after exposure to catecholamines. Hypertension 34: 151–159, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25: 893–897, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 2: 266–273, 1980 [DOI] [PubMed] [Google Scholar]
- 21.Kompanowska-Jezierska E, Walkowska A, Johns EJ, Sadowski J. Early effects of renal denervation in the anaesthetised rat: natriuresis and increased cortical blood flow. J Physiol 531: 527–534, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275–1281, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Lohmeier TE. The sympathetic nervous system and long-term blood pressure regulation. Am J Hypertens 14: 147S–154S, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension 33: 487–492, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59: 673–679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathis KW, Venegas-Pont MR, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. Am J Physiol Regul Integr Comp Physiol 301: R1281–R1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Mulder J, Hokfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 304: R675–R682, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers BD, Deen WM, Brenner BM. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res 37: 101–110, 1975 [DOI] [PubMed] [Google Scholar]
- 30.Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, Kashihara N. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant 25: 2889–2898, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Ebihara I, Tomino Y, Koide H. Effect of a specific endothelin A receptor antagonist on murine lupus nephritis. Kidney Int 47: 481–489, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol 34: 1212–1216, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen MH, Wachtell K, Bella JN, Palmieri V, Gerdts E, Smith G, Nieminen MS, Dahlof B, Ibsen H, Devereux RB. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 18: 453–459, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Reinhold U, Kukel S, Goeden B, Neumann U, Wehrmann W, Kreysel HW. Interleukin-4 promotes the expansion of skin-infiltrating lymphocytes from atopic dermatitis in vitro. J Invest Dermatol 96: 370–375, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am J Pathol 116: 107–114, 1984 [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan MJ, McLemore GR., Jr Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–R742, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension 48: 988–993, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jimenez-Jaimez J, Diaz-Chamorro A, Jimenez-Alonso J. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol Renal Fluid Electrolyte Physiol 263: F601–F606, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, Neuhuber W, Tiegs G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol 19: 1371–1378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover PH, Ryan MJ. Blood pressure and renal hemodynamic responses to acute angiotension II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 301: R1286–R1292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1282–R1289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff DW, Gesek FA, Strandhoy JW. In vivo assessment of rat renal alpha adrenoceptors. J Pharmacol Exp Ther 241: 472–476, 1987 [PubMed] [Google Scholar]
- 46.Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 56: 691–698, 1977 [DOI] [PubMed] [Google Scholar]
- 47.Zietz B, Reber T, Oertel M, Gluck T, Scholmerich J, Straub RH. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepiandrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol 27: 911–918, 2000 [PubMed] [Google Scholar]