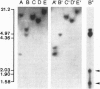
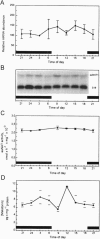
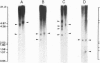
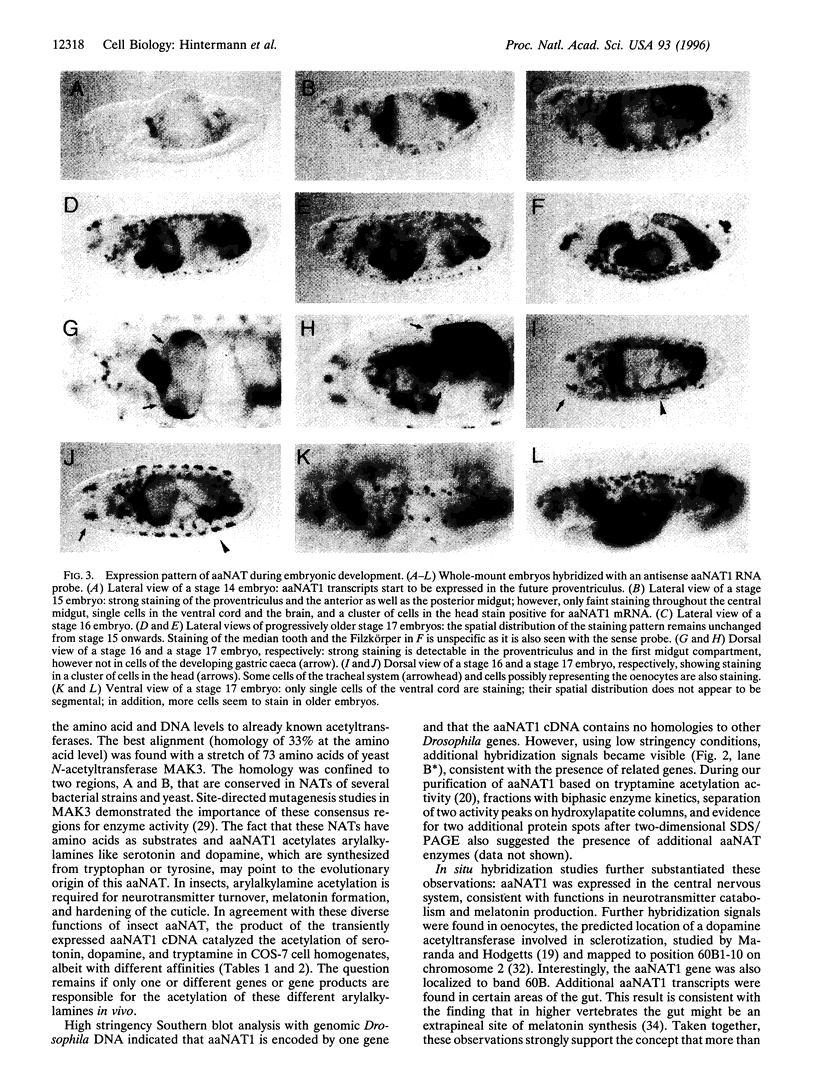
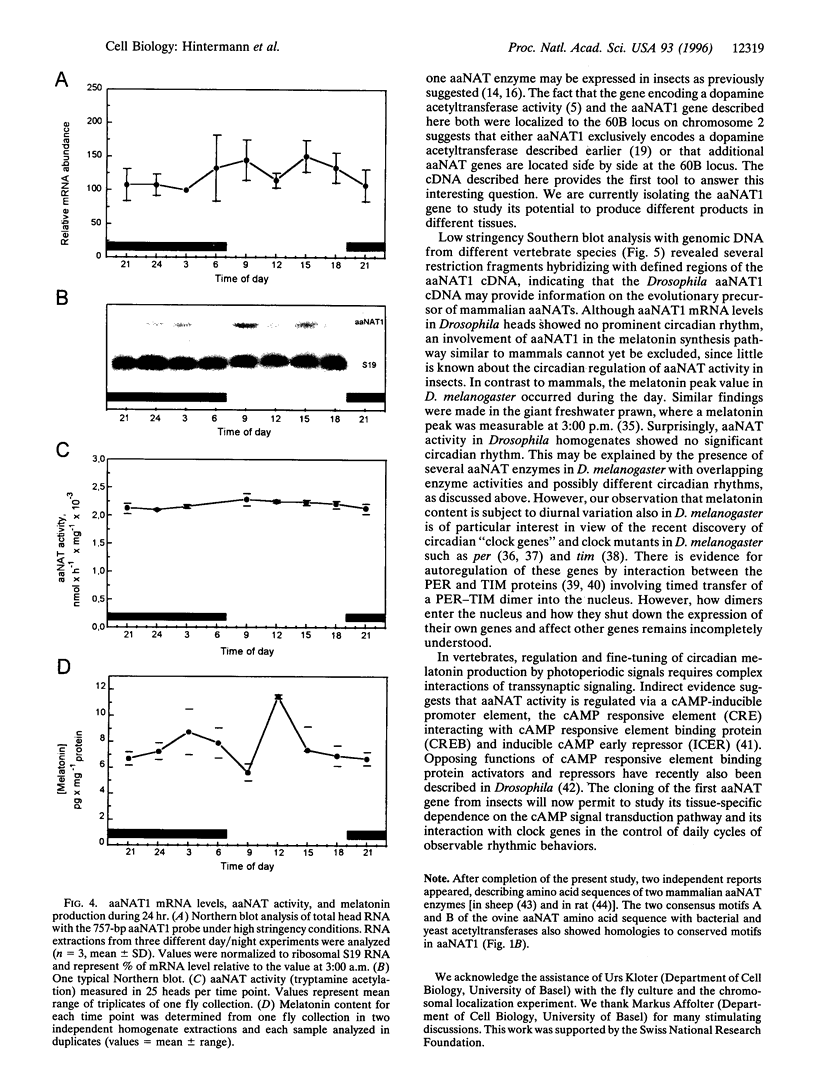
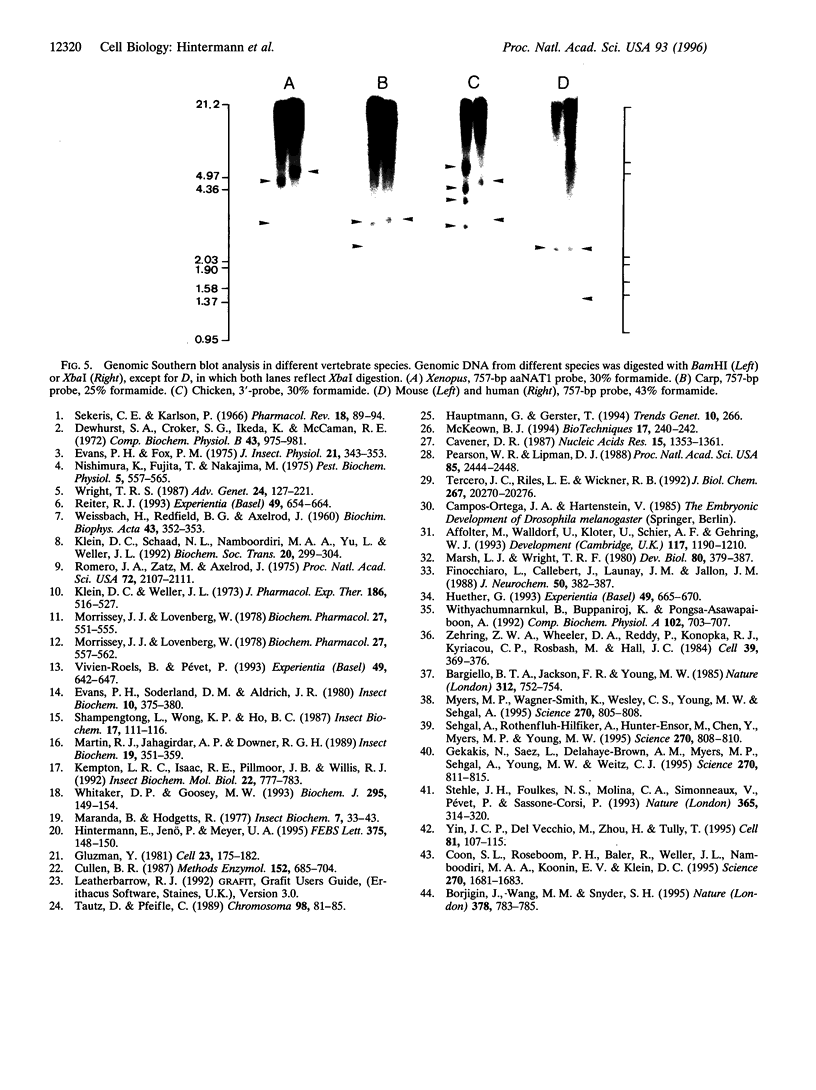
Abstract
In insects, neurotransmitter catabolism, melatonin precursor formation, and sclerotization involve arylalkylamine N-acetyltransferase (aaNAT, EC 2.3.1.87) activity. It is not known if one or multiple aaNAT enzymes are responsible for these activities. We recently have purified an aaNAT from Drosophila melanogaster. Here, we report the cloning of the corresponding aaNAT cDNA (aaNAT1) that upon COS cell expression acetylates dopamine, tryptamine, and the immediate melatonin precursor serotonin. aaNAT1 represents a novel gene family unrelated to known acetyl-transferases, except in two weakly conserved amino acid motifs. In situ hybridization studies of aaNAT1 mRNA in embryos reveal hybridization signals in the brain, the ventral cord, the gut, and probably in oenocytes, indicating a broad tissue distribution of aaNAT1 transcripts. Moreover, in day/ night studies we demonstrate a diurnal rhythm of melatonin concentration without a clear-cut change in aaNAT1 mRNA levels. The data suggest that tissue-specific regulation of aaNAT1 may be associated with different enzymatic functions and do not exclude the possibility of additional aaNAT genes.
Full text
PDF

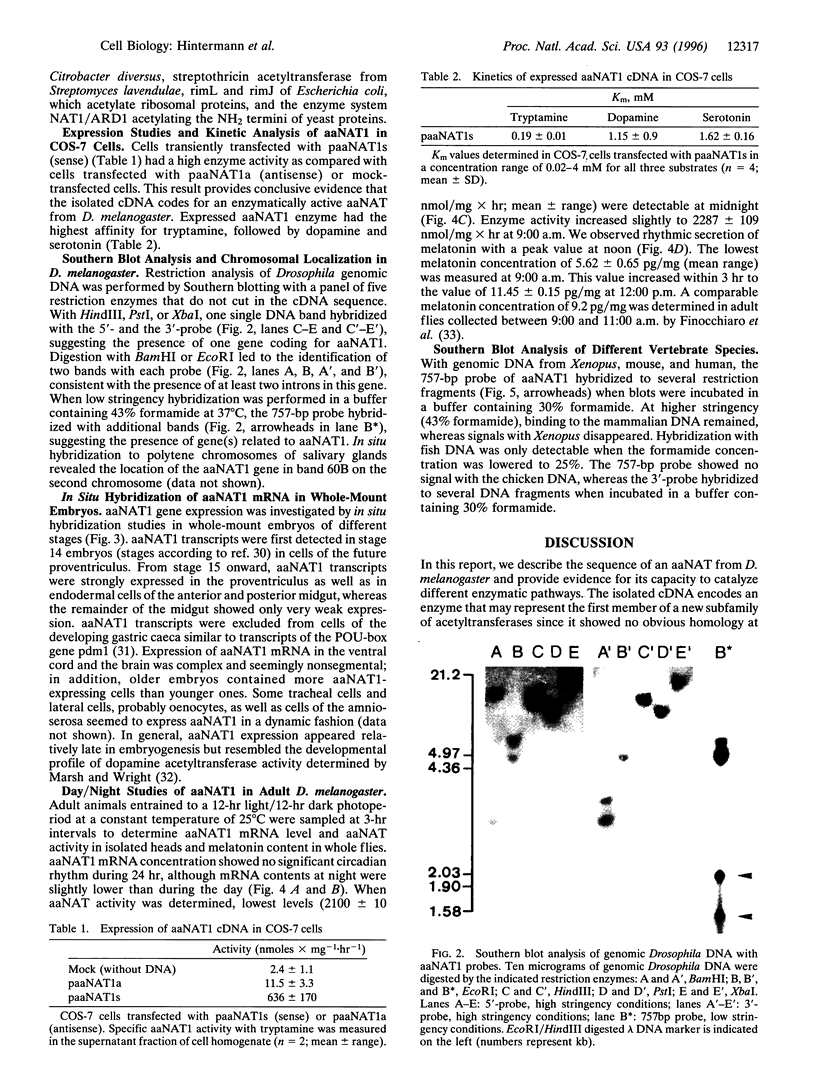
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Walldorf U., Kloter U., Schier A. F., Gehring W. J. Regional repression of a Drosophila POU box gene in the endoderm involves inductive interactions between germ layers. Development. 1993 Apr;117(4):1199–1210. doi: 10.1242/dev.117.4.1199. [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Jackson F. R., Young M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984 Dec 20;312(5996):752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- Borjigin J., Wang M. M., Snyder S. H. Diurnal variation in mRNA encoding serotonin N-acetyltransferase in pineal gland. Nature. 1995 Dec 21;378(6559):783–785. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon S. L., Roseboom P. H., Baler R., Weller J. L., Namboodiri M. A., Koonin E. V., Klein D. C. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science. 1995 Dec 8;270(5242):1681–1683. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dewhurst S. A., Croker S. G., Ikeda K., McCaman R. E. Metabolism of biogenic amines in Drosophila nervous tissue. Comp Biochem Physiol B. 1972 Dec 15;43(4):975–981. doi: 10.1016/0305-0491(72)90241-6. [DOI] [PubMed] [Google Scholar]
- Finocchiaro L., Callebert J., Launay J. M., Jallon J. M. Melatonin biosynthesis in Drosophila: its nature and its effects. J Neurochem. 1988 Feb;50(2):382–387. doi: 10.1111/j.1471-4159.1988.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Gekakis N., Saez L., Delahaye-Brown A. M., Myers M. P., Sehgal A., Young M. W., Weitz C. J. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science. 1995 Nov 3;270(5237):811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hauptmann G., Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994 Aug;10(8):266–266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- Hiltunen T., Raja-Honkala M., Nikkari T., Ylä-Herttuala S. A PCR artifact under low-stringency conditions due to amplification by only one primer. Biotechniques. 1994 Aug;17(2):240–242. [PubMed] [Google Scholar]
- Hintermann E., Jenö P., Meyer U. A. Isolation and characterization of an arylalkylamine N-acetyltransferase from Drosophila melanogaster. FEBS Lett. 1995 Nov 13;375(1-2):148–150. doi: 10.1016/0014-5793(95)01198-n. [DOI] [PubMed] [Google Scholar]
- Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993 Aug 15;49(8):665–670. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Schaad N. L., Namboordiri M. A., Yu L., Weller J. L. Regulation of pineal serotonin N-acetyltransferase activity. Biochem Soc Trans. 1992 May;20(2):299–304. doi: 10.1042/bst0200299. [DOI] [PubMed] [Google Scholar]
- Klein D., Weller J. L. Adrenergic-adenosine 3',5'-monophosphate regulation of serotonin N-acetyltransferase activity and the temporal relationship of serotonin N-acetyltransferase activity synthesis of 3H-N-acetylserotonin and 3H-melatonin in the cultured rat pineal gland. J Pharmacol Exp Ther. 1973 Sep;186(3):516–527. [PubMed] [Google Scholar]
- Marsh J. L., Wright T. R. Developmental relationship between dopa decarboxylase, dopamine acetyltransferase, and ecdysone in Drosophila. Dev Biol. 1980 Dec;80(2):379–387. doi: 10.1016/0012-1606(80)90412-1. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Lovenberg W. Synthesis of RNA in the pineal gland during N-acetyltransferase induction. The effects of actinomycin D, alpha-amanitin and cordycepin. Biochem Pharmacol. 1978 Feb 15;27(4):557–562. doi: 10.1016/0006-2952(78)90394-5. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Lovenberg W. Synthesis of RNA in the pineal gland during N-acetyltransferase induction. Biochem Pharmacol. 1978 Feb 15;27(4):551–555. doi: 10.1016/0006-2952(78)90393-3. [DOI] [PubMed] [Google Scholar]
- Myers M. P., Wager-Smith K., Wesley C. S., Young M. W., Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995 Nov 3;270(5237):805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R. J. The melatonin rhythm: both a clock and a calendar. Experientia. 1993 Aug 15;49(8):654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Zatz M., Axelrod J. Beta-adrenergic stimulation of pineal N-acetyltransferase: adenosine 3':5'-cyclic monophosphate stimulates both RNA and protein synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2107–2111. doi: 10.1073/pnas.72.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Rothenfluh-Hilfiker A., Hunter-Ensor M., Chen Y., Myers M. P., Young M. W. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science. 1995 Nov 3;270(5237):808–810. doi: 10.1126/science.270.5237.808. [DOI] [PubMed] [Google Scholar]
- Sekeris C. E., Karlson P. Biosynthesis of catecholamines in insects. Pharmacol Rev. 1966 Mar;18(1):89–94. [PubMed] [Google Scholar]
- Stehle J. H., Foulkes N. S., Molina C. A., Simonneaux V., Pévet P., Sassone-Corsi P. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature. 1993 Sep 23;365(6444):314–320. doi: 10.1038/365314a0. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tercero J. C., Riles L. E., Wickner R. B. Localized mutagenesis and evidence for post-transcriptional regulation of MAK3. A putative N-acetyltransferase required for double-stranded RNA virus propagation in Saccharomyces cerevisiae. J Biol Chem. 1992 Oct 5;267(28):20270–20276. [PubMed] [Google Scholar]
- WEISSBACH H., REDFIELD B. G., AXELROD J. Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim Biophys Acta. 1960 Sep 23;43:352–353. doi: 10.1016/0006-3002(60)90453-4. [DOI] [PubMed] [Google Scholar]
- Whitaker D. P., Goosey M. W. Purification and properties of the enzyme arylamine N-acetyltransferase from the housefly Musca domestica. Biochem J. 1993 Oct 1;295(Pt 1):149–154. doi: 10.1042/bj2950149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. R. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]
- Yin J. C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995 Apr 7;81(1):107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984 Dec;39(2 Pt 1):369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]