Abstract
The sensitization of capsaicin-sensitive lung vagal (CSLV) afferents by inflammatory mediators is important in the development of airway hypersensitivity. Hydrogen sulfide (H2S) is an endogenous mediator inducing hyperalgesia through transient receptor potential ankyrin 1 (TRPA1) receptors located on nociceptors. We conducted this study to determine whether H2S elevates the sensitivity of rat CSLV afferents. In anesthetized, artificially ventilated rats, the inhalation of aerosolized sodium hydrosulfide (NaHS, a H2S donor) caused no significant changes in the baseline activity of CSLV afferents. However, the afferent responses to right atrial injection of capsaicin or phenylbiguanide and to lung inflation were all markedly potentiated after NaHS inhalation. By contrast, the inhalation of its vehicle or NaOH (with a similar pH to NaHS) failed to enhance the afferent responses. Additionally, the potentiating effect on the afferent responses was found in rats inhaling l-cysteine (a substrate of H2S synthase) that slowly releases H2S. The potentiating effect of NaHS on the sensitivity of CSLV afferents was completely blocked by pretreatment of HC-030031 (a TRPA1 receptor antagonist) but was unaffected by its vehicle. In isolated rat CSLV neurons, the perfusion of NaHS alone did not influence the intracellular Ca2+ concentration but markedly potentiated the Ca2+ transients evoked by capsaicin. The NaHS-caused effect was totally abolished by HC-030031 pretreatment. These results suggest that H2S induces a nonspecific sensitizing effect on CSLV fibers to both chemical and mechanical stimulation in rat lungs, which appears mediated through an action on the TRPA1 receptors expressed on the nerve endings of CSLV afferents.
Keywords: lung, lung vagal C fibers, afferent sensitization, H2S, TRPA1 receptors
capsaicin-sensitive lung vagal (CSLV) afferents are nociceptive-like free nerve endings innervating all levels of the respiratory tract. CSLV afferents can detect several inhaled irritants (22, 25) and inflammatory mediators (16, 25, 26, 39) that might in turn trigger various respiratory reflexes such as cough, mucus secretion, and bronchoconstriction (7, 22). The afferents are sensitized by several mediators released because of lung inflammation (2, 7, 15, 27), which might then exaggerate these respiratory reflexes. Therefore, the sensitization of CSLV afferents is probably involved in the pathogenesis of airway hypersensitivity in diseases such as chronic cough and asthma (7, 26, 47).
Hydrogen sulfide (H2S), an irritant gas with the smell of rotten eggs, is emitted principally from volcanoes, hot springs, and numerous industrial sites. Lung exposure to H2S might lead to adverse respiratory effects, such as cough, airway irritation, airway hypersensitivity, and lung inflammation (16, 37, 41). Therefore, for the past decades, H2S was generally considered only an exogenous irritant. However, beginning with a report in 1996, H2S was suggested to act as an endogenous neuromodulator facilitating the hippocampal long-term potentiation in rats (1). Since then, H2S has been recognized as a signaling molecule involved in a number of physiological processes, such as vasodilation (17), neuronal protection from oxidative stress (20), the regulation of insulin release (49), and the facilitation of bladder contraction (35). Recently, H2S has been suggested as a pro-inflammatory mediator under various pathophysiological conditions such as hind paw inflammatory edema (50) and endotoxin-induced systemic inflammation (3, 24).
The application of sodium hydrosulfide (NaHS, a donor of H2S) has been demonstrated to induce the sensitization of colon nociceptive neurons (44, 48), a counterpart of CSLV neurons in visceral tissues. Moreover, one study showed that the intratracheal instillation of NaHS triggered airway neurogenic inflammation that was nearly abolished by the desensitization of CSLV afferents (41), which implied the effects of H2S on the afferents. However, whether H2S acts on CSLV afferents is unknown; if it does, then the H2S-induced effect and its underlying mechanism remain to be explored. The application of NaHS has been reported to induce somatic hyperalgesia and the depolarization of dorsal root ganglion neurons, which are dependent on transient receptor potential ankyrin 1 (TRPA1) receptors (2, 28, 48). The TRPA1 receptor, a Ca2+-permeable, nonselective cation channel, is predominantly expressed on nociceptive sensory neurons that include CSLV neurons (31, 40). From this information, we conducted this study 1) to investigate whether H2S sensitizes CSLV afferents to chemical and mechanical stimulation by using a single-fiber recording technique in anesthetized, artificially ventilated rats; 2) to determine whether the H2S-induced effect is also present in isolated CSLV neurons by using a Ca2+ image technique; and 3) to delineate the role of the TRPA1 receptors in H2S-induced sensitization.
METHODS
The following procedures were performed in accordance with the recommendations found in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Taipei Medical University.
In Vivo Study
Male SD rats (weighing 320–420 g) were initially anesthetized with an intraperitoneal injection of α-chloralose (100 mg/kg) and urethane (500 mg/kg) dissolved in a borax solution (2%). Supplemental doses of these anesthetics were administrated intravenously to sustain the elimination of pain reflexes produced by pinching the rat's tail throughout the experiment. For the application of anesthetics and pharmacological agents, the left jugular vein was cannulated and a catheter was advanced until its tip was positioned near the right atrium. The right femoral artery was cannulated to measure the arterial blood pressure. Body temperature was maintained at ∼36°C throughout the experiment by using a servo-controlled heating pad. At the end of the experiment, the animals were euthanzed using an intravenous injection of KCl.
Measurement of activity of CSLV afferents.
Fiber activity arising from CSLV afferents was recorded in the anesthetized, artificially ventilated rats by using the techniques described in our previous studies (14, 25–27). In brief, the trachea was cannulated and tracheal pressure was measured via a sideport of the cannula. The rats were artificially ventilated with a respirator (683, Harvard, South Natick, MA); the tidal volume and respiratory frequency were set at 8 ml/kg and 50 breaths/min, respectively. The expiratory outlet of the respirator was placed under 3- cmH2O pressure after the chest was opened to identify the locations of the sensory nerve endings. The right cervical vagus nerve was separated and placed on a small dissecting platform. A thin filament was teased away from the desheathed nerve trunk and placed on a platinum-iridium hook electrode. To search for these afferent fibers, the lungs were hyperinflated in a stepwise manner to 3 or 4 tidal volume; the CSLV fibers are activated by lung inflation at this high volume level (22). The thin filament was split until the afferent activity arising from a single unit was electrically isolated. Once the presence of a single unit was detected, capsaicin (1.5 μg/kg) was injected intravenously into the right atrium. Only afferent fibers that met the following criteria were studied: 1) fibers with a short latency (<2 s) and intense response to the capsaicin injection, and 2) fibers where the general locations of the receptors could be identified by their responses to the gentle pressing of the lungs with a wet cotton swab at the end of the experiment.
Experimental protocols.
Rats were divided into 13 groups to conduct 6 series of experiments. Each group contained 8 rats, and only one CSLV fiber was tested in each rat. The H2S was delivered by its donor NaHS. To minimize the systemic effect, NaHS solution (5 mg/ml) was provided by aerosol generated through a vibrating plate nebulizer (Aeroneb Pro; Aerogen Nektar, Galway, Ireland). The nebulizer was connected to the breathing circuit between the inspiratory outlet of the respirator and the tracheal cannula. The particle sizes of the aerosol were ∼2.1 μm, and the solution volume delivered from nebulizer over 3 min was ∼0.25 ml under the experimental conditions. For each NaHS inhalation, the bolus injections of chemical stimulants of CSLV fibers or lung inflations were performed 15 min before and 5 and 30 min after the termination of the inhalation. In series 1, to determine the sensitizing effect of H2S on CSLV fibers, the afferent responses to capsaicin (a selective stimulant of C fibers; 0.5–1.0 μg/kg) were compared between before and after airway exposure to NaHS or its vehicle (saline) in a group of rats (group 1). The inhalation of NaHS and its vehicle was performed in alternate order between fibers to achieve a balanced design, and 80 min elapsed between the 2 inhalations for recovery. In series 2, to determine whether the H2S-induced sensitizing effect on CSLV fibers was limited to capsaicin as a stimulant, 2 other stimulants of the fibers were chosen [phenylbiguanide (3–8 μg/kg) (group 2) and lung inflation (tracheal pressure = 30 cmH2O for 10 s) (group 3)]. The protocol of this study series was the same as that of series 1, except that the stimulant was replaced by phenylbiguanide injection or lung inflation. In series 3, because alkalinity has been reported to induce hyperalgesia in mice through TRPA1 receptors (13), to determine whether the H2S-induced sensitization of CSLV fibers resulted from the alkaline nature of the NaHS solution, the afferent responses to capsaicin (group 4) or lung inflation (group 5) were compared between the airway exposure to NaHS and its vehicle (saline) in the same group of rats; the pH value of the later was adjusted to 11.3 by adding NaOH (0.1 N). In series 4, because the effect of H2S generated from NaHS (a fast releasing donor) on inflammation was reported to be opposite to that produced from slow releasing donors (45), to determine whether CSLV fibers were also sensitized by slow-releasing agents of H2S, l-cysteine (a metabolic precursor of H2S) was chosen as a slow-releasing agent of H2S (43). The afferent responses to capsaicin (group 6) or lung inflation (group 7) were compared between the airway exposure to l-cysteine (5 mg/ml for 3 min) and its vehicle (saline) in the same group of rats. Series 5 was used to evaluate the role of TRPA1 receptors in the H2S-induced sensitization of the CSLV fibers. As controls, either capsaicin injection or lung inflation was performed before and 5 and 30 min after the NaHS inhalation in two different groups. Subsequently, the experiments were repeated 15 min after pretreatment with HC-030031 (an antagonist of TRPA1 receptors; 8 mg/kg iv) (groups 8 and 9) or its vehicle (groups 10 and 11). The dose of HC-030031 was shown to exert a complete blocking effect on the stimulation of the CSLV fibers elicited by the TRPA1 receptor agonist in our previous study (25). The purpose of series 6 was to verify the effectiveness and specificity of the antagonizing effect of HC-030031 on the TRPA1 receptors under the present experimental conditions. The sensitizing responses of the CSLV afferents to capsaicin were compared before and after HC-030031 pretreatment. Protocols same to series 5 were used, except that the fibers were sensitized using allyl isothiocyanate (AITC, a selective agonist of TRPA1 receptors; 0.4 mg·kg−1·min−1, 2 min) (group 12) and using prostaglandin E2 (PGE2; 3 μg·kg−1·min−1, 2 min) (group 13). The PGE2 has been demonstrated to induce hypersensitivity of CSLV afferents in our previous study (21).
In Vitro Study
In addition to its primary expression on the sensory neurons, TRPA1 receptors are also expressed on nonneuronal cells in the lung (12, 32). Mediators released by these nonneuronal cells after NaHS exposure might lead to a secondary sensitizing effect on the in vivo preparation of CSLV afferents (15, 22, 25, 26). To determine whether H2S exerts a sensitizing effect on the CSLV neurons, the following experiments were performed in vitro preparation.
Labeling CSLV neurons with DiI.
Sensory neurons innervating the lungs and airways were identified by retrograde labeling from the lungs with the fluorescent tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), as described previously (14, 18, 21). In brief, young male SD rats (∼150 g) were anesthetized by aerosolized isoflurane (2% in O2) through a nose cone connected to a vaporizing machine (AM Bickford, New York City, NY). To expose the trachea, a small midline incision was made on the ventral neck skin. The DiI (0.2 mg/ml; 0.05 ml) was instilled into the lungs through a needle (30 gauge) inserted into the trachea lumen, and the incision was then closed. To allow the DiI to be transported toward the soma of CSLV neurons, the animals were kept undisturbed for 7–10 days until they were euthanized for the cell culture.
Isolation and culture of nodose and jugular ganglion neurons.
The methodology was described in detail in our previous studies (14, 18, 21). Male SD rats were anesthetized with 5% isoflurane and were decapitated. The head was quickly immersed in an ice-cold DMEM/F-12 solution, followed by the extraction of the nodose and jugular ganglia. Each ganglion was desheathed, cut, placed into a mixture of type IV collagenase (0.04%) and dispase II (0.02%), and incubated for 80 min in 5% CO2 in air at 37°C. The ganglion suspension was centrifuged (150 g, 5 min), and the supernatant was aspirated. The pellet was then resuspended in a modified DMEM/F12 solution and gently triturated. The dispersed cell suspension was centrifuged (500 g, 8 min) through a layer of bovine serum albumin (15%) to separate the cells from the myelin debris. The pellets were resuspended in the modified DMEM/F12 solution plated onto poly-l-lysine-coated glass coverslips and were then incubated overnight (5% CO2 in air at 37°C).
Measurement of Ca2+ transients.
Cells were washed or maintained with an extracellular solution in a small-volume (0.2 ml) perfusion chamber at room temperature. The Ca2+ transients were measured in these cells by using a Zeiss digital fluorescence microscope (Axiovert 100; Carl Zeiss, Thornwood, NY) equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and a digital CCD camera (Princeton Instruments, Trenton, NJ), as previously described (14, 18). Before the Ca2+-imaging experiments, cells were incubated with 5 μM Fura-2 acetoxymethyl ester for 30 min at 37°C, then rinsed with an extracellular solution, and allowed to deesterify for at least 30 min before use. Dual images (340 and 380 nm excitation, 510 nm emission) were collected, and the pseudocolored ratiometric images were monitored during the experiments. The imaging system was standardized with a 2-point calibration by using a Ca2+-free standard (−) and a Ca2+-saturated standard (+). Both standards contained 11 μM Fura-2 [44 μl of 10 mM Fura-2 penta K+ salt, 8 ml of 20 mM sodium HEPES (pH 7.4), and 32 ml H2O] and were prepared as follows: − standard, 18 ml Fura-2 and 1.98 ml of 10 mM sodium EGTA (pH 7.6); and + standard, 18 ml Fura-2 and 1.98 ml of 10 mM CaCl2. The parameters used for the 2-point calibration included the dissociation constant of Fura-2 (Kd 285), the ratio values for the (−) and (+) concentration standards (Rmin and Rmax, respectively) and the denominator wavelength intensities for the (−) and (+) concentration standards (Denmin and Denmax, respectively). The intracellular concentration of Ca2+ ([Ca2+]i, in nM) was calculated according to the following equation: [Ca2+]i = Kd(R − Rmin)/(Rmax − R)(Denmin/Denmax). Typical Rmin and Rmax values were 0.225 and 1.45, respectively.
Experimental protocols.
After the incubation period with Fura-2 AM, the coverslip containing the cells was mounted into a chamber placed on the stage of the microscope. The entire chamber was continuously perfused with an extracellular solution during the experiment by a gravity-fed valve-controlled system (VC-66CS, Warner Instruments, Hamden, CT) at a constant rate of ∼2 ml/min. The KCl solution (60 mM, 20 s) was perfused at the end of each experimental run to test for cell viability. The CSLV neurons were selected from the cultured cells for analysis that met the following criteria: 1) a spherical shape with no neurite outgrowths, 2) activated by capsaicin (200 nM, 30 s), and 3) labeled with DiI fluorescence. A total of 112 neurons from 11 rats were studied in three separate series of experiments. Series 1 was performed to examine the sensitizing effect of H2S on the CSLV neurons; Ca2+ transients elicited by capsaicin (200 nM, 30 s) were determined before and 1 min after the onset of NaHS perfusion (200 μM, 2.5 min). Series 2 was performed to evaluate the role of the TRPA1 receptors; the NaHS-induced potentiation of capsaicin-evoked Ca2+ transients was determined after the pretreatment of HC-030031 (20 μM, 16.5 min). Series 3 was performed to investigate whether the inhibitory effect of HC-030031, if any, on the potentiation of Ca2+ transients resulted from its suppressive action on capsaicin. The Ca2+ transients evoked by capsaicin alone were determined before and after HC-030031 pretreatment.
Pharmacological agents.
In the in vivo study, a stock solution of capsaicin (250 μg/ml) was prepared in 1% Tween 80, 1% ethanol, and 98% saline; and a stock solution of phenylbiguanide (400 μg/ml) was prepared in saline. The solutions of capsaicin and phenylbiguanide for injection at the desired concentrations were prepared daily by dilution with saline based on the animal's body weight. A stock of HC-030031 (30 mg/ml) was dissolved in dimethyl sulfoxide and further diluted to a final concentration of 2 mg/ml with a vehicle (10% Tween 80, 10% ethanol, and 80% saline) before use. In the in vitro study, desired concentrations of the pharmacological agents were prepared in a similar manner, except that the extracellular solution, instead of saline, was used as the vehicle. An extracellular solution was prepared with 5.4 mM KCl, 136 mM NaCl, 1 mM MgCl2, 1.8 mM CaCl2, 0.33 mM NaH2PO4, 10 mM glucose, 10 mM HEPES, and a pH level adjusted to 7.4 with NaOH and the osmolarity to 300 mosM. A modified DMEM/F12 solution was prepared using DMEM/F-12 supplemented with a 10% vol/vol heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM MEM nonessential amino acids. The pH value of the extracellular solution containing NaHS (200 μmol/l) was 7.42. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except HC-030031 (Tocris, Ellisville, MO), dispase II (Roche, Indianapolis, IN), DMEM/F12 (Invitrogen, Carlsbad, CA), and Fura-2 AM and DiI (Molecular Probes, Eugene, OR).
Data Analysis
In the in vivo studies, the fiber activity of CSLV fibers, heart rate, and mean arterial blood pressure were continually analyzed at 1-s intervals over an interval of at least 20 s before and 60 s after the challenges of the chemical or mechanical stimulants. The baseline of these physiological parameters was calculated as the average value over the 10-s period immediately preceding a challenge. The peak response was defined as the maximum 3-s average within 20 s following the injection of the chemical stimulant, or over 5 s after the lung inflation. A fiber was considered activated when the increase in fiber activity exceeded 0.5 impulses/s. These physiological parameters were analyzed using a computer equipped with an A/D converter (DASA 4600, Gould, Columbus, OH) and software (BioCybernatics, 1.0, Taipei, Taiwan). In the in vitro studies, the intracellular Ca2+ concentration ([Ca2+]i) was continually analyzed at 2-s intervals during the experiments by using the Axon Imaging Workbench software (Axon Instruments, Union City, CA). An increase in intracellular Ca2+ concentration (Δ[Ca2+]i) was measured as the difference between the peak amplitude of Ca2+ transients (4 s average) and the 30-s average at baseline. Data were compared using a paired t-test or a two-way repeated-measures analysis of variance (ANOVA). When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Newman-Keuls test). A value of P < 0.05 was considered significant. All data are reported as means ± SE.
RESULTS
In Vivo Study
A total of 104 CSLV afferents were studied in 104 anesthetized, artificially ventilated rats. The locations of the CSLV nerve endings were as follows: 19, 41, 32, and 10 in the upper, middle, lower, and accessory lobes of the right lung, respectively. The locations of the remaining 2 fibers could not be identified. The 104 fibers were divided evenly into 13 groups for the studies in 6 series of experiments.
Effects of NaHS inhalation on baseline CSLV afferent activity.
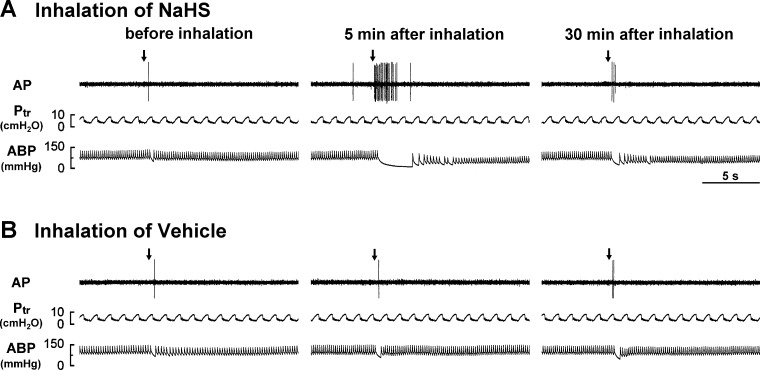
CSLV afferents had a very low and irregular baseline activity during eupneic breathing (Figs. 1 and 2; Table 1). Eighteen of these CSLV fibers had sparse activity, whereas the rest of the 86 were silent under basal condition (Fig. 1). The averaged baseline activity of the CSLV fibers was 0.04 ± 0.01 impulses/s. Five minutes after exposure to NaHS or its vehicle, no significant change was observed in the average baseline activity of these fibers (Fig. 1; Table 1), despite a slight increase in 8 fibers. In addition, the inhalation of NaHS or its vehicle had no detectable effect on the baseline heart rate and mean arterial blood pressure (Fig. 1; Table 1).
Fig. 1.
Experimental records illustrating the responses of a capsaicin-sensitive lung vagal (CSLV) afferent to the right atrial bolus injection of capsaicin (0.75 μg/kg, arrows) before and 5 and 30 min after the termination of airway exposure to aerosolized sodium hydrosulfide (NaHS, a donor of H2S) in an anesthetized, artificially ventilated rat. A and B: inhalation of aerosolized NaHS (5 mg/ml, 3 min) and its vehicle (saline), respectively. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure. Note that the afferent response to capsaicin was enhanced 5 min after NaHS inhalation but was not affected after vehicle inhalation.
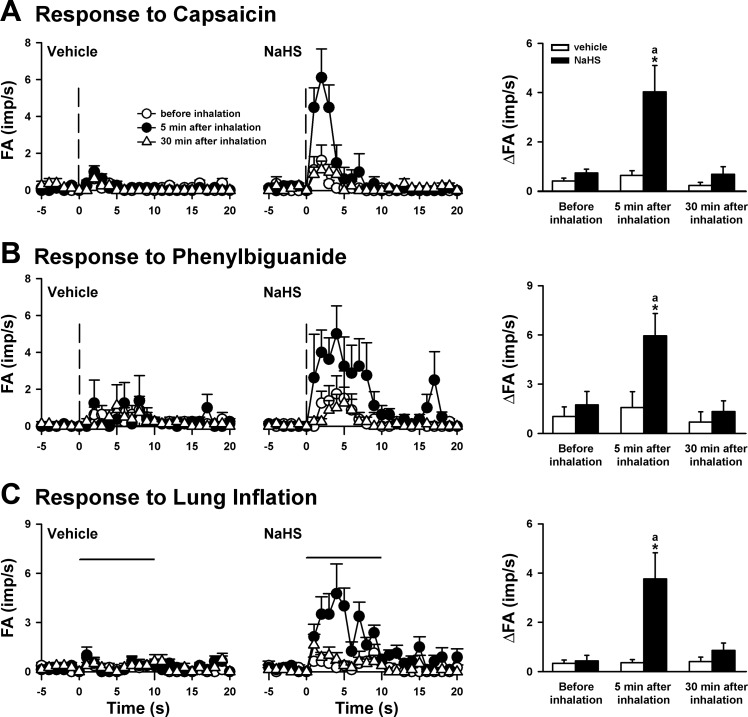
Fig. 2.
The afferent responses of CSLV fibers to the right atrial bolus injection of capsaicin (A) and phenylbiguanide (B) and to lung inflation (C) before and 5 and 30 min after the termination of airway exposure to aerosol in 3 groups of rats. Middle and left: average afferent responses at groups of inhalation of NaHS aerosol (5 mg/ml, 3 min) and its vehicle (saline), respectively. Vertical dashed lines in A and B are the onset time of the right atrial injection of capsaicin (0.5–1.0 μg/kg) and phenylbiguanide (3–8 μg/kg), respectively. The horizontal lines in C are the duration of lung inflation (Ptr = 30 cmH2O, 10 s). FA, fiber activity; imp, impulses. Right: average peak afferent responses to inhalation of NaHS aerosol and its vehicle (saline). In A and B: the increase in fiber activity (ΔFA) was measured as the difference between peak FA (averaged over 3-s intervals) and baseline FA in each fiber. In C: ΔFA was measured as the difference between the peak FA (averaged over a 5-s interval) during inflation and the baseline FA. aSignificantly different from the value before inhalation in the same group (P < 0.05). *Significantly different from the value at the corresponding time period in the vehicle group (P < 0.05). Data in each group are means ± SE of 8 fibers recorded from 8 rats.
Table 1.
Effects of inhalation of NaHS and its vehicle on the baselines of CSLV fiber activity, mean arterial blood pressure, and heart rate in anesthetized, artificially ventilated rats
| Vehicle of NaHS (n = 24) | NaHS (n = 72) | |
|---|---|---|
| CSLV fiber activity, impulses/s | ||
| Before inhalation | 0.05 ± 0.02 | 0.04 ± 0.01 |
| 5 Min after inhalation | 0.06 ± 0.02 | 0.06 ± 0.01 |
| 30 Min after inhalation | 0.05 ± 0.02 | 0.04 ± 0.01 |
| Mean arterial blood pressure, mmHg | ||
| Before inhalation | 85 ± 5 | 81 ± 3 |
| 5 Min after inhalation | 78 ± 5 | 83 ± 3 |
| 30 Min after inhalation | 83 ± 5 | 84 ± 3 |
| Heart rate, beats/min | ||
| Before inhalation | 349 ± 14 | 334 ± 9 |
| 5 Min after inhalation | 337 ± 16 | 317 ± 9 |
| 30 Min after inhalation | 337 ± 15 | 333 ± 10 |
Data (means ± SE) are values averaged over 10-s periods before and 5 min and 30 min after the termination of inhalation. n represents fiber numbers. Lungs were exposed to aerosolized NaHS (5 mg/ml) or its vehicle for 3 min. Only one capsaicin-sensitive lung vagal (CSLV) fiber was studied in each rat. No statistical significance was found between any 2 groups of mean.
Sensitization of CSLV afferents by NaHS.
During the control experiments, the injection of low-dose capsaicin abruptly triggered a mild and short burst of discharge in the CSLV afferents (Figs. 1 and 2A); the difference between the mean peak response and the baseline fiber activity was 0.74 ± 0.15 impulses/s (Fig. 2A). However, the stimulatory effect by the same dose of capsaicin on these fibers was markedly potentiated (5.4-fold relative to its control) 5 min after NaHS inhalation; the response to capsaicin returned to control levels 30 min after inhalation (Fig. 2A). In contrast, inhalation of the vehicle did not significantly change the afferent response to capsaicin (Figs. 1 and 2A). The potentiating effect of NaHS was not limited only to the response triggered by capsaicin injection. Similarly, the afferent responses (increase in fiber activity: 1.74 ± 0.81 impulses/s) elicited by phenylbiguanide injection were enhanced (3.4-fold relative to its control) 5 min after NaHS inhalation, whereas the inhalation of its vehicle did not have such an enhancing effect (Fig. 2B). At 5 min after NaHS inhalation, the fiber responses to these chemical stimulants increased significantly in both the peak activity and the duration of the firing (Figs. 1A, and 2, A and B). The durations of the firing evoked by capsaicin before and 5 min after NaHS inhalation were 2.4 ± 0.8 and 6.3 ± 2.0 s (n = 8, P < 0.05), respectively. The durations of the firing evoked by phenylbiguanide before and 5 min after NaHS inhalation were 4.0 ± 2.2 and 13.4 ± 2.1 s (n = 8, P < 0.05), respectively. Furthermore, the potentiating effect of NaHS was found in the afferent response to lung inflation. Before NaHS inhalation (in the control group), the CSLV fibers were relatively insensitive to lung inflation (Fig. 2C); only 5 of the 8 CSLV fibers were slightly activated by constant-pressure lung inflation (averaged increase in fiber activity: 0.44 ± 0.23 impulses/s, n = 8). At 5 min after inhalation, NaHS significantly potentiated the stimulatory effect of lung inflation (8.5-fold relative to its control), whereas the vehicle failed to do so (Fig. 2C). The NaHS-induced potentiation to lung inflation was reversed 30 min after the inhalation (Fig. 2C).
NaOH failed to mimic the sensitization of CSLV afferents by NaHS.
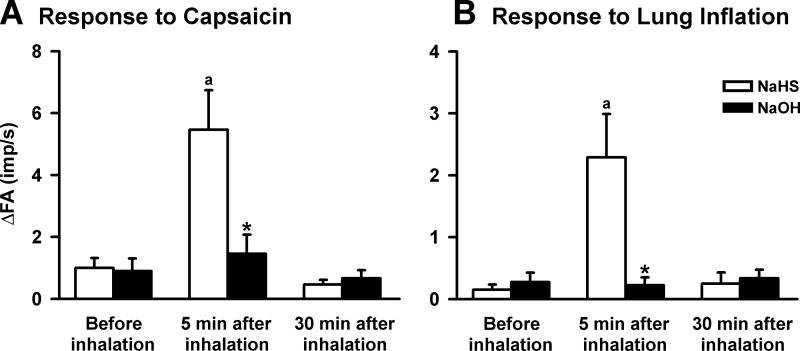
Five minutes after the inhalation of an aerosolized NaOH solution with a similar pH (value = 11.3) to NaHS, the baseline fiber activity of the CSLV fibers did not change significantly (before NaOH: 0.02 ± 0.01 impulses/s, 5 min after NaOH: 0.04 ± 0.02 impulses/s; n = 16, P > 0.05). Similarly, NaOH inhalation did not affect the afferent responses to chemical stimulation provided by capsaicin injection (Fig. 3A). In addition, NaOH inhalation did not affect the afferent responses to lung inflation (Fig. 3B). However, in the same group of CSLV fibers, NaHS still exerted a markedly sensitizing effect as shown previously (Fig. 3).
Fig. 3.
The effects of inhalations of NaHS and sodium hydroxide (NaOH) on the afferent responses of CSLV fibers to the right atrial bolus injection of capsaicin (A) and to lung inflation (B) in 2 groups of rats. The NaOH was dissolved in the saline (NaHS vehicle) at pH 11.3 to mimic the pH effect of a NaHS solution. aSignificantly different from the value before inhalation in the same group (P < 0.05). *Significantly different from the value at the corresponding time period in the NaHS group (P < 0.05). Data in each group are means ± SE of 8 fibers recorded from 8 rats. See Fig. 2 for further explanation.
CSLV afferents were also sensitized by l-cysteine.
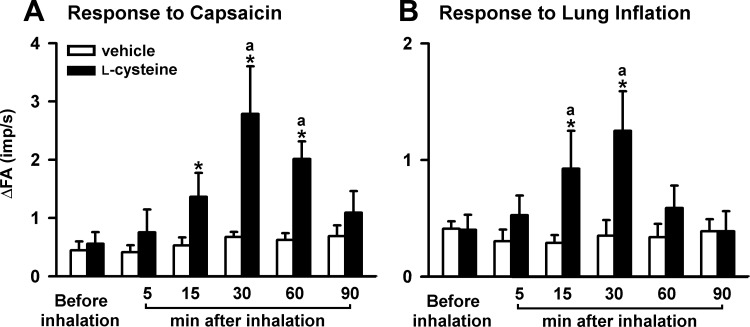
Five minutes after the inhalation of an aerosolized l-cysteine solution, the baseline fiber activity of the CSLV fibers did not change significantly (before l-cysteine: 0.04 ± 0.02 impulses/s, after l-cysteine: 0.03 ± 0.02 impulses/s; n = 16, P > 0.05). However, l-cysteine enhanced the afferent responses to chemical stimulation by capsaicin injection (Fig. 4A) and by lung inflation (Fig. 4B). The sensitizing effect of l-cysteine returned to control levels within 90 min after inhalation (Fig. 4). In contrast, inhalation of the vehicle did not significantly change the afferent responses (Fig. 4).
Fig. 4.
The afferent responses of CSLV fibers to the right atrial bolus injection of capsaicin (A) and to lung inflation (B) before and 5, 15, 30, 60, and 90 min after the termination of airway exposure to aerosolized l-cysteine (5 mg/ml for 3 min) in 2 groups of rats. aSignificantly different from the value before inhalation in the same group (P < 0.05). *Significantly different from the value at the corresponding time period in the vehicle group (P < 0.05). Data in each group are means ± SE of 8 fibers recorded from 8 rats. See Fig. 2 for further explanation.
Role of TRPA1 receptors in the sensitization of CSLV afferents by NaHS.
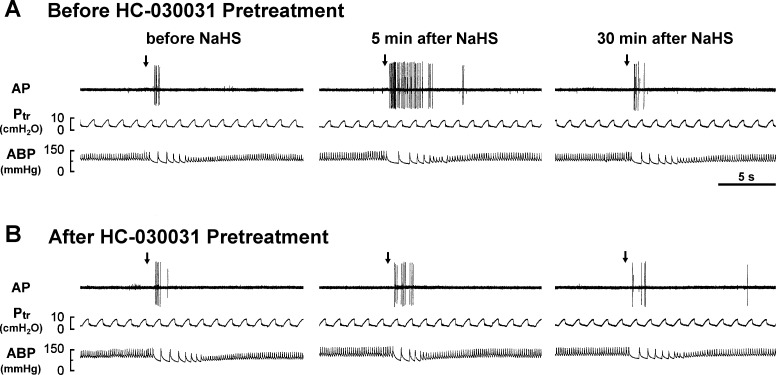
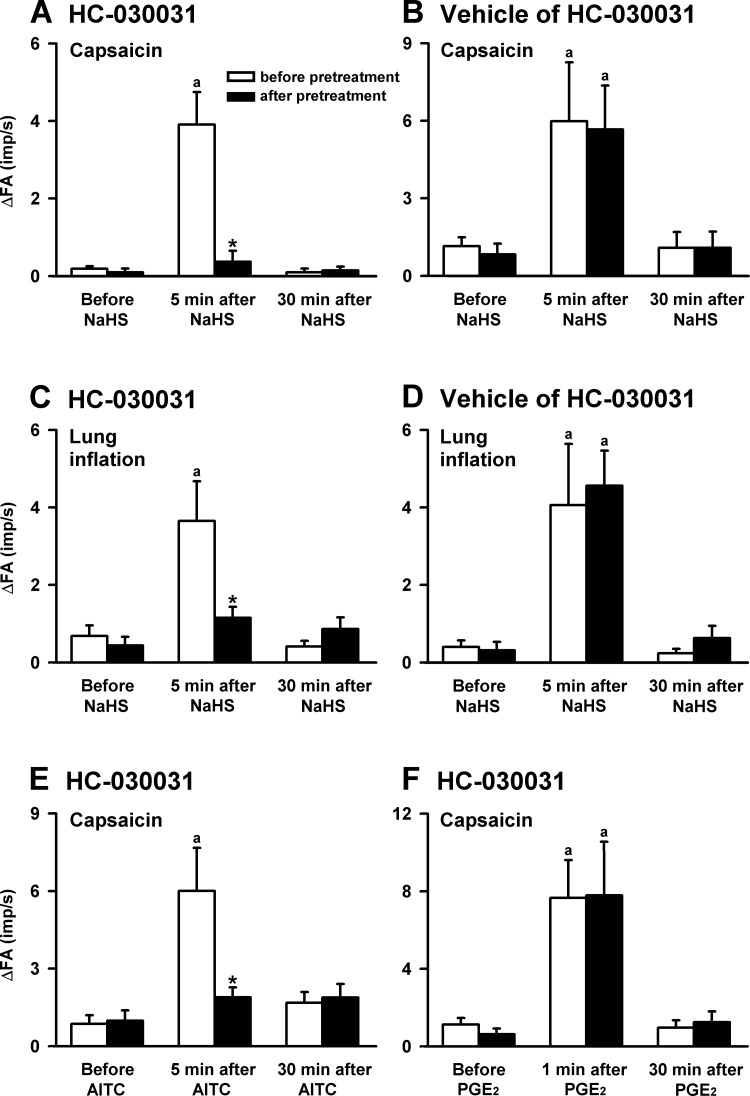
The pretreatment of HC-030031 did not alter the baseline fiber activity of the CSLV afferents. As a group, in the control experiments, the baseline fiber activities before and after HC-030031 pretreatment were 0.02 ± 0.01 and 0.03 ± 0.03 impulses/s (n = 16; P > 0.05), respectively. Nevertheless, the HC-030031 pretreatment completely blocked the NaHS-induced potentiating effect on the fiber responses to capsaicin injection (Figs. 5 and 6A) and to lung inflation (Fig. 6C). Pretreatment with the HC-030031 vehicle did not affect the potentiating effect caused by NaHS on the fiber response to either capsaicin injection (Fig. 6B) or lung inflation (Fig. 6D).
Fig. 5.
Experimental records illustrating the effect of pretreatment with HC-030031 on the responses of a CSLV afferent to capsaicin injection (1 μg/kg, arrows) before and 5 and 30 min after the termination of the airway exposure to aerosolized NaHS (5 mg/ml, 3 min) in an anesthetized, artificially ventilated rat. A and B: before and after pretreatment with HC-030031 (an antagonist of TRPA1 receptors; 8 mg/kg), respectively. Note that pretreatment with HC-030031 blocked the sensitizing effect of NaHS on the fiber response to the capsaicin injection.
Fig. 6.
The effects of pretreatment with HC-030031 on the CSLV-fiber sensitization induced by NaHS, allyl isothiocyanate (AITC), or prostaglandin E2 (PGE2) in 6 groups of rats. The NaHS (A–D; 5 mg/ml, 3 min) was provided through aerosol inhalation, and AITC (E; an agonist of TRPA1 receptors; 0.4 mg·kg−1·min−1, 2 min) and PGE2 (F; a sensitizer of CSLV afferents; 3 μg·kg−1·min−1, 2 min) were applied by intravenous infusion. Top and bottom: responses to capsaicin injections; middle: response to lung inflation. aSignificantly different from the value before the sensitizing agents in the same group (P < 0.05). *Significantly different from the value at the corresponding time period before pretreatment (P < 0.05). Data in each group are means ± SE of 8 fibers recorded from 8 rats. See Fig. 2 for further explanation.
The effectiveness and specificity of the antagonizing effect of HC-030031 were evaluated with AITC (a selective agonist of TRPA1 receptors) and PGE2 (a sensitizer of CSLV fibers), respectively. Five minutes after AITC infusion, no significant change was noted in the baseline fiber activity of the CSLV fibers (before infusion: 0.03 ± 0.02 impulses/s; after infusion: 0.03 ± 0.03 impulses/s; n = 8, P > 0.05). However, AITC markedly elevated the excitability of the CSLV fibers to capsaicin (Fig. 6E). Pretreatment with HC-030031 completely blocked the hypersensitivity of the CSLV fibers caused by AITC infusion (Fig. 6E). In another group of fibers, the blocking effect of HC-030031 on the sensitization of the CSLV fibers was absent if the fibers were sensitized by PGE2 infusion (Fig. 6F).
In Vitro Study
In total, 112 isolated CSLV neurons cultured from both nodose and jugular ganglia in 11 rats were studied. The average baseline [Ca2+]i was 112.3 ± 5.4 nM.
Enhancement of capsaicin-evoked Ca2+ transients by NaHS.
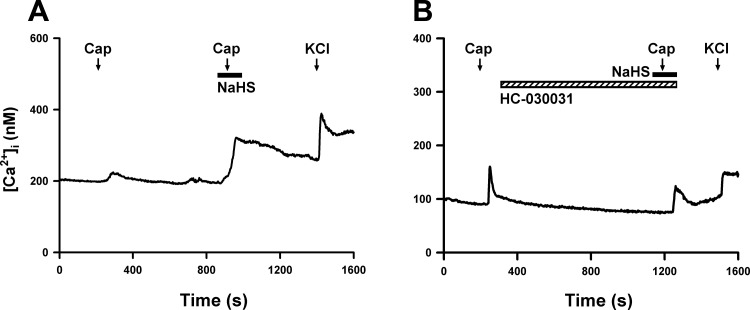
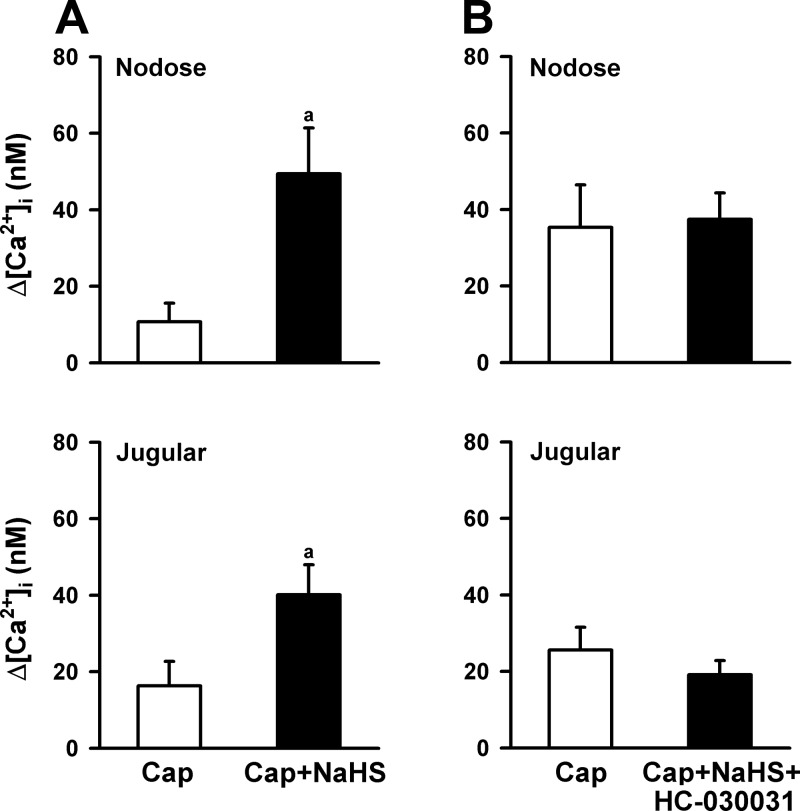
The application of capsaicin (200 nM, 30 s) evoked a rapid and transient increase in [Ca2+]i in isolated rat CSLV neurons from both the nodose and jugular ganglia. As shown in Fig. 7A, NaHS application alone did not have a detectable effect on the basal [Ca2+]i. In the nodose neurons, the baseline [Ca2+]i was 105.8 ± 16.6 and 105.6 ± 16.6 nM (n = 18, P > 0.05) before and 1 min after NaHS perfusion, respectively. In the jugular neurons, the basal [Ca2+]i was 99.2 ± 9.3 and 99.4 ± 9.7 nM (n = 24, P > 0.05) before and 1 min after NaHS perfusion, respectively. However, without a detectable effect on baseline [Ca2+]i, the NaHS pretreatment markedly enhanced the peak Ca2+ transients evoked by capsaicin (Figs. 7A and 8A). The NaHS-induced enhancing effect gradually declined after washout but remained higher than in the control for > 4 min after NaHS application in all tested CSLV neurons (Fig. 7A). In addition to its effect on the amplitude, NaHS pretreatment markedly prolonged the duration of the Ca2+ transients triggered by capsaicin (Fig. 7A). The durations were 11.4 ± 3.3 and 134.4 ± 21.3 s (n = 18, P < 0.05) in the control and NaHS-treated groups, respectively, in the nodose neurons. The durations were 31.7 ± 12.2 and 172.6 ± 17.3 s (n = 24, P < 0.05) in the control and NaHS-treated groups, respectively, in the jugular neurons.
Fig. 7.
Experimental records illustrating the potentiating effect of NaHS on capsaicin-evoked Ca2+ transients in 2 rat CSLV neurons. A and B: without and with HC-030031 pretreatment (20 μM, 16.5 min; hatched horizontal bar), respectively. Both neurons were isolated from nodose ganglia. Capsaicin (Cap, 200 nM, 30 s; arrows) was applied 1 min after the onset of NaHS perfusion (200 μM, 2.5 min; filled horizontal bars), and a KCl solution (60 mM, 20 s; arrows) was applied to test the cell vitality at the end of both experiments. [Ca2+]i, intracellular concentration of Ca2+. Note that pretreatment with HC-030031 totally abolished the potentiating effect of NaHS on the Ca2+ transients evoked by capsaicin.
Fig. 8.
The effects of HC-030031 pretreatment on the potentiation of capsaicin (Cap)-evoked Ca2+ transients by NaHS (200 μM, 2.5 min) in CSLV neurons. A: without HC-030031 pretreatment (20 μM, 16.5 min) in nodose (n = 18) and jugular (n = 24) ganglion neurons. B: pretreatment with HC-030031 in nodose (n = 22) and jugular (n = 18) ganglion neurons. See Fig. 7 for further explanation. An increase in intracellular Ca2+ concentration (Δ[Ca2+]i) was measured as the difference between the peak amplitude of Ca2+ transients (4-s average) and the 30-s average at baseline. aSignificantly different from the value of Cap only (P < 0.05). Data in each group are means ± SE.
Role of TRPA1 receptors in the NaHS-induced enhancement of Ca2+ transients.
HC-030031 pretreatment did not affect the Ca2+ transients evoked by capsaicin alone (300 nM, 30 s) in CSLV neurons, which were 68.4 ± 15.8 and 92.6 ± 22.7 nM (n = 30, P > 0.05) in the control and HC-030031-pretreated groups, respectively. However, the NaHS-induced enhancement of Ca2+ transients evoked by capsaicin was absent if NaHS was applied after the HC-030031 pretreatment (Figs. 7B and 8B).
DISCUSSION
The results of this study demonstrate that, in anesthetized and artificially ventilated rats, the inhalation of aerosolized NaHS, a donor of H2S, caused no detectable change in the baseline parameters of CSLV fiber activity or in the mean arterial blood pressure and the heart rate. However, the afferent responses of CSLV fibers to the chemical stimulation by the right atrial bolus injection of capsaicin or phenylbiguanide were all markedly potentiated. The sensitizing effect of NaHS was also found if the CSLV fibers were activated by lung inflation. In addition, the NaHS-induced sensitizing effect on the CSLV fiber response to capsaicin injection or lung inflation was totally abolished by pretreatment with a selective TRPA1 receptor antagonist, HC-030031, but was unaffected by its vehicle. In isolated rat CSLV neurons, we demonstrated that NaHS perfusion did not influence the baseline [Ca2+]i but significantly potentiated the Ca2+ transients evoked by capsaicin. Consistent with our findings from the in vivo electrophysiological experiment, pretreatment with HC-030031 fully reversed the NaHS-induced elevation of Ca2+ transients. Taken together, these results suggest that H2S induces a nonspecific sensitizing effect on CSLV fibers to both chemical and mechanical stimulation in rat lungs, and this effect is mediated, at least in part, through an action on the TRPA1 receptors expressed in the nerve endings of CSLV fibers.
In this study, H2S was applied by its donor, NaHS. The formation of HS− from the dissociation of NaHS to Na+ + HS− and the subsequent production of H2S were expected to elevate pH value. An alkaline solution has been shown to induce hyperalgesia in mouse hind paws through an activation of TRPA1 receptors (13). The sensitizing effect of NaHS possibly results from its alkalinity. However, the inhalation of an aerosolized NaOH solution (with a similar pH to NaHS) did not cause any effect on the basal activity and the excitability of the CSLV fibers under our experimental conditions. Therefore, we can rule out the possibility that the alkaline property of NaHS is responsible for the sensitization of CSLV fibers. In addition to NaHS, the sensitizing effect on CSLV fibers was also found in rats pretreated with l-cysteine, which is a common substrate for endogenous H2S generating enzyme in mammalian cells. However, the exogenous application of l-cysteine could also exert effects independent of H2S. For example, l-cysteine enters many metabolic pathways, such as metabolism of glutathione, methionine, and coenzyme A (30). The cysteine metabolites are involved in attenuating oxidative stress (4). Thus we cannot totally rule out the possibility that l-cysteine-induced sensitization resulted from the effects of cysteine metabolites.
Several studies have suggested that H2S is involved in the pathogenesis of airway neurogenic inflammation through an action on the CSLV fibers (5, 42); however, there is no direct evidence of the effect of H2S on these afferents. Thus this study reported the first evidence that H2S applied exogenously exerts a sensitizing effect on the CSLV fibers in response to various types of stimuli. Our findings were consistent with other studies that have shown that, in trigeminal ganglia (20) and colonic dorsal root ganglia (48), the exogenous application of H2S by its donor elevates the excitability of rat nociceptive neurons. Our observations also gain support from the findings that, in temporomandibular joints (11), hind paws (28), and the colon (48), the local application of NaHS induces hyperalgesia, which is a behavioral consequence of the sensitization of nociceptive neurons. In addition to exogenous H2S, endogenous H2S has also been suggested to play a vital role in inflammation-induced visceral (11) and somatic (12) hyperalgesia, which is substantiated because the hyperalgesia was reduced by the systemic pretreatment of the inhibitor of H2S-producing enzymes. However, in these studies, because the enzyme inhibitor was applied systemically, a possibility that the hyperalgesic effect of endogenous H2S results from its action on the central nervous system but not on peripheral nociceptive nerve endings cannot be ruled out. Thus, whether endogenous H2S contributes to the sensitization of peripheral nociceptive neurons, including CSLV neurons, is still unknown. Our study provides evidence that, without a significant influence on the basal activity, exogenous H2S exerts a sensitizing effect on CSLV neurons in the lungs. This finding suggests a potential environmental impact of H2S, but the role of endogenous H2S in relation to inflammation of the airways remains to be determined.
We demonstrated that H2S sensitizes CSLV neurons mediated through the activation of the TRPA1 receptor. In addition to TRPA1 receptors, H2S has been identified as an endogenous ligand of several receptors/channels that might also participate in nociceptor sensitization and hyperalgesia under various experimental conditions (8, 11, 33, 38). For example, the suppression of sustained potassium channel currents has been suggested to be responsible for the NaHS-induced sensitization of rat trigeminal ganglion neurons innervating the temporomandibular joints (11). It has been shown that the Cav3.2 T-type Ca2+ channels involves the somatic hyperalgesia induced by the intraplantar injection of NaHS (28, 33) and also contributes to the bladder hyperalgesia induced by the intraperitoneal injection of cyclophosphamide (29). Moreover, a recent study concluded that the activation of voltage-gated sodium channels by endogenous H2S is vital to the sensitization of colonic dorsal root ganglion neurons induced by heterotypic intermittent stress (44). In summary, these results suggest that H2S-induced hyperalgesia and nociceptive hypersensitivity appear to be related to the contribution of potassium channels, T-type Ca2+ channels, and voltage-gated sodium channels. In this study, we suggest that the H2S-induced sensitization of CSLV neurons relies on the activation of TRPA1 receptors. Our observation is in general agreement with two recent reports that have demonstrated the vital role of TRPA1 receptors in H2S-induced somatic hyperalgesia (2, 33). The receptors/channels responsible for H2S-induced hyperalgesia are diverse and might vary with different inducers/locations of the hyperalgesic responses. This notion is supported by a study showing that H2S-induced hyperalgesia in somatic tissue is dependent on both T-type Ca2+ channels and TRPA1 receptors, whereas that in the colon is mediated through T-type Ca2+ channels but not through TRPA1 receptors (2). In this study, the significance of TRPA1 receptors was substantiated by the full blockade of HC-030031 pretreatment on the NaHS-induced sensitization. To verify that this conclusion is accurate, we examined the effectiveness and specificity of the antagonizing effect of HC-030031 on TRPA1 receptors under the present experimental conditions. The effectiveness of HC-030031 was demonstrated by its total blockade of CSLV-fiber sensitization induced by AITC, a selective activator of TRPA1 receptors (Fig. 6E), whereas the specificity of HC-030031 was determined by its ineffectiveness on CSLV-fiber sensitization caused by PGE2 (Fig. 6F), which sensitizes the fibers through EP prostanoid receptors (21).
The cellular mechanism by which H2S sensitizes CSLV fibers remains unknown. In this study, the stimulation of the fibers elicited by injection of capsaicin or phenylbiguanide and by lung inflation was all potentiated by H2S inhalation. Capsaicin and phenylbiguanide are known to be mediated through their activation of the TRP vanilloid 1 (TRPV1) receptor and the serotonin 5-HT3 receptor, respectively (39), whereas mechanical stimulation of the CSLV fibers possibly involves as yet unidentified cationic channels gated by mechanical stress in the membrane of the nerve terminals (39). Because the afferent responses of CSLV fibers to injection of capsaicin or phenylbiguanide and to lung inflation were all potentiated, this suggests a nonspecific increase in the electrical excitability of CSLV fibers induced by NaHS inhalation. Alternatively, the H2S-induced activation of TRPA1 receptors probably shares a common cellular mechanism that enhances the function of these chemosensitive and mechanosensitive receptors. Our observations gain support from findings showing that, in rodent dorsal root ganglion neurons, the activation of TRPA1 receptors by AITC enhanced the inward currents evoked by repeated applications of AITC (36) and by the mechanical stimulation of neurites (6). The cellular mechanisms by which TRPA1 activation leads to the sensitization of CSLV fibers is unclear, but they are probably related to changes in the membrane conductance or to the resting membrane potential (19), or both. Activation of TRPA1 receptors evokes the influx of cations, such as calcium and sodium ions, which in turn can trigger the opening of voltage-gated cation channels (19) and lead to elevation of cell excitability of CSLV neurons. In addition to its abundant location on sensory neurons, TRPA1 receptors are also expressed on nonneuronal tissues including in mice and human airways (12, 32). The release of inflammatory mediators caused by the activation of nonneuronal TRPA1 receptors (12, 32), which might then lead to the sensitization of CSLV fibers (7, 22, 39).Furthermore, in mouse airways, H2S was reported to evoke releases of pro-inflammatory neuropeptides, such as substance P (3, 5). Thus a possibility that the sensitizing effect of H2S is due to its indirect action on the releases inflammatory mediators should be considered. However, we demonstrated that NaHS-induced sensitization was also found in the isolated rat CSLV neurons, which suggests, at least in part, a direct action of H2S on the nerve endings of CSLV fibers.
The elevation of plasma H2S levels has been reported in certain lung inflammatory conditions, such as in patients with chronic obstructive pulmonary disease (10) and in mice with lipopolysaccharide pretreatment (24). Furthermore, pharmacological inhibition or the genetic ablation of endogenous H2S-producing enzymes reduces lung inflammation caused by lipopolysaccharides (3, 24). Thus H2S is believed to act as a pro-inflammatory mediator. However, the role of H2S in inflammatory reaction remains controversial, with anti-inflammatory effects having been documented (9, 42, 43, 51). The controversy might be related to several factors such as the concentration, the duration of presence, and site of production (15, 46). Regardless of its role in the inflammatory reaction, H2S exerts a distinct sensitizing effect on CSLV afferents. The concept was supported by our results that sensitizing effect of CSLV-afferents was observed in rats treated with NaHS or l-cysteine; treatments with the former and the latter were reported to produce pro-inflammatory and anti-inflammatory effects, respectively (45). The stimulation of CSLV afferents is well documented to elicit various reflex responses, such as cough, bronchoconstriction, and airway hypersecretion (7, 22). Once the afferents are sensitized by mediators, this result might in turn exaggerate these respiratory reflexes (7, 15, 23, 26). Accordingly, during lung inflammation, we propose that H2S might contribute to the pathogenesis of airway hypersensitivity such as chronic cough. However, whether the endogenous H2S and its sensitizing effect on CSLV afferents play any part in the regulation of airway functions during lung inflammation remains to be determined.
Perspectives and Significance
Our results have demonstrated that in anesthetized and artificially ventilated rats, airway exposure to H2S causes an increase in the excitability of CSLV afferents. Furthermore, H2S pretreatment markedly enhanced chemical stimulation-evoked Ca2+ transients in isolated rat CSLV neurons. The H2S-induced sensitizing effect seems to be mediated through an action on TRPA1 receptor, which is probably located at terminals of CSLV fibers. Thus our findings provide new information for understanding of the physiological effects of H2S in the respiratory tract.
GRANTS
This study was supported by Grant NTUT-TMU-98-15 from the National Taipei University of Technology-Taipei Medical University Joint Research Program of Taiwan (to Y. S. Lin), Grant HL-96914 from National Institutes of Health (to L. Y. Lee), and W81XWH-10-2-0189 from the United States Department of Defense DMRDP/USAMRMC/TATRC award (to L. Y. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.-C.H. and Y.S.L. conception and design of research; C.-C.H., R.-L.L., and Y.S.L. performed experiments; C.-C.H. and R.-L.L. analyzed data; C.-C.H., L.-Y.L., and Y.S.L. interpreted results of experiments; C.-C.H. and Y.S.L. prepared figures; C.-C.H. and Y.S.L. drafted manuscript; C.-C.H., L.-Y.L., and Y.S.L. edited and revised manuscript; C.-C.H. and Y.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Maria Wasilewska for help with language editing.
REFERENCES
- 1.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson DA, Gentry C, Bevan S. TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide. PLos One 7: e46917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang SF, Moochhala SM, MacAry PA, Bhatia M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: involvement of substance P and ERK-NF-κB signaling. PLos One 6: e24535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardwell JCA, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell 67: 581–589, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Zhi L, Zhang H, Ng SW, Moore PK. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am J Physiol Lung Cell Mol Physiol 291: L896–L904, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol 589: 3575–3593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology 8: 291–301, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wang R. The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol 184: 130–138, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Chen YH, Wang PP, Wang XM, He YJ, Yao WZ, Qi YF, Tang CS. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 53: 334–341, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, Tang CS. Endogenous hydrogen sulfide in patients with COPD. Chest 128: 3205–3211, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Zhou YL, Meng X, Qi FH, Chen W, Jiang X, Xu GY. Hydrogen sulfide increases excitability through suppression of sustained potassium channel currents of rat trigeminal ganglion neurons. Mol Pain 9: 4, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 166: 510–521, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T, Tominaga M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 118: 4049–4057, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Q, Lin YS, Lee LY. Epinephrine enhances the sensitivity of rat vagal chemosensitive neurons: role of β3-adrenoceptor. J Appl Physiol 102: 1545–1555, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegde A, Bhatia M. Hydrogen sulfide in inflammation: friend or foe? Inflamm Allergy Drug Targets 10: 118–122, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Hessel PA, Herbert FA, Melenka LS, Yoshida K, Nakaza M. Lung health in relation to hydrogen sulfide exposure in oil and gas workers in Alberta, Canada. Am J Ind Med 31: 554–557, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-α in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 299: L483–L492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue T, Bryant BP. Multiple cation channels mediate increases in intracellular calcium induced by the volatile irritant, trans-2-pentenal in rat trigeminal neurons. Cell Mol Neurobiol 30: 35–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kwong K, Lee LY. PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol 93: 1419–1428, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lee LY, Undem BJ. Mechanisms of chronic cough. Pulm Pharmacol Ther 17: 463–464, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lin YS, Hsu CC, Bien MY, Hsu HC, Weng HT, Kou YR. Activations of TRPA1 and P2X receptors are important in ROS-mediated stimulation of capsaicin-sensitive lung vagal afferents by cigarette smoke in rats. J Appl Physiol 108: 1293–1303, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J Physiol 539: 947–955, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YS, Lin RL, Bien MY, Ho CY, Kou YR. Sensitization of capsaicin-sensitive lung vagal afferents by anandamide in rats: the role of transient receptor potential vanilloid 1 receptors. J Appl Physiol 102: 1545–1555, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Maeda Y, Aoki Y, Sekiguchi F, Matsunami M, Takahashi T, Nishikawa H, Kawabata A. Hyperalgesia induced by spinal and peripheral hydrogen sulfide: evidence for involvement of Cav3.2 T-type calcium channels. Pain 142: 127–132, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Matsunami M, Miki T, Nishiura K, Hayashi Y, Okawa Y, Nishikawa H, Sekiguchi F, Kubo L, Ozaki T, Tsujiuchi T, Kawabata A. Involvement of the endogenous hydrogen sulfide/Cav3.2 T-type Ca2+ channel pathway in cystitis-related bladder pain in mice. Br J Pharmacol 167: 917–928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherson RA, Hardy G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr Opin Clin Nutr Metab Care 14: 562–568, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, Svitacheva N, McGarvey L, Patacchini R, Damholt AB, Geppetti P, Materazzi S. Transient receptor potential ankyrin 1 channel localized to nonneuronal airway cells promotes non-neurogenic inflammation. PLos One 7: e42454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okubo K, Matsumura M, Kawaishi Y, Aoki Y, Matsunami M, Okawa Y, Sekiguchi F, Kawabata A. Hydrogen sulfide-induced mechanical hyperalgesia and allodynia require activation of both Cav3.2 and TRPA1 channels in mice. Br J Pharmacol 166: 1738–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo K, Takahashi T, Sekiguchi F, Kanaoka D, Matsunami M, Ohkubo T, Yamazaki J, Fukushima N, Yoshida S, Kawabata A. Inhibition of T-type calcium channels and hydrogen sulfide-forming enzyme reverses paclitaxel-evoked neuropathic hyperalgesia in rats. Neuroscience 188: 148–156, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol 142: 31–34, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raisinghani M, Zhong L, Jeffry JA, Bishnoi M, Pabbidi RM, Pimentel F, Cao DS, Evans MS, Premkumar LS. Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception. Am J Physiol Cell Physiol 301: C587–C600, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32: 109–134, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753–763, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol 101: 950–959, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 178: 406–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123–1131, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandiver M, Snyder SH. Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med 90: 255–263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Qu R, Hu S, Xiao Y, Jiang X, Xu GY. Upregulation of cystathionine β-synthetase expression contributes to visceral hyperalgesia induced by heterotypic intermittent stress in rats. PLos One 7: e53165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 12: 1147–1154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol 4: 13–32, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Widdicombe JG. Overview of neural pathways in allergy and asthma. Pulm Pharmacol Ther 16: 23–30, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Xu GY, Winston JH, Shenoy M, Zhou S, Chen JD, Pasricha PJ. The endogenous hydrogen sulfide producing enzyme cystathionine-beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain 5: 44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol 569: 519–531, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Zhang G, Wang P, Yang G, Cao Q, Wang R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol 182: 1188–1195, 2013 [DOI] [PubMed] [Google Scholar]