Abstract
We examined the impact of strength fitness and body weight on the redox properties of high-density lipoprotein (HDL) and associations with indices of vascular and metabolic health. Ninety young men were categorized into three groups: 1) overweight untrained (OU; n = 30; BMI 30.7 ± 2.1 kg/m2); 2) overweight trained [OT; n = 30; BMI 29.0 ± 1.9; ≥4 d/wk resistance training (RT)]; and 3) lean trained (LT; n = 30; BMI 23.7 ± 1.4; ≥4 d/wk RT). Using a novel assay on the basis of the HDL-mediated rate of oxidation of dihydrorhodamine (DOR), we determined the functional (redox) properties of HDL and examined correlations between DOR and indices of vascular and metabolic health in the cohort. DOR was significantly lower in both trained groups compared with the untrained group (LT, 1.04 ± 0.49; OT, 1.39 ± 0.57; OU, 1.80 ± 0.74; LT vs. OU P < 0.00001; OT vs. OU P = 0.02), however, DOR in the OT group was not significantly different from that of the LT group. DOR was negatively associated with HDL-cholesterol (R = −0.64), relative strength (R = −0.42), sex hormone-binding globulin (R = −0.42), and testosterone (R = −0.35) (all P ≤ 0.001); whereas DOR was positively associated with triglycerides (R = 0.39, P = 0.002), oxidized low-density lipoprotein (R = 0.32), body mass index (R = 0.43), total mass (R = 0.35), total fat mass (R = 0.42), waist circumference (R = 0.45), and trunk fat mass (R = 0.42) (all P ≤ 0.001). Chronic RT is associated with improved HDL redox activity. This may contribute to the beneficial effects of RT on reducing cardiovascular disease risk, irrespective of body weight status.
Keywords: dysfunctional hdl, dihydrorhodamine, resistance training, exercise, obesity
the impact of high-density lipoprotein cholesterol (HDL-C) as a marker and mediator of coronary heart disease (CHD) risk is well established. High levels of HDL-C appear to be protective against CHD and the National Cholesterol Education Program has emphasized increasing HDL-C to help reduce CHD risk (7). However, given that a large portion of CHD events occur in patients with normal HDL-C levels (3), it is apparent that the quantitative level of HDL-C may not be a suitable indicator of the true antiatherosclerotic capacity of HDL. For example, Ansell et al. (1) tested the ability of patients' HDL-C to inhibit low-density lipoprotein (LDL) oxidation and observed that HDL-C from patients with CHD and very high HDL-C (mean HDL-C 95 mg/dl) was not protective against LDL oxidation. Thus, although at a population-level higher plasma HDL-C levels are associated with lower risk for CHD, at an individual level, the functional properties of HDL, such as its anti-inflammatory and antioxidative properties may be more important (24). Dysfunctional HDL has increased redox and proinflammatory activity (15), reduced antioxidant and anti-inflammatory properties (19, 34), including increased lipid hydroperoxide content (22), diminished ability to prevent LDL oxidation (21) and cholesterol efflux (9), and thus contributes to atherogenesis (20, 21). Most assays of HDL function have relied on examining biologic effects, but such bioassays are technically challenging, and therefore difficult to apply to clinical studies and to standardize between laboratories. We recently developed a novel, fluorescence-based, cell-free assay that quantifies the redox activity of HDL on the basis of the oxidation rate of the fluorogenic probe dihydrorhodamine 123 (DHR), which can be used in large-scale studies to measure HDL function (15).
In addition to this, much debate exists regarding the relative roles of body weight and fitness on the risk for CHD. Many studies have suggested that the effects of obesity on CHD risk are in fact mediated largely by fitness. For example, in the Aerobics Center Longitudinal Study (ACLS), the impact of metabolic syndrome on cardiovascular disease (CVD) mortality risk was no longer significant when accounting for fitness levels (14). Jurca et al. (11, 12) noted that strength fitness was inversely associated with risk for metabolic syndrome in men in the ACLS. Additionally, exercise interventions may improve CVD risk in the absence of changes in weight. Along these lines, we demonstrated that a short-term lifestyle intervention could improve the anti-inflammatory function of HDL in the face of a quantitative decrease in HDL-C, and that these changes occurred despite subjects remaining obese after the intervention (29).
In view of these preliminary studies and the emerging role of HDL function in the pathophysiology of CHD, using a novel assay (15) we investigated the redox activity of HDL cross-sectionally in 90 young men categorized into 3 groups on the basis of their body weight status (i.e., normal weight vs. overweight/obese) and strength training status: overweight untrained (OU); overweight trained (OT); and lean trained (LT). In addition, we investigated associations of dysfunctional HDL with indices of vascular and metabolic disease risk factors. We hypothesized that the redox properties of HDL would be improved in trained subjects, irrespective of body weight status.
METHODS
Study Participants
In this cross-sectional study, 90 young adult men aged 18–30 were recruited and categorized into 3 phenotypes on the basis of training status and body mass index (BMI): lean trained [LT; n = 30; ≥4 days/wk resistance training (RT); BMI <25 kg/m2]; overweight trained (OT; n = 30; ≥4 days/wk RT; BMI >27 kg/m2); and overweight untrained (OU; n = 30; no structured exercise regimen; BMI >27 kg/m2). OU subjects participated only in light-intensity physical activity ≤2 times/wk. Any participants with overt chronic disease symptoms as indicated by a screening, comprehensive history, and physical examination were excluded from the study. Potential participants who had documented CVD or heart arrhythmia found on an electrocardiogram reading or who used tobacco products or medications that influence cardiovascular function, body composition, or insulin indices in the prior 6 mo were also excluded. All of the study protocols were approved by the University of California, Los Angeles Institutional Review Board, and were performed according to the Declaration of Helsinki.
Outpatient Visit Procedures
Subjects were reminded to abstain from the consumption of food, caffeine, alcohol, and vitamin supplements for at least 12 h prior to testing. They also did not engage in any moderate to vigorous physical activity within 36 h of the visit. Height, weight (for BMI calculation), and waist circumference (WC) were measured in duplicate. Body composition was determined by dual energy X-ray absorptiometry scan (Hologic QDR4500 Fan Beam X-ray Densitometer; Hologic, Waltham, MA) and blood pressure measurements (i.e., brachial systolic and diastolic pressure, bSBP and bDBP, respectively) taken from the right arm using an automated oscillometric cuff (Prevention DS2200; Ultima). Subsequently, subjects underwent a blood draw. Serum and plasma aliquots were acquired immediately and systematically in all subjects after the blood draw, and samples were stored at −80°C until assayed.
Muscular Strength Testing
Maximal strength testing consisted of 1-repetition maximum (1-RM) lifts for the barbell bench press, 45° incline leg press, and machine-seated row carried out by certified trainers at the John Wooden Recreation Center. Participants first warmed up each muscle group by performing 8–10 repetitions with weight equivalent to 40–60% of their estimated 1-RM. The weight was progressively increased while repetitions were decreased until participants could safely attempt an estimated 1-RM for each exercise. A successful 1-RM occurred on the penultimate set, having failed their last attempt. Participants were allowed 3–4 min of rest between all sets. The strength score was determined by the sum of the three strength measures. Relative strength was calculated by dividing strength score by participant body weight.
Carotid Intima-Media Thickness
Subclinical estimation of carotid atherosclerosis was obtained by assessment of carotid intima-media thickness (IMT) of the right and left carotid arteries. Participants rested supine with their neck in neutral position and rotated 45° from the midline. Two-dimensional ultrasound images of the carotid artery were obtained using a 12-MHz linear array transducer, and data were digitally recorded on an external computer for off-line analysis. Automated edge detection software (Medical Imaging Applications, Iowa) was utilized to measure the carotid artery diameter (intima-intima) and the right and left IMT at ∼1 cm distal to the carotid bulb. All IMT outcome measures were determined by averaging data from a minimum of 10 cardiac cycles obtained during end-diastole. Both near- and far-wall IMTs were obtained and averaged between right and left carotid arteries. Intraobserver coefficients of variability for near- and far-wall IMTs were 2.9% and 1.8%, respectively.
Blood Chemistry Assays
Samples were assayed for total cholesterol, HDL-C, non-HDL cholesterol (non-HDL-C), and triglycerides (TGs) using the Olympus AU400 Chemistry Analyzer. LDL cholesterol (LDL-C) was calculated using the Friedewald equation (8). Serum glucose was assayed via the hexokinase method (Olympus AU400 Chemistry Immuno Analyzer; Olympus America, Center Valley, PA). Serum insulin was measured by solid-phase, enzyme-labeled chemiluminescent immunometric assay (Immulite 2000; Diagnostic Products, Los Angeles, CA). Homeostasis model assessment for insulin resistance was calculated by the formula [fasting insulin (μU/ml) × fasting glucose (mM)/22.5]. Adiponectin (R&D Systems, Minneapolis, MN), oxidized LDL (oxLDL; Mercodia Laboratories, Upsala, Sweden), and high-sensitivity C-reactive protein (Alpco, Salem, NH) concentrations were determined by ELISA. Interleukin-8, tumor necrosis factor-α, myeloperoxidase, total plasminogen activator inhibitor-1, soluble intercellular adhesion molecule-1, total amylin, and leptin were determined using Millipore Multiplex assays (Billerica, MA). All analytes were measured in duplicate. Plasma levels of sex hormone binding globulin (SHBG), cortisol, and testosterone were measured by an electrochemiluminescent immunoassay on an Elecsys 2010 autoanalyzer (Roche Diagnostics, Indianapolis, IN). Free testosterone was calculated via the Sodergard method (30a). Free androgen index was calculated by 100·(Total Testosterone/SHBG).
Dysfunctional HDL Assessment
Reagents.
DHR was obtained from Molecular Probes (Eugene, OR). DHR was prepared as concentrated stock of 50 mM in dimethyl sulfoxide as previously described (15). Iron-free N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered saline (HBS; HEPES 20 mM, NaCl 150 mM, pH 7.4) was prepared as previously described (15). The DHR stock was diluted 1:1,000 in HEPES saline solution to prepare a working solution of 50 μM.
HDL isolation.
HDL was isolated from cryopreserved human serum by ultracentrifugation, as previously described (15).
Determination of HDL-C concentration.
HDL-C was quantified using a standard colorimetric assay (Thermo DMA, San Jose, CA) as previously described (15).
DHR-based cell-free assay of HDL function.
HDL function was determined on the basis of a previously established biochemical cell-free assay that measures HDL redox activity based on oxidation of the fluorogenic probe DHR (15). Briefly, quadruplicates of 1.25 μg of HDL were added to 96-well plates (polypropylene, flat bottom, black, Fisher Scientific). HBS was added to each well to a final volume of 150 μl, followed by addition of 25 μl of the working solution of 50 μM DHR, for a total volume of 175 μl (final DHR concentration of 7 μM). Immediately following DHR addition, the plate was protected from light and placed in a fluorescence plate reader. The fluorescence of each well was assessed at 2-min intervals for 1 h with a Synergy 2 multimode microplate reader (Biotek, VT) using a 485/538 nm excitation/emission filter pair with the photomultiplier sensitivity set at medium. The oxidation rate was calculated for each well as the slope for the linear regression of fluorescence intensity between 10 and 50 min (DHR oxidation rate or DOR), expressed as fluorescence units per minute (FU/min). HDL redox activity was calculated as the mean of quadruplicates for the wells containing the HDL sample. The DOR of each sample was normalized as a ratio of the DOR of a control HDL isolated from pooled serum from all study subjects. All the DOR values were also normalized to the respective HDL-C concentration from the clinical laboratory (16).
Statistical Analyses
Robust measures of statistical significance were obtained by running a Monte Carlo permutation for each respective test statistic (e.g., F-statistic) 10,000 times from which a test for significance was obtained at an alpha value of <0.05 using one-way ANOVA analyses. Permutation and bootstrap analyses were used to avoid making any assumptions on the distribution of the data. Post hoc permuted t-tests were used to test significance between groups. Bootstrapped post hoc Pearson correlation analyses were used to determine the relationships between DOR and cardiovascular and metabolic disease risk biomarkers within the full cohort. To account for multiple exploratory correlations, a Bonferroni correction was applied resulting in a critical alpha value of 0.002. Covariate analyses were performed to assess the role of strength or fat in driving significance in outcome variables. Data from one OU subject was not used because the subject had an acute skin rash. Additionally, a Grubbs test was used to assess outliers in the data. Data for one OT and one LT subject were not used due to the presence of outliers for the main variable in question as confirmed by the Grubbs outlier test (GraphPad Prism 5). Thus data from 87 subjects were used in the final data analysis (29 LT, 29 OT, and 29 OU). Statistical analyses were performed with the use of Stata statistical software 12 (StataCorp, College Station, TX). Data are reported as means ± SD.
RESULTS
Anthropometrics, Strength, Blood Pressure, and Lipids
There were no significant differences in weight or BMI among OT and OU subjects; however, OT subjects displayed lower WC compared with OU subjects (P < 0.001). LT subjects had lower WC, fat mass, and lean body mass compared with OT subjects (Table 1).
Table 1.
Anthropometrics, strength, blood pressure, and lipids
| Outcomes | LT | OT | OU | P |
|---|---|---|---|---|
| Age (yr) | 23 (2.9) | 22 (2.7) | 22 (2.1) | 0.29 |
| Height (m) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 0.17 |
| Weight (kg) | 73.8 (7.2)*† | 93.1 (11.1) | 94.9 (9.4) | <0.00001 |
| BMI (kg/m2) | 23.7 (1.4)*† | 29.0 (1.9)* | 30.7 (2.1) | <0.00001 |
| Total lean (kg) | 64.1 (6.3)† | 76.5 (7.4)* | 68.3 (6.3) | <0.00001 |
| Total fat (kg) | 9.8 (2.3)*† | 16.6 (5.7)* | 26.6 (5.6) | <0.00001 |
| Relative strength | 6.7 (0.9)* | 6.5 (1.2)* | 4.5 (0.7) | <0.00001 |
| WC (cm) | 80.1 (4.4)*† | 90.8 (6.7)* | 101.7 (7.1) | <0.00001 |
| bSBP (mmHg) | 120.6 (10.0)* | 124.7 (12.0)* | 131.0 (10.7) | 0.002 |
| bDBP (mmHg) | 72.3 (8.0)* | 75.9 (7.1) | 80.7 (10.2) | 0.001 |
| TG (mg/dl) | 69.7 (33.8)* | 80.9 (31.0)* | 140.3 (72.4) | <0.00001 |
| Total cholesterol (mg/dl) | 146.2 (28.0)* | 149.4 (23.7) | 163.9 (33.2) | 0.05 |
| HDL-C (mg/dl) | 51.6 (7.8)* | 49.5 (8.3)* | 40.2 (6.3) | <0.00001 |
| LDL-C (mg/dl) | 73.3 (21.3)* | 81.7 (21.0) | 94.9 (32.7) | 0.004 |
LT, lean-trained; OT, overweight-trained; OU, overweight-untrained; BMI, body mass index; WC, waist circumference; bSBP, brachial systolic blood pressure; bDBP, brachial diastolic blood pressure; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. Group significance was calculated using Monte Carlo permutations coupled with one-way ANOVA analyses. Between-group significance was calculated by post hoc permuted regression analyses.
Significance (P < 0.05) between LT vs. OU and OT vs. OU; †significance between LT vs. OT.
Brachial SBP and bDBP were lower in the LT group compared with the OU group (all P < 0.001) and trended toward being lower (bSBP P = 0.05; bDBP P = 0.053) in the OT group compared with the OU group. Subjects in the LT group exhibited lower total cholesterol (P = 0.02) and LDL-C (P = 0.001), whereas those in the OT group exhibited a trend toward lower total cholesterol (P = 0.06) and LDL-C (P = 0.054) compared with the OU group. The LT and OT groups had lower TG (both P < 0.00001) and higher HDL-C (both P < 0.001) than the OU group. No differences were noted between the LT and OT groups for any blood pressure or lipid parameter.
Dysfunctional HDL
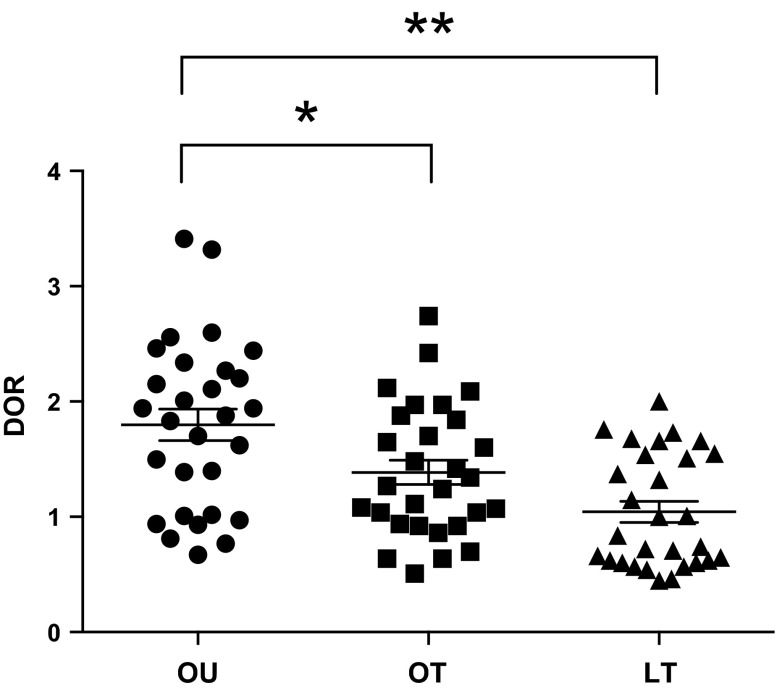
As a marker of HDL redox activity, we noted higher DOR in the OU group (1.80 ± 0.74) compared with both OT (1.39 ± 0.57, P = 0.02) and LT (1.04 ± 0.49, P < 0.0001) groups, and no difference was noted between the LT and OT groups (Fig. 1). In addition, in view of the limited data on the associations of HDL redox function with other cardiovascular and metabolic phenotypes (25), we correlated dysfunctional HDL with several common indices of metabolic and cardiovascular health within the full cohort (Table 2 and Table 3). DOR was negatively associated with HDL-C (R = −0.64, P < 0.00001), relative strength (R = −0.42, P < 0.00001; Fig. 2A), SHBG (R = −0.42, P < 0.00001; Fig. 2B), and testosterone (R = −0.35, P = 0.001), whereas DOR was positively associated with TG (R = 0.39, P = 0.002), oxLDL (R = 0.32, P = 0.001; Fig. 2C; Table 2), BMI (R = 0.43, P < 0.00001), WC (R = 0.45, P < 0.00001), trunk fat mass (R = 0.42, P < 0.00001; Fig. 2D), total fat mass (R = 0.42, P < 0.00001), and total mass (R = 0.35, P = 0.0004).
Fig. 1.
Dihydrorhodamine oxidation rate (DOR) in overweight-untrained (OU), overweight-trained (OT), and lean-trained (LT) subjects. Between-group significance was calculated by permuted regression post hoc tests. Between-group comparisons of DOR demonstrates higher dysfunctional HDL in OU than OT (P = 0.02) and LT (P = <0.00001) subjects. *P < 0.05; **P < 0.00001.
Table 2.
Dihydrorhodamine correlations with indices of vascular and metabolic health
| Outcome | R | P |
|---|---|---|
| BMI (kg/m2) | 0.43 | <0.00001 |
| WC (cm) | 0.45 | <0.00001 |
| Trunk fat (kg) | 0.46 | <0.00001 |
| Total fat (kg) | 0.44 | <0.00001 |
| Total mass (kg) | 0.35 | 0.0004 |
| Total lean (kg) | 0.11 | 0.32 |
| Relative strength | −0.42 | <0.00001 |
| bSBP (mmHg) | <0.00001 | 0.98 |
| bDBP (mmHg) | <0.00001 | 0.96 |
| Average IMT (mm) | −0.13 | 0.23 |
| Adiponectin (ng/ml) | −0.24 | 0.01 |
| Total amylin (pmol/L) | 0.27 | 0.03 |
| CRP (mg/L) | 0.13 | 0.23 |
| sICAM-1 (ng/ml) | 0.20 | 0.03 |
| PAI-1 total (ng/ml) | 0.15 | 0.16 |
| MPO (ng/ml) | 0.13 | 0.27 |
| IL-8 (pg/ml) | 0.11 | 0.49 |
| TNF-α (pg/ml) | 0.02 | 0.80 |
| Fasting glucose (mg/dl) | 0.25 | 0.02 |
| Fasting insulin (uIU/ml) | 0.27 | 0.01 |
| HOMA | 0.07 | 0.40 |
| Leptin (pM) | 0.37 | 0.08 |
| Total cholesterol (mg/dl) | 0.05 | 0.59 |
| HDL-C (mg/dl) | −0.64 | <0.00001 |
| OxLDL (U/l) | 0.32 | 0.001 |
| LDL-C (mg/dL) | 0.18 | 0.08 |
| Non-HDL-C (mg/dL) | 0.25 | 0.01 |
| TG (mg/dL) | 0.39 | 0.002 |
| Cortisol (μg/dl) | <0.00001 | 0.96 |
| SHBG (nmol/l) | −0.42 | <0.00001 |
| Testosterone (ng/ml) | −0.35 | 0.001 |
| FAI | 0.34 | 0.03 |
CRP, C-reactive protein; sICAM-1, soluble intercellular adhesion molecule-1; PAI-1, plasminogen activator inhibitor-1; MPO, myeloperoxidase; IL-8, interleukin-8; TNF-α, tumor necrosis factor alpha; HOMA, homeostasis model assessment for insulin resistance; FAI, free androgen index. Correlations were determined using post hoc Pearson correlation analyses. P values in bold represent significance at the Bonferroni corrected alpha value of 0.002.
Table 3.
Dihydrorhodamine correlations with indices of vascular and metabolic health controlled for strength and trunk fat
| Strength controlled |
Trunk fat controlled |
|||||
|---|---|---|---|---|---|---|
| Outcome | R | P | R | P | R | P |
| BMI (kg/m2) | 0.43 | <0.00001 | 0.47 | <0.00001 | — | — |
| WC (cm) | 0.45 | <0.00001 | 0.46 | <0.00001 | — | — |
| Trunk fat (kg) | 0.46 | <0.00001 | 0.50 | <0.00001 | — | — |
| Total fat (kg) | 0.44 | <0.00001 | 0.49 | <0.00001 | — | — |
| Total mass (kg) | 0.35 | 0.0004 | 0.45 | <0.00001 | — | — |
| Relative strength | −0.42 | <0.00001 | — | — | −0.74 | 0.21 |
| HDL-C (mg/dl) | −0.64 | <0.00001 | −0.65 | <0.00001 | −0.70 | <0.00001 |
| Ox-LDL (U/L) | 0.32 | 0.001 | 0.39 | 0.01 | 0.53 | 0.31 |
| TG (mg/dl) | 0.39 | 0.002 | 0.43 | 0.01 | 0.58 | 0.29 |
| SHBG (nmol/L) | −0.42 | <0.00001 | −0.45 | <0.00001 | −0.63 | 0.06 |
| Testosterone (ng/ml) | −0.35 | 0.001 | −0.35 | 0.001 | −0.42 | 0.04 |
Correlations were determined using post hoc Pearson correlation analyses.
Fig. 2.
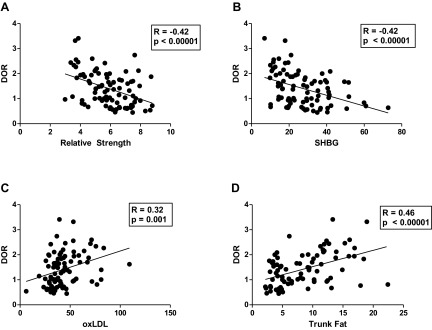
Correlation of DOR with indices of metabolic and cardiovascular health, including relative strength (A), sex hormone-binding globulin (SHBG) (B), oxidized low-density lipoprotein (oxLDL) (C), and trunk fat (D).
Additionally, to further investigate the relative roles of muscular fitness and trunk fat mass with HDL redox activity, we controlled for a composite strength score and trunk fat (as well as total fat and body fat percentage; data not shown). Controlling for strength and trunk fat mass indicated that both appear to contribute to the relationships between HDL redox activity and cardiovascular/metabolic phenotypes (Table 3).
DISCUSSION
We set out to compare HDL redox activity using a novel, fluorescence-based, cell-free assay in young adult men classified by strength training and body weight status. Our primary findings demonstrate that 1) the rate of DHR oxidation (or DOR) was significantly lower in LT and OT subjects compared with OU subjects; and 2) we noted that HDL redox activity exhibited associations with several indices, including strength, body composition, lipids (HDL-C, TG, oxLDL) and steroid hormones (SHBG, testosterone).
HDL-C is an established marker and mediator of CHD risk. Although high HDL-C appears to be protective against CHD, a large portion of CHD events occur in patients with normal HDL-C levels (3). Thus, the quantitative level of HDL-C does not by itself indicate the pro- or antiatherosclerotic capacity of HDL. Although the classical protective property of HDL-C has been described as the promotion of total cholesterol efflux, the anti-inflammatory and antioxidative properties of HDL are critically important. Oxidative damage has been proposed to deprive HDL of its cardioprotective effects (31). Prior studies have noted that in patients with CHD, HDL-C fails to inhibit LDL oxidation despite being quantitatively high (1). This has led to the hypothesis that the qualitative properties of HDL are critical for individual prevention and treatment of CHD, and this phenomenon has been coined by Navab et al. (21) as the double jeopardy of HDL.
Along these lines, recent interest has focused on the functional properties of HDL oxidation (5, 25). Oxidation contributes to the formation of dysfunctional HDL, proposed to be present in humans with cardiovascular disease (25). However, due to the complexity of HDL particles, which have multiple functional properties such as antioxidant and anti-inflammatory activities (24, 25), and technical challenges of measuring its function, human data have been limited (17, 26). Most assays of function have relied on examining biologic effects, but such bioassays are labor-intensive, technically challenging, prone to variability, and therefore difficult to apply to large clinical studies and standardize between laboratories (23, 27, 33, 35). We have demonstrated that oxidized HDL, which has increased HDL redox activity, is dysfunctional in terms of its modulatory effect on monocyte chemotaxis, a key process in atherogenesis (15). Thus HDL redox status is a biochemical property that may be a good indicator of HDL function. This HDL redox activity can be measured by a fluorometric biochemical assay on the basis of the effect of HDL on the oxidation of DHR that may offer a convenient tool for studies of the role of HDL functional phenotype in the development of atherosclerosis in vivo (15).
In the present study, DOR was lower in both trained groups (LT and OT) compared with the OU group, suggesting that HDL redox function is better in those with higher muscular fitness irrespective of body weight status. The potential significance of this finding includes the possibility that regular RT may be associated with improved HDL redox function and be a potential mechanism by which RT may decrease cardiovascular disease risk. Interestingly, we noted that some metabolic and cardiovascular phenotypes were related to HDL redox activity. The correlation with oxLDL is logical given the redox function of HDL to inhibit LDL oxidation. The inverse correlations with SHBG and testosterone are also of interest. Low SHBG (13) and testosterone (2, 37) are associated with increased cardiovascular disease risk, and the present study is the first to demonstrate a relationship between HDL redox activity and steroid hormones.
Our finding of less dysfunctional HDL with high muscular fitness irrespective of weight status is not surprising because it has become apparent that the role of obesity in the risk of CHD may indeed be largely accounted for by differences in fitness (14). Although the mechanisms are poorly understood, strength training/fitness is inversely associated with metabolic syndrome (11, 12) and CHD (32). Previously, we noted that exercise interventions may improve CVD risk factors in the absence of obesity reversal. For example, a short-term lifestyle intervention could improve the anti-inflammatory properties of HDL in the face of a quantitative decrease in HDL-C, and these changes occurred despite subjects remaining obese after the intervention (29). We also noted that several other aspects of metabolic health improve with lifestyle modification despite the lack of obesity reversal and despite no change or decreases in HDL-C in children (4, 10, 28), women (38), and men (29, 30). Interestingly, no prior studies have used RT as the exercise modality for the assessment of the functional properties of HDL, although it has been noted that RT may increase LDL-C clearance (6). However, consistent with these findings, Kosola et al. (18) demonstrated that the high oxLDL commonly noted in people who are overweight and obese (39) is attenuated in subjects with higher muscular fitness. In addition, low physical activity was associated with dysfunctional HDL in patients with systemic lupus erythematosus (36). Interestingly, when we controlled for a composite strength score or trunk fat mass, in the adjusted analyses, both trunk fat mass and strength appeared to contribute to the relationships between HDL redox activity and cardiovascular and metabolic phenotypes. These analyses suggest that although strength fitness impacts HDL redox function irrespective of body weight status, anthropometric variables still contribute to the relationships noted, and the relative roles of strength and fat mass should be addressed in future studies.
In summary, HDL exhibits higher antioxidative potential in strength-trained young men irrespective of overweight/obesity status. One limitation of our findings is that we did not include subjects with BMI >35, so whether this relationship holds for these individuals is unknown. Future studies can use this novel cell-free assay to estimate the redox functional properties of HDL and further investigate the roles of fitness and body weight in the context of HDL's antiatherosclerotic properties.
GRANTS
This study was supported by American Heart Association Grant BGIA 0765139Y to C.K.R., National Heart, Lung, and Blood Institute Grant P50 HL105188 to C.K.R., and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK090406 to C.K.R. This study was also supported by National Institute of Allergy and Infectious Diseases Grants AI-068634 and AI-056933, the UCLA AIDS Institute, and the UCLA Center for AIDS Research Grant AI28697. Partial funding for laboratory work was also provided by University of Washington CVD and Metabolic Complications of HIV/AIDS Data Coordinating Center Grant 5R01 HL-095126.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.K.R. and T.K. conception and design of research; D.M.C. and T.K. performed experiments; C.K.R., M.K., D.M.C., and T.K. analyzed data; C.K.R., M.K., and D.M.C. interpreted results of experiments; M.K. prepared figures; C.K.R., M.K., and T.K. drafted manuscript; C.K.R., M.K., D.M.C., O.O.Y., and T.K. edited and revised manuscript; C.K.R., M.K., D.M.C., O.O.Y., and T.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mary M. Lee, Shannon L. Krell, and the entire Exercise and Metabolic Disease Research team for their commitment to this study. We thank the Gonda (Goldschmied) Diabetes Center, Elisa Terry, Mick DeLuca, and colleagues at the John Wooden Recreation Center, and the UCLA Academic Technology Services Statistical Consulting Group for their statistical support. Furthermore, we thank all participants for their time and effort.
REFERENCES
- 1.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108: 2751–2756, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bhasin S. Effects of testosterone administration on fat distribution, insulin sensitivity, and atherosclerosis progression. Clin Infect Dis 37, Suppl 2: S142–S149, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 256: 2835–2838, 1986 [PubMed] [Google Scholar]
- 4.Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism 55: 871–878, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res 158: 369–384, 2011 [DOI] [PubMed] [Google Scholar]
- 6.da Silva JL, Vinagre CG, Morikawa AT, Alves MJ, Mesquita CH, Maranhao RC. Resistance training changes LDL metabolism in normolipidemic subjects: a study with a nanoemulsion mimetic of LDL. Atherosclerosis 219: 532–537, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 9.Hayek T, Oiknine J, Brook JG, Aviram M. Role of HDL apolipoprotein E in cellular cholesterol efflux: studies in apo E knockout transgenic mice. Biochem Biophys Res Commun 205: 1072–1078, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Izadpanah A, Barnard RJ, Almeda AJ, Baldwin GC, Bridges SA, Shellman ER, Burant CF, Roberts CK. A short-term diet and exercise intervention ameliorates inflammation and markers of metabolic health in overweight/obese children. Am J Physiol Endocrinol Metab 303: E542–E550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc 37: 1849–1855, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc 36: 1301–1307, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kalme T, Seppälä M, Qiao Q, Koistinen R, Nissinen A, Harrela M, Loukovaara M, Leinonen P, Tuomilehto J. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab 90: 1550–1556, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med 164: 1092–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kelesidis T, Currier JS, Huynh D, Meriwether D, Charles-Schoeman C, Reddy ST, Fogelman AM, Navab M, Yang OO. A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res 52: 2341–2351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelesidis T, Yang O, Kendall M, Hodis H, Currier J. Dysfunctional HDL and progression of atherosclerosis in HIV-1-infected and -uninfected adults. Lipids Health Dis 12: 23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosola J, Ahotupa M, Kyrolainen H, Santtila M, Vasankari T. Good aerobic or muscular fitness protects overweight men from elevated oxidized LDL. Med Sci Sports Exerc 44: 563–568, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Mackness MI, Durrington PN, Mackness B. The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am J Cardiovasc Drugs 4: 211–217, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. Thematic review series: the pathogenesis of atherosclerosis the oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 45: 993–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Hama S, Hough G, Bachini E, Grijalva VR, Wagner AC, Shaposhnik Z, Fogelman AM. The double jeopardy of HDL. Ann Med 37: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, Reddy S, Shih D, Shi W, Watson AD, Van Lenten BJ, Vora D, Fogelman AM. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol 21: 481–488, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 42: 1308–1317, 2001 [PubMed] [Google Scholar]
- 24.Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, Fogelman AM. The role of dysfunctional HDL in atherosclerosis. J Lipid Res 50: S145–S149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 8: 222–232, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol 58: 2068–2075, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol 53: 962–971, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Roberts CK, Chen AK, Barnard RJ. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis 191: 98–106, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol 101: 1727–1732, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation 106: 2530–2532, 2002 [DOI] [PubMed] [Google Scholar]
- 30a.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 16: 801–810, 1982 [DOI] [PubMed] [Google Scholar]
- 31.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem 287: 6375–6386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA 288: 1994–2000, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem 284: 30825–30835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 96: 2758–2767, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res 48: 2344–2353, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Volkmann ER, Grossman JM, Sahakian LJ, Skaggs BJ, FitzGerald J, Ragavendra N, Charles-Schoeman C, Chen W, Gorn A, Karpouzas G, Weisman M, Wallace DJ, Hahn BH, McMahon M. Low physical activity is associated with proinflammatory high-density lipoprotein and increased subclinical atherosclerosis in women with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 62: 258–265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Von Eckardstein A, Wu FC. Testosterone and atherosclerosis. Growth Horm IGF Res 13, Suppl A: S72–S84, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism 53: 377–381, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Weinbrenner T, Schroder H, Escurriol V, Fito M, Elosua R, Vila J, Marrugat J, Covas MI. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr 83: 30–35, 2006 [DOI] [PubMed] [Google Scholar]