Abstract
Children with heart failure are treated with similar medical therapy as adults with heart failure. In contrast to adults with heart failure, these treatment regiments are not associated with improved outcomes in children. Recent studies have demonstrated age-related pathophysiological differences in the molecular mechanisms of heart failure between children and adults. There are no animal models of pediatric cardiomyopathy to allow mechanistic studies. The purpose of the current experiments was to develop a mouse model of pediatric heart disease and test whether the influence of β-adrenergic receptor (β-AR) antagonism could be modeled in this system. We hypothesized that isoproterenol treatment of young mice would provide a model system of cardiac pathology, and that nonselective β-AR blockade would provide benefit in adult, but not young, mice, similar to clinical trial data. We found that isoproterenol treatment (through osmotic minipump implantation) of young and adult mice produced similar degrees of cardiac hypertrophy and recapitulated several age-related molecular abnormalities in human heart failure, including phospholamban phosphorylation and β-AR expression. We also found that nonselective β-AR blockade effectively prevented pathological cardiac growth and collagen expression in the adult but not young mice, and that selective β1-AR blockade was effective in both young and adult isoproterenol-treated mice. In conclusion, we have developed the first model system for β-AR-mediated pediatric heart disease. Furthermore, we have generated novel data suggesting beneficial effects of selective β1-AR blockade in the pediatric heart.
Keywords: β-adrenergic receptor, antagonist, pediatric, heart failure, phospholamban
clinical data have demonstrated that children with heart failure (HF) have different molecular and clinical responses to the disease than their adult counterparts. The Pediatric Carvedilol Trial demonstrated no benefit to the nonselective β-adrenergic receptor (AR) blocker, carvedilol, in children with HF (29). Although there were several limitations to this trial, it remains the only multicenter randomized clinical pediatric HF experience available. Importantly, there is complementary retrospective outcomes data from two sources that further demonstrate no improvement in pediatric HF outcomes between the digoxin and diuretics treatment era of the 1980's to the current angiotensin-converting enzyme inhibitor and β-AR blocker regimens (17, 33). Using similar methodology, significant benefit has been demonstrated in comparing these treatment paradigms in adults (10, 18). Despite these clinical data, many pediatric cardiologists continue to prescribe off-label carvedilol for their patients with HF with the rationale that the trial demonstrated no harm.
In contrast to the differences in clinical outcomes, plasma norepinephrine levels are elevated to a similar extent in both children and adults with HF. Furthermore, elevated norepinephrine appears to have a direct prognostic relationship, regardless of age (15, 34). In contrast to the age-related similarities in circulating catecholamines, our laboratory has recently demonstrated that expression of cardiac adrenergic receptors and a number of downstream signaling molecules are different between adults and children in the setting of HF (21). One of the principal differences is in the remodeling of specific adrenergic receptor subtypes. In adults, it has been well established that HF produces downregulation of the β1-AR without change in β2-AR expression (5–7, 21). In contrast, HF in children produces a downregulation of both receptors (21).
There is similar activation of calcium-calmodulin kinase (CaMK) in both adults and children with HF, indicative of persistent pathological signaling through the β1-AR intracellular cascade (21). The detrimental effect of CaMK activation provides a rationale for the importance of β1-AR selective blockade in both children and adults with HF. Although the beneficial effects of β1-selective adrenergic receptor blockade have been clearly demonstrated in randomized clinical trials in adults (1, 2, 14), the pediatric experience consists mainly of retrospective cohort studies (30, 35), confounded by a 50% spontaneous recovery rate (29). The age-related difference in β2-AR expression may contribute to myocardial remodeling due to the prosurvival/antiapoptotic nature of the β2-AR signaling cascade (8, 9, 31, 36, 38, 39). Importantly, the blockade of the β2-AR with carvedilol in the pediatric clinical trial may be responsible, in part, for the lack of clinical benefit in the pediatric population. Unfortunately the pathophysiological importance of these differences in myocardial adaptation of the β-AR system is virtually impossible to determine in children due to population-specific protections and characteristics (25).
Therefore, the purpose of the present investigation was to develop an animal model of pediatric cardiac disease and test a physiological response to pharmacological blockade in this model. Isoproterenol (ISO) treatment in adult mice is a well-established method to produce pathological adaptation as a model for adult heart disease through chronic β-AR activation (4, 13, 16). We hypothesized that ISO treatment in young and adult mice would reproduce catecholamine-mediated human age-related differences in myocardial remodeling of the β-AR. In addition, we hypothesized that nonselective β-AR blockade with carvedilol would blunt pathophysiological changes in adult mice, but not young mice, and that β1-selective adrenergic receptor blockade would block these changes in both the young and adult mice.
MATERIALS AND METHODS
Drug preparation.
Isoproterenol hydrochloride (ISO, Sigma-Aldrich, St. Louis, MO) was dissolved in either 0.57 mM l-ascorbic acid (Fisher Scientific, Fair Lawn, NJ) in 0.9% wt/vol saline solution or 60:40 l-ascorbic acid saline-DMSO, depending on the solubility characteristics of the β-blocker being delivered to the cohort. Carvedilol and metoprolol were dissolved in sterile drinking water. Bisoprolol was dissolved in saline vehicle (Veh), nebivolol and CGP-20712A were dissolved in 60:40 saline-DMSO, and each was delivered via osmotic minipump.
Experimental animals.
FVB mice were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in the animal facility at the University of Colorado. To model a pediatric population, young (Y) mice were ∼4 wk of age at the time of pump implantation to allow the completion of the experimental protocol before the time of sexual maturity [∼6 wk of age (20, 27, 37)]. Adult (A) mice were ∼5 mo of age. Mice were individually housed and allowed ad libitum access to standard diet chow and water during the experimental protocols. Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver and adhered to the American Physiological Society's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.
Disease model.
Y and A mice were treated with ISO (30 mg·kg−1·day−1; n: 72 Y, 43 A) or Veh (Y: 92; A: 38) delivered subcutaneously via osmotic minipump (model 1007D or 2001, Alzet, Durect, Cupertino, CA) for 7 days. Minipumps were inserted subcutaneously through a midline interscapuar incision (caudal to the ears) under 1.5% inhaled isoflurane anesthesia (VetOne, Meridian, ID). Mice received perioperative bupivacaine (Hospira, Lake Forest, IL) on the wound, as well as postoperative carprofen (5 mg/kg sc) (Pfizer, New York, NY) for 1 day. At the end of the 1-wk treatment course, study mice were killed. Cardiac tissue was isolated immediately, weighed, frozen in liquid N2, and stored at −80°C for further analysis.
Echocardiography.
Cardiac ultrasound was performed on a subpopulation of the ISO- and Veh-treated Y (n = 6/group) and A (Veh: n = 18, ISO: n = 19) mice. On day 7 of the treatment paradigm, parasternal short-axis M-mode was obtained under 1.5% isoflurane anesthesia using a high-resolution Visual Sonics Vevo 770 (VisualSonics, Toronto, Ontario, Canada) platform with a 30-MHz mechanical transducer. Ventricular parameters were assessed using short-axis M-mode and long- and short-axis B-mode images of the left ventricle (LV). LV dimensions were measured, and LV mass was calculated.
β-AR expression.
β-AR density was determined in crude LV tissue membranes (n: 5 veh, 8 ISO), as previously described (21). Kudej et. al. (19) have previously demonstrate β-AR downregulation with ISO treatment in A mice. For this reason, β-AR expression was only determined in the Y mice. A 100–300 μg/ml membrane protein solution was radiolabeled with [125I]cyanopindolol. Binding displaceable by 1 μM (−)-propranolol was considered specific binding. Percent β1-AR was determined by using 1 μM CGP-20712A and subtracting the percentage of β2-AR binding from total specific binding. All experiments were conducted at 30°C for 120 min. LV samples from age- and treatment-matched groups were pooled from three to four hearts to generate sufficient membrane protein for a single assessment (n values represent pooled samples, not individual hearts used).
Gene expression.
Total RNA was extracted from LV tissue by mirVana kit (Ambion), 0.5 μg of RNA were reverse transcribed into cDNA using I-script (Bio-Rad). Typically, 5–10 ng of cDNA, 12.5 nM of each primer, and Power Syber Green PCR Master Mix (ABI) were used in the RT-PCR reactions. Reactions were performed using the ABI7300 system and normalized to 18s. The primers for 18s, atrial natriuretic peptide (ANP), α-myosin heavy chain (MHC), and β-MHC have been previously described (12, 32). The collagen 1A primers are as follows: forward, 5′-GACGCCATCAAGGTCTACTG-3′, and reverse, 5′-ACGGGAATCCATCGGTCA-3′. Change in expression was determined by the ΔΔCt method (21).
Protein expression.
Protein expression was evaluated by Western blot, as previously described (21). Protein was isolated from 10- to 25-mg frozen LV tissue in isoelectric focusing buffer homogenized at 4°C. A 7.5% Tris·HCl gel containing 10 μg of protein extract per well was used for electrophoresis. Resolved protein was transferred to a polyvinyldene difluoride membrane for 80 min at 80 V for phospholamban (PLB) and overnight at 30 V for both MHCs in 20% methanol. The membrane was blocked for 1 h in 1 × Tris-buffered saline containing 5% wt/vol low-fat dry milk and 0.1% vol/vol Tween 20. Primary antibodies [β-MHC: 1:10,000, M8421, Sigma-Aldrich, St. Louis, MO, and as previously described (21)] were diluted in 1 × Tris-buffered saline containing 5% wt/vol bovine serum albumin and 0.1% vol/vol Tween 20. Primary antibodies were incubated overnight at 4°C. Secondary antibodies were incubated for 90 min at room temperature. PICO SuperBright enhanced chemiluminescence reagent, Kodak autoradiography paper, and ImageJ were used to visualize and quantify bound antibody. All protein data were normalized to glyceraldehyde-3-phosphate dehydrogenase (1:5,000, sc-20357, Santa Cruz Biotechnology, Dallas, TX) and the age-matched Veh control group.
β-Blocker treatment.
Littermate mice were divided into three groups for each β-blocker cohort: Veh, ISO, and ISO + β-blocker (ISO/BB). The five independent β-blocker cohorts were treated with either the nonselective β-AR antagonist carvedilol [dose: 30 mg·kg−1·day−1; n = 33 (Y Veh), 16 (Y ISO), 18 (Y ISO/BB), 17 (A Veh), 9 (A ISO), 10 (A ISO/BB)], or one of four β1-AR-selective antagonists: 1) metoprolol [300 mg·kg−1·day−1; n = 32 (Y Veh), 15 (Y ISO), 14 (Y ISO/BB), 22 (A Veh), 14 (A ISO), 11 (A ISO/BB)]; 2) bisoprolol [2.5 mg·kg−1·day−1; n = 21 (Y Veh), 13 (Y ISO), 16 (Y ISO/BB), 11 (A Veh), 14 (A ISO), 8 (A ISO/BB)]; 3) nebivolol [10 mg·kg−1·day−1; n = 17 (Y Veh), 14 (Y ISO), 20 (Y ISO/BB), 4 (A Veh), 4 (A ISO), 4 (A ISO/BB)]; or 4) CGP-20712A [10 mg·kg−1·day−1; n = 34 (Y Veh), 34 (Y ISO), 19 (Y ISO/BB), 40 (A Veh), 35 (A ISO), 15 (A ISO/BB)]. The β-blocker was started at the same time as the ISO treatment in all groups. No difference in ISO-induced hypertrophy was observed between the standard Veh group and the DMSO Veh group (data not shown). A subset of mice was treated with Veh + β-blocker and demonstrated no differences in heart weight (HW)/body weight (BW) (data not shown). At the end of the 1-wk treatment course, mice were killed. Cardiac tissue was isolated immediately, weighed, frozen in liquid N2, and stored at −80°C for further analysis.
Data analysis and presentation.
All statistical analyses were performed using StatView (SAS Institute, Cary, NC). Statistical significance was set a priori at P < 0.05. All data in the text and Tables 1 and 2 are presented as mean (SD), while the data in Figs. 1–7 are means ± SE. Comparisons between Veh and ISO treatments within each age group were performed by unpaired Student's t-test. Data not normally distributed by Shapiro-Wilk test were log transformed and retested to assure normality before statistical analysis. Nonnormally distributed data are presented using the raw values in the text and Figs. 1–7 for visual clarity. Comparisons for each β-blocker intervention were performed with an ANOVA and Fisher's post hoc testing for between-group differences in the presence of a significant main effect.
Table 1.
Gross morphometric and echocardiogram indexes
| Young |
Adult |
|||||
|---|---|---|---|---|---|---|
| Veh | ISO | P Value | Veh | ISO | P Value | |
| Body weight, g | 16.7 (2.9) | 17.3 (2.9) | 0.14 | 28.6 (3.9) | 28.6 (4.3) | 0.94 |
| Heart weight, mg | 88 (14) | 103 (15) | <0.0001 | 117 (20) | 142 (22) | <0.0001 |
| LV mass/body weight, mg/g | 4.5 (0.4) | 5.4 (0.6) | <0.05 | 4.1 (0.8) | 4.9 (0.8) | <0.005 |
| LVIDd, mm | 3.6 (0.3) | 3.5 (0.2) | 0.68 | 3.6 (0.3) | 3.7 (0.2) | 0.17 |
Values are means (SD). Veh, vehicle treated; ISO, isoproterenol; LV, left ventricle; LVIDd, LV internal diameter in diastole.
Table 2.
Cardiac growth with β-blocker intervention
| Young |
Adult |
|||||
|---|---|---|---|---|---|---|
| BB | Veh | ISO | ISO/BB | Veh | ISO | ISO/BB |
| Carvedilol | ||||||
| Body weight, g | 18.1 (3.0) | 18.5 (3.2) | 18.4 (3.1) | 27.9 (3.8) | 29.1 (5.4) | 28.4 (4.6) |
| Heart weight, mg | 89 (9.4) | 105 (15) | 101 (13) | 112 (15) | 138 (18) | 119 (21) |
| Heart weight/body weight, mg/g | 5.0 (0.8) | 5.7 (0.7) | 5.5 (0.6) | 4.0 (0.3) | 4.8 (0.5) | 4.2 (0.3) |
| Metoprolol | ||||||
| Body weight, g | 19.3 (3.0) | 19.6 (2.1) | 19.5 (4.1) | 27.5 (5.2) | 28.0 (5.0) | 25.2 (3.5) |
| Heart weight, mg | 90 (13) | 100 (22) | 111 (13) | 110 (19) | 116 (16) | 138 (19) |
| Heart weight/body weight, mg/g | 4.7 (0.4) | 5.6 (0.3) | 5.1 (0.3) | 4.0 (0.4) | 5.0 (0.4) | 4.6 (0.5) |
| Bisoprolol | ||||||
| Body weight, g | 14.7 (2.5) | 15.4 (3.1) | 16.9 (2.6) | 28.8 (2.8) | 28.3 (3.0) | 28.7 (2.1) |
| Heart weight, mg | 80 (16) | 100 (15) | 97 (16) | 119 (20) | 143 (23) | 128 (17) |
| Heart weight/body weight, mg/g | 5.5 (1.0) | 6.6 (0.7) | 5.7 (0.6) | 4.1 (0.4) | 5.1 (0.7) | 4.5 (0.4) |
| Nebivolol | ||||||
| Body weight, g | 19.6 (3.1) | 18.0 (2.7) | 20.4 (3.0) | 28.1 (4.0) | 29.1 (5.6) | 27.1 (3.3) |
| Heart weight, mg | 93 (15) | 110 (15) | 116 (17) | 125 (20) | 164 (39) | 134 (19) |
| Heart weight/body weight, mg/g | 4.8 (0.5) | 6.2 (0.5) | 5.7 (0.4) | 4.4 (0.3) | 5.6 (0.3) | 5.0 (0.3) |
| CGP-20712A | ||||||
| Body weight, g | 17.8 (2.6) | 18.3 (2.6) | 18.5 (2.3) | 28.9 (4.2) | 28.1 (3.5) | 29.5 (4.1) |
| Heart weight, mg | 94 (10) | 112 (16) | 106 (12) | 123 (19) | 148 (23) | 147 (22) |
| Heart weight/body weight, mg/g | 5.4 (0.7) | 6.2 (0.8) | 5.8 (0.3) | 4.3 (0.4) | 5.3 (0.5) | 5.0 (0.3) |
Values are means (SD). Cardiac growth is in mg heart weight/g body weight. BB, β-blocker.
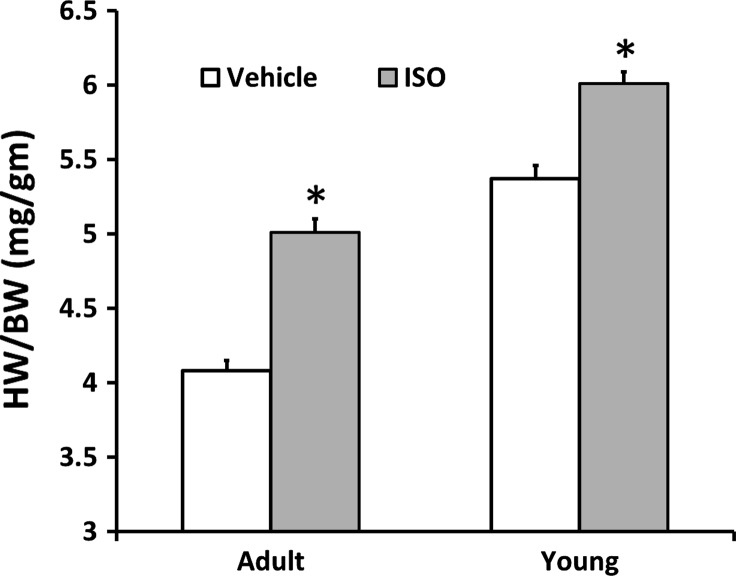
Fig. 1.
Cardiac growth was higher in response to isoproterenol (ISO) in both young (Y) and adult (A) mice. Values are means ± SE. *P < 0.0001 vs. age-matched vehicle treated (V). HW/BW, heart weight-to-body weight ratio.
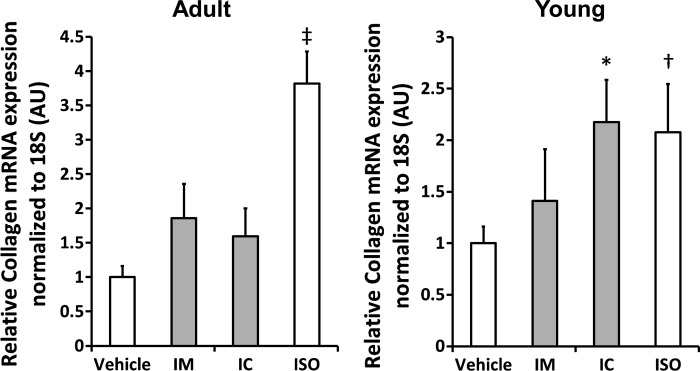
Fig. 7.
Cardiac collagen 1A mRNA expression in Y (right) and A (left) mice. Collagen expression was lower in response to Met and Car treatment in the A mice (left), but only in response to Met treatment in the Y mice. Car treatment of Y mice had no effect on collagen expression. Values are means ± SE. *P < 0.05, †P < 0.01, ‡P < 0.0001. IM, ISO plus Met treatment; IC, ISO plus Car treatment.
RESULTS
Cardiac growth.
To mimic catecholamine stimulation of the β-ARs observed in human heart disease, Y and A mice were treated with ISO and compared with Veh-treated controls. HW was ∼20% higher (P < 0.0001) in the ISO-treated Y and A mice. The hearts from female mice, regardless of age or treatment, were ∼15% smaller than the age- and treatment-matched males (P < 0.0001). This sex difference in HW disappeared (P = 0.66) when normalized to BW, but the HW-to-BW ratios (HW/BW) remained lower in the A mice, regardless of sex or treatment (ANOVA main effect, P < 0.0001, Fig. 1). Since BW did not adequately normalize HW in response to age, it is important to note that there was no significant difference in age between the Veh- and ISO-treated Y [Veh: 35.6 (3.8) days; ISO: 35.6 (3.1) days, P = 0.95] or A [Veh: 151 (20) days; ISO: 148 (18) days, P = 0.48] mice. In addition, BW was not significantly different between the ISO- and Veh-treated mice within each age group (Table 1). Significantly higher HW/BW (Fig. 1) are demonstrated in Y (∼12%, P < 0.0001) and A (∼25%, P < 0.0001) ISO-treated mice compared with age-matched Veh-treated controls. The degree of ISO-induced hypertrophy was similar between the age groups (ANOVA age × treatment interaction, P = 0.15). Cardiac ultrasound demonstrated significantly higher LV mass/BW in both the Y and A ISO-treated mice compared with the age-matched control group (Table 1). There was no effect of ISO on chamber dimension in diastole (LV internal diameter in diastole).
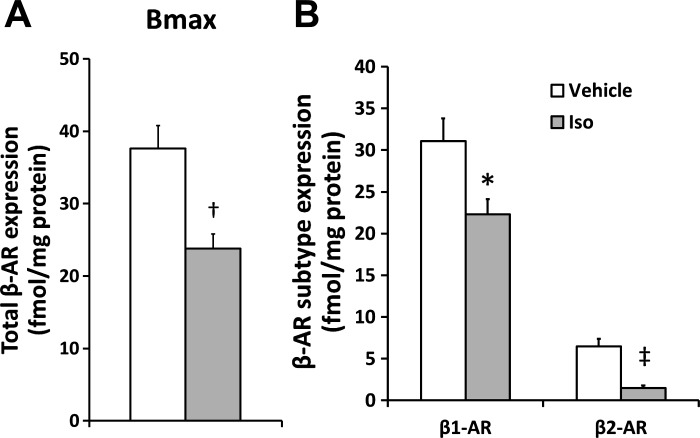
β-AR expression.
β-AR protein expression was determined in the heart of Y mice to determine whether the model recapitulated expression in the human pediatric heart (Fig. 2). Total β-AR expression (Fig. 2A) was ∼40% lower in the ISO-treated mice (P < 0.01). Similar to the clinical data (21), the lower total receptor number in response to ISO was due to a lower expression of both the β1-AR [Veh: 31.1 (6.1) vs. ISO: 22.3 (3.9) fmol/mg protein, P < 0.05] and β2-AR [Veh: 6.5 (2.0) vs. ISO: 1.5 (0.8) fmol/mg protein, P < 0.001] subtypes (Fig. 2B).
Fig. 2.
Lower total β-adrenergic receptor (β-AR) number (A) with ISO treatment in Y mice was associated with lower expression of the β1- and β2-AR subtypes (B). Bmax, total β-AR expression. Values are means ± SE. *P < 0.05, †P < 0.01, ‡P < 0.001 vs. V.
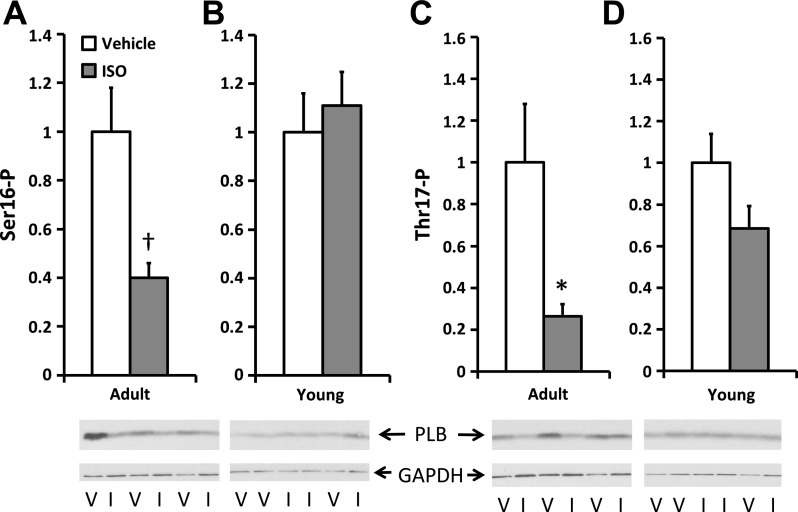
PLB phosphorylation.
PLB, a protein that regulates the sarcoplasmic reticulum ATPase, has an age-dependent difference in phosphorylation in the setting of HF (21). Phosphorylation at the protein kinase A-dependent site, serine 16 (Ser16), and at the CaMK dependent site, threonine 17 (Thr17), was determined in extracts from Y and A mice (Fig. 3). Similar to what is found in adult humans with HF, PLB phosphorylation at both the Ser16 [1.0 (0.7) vs. 0.4 (0.2) arbitrary units (AU), Fig. 3A] and Thr17 [1.0 (1.0) vs. 0.3 (0.2) AU, Fig. 3B] sites was significantly lower in the ISO-treated A mice than the controls (both P < 0.05). In contrast, no difference in PLB phosphorylation was found in the Y mice at either site [Ser16: 1.0 (0.6) vs. 1.1 (0.8) AU; Thr17: 1.0 (0.5) vs. 0.7 (0.4) AU]. In addition, there was no difference in total PLB expression in either group (data not shown). These data recapitulate the age-dependent difference in PLB phosphorylation in humans in response to HF.
Fig. 3.
Phosphorylation at serine 16 (Ser16-P; A and B) and threonine 17 (Thr17-P; C and D) of phospholamban (PLB) normalized to GAPDH with representative blots. A and C: A mice. B and D: Y mice. Phosphorylation is lower in the ISO-treated (I) A mice at both sites, but unchanged in the Y mice. Values are means ± SE. *P < 0.05, †P < 0.005 vs. age-matched V.
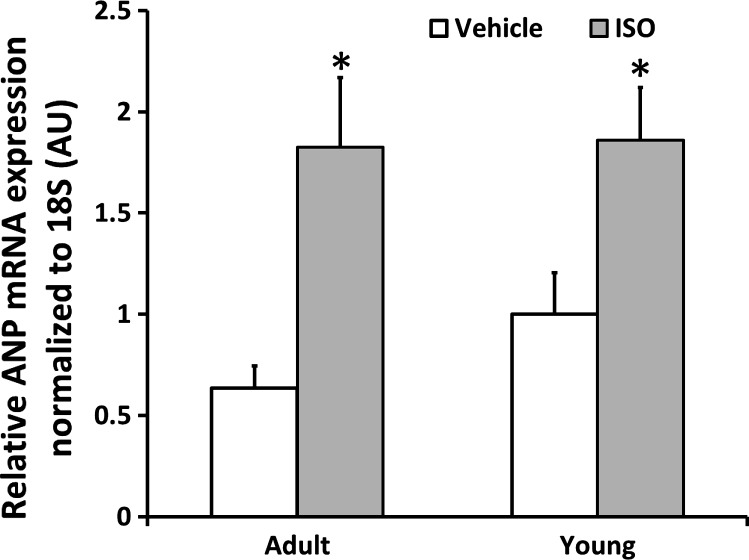
ANP expression.
An increase in ANP mRNA is a marker of cardiac disease in human HF, as well as in many animal models of heart disease. ANP mRNA expression was significantly higher in the ISO-treated Y and A mice compared with their age-match Veh controls [Y: 1.0 (0.9) vs. 1.9 (1.2) AU; A: 0.6 (0.4) vs. 1.8 (1.4) AU; Fig. 4], reproducing the changes observed in adult and pediatric human HF.
Fig. 4.
Atrial natriuretic peptide (ANP) mRNA expression is higher in I Y and A mice. Values are means ± SE. *P < 0.005 vs. age-matched V. AU, arbitrary units.
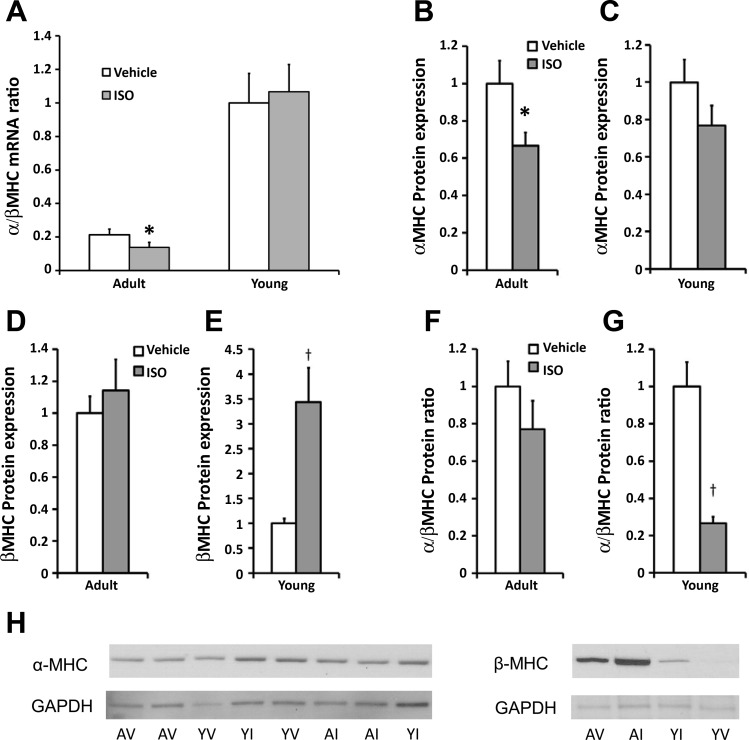
MHC mRNA and protein expression.
MHC isoforms are different between rodents and humans. The mouse heart contains predominantly α-MHC, and the human heart contains predominantly β-MHC. Despite these species differences, switching from the α- to the β-isoform is a universal marker of cardiac pathology. Expression of both mRNA and protein is altered with disease. Although the individual changes in α- or β-MHC mRNA expression in response to ISO were not statistically different in the Y or A mice (data not shown), the α-to-β ratio, a composite of change in the individual isoforms, was significantly lower with ISO treatment in the A mice (Fig. 5A). At the protein level, α-MHC was significantly lower in the A mice [1.0 (0.5) vs. 0.7 (0.3) AU, Fig. 5B], while the β-MHC was significantly higher in the Y mice [1.0 (0.5) vs. 3.4 (2.7) AU, Fig. 5E] in response to ISO treatment. The α-to-β-protein ratio was significantly lower with ISO treatment in the Y mice (Fig. 5G). There was no change in α-MHC expression in the Y mice (Fig. 5C) and β-MHC (Fig. 5D) or the α-to-β-protein ratio (Fig. 5F) in the A mice. A subset of Y and A samples could be directly compared and demonstrated that β-MHC is significantly more abundant in the A mice, while α-MHC is slightly more abundant in the Y mice (Fig. 5H).
Fig. 5.
Expression of α- and β-myosin heavy chain (MHC) mRNA (A) and protein [α-MHC protein in A (B) and Y mice (C), β-MHC protein in A (D) and Y mice (E), α/β-MHC protein in A (F) and Y (G) mice, and H]. Lower α/β-MHC mRNA ratio in the A mice (A) and α/β-MHC protein ratio in the Y mice (G) in response to ISO treatment are consistent with the isoform switching that occurs in a diseased heart. Lower α-MHC expression in the A mice (B) and higher β-MHC expression in the Y mice (E) in response to ISO are consistent with expected changes with cardiac disease. H: qualitatively higher β-MHC and lower α-MHC are found in the A mice compared with the Y mice. Values are means ± SE. *P < 0.05, †P < 0.01, vs. age-matched V.
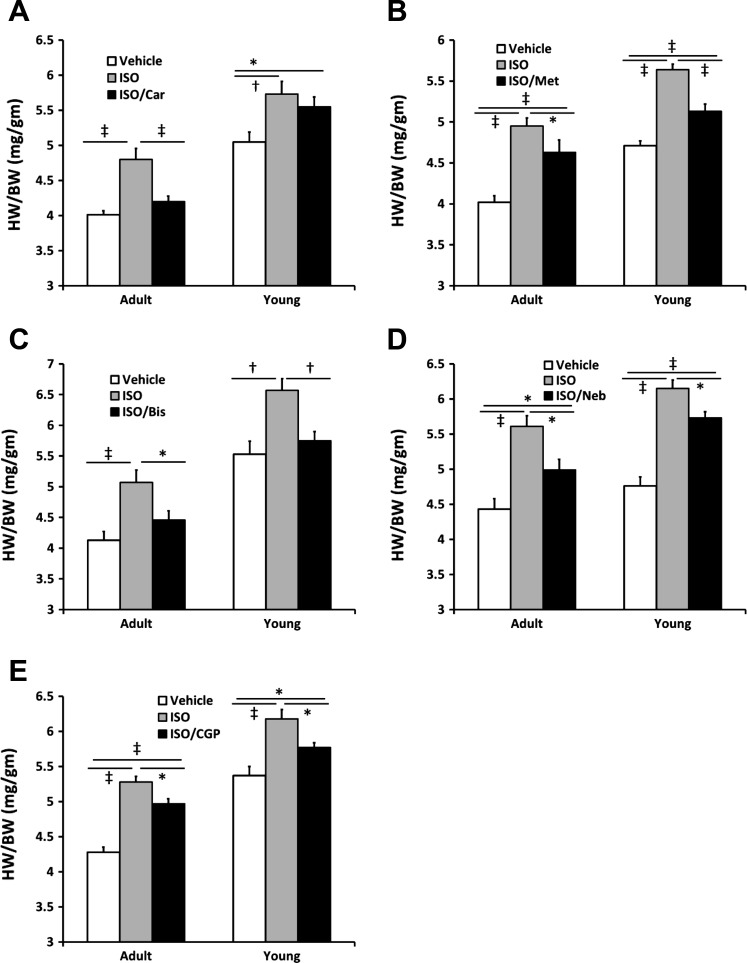
β-Blocker treatment.
To model human pathophysiology, the mice were treated with a number of β-blockers used in clinical practice (carvedilol, metoprolol, bisoprolol, nebivolol), as well as a highly β1-AR-selective blocker not used clinically (CGP-20712A). Similar to the data presented above, ISO treatment produced significant growth in all β-blocker treatment cohorts, regardless of age (all P < 0.005). Hypertrophy was not influenced by the use of saline Veh or 60:40 saline-DMSO Veh (data not shown). Treatment with the nonselective β-blocker carvedilol significantly blunted the pathological cardiac growth (HW/BW) in the A but not in the Y mice (Table 2, Fig. 6A). In contrast, all of the β1-AR-selective (Table 2, Fig. 6, B–E) blockers significantly blunted the cardiac hypertrophy in both the Y and A mice.
Fig. 6.
Cardiac growth in response to β-pageer treatment in Y and A mice. A: the nonselective β-AR antagonist carvedilol (Car) blocks pathological cardiac growth in response to ISO in A but not Y mice. The selective β1-AR antagonists, metoprolol (Met; B), bisoprolol (Bis; C), nebivolol (Neb; D), and CGP-20712A (CGP; E) block cardiac growth in response to ISO in both Y and A mice. Values are means ± SE. *P < 0.05, †P < 0.01, ‡P < 0.0005.
Collagen 1A expression.
Since fibrosis is integral to cardiac pathology in humans and ISO induces collagen expression and fibrosis (19), we assessed collagen 1A expression (Fig. 7) with ISO and the response to the nonselective β-blocker, carvedilol, and the prototypical β1-selective blocker, metoprolol. Collagen expression was significantly higher in both the Y (n = 16) and A (n = 14) ISO-treated mice compared with age-matched Veh controls (n = 16/each group). In the Y mice (Fig. 7), collagen expression remained significantly higher in carvedilol-treated group (n = 8) than the Veh controls while collagen expression in the metoprolol treated mice (n = 8) was not different from the controls. In contrast, both metoprolol (n = 8) and carvedilol (n = 7) treatment of ISO mice significantly suppressed collagen expression (both P < 0.005).
DISCUSSION
To our knowledge, these are the first data to directly examine the potential for a mouse to model pediatric vs. adult differences in human cardiac disease. First, we have demonstrated that ISO treatment produces similar cardiac pathology independent of age. Second, we have demonstrated that this rodent model system recapitulates a number of the molecular changes in response to HF in children and adult humans. Finally, we have demonstrated that the response to β-blocker therapy in the animal model has parallels to the outcomes data in pediatric and adult clinical β-blocker trials. Taken together, these data demonstrate that a mouse model may be useful to improve our understanding of pediatric heart disease.
Recently, age-related differences in myocellular remodeling in response to HF have been demonstrated between children and adult humans (21). Studies in clinical pediatric populations are limited by heterogeneity of the disease, a small population at any one institution, as well as ethical restrictions on a vulnerable population (25). These factors are sufficient to significantly restrict the scientific community's ability to address myocellular factors in pediatric HF. Although there are models for a few specific congenital cardiac diseases, animal models are lacking for most pediatric heart diseases, including idiopathic cardiomyopathies. Thus the development of animal models for these diseases will greatly facilitate pediatric-specific discovery and therapeutics.
Since sympathetic activation is common to HF in both children (34) and adults (15), and the clinical response to the nonselective β-blocker, carvedilol, is divergent between these clinical populations (11, 23, 24, 29), the present investigation focused on age differences in β-AR activation. The study adapted an established mouse model of pathological hypertrophy in adults (4, 13, 16, 19) to explore the pediatric pathophysiology by treating weanling mice with ISO and determining the response of adrenergic receptor expression and select downstream signaling pathways. The increase in ANP expression and MHC isoform switching demonstrates that ISO produces cardiac pathology, regardless of age. This age-independent sensitivity to β-AR stimulation may explain the correlation between circulating catecholamine levels and mortality in both pediatric and adult human HF populations (15, 34). To further support the validity of this model for the pediatric population, ISO treatment of the Y mice produced a downregulation of both β1- and β2-AR subtypes. This expression profile is similar to that of human pediatric patients with HF. It is possible that this difference in β-AR expression from adults, where only the β1-AR is downregulated in response to HF (5–7, 21), contributes to the difference in clinical response to carvedilol therapy (11, 23, 24, 29).
The primary goal of the present investigation was to understand and model the response of the β-adrenergic system with HF in the pediatric population. The results of this study have demonstrated that the Y mouse models the β-adrenergic findings of the clinical pediatric HF population. It is important to note that few animal models are able to reproduce all aspects of a clinical disease. Most models are useful for specific attributes of a disease process. Since serum catecholamine levels are predictive of death in the pediatric HF population, the present investigation has likewise modeled an important pathophysiological aspect of pediatric HF that is associated with increased mortality (34). This model should provide an important tool to understand how to mitigate these detrimental effects of β-adrenergic activation. The hypertrophy demonstrated in the model is certainly related to pathological function due to the similarities in the downstream signaling between the human pediatric heart and Y mouse heart, including the β-AR downregulation, PLB phosphorylation abnormalities, natriuretic peptide, MHC, and collagen expression abnormalities. Furthermore, the blunting of collagen expression with selective β1-AR, but not nonselective blockade, in the Y mice further suggests that the type of β-blocker may be important for a clinical response in children.
It is interesting to note that the ISO-induced hypertrophy in the Y mice was less than that in the A mice. The mechanism underlying this difference is unclear, but the difference does suggest that the model reflects age-dependent differences in the myocardial response to β-AR stimulation. It is possible that the effect is blunted due to augmented physiological or developmental growth of the young heart in the Veh-treated Y mice. Alternatively, the age-related differences in β-AR expression or other downstream signaling may be responsible for the observed differences. Further work will be necessary to determine the cause for this difference.
Regulation of myocellular calcium handling proteins is another hallmark of HF adaptation in humans. Chronic HF in adults results in a decrease in PLB phosphorylation at both the protein kinase A (Ser16) and CaMK (Thr17) dependent sites (21, 28). In contrast, no change in PLB phosphorylation at either site is noted in the failing pediatric heart (21). ISO treatment of young and old mice has recapitulated this PLB phenotype, demonstrating lower PLB phosphorylation at both the Ser16 and Thr17 sites in the adult mice, but no change in the younger mice. Due to limitations in the ability to perform studies from freshly isolated tissue in humans and difficulties generating isolated human cardiomyocytes, this model may facilitate an improved understanding of age-dependent differences in myocellular sarcoplasmic reticulum calcium cycling.
Clinical studies examining the use of β-AR blockade in adult humans have been overwhelmingly positive. Based on numerous randomized clinical trials, carvedilol (11, 23, 24), metoprolol (2), bisoprolol (1), and nebivolol (14) have all been approved for use in HF populations in the US or Europe. The only randomized clinical trial of β-AR blockade in children with HF demonstrated no clinical benefit to the use of carvedilol (29). In light of the more recent data demonstrating age-dependent differences in β-AR expression, it is tempting to speculate that the results of this trial were influenced by this molecular change. Since the pathological CaMK pathway remains activated in the failing pediatric heart, we hypothesized that selective β1-AR blockade may be more beneficial than nonselective β-AR blockade, as was performed in the pediatric clinical trial. To model the clinical trial data, we expected that both nonselective β-AR blockade and selective β1-AR blockade would be beneficial in the A mice, while nonselective β-AR blockade would have a blunted effect and selective β1-AR blockade would be beneficial in the Y mice.
Indeed, the present investigation demonstrated that carvedilol effectively blocked the pathological effect of ISO on cardiac growth in the A mice, but found no effect in the Y mice. In addition, collagen expression, a marker of fibrosis and pathological hypertrophy, was blunted by both carvedilol and metoprolol in the A ISO-treated mice, but only by metoprolol in the Y animals. To support the hypothesis of a beneficial effect of β1-AR blockade in the absence of β2-AR blockade, the β-blocker intervention was repeated with the increasingly β1-AR-selective blockers utilized in adult clinical practice; first metoprolol [β1-to-β2 ratio (β1/β2): 74:1] followed by bisoprolol (β1/β2: 103:1) and then with nebivolol (β1/β2: 321:1) (22). In addition, CGP-20712A, a highly β1-AR-selective (β1/β2: 500:1) compound, was used to block the β1-AR without the pleomorphic effects attributed to nebivolol, which might confound the outcomes (3). Similar to the MERIT HF trial [metoprolol (2)], the CIBIS trial [bisoprolol (1)], and the SENIORS trial [nebivolol (14)], selective β1-AR blockade was efficacious in the A mice. In addition, CGP-20712A treatment blunted the cardiac growth in the A mice, suggesting that the beneficial effect is independent of the degree of β1-AR selectivity. We also found a similar degree of benefit in the Y mice following all β1-AR-selective therapies. There have been no multicenter, randomized clinical trial of these therapies in children with HF, but this data may provide proof of principle for further investigation. The need for additional clinical studies specifically in children is particularly important, as the clinical benefits to improvements in medical therapies noted in adult HF patients have not been realized in children. In fact, the data suggest that the lack of improvement in clinical outcomes in children with HF treated with adult regimens may be due to age-related effects that influence the treatment response in this orphan population (17, 33).
The mechanisms underlying this age difference in response to selective vs. nonselective β-AR blockade remain elusive. The β2-AR couples with both the stimulatory and inhibitory G protein. Therefore, evaluation of the end results of activation or blockade of this receptor is more difficult than that of the β1-AR, which only couples with the stimulatory G protein. Activation of the β2-AR is antiapoptotic and prosurvival through the inhibitory G protein (8, 9, 31, 36, 38, 39). Although purely speculative, it is possible that blockade of this beneficial pathway, in addition to the receptor downregulation in the Y mice, contributes to the lack of benefit with nonselective β-AR blockade. Alternatively, age-related differences in the interaction of the β2-AR with the β1-AR or subsequent downstream signals could contribute. Hopefully, with the assistance of this model, future studies can improve our understanding of these mechanisms.
There are several limitations to the present investigation. First, the ISO treatment paradigm was not of sufficient dose or duration to produce systolic dysfunction. The current dosing regimen has been well established to generate cardiac pathology through the β-AR system, which was the primary goal for the present study. While higher doses and longer duration have been demonstrated to reliably produce HF in mice (26), the dosing duration was limited by the age of the Y mice and the need to complete studies before the animals completed puberty. Additional studies will be necessary to evaluate alternative dosing regimens and longer term outcomes. Second, we only assessed a small number of molecules in the cardiac β-AR system. While a more comprehensive assessment will be necessary to completely understand which aspects will be useful to model the clinical disease, we started with those molecules known to validate the disease process in the young and recapitulate age-dependent differences in the human heart. Finally, the molecular signatures associated with β-blockade have not been addressed in the present study. The molecular mechanisms underlying the beneficial effects of β-AR blockade in the clinical HF population are incompletely understood, and, therefore, the ability to compare the mouse data following the β-blocker interventions to established molecular changes in humans is limited. The primary objective for the current experiments was to determine whether there are pathophysiologically appropriate features to use the mouse as a model for pediatric heart disease. Further work will be necessary to assess the molecular mechanisms responsible for the age-dependent difference in the β-AR blocker response.
In conclusion, these data demonstrate, for the first time, that a mouse model of β-AR stimulation can recapitulate a number of age-dependent differences in the molecular remodeling that occurs in response to HF in humans. In addition, β-AR blocker therapy elicits similarities in the mouse model to those observed in clinical populations. Future work will be necessary to more completely develop this model system; however, the mouse model may provide a tool to improve our understanding of unique aspects of HF in children and translate these finding into clinical therapies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants K01 HL-088708 (C. C. Sucharov), K08 HL-080212 (B. L. Stauffer), R21 HL-097123 (C. C. Sucharov, S. D. Miyamoto, B. L. Stauffer), R01 HL-107715 (B. L. Stauffer), and State of Colorado, Bioscience Discovery and Evaluation Grant (C. C. Sucharov, S. D. Miyamoto, B. L. Stauffer).
DISCLOSURES
C. C. Sucharov has equity in miRagen. B. L. Stauffer received clinical research support from Forest Laboratories. No funding from Forest Laboratories supported the current paper.
AUTHOR CONTRIBUTIONS
Author contributions: C.C.S., J.G.H., W.F.A.M., S.D.M., and B.L.S. conception and design of research; C.C.S., J.G.H., R.D.S., W.F.A.M., and B.L.S. performed experiments; C.C.S., J.G.H., R.D.S., W.F.A.M., S.D.M., and B.L.S. analyzed data; C.C.S., J.G.H., R.D.S., W.F.A.M., S.D.M., and B.L.S. interpreted results of experiments; C.C.S., J.G.H., R.D.S., S.D.M., and B.L.S. drafted manuscript; C.C.S., J.G.H., R.D.S., W.F.A.M., S.D.M., and B.L.S. edited and revised manuscript; C.C.S., J.G.H., R.D.S., W.F.A.M., S.D.M., and B.L.S. approved final version of manuscript; J.G.H., R.D.S., W.F.A.M., and B.L.S. prepared figures.
REFERENCES
- 1.Anonymous. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353: 9–13, 1999 [PubMed] [Google Scholar]
- 2.Anonymous. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007, 1999 [PubMed] [Google Scholar]
- 3.Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 144: 317–322, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balakumar P, Singh AP, Singh M. Rodent models of heart failure. J Pharmacol Toxicol Methods 56: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson EB. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Hershberger RE, Port JD, Minobe W, Rasmussen R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol 35: 295–303, 1989 [PubMed] [Google Scholar]
- 8.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res 87: 1172–1179, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation 100: 2210–2212, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, Rajwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail 4: 396–403, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 357: 1385–1390, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Dockstader K, Nunley K, Karimpour-Fard A, Medway A, Nelson P, Port JD, Liggett SB, Bristow MR, Sucharov CC. Temporal analysis of mRNA and miRNA expression in transgenic mice overexpressing Arg- and Gly389 polymorphic variants of the beta1-adrenergic receptor. Physiol Genomics 43: 1294–1306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Demerdash E, Awad AS, Taha RM, El-Hady AM, Sayed-Ahmed MM. Probucol attenuates oxidative stress and energy decline in isoproterenol-induced heart failure in rat. Pharm Res 51: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIORS Investigators Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26: 215–225, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation 87: VI40–VI48, 1993 [PubMed] [Google Scholar]
- 16.Galindo CL, Skinner MA, Errami M, Olson LD, Watson DA, Li J, McCormick JF, McIver LJ, Kumar NM, Pham TQ, Garner HR. Transcriptional profile of isoproterenol-induced cardiomyopathy and comparison to exercise-induced cardiac hypertrophy and human cardiac failure. BMC Physiol 9: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol 55: 1377–1384, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Konstam MA. Improving clinical outcomes with drug treatment in heart failure: what have trials taught? Am J Cardiol 91: 9D–14D, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF. Effects of chronic beta-adrenergic receptor stimulation in mice. J Mol Cell Cardiol 29: 2735–2746, 1997 [DOI] [PubMed] [Google Scholar]
- 20.MacLennan MB, Anderson BM, Ma DW. Differential mammary gland development in FVB and C57Bl/6 mice: implications for breast cancer research. Nutrients 3: 929–936, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol 54: 1491–1499, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 334: 1349–1355, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651–1658, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Park SS, Grayson MjH. Clinical research: protection of the “vulnerable”? J Allergy Clin Immunol 121: 1103–1107, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, Kobilka B. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med 32: 1041–1048, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Pritchett KR, Taft RA. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. New York: Academic, 2006, p. 816 [Google Scholar]
- 28.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol 31: 479–491, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 298: 1171–1179, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Shaddy RE, Tani LY, Gidding SS, Pahl E, Orsmond GS, Gilbert EM, Lemes V. Beta-blocker treatment of dilated cardiomyopathy with congestive heart failure in children: a multi-institutional experience. J Heart Lung Transplant 18: 269–274, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Shizukuda Y, Buttrick PM. Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol 34: 823–831, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell 19: 4141–4153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296: 1867–1876, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Venugopalan P, Agarwal AK. Plasma catecholamine levels parallel severity of heart failure and have prognostic value in children with dilated cardiomyopathy. Eur J Heart Fail 5: 655–658, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Williams RV, Tani LY, Shaddy RE. Intermediate effects of treatment with metoprolol or carvedilol in children with left ventricular systolic dysfunction. J Heart Lung Transplant 21: 906–909, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, Cheng H. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci 25: 358–365, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Yuan R, Meng Q, Nautiyal J, Flurkey K, Tsaih SW, Krier R, Parker MG, Harrison DE, Paigen B. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci U S A 109: 8224–8229, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation 102: 344–350, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A 98: 1607–1612, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]