Abstract
A current and major unanswered question is why the highly sensitive central CO2/H+ chemoreceptors do not prevent hypoventilation-induced hypercapnia following carotid body denervation (CBD). Because perturbations involving the carotid bodies affect central neuromodulator and/or neurotransmitter levels within the respiratory network, we tested the hypothesis that after CBD there is an increase in inhibitory and/or a decrease in excitatory neurochemicals within the ventrolateral medullary column (VMC) in awake goats. Microtubules for chronic use were implanted bilaterally in the VMC within or near the pre-Bötzinger Complex (preBötC) through which mock cerebrospinal fluid (mCSF) was dialyzed. Effluent mCSF was collected and analyzed for neurochemical content. The goats hypoventilated (peak +22.3 ± 3.4 mmHg PaCO2) and exhibited a reduced CO2 chemoreflex (nadir, 34.8 ± 7.4% of control ΔV̇E/ΔPaCO2) after CBD with significant but limited recovery over 30 days post-CBD. After CBD, GABA and glycine were above pre-CBD levels (266 ± 29% and 189 ± 25% of pre-CBD; P < 0.05), and glutamine and dopamine were significantly below pre-CBD levels (P < 0.05). Serotonin, substance P, and epinephrine were variable but not significantly (P > 0.05) different from control after CBD. Analyses of brainstem tissues collected 30 days after CBD exhibited 1) a midline raphe-specific reduction (P < 0.05) in the percentage of tryptophan hydroxylase–expressing neurons, and 2) a reduction (P < 0.05) in serotonin transporter density in five medullary respiratory nuclei. We conclude that after CBD, an increase in inhibitory neurotransmitters and a decrease in excitatory neuromodulation within the VMC/preBötC likely contribute to the hypoventilation and attenuated ventilatory CO2 chemoreflex.
Keywords: carotid body, breathing, neuromodulation
in the 1960s, the elegant studies of Fencl et al. in awake goats demonstrated that alveolar ventilation was linearly correlated with the [H+] of the cerebral spinal fluid (CSF) (12). Fencl et al. concluded that “resting ventilation is a single function of [H+] in the cerebral interstitial fluid” and that the carotid bodies play “no significant role in the respiratory response to acid-base disruptions” (12). However, Bisgard et al. subsequently found in awake ponies that carotid body denervation (CBD) resulted in hypoventilation and a secondary decrease in the pH of the CSF by ∼0.03, which on the basis of studies of Fencl et al, should have robustly stimulated the central chemoreceptors (3). Accordingly, a major unanswered question regarding the effects of CBD is why the intact and highly responsive central chemoreceptors do not prevent the marked hypercapnia post-CBD. Major insight into this question was provided by the isolated carotid body perfusion studies by Blain et al. in awake dogs, which suggested that the sensitivity of central chemoreceptors is directly related to carotid chemoreceptor activity (4). This carotid afferent effect does not appear to be specific to central chemosensitivity because there is a uniform reduction in ventilatory responses to other stimuli after CBD (14, 15).
One pathway for carotid chemoreceptor effects on the control of breathing is through second-order projections from the nucleus of the solitary tract (NTS) to the parafacial respiratory group/retrotrapezoid nucleus (pFRG/RTN) (17). The pFRG/RTN integrates multiple sources of excitatory drive to breathe and provides a major source of excitation (17) to the pre-Bötzinger Complex (preBötC) and many other brainstem respiratory neurons. An alternative pathway for carotid afferent effects on breathing is suggested by studies demonstrating that perturbations involving the carotid bodies (such as CBD, intracarotid perfusion of CO2-enriched saline/KCN, and hypoxia) can alter central neuromodulator and/or neurotransmitter levels at multiple sites that contribute to ventilatory control, including the medullary raphe nuclei (16, 21, 22, 27, 33, 42, 46). These findings suggest that carotid afferents may affect breathing by determining the excitability of ventral respiratory column neurons, including those in the preBötC, via alterations in central neuromodulators and/or neurotransmitters.
The major objective of the current study was to test the hypothesis that after CBD, there is an increase in inhibitory and/or a decrease in excitatory neurochemicals within the preBötC/ventrolateral medullary column (VMC). A secondary objective was to test the hypothesis that the time-dependent recovery of ventilation following CBD is associated with a return of central neuromodulation and/or neurotransmission to near control levels within the preBötC/VMC.
METHODS
Data were obtained from 21 female adult goats weighing 42.5 ± 8.3 kg. The goats were housed in an environmental chamber with a fixed ambient temperature and photoperiod (6:00 AM–6:00 PM). All goats were allowed access to feed and water ad libitum except for study periods and 24-h fasting periods prior to surgeries. All aspects of the study were reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Experimental Design
Five of 21 goats were euthanized immediately upon arrival at the laboratory and served as controls for histological analysis. The remaining 16 goats underwent an instrumentation surgery for subcutaneous elevation of the carotid arteries. After 2 wk of recovery and familiarization with study equipment and baseline study procedures, 10 of the 16 experimental goats underwent a second surgery for bilateral microtubule (MT) implantation targeting the preBötC area of the VMC. These goats were subsequently allowed an additional 2 wk for recovery and training followed by a series of baseline physiologic studies (described below).
Upon completion of baseline studies, the carotid bodies were bilaterally denervated (CBD) in 9 MT-implanted goats and 4 non-MT-implanted goats. In addition, one MT-implanted goat and two non-MT-implanted goats underwent sham CBD. During preliminary studies, some goats required additional ventilatory support following recovery from CBD surgery. Accordingly, in these and all subsequent goats, we created a tracheostomy at the time of the CBD surgery. Studies resumed within 24–48 h postsurgery, and most goats were studied up to 30 days post-CBD. Two goats were euthanized at 5 days post-CBD and one was euthanized 13 days post-CBD. Upon completion of studies, each goat was euthanized, the head perfused and fixed, and the medulla harvested.
Surgical Procedures
For all surgeries, the goats were anesthetized with ketamine, intubated, and mechanically ventilated with 2% isoflurane in 100% O2. All procedures were performed under sterile conditions. For the initial instrumentation surgery, the carotid arteries were isolated from the vagi, elevated superficially to the muscle, and skin was sutured. For this and all other surgeries, flunixin meglumine (1 mg/kg, IM) was administered once before surgery followed by buprenorphine hydrochloride (0.005 mg/kg, IM, twice daily) for 2 days postoperatively for analgesic purposes. To minimize infection, the goats received ceftiofur sodium (2 mg/kg, every day) as an antibiotic for 1 wk.
Implantation of stainless steel MTs into the preBötC region occurred as described previously (25, 35, 49). Under anesthesia, a dorsal midline incision was made at the base of the skull and neck bisecting the nuchal ligament. Rostral to the foramen magnum, a rotary drill was used to remove bone until a segment of dura mater was exposed. A small patch of the dura was removed to expose obex. Obex was then used as a reference point for placement of MTs (70 mm length, 1.27 mm outer diameter, 0.84 mm inner diameter) in or near the target site, the preBötC (4.5 mm lateral to midline, 2 mm rostral to obex, 4 mm ventral to the dorsal surface). However, to avoid blood vessels on the dorsal medullary surface, the implantation site was adjusted slightly in some goats. After placement, the MTs were secured to the surrounding bone and tissue, and the craniotomy was sealed using dental acrylic.
Experienced laboratory personnel monitored the goats continuously for 24 h after implantation surgery. In most cases, the goats were able to stand and/or maintain sternal recumbence within 2–3 h postsurgery. Acute nystagmus was minimal in all goats. Food and water intake were restricted during the first 24 h postsurgery and were closely monitored for several days thereafter. Brain edema was minimized with dexamethasone injections (starting at 0.4 mg/kg and decreasing by 0.05 mg/kg each day, IV, three times a day) for 1 wk. Infection was minimized with chloramphenicol injections (20 mg/kg, IV, three times a day) for 3 days postsurgery and with daily injections of ceftiofur sodium (2 mg/kg) and gentamicin (3 mg/kg) throughout the protocol.
CBD surgery.
CBD or sham-CBD surgery was performed as previously described (20, 40). Under anesthesia, the head was rotated to a supine position for a ventral midline incision. After gentle retraction of the airway, the carotid arteries and carotid bifurcations were exposed. Proximal to the bifurcation and dorsal to the carotid artery is a small bundle of nerves and vessels containing the carotid sinus nerve. Once identified, two sutures were placed around the carotid sinus nerve and a small segment was excised. This procedure was performed bilaterally, and both chemoreceptors and baroreceptors were denervated; thus, attenuation/absence of both may have contributed to the effects of CBD. For sham CBD, the carotid bifurcation was exposed and identified, but no nerves were sectioned. After completion, the midline incision was then sutured closed.
Tracheostomy.
Immediately following CBD surgery, a small midline incision was made caudal to the larynx to create a tracheostomy. A segment of cartilage from the trachea was then removed, creating an opening ∼1 cm in diameter, and the surrounding skin was sutured to the opening in the trachea. The goats then recovered and were later fitted with an endotracheal tube. All goats were monitored continuously for 24 h following surgery.
Physiologic Studies
All experimental protocols and procedures are standard in our laboratory (35, 49). During experimental procedures, the goats stood comfortably while loosely secured in a stanchion. A custom-fitted airtight mask was secured to the goats' snout and a two-way breathing valve was attached. In tracheostomized goats, the breathing valve was fitted directly to the endotracheal tube. Inspiratory flow was measured with a pneumotach and computerized data acquisition software. Expired air was collected in a spirometer for measurement of pulmonary ventilation (V̇E), breathing frequency (f), tidal volume (VT), and expired O2 and CO2, which permitted calculation of O2 consumption and CO2 excretion. A chronically placed catheter in the elevated carotid artery was used for arterial blood sampling to measure pH, PaCO2, and PaO2 (model 248; Bayer Health Care). Respiratory dead space was calculated using the Bohr equation, and alveolar ventilation (V̇A) was calculated subtracting dead space ventilation from V̇E. Rectal temperature of the animals was measured at regular intervals.
Assessment of CBD.
Pre- and post-CBD, the goats were exposed to hypoxia to evaluate the peripheral chemoreflex. Inspiratory flow (V̇I) was measured during 30 min of room air breathing followed by exposure to a hypoxic gas mixture for 5 min. The objective was to decrease PaO2 to ∼30 torr, which in intact goats required an FiO2 of 0.10 (N2 balance) and post-CBD required an FiO2 of 0.12 (N2 balance).
Assessment of the CO2 chemoreflex.
The ventilatory CO2 chemoreflex was assessed every 1 to 3 days throughout the duration of the study protocol. V̇E was measured during 30 min of room air breathing followed by exposure to three increasing concentrations of inspired CO2 (FiCO2 = 0.03, 0.05, 0.07 in room air) for 5 min each. Arterial blood was sampled twice during room-air breathing and once during each of the last 2 min of each level of elevated inspired CO2. The CO2 chemoreflex was expressed as ΔV̇E/ΔPaCO2 when progressing from room-air breathing to all three levels of inspired CO2.
Injection studies.
Glutamate receptor agonist injection into the preBötC region of awake goats has previously been shown to elicit a robust hyperpnea (25, 49). Accordingly, N-methyl-d-aspartic acid (NMDA), which transiently stimulates glutamate receptors, was injected into the MTs of each implanted goat to gain insight into whether the MT placement was in or near the preBötC. Inspiratory flow was measured for 30 min during room air breathing and for 30 min following unilateral control injections of 500 nl of mock CSF (mCSF), containing 124 mM NaCl, 2.0 mM KCl, 2.0 mM MgCl2, 1.3 mM K2PO4, 2.0 mM CaCl2, 11 mM glucose, 26 mM NaHCO3, pH 7.32 in sterile distilled H2O equilibrated with 6.4% CO2, 21% O2, balance N2. Subsequently, 500 nl of 100 mM NMDA in mCSF was unilaterally injected and V̇I was measured for 30 min postinjection.
Microdialysis studies.
For assessment of central neuromodulator and/or neurotransmitter levels pre- and post-CBD within the preBötC region of the VMC, a microdialysis probe (CMA 12;70 mm shaft length, 2 mm membrane length, 0.5 mm membrane diameter, 20 kDa molecular mass limit) was inserted into an implanted MT for dialysis of mCSF and collection of the effluent dialysate (25, 35). After a 60-min stabilization period following probe insertion, mCSF was dialyzed (10 μl/min; Harvard Apparatus syringe pump) continuously for 3 h while the goats were breathing room air. The effluent mCSF was collected in modified crytotubes; frozen at −80°C; and later analyzed for glutamine, glycine, GABA, serotonin (5-HT) substance P, epinephrine, norepinephrine, and dopamine concentrations. During dialysis, inspiratory flow was monitored continuously and arterial blood gases were measured during the last 5 min of each hour; however, dialysis of mCSF had no effect on these variables (data not shown). At least two dialysis studies were performed prior to CBD (once on each side), and one or two dialysis studies were performed within every 10-day period post-CBD (once on each side) until goats were euthanized.
Neurochemical analyses.
Glutamine, glycine, and GABA were measured by reverse-phase HPLC with fluorescent detection using the following parameters: Waters Resolve C18 column (150 × 3.9), a fluorescent detector with excitation at 229 nm and emission at 470 nm, β-alanine internal standard, and O-phthaldialdehyde derivitization. For measurement of 5-HT, the same type of column was used, but with a potential setting of 0.6 V vs. a reference electrode of Ag/AgCl and an N-methylserotonin internal standard. Measurement of norepinephrine, epinephrine, and dopamine was accomplished by using a Waters uBondapak C18 column (300 × 3.9), potential setting of 0.65 V vs. Ag/AgCl reference electrode and a DHBA internal standard. A commercially available immunoassay (Assay Designs 900-018; range, 9.76–10,000 pg/ml) was used to measure substance P via a microplate reader at 405 nm.
Histological Studies
Upon completion of physiologic studies and in histological control goats, the goats were anesthetized with a ketamine/xylazine mixture (24:1 vol/vol) and euthanized with B-euthanasia. The head was then flushed (10% sucrose in PBS) and fixed (4% paraformaldehyde in PBS), and the medullary tissue was extracted. The tissue was cryoprotected in 20% and 30% sucrose for at least 72 h, and was then frozen and serial sectioned (25 μm) in the transverse plane from obex to the pontomedullary junction (∼10 mm). Each section was adhered to electrostatically treated slides and was either Nissl-stained for identification of gross anatomical structures or immunostained for tryptophan hydroxylase (TPH) or the serotonin transporter (SERT). For TPH and SERT staining, CBD and sham-CBD goat tissue was always processed paired with tissue of a control, naïve goat, or sham-CBD goat.
Nissl staining.
After a 10-min drying period, the tissue was cleared in xylene for 1 h followed by sequential rehydration in 100%, 95%, and 70% ethanol before a 2-min distilled, deionized water rinse. The tissue was exposed to 4% cresyl violet for 10 min followed by sequential dehydration in ethanol, including exposure to 0.5% acid ethanol. The tissue was again cleared in a graded xylene series for 1 h followed by coverslipping. Tissue was stained at 100-μm intervals.
TPH immunostaining.
After a 2-h drying period, the tissue underwent heat antigen retrieval (DAKO) for 12 min at 92°C and was rinsed in PBS for 1 h. The tissue was then washed in a 0.4% Triton X-100 in PBS solution for 10 min and blocked with 5% normal horse serum in 0.1% Triton X-100 PBS for 1 h. The tissue was incubated with the primary antibody (1:2,000 in 2.5% normal horse serum, 0.1% Triton X-100 PBS) for up to 72 h, followed by rinsing with 0.1% Triton X-100 PBS for 10 min three times. The tissue was then incubated with a biotinylated anti-mouse secondary antibody (1:500 in 0.1% Triton X-100 PBS) for 30 min, rinsed with PBS for 10 min three times, and incubated with ABC reagents (Vector Labs) for 30 min. After a final rinse with PBS, the tissue was exposed to 3,3′ diaminobenzidine (∼3 min) or ImmPACT VIP (Vector Labs). The tissue was then dehydrated with 95% ethanol, cleared with xylene, and coverslipped. Tissue was stained at 400-μm intervals.
SERT immunostaining.
After a 2-h drying period, the tissue underwent heat antigen retrieval (DAKO) for 12 min and was rinsed in PBS for 1 h. The tissue was then washed in a 0.4% Triton X-100 PBS solution for 10 min and blocked with 5% normal horse serum, 5% normal goat serum in 0.1% Triton X-100 PBS for 1 h. The tissue was incubated with the primary antibody (1:2,000 in 1.25% normal horse serum, 1.25% normal goat serum, 0.1% Triton X-100 PBS) for up to 72 h followed by rinsing with 0.1% Triton X-100 PBS for 10 min three times. The tissue was then incubated with a biotinylated anti-rabbit secondary antibody (1:500 in 0.1% Triton X-100 PBS) for 30 min, rinsed with PBS for 10 min three times, and incubated with ABC reagents (Vector Labs) for 30 min. After a final rinse with PBS, the tissue was exposed to ImmPACT VIP (Vector Labs) (∼6 min), dehydrated with Dryrite for 72 h, cleared with a graded xylene series, and coverslipped. Tissue was stained at 400-μm intervals.
MT location.
Four thousand DPI scanned images of Nissl-stained tissue sections from obex to the pontomedullary junction were captured (Nikon Super Coolscan 9000) for identification of MT location. Metamorph imaging software was used to spatially calibrate each image and measure MT placement in millimeters relative to midline, the ventral medullary surface, and obex. MT placement was identifiable as absent or disrupted tissue, and the implantation site was defined as the ventral-most aspect and center of the rostral-caudal MT tissue disruption (19, 25, 49).
Neuronal count regions.
The medullary raphe count regions were within areas defined by previous studies (19), including a midline raphe (MR) region (1.0 mm lateral to the midline bilaterally from the dorsal to ventral surfaces), or a ventrolateral region (all other TPH+ neurons >1.0 mm lateral to the midline). Neurons within these regions were manually counted by an experienced technician who was unaware of whether goats had been an unoperated control, or underwent sham-CBD or unimplanted CBD. However, because of the MT tract, the microscopist was aware of whether the goats had MTs implanted.
SERT density quantification.
SERT staining was quantified within several respiratory-related nuclei, including the NTS, dorsal motor nucleus of the vagus, hypoglossal motor nucleus, facial nucleus (FN), and a region ventral to FN in which there is a population of Phox2b+ neurons (31) (a marker for pFRG/RTN) (18). SERT staining was also quantified within the cuneate nucleus, which is not considered part of the respiratory network. A 296 × 222 μm image centered within the nucleus of interest was acquired (40× objective lens, 0.75 NA) for processing with ImageJ software. Processing included background subtraction and objective threshold setting (Renyi entropy algorithm) of positively labeled pixels for conversion to a binary image. The percent area of positively labeled pixels in each image was measured as an index of SERT density. In CBD goats, SERT density was normalized to a control or a sham-CBD animal.
Data and Statistical Analysis
Hypoxic response studies.
Inspiratory minute ventilation was calculated and binned into 1-min periods for the entire study protocol. These values were then subjected to a one-way ANOVA with repeated measures to compare V̇I during each minute of room air and hypoxic gas breathing. This analysis was performed before and after CBD surgery on the raw values for V̇I; however, data are expressed as percent of mean V̇I during the last 5 min of room-air breathing.
Ventilatory variables, blood gases, and CO2 sensitivity.
The effect of CBD on ventilatory variables, blood gases, and CO2 sensitivity varies temporally between goats, and thus binning these data day-by-day masks the peak effects of CBD. Accordingly, in each goat, these variables were during which the following occurred: peak hypoventilation, nadir of CO2 sensitivity, transient recovery of CO2 sensitivity, and 20–30 days post. These values and the pre-CBD values were subjected to a one-way ANOVA with repeated measures to establish effects of CBD. The threshold for significance for this analysis and all subsequent statistical analyses was set as P < 0.05.
NMDA injection studies.
Breathing frequency (f) was calculated and binned into 1-min periods for the entire study protocol. Control f was then calculated by averaging f during the last 15 min of the control period, and the peak f after injection was calculated by averaging f during the 5-min span that encompassed the peak value. A paired t-test was used to compare control f with peak f.
Microdialysis studies.
The measured values of neurochemicals were binned as follows: pre-CBD, 0–10 days post-CBD, 11–20 days post-CBD, and 21–30 days post-CBD. The rationale for the time intervals was based on the temporal pattern of CBD effects on ventilatory variables, blood gases, and the CO2 chemoreflex post-CBD. The absolute values for each interval were subjected to a one-way ANOVA with repeated measures to establish whether CBD affected neurochemicals.
Neuron counts.
TPH+ neurons and total neurons (Nissl-stained) were counted and binned every 400 μm. No difference was observed between control (naïve) goats and sham-CBD goats, thus these groups were combined. Measured values were subjected to a two-way ANOVA with repeated measures (distance and condition) to compare CBD goats with control/sham-CBD goats. Average number of neurons per tissue section was also calculated by averaging counts at each rostrocaudal distance for each goat. These values were subjected to a Student's t-test to compare CBD goats with control/sham goats.
SERT analysis.
SERT density was quantified and binned every 400 μm. Average control or sham-CBD SERT density for each nucleus was calculated by averaging SERT density across each rostrocaudal distance, and individual values were then normalized to the average control or sham-CBD SERT density. No difference was observed between control goats and sham-CBD goats, thus these groups were combined. Normalized values were subjected to a two-way ANOVA with repeated measures (distance and condition) to compare CBD goats with control/sham-CBD goats.
RESULTS
Verification of CBD
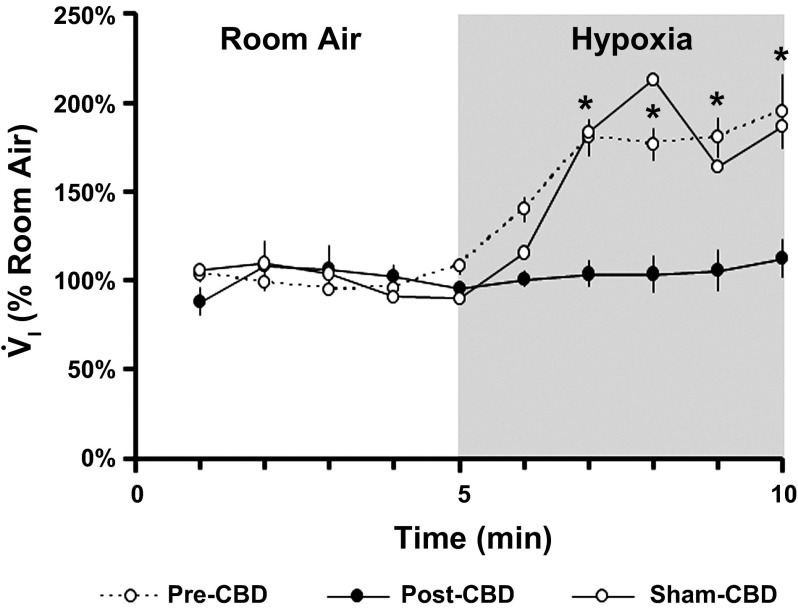
Exposure to 5 min of hypoxic gas before bilateral CBD resulted in a significant increase (P < 0.001) in V̇I to 174.11 ± 8.64% of room air breathing, whereas hypoxia post-CBD had no significant effect (P = 0.601) on V̇I in all except two goats (Fig. 1). These two goats (one with implanted MTs and one without) exhibited an attenuated but not eliminated hypoxic ventilatory response post-CBD (V̇I = 133.66%, data not shown) and were thus considered partially denervated (partial-CBD). The hypoxic response was not measured in one goat euthanized at 30 days post-CBD and in the goats euthanized at 5 and 13 days post-CBD; however, these goats exhibited hypoventilation and a reduced CO2 chemoreflex consistent with bilateral CBD (data not shown). The hypoxic ventilatory response in sham-CBD animals was unchanged relative to carotid body intact animals (V̇I = 172.12%; n = 3). In total, 8 goats euthanized at 30 days post-CBD were considered to have had bilateral CBD, and 2 were considered to have had partial-CBD. The goats euthanized at 5 (n = 2) and 13 days post-CBD (n = 1) were also considered to have had bilateral CBD, and 3 goats were considered to be sham-CBD.
Fig. 1.
Carotid body denervation (CBD) attenuates the ventilatory response to hypoxia. The y-axis depicts mean inspired minute ventilation (V̇I ± SEM) expressed as a percent of room-air breathing; the x-axis depicts time in minutes during room air and hypoxic gas breathing pre-CBD (n = 7), post-CBD (n = 7), and after sham-CBD (n = 3). *Statistically significance differences (P < 0.001, one-way repeated-measures ANOVA).
Effects of CBD on Ventilation, Blood Gases, and the CO2 Chemoreflex
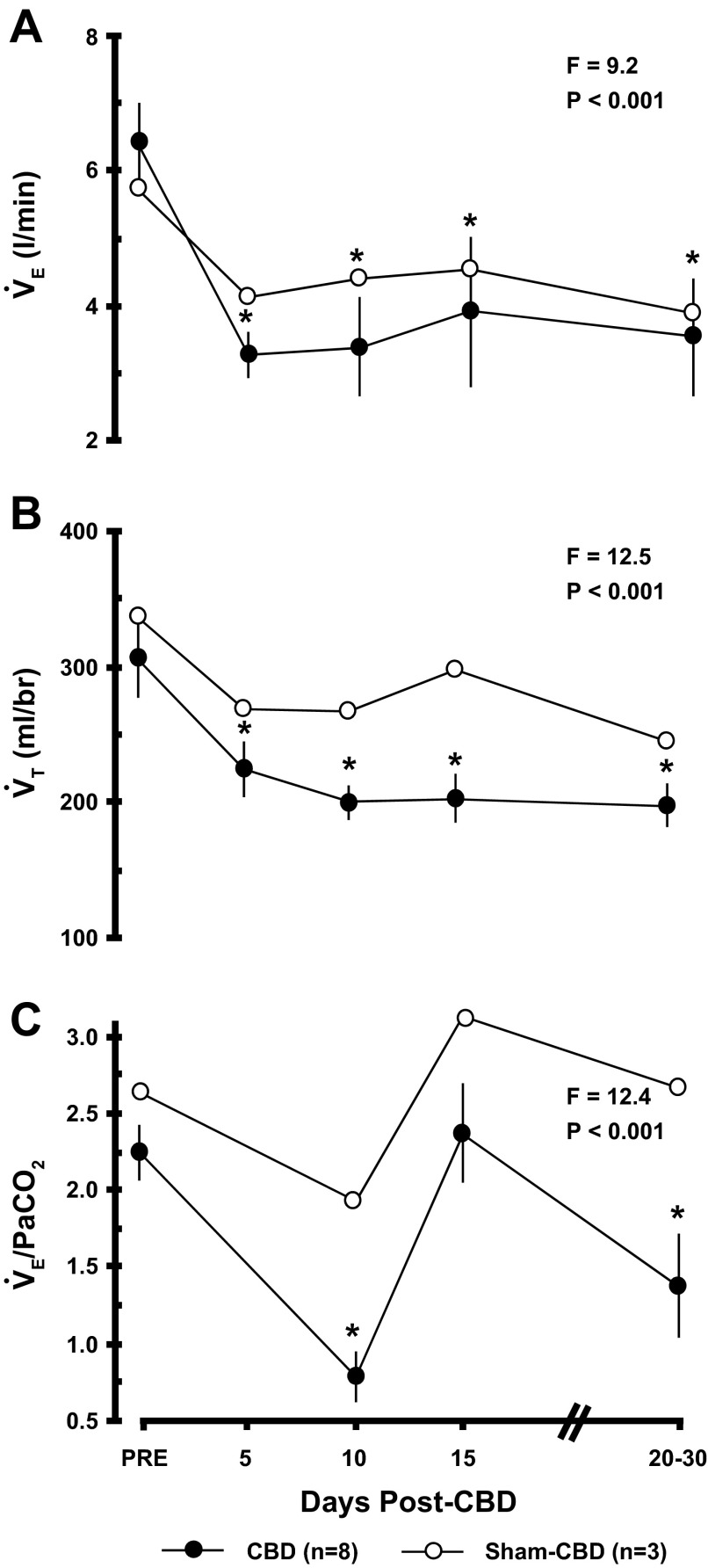
While breathing room air (eupnea) before bilateral CBD, PaCO2 (42.3 ± 1.0 mmHg), PaO2 (96.6 ± 2.7 mmHg), and arterial pH (7.444 ± .004) (Fig. 2) were consistent with past studies on goats (20, 40, 50). Significant hypoventilation was evident following bilateral CBD as shown particularly by the marked increase in eupneic PaCO2 (Fig. 2), which peaked (22.3 ± 3.4 mmHg; P < 0.001) ∼3 days post-CBD. By 10 days post-CBD, PaCO2 had partially but significantly (P < 0.05) recovered. Also consistent with hypoventilation was a decrease (P < 0.05) in PaO2 and arterial pH (Fig. 2), and a decrease in the ratio of alveolar ventilation to CO2 excretion (V̇A/V̇CO2) (data not shown). Pulmonary ventilation (V̇E) and tidal volume (VT) (Fig. 3) were also significantly (P < 0.05) below control after CBD, but breathing frequency did not change from control after CBD (data not shown).
Fig. 2.
Bilateral CBD results in hypoventilation while breathing room air. The y-axis depicts eupneic PaCO2 (mmHg ± SEM), PaO2 (mmHg ± SEM), and arterial pH in A, B, and C, respectively; the x-axis depicts pre-CBD and days post-CBD in CBD (n = 8, closed symbols) and sham-CBD (n = 3, open symbols). The F and P values are from one-way ANOVA on data obtained from the CBD goats (*P < 0.05 vs. pre-CBD, **P < 0.05 vs. peak hypoventilation). Note that there was significant but partial recovery of PaCO2 10 days after CBD after reaching a peak at 5 days post-CBD. All PaO2 and pH values after CBD differed (P < 0.05) from control values but no post-CBD values differed from each other. Sham-CBD had no effect on any variable.
Fig. 3.
Bilateral CBD in goats (n = 8) decreased pulmonary ventilation (V̇E), tidal volume (VT), and the ventilatory CO2 chemoreflex (V̇E/PaCO2). The F and P values are from one-way ANOVA from data obtained on CBD goats. All values for V̇E and VT post-CBD differed from pre-CBD (P < 0.05). V̇E and VT also decreased after sham-CBD, reflecting the effect of a chronic tracheostomy. The CO2 chemoreflex differed (P < 0.05) from control on 10 and 20–30 days post-CBD but not 15 days post-CBD.
The CO2 chemoreflex (ΔV̇E/ΔPaCO2 = 2.24 ± 0.18) was within the normal range for goats before CBD (Fig. 3) (20, 40). A nadir in CO2 sensitivity (34.8 ± 7.8% of pre-CBD) occurred ∼10 days post-CBD (P < 0.05) followed by a transient recovery (105.8 ± 14.2% of pre-CBD) similar to that in previous reports (20). Thereafter, there was a secondary reduction in the CO2 chemoreflex (61.2 ± 14.7% of pre-CBD; P < 0.05). This temporal pattern of the CO2 chemoreflex has been observed in CBD goats previously (20, 40).
For sham-CBD goats, V̇E and VT (Fig. 3) were reduced from control as expected from the tracheostomy, but all other ventilatory and blood gas values and the CO2 chemoreflex were unchanged from control (Figs. 2 and 3). In partial-CBD goats, there was a transient, mild hypoventilation (4 mmHg increase in PaCO2) and a 50% decrease in the CO2 chemoreflex (data not shown).
Location of Implanted MTs
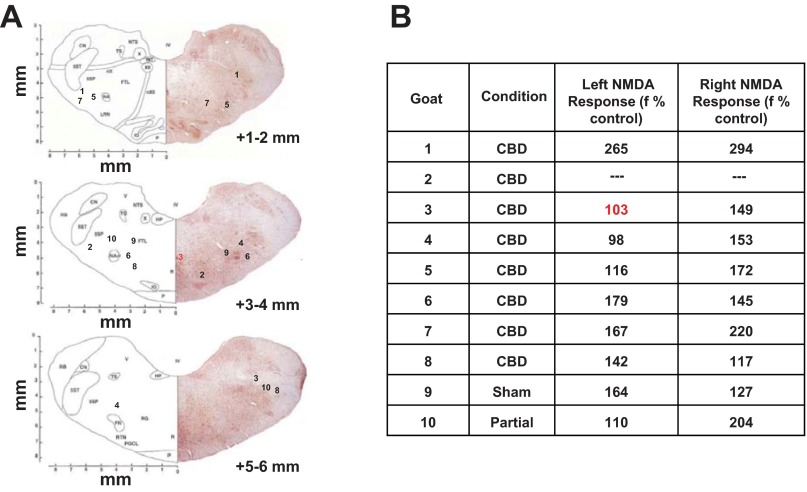
In all but one MT-implanted CBD goat, the histologic analysis of medullary tissue indicated both MTs were within the VMC (Fig. 4). The single exception was in goat 3, in which one MT was in the midline medullary raphe; thus, neurochemical data from dialysis at this site are not included in computed average values. The average location for the left MT was 3.29 ± 0.56 mm rostral to obex, 4.60 ± 0.44 mm lateral from midline, and 2.33 ± 0.35 mm from the ventral surface of the medulla. The right MTs were located on average 3.58 ± 0.49 mm rostral to obex, 4.41 ± 0.41 lateral from midline, and 2.75 ± 0.36 mm from the ventral surface of the medulla. These coordinates are in close proximity to the presumed location of the of the preBötC region in goats (25, 49).
Fig. 4.
The microtubule locations and NMDA injections suggested microtubule placement within the pre-Bötzinger Complex (preBötC)/ventrolateral medullary column (VMC). A: sections from the goat brainstem atlas (8) in which inserted numbers are assigned to each goat identifying the site of implanted microtubules. B: breathing frequencies (% preinjection values) after NMDA injection into the microtubules. Note the red 3, which indicates microtubule placement at the midline; thus neurochemicals from this site were not included in any averages.
Physiologic evidence of MT placement within or near the preBötC was also provided by an increase in f by 161.24 ± 29.15% (left MT; P = 0.03) and 188.77 ± 23.83% (right MT; P = 0.006) after NMDA injection (Fig. 4). In contrast, f after mCSF injection was 105.08 ± 4.13% and 106.24 ± 4.60% (P > 0.05). Overall, the f response to glutamate receptor stimulation is consistent with MT placement within or near the preBötC region (25, 30, 44, 49). Note, however, that there was a minimal response to NMDA in some goats, including goat 3, in which one of the injections was in the caudal midline raphe. In the CBD goat euthanized 13 days post-CBD (goat 2), NMDA injection studies were not performed, but the MT locations were found to be within the normal range of the preBötC/VMC in goats.
Effect of CBD on Neurochemicals within the preBötC/VMC
In goats with both MTs considered to be within the VMC, neurochemical levels from the left and right MTs did not differ significantly (P > 0.10), and the mean differences between pairs were all less than 5% of the grand means. Accordingly, data from the left and right MTs in these goats were averaged. The pre-CBD concentrations of the neurochemicals are in agreement with previous data on awake goats (35), including absence of norepinephrine in all samples.
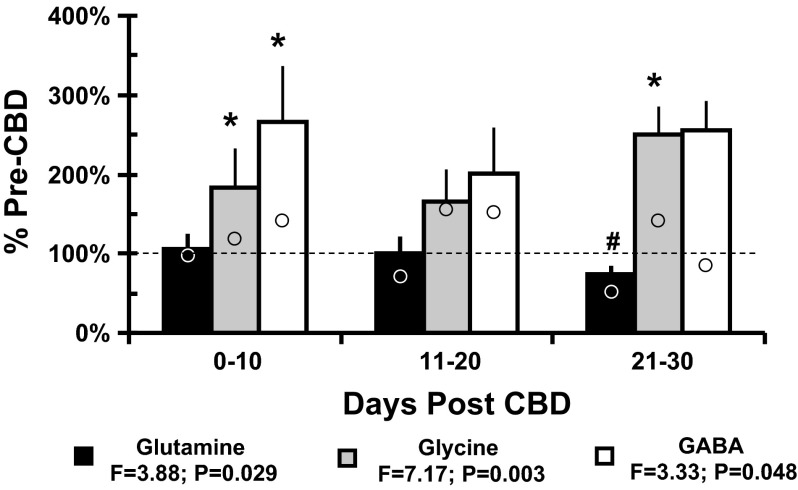
After CBD, the inhibitory neurotransmitters GABA and glycine were elevated to 265.6 ± 71.2% (P = 0.048) and 183.4 ± 50.7% (P = 0.003), respectively, from pre-CBD values, and glutamine was decreased (P = 0.029) compared with pre-CBD (Fig. 5). The greatest change from pre-CBD tended to be during the intervals 0 to 10 and 21 to 30 days post-CBD. In the single partial-CBD goat with implanted MTs, the level of glutamine was not affected by partial-CBD, but GABA and glycine were increased by nearly 100% 10–20 and 21–30 days after partial-CBD (data not shown). In the single sham-CBD goat, average glutamine, glycine, and GABA levels were 73, 138, and 125% of control, respectively, over the 30 days after surgery (Fig. 5).
Fig. 5.
CBD results in significant (P < 0.05) increases in GABA and glycine and decreased glutamine in mCSF dialyzed into the preBötC/VMC. The y-axis shows the level of each variable at three time intervals after CBD expressed as the percent of the pre-CBD value (mean ± SE, n = 5–8 goats). *Differences (one-way ANOVA, P < 0.05) from control values. #Differences between 21–30 and 0–10 days post-CBD. Open circles are values from a single sham-CBD goat whose values for glycine and GABA were near control 0–10 and 21–30 days post sham surgery, suggesting that the increases in the CBD group was directly or indirectly due to loss of carotid afferents.
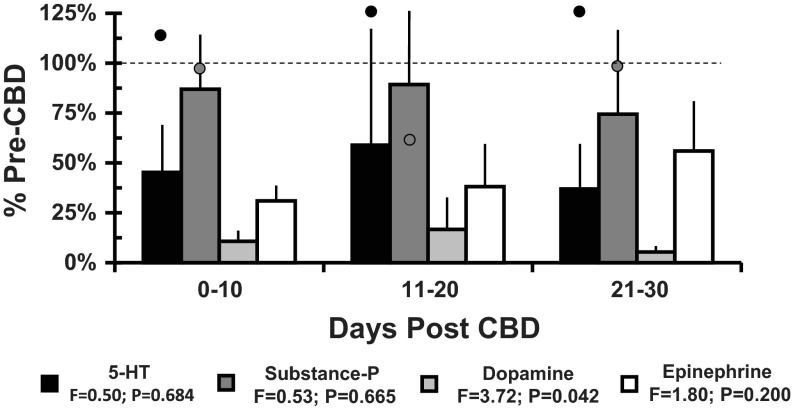
There was a significant (P < 0.05) decrease in dopamine after CBD (Fig. 6). There was variation among goats in the pre-CBD values and the effects of CBD on 5-HT, epinephrine, and substance P; thus there was no significant (P > 0.05) effect of CBD on these neurochemicals. However, there was decreased 5-HT and epinephrine in four of six goats 11–20 post-CBD and in four of five goats 21–30 days post-CBD. The trend in the partial-CBD goat was for less of a reduction in these neuromodulators than in the CBD goats (data not shown). In the single sham-CBD goat, 5-HT did not decrease, and there was no consistent trend in substance P after surgery (Fig. 6).
Fig. 6.
Bilateral CBD results in significantly (P < 0.05) decreased dopamine and but not significantly changed epinephrine, serotonin, and substance P in mCSF dialyzed into the preBötC/VMC. The y-axis shows the level of each variable at three time intervals after CBD expressed as the percent of the pre-CBD value (mean ± SE, n = 5–8 goats). The open symbols for 5-HT and substance P are values from a single sham-CBD goat. The near normal values for 5-HT throughout the period post sham CBD surgery suggest that the tendency toward reduced values in the CBD goats was due to loss of carotid afferents. Prior to sham surgery, dopamine and epinephrine were not detectable in this goat.
Medullary TPH+ Neuron Counts
To further evaluate the effect of CBD on the serotonin neuromodulatory system, we investigated potential changes in tryptophan hydroxylase (TPH) expression in the midline raphe (MR) and ventrolateral medulla (VLM). An example of immunolabeled TPH+ neurons within the MR in a naïve goat (control) and a CBD goat is shown in Fig. 7A. In CBD goats euthanized at 30 days post-CBD, there was a significant reduction in the number of immunolabeled TPH+ neurons within the MR over the entire rostrocaudal distance of the medulla relative to control/sham-CBD goats (P < 0.05; Fig. 7B). The average number of MR TPH+ neurons/tissue section was 26.55 ± 2.91 in CBD goats euthanized at 30 days post-CBD (n = 7) and 37.31 ± 2.09 in control/sham-CBD goats (P = 0.009; n = 8; Fig. 7C).
Fig. 7.

Goats euthanized 30 days post bilateral CBD exhibit a midline raphe (MR)-specific reduction in the percent of tryptophan hydroxylase (TPH+) neurons. A: examples of the MR of a control, naïve goat (top), and a CBD goat (bottom). Note the qualitative reduction in the number of TPH+ neurons in the MR of the CBD goat. The y-axis in B depicts neuron counts ± SEM for MR TPH+ neurons (top), MR total neurons (middle), and ventrolateral medullary (VLM) TPH+ neurons (bottom); the x-axis depicts distance rostral from obex (mm). The bar graphs in C show the average counts per tissue section for MR TPH+ neurons (top), MR total neurons (middle), and VLM TPH+ neurons (bottom) for control/sham-CBD and CBD goats. *Statistically significant differences between groups (P < 0.05, Student's t-test). The closed and open symbols for the control group represent individual values for five unoperated goats and two sham CBD goats, respectively. The red symbol is a sham-CBD goat with microtubules implanted in the preBötC/VMC. Note the close agreement between unoperated and sham-CBD goats. The closed and open symbols for the CBD groups represent individual values for goats that did or did not, respectively, have microtubules implanted into the preBötC/VMC. Note that values for goats with implanted microtubules in the sham-CBD and CBD goats did not differ systematically from those that did not have implanted microtubules.
Nissl-stained neuron counts indicated that there was no change in the total number of neurons within the MR in both CBD and sham-CBD goats relative to control goats (P > 0.05) throughout the entire rostrocaudal distance of the medulla (Fig. 7B, middle). The average number of MR Nissl-stained neurons/section was 176.76 ± 10.37 in CBD goats euthanized at 30 days post-CBD (n = 7) and 179.82 ± 5.84 in control/sham-CBD goats (P = 0.795; n = 8; Fig. 7C, middle). Accordingly, 15.29 ± 1.60% of total neurons in CBD goats were TPH+ compared with 21.17 ± 1.67% in control/sham-CBD goats (P = 0.01; n = 8; data not shown). CBD goats euthanized 5 to 13 days post-CBD and partial-CBD goats did not exhibit a reduced percentage of TPH+ neurons within the MR relative to control/sham-CBD goats (data not shown).
The decreased number of TPH+ neurons in CBD goats euthanized at 30 days post-CBD was specific to the midline regions of the medullary raphe, because there was no significant difference in the number of ventrolateral TPH+ neurons between CBD and control/sham-CBD goats at each rostrocaudal distance within the medulla (P > 0.05; Fig. 7, B and C, bottom).
SERT Density Quantification
The SERT protein located presynaptically has been used as a marker for 5-HT-releasing terminals. An example of immunolabeled SERT terminals and the quantification of SERT density within the NTS of a control, naïve goat and a CBD goat are shown in Fig. 8, A and B, respectively. Relative to control/sham goats, in the CBD goats, there was a 50% reduction (P < 0.001) in SERT density (Fig. 8C) in the NTS, dorsal motor nucleus of the vagus, hypoglossal nucleus, facial nucleus, and ventral to the facial nucleus. The reduction in SERT was not restricted to respiratory nuclei because SERT was also reduced (P < 0.05) in the cuneate nucleus and the reduction did not differ (P > 0.05) from the respiratory sites. Partial-CBD goats (n = 2) exhibited a similar uniform reduction in SERT density as CBD goats (data not shown). In the goats euthanized at 5 (n = 2) and 13 (n = 1) days post-CBD, a consistent reduction in SERT density was only observed ventral to the facial nucleus.
Fig. 8.

Goats euthanized 30 days post-CBD exhibited a significantly (P < 0.05) reduced serotonin transporter (SERT) density at all six medullary sites studied, but there were no significant (P > 0.05) differences between sites. A: examples of immunolabeled SERT terminals from the nucleus of solitary tract (NTS) of a control, naïve goat (top) and a CBD goat (bottom). Left: raw SERT images; right: images processed and quantified with ImageJ software. Note the qualitative reduction in SERT density in the NTS of the CBD goat. B: an example of the quantification of NTS SERT density ± SEM in CBD and control/sham goats. The y-axis depicts SERT density; the x-axis depicts distance rostral from obex (mm). C: bar graphs represent average SERT density ± SEM in six different nuclei in CBD goats euthanized 30 days post-CBD. The closed and open squares represent values for individual goats that did or did not, respectively, have microtubules implanted into the preBötC/VMC. Note there was no systematic difference between implanted and unimplanted goats. DMNV, dorsal motor nucleus of the vagus; XII, hypoglossal motor nucleus; FN, facial nucleus; ventral to facial, presumed parafacial/retrotrapezoid nucleus; CN, cuneate nucleus.
DISCUSSION
The major finding of this study was that the hypoventilation and reduced ventilatory CO2 chemoreflex for several days following CBD were associated with changes in neurochemicals, suggesting increased inhibition and decreased excitatory neuromodulation within the respiratory network. Specifically there was 1) a significant (P < 0.05) increase in GABA and glycine, and a decrease in glutamine and dopamine in mCSF dialyzed within the preBötC/VMC; and 2) a significant (P < 0.05) reduction in detectable TPH expression in midline raphe neurons, and 3) a significant (P < 0.05) reduction in SERT expression in multiple medullary nuclei. We also noted that the significant but partial return of resting ventilation and arterial blood gases following CBD were not associated with a normalization of neurochemicals within the respiratory control network.
Effects of CBD on VMC Neurochemicals
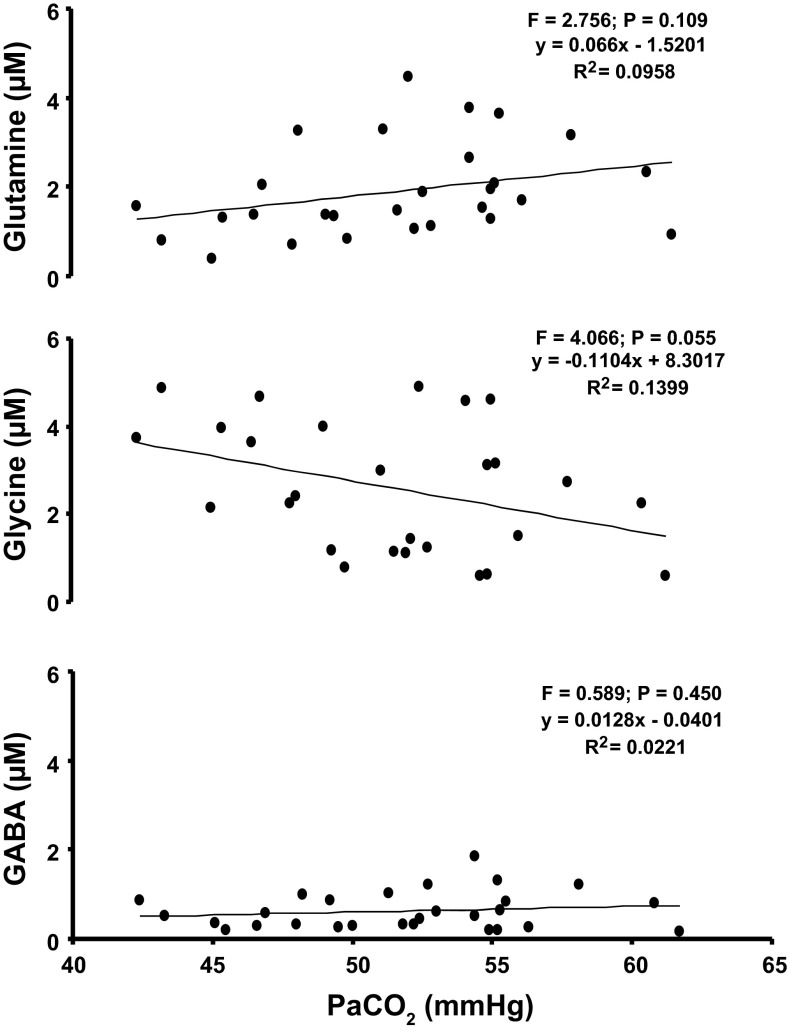
Herein we tested the hypothesis that after CBD there would be a decrease in excitatory neuromodulators and/or an increase in inhibitory neurotransmitters within the preBötC/VMC after CBD. Our current data support this hypothesis. However, CBD causes hypercapnia and hypoxemia, which may have caused rather than resulted from these changes in neurochemicals. We show in Fig. 9 that there was no clear correlation between the hypercapnia induced by CBD and the changes in GABA, glycine, and glutamine (or any other neurochemical). These data suggest that the observed changes in neurochemicals were not driven by the hypercapnia. These data are in agreement with data of Hoop et al. who found that GABA and glycine levels in effluent mCSF dialyzed into the VMC were 400% higher in anesthetized, ventilated rats following CBD compared with carotid-intact rats in which PaCO2 was maintained (22). In addition, other studies found that acute, moderate hypercapnia does not change neurochemicals in mCSF dialyzed into brainstem respiratory nuclei (16, 33), although more severe (PaCO2 = 80 mmHg) hypercapnia increased glutamate and GABA ∼20% after 20 min and was maintained for up to 21 days (51). Accordingly, the bulk of the evidence suggests that the CBD-induced hypercapnia was not the primary cause of the changes in neurochemicals after CBD.
Fig. 9.
As indicated by the F and P values, there was no significant relationship between hypercapnia after CBD and GABA, glycine, or glutamine in mock cerebrospinal fluid dialyzed into or near the pre-BötC of the ventrolateral medullary column. Data points are from five bilateral CBD goats studied repeatedly over 30 days after CBD.
The changes in neurochemicals within the preBötC/VMC following CBD may reflect changes in the activity of local (21) or distant neurons resulting from a loss of tonic excitatory carotid input. The first synapse of carotid afferents is in the NTS, which projects to multiple sites, including the pFRG/RTN and the serotonergic raphe nuclei (39), both of which project to the pre-BötC/VMC. Data from several studies suggest that neurons in the pFRG/RTN are inherently CO2/H+ sensitive, integrate input from multiple sources, and provide excitatory (glutamatergic) drive to multiple sites of respiratory neurons, including the preBötC (17). Thus the reduction in pFRG/RTN activity after CBD would contribute to the reduced eupneic breathing and ventilatory CO2 chemoreflex.
Similarly, 5-HT neurons in the raphe are believed to affect ventilation through inherent CO2/H+ sensitivity and the release of excitatory neuromodulators within the respiratory network. The neuromodulatory effects of 5-HT neurons occur mainly through presynaptic and postsynaptic G protein-coupled receptors (41), including the 5-HT1A and 5-HT2A receptor subtypes. 5-HT1A receptors are coupled to inhibitory Gi proteins leading to inhibition of adenylyl cyclases and neuronal excitability, whereas the 5-HT2A receptors are coupled to Gq proteins, which increase neuronal excitability. The 5-HT1A and 5-HT2A receptors are both expressed on respiratory neurons, including within the preBötC (29, 48), where postsynaptic 5-HT1A receptors are heavily expressed on glycinergic neurons (10, 28). Our observations that TPH-expressing neurons and SERT density are reduced following CBD suggest that excitatory drive to the raphe is also reduced, and potentially, the capacity for 5-HT release is also reduced after CBD. A reduction in 5-HT release within the preBötC/VMC may reduce neuronal excitability of some respiratory neurons, and disinhibit local glycinergic and/or GABAergic neurons via reduced 5-HT1A receptor activity. Moreover, reduced 5-HT release may also directly affect pFRG/RTN activity, which is likely already reduced after CBD (37). A postulated reduction in 5-HT release is supported by the trends in reductions in 5-HT in our measurements of the effluent dialysate from the pre-BötC/VMC. The trend in reduced 5-HT was apparent 0–10 days after CBD, but TPH expression was not reduced during this time frame, which seems to indicate that there was a decrease in 5-HT release before there was an effect on the enzyme that synthesizes 5-HT.
There are multiple other factors that could have affected glycine and GABA levels in the extracellular fluid, including changes in cerebral blood flow and/or glial function, and which may be locally or globally altered in our CBD model. In addition, carotid sinus nerve sectioning also attenuates the baroreflex, and thus the observed effects could also be relevant to the cardiovascular control mechanisms. Finally, the neurochemical changes observed may also be secondary to alterations in other neurochemicals and/or receptor activity not measured in these studies, because we have previously shown with pharmacological antagonism of muscarinic cholinergic receptors dramatically alters local 5-HT, substance P, glycine, and GABA levels (35). Regardless, the present study provides evidence that the reductions in ventilation and high tolerance for hypercapnia after CBD might be caused in part by increased glycine and GABA inhibitory modulation of preBötC/VMC neurons.
Respiratory Neuroplasticity Following CBD
We observed a partial recovery (attenuation) from the CBD-induced hypoventilation and a transient return of ventilatory CO2 sensitivity (Fig. 2). This modest recovery likely represents a “manifestation of plasticity within the regulatory system” (13). Variable recovery of hypoventilation following CBD has been documented in multiple species (2, 3, 34). Humans studied after carotid body resection also demonstrate limited recovery of hypoventilation (1, 6, 11), suggesting that goats are an appropriate model for the study of plasticity within the respiratory network.
Although data show increases in peripheral chemosensitivity following CBD at sites other than the carotid bodies, these sites likely do not contribute to the recovery of hypoventilation (2, 43). Indeed, the recovery of peripheral chemosensitivity after CBD in ponies was eliminated by denervation of aortic chemoreceptors (2). However, aortic denervation after recovery from CBD did not cause further hypoventilation; thus the recovery of eupneic PaCO2 after CBD must have been due to a form of plasticity at another site, likely within the brainstem respiratory network. Several types of central neuroplasticity involve alterations in neuromodulatory systems such as the serotonergic system (7, 15, 24, 32, 47). TPH, the rate-limiting enzyme in serotonin production, exhibits activity-dependent expression by altering expression appropriately to normalize neuronal activity in response to altered afferent input (5, 9, 26, 45). Also, structural plasticity of the serotonergic system, including increased 5-HT terminal density, has been observed following several types of peripheral sensory deafferentation such as thoracic dorsal rhizotomy (32) and cervical dorsal rhizotomy (23, 24).
However, despite a partial recovery from hypoventilation after CBD, we observed a decreased number of neurons that express detectable levels of TPH. Furthermore, the density of SERT staining was globally reduced. Collectively, these data likely indicate a reduced capacity of medullary raphe neurons to produce and release 5-HT following CBD in goats. Throughout the 30 days following CBD, the inhibitory neurotransmitters GABA and glycine remained significantly elevated, which may be related to disinhibition of their release via reduced 5-HT (by reduced activation of 5-HT1A receptors). The sum of our neurochemical and immunohistochemical data do not aid in providing evidence of specific mechanisms driving the limited plasticity post-CBD.
We found that the ventilatory CO2 chemoreflex showed a significant but transient recovery followed by another period of attenuation up to 30 days post-CBD (Fig. 3). These data point to a dynamic process affecting CO2 sensitivity during the week following CBD. Given the downregulated serotonergic system and increased inhibitory neurotransmitters, one might hypothesize an overall reduction in the CO2 chemoreflex. However, the divergence in the pattern and/or timing of the recovery of hypoventilation and the chemoreflex suggest physiologically distinct control mechanisms for room-air breathing and the chemoreflex. This divergence has been demonstrated under multiple conditions (14, 34, 38, 50). Collectively, the data suggest that intact input from the carotid bodies, and not the ventilatory CO2 chemoreflex, is a primary determinant of room-air breathing.
GRANTS
Funding for this study was provided by National Heart, Lung, and Blood Institute Grants HL25739 and HL007852 and by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.M. and H.V.F. conception and design of research; J.R.M., S.E.N., C.M., S.J.O., and L.G.P. performed experiments; J.R.M., S.E.N., S.J.O., and H.V.F. analyzed data; J.R.M., M.R.H., and H.V.F. interpreted results of experiments; J.R.M. and S.E.N. prepared figures; J.R.M. drafted manuscript; S.E.N., C.M., M.R.H., and H.V.F. edited and revised manuscript.
REFERENCES
- 1.Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA, Wiberg DM. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol 46: 843–853, 1979 [DOI] [PubMed] [Google Scholar]
- 2.Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol 49: 964–970, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA, Rasmussen B. Hypoventilation in ponies after carotid body denervation. J Appl Physiol 40: 184–190, 1976 [DOI] [PubMed] [Google Scholar]
- 4.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2). J Physiol 588: 2455–2471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown HJ, Henderson LA, Keay KA. Hypotensive but not normotensive haemorrhage increases tryptophan hydroxylase-2 mRNA in caudal midline medulla. Neurosci Lett 398: 314–318, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med 4: e239, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean C, Geiger LK, Sprtel BM, Ohtake PJ, Forster HV. An anatomic atlas of the medulla oblongata of the adult goat. J Appl Physiol 87: 1220–1229, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Demarque M, Spitzer NC. Neurotransmitter phenotype plasticity: an unexpected mechanism in the toolbox of network activity homeostasis. Dev Neurobiol 72: 22–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JF. The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc Lond B Biol Sci 364: 2611–2623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatemian M, Nieuwenhuijs DJ, Teppema LJ, Meinesz S, van der Mey AG, Dahan A, Robbins PA. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J Physiol 549: 965–973, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fencl V, Miller TB, Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol 210: 459–472, 1966 [DOI] [PubMed] [Google Scholar]
- 13.Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol 94: 784–794, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Forster HV, Martino P, Hodges M, Krause K, Bonis J, Davis S, Pan L. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupneic breathing. Adv Exp Med Biol 605: 322–326, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119: 199–208, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Goiny M, Lagercrantz H, Srinivasan M, Ungerstedt U, Yamamoto Y. Hypoxia-mediated in vivo release of dopamine in nucleus tractus solitarii of rabbits. J Appl Physiol 70: 2395–2400, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Guyenet P. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Resp Physiol Neurobiol 173: 244–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol 97: 2236–2247, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol 98: 1234–1242, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hoop B, Beagle JL, Maher TJ, Kazemi H. Brainstem amino acid neurotransmitters and hypoxic ventilatory response. Respir Physiol 118: 117–129, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hoop B, Masjedi MR, Shih VE, Kazemi H. Brain glutamate metabolism during hypoxia and peripheral chemodenervation. J Appl Physiol 69: 147–154, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Johnson RA, Okragly AJ, Haak-Frendscho M, Mitchell GS. Cervical dorsal rhizotomy increases brain-derived neurotrophic factor and neurotrophin-3 expression in the ventral spinal cord. J Neurosci 20: RC77, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol 106: 605–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. J Neurosci 32: 8521–8531, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindefors N, Yamamoto Y, Pantaleo T, Langercrantz H, Brodin E, Ungerstedt U. In vivo release of substance P in the nucleus tractus solitarii increases during hypoxia. Neurosci Lett 69: 94–97, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Manzke T, Dutschmann M, Schlaf G, Morschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci 364: 2589–2602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226–229, 2003 [DOI] [PubMed] [Google Scholar]
- 30.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Miller J, Neumueller S, Qian B, Hodges MR, Pan L, Forster HV. Hypoventilation and reduced CO2 sensitivity with sustained expiratory muscle activity in the awake goat. FASEB J 25: 847, 2011 [Google Scholar]
- 32.Mitchell GS, Bach KB, Martin PA, Foley KT, Olson EB, Brownfield MS, Miletic V, Behan M, McGuirk S, Sloan HE. Increased spinal monoamine concentrations after chronic thoracic dorsal rhizotomy in goats. J Appl Physiol 89: 1266–1274, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol 478, Pt 1: 55–66, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J Physiol 590: 3335–3347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Bötzinger Complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol 114: 694–704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattie E, Julius H., Comroe Jr., Distinguished Lecture: Central chemoreception: then . . . and now. J Appl Physiol 110: 1–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuding SC, Segers LS, Baekey DM, Dick TE, Solomon IC, Shannon R, Morris KF, Lindsey BG. Pontine-ventral respiratory column interactions through raphe circuits detected using multi-array spike train recordings. J Neurophysiol 101: 2943–2960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol 85: 1299–1306, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med 9: 542–548, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514, Pt 2: 567–578, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serra A, Brozoski D, Hodges M, Roethle S, Franciosi R, Forster HV. Effects of carotid and aortic chemoreceptor denervation in newborn piglets. J Appl Physiol 92: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol 81: 1150–1161, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci 13: 94–106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan M, Goiny M, Pantaleo T, Lagercrantz H, Brodin E, Runold M, Yamamoto Y. Enhanced in vivo release of substance P in the nucleus tractus solitarii during hypoxia in the rabbit: role of peripheral input. Brain Res 546: 211–216, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Turner DL, Bach KB, Martin PA, Olsen EB, Brownfield M, Foley KT, Mitchell GS. Modulation of ventilatory control during exercise. Respir Physiol 110: 277–285, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol 95: 2070–2082, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol 97: 1629–1636, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Wenninger JM, Pan LG, Martino P, Geiger L, Hodges M, Serra A, Feroah TR, Forster HV. Multiple rostral medullary nuclei can influence breathing in awake goats. J Appl Physiol 91: 777–788, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Weyne J, Van Leuven F, Kazemi H, Leusen I. Selected brain amino acids and ammonium during chronic hypercapnia in conscious rats. J Appl Physiol 44: 333–339, 1978 [DOI] [PubMed] [Google Scholar]