Abstract
Inflammation is an important contributor to pediatric and adult neurodegeneration. Understanding the genetic determinants of neuroinflammation provides valuable insight into disease mechanism. We characterize a disorder of recurrent immune-mediated neurodegeneration. We report two sisters who presented with neurodegeneration triggered by infections. The proband, a previously healthy girl, presented at 22.5 months with ataxia and dysarthria following mild gastroenteritis. MRI at onset showed a symmetric signal abnormality of the cerebellar and peritrigonal white matter. Following a progressive course of partial remissions and relapses, she died at 5 years of age. Her older sister had a similar course following varicella infection, she died within 13 months. Both sisters had unremarkable routine laboratory testing, with exception of a transient mild cytopenia in the proband 19 months after presentation. Exome sequencing identified a biallelic perforin1 mutation (PRF1; p.R225W) previously associated with familial hemophagocytic lymphohistiocytosis (FHL). In contrast to FHL, these girls did not have hematopathology or cytokine overproduction. However, 3 years after disease onset, the proband had markedly deficient interleukin-1 beta (IL-1β) production. These observations extend the spectrum of disease associated with perforin mutations to immune-mediated neurodegeneration triggered by infection and possibly due to primary immunodeficiency.
Keywords: exome sequencing, neurodegeneration, cerebellar white matter, familial hemophagocytic lymphohistiocytosis, interleukin-1 beta
Introduction
Childhood neurodegeneration is a diagnostic challenge because it arises as a primary or secondary consequence of many genetic and acquired etiologies, particularly infectious causes.1 Genetic bases include disorders of metabolism, myelination, vascular integrity and inflammation.2, 3 Dysfunction of the innate immune system is the most common primary inflammatory cause of neurodegeneration.4 The aberrant release of cytokines by activated microglia and circulating macrophages in disorders of the innate immune system damage the myelin sheath directly, and also enhance blood-brain barrier permeability, allowing further recruitment of leukocytes to the CNS.
The myriad of inflammatory neurodegenerative disorders rarely have pathognomonic clinical or radiographic features, and are generally subclassified as affecting primarily the white matter, the gray matter, or both. Even when specific neuroradiological signs suggest a particular subset, diagnosis remains challenging and requires additional investigations. Consequently, many neurodegenerative disorders defy a precise diagnosis.
Pro- and anti-inflammatory cytokines can both cause and mitigate neurodegeneration.5, 6 Interleukin-1 beta (IL-1β) is a potent pro-inflammatory cytokine associated with the induction and maintenance of the innate immune response. It has been implicated in neurodegeneration as well as neuroprotection (reviewed by Fogal and Hewett7). IL-1β is overexpressed within the central nervous system of patients with multiple sclerosis, in animal models of experimental autoimmune encephalomyelitis, and systemically in some patients with active hemophagocytic lymphohistiocytosis (HLH) with CNS involvement.8, 9, 10
Familial hemophagocytic lymphohistiocytosis (FHL) is an autosomal recessive disorder of immune dysregulation, resulting in hypercytokinemia associated with fever, hepatosplenomegaly, and cytopenia.10, 11 Identified genetic causes of FHL include mutations in perforin1 (PRF1; FHL2),12 UNC13D (FHL3),13 STX11 (FHL4),13 and STXBP2 (FHL5).14 The diagnostic criteria for HLH include fever, cytopenia (≥2 cell lineages), hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis in the bone marrow, spleen, or lymph nodes. Additional diagnostic criteria include decreased NK-cell activity, hyperferritinemia, and elevated secretion of soluble IL-2 receptor.15 Treatment includes chemo-immunotherapy and hematopoietic stem cell transplantation.
We present a family with two daughters who died of a previously undescribed neurodegenerative disorder triggered by infection. Both girls had biallelic mutations in PRF1 without diagnostic features of FHL2.
Materials and methods
Patient data
Clinical data on each affected individual were obtained through retrospective chart review and interviews of the parents. Data on the proposita were also collected prospectively.
Standard protocol approvals, registrations, and patient consents
Family members gave informed consent/assent for protocol H09-01228 (University of British Columbia, Vancouver, Canada) and for protocol 76-HG-0238 (National Institutes of Health, Bethesda, MD, USA).
Exome sequencing and analysis
Exome sequencing was performed on the nuclear family (both affected sisters, their three unaffected siblings, and both parents – Figure 1) as previously described.16 We used NextGENe software version 2.2.2 (SoftGenetics, LLC, State College, PA, USA) to align sequences to the human reference genome (NCBI, Build 37 v2) and call variants. Each sample alignment was performed allowing one mismatched base and ≥85% of the read matching the reference sequence. Variant calling required that the variant was observed in ≥20% and ≥3 short reads, with a minimum of 5 × coverage.
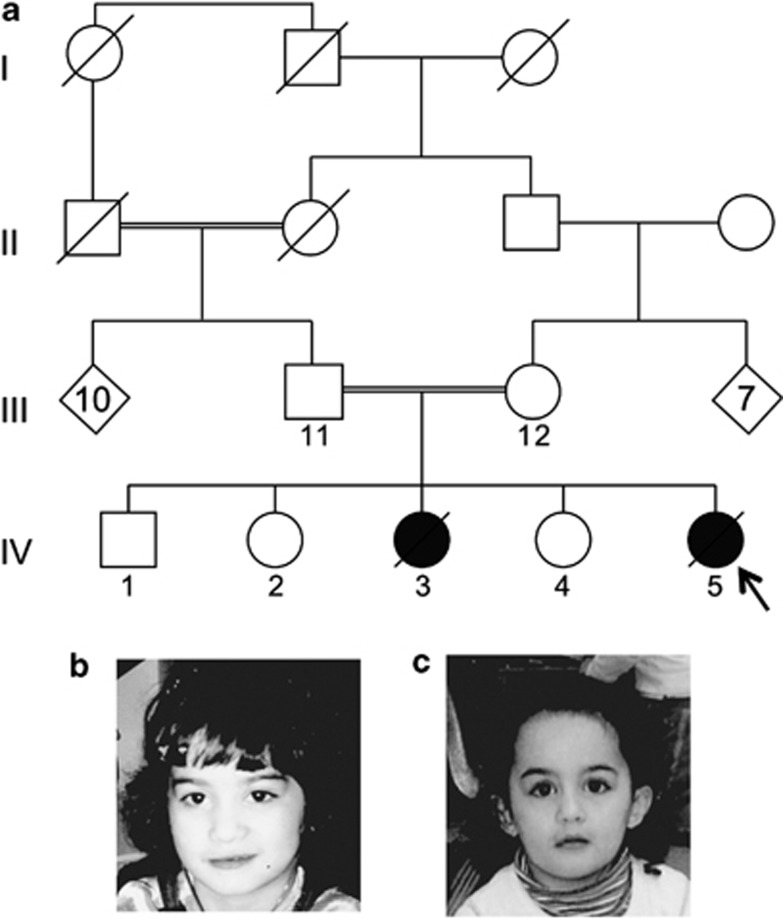
Figure 1.
Familial pedigree and photographs of the affected girls. (a) The pedigree shows two loops of consanguinity; however, molecular studies and further family history analysis confirmed additional loops. The affected girls are noted by shaded shapes and the proband by an arrow. (b) Photograph of the elder affected girl. (c) Photograph of the proband.
Following generation of a variant list fitting autosomal recessive inheritance by NextGENe Viewer's Variant Comparison Tool, we ranked the variants using VAR-MD as described.17 To accept variants for ranking, we required a variant to meet the following criteria in at least four of the seven exomes: a cutoff for genotype confidence score of ≥16, or for variants that had scores between 7 and 16, a genotype confidence score of at least one-fourth the coverage value.
Cell stimulation and measurement of cytokines
Peripheral blood mononuclear cells (PBMCs) were purified over a gradient of Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ, USA). PBMCs were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum and 2 mM L-glutamine.
For evaluation of inflammasome activation, extracellular IL-1β was detected in culture supernatants after stimulation of PBMCs with lipopolysaccharide (LPS) in the presence or absence of exogenous ATP.18 An LPS challenge was performed (in triplicate) on patient IV-5, unaffected siblings, parents, and seven healthy controls. Briefly, 2 × 105 PBMCs/well were added to a 96-well plate and treated in triplicate with 10 ng/ml E. coli Ultra-Pure LPS (InvivoGen, San Diego, CA, USA) for 4 h with or without addition of 5 mM ATP for the last hour of incubation. The IL-1β concentrations were measured using an ELISA Ready-SET-Go (eBioscience, San Diego, CA, USA). Statistical analysis was performed using SPSS v.19 (IBM, North Castle, NY, USA), applying independent sample t-tests.
A Luminex-based cytokine/chemokine assessment was performed on patient and healthy adult control whole blood.19 Briefly, 2.2 μl of a 10 × TLR ligand for stimulation of whole blood (final working concentrations are 10 ng/ml LPS, 1 μg/ml PAM, 100 μg/ml pIC, 10 μg/ml PGN, and 10 μM R848) was added to a well of a 96-well culture plate. Assays were performed in duplicate. Two hundred microliters of a 1:1 whole blood:RPMI 1640 (Invitrogen, Carlsbad, CA, USA) mixture was then added to each well. After a 24-hour incubation, cytokine levels (IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, IP-10, MIP-1α, MIP-1β, TNF-α, IFN- α2, and IFN-γ) were measured in the supernatants using a Luminex immunobead-based 12-plex assay (Luminex running the Masterplex software, MiraiBio, Alameda, CA, USA).
Results
Clinical features
The proband and her sister (Table 1) were daughters of a consanguineous couple of Lebanese origin. The parents were first cousins but had additional loops of consanguinity such that the estimated coefficient of inbreeding for the proband was 0.13 (Supplementary data), the equivalent of the offspring of half-sibs.
Table 1. Summary of key laboratory investigations.
| Patient IV-5 |
Age (months) |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
|
22.5 |
23.5 |
32.5 |
44–44.5 |
45 |
59 |
60–61 |
|
Time after onset (months) |
Onset |
1 |
10 |
21.5–22 |
22.5 |
36.5 |
37.5–38.5 |
|
| Analyte and units | Diagnostic cutoff for HLH15 | Test result (reference range) | ||||||
| Ferritin μg/l | >500 | 7 (6–30) | — | — | — | 27 (12–30) | — | — |
| Hemoglobin g/l | <90 | 114 (103–135) | 113 (103–135) | — | 79→96 (105–135) | 127 (105–135) | 127 (107–131) | 128→124 (105–147) |
| Neutrophils × 109/l | <1.0 | 2.05 (1.5–8.5) | 1.72 (1.5– 8.5) | — | 0.96→1.53 (1.5–8.5) | 3.34 (1.5–8.5) | 3.4 (2.0–5.5) | 1.04→1.4 (1.5–8.5) |
| Platelets × 109/l | <100 | 219 (200–550) | 253 (200–550) | — | 77→108 (200–550) | 187 (200–490) | 102 (180–440) | 123→106 (200–490) |
| Fibrinogen g/l | <1.5 | — | — | — | 2.75 | — | — | — |
| ALT U/l | Elevated | 25 (5–45) | 23 (5–45) | — | 703→321 (5–45) | 67 | 27 (<36) | — |
| AST U/l | Elevated | 46 (20–60) | 54 (20–60) | — | 740→117 (20–60) | 77 | 35 (<36) | — |
| Serum protein g/l | Low | Total 74 (56–75) | — | — | Total 64 (59–78) | — | — | — |
| Triglycerides mmol/l | >3.0 | 0.67 (0.30–1.41) | — | — | — | — | — | — |
| Cholesterol mmol/l | — | 3.96 (1.15–4.70) | — | — | — | — | — | — |
| HDL mmol/l | Low | 1.70 | — | — | — | — | — | — |
| CSF protein g/l | Elevated | 0.56 (0.10–0.35) | 0.28 (0.10–0.35) | 0.28 (0.10–0.35) | — | — | — | — |
| CSF-nucleated cell × 106/l | Elevated | 12 (0–20) | 12 (0–20) | 4 (0–20) | — | — | — | — |
| CSF microbiology | — | Negativea | — | Negative | — | — | — | — |
| Bone marrow aspirate and biopsy | — | Normocellular for age. No abnormal cells. Iron deficiency. (Bilateral iliac crests) | — | — | Normocellular for age. Mild dyserythropoiesis consistent with iron deficiency (Right iliac crest) | — | — | — |
|
Patient IV-3 |
|
Age (months) |
||||||
|
75.5 |
82.5 |
83 |
88 |
|||||
|
Time after onset (months) |
|
Onset |
6.5 |
7 |
12 |
|
|
|
|
Analyte and units |
Diagnostic cutoff for HLH15 |
Test result (reference range) |
||||||
| Hemoglobin g/l | <90 | — | 133 (118–146) | 121 (118–146) | 132 (118–146) | — | — | |
| Neutrophils × 109/l | <1.0 | — | 1.49 (1.8–5.4) | 1.16 (1.8–5.4) | 3.1 (1.7–5.0) | — | — | |
| Platelets × 109/l | <100 | — | 254 (180–440) | 230 (180–440) | 406 (180–440) | — | — | |
| Fibrinogen g/l | <1.5 | — | 3.24 (1.68–4.40) | — | — | — | — | |
| ALT U/l | Elevated | — | 5 (10–25) | — | — | — | — | |
| AST U/l | Elevated | — | 21 (15–50) | — | — | — | — | |
| protein g/l | Low | — | Albumin 43 (35–52) | — | — | — | — | |
| HDL mmol/l | Low | — | — | — | — | — | — | |
| CSF protein g/l | Elevated | — | 0.30 (0.15–0.45) | — | — | — | — | |
| CSF-nucleated cell × 106/l | Elevated | — | 4 (<1) | — | — | — | ||
Abbreviation: HLH, hemophagocytic lymphohistiocytosis.
Values fulfilling diagnostic criteria for HLH are shown in bold.
PCR for Cytomegalovirus, Herpes virus type 6, Varicella-Zoster, Epstein–Barr virus, Adenovirus, West Nile virus, Enterovirus, Herpes simplex virus 1 and 2, and toxoplasmosis. It is performed on a cryopreserved sample.
PCR for Cytomegalovirus, Herpes virus type 6, Varicella-Zoster, CSF bacterial and viral culture.
Patient IV-5
The proband (IV-5, Figure 1) was born at term after an unremarkable pregnancy and delivery. She had normal early growth and development and good health aside from upper respiratory and middle ear infections. Beginning at 18 months, her weight gain declined, and at 22.5 months, a week after a bout of gastroenteritis with an eczematous rash, she developed ataxia, abnormal eye movements and dysarthria as well as brain white matter abnormalities (see below; Figure 2). She had elevated CSF protein concentration (0.56 g/l; ref.: 0.10–0.35 g/l). Bone marrow biopsy showed iron depletion. Additional testing was otherwise unremarkable (Table 1 and Supplementary Table 1).
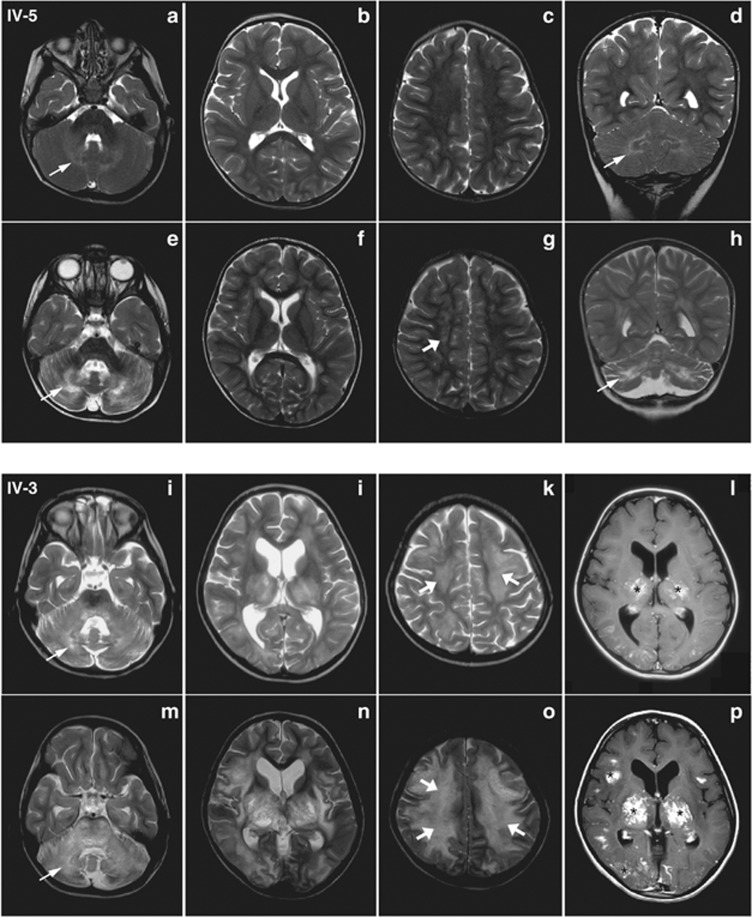
Figure 2.
Brain MRI of patients IV-5 and IV-3, comparing the initial and follow-up studies. Patient IV-5: axial and coronal T2 images (a–d, 22.5 months of age, 2 weeks after onset; e–h, 32.5 months of age, 10 months after onset) show progression of signal abnormality in the cerebellar white matter (fine arrows) with atrophy of the cerebellum and subsequent involvement of the cerebral white matter (thick arrows). Patient IV-3: axial T2 and axial post-contrast T1 images (i–l, 79 months of age, 3 months after onset; m–p, 88 months of age, 12 months after onset) show extensive and progressive signal abnormality involving the cerebellar (thin arrow) and cerebral (thick arrows) white matter, thalami and basal ganglia, with some lesions showing post-contrast enhancement (asterisks).
Soon after partial resolution of the ataxia and dysarthria, she developed progressive disease. At age 32.5 months, her brain MRI documented further white matter degeneration, and a skeletal muscle biopsy showed mild variation in fiber size and type II fiber atrophy on light microscopy; fiber and mitochondrial ultrastructure as well as mitochondrial respiratory chain activity were normal. Also, her CSF had unremarkable chemistry and cytology, and by PCR, no detectable nucleic acid indicative of infection with Cytomegalovirus, Herpes virus type 6, Varicella-Zoster, Epstein–Barr virus, Adenovirus, West Nile virus, Enterovirus, Herpes simplex viruses 1 and 2, and toxoplasmosis (Table 1). By age 42 months, she developed mild splenomegaly and lymphadenopathy without hepatomegaly or detectable acute viral infection. Two months later, she was admitted to hospital for vomiting, tachycardia, tachypnea and fever (39 °C); she had hypertrophic cardiomyopathy with mild diastolic dysfunction, mild pancytopenia, and elevated AST and ALT levels. Again her bone marrow biopsy was unremarkable except for iron depletion (Table 1). Her immune studies showed normal numbers and distribution of T, B, and NK cells as well as unremarkable responses to mitogens and normal complement activity (Supplementary Table 1). No viral or bacterial pathogens were identified. During her recovery, she manifested persistent neck rigidity suggestive of meningeal inflammation.
Shortly after this hospitalization, she developed seizures. Over the ensuing 17 months, she gradually became blind, lost cognitive and most motor skills, and developed dysphagia. Throughout this period, she manifested neck rigidity following each of many infections but had no more recorded fevers until the week before her death when her temperature was 38.5 °C. She died at 61 months of age with upper respiratory infection and cardiac failure secondary to left ventricular hypertrophy with outlet obstruction and mitral regurgitation. The family declined an autopsy.
Patient IV-3
The proband's sister (IV-3; Figure 1) was the product of an uneventful pregnancy. She had mild developmental delay and hearing loss. At age 75.5 months, concurrent with varicella infection, she had a seizure in the context of fever, deterioration of mental status and was diagnosed with varicella meningitis. CT brain imaging showed dilated ventricles and cerebellar swelling. Following 2 weeks of treatment with acyclovir and steroids, she regained baseline neurological function. Subsequently, however, she became ataxic and was unable to walk. Three months after initial presentation her brain MRI showed diffuse white matter changes (Figure 2). She gradually lost additional motor skills and strength; eight months after presentation the nerve conduction velocities of her sensory and motor evoked responses were at the lower limits of normal in the right upper and lower limbs (Right Tibial Motor at ankle: latency 3.22 ms, conduction 40.97 m/s). Her CSF showed normal protein, glucose and amino acid levels. CSF neurotransmitters were normal except for an elevated neopterin level; her skin fibroblasts had normal 6-pyruvoyltetrahydropterin synthase activity, excluding 6-pyruvoyltetrahydropterin synthase deficiency as the cause. All other testing was unremarkable (Table 1 and Supplementary Table 1). At age 88.5 months, her brain MRI showed progression of the white matter changes (see below; Figure 2). She died at 91 months of age, 1 year before the birth of her younger sister, the proband. No autopsy was performed.
Brain imaging
Patient IV-5
Two weeks after the onset of ataxia, a CT scan showed abnormal hyperintense areas in the cerebellar white matter. A subsequent brain MRI (Figures 2a–d) showed symmetrical areas of T2 hyperintense signal abnormality in the cerebellar white matter and the cerebral white matter of the frontal lobe, as well as in a peritrigonal distribution.
Ten months later a repeat brain MRI (Figure 2e–h) showed worsening of the cerebellar changes with atrophy and involvement of the gray matter. In addition, new areas of signal abnormality distributed along an anterior to posterior gradient were seen in the periventricular cerebral white matter.
Patient IV-3
Three months after her first seizure, a brain MRI (Figure 2i–l) showed cerebellar volume loss, diffuse symmetrical T1 hypointense and T2 hyperintense signal abnormalities in the cerebellar white matter, mild symmetrical ventriculomegaly and signal abnormalities in the periventricular white matter, thalami, basal ganglia, internal capsule, and midbrain. Some of these lesions enhanced after contrast, suggesting disruption of the blood-brain barrier.
Nine months later, a brain CT confirmed the prior findings, and a repeat brain MRI identified worsening of the T1 and T2 signal abnormalities involving the cerebellar white matter and extending into the gray matter, and greater involvement of the cerebral white matter, thalami, basal ganglia, internal and external capsules (Figure 2m–p), and brainstem. Many lesions in the subcortical white matter, basal ganglia, cerebellar peduncles and brainstem enhanced with contrast.
Defective production of IL-1 β
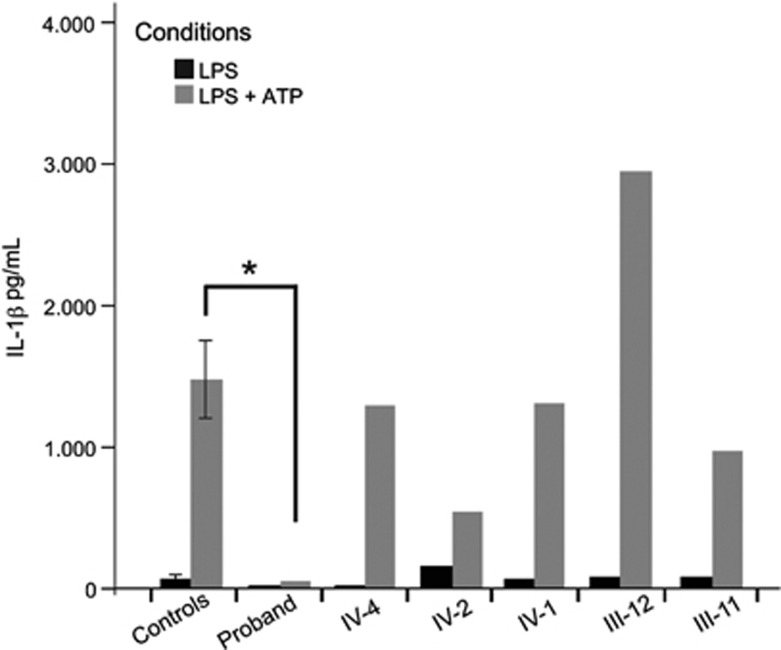
In the context of her normal B- and T-cell studies, the absence of fever with apparent infection and neurodegeneration triggered by minor infections, we suspected that the proband had a defect in pyrogen generation mediated by innate immunity. To assess this, we tested if her monocytes could produce the pyrogen IL-1β in response to an LPS challenge in the presence of ATP (inflammasome activation assay).18 At 59 months (37 months after onset), the proband's PBMCs failed to produce IL-1β, in contrast to healthy controls, the asymptomatic siblings, and the parents (Figure 3). Further testing using the Luminex cytokine assay confirmed the minimal IL-1β production and revealed a broad decrease in inflammatory cytokines, following stimulation with a panel of innate immune activators (Supplementary Figure 1). The proband died before these abnormalities could be investigated further.
Figure 3.
Inflammasome activation assay. Concentration of IL-1β excreted by PBMCs measured by ELISA. The cells were treated with 10 ng/ml LPS, with or without treatment with ATP. All experiments were performed in triplicate. ‘Controls' represents the mean of seven biological replicates in healthy controls; error bars indicate the standard error of the mean. Proband IL-1β after stimulation with LPS and ATP is significantly decreased in comparison to other healthy controls, and to all five unaffected family members (P<0.05). All heterozygous family members showed no significant difference from controls. Control experiments with media only, or media with ATP, yielded negligible concentration of IL-1β in all samples (data not shown). An asterisk indicates a statistically significant difference between the patient and controls (independent samples t-test, P≤0.05).
Exome sequencing identifies a homozygous pathogenic PRF1 mutation
To determine the molecular etiology for this unusual innate immune response, we performed exome sequencing on the DNA of the affected girls and immediate family members (parents and unaffected siblings; Figure 1). Analysis for homozygous variants unique to the affected siblings identified a mutation (NM_005041.4: c.673C>T, p.R225W) in exon 3 of the PRF1 gene (Supplementary Figure 2). Both parents and all of the unaffected siblings were heterozygous for the same variant. The p.R225W mutation is in the transmembrane region of perforin and has been associated with FHL2.11, 12, 20, 21 In an animal cell model, p.R225W causes decreased cytotoxicity and impaired trafficking of secretory granules.22
Discussion
We present two sisters with fatal neurodegeneration showing similar patterns of white matter cerebellar degeneration. We also found that the proband had a defective innate immune response, and that both she and her affected sister had pathogenic biallelic mutations in PRF1 as a possible cause of their disease.
The PRF1 mutation identified in our patients has been associated with FHL2,11 but the sisters did not manifest diagnostic features of FHL2.15 Specifically, they had minimal or no fevers, no hypertriglyceridemia, no hypofibrinogenemia, and no hyperferritinemia. The youngest had only mild transient cytopenia. Because both sisters died before the PRF1 mutation was identified, they were not tested for reduced NK-cell activity or elevated soluble IL-2 receptor levels. Also, two bone marrow biopsies in the proband did not demonstrate increased hemophagocytosis found in 82% of FHL2 patients.23
Neurodegeneration has been reported as a possible feature of FHL2 and sporadic HLH,24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 with a frequency of 37–69%.23, 29, 39, 40 A third of patients have neurological symptoms at diagnosis,40 and 36% of those with PRF1 mutations have some CNS involvement.23 Although there is an association of variations in PRF1 with susceptibility to multiple sclerosis,41 very few patients with HLH have had neurological symptoms as the sole presenting feature, and consistent with the expected ascertainment and reporting bias,29, 30, 32, 39 all developed the diagnostic features of FHL2-HLH, with the exception of one individual who was diagnosed by brain histopathology but without identification of a mutation.25, 26, 29, 31, 32, 33, 36, 42 The sisters reported herein are therefore the first individuals reported with neurodegeneration, biallelic pathogenic PRF1 mutations and no diagnostic features suggestive of FHL–HLH (Table 2).
Table 2. Comparison of neurological features of patients reported with PRF1 mutations for whom MRI images or detailed description was provided.
| Reference | PRF1 mutation | Age onset/death | Infectious trigger | Features onset | Systemic features of HLH | Clinical Dx at onset | MRI |
|---|---|---|---|---|---|---|---|
| Present case IV-5 | p.R225W | 22.5 m/ 61 m | Unidentified GI virus at onset | Ataxia, Dysarthria, Nystagmus | Inconsistenta | Metabolic neurodegeneration | Progressive symmetrical cerebellar white matter signal abnormality with cerebellar atrophy. Less involvement of the cerebral white matter |
| Present case IV-3 | p.R225W | 5 y and 8 m/ 7 y and 7 m | Varicella at onset | Seizure, Ataxia | No | Encephalomyelitis | Progressive symmetrical signal abnormality of the cerebellar and cerebral white matter, cortex, gray matter structures and brain stem, with multiple enhancing lesions. |
| Chiapparini et al26 | p.R225W | 13 y/ alive 18 m post-HSCT | Not reported | Headache, Gait imbalance | Yes | Inflammatory immune-mediated disorder | Symmetrical cerebral and cerebellar gray and white matter signal abnormality, worse in the cerebellum, with multiple enhancing lesions. |
| ME van Egmond et al36 | p.G149S+p.E253K | 11 m/ alive 18 m post-HSCT | Not reported | Regression in motor and language skills | Yes | Suspected acute disseminating encephalomyelitis (ADEM). | Signal abnormalities in the cortex, cerebral and cerebellar white matter, with multiple enhancing perivascular foci. |
| Astigarraga et al24 | p.L215I+p.A262D | 4 y/ alive16 m post-HSCT | varicella | Ataxia, nystagmus, dysmetria | Yes | Cerebellitis | Signal abnormalities in the periventricular and cerebellar white matter. |
| Moshous et al44 | p.E6X+p.A91Val/p.R119W | 3 y and 6 m/ alive 5 m post-HSCT | URTI at exacerbation 3 months after onset | Headaches, ataxia, visual disturbances | Yes | CNS vasculitis | Asymmetrical cerebellar and cerebral gray and white matter signal abnormality, with multiple supra and infratentorial lesions with peripheral post-contrast enhancement and central necrosis. |
| Feldman et al43 | p.P459L | 5 m/44 m | Upper respiratory infection at onset | Poor head control, tremor, abnormal tone | Inconsistenta | Chronic lymphocytic meningitis | Signal abnormalities in the periventricular, deep supratentorial and cerebellar white matter, corpus callosum and brainstem with some enhancing lesions. |
| Beaty et al25 | p.R356W + T450Mb | 16 y/18 y | Not reported | Unilateral upper extremity weakness and sensory deficits | Inconsistenta | Multiple sclerosis | Patchy areas of focal demyelination |
Abbreviations: GI, gastrointestinal; HLH, hemophagocytic lymphohistiocytosis; HSCT, hematopoietic stem cell transplantation; m, months; PRF1, perforin1 mutation; URTI, upper respiratory tract infection; y, years.
The p.R225W mutation is highlighted in bold.
Mild systemic features that did not fulfill diagnostic criteria for FHL, subsequently interpreted as possibly related to PRF1 in the context of the genetic diagnosis.
Predicted protein alteration according to the report of 1066C>T and 1349C>T mutations in the coding sequence; gene accession number not provided.
Potential explanations for this lack of features include the nature of the triggering pathogen, pathogen–host interaction, the immunological landscape defined by prior infectious exposures, genetic background or another primary cause of neurodegeneration. By history, however, the sisters did not have common triggering pathogens or common environmental exposures. Thus, this precludes ready acceptance of the first three possible explanations. Notwithstanding the limitations of exome sequencing,16 extensive evaluation of all exome variants in the siblings failed to identify an alternative primary causal mutation for neurodegeneration or candidate modifier variant even though the high degree of consanguinity suggests that the affected children may both have inherited such modifier variants for the phenotype associated with the PRF1 mutation (Supplementary Table 2 to 4). A genome-wide analysis of a larger cohort of patients harboring PRF1 mutations, presenting with and without neurodegeneration might, however, identify such modifiers.
In contrast to the febrile cytokine storms observed in FHL2, the proband stopped developing fevers with infection during the course of her disease. Consistent with this, her PBMCs did not produce IL-1β or other pro-inflammatory cytokines overexpressed in FHL2.10 This observation, consistent with a defect in the innate immune response, suggests that the link between PRF1 mutations and cytokine overexpression may be indirect or complex. The discrepant cytokine phenotype between FHL2 patients and our patients can be explained by genetic modifiers or by a difference in the timing of cytokine testing. Studies of cytokine expression in patients with classical FHL have been performed early in the disease course, most typically before treatment,10 whereas our proband was tested long after onset of symptoms. Determining whether this cytokine profile is a distinguishing feature of the PRF1-related neurodegenerative disorder manifest by these girls, or whether loss of cytokine production or cytokine depletion is a feature common to the later stages of FHL2 will require testing of additional FHL2 patients.
The brain MRI findings in HLH are nonspecific. They include multiple focal or diffuse areas of signal abnormality within the cerebral and cerebellar white and gray matter.28, 29, 36 The lesions can have a nodular appearance and enhance after contrast, a finding suggestive of perivascular involvement.29, 30 The involvement of the cerebellum with development of cerebellar atrophy is a prominent feature observed in the majority of HLH patients. Cerebellar gray matter involvement usually appears later in the disease, and thus is not constant. Involvement of the cerebral gray matter is less common. Even when the patient cohort is limited to those with perforin mutations, the MRI findings are also not consistent (Table 2), although cerebellar disease predominates.24, 25, 26, 36, 43, 44 In the absence of systemic findings of HLH, therefore, one is unable to make a clinical diagnosis of PRF1-related neurodegeneration, and molecular testing is required.
In summary, we extend the neurodegenerative phenotype associated with biallelic mutations of PRF1 and show that the neurodegenerative phenotype can occur in the absence of the hematological and immune signs of FHL2-HLH. We hypothesize that PRF1-related neurodegeneration is an under-recognized condition, and suggest that when suspicion of immune-mediated neurodegeneration arises, this diagnosis should be considered because it is potentially curable with hematopoietic stem cell transplantation.15, 44
Web resources
Acknowledgments
We are grateful to Dr Camilo Toro for manuscript review and critical analysis, and acknowledge Drs Patrick Tang and Rusang Tan for their collaboration. We would like to express a special thanks to the patients' family. This study was primarily funded by the Intramural Research Program of the NHGRI and in part by the Rare Disease Foundation. Funding was supplied by the Canadian Institutes for Health Research (DPS). DPS is the Sauder Family Professor of Pediatric Infectious Diseases. CD is supported by the Canadian Child Health Clinician Scientist Program and the Child and Family Research Institute. AM is supported by the University of British Columbia.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Phelan J, Lowe L, Glasier C. Pediatric neurodegenerative white matter processes: leukodystrophies and beyond. Pediatr Radiol. 2008;38:729–749. doi: 10.1007/s00247-008-0817-x. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Saudubray J-M, Gvd Berghe, Walter JH.(eds): Inborn Metabolic Diseases: Diagnosis and Treatment Heidelberg, DEU, Germany: Springer, London, Ltd.2006 [Google Scholar]
- Schlüter A, Espinosa L, Fourcade S, et al. Functional genomic analysis unravels a metabolic-inflammatory interplay in adrenoleukodystrophy. Hum Mol Genet. 2011;21:1062–1077. doi: 10.1093/hmg/ddr536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Differential effects of pro- and anti-inflammatory cytokines alone or in combinations on the metabolic profile of astrocytes. J Neurochem. 2011;116:564–576. doi: 10.1111/j.1471-4159.2010.07135.x. [DOI] [PubMed] [Google Scholar]
- Fogal B, Hewett SJ. Interleukin-1β: a bridge between inflammation and excitotoxicity. J Neurochem. 2008;106:1–23. doi: 10.1111/j.1471-4159.2008.05315.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Furlan R, De Chiara V, et al. Interleukin-1β causes synaptic hyperexcitability in multiple sclerosis. Ann Neurol. 2012;71:76–83. doi: 10.1002/ana.22512. [DOI] [PubMed] [Google Scholar]
- de Jong BA, Huizinga TWJ, Bollen ELEM, et al. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126:172–179. doi: 10.1016/s0165-5728(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Sumegi J, Barnes MG, Nestheide SV, et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 2011;117:e151–e160. doi: 10.1182/blood-2010-08-300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp SE, Dufourcq-Lagelouse Rm, Deist FoL, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. [PubMed] [Google Scholar]
- Molleran Lee S, Villanueva J, Sumegi J, et al. Characterisation of diverse PRF1 mutations leading to decreased natural killer cell activity in North American families with haemophagocytic lymphohistiocytosis. J Med Genet. 2004;41:137–144. doi: 10.1136/jmg.2003.011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Stadt U, Schmidt S, Kasper B, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- Cetica V, Santoro A, Gilmour KC, et al. STXBP2 mutations in children with familial haemophagocytic lymphohistiocytosis type 5. J Med Genet. 2010;47:595–600. doi: 10.1136/jmg.2009.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- Dias C, Sincan M, Cherukuri PF, et al. An analysis of exome sequencing for diagnostic testing of the genes associated with muscle disease and spastic paraplegia. Hum Mutat. 2012;33:614–626. doi: 10.1002/humu.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincan M, Simeonov D, Adams D, et al. VAR-MD: A tool to analyze whole exome/genome variants in small human pedigrees with Mendelian inheritance. Hum Mutat. 2012;33:593–598. doi: 10.1002/humu.22034. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralitharan S, Wali YA, Dennison D, et al. Novel spectrum of perforin gene mutations in familial hemophagocytic lymphohistiocytosis in ethnic omani patients. Am J Hematol. 2007;82:1099–1102. doi: 10.1002/ajh.21009. [DOI] [PubMed] [Google Scholar]
- Clementi R, zur Stadt U, Savoldi G, et al. Six novel mutations in the PRF1 gene in children with haemophagocytic lymphohistiocytosis. J Med Genet. 2001;38:643–646. doi: 10.1136/jmg.38.9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboinik I, Thia M-C, De Bono A, et al. The functional basis for hemophagocytic lymphohistiocytosis in a patient with co-inherited missense mutations in the perforin (PFN1) gene. J Exp Med. 2004;200:811–816. doi: 10.1084/jem.20040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trizzino A, Uz Stadt, Ueda I, et al. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008;45:15–21. doi: 10.1136/jmg.2007.052670. [DOI] [PubMed] [Google Scholar]
- Astigarraga I, Prats JM, Navajas A, Fernández-Teijeiro A, Urberuaga A. Near fatal cerebellar swelling in familial hemophagocytic lymphohistiocytosis. Pediatr Neurol. 2004;30:361–364. doi: 10.1016/j.pediatrneurol.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Beaty AD, Weller C, Levy B, et al. A teenage boy with late onset hemophagocytic lymphohistiocytosis with predominant neurologic disease and perforin deficiency. Pediatr Blood Cancer. 2008;50:1070–1072. doi: 10.1002/pbc.21438. [DOI] [PubMed] [Google Scholar]
- Chiapparini L, Uziel G, Vallinoto C, et al. Hemophagocytic lymphohistiocytosis with neurological presentation: MRI findings and a nearly miss diagnosis. Neurol Sci. 2011;32:473–477. doi: 10.1007/s10072-010-0467-2. [DOI] [PubMed] [Google Scholar]
- Chung TW. CNS Involvement in Hemophagocytic Lymphohistiocytosis: CT and MR Findings. Korean J Radiol. 2007;8:78–81. doi: 10.3348/kjr.2007.8.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaminada N, Cappellini M, Mortilla M, et al. Familial hemophagocytic lymphohistiocytosis: clinical and neuroradiological findings and review of the literature. Child's Nerv Syst. 2010;26:121–127. doi: 10.1007/s00381-009-0957-9. [DOI] [PubMed] [Google Scholar]
- Goo H, Weon Y. A spectrum of neuroradiological findings in children with haemophagocytic lymphohistiocytosis. Pediatr Radiol. 2007;37:1110–1117. doi: 10.1007/s00247-007-0569-z. [DOI] [PubMed] [Google Scholar]
- Gurgey A, Aytac S, Balta G, Oguz KK, Gumruk F. Central nervous system involvement in Turkish children with primary hemophagocytic lymphohistiocytosis. J Child Neurol. 2008;23:1293–1299. doi: 10.1177/0883073808319073. [DOI] [PubMed] [Google Scholar]
- Haddad E, Sulis M-L, Jabado N, Blanche S, Fischer A, Tardieu M. Frequency and severity of central nervous system lesions in hemophagocytic lymphohistiocytosis. Blood. 1997;89:794–800. [PubMed] [Google Scholar]
- Henter J-I, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr. 1997;130:358–365. doi: 10.1016/s0022-3476(97)70196-3. [DOI] [PubMed] [Google Scholar]
- Puliyel MM, Rose W, Kumar S, Moses PD, Gibikote S. Prolonged neurologic course of familial hemophagocytic lymphohistiocytosis. Pediatr Neurol. 2009;41:207–210. doi: 10.1016/j.pediatrneurol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Kolb R, Pohl D, et al. CNS disease as the main manifestation of hemophagocytic lymphohistiocytosis in two children. Neuropediatrics. 2004;35:45–49. doi: 10.1055/s-2004-815791. [DOI] [PubMed] [Google Scholar]
- Sieni E, Cetica V, Santoro A, et al. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis type 3. J Med Genet. 2011;48:343–352. doi: 10.1136/jmg.2010.085456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Egmond ME, Vermeulen RJ, Peeters-Scholte CMPCD, et al. Familial hemophagocytic lymphohistiocytosis in a pediatric patient diagnosed by brain magnetic resonance imaging. Neuropediatrics. 2011;42:191–193. doi: 10.1055/s-0031-1287788. [DOI] [PubMed] [Google Scholar]
- Wada T, Nishiura K, Kuroda M, et al. A case of acute encephalopathy with hemophagocytic lymphohistiocytosis and clonal T-cell expansion. Brain Dev. 2011;34:376–379. doi: 10.1016/j.braindev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang L, Jia C, Ma H, Henter J-I, Shen K. Frequency and development of CNS involvement in Chinese children with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2010;54:408–415. doi: 10.1002/pbc.22239. [DOI] [PubMed] [Google Scholar]
- Deiva K, Mahlaoui N, Beaudonnet F, et al. CNS involvement at the onset of primary hemophagocytic lymphohistiocytosis. Neurology. 2012;78:1150–1156. doi: 10.1212/WNL.0b013e31824f800a. [DOI] [PubMed] [Google Scholar]
- Horne A, Trottestam H, Aricò M, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140:327–335. doi: 10.1111/j.1365-2141.2007.06922.x. [DOI] [PubMed] [Google Scholar]
- Cappellano G, Orilieri E, Comi C, et al. Variations of the perforin gene in patients with multiple sclerosis. Genes Immun. 2008;9:438–444. doi: 10.1038/gene.2008.35. [DOI] [PubMed] [Google Scholar]
- Shinoda J, Murase S, Takenaka K, Sakai N. Isolated central nervous system hemophagocytic lymphohistiocytosis: case report. Neurosurgery. 2005;56:187. [PubMed] [Google Scholar]
- Feldmann J, Ménasché G, Callebaut I, et al. Severe and progressive encephalitis as a presenting manifestation of a novel missense perforin mutation and impaired cytolytic activity. Blood. 2005;105:2658–2663. doi: 10.1182/blood-2004-09-3590. [DOI] [PubMed] [Google Scholar]
- Moshous D, Feyen O, Lankisch P, et al. Primary necrotizing lymphocytic central nervous system vasculitis due to perforin deficiency in a four-year-old girl. Arthritis Rheum. 2007;56:995–999. doi: 10.1002/art.22442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.