Abstract
Aflatoxin B1 (AFB1) is a potent carcinogen that causes growth stunting, immunosuppression and liver cancer in multiple species. The recent trend of replacing fishmeal with plant-based proteins in fish feed has amplified the AFB1 exposure risk in farm-raised fish. NovaSil (NS), a calcium montmorillonite clay, has previously been shown to reduce AFB1 bioavailability safely and efficaciously in several mammalian species. This study was designed to: (1) evaluate AFB1 impact on cultured red drum, Sciaenops ocellatus, over the course of seven weeks; and (2) assess NS supplementation as a strategy to prevent aflatoxicosis. Fish were fed diets containing 0, 0.1, 0.25, 0.5, 1, 2, 3, or 5 ppm AFB1. Two additional treatment groups were fed either 5 ppm AFB1 + 1% NS or 5 ppm AFB1 + 2% NS. Aflatoxin B1 negatively impacted red drum weight gain, survival, feed efficiency, serum lysozyme concentration, hepatosomatic index (HSI), whole-body lipid levels, liver histopathological scoring, as well as trypsin inhibition. NovaSil inclusion in AFB1-contaminated diets improved weight gain, feed efficiency, serum lysozyme concentration, muscle somatic index, and intraperitoneal fat ratios compared to AFB1-treated fish. Although not significant, NS reduced AFB1-induced histopathological changes in the liver and decreased Proliferating Cell Nuclear Antigen (PCNA) staining. Importantly, NS supplementation improved overall health of AFB1-exposed red drum.
Keywords: red drum, aflatoxin, calcium montmorillonite, NovaSil, histopathology, immune
1. Introduction
Mycotoxins are toxic metabolites produced by a diverse group of fungi that contaminate agricultural crops prior to harvest or during storage post-harvest [1,2]. Aflatoxin B1 (AFB1), a mycotoxin produced by Aspergillus flavus and A. parasiticus, is one of the most potent, naturally-occurring carcinogens known to mankind. Aflatoxin B1 exposure causes decreases in weight gain, growth stunting and immunosuppression in animals, while increasing hepatocellular carcinoma incidence [3]. Different species including humans, poultry, swine, and fish all exhibit varying levels of mortality and morbidity upon exposure to AFB1 [4,5,6]. However, because the damaging AFB1 effects are largely species and dose-specific, additional studies are necessary to determine AFB1 susceptibility for at-risk unevaluated species.
As a vital part of the global food industry, aquaculture contributes nearly half of all food of aquatic origin intended for human consumption [7]. Fishmeal, one the most expensive fish feed ingredients, is widely used in the aquaculture industry as the major protein source for farm-raised fish [8]. Menhaden (Brevoortia sp.) is a clupeid fish species and the most prevalent form of fishmeal used in North America [9]. Recent studies have been directed toward the development of plant-based alternative protein sources such as soybean, peanut, corn and cottonseed meal [10,11,12,13]. However, incorporation of plant-based ingredients into feed increases the risk for AFB1 contamination and subsequent exposure. Aflatoxin B1 presence in aquaculture feeds and fish feed ingredients has been well-documented, especially in developing countries [14,15,16].
One strategy to reduce aflatoxin exposure in humans and animals is the use of enterosorption therapy. NovaSil (NS), a calcium montmorillonite clay, binds AFB1 in the gastrointestinal tract, thereby reducing overall AFB1 bioavailability [17]. With a dioctahedral-layered structure and negatively charged interlayer, NS has high affinity and capacity for AFB1 molecules, which exhibit a partial positive charge [18]. Numerous in vivo studies have demonstrated the safety and efficacy of this technology [19,20,21], although additional studies are needed to determine the efficacy and proper dosage for farm-raised fish [22].
Red drum, Sciaenops ocellatus, is a common recreational and commercial fish native to the Atlantic and Gulf Coast regions of the United States [23]. Red drum is currently farmed in China, Israel, Ecuador and North America [24]. Despite its prevalence and economic importance to the food industry, no studies have evaluated red drum AFB1 susceptibility. The study presented here was designed to address two objectives: (1) to evaluate red drum susceptibility to AFB1 using a multi-level AFB1 challenge incorporated into the feed; and (2) to assess the ability of NS to prevent AFB1 toxicity in red drum.
2. Results
2.1. Growth Parameters
Aflatoxin B1 treatment effects, including weight gain (%), survival (%), and feed efficiency, did not result in linear trends, with R2 values of 0.22, 0.01 and 0.1, respectively. Weight gain of individual treatment means were significantly different, and varied with the 0 ppm AFB1 group experiencing the highest weight gain and the 2, 3, and 5 ppm exposure groups exhibiting the least amount of weight gain (Table 1). Likewise, AFB1 significantly reduced feed efficiency in a non-linear manner, with the 0 ppm AFB1 treatment group demonstrating the highest feed efficiency (0.91) and treated groups ranging from 0.49–0.75. Survival also greatly varied across treatments with 0 ppm AFB1 having the highest survival rate.
Table 1.
Growth performance of red drum fed different concentrations of Aflatoxin B1 (AFB1) 1 and AFB1 + NovaSil (NS) 2,3,4.
| Variable | Weight gain 5 (%) | Survival (%) | Feed efficiency | Variable | Weight gain (%) | Survival (%) | Feed efficiency | |
|---|---|---|---|---|---|---|---|---|
| AFB1 (ppm) | Individual treatment means | AFB1 (ppm) | NS (%) | Individual treatment means | ||||
| 0 | 332 a | 80.0 a | 0.91 a | 0 | 0 | 332 ab | 80.0 | 0.91 a |
| 0.1 | 223 bc | 46.6 b | 0.62 bc | 5 | 0 | 188 c | 55.5 | 0.62 c |
| 0.25 | 224 bc | 55.5 b | 0.65 bc | 5 | 1 | 339 a | 73.3 | 0.82 ab |
| 0.5 | 254 ab | 60.0 ab | 0.75 ab | 5 | 2 | 218 bc | 57.7 | 0.71 bc |
| 1 | 212 bc | 60.0 ab | 0.73 abc | p-value | 0.039 | 0.261 | 0.005 | |
| 2 | 136 c | 60.0 ab | 0.49 c | Pooled Std. Error | 7.047 | 1.801 | 0.008 | |
| 3 | 183 bc | 62.2 ab | 0.67 bc | AFB1 (ppm) | NS (%) | Means of main effect | ||
| 5 | 188 bc | 55.5 b | 0.62 bc | 0 | 332 | 80.0 | 0.91 a | |
| R2 | 0.229 | 0.010 | 0.100 | 5 | 249 | 62.2 | 0.72 b | |
| p-value | 0.005 | 0.132 | 0.030 | 0 | 260 a | 67.7 | 0.77 | |
| Pooled Std. Error | 5.189 | 1.309 | 0.013 | 1 | 339 ab | 73.3 | 0.82 | |
| 2 | 218 b | 57.7 | 0.71 | |||||
| ANOVA: p-values | ||||||||
| AFB1 | 0.083 | 0.138 | 0.003 | |||||
| NS | 0.043 | 0.387 | 0.029 | |||||
1 Aflatoxin B1; 2 NovaSil; 3 Values are means of three replicate groups of fish (n = 3); 4 Values in a column that do not have the same superscript are significantly different according to Duncan’s multiple range test (p < 0.05); 5 Initial average weight was 2.1 ± 0.1 g/fish.
Among the NS-supplemented treatment groups, only weight gain and feed efficiency were significantly different compared to AFB1 controls, with p-values of 0.039 and 0.005, respectively. In the case of feed efficiency, 0 ppm AFB1 and 5 ppm AFB1 were the most significantly different. NovaSil inclusion at both 1% and 2% positively affected weight gain, feed efficiency, and survival after AFB1 exposure, although not in a dose-dependent manner.
2.2. Immune Response
A summary of immune parameters evaluated for each group is shown in Table 2. The 0.1 ppm AFB1-exposed fish exhibited the highest plasma lysozyme values (246 units/mL), while the 5 ppm-exposed fish displayed the lowest levels (45 units/mL). Trypsin inhibition (%) results indicated that 1, 2, 3, and 5 ppm AFB1-exposed groups had the lowest percent inhibition and 0.25 ppm AFB1 the highest. Additionally, neither the lysozyme nor the trypsin results suggested linearity with an R2 of 0.3947 and 0.109, respectively. The nitro blue tetrazolium (NBT) test showed no significant differences among any of the AFB1-exposed groups.
Table 2.
Immune parameters of red drum 1.
| Variable | Serum lysozyme (units/mL) | NBT (mg/mL blood) 2 | Trypsin inhibition (%) | Variable | Serum Lysozyme (units/mL) | NBT (mg/mL blood) 2 | Trypsin inhibition (%) | |
|---|---|---|---|---|---|---|---|---|
| AFB1 (ppm) | Individual treatment means | AFB1 (ppm) | NS (%) | Individual treatment means | ||||
| 0 | 165 ab | 3.52 | 83.6 ab | 0 | 0 | 165 ab | 3.52 | 83.6 |
| 0.1 | 246 a | 3.35 | 82.4 b | 5 | 0 | 45 c | 3.07 | 81.9 |
| 0.25 | 131 bcd | 2.54 | 86.3 a | 5 | 1 | 76b c | 3.32 | 81.3 |
| 0.5 | 155 abc | 3.30 | 83.2 b | 5 | 2 | 185 a | 3.21 | 79.4 |
| 1 | 106 bcd | 1.78 | 81.9 b | p-Value | 0.024 | 0.944 | 0.577 | |
| 2 | 82 bcd | 3.05 | 80.5 b | Pooled Std. Error | 5.550 | 0.104 | 0.395 | |
| 3 | 63 cd | 2.21 | 82.7 b | AFB1 (ppm) | NS (%) | Means of main effect | ||
| 5 | 45 d | 3.07 | 81.9 b | 0 | 165 | 3.52 | 83.6 | |
| R 2 | 0.394 | 0.015 | 0.109 | 5 | 102 | 3.20 | 80.9 | |
| p-Value | 0.004 | 0.250 | 0.038 | 0 | 105 | 3.30 | 82.7 | |
| Pooled Std. Error | 5.705 | 0.102 | 0.192 | 1 | 76 | 3.32 | 81.3 | |
| 2 | 185 | 3.21 | 79.4 | |||||
| ANOVA: p-Values | ||||||||
| AFB1 | 0.018 | 0.622 | 0.291 | |||||
| NS | 0.021 | 0.948 | 0.674 | |||||
1 Values in a column that do not have the same superscript letters are significantly different according to Duncan’s multiple range test (p < 0.05); 2 Values are means of determinations on two fish from each of three replicate groups (6 fish/treatment, n = 6).
NovaSil had a significant impact (p = 0.021) on the plasma lysozyme concentration with 5 ppm AFB1 + 2% NS outperforming all other treatments. NovaSil did not significantly alter levels of NBT or trypsin inhibition.
2.3. Somatic Indexes
Somatic indexes for spleen, MSI and IPF did not vary within the AFB1-treated groups; however, HSI varied slightly between treatments. The highest HSI levels were recorded in the 0.1 AFB1-treated group, while the 2 ppm and 5 ppm exposure groups exhibited the lowest values (Table 3). A linear trend was not present in any of the groups.
Table 3.
Somatic indices of red drum fed different concentrations of AFB1 1 and AFB1 + NS 2,3,4.
| Variable | Spleen | MSI 5 | HSI 6 | IPF 7 | Variable | Spleen | MSI 5 | HSI 6 | IPF 7 | |
|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 (ppm) | Individual treatment means | AFB1 (ppm) | NS (%) | Individual treatment means | ||||||
| 0 | 0.04 | 28.94 | 1.67 abc | 0.32 | 0 | 0 | 0.04 | 28.94 a | 1.67 | 0.32 a |
| 0.1 | 0.04 | 27.42 | 1.98 a | 0.11 | 5 | 0 | 0.04 | 26.06 b | 0.88 | 0.01 b |
| 0.25 | 0.04 | 26.18 | 1.79 ab | 0.20 | 5 | 1 | 0.09 | 28.33 ab | 0.82 | 0.10 b |
| 0.5 | 0.05 | 27.87 | 1.20 abc | 0.26 | 5 | 2 | 0.20 | 29.70 a | 1.56 | 0.46 a |
| 1 | 0.03 | 27.79 | 1.15 abc | 0.07 | p-Value | 0.528 | 0.031 | 0.292 | 0.003 | |
| 2 | 0.18 | 26.25 | 0.72 c | 0.18 | Pooled Std. Error | 0.015 | 0.135 | 0.070 | 0.012 | |
| 3 | 0.05 | 25.72 | 0.94 abc | 0.06 | AFB1 (ppm) | NS (%) | Means of main effect | |||
| 5 | 0.04 | 26.06 | 0.88 bc | 0.01 | 0 | 0.04 | 28.94 | 1.67 | 0.32 | |
| R2 | 0.004 | 0.141 | 0.267 | 0.152 | 5 | 0.11 | 28.03 | 1.09 | 0.19 | |
| p-Value | 0.503 | 0.417 | 0.091 | 0.466 | 0 | 0.04 | 27.50 | 1.27 | 0.17 a | |
| Pooled Std. Error | 0.015 | 0.315 | 0.091 | 0.03 | 1 | 0.09 | 28.33 | 0.82 | 0.10 a | |
| 2 | 0.20 | 29.70 | 1.56 | 0.46 b | ||||||
| ANOVA: p-Values | ||||||||||
| AFB1 | 0.494 | 0.298 | 0.205 | 0.112 | ||||||
| NS | 0.429 | 0.019 | 0.332 | 0.002 | ||||||
1 Aflatoxin B1; 2 NovaSil; 3 Values in a column that do not have the same superscript letters are significantly different according to Duncan’s multiple range test (p < 0.05); 4 Values are means of determinations on two fish from each of three replicate groups (6 fish/treatment, n = 6); 5 Muscle somatic index; 6 Hepatosomatic index; 7 Intraperitoneal fat.
In the NS-supplemented groups, muscle and IPF levels recovered to control levels in the treatment group administered 2% NS. Likewise, the means of main effect data indicate that NS inclusion at either 0% and 1% was statistically different than 2%.
2.4. Proximate Composition
No linear trends were present in the AFB1-treated groups (Table 4). Percent lipid composition was highest in the 0 ppm AFB1 group and the lowest at 2 ppm AFB1, but varied among other treatments. There were some variations in ash values as well; however, these results were not linearly correlated. Inclusion of NS in the diets did not exhibit any statistically significant changes in whole-body proximate composition.
Table 4.
Proximate composition of red drum (fresh-weight basis) 1,2.
| Variable | % Lipid | % Protein | % Moisture | % Ash | Variable | % Lipid | % Protein | % Moisture | % Ash | |
|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 (ppm) | Individual treatment means | AFB1 (ppm) | NS (%) | Individual treatment means | ||||||
| 0 | 2.70 a | 76.01 | 78.38 | 16.38 ab | 0 | 0 | 2.21 | 76.01 | 78.30 | 3.54 |
| 0.1 | 2.37 ab | 74.45 | 79.34 | 17.56 a | 5 | 0 | 1.98 | 76.52 | 79.29 | 3.73 |
| 0.25 | 1.97 bcd | 70.06 | 79.67 | 13.64 b | 5 | 1 | 2.20 | 73.92 | 76.91 | 4.28 |
| 0.5 | 2.42 ab | 74.33 | 77.69 | 16.72 ab | 5 | 2 | 2.19 | 72.93 | 79.04 | 4.35 |
| 1 | 2.17 abc | 71.64 | 78.71 | 18.04 a | p-Value | 0.510 | 0.723 | 0.173 | 0.629 | |
| 2 | 1.45 d | 74.20 | 84.55 | 19.43 a | Pooled Std. Error | 0.022 | 0.488 | 0.140 | 0.098 | |
| 3 | 1.77 cd | 71.64 | 80.60 | 17.22 a | AFB1 (ppm) | NS (%) | Means of main effect | |||
| 5 | 1.98 bcd | 76.52 | 79.29 | 18.02 a | 0 | 2.21 | 76.01 | 78.38 | 3.54 | |
| R2 | 0.211 | 0.021 | 0.024 | 0.109 | 5 | 2.12 | 74.46 | 78.41 | 4.12 | |
| p-Value | 0.002 | 0.476 | 0.452 | 0.038 | 0 | 2.10 | 76.20 | 78.83 | 3.64 | |
| Pooled Std. Error | 0.033 | 0.441 | 0.402 | 1.728 | 1 | 2.20 | 73.90 | 76.91 | 4.28 | |
| 2 | 2.19 | 72.90 | 79.04 | 4.35 | ||||||
| ANOVA: p-Values | ||||||||||
| AFB1 | 0.534 | 0.611 | 0.964 | 0.357 | ||||||
| NS | 0.394 | 0.604 | 0.095 | 0.663 | ||||||
1 Values are means of determinations on three fish from each of the three replicates (n =3). 2 Values in a column that do not have the same superscript letters are significantly different according to Duncan’s multiple range test (p < 0.05).
2.5. Histopathological Response and Immunohistochemistry
Significant histological changes were observed between treatments (Table 5), with 3 and 5 ppm AFB1 eliciting the most severe hepatic alterations. Although some samples revealed significant hepatic lesions in groups treated with 5 ppm AFB1 + 1% or 2% NS, the findings in these fish were considered mild when compared to the 5 ppm AFB1 without NS. There were no significant differences in Proliferating Cell Nuclear Antigen (PCNA) values among all treatments, nor did PCNA staining exhibit a positive linear correlation. Histological changes, characterized by restoration of hepatocellular macrovacuolation and reduced megalocytosis and karyomegaly, were noted with the addition of NS in the diet; however, these results were not statistically significant (Figure 1). A decrease in PCNA staining as compared to the 5 ppm inclusion level was noted, but also did not achieve significant levels with 1% or 2% NS inclusion in the diet (Figure 2).
Table 5.
Histopathology and immunohistochemistry.
| Variable | Histology Score 1 | PCNA | Variable | Histology Score | PCNA | |
|---|---|---|---|---|---|---|
| AFB1 (ppm) | Individual treatment means | AFB1 (ppm) | NS (%) | Individual treatment means | ||
| 0 | 5.25 a | 6.27 | 0 | 0 | 13.16 ab | 6.27 |
| 0.1 | 10.67 a | 8.59 | 5 | 0 | 19.00 b | 10.49 |
| 0.25 | 17.33 ab | 9.35 | 5 | 1 | 9.16 a | 9.11 |
| 0.5 | 30.16 c | 11.34 | 5 | 2 | 7.66 a | 9.72 |
| 1 | 25.83 bc | 9.52 | p-Value | 0.0925 | 0.7542 | |
| 2 | 31.83 c | 9.06 | Pooled Std. Error | 0.838 | 0.836 | |
| 3 | 37.00 c | 10.30 | AFB1 (ppm) | NS (%) | Means of main effect | |
| 5 | 37.00 c | 10.49 | 0 | 13.16 | 6.27 | |
| R 2 | 0.2353 | 0.0204 | 5 | 11.94 | 9.78 | |
| p-Value | 0.0001 | 0.5059 | 0 | 16.08 | 8.38 | |
| Pooled Std. Error | 1.130 | 0.815 | 1 | 9.16 | 9.11 | |
| 2 | 7.66 | 9.72 | ||||
| ANOVA: p-Values | ||||||
| AFB1 | 0.7248 | 0.3251 | ||||
| NS | 0.0491 | 0.9454 | ||||
1 Values in a column that do not have the same superscript letters are significantly different according to Duncan’s multiple; range test (p < 0.05).
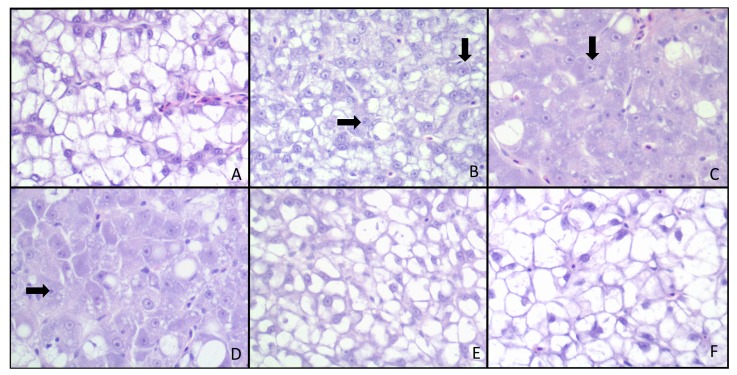
Figure 1.
Liver histopathology in AFB1-exposed red drum. Liver sections were stained with hematoxylin and eosin. Treatments were as follows: (A) 0 ppm AFB1 (B) 1 ppm AFB1 (C) 3 ppm (D) 5 ppm AFB1 (E) AFB1 + 1% NS and (F) 5 ppm AFB1 + 2% NS. Marked pleomorphism, megalokaryosis with prominent nucleoli (arrows) and loss of hepatocellular cytoplasmic macrovacuolation was observed in the treatment groups that received large amounts of aflatoxin (B,C,D). Although not significant, inclusion of NS resulted in decreased histopathological scores attributable to increased cytoplasmic vacuolation and reduced cellular pleomorphism.
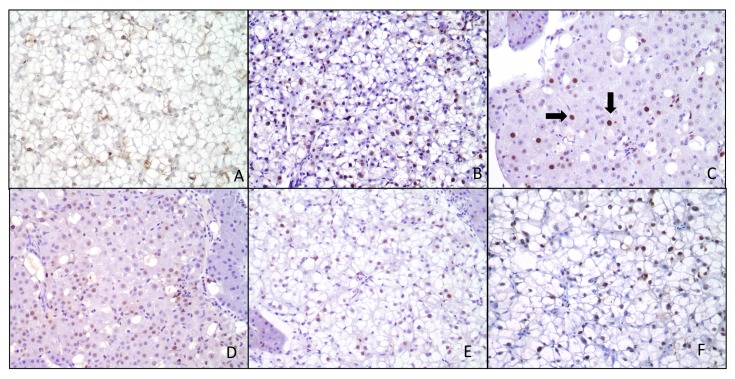
Figure 2.
Proliferating Cell Nuclear Antigen (PCNA) positive cells in red drum hepatocytes. Liver sections were stained with PCNA (arrows) and hematoxylin counterstain. Treatments were as follows: (A) 0 ppm AFB1 (B) 1 ppm AFB1 (C) 3 ppm AFB1 (D) 5 ppm AFB1 (E) 5 ppm AFB1 + 1% NS (F) 5 ppm AFB1 + 2% NS. Although not significant, inclusion of NS resulted in a decrease of PCNA-positive hepatocytes. Reduction in cell proliferation suggests that NS afforded some protection from AFB1 toxicity and cellular proliferation.
3. Discussion
Aflatoxin B1 displayed a significant effect across multiple treatment levels. The survival rate for the basal diet group (0 ppm AFB1) was similar to control survival results reported in other red drum studies [25,26], although survival was negatively affected by AFB1 presence. Likewise, the impact on feed efficiency and weight gain found in this study has been similarly documented in other AFB1-exposure publications, including research analyzing the effects of aflatoxins on several different farmed aquatic species [27,28,29,30,31]. The majority of AFB1-sensitive ichthyoids are cold-water species and our findings suggest that red drum may be one of the first identified AFB1-sensitive warm-water species. However additional studies are necessary to determine the specific metabolic mechanisms responsible for this sensitivity.
The published aquaculture literature indicates that incremental increases in AFB1 exposure do not typically result in dose-dependent, linear responses [32,33,34]. Herein, analysis of growth performance factors indicated that some of the most significant AFB1 effects were present at the lowest level of AFB1-exposure (0.1 ppm) for feed efficiency, survival, and weight gain. Hormetic responses for growth and immunological parameters have been observed in several species [35]. Hormesis is defined as a biphasic response to a xenobiotic, characterized by a low-dose stimulatory effect and high-dose inhibitory or toxic effect in which a U-shaped or J-shaped model is apparent [36]. Instances of AFB1-associated hormesis have also been documented in multiple species [32,37,38]. Several measured parameters in the current study suggest that AFB1-exposed red drum exhibited an “inverted U-shaped” immunological hormetic response to AFB1 as suggested by plasma lysozyme at the 0.1 ppm level and trypsin inhibition at the 0.25 ppm level. Additionally, HSI results indicated a similar increase at the 0.1 ppm level followed by subsequent decreases at higher AFB1 levels.
Several studies have indicated that PCNA is a suitable marker for cellular proliferation in fish [39,40] as well as other species [41,42]. However, our study did not indicate any significant differences in PCNA staining among the treatments. It is possible that the levels of AFB1 used in this study were not capable of inducing significant cellular proliferation as observed with other species. While there was a slight increase in PCNA with the presence of AFB1, there was a decrease in HSI. The increase in PCNA is due to liver damage and mitotic activity from AFB1-exposure, while the overall decrease in HSI is likely attributed to the loss of vacuolation and fat in the liver. Histological evaluation indicated liver changes characterized by anisokaryosis, megalocytosis and karyomegaly in AFB1-exposed red drum, which have been noted in a series of AFB1 studies with other fish species [43,44,45]. Hepatocellular lipid deposition, a well-documented classical sign of aflatoxicosis in fish [46,47], was present in red drum exposed to AFB1. However, red drum kept in captivity typically display fatty deposition and hepatocellular macrovacuolation [48], which should be taken into consideration for accurate red drum liver evaluation. The hepatocellular vacuolation seen in control livers was markedly reduced, as anisocytosis and karyomegaly increased, especially in fish exposed to higher levels of aflatoxin. Interestingly, hepatocellular vacuolation and liver fat were restored in fish treated with NS. Ideally, further red drum AFB1 studies should pair liver histological evaluation with other molecular markers to confirm liver damage, such as inducible nitric oxide synthase ([49] or γ-glutamyl transpeptidase [50,51]. Additionally, because feed efficiency, IPF and liver fat decreased with AFB1 exposure, it is possible that there was increased energy expenditure in these fish because less food was utilized. However, more research is needed to determine the exact mechanism of fat loss in AFB1-exposed red drum.
In this study, NS supplementation in the diets of AFB1-exposed fish resulted in a protective effect, which was evident by the significant improvement in many of the tested parameters. Other studies have reported that a 2% inclusion level of bentonite, a common clay containing montmorillonite, in trout feed reduced toxic AFB1 effects [52]. Yet other studies suggest that a 0.5% inclusion level was sufficient to protect tilapia from 1.5 ppm AFB1 [45]. Bentonites have been added into fish feed at concentrations up to 10% with no alteration in whole-body proximate composition [53]. Discrepancies in the aquaculture literature concerning the proper inclusion level of clay-based binders indicate a need to establish a clay dosing regimen for fish at risk for AFB1 exposure.
4. Materials and Methods
4.1. Experimental Diets
The control basal diet was composed of 400 g protein kg−1 and 110 g lipid kg−1, containing an estimated 3.5 kcal digestible energy kg−1 (Table 6) and fulfilling all documented nutrient requirements of red drum [54]. Aflatoxin B1 (Sigma-Aldrich, St. Louis, MO, USA) was incorporated into the diet by first dissolving the AFB1 in chloroform and subsequently adding it to Celufil, a non-nutritive bulking agent (USB Corporation, Cleveland, OH, USA). The chloroform was evaporated to dryness from the mixture in a dark room under a fume hood, leaving the Celufil amended with AFB1. A V-mixer was used to blend all dry ingredients, with the exception of the AFB1-spiked Celufil, for 20 min. The dry ingredients were then mixed with the AFB1-spiked Celufil in a Hobart mixer until homogeneity was achieved. The oil component and 700 mL of H2O were further added to the dry ingredients and mixed for 1 h. Aflatoxin-free Celufil was incorporated into the basal diet for comparison. The moist feed was cold-pelleted through a 3-mm die on a meat grinder attachment and dried in a dark room for 24 h. Diets were subsequently bagged and stored at −20 °C until needed. The ten diets contained the following: 0 ppm AFB1 (i.e., 0 ppm AFB1 + 0% NS), 0.1 ppm AFB1, 0.25 ppm AFB1, 0.5 ppm AFB1, 1 ppm AFB1, 2 ppm AFB1, 3 ppm AFB1, 5 ppm AFB1, 5 ppm AFB1 + 1% NS and 5 ppm AFB1 + 2% NS. A NS control group was not included since its safety was previously evaluated over the course of 10 weeks in a similar warm-water species [22].
Table 6.
Ingredient and proximate composition of experimental diets (g/100 g of dry weight).
| Level of AFB1 (ppm) | 0 | 0.1 | 0.25 | 0.5 | 1 | 2 | 3 | 5 | 5 | 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Level of NS (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Menhaden Meal a | 34.9 | 34.9 | 34.9 | 34.9 | 34.9 | 34.9 | 34.9 | 34.9 | 34.9 | 34.9 |
| Soybean Meal b | 27.3 | 27.3 | 27.3 | 27.3 | 27.3 | 27.3 | 27.3 | 27.3 | 27.3 | 27.3 |
| Dextrinized Starch c | 16.0 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 |
| Menhaden Oil a | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 |
| Vitamin Premix d | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Mineral Premix c | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| CMC c | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Glycine e | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Lysine e | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| NS f | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 | 2.3 |
| AFB1-spiked Celufil g | 0.0 | 0.2 | 0.7 | 1.6 | 4.5 | 0.5 | 0.8 | 1.6 | 1.6 | 1.6 |
| Celufil e | 5.5 | 5.3 | 4.8 | 3.9 | 1.0 | 5.0 | 4.7 | 3.9 | 2.8 | 1.7 |
| Proximate Composition (% dry matter) | ||||||||||
| Protein | 36.2 | 35.8 | 35.7 | 35.2 | 35.6 | 35.5 | 36.1 | 35.6 | 35.2 | 35.5 |
| Lipid | 9.5 | 9.3 | 10.3 | 10.4 | 10.7 | 10.6 | 10.6 | 10.7 | 10.7 | 11.1 |
| Dry Matter | 94.5 | 94.7 | 94.9 | 93.6 | 94.3 | 94.8 | 95.2 | 95.4 | 95.4 | 95.0 |
| Ash | 11.1 | 10.9 | 10.9 | 11.3 | 11.1 | 10.9 | 10.9 | 11.1 | 11.8 | 12.9 |
a Special Select, Omega Protein, Houston, TX, USA; b De-hulled, roasted/cooked and solvent extracted, Producers Cooperative Association, Bryan, TX; c MP Biomedicals LLC, Solon, OH; d Contains (as g kg−1): Ca(C6H10O6)·5H2O, 348.49; Ca(H2PO4)·2H2O, 136.0; FeSO4·7H2O, 5.0; MgSO4·7H2O, 132.0; K2HPO4, 240.0; NaH2PO4·H2O, 88.0; NaCl, 45.0; AlCl3 6H2O, 0.15; KI, 0.15; CuSO4·5H2O, 0.5; MnSO4·H2O, 0.7; CoCl2·6H2O, 1.0; ZnSO4·7H2O, 3.0; Na2SeO3, 0.011; e USB Corporation, Cleveland, OH; f Englehard Corporation, Jackson, MS; g Sigma-Aldrich, St. Louis, MO, USA.
4.2. Fish Stock and Culture Conditions
Fingerling red drum were transported from the Texas Parks and Wildlife hatchery located at Lake Jackson, TX to the Texas A&M Aquacultural Research and Teaching Facility. Fish were stocked and conditioned in round tanks with a commercial diet (Rangen, Inc., Angelton, TX, USA) for 2 weeks, then transferred to aquaria and conditioned for 1 week on the basal diet. A closed, re-circulating system was composed of 110 L aquaria with water flowing at 1 L/min. Biofiltration was used to maintain ammonia, nitrate and nitrite concentrations at non-toxic levels. Salinity was maintained at 7 ppt with artificial salts and water temperature was kept constant at 37 ± 2 °C by controlling air temperature in the wet laboratory. Supplemental aeration provided an adequate dissolved oxygen level of at least 80% air saturation. A 12:12 h light:dark cycle was maintained throughout the conditioning and trial period and water quality was monitored on a daily basis. Fifteen fish (2.1 ± 0.1 g) were stocked in each aquarium. The 10 dietary treatments were randomly assigned to triplicate aquaria, requiring a total of 30 tanks. Fish were fed a morning and afternoon ration over the course of 7 weeks. The diets were fed to fish beginning at a rate of 6% of the initial body weight and tapered to 3% over the span of the trial to prevent overfeeding and to approach apparent satiation. The system was monitored for mortalities and any deceased fish were immediately removed and evaluated for cause of death. With the exception of weight gain and survival, which were monitored on a weekly and daily basis, respectively, all other parameters were evaluated at the end of 7 weeks.
4.3. Fish Growth and Health Responses
Weight gain (% of initial weight), feed efficiency (g weight gain/g dry diet fed), and survival rate (% per treatment group) were calculated at the end of the trial. Two fish were sampled from each aquaria and homogenized together using a blender. Whole-body analysis was performed by evaluating moisture, ash, protein and lipid content according to previously established procedures [55]. Somatic indexes including spleen, liver (HSI), intraperitoneal (IPF) fat and muscle (MSI) were averaged based on 2 fish per aquaria (n = 6). Each somatic index was calculated as follows: (organ weight/body weight) × 100. Only the dextral side of each fish was filleted, weighed, and then doubled to obtain MSI.
4.4. Immunological Responses
Immunological parameters were evaluated including plasma lysozyme of white blood cell origin, neutrophil oxidative radical production in whole blood, and % trypsin inhibition. Two fish were randomly selected and bled from each tank, then pooled according to treatment (6 fish per treatment). A total of approximately 1–2 mL of blood was collected per treatment group using heparinized syringes. Plasma lysozyme was analyzed by employing a turbidimetric method [56,57]. Blood neutrophil oxidative radical production was measured utilizing a nitro blue tetrazolium (NBT) assay [56,58]. Plasma was also used to determine % trypsin inhibition according to a previously established method [59].
4.5. Histological Response
Livers were dissected from two fish per tank, or six per treatment. Immediately after dissection, livers were fixed in 10% formalin overnight. Livers were subsequently rinsed with 70% ethanol solution and transferred to vials containing 10 mL fresh 70% ethanol. Samples were processed and paraffin embedded within 48 h for routine histopathology at the Texas A&M Veterinary Pathobiology Histology Laboratory (College Station, TX, USA). Samples were sectioned at a thickness of 5 µm and stained with hematoxylin and eosin (H&E). Lesions were blindly examined and scored according to the criteria listed in Table 7.
Table 7.
Histological evaluation criteria.
| Score | Evaluation | Description |
|---|---|---|
| 0 | Normal | Intracytoplasmic vacuolation, mostly macrovacuolar with one of the control livers also having micro and macrovesiculation. Nuclei are small and pushed to the periphery with small nucleoli. |
| 1+ | Minimal | Scattered increase in nuclear size and mostly inconspicuous nucleoli. |
| 2+ | Mild | Mild hypertrophy and pleomorphism with slightly prominent nuclei and more evident nucleoli. Some loss of intracytoplasmic macrovacuoles, and formation of microvacuoles. |
| 3+ | Moderate | Moderate cellular pleomorphism, with anisocytosis, anisokaryosis, megalocytosis and megalokaryosis. Sparse intracytoplasmic vacuoles. |
| 4+ | Marked | Diffuse loss of cytoplasmic vacuolation, mostly solid cytoplasm. Marked pleomorphism, anisocytosis, anisokaryosis, megalocytosis and megalokaryosis. |
4.6. Immunohistochemistry
Immunohistochemistry for proliferating cell nuclear antigen (PCNA) was performed on deparaffinized sections of liver mounted on positively charged, silanized slides using an automated staining system for immunohistochemistry (Lab Vision Autostainer 360, Runcom, Cheshire, UK). Briefly, slides were placed in a heated chamber with DIVA decloaking solution (Biocare Medical LLC., Concord, CA, USA) and heated to 121 °C for antigen retrieval. The slides were incubated with a 1:200 dilution of PCNA (Fisher Scientific, Walther, MA, USA) for 20 min followed by a secondary antibody, ImmpRESS (Vector Scientific, Burlingame, CA, USA) for 30 min. The primary antibody was omitted on negative control tissues. Slides were then stained with DAB Quanto (Vector Scientific, Burlingame, CA, USA) for 5 min, followed by counterstaining with hematoxylin (Biocare Medical LLC., Concord, CA, USA) for 1.5 min. Slides were further dehydrated and mounted. Negative and positive control tissues were stained together with all fish livers. Canine and mouse small intestine, bronchial epithelium and tonsils were used as positive control tissues. All photographs were taken at 400× magnification. Stained nuclei were counted, averaged and evaluated for each treatment using CellProfiler software [60]. The percentage of PCNA positive cells ((positive/total nuclei) ×100) was calculated based on 4 fields/fish × 6 fish/treatment (24 fields/treatment).
4.7. Statistical Analysis
All statistics were computed using Statistical Analysis System (SAS) version 9.2 (SAS Institute, Cary, NC, USA). Data from groups exposed to 0–5 ppm AFB1 were subject to a general linear model regression, while 0 ppm AFB1, 5 ppm AFB1, 5 ppm AFB1 + 1% NS and 5 ppm AFB1 + 2% NS group data were subject to an incomplete factorial ANOVA for all parameters except histopathological scoring. Histopathological scores were first subject to Aligned Rank Transformation [61] and then further analyzed using a general linear model regression or incomplete factorial ANOVA. All differences among treatment means were determined using Duncan’s multiple range test. Treatment differences were considered significant at p < 0.05.
5. Conclusions
These findings indicate that red drum are susceptible to AFB1 in levels as low as 0.1 ppm. Other unevaluated species should be tested for AFB1 susceptibility, especially warm-water species raised in tropical and subtropical environments where the mycotoxin contamination risk is high. NovaSil supplementation at levels between 1%–2% may be used in fish feed safely to effectively reduce AFB1 toxicity. Therefore, this technology could be used by the aquaculture industry as a strategy to reduce aflatoxin-related morbidity and mortality in fish.
Acknowledgements
A special thank-you to Brian Ray, Manager of the Aquacultural Research and Teaching Facility, Texas A&M University. This research was funded by CONACyT 2010-020 and BASF Corporation and was conducted as part of Katherine Zychowski’s Toxicology program through the Texas A&M University Veterinary Integrative Biosciences program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cardwell K.F. Mycotoxin Contamination in Foods: Anti-Nutritional Factors; Proceedings on Improving Human Nutrition through Agriculture; Los Banos, CA, USA. 1999; pp. 27–34. [Google Scholar]
- 2.Council for Agricultural Science and Technology (CAST) Mycotoxins: Economic and Health Risks. CAST; Ames, IA, USA: 1989. [Google Scholar]
- 3.Mishra H.N., Das C. A review on biological control and metabolism of aflatoxin. Crit. Rev. Food Sci. Nutr. 2003;43:245–264. doi: 10.1080/10408690390826518. [DOI] [PubMed] [Google Scholar]
- 4.Quist C.F., Bounous D.I., Kilburn J.V., Nettles V.F., Wyatt R.D. The effect of dietary aflatoxin on wild Turkey poults. J. Wildlife Dis. 2000;36:436–444. doi: 10.7589/0090-3558-36.3.436. [DOI] [PubMed] [Google Scholar]
- 5.Florentin E.R., Cottier G.J., Diener U.L., Davis N.D. Effect of aflatoxin on different breeds and crosses of chickens. Poult. Sci. 1969;48:1807. [Google Scholar]
- 6.Jiang Y., Jolly P.E., Ellis W.O., Wang J.S., Phillips T.D., Williams J.H. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int. Immunol. 2005;17:807–814. doi: 10.1093/intimm/dxh262. [DOI] [PubMed] [Google Scholar]
- 7.Bostock J., McAndrew B., Richards R., Jauncey K., Telfer T., Lorenzen K., Little D., Ross L., Handisyde N., Gatward I. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B. 2010;365:2897–2912. doi: 10.1098/rstb.2010.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bimbo A.P., Crowther J.B. Fish meal and oil: Current uses. J. Am. Oil Chem. Soc. 1992;69:221–227. doi: 10.1007/BF02635890. [DOI] [Google Scholar]
- 9.Pauly D. The most important fish in the sea—Menhaden and America. Science. 2007;318:750–751. doi: 10.1126/science.1147800. [DOI] [Google Scholar]
- 10.Davis D.A., Jirsa D., Arnold C. Evaluation of soybean proteins as replacements for menhaden fish meal in practical diets for the red drum Sciaenops ocellatus. J. World Aquac. Soc. 1995;26:48–58. doi: 10.1111/j.1749-7345.1995.tb00208.x. [DOI] [Google Scholar]
- 11.Yue Y.R., Liu Y.J., Tian L.X., Gan L., Yang H.J., Liang G.Y. Effects of replacing fish meal with soybean meal and peanut meal on growth, feed utilization and haemolymph indexes for juvenile white shrimp Litopenaeus vannamei, Boone. Aquac. Res. 2012;43:1687–1696. doi: 10.1111/j.1365-2109.2011.02976.x. [DOI] [Google Scholar]
- 12.Li M.H., Robinson E.H., Oberle D.F., Lucas P.M., Bosworth B.G. Evaluation of corn gluten feed and cottonseed meal as partial replacements for soybean meal and corn in diets for pond-raised hybrid catfish, Ictalurus punctatus X I. furcatus. J. World Aquac. Soc. 2012;43:107–113. doi: 10.1111/j.1749-7345.2011.00542.x. [DOI] [Google Scholar]
- 13.13. Naylor R.L., Hardy R.W., Bureau D.P., Chiu A., Elliott M., Farrell A.P., Forster I., Gatlin D.M., Goldburg R.J., Hua K., et al. Feeding aquaculture in an era of finite resources. Proc.Natl. Acad. Sci. USA. 2009;106:15103–15110, 18040. doi: 10.1073/pnas.0905235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bautista M.N., Lavillapitogo C.R., Subosa P.F., Begino E.T. Aflatoxin B-1 contamination of shrimp feeds and its effect on growth and hepatopancreas of pre-adult Penaeus monodon. J. Sci. Food Agric. 1994;65:5–11. doi: 10.1002/jsfa.2740650103. [DOI] [Google Scholar]
- 15.Abdelhamid A.M., Khalil F.F., Ragab M.A. Problem of mycotoxins in fish production. Egyp. J. Nutr. Feeds. 1998;1:63–71. [Google Scholar]
- 16.Barbosa T., Pereyra C., Soleiro C., Dias E., Oliveira A., Keller K., Silva P., Cavaglieri L., Rosa C. Mycobiota and mycotoxins present in finished fish feeds from farms in the Rio de Janeiro State, Brazil. Int. Aquat. Res. 2013;5:1–9. doi: 10.1186/2008-6970-5-1. [DOI] [Google Scholar]
- 17.Phillips T., Afriyie-Gyawu E., Williams J., Huebner H., Ankrah N.A., Ofori-Adjei D., Jolly P., Johnson N., Taylor J., Marroquin-Cardona A. Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. 2008;25:134–145. doi: 10.1080/02652030701567467. [DOI] [PubMed] [Google Scholar]
- 18.Grant P.G., Phillips T.D. Isothermal adsorption of aflatoxin B-1 on hscas clay. J. Agric. Food Chem. 1998;46:599–605. doi: 10.1021/jf970604v. [DOI] [PubMed] [Google Scholar]
- 19.Marroquín-Cardona A., Deng Y., Garcia-Mazcorro J., Johnson N., Mitchell N., Tang L., Robinson A., Taylor J., Wang J.S., Phillips T. Characterization and safety of uniform particle size NovaSil clay as a potential aflatoxin enterosorbent. Appl. Clay Sci. 2011;54:248–257. doi: 10.1016/j.clay.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afriyie-Gyawu E., Mackie J., Dash B., Wiles M., Taylor J., Huebner H., Tang L.L., Guan H.X., Wang J.S., Phillips T. Chronic toxicological evaluation of dietary NovaSil clay in Sprague-Dawley rats. Food Addit. Contam. 2005;22:259–269. doi: 10.1080/02652030500110758. [DOI] [PubMed] [Google Scholar]
- 21.Mayura K., Abdel-Wahhab M.A., McKenzie K.S., Sarr A.B., Edwards J.F., Naguib K., Phillips T.D. Prevention of maternal and developmental toxicity in rats via dietary inclusion of common aflatoxin sorbents: Potential for hidden risks. Toxicol. Sci. 1998;41:175–182. doi: 10.1093/toxsci/41.2.175. [DOI] [PubMed] [Google Scholar]
- 22.Zychowski K.E., Pohlenz C., Mays T., Romoser A., Hume M., Buentello A., Gatlin Iii D.M., Phillips T.D. The effect of NovaSil dietary supplementation on the growth and health performance of Nile tilapia (Oreochromis niloticus) fed aflatoxin-B1 contaminated feed. Aquaculture. 2013;376–379:117–123. doi: 10.1016/j.aquaculture.2012.11.020. [DOI] [Google Scholar]
- 23.Peters K.M., McMichael R.H., Jr. Early life history of the red drum, Sciaenops ocellatus (Pisces: Sciaenidae), in Tampa Bay, Florida. Estuaries. 1987;10:92–107. doi: 10.2307/1352173. [DOI] [Google Scholar]
- 24.Food and Agriculture Organization (FAO) Cultured Aquatic Species Information Programme: Sciaenops ocellatus. [(accessed on 2 May 2013)]. Available online: http://www.fao.org/fishery/en.
- 25.Ellis S.C., Reigh R.C. Effects of dietary lipid and carbohydrate levels on growth and body composition of juvenile red drum, Sciaenops ocellatus. Aquaculture. 1991;97:383–394. doi: 10.1016/0044-8486(91)90330-A. [DOI] [Google Scholar]
- 26.McGoogan B., Gatlin D. Effects of replacing fish meal with soybean meal in diets for red drum sciaenops ocellatus and potential for palatability enhancement. J. World Aquac. Soc. 1997;28:374–385. doi: 10.1111/j.1749-7345.1997.tb00284.x. [DOI] [Google Scholar]
- 27.Panangala V.S., Giambrone J.J., Diener U.L., Davis N.D., Hoerr F.J., Mitra A., Schultz R.D., Wilt G.R. Effects of aflatoxin on the growth performance and immune responses of weanling swine. Am. J. Vet. Res. 1986;47:2062–2067. [PubMed] [Google Scholar]
- 28.Dalvi R. An overview of aflatoxicosis of poultry: Its characteristics, prevention and reduction. Vet. Res. Commun. 1986;10:429–443. doi: 10.1007/BF02214006. [DOI] [PubMed] [Google Scholar]
- 29.Deng S.X., Tian L.X., Liu F.J., Jin S.J., Liang G.Y., Yang H.J., Du Z.Y., Liu Y.J. Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquaculture. 2010;307:233–240. doi: 10.1016/j.aquaculture.2010.07.029. [DOI] [Google Scholar]
- 30.Anh Tuan N., Grizzle J.M., Lovell R.T., Manning B.B., Rottinghaus G.E. Growth and hepatic lesions of Nile Tilapia (Oreochromis niloticus) fed diets containing aflatoxin B1. Aquaculture. 2002;212:311–319. doi: 10.1016/S0044-8486(02)00021-2. [DOI] [Google Scholar]
- 31.Santacroce M.P., Conversano M., Casalino E., Lai O., Zizzadoro C., Centoducati G., Crescenzo G. Aflatoxins in aquatic species: Metabolism, toxicity and perspectives. Rev. Fish Biol. Fish. 2008;18:99–130. doi: 10.1007/s11160-007-9064-8. [DOI] [Google Scholar]
- 32.Han D., Xie S., Zhu X., Yang Y., Guo Z. Growth and hepatopancreas performances of gibel carp fed diets containing low levels of aflatoxin B1. Aquac. Nutr. 2010;16:335–342. [Google Scholar]
- 33.Raghavan P.R., Zhu X., Lei W., Han D., Yang Y., Xie S. Low levels of Aflatoxin B1 could cause mortalities in juvenile hybrid sturgeon, Acipenser ruthenus ♂×A. baeri♀. Aquac. Nutr. 2011;17:e39–e47. doi: 10.1111/j.1365-2095.2009.00725.x. [DOI] [Google Scholar]
- 34.Cagauan A.G., Tayaban R.H., Somga J.R., Bartolome R.M. Effect of Aflatoxin-Contaminated Feeds in Nile Tilapia (Oreochromis Niloticus L.); Proceedings of 6th International Symposium on Tilapia in Aquaculture (ISTA 6) Section: Health Management and Diseases; Manila, Philippines. 2004. [Google Scholar]
- 35.Calabrese E.J. Hormetic dose-response relationships in immunology: Occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit. Rev. Toxicol. 2005;35:89–295. doi: 10.1080/10408440590917044. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese E.J. Hormesis: A revolution in toxicology, risk assessment and medicine. EMBO Rep. 2004;5:S37–S40. doi: 10.1038/sj.embor.7400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz G., Calabrese E., Blain R. Aflatoxicosis in chickens (Gallus gallus): An example of hormesis? Poult. Sci. 2008;87:727–732. doi: 10.3382/ps.2007-00403. [DOI] [PubMed] [Google Scholar]
- 38.Chávez-Sánchez M.C., Martínez Palacios C.A., Osorio Moreno I. Pathological effects of feeding young Oreochromis niloticus diets supplemented with different levels of aflatoxin B1. Aquaculture. 1994;127:49–60. doi: 10.1016/0044-8486(94)90191-0. [DOI] [Google Scholar]
- 39.Ostrander G.K., Blair J.B., Stark B.A., Marley G.M., Bales W.D., Veltri R.W., Hinton D.E., Okihiro M., Ortego L.S., Hawkins W.E. Long-term primary culture of epithelial cells from rainbow trout (Oncorhynchus mykiss) liver. In Vitro Cell. Dev. Biol. Anim. 1995;31:367–378. doi: 10.1007/BF02634286. [DOI] [PubMed] [Google Scholar]
- 40.Borucinska J.D., Schmidt B., Tolisano J., Woodward D. Molecular markers of cancer in cartilaginous fish: Immunocytochemical study of PCNA, p-53, myc and ras expression in neoplastic and hyperplastic tissues from free ranging blue sharks, Prionace glauca (L.) J. Fish Dis. 2008;31:107–115. doi: 10.1111/j.1365-2761.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 41.Banlunara W., Bintvihok A., Kumagai S. Immunohistochemical study of proliferating cell nuclear antigen (PCNA) in duckling liver fed with aflatoxin B1 and esterified glucomannan. Toxicon. 2005;46:954–957. doi: 10.1016/j.toxicon.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Oznurlu Y., Celik I., Sur E., Ozaydin T., Oguz H., Altunbas K. Determination of the effects of aflatoxin B-1 given in ovo on the proximal tibial growth plate of broiler chickens: Histological, histometric and immunohistochemical findings. Avian Pathol. 2012;41:469–477. doi: 10.1080/03079457.2012.712673. [DOI] [PubMed] [Google Scholar]
- 43.Bailey G.S., Williams D.E., Wilcox J.S., Loveland P.M., Coulombe R.A., Hendricks J.D. Aflatoxin B1 carcinogenesis and its relation to DNA adduct formation and adduct persistence in sensitive and resistant salmonid fish. Carcinogenesis. 1988;9:1919–1926. doi: 10.1093/carcin/9.11.1919. [DOI] [PubMed] [Google Scholar]
- 44.Mohapatra S., Sahu N.P., Pal A.K., Prusty A.K., Kumar V., Kumar S. Haemato-immunology and histo-architectural changes in Labeo rohita fingerlings: Effect of dietary aflatoxin and mould inhibitor. Fish Physiol. Biochem. 2011;37:177–186. doi: 10.1007/s10695-010-9428-1. [DOI] [PubMed] [Google Scholar]
- 45.Hassan A.M., Kenawy A.M., Abbas W.T., Abdel-Wahhab M.A. Prevention of cytogenetic, histochemical and biochemical alterations in Oreochromis niloticus by dietary supplement of sorbent materials. Ecotoxicol. Environ. Saf. 2010;73:1890–1895. doi: 10.1016/j.ecoenv.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton P., Garlich J. Aflatoxin as a possible cause of fatty liver syndrome in laying hens. Poult. Sci. 1971;50:800–804. doi: 10.3382/ps.0500800. [DOI] [PubMed] [Google Scholar]
- 47.Rogers A.E., Newberne P.M. Diet and aflatoxin B1 toxicity in rats. Toxicol. Appl. Pharmacol. 1971;20:113–121. doi: 10.1016/0041-008X(71)90095-0. [DOI] [PubMed] [Google Scholar]
- 48.Tucker J.W., Jr., Lellis W.A., Vermeer G.K., Roberts D.E., Jr., Woodward P.N. The effects of experimental starter diets with different levels of soybean or menhaden oil on red drum (Sciaenops ocellatus) Aquaculture. 1997;149:323–339. doi: 10.1016/S0044-8486(96)01448-2. [DOI] [Google Scholar]
- 49.Karaman M., Ozen H., Tuzcu M., Cigremis Y., Onder F., Ozcan K. Pathological, biochemical and haematological investigations on the protective effect of α-lipoic acid in experimental aflatoxin toxicosis in chicks. Br. Poult. Sci. 2010;51:132–141. doi: 10.1080/00071660903401839. [DOI] [PubMed] [Google Scholar]
- 50.Godlewski C.E., Boyd J.N., Sherman W.K., Anderson J.L., Stoewsand G.S. Hepatic glutathione S-transferase activity and aflatoxin B1-induced enzyme altered foci in rats fed fractions of brussels sprouts. Cancer Lett. 1985;28:151–157. doi: 10.1016/0304-3835(85)90070-9. [DOI] [PubMed] [Google Scholar]
- 51.Hanigan M.H., Pitot H.C. Gamma-glutamyl transpeptidase—Its role in hepatocarcinogenesis. Carcinogenesis. 1985;6:165–172. doi: 10.1093/carcin/6.2.165. [DOI] [PubMed] [Google Scholar]
- 52.Ellis R., Clements M., Tibbetts A., Winfree R. Reduction of the bioavailability of 20 μg/kg aflatoxin in trout feed containing clay. Aquaculture. 2000;183:179–188. doi: 10.1016/S0044-8486(99)00292-6. [DOI] [Google Scholar]
- 53.Eya J.C., Parsons A., Haile I., Jagidi P. Effects of dietary zeolites (bentonite and mordenite) on the performance juvenile rainbow trout onchorhynchus myskis. Austr. J. Basic Appl. Sci. 2008;2:961–967. [Google Scholar]
- 54.Gatlin D.M. Red Drum Sciaenops Ocellatus. In: Webster C.D., Lim C., editors. Nutrient Requirements and Feeding of Fish for Aquaculture. CABI Publishing; Wallingford, Oxon, UK: 2002. pp. 147–158. [Google Scholar]
- 55.Webb K.A., Gatlin D.M. Effects of dietary protein level and form on production characteristics and ammonia excretion of red drum sciaenops ocellatus. Aquaculture. 2003;225:17–26. doi: 10.1016/S0044-8486(03)00274-6. [DOI] [Google Scholar]
- 56.Li P., Gatlin D.M. Evaluation of brewers yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysops × M-saxatilis) Aquaculture. 2003;219:681–692. doi: 10.1016/S0044-8486(02)00653-1. [DOI] [Google Scholar]
- 57.Jorgensen J.B., Sharp G.J.E., Secombes C.J., Robertsen B. Effect of a yeast-cell-wall glucan on the bactericidal activity of rainbow-trout macrophages. Fish Shellfish Immunol. 1993;3:267–277. doi: 10.1006/fsim.1993.1026. [DOI] [Google Scholar]
- 58.Siwicki A.K., Anderson D.P., Rumsey G.L. Dietary intake of immunostimulants by rainbow trout affects nonspecific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994;41:125–139. doi: 10.1016/0165-2427(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 59.Lange S., Guđmundsdottir B.K., Magnadottir B. Humoral immune parameters of cultured atlantic halibut (Hippoglossus Hippoglossus) Fish Shellfish Immunol. 2001;11:523–535. doi: 10.1006/fsim.2000.0333. [DOI] [PubMed] [Google Scholar]
- 60.Lamprecht M.R., Sabatini D.M., Carpenter A.E. Cellprofiler™: Free, versatile software for automated biological image analysis. Biotechniques. 2007;42:71. doi: 10.2144/000112257. [DOI] [PubMed] [Google Scholar]
- 61.Wobbrock J.O., Findlater L., Gergle D., Higgins J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only Anova Procedures; Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI '11); Vancouver, Canada. 7–12 May 2011; pp. 143–146. [Google Scholar]