Abstract
Observing the actions of others has been shown to affect motor learning, but does it have effects on sensory systems as well? It has been recently shown that motor learning that involves actual physical practice is also associated with plasticity in the somatosensory system. Here, we assessed the idea that observational learning likewise changes somatosensory function. We evaluated changes in somatosensory function after human subjects watched videos depicting motor learning. Subjects first observed video recordings of reaching movements either in a clockwise or counterclockwise force field. They were then trained in an actual force-field task that involved a counterclockwise load. Measures of somatosensory function were obtained before and after visual observation and also following force-field learning. Consistent with previous reports, video observation promoted motor learning. We also found that somatosensory function was altered following observational learning, both in direction and in magnitude, in a manner similar to that which occurs when motor learning is achieved through actual physical practice. Observation of the same sequence of movements in a randomized order did not result in somatosensory perceptual change. Observational learning and real physical practice appear to tap into the same capacity for sensory change in that subjects that showed a greater change following observational learning showed a reliably smaller change following physical motor learning. We conclude that effects of observing motor learning extend beyond the boundaries of traditional motor circuits, to include somatosensory representations.
Keywords: observational learning, somatosensory plasticity, motor learning, force-field learning
observing others while they learn a motor task has been shown to engage the motor system and to result in reliable changes to motor learning. Here, we assess the possibility that the effects of observing motor learning are not solely confined to the motor system but spread as well to somatosensory representations. We show that there are changes to sensed limb position following observational learning that are similar to those that occur following actual motor learning.
There have been a number of demonstrations that motor learning can occur even in the absence of overt physical practice, as is the case of when one observes motor learning. A series of studies (Brown et al. 2009; Mattar and Gribble 2005) have shown that subjects who observed a video depicting another person learning to reach in a novel mechanical environment performed better when later tested in the same environment than subjects who observed similar movements that did not involve learning. Similarly, the observation of another individual performing repetitive thumb movements has been shown to alter both the movements and the motor potentials evoked from the stimulation of motor cortex (Stefan et al. 2005). Several studies have shown that similar brain networks are activated during the observation and execution of movement, in particular, ventral premotor cortex and supplementary motor area and inferior parietal lobule and superior temporal sulcus (see Kilner 2011 for review).
Several studies have also shown that motor learning is accompanied by adaptation in sensory systems. Learning tasks involving arm movements have been shown to change attributes of sensory function such as sensed limb position (Cressman and Henriques 2009; Haith et al. 2008; Ostry et al. 2010) and perceptual acuity (Wong et al. 2011). At the neural level, a network has been identified that is associated with the perceptual changes that occur in conjunction with motor learning. This comprises second somatosensory cortex, ventral premotor cortex, and supplementary motor area (Vahdat et al. 2011).
Taken together, these observations raise the possibility that changes in sensory perception could be triggered not only by actual motor learning, but also by observing someone else engaged in a motor-learning task. We tested this hypothesis by assessing somatosensory perception before and after a task that involved observation of motor learning. The test involved two groups of subjects that watched a video depicting an actor learning to reach in a novel mechanical environment. The direction of the perturbation applied to the actor's arm was opposite for the two groups. We found that watching someone else learn not only affected the characteristics of motor learning, but also was associated with changes in somatosensory perception. Moreover, depending on the direction of the force field during the observed learning, the two groups showed changes in sensory perception in opposite directions. The perceptual changes observed here are in the same direction as those previously described following actual motor learning. We conclude that observational learning has effects that spread beyond motor circuits of the brain and contributes to plasticity in sensory systems.
METHODS
Subjects and experimental tasks.
Twenty-eight subjects of either sex were randomly assigned to two experimental conditions (n = 14 each; mean age ± standard deviation: 20.2 ± 2.5). The conditions differed only in terms of the direction of the force field observed in the video recording (see below). An additional group of 14 subjects (mean age ± standard deviation: 21.4 ± 3.1) was recruited and assigned to a scrambled-video control condition (see below). The subjects were all right-handed and reported no history of sensorimotor disorders. All procedures were approved by the McGill University Research Ethics Board.
Subjects were tested individually in a single session lasting 2 h. The session comprised perceptual tests, reaching movements, and video observation (Fig. 1). In all tasks, subjects held the handle of a two degree-of-freedom planar robotic arm with their right hand (InMotion2; Interactive Motion Technologies). Subjects were seated, and, in conditions involving movement, the arm movements occurred in a horizontal plane at shoulder height. Vision of the arm was blocked.
Fig. 1.
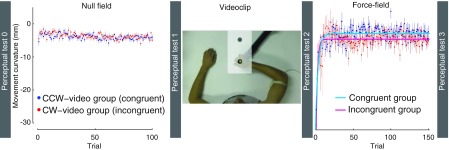
Sequence of procedures and experimental data showing changes in movement curvature [lateral deviation at maximum velocity (PDmaxv)] during training averaged across subjects (±SE). Subjects that observed and practiced movements in a counterclockwise (CCW) field are in blue (congruent group, N = 14). Subjects that observed a CW field and then trained with a CCW load are in red (incongruent group, N = 14). The cyan and magenta lines show exponential fits to the data for the congruent and incongruent groups, respectively.
At first, subjects were familiarized with the perceptual test and the reaching task. Afterward, the experiment began with a baseline estimate of sensed limb position (Perceptual test 0). Subjects then performed 100 straight-out reaching movements during which the robot applied no force to the hand (null condition). Immediately following null-field training, another baseline estimate of sensed limb position was obtained (Perceptual test 1). Subjects were subsequently asked to watch a video recording of another individual performing reaching movements in a velocity-dependent force field (see below). Following the video observation, another estimate of sensed limb position was taken (Perceptual test 2). Finally, subjects made 150 movements straight-out from the body, in a velocity-dependent force field, followed by a final estimate of sensed limb position (Perceptual test 3). Subjects were naïve with regard to the purpose of the study and received no information about the force applied by the robot in any stage of the experiment.
Perceptual judgments.
Subject's perception of the boundary between left and right was estimated using an adaptive procedure (Parameter Estimation by Sequential Testing, PEST; Taylor and Creelman 1967), as described previously (Ostry et al. 2010; Vahdat et al. 2011). The perceptual tests were conducted with the eyes closed. The robot was programmed to move the subjects' hand outward from a start position following a fork-shaped trajectory (Fig. 2A). Subjects were instructed not to resist the action of the robot. At the end of each movement, the subjects' hand was either to the left or the right of the midline, by an amount that was computed on a trial-by-trial basis. When the robot reached its final position, subjects were asked to indicate whether the hand had been moved to the left or to the right. The sagittal plane movement amplitude in the perceptual tests was 15 cm for all trials. The lateral displacement on the first movement of each run was randomly selected from a uniform distribution with values ranging from 20 to 30 mm (in both directions). All participants were able to discriminate correctly the direction of the first arm deflection. On the next trial, the deflection was reduced by 10 mm, and this was repeated on successive trials until the subject reported a change in the direction of lateral displacement. At this point, we reduced the step size by half, and the next displacement was in the opposite direction. The algorithm terminated whenever the step size for the upcoming movement fell <1 mm. Thus, on each trial, the magnitude of the lateral deviation of the hand was modified in an adaptive manner (Taylor and Creelman 1967) until an estimate of the perceived boundary between left and right was obtained. Each block of perceptual tests involved six runs. Occasionally, four runs were collected if the perceptual estimates converged slowly. This procedure yielded a corresponding number of estimates of the right-left boundary. On successive runs, the initial displacement direction alternated between left and right.
Fig. 2.
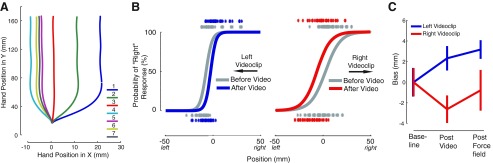
Assessment of somatosensory function. A: representative hand paths during perceptual tests. The color code gives the trial number in the testing sequence. B: fitted psychometric functions for 2 representative subjects showing perceptual classification before (gray) and after (red or blue) observational force-field learning. As in previous studies of force-field learning with physical practice, following motor learning by observing the perceptual boundary shifts in a direction opposite to the observed-applied force. C: mean change (±SE) in the perceptual boundary (bias) following observational motor learning and following actual motor learning for observation of a CCW (blue; N = 14) or CW (red; N = 14) force field. For visualization purposes, the 2 groups have been aligned at baseline (Perceptual test 1).
To exclude the possibility of perceptual changes related to active motor outflow (force production) during the perceptual testing phase, we measured the average lateral force applied by the subjects to the robot handle during the final trial of each PEST run. This is the trial in which the PEST algorithm converges, providing an estimate of the perceptual boundary. To calculate this force, we took the difference between the sensed force in the 500 ms before and the final 500 ms of the plateau phase of servo displacement. The average displacement for the arm in this time window was measured as 4.4 mm, and the average lateral force, across all subjects and all conditions, was 1.14 N (∼110 g). A force of this magnitude would be expected simply due to the passive stiffness of the arm. This is consistent with the idea that active force production was not a significant factor in the obtained perceptual estimates.
Reaching movements.
In the dynamics-learning task, subjects made reaching movements to a single visual target. The start point was situated in the center of the workspace, ∼25 cm from the subject's chest along the body midline. The target was located 15 cm directly in front of the start position in the sagittal plane. The start and target positions were represented by white circles, 20 mm in diameter. A yellow circle, 12 mm in diameter, provided the subject with visual feedback on the current position of the hand. Note that visual feedback was present during reaching movements and was not provided during the perceptual testing phase. Subjects were also asked to move as straight as possible. Visual feedback of movement duration was provided at the end of each reaching movement by a target color change. The feedback was used to help subjects achieve the desired movement duration, but no trials were removed from analysis if subjects failed to comply with the speed requirement. At the end of each trial, the robot returned the subject's hand to the start position. In the force-field-learning phase, the robot applied a counterclockwise (CCW) load to the hand that primarily acted to deflect the limb to the left. The force was applied to the hand according to the following equation:
where x and y are the lateral and sagittal directions, fx and fy are the commanded force to the robot in Newtons, vx and vy are hand velocities in Cartesian coordinates in meters per second, and D defines the direction of the force field. For the CCW force field, D is −1.
Video recordings.
Video recordings provided subjects with a screen-centered, top-down view of another individual's right arm and the workspace within which movements to the target were made. The recording depicted an individual moving to the target as the robot applied a perturbing force to the arm. In the CCW video recording, the forces were the same as those later experienced by the observer (congruent group); in the CW video recording, the forces applied in the observational phase were opposite to those later experienced by the observer (incongruent group). These recordings showed the progression from highly perturbed to straight movements typically associated with motor learning. Superimposed on the video image were images of the visual target and a cursor representing the position of the hand (Fig. 1). Each recording was ∼3 min in duration and demonstrated a series of 28 movements from the beginning of the force-field training sequence.
A 3rd video was developed for the control experiment. This video comprised the 28 original movements from the CCW video that we used for the congruent group, but in this case the movements were presented in random order. The order was further edited to minimize information potentially relevant to learning. Thus high-error movements were not presented in the first three trials, repetitive sequences of low-error movements were not presented at the end, and homogeneous blocks of high- or low-error trials were avoided.
All video presentations were repeated five times. The subject's task was to observe attentively. No mention was made of the forces applied. To ensure that subjects paid attention to the video recordings, we asked them to monitor the depicted movements and report to the experimenter when movements made by the subject in the video were too fast or slow, as indicated by the targets changing color. We found that subjects were highly accurate (mean score > 90% correct), which provides support for the idea that adequate attention was given to the observational phase of the experiment. During observation, subjects were instructed to keep hold of the robot handle, which was positioned to correspond to the starting position of the actor on the screen.
Data analysis.
The data from all perceptual runs in each phase of the experiment were used to estimate the perceived boundary between left and right. The entire set of measured lateral deviations and associated binary responses were fitted on a per-subject basis with a logistic function that gave the probability of responding “the hand was deflected to the right” as a function of the lateral position of the hand. We used a least-squares error criterion (glmfit in MATLAB) to obtain the fit. The 50% point of the fitted function was taken as the perceptual boundary and used for purposes of statistical analysis.
We assessed motor learning by calculating the perpendicular deviation of the hand from a straight line connecting the start point and the target at the movement peak velocity (PDmaxv). We assessed the change in PDmaxv over trials by fitting a single exponential function to the data averaged across subjects. The equation takes the form P = c − ae−bn, where P is the PDmaxv on trial n. This function is well-approximated in the discrete domain by P(n) = c − a(1 − b)n, where b is the rate of learning and c is the asymptotic performance level. Separate fits were conducted for subjects that experienced force fields congruent with their visual observation and those for which the force-field training was incongruent.
To investigate further the potential effects of the video recording on motor performance, we also computed the perpendicular deviation of the hand from the same straight line in an early stage of the movement, 100 ms following movement onset (PD100). This particular variable was chosen for this test because it minimizes the likelihood of feedback-based corrections in limb trajectory measures. For both PDmaxv and PD100, we quantified motor learning as the difference in movement curvature between the final 5 and the 1st 5 movements in the force-field condition. In addition to mean movement curvature, we evaluated the between-subjects variability of motor performance in the 1st part (10 movements) of the force-field-learning task.
Changes in somatosensory perception were evaluated statistically using ANOVA. To compare motor learning in subjects that viewed CW vs. CCW force-field-learning videos, we employed independent-samples t-tests. Differences in the variability of motor performance were assessed by using Bartlett's test. The two groups showed similar baseline estimates of sensed limb position, and no differences were found in the two baselines in either of the two groups (P > 0.1). The second baseline was therefore taken as the reference point for subsequent analyses.
RESULTS
Participants were tested for somatosensory perception at the beginning of the experimental session as well as at several points in the experimental sequence: following reaching movements in the absence of any mechanical load (null condition), following video observation, and following force-field learning (Fig. 1).
Figure 2B shows estimates of sensed limb position obtained for the two video observation conditions. It is seen that there are shifts in sensed limb position that vary with the pattern of force-field learning observed in the video. In both cases, there is a shift in the perceptual boundary in a direction opposite to the observed force. Thus subjects that watched a video of adaptation to a rightward force field showed a leftward shift in the perceptual boundary and vice versa. This same pattern of perceptual change is observed under actual force-field-learning conditions. When subjects were subsequently required to train under actual force-field conditions, further perceptual change was observed. For subjects in which the force field was congruent with the observed learning, we saw a further shift in the perceptual boundary, in the same direction as that obtained during observational learning. In contrast, when the learned force field was incongruent with the observed learning, the subsequent perceptual shift was in the direction one would expect on the basis of the mechanical load (and resulted in the elimination of the previous perceptual change). These effects are summarized in Fig. 2C, which shows changes in the perceptual boundary relative to the pre-video-clip baseline.
To test the hypotheses of the study, we designed statistical analyses that could assess the specific effect of each manipulation separately. ANOVA was therefore employed to assess perceptual change following video observation (Perceptual test 2 − 1) and following actual force-field learning (Perceptual test 3 − 2). ANOVA revealed that the pattern of perceptual changes differed for subjects in the congruent and incongruent experimental conditions [F(1,27) = 5.75, P < 0.03]. Following video observation, sensed limb position was different for participants who watched the CW video clip and those who watched the CCW video clip (post hoc comparison: P < 0.01). Watching opposite forces led to opposite changes in sensed limb position. The absolute change in sensed limb position due to video observation was reliably different from zero [t(27) = 2.82, P < 0.01].
The force-field learning followed video observation and resulted in changes in sensed limb position that were in the same direction and of similar magnitude in the two groups (P > 0.4). When comparing this change in perception with the previous change, following video-clip observation, differences emerged for the two groups. The group that watched a CW force in the video clip and then experienced a force field in the opposite, CCW direction (shown in red in Fig. 2C), showed a significant difference in perceptual change scores (post hoc comparison: P < 0.02). In particular, whereas the CW video clip resulted in a leftward shift in the perceptual boundary, subsequent training in a CCW field served to create a perceptual change in the opposite direction. In contrast, for the group who first watched and then experienced a CCW force field (shown in blue in Fig. 2C), both manipulations resulted in rightward shifts in the perceptual boundary. The increased shift in the rightward direction was not reliably different in magnitude from that which occurred due to visual observation alone (P > 0.4).
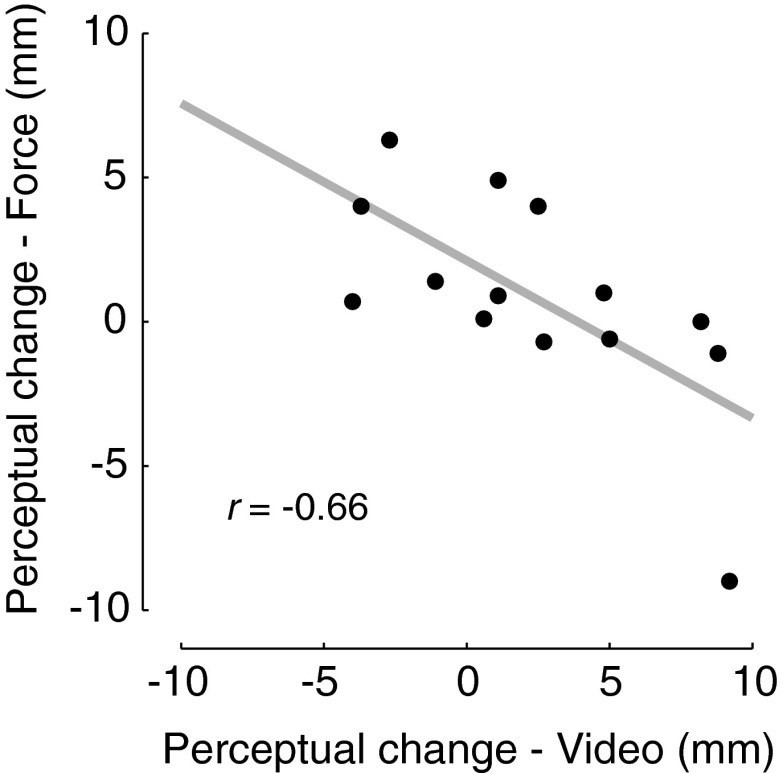
We assessed whether the change in sensed limb position following actual force-field learning was related to that experienced following video observation. For the group that observed and experienced forces that acted in the same direction (CCW), a highly significant inverse relationship was observed (Fig. 3). Subjects that experienced larger changes in sensed limb position following video observation had smaller subsequent changes following force-field learning [r(13) = −0.66, P < 0.01]. Subjects that watched learning in one direction and then trained in an opposite force field showed no reliable correlation in changes in sensed limb position due to the video and the actual force field [r(13) = 0.29, P > 0.3].
Fig. 3.
For subjects that both observed and practiced a force field in a CCW direction (N = 14), the amount of shift in the perceptual boundary following observational learning is inversely correlated with the change following actual motor learning.
We compared changes in sensory perception following observational learning with those reported previously in the context of actual force-field learning. For this analysis, we used the data from a previous study (Vahdat et al. 2011) in which we used a similar experimental protocol (with n = 13) and the same perceptual testing procedure as employed here. The analysis focused on changes in sensed limb position in the perceptual tests that were conducted following the primary experimental manipulation, that is, immediately following actual vs. observational learning. A comparison of the two data sets revealed no differences in the magnitudes of perceptual change between the observational and physical learning conditions [t(25) = 0.65, P > 0.5]. However, sensory change following actual motor learning showed significantly less between-subjects variability compared with motor learning by observing [t(12) = 10.51, P < 0.002].
All subjects were tested for motor learning using a CCW force field. Subjects who had previously watched a movie showing a CCW force (congruent condition) showed better performance in the motor-learning task than subjects that watched a CW force field (incongruent directions; Fig. 1). Asymptotic performance [mean ± 99% confidence interval (CI)] based on exponential fits to the PDmaxv was reliably better for subjects in the congruent (−2.3 ± 0.34 mm) than in the incongruent (−4.3 ± 0.4 mm) group. The overall goodness of fit was similar in the two groups (r2 = 0.69 and 0.61, for congruent and incongruent conditions, respectively).
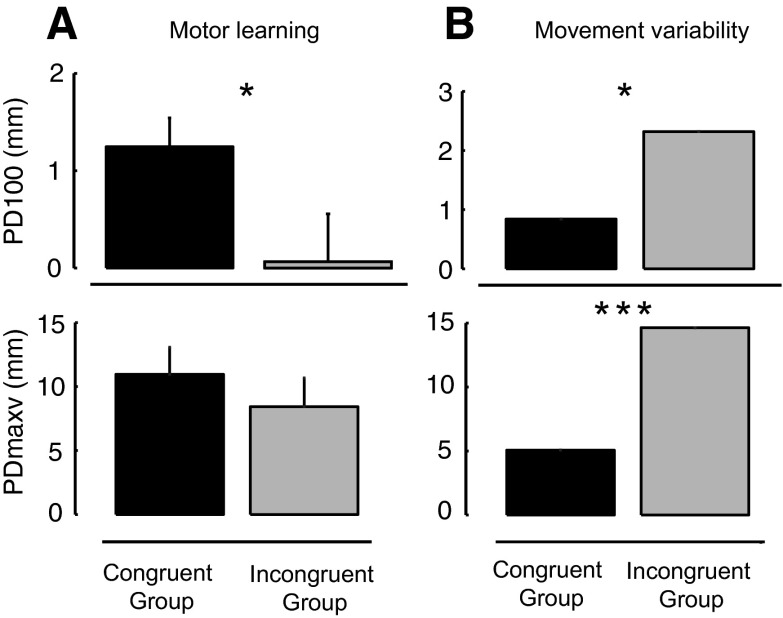
We also evaluated the lateral deviation of the limb at PD100. Figure 4 shows that the amount of learning (that is, the decrease following learning in the magnitude of lateral deviation 100 ms into the reaching movement) was greater for subjects who experienced the same force in the observational and actual learning tasks [t(26) = 2.16, P < 0.05]. Measures of PDmaxv showed similar patterns, although the difference was not statistically reliable. We observed differences in variability of movement between the two conditions as well. The group exposed to a congruent force in the observational and actual learning task showed less variability in movements in the initial motor-learning trials [PDmaxv: t(13) = 12.64, P < 0.001; PD100: t(13) = 6.49, P < 0.02].
Fig. 4.
Assessment of motor learning. A: subjects that observed and practiced a force field in the same direction (congruent group, N = 14) show greater motor learning than the group that observed and practiced force fields in opposite directions (incongruent group, N = 14). PD100 gives measures of lateral deviation 100 ms into the movement. For both PD100 and PDmaxv, motor learning is expressed as the mean difference in deviation scores between the last 5 and 1st 5 trials (±SE). B: the congruent group shows less variable movements at the beginning of the force-field task compared with the incongruent group. Variability is expressed as standard deviation across subjects in millimeters. *P < 0.05; ***P < 0.001.
The results show that video observation produces reliable changes in both sensed limb position and in motor performance. However, it is unclear whether the effects depend specifically on the observation of learning or whether they are attributable to the statistical distribution of the events in the visual display. In particular, the video clips show trajectories that are curved in a single direction, to the left for the CCW video clip and to the right for the CW clip. Thus it is possible that the asymmetric distribution of the visual input, rather than the observation of learning, biases subjects toward one side of the workspace, thus producing changes in sensed limb position.
As a control, we tested a further group of subjects that were exposed to the same CCW video clip employed before, except that in this case the order of the movements in the video was randomized. In this way, the overall visual information presented to subjects in the two experiments was the same. However, the video sequence did not show learning but rather a random mixture of high- and low-error trials. If the distributional properties of the visual input are sufficient to induce the effects described above, we would expect subjects to show a pattern of change in sensed limb position similar to that observed for subjects in the congruent condition. A comparable level of motor learning should also be observed.
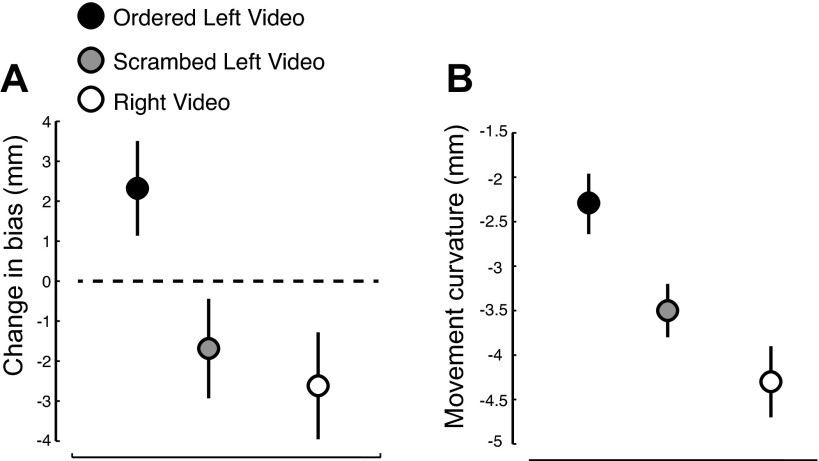
Figure 5A shows estimates of sensed limb position for the scrambled CCW video observation condition along with the data from the original video clips. The change in sensed limb position due to scrambled CCW video observation was not reliably different from zero [t(13) = −1.35, P > 0.19], with half of the sample showing changes in one direction and half in the other. Indeed, the overall pattern was opposite to that of the CCW video group and not significantly different from the pattern of the CW video group (t = −0.51, P > 0.6).
Fig. 5.
Watching a video in which the order of the movements was randomized resulted in no change in somatosensory perception and a reduced benefit to motor learning. A: mean perceptual change (±SE) following observation of a standard-order CCW video (black; N = 14), a scrambled-order CCW video (gray; N = 14), or a standard-order CW video (white; N = 14). B: asymptotic performance (±99% confidence interval) in force-field-learning trials for the same 3 groups derived from exponential fits to the motor-learning data (PDmaxv).
We conducted further tests for changes to sensed limb position following video observation using bootstrap procedures (bootstrp and bootci in MATLAB). We estimated the 95% CI for the mean change in sensed limb position (bias) following observation of the video clip in the CCW, CW, and the scrambled CCW video control condition, using 100,000 iterations each. For each of the 2 experimental groups, the estimated change in sensed limb position was reliably different from 0 [CI: (0.0002, 0.0046) for the CCW video group, (−0.0053, −0.0003) for the CW video group]. This was not the case for the scrambled CCW video control condition [CI: (−0.0042, 0.0005)].
Figure 5B shows motor-learning data for the scrambled CCW video group along with learning data for the two original groups of subjects. Subjects in the scrambled CCW video group exhibited asymptotic levels of motor learning that were intermediate to the two other groups. Asymptotic performance based on exponential fits to the PDmaxv (−3.5 ± 0.3 mm, mean ± 99% CI; r2 = 0.73) was reliably better than that of the group that observed an incongruent, CW video but reliably worse than subjects that observed the original CCW video (P < 0.01 in each case). Comparisons of motor learning based on the raw data resulted in the same overall pattern but without statistical significance.
DISCUSSION
The present investigation tested the idea that observational motor learning produces changes to somatosensory function in addition to its effects on motor learning. We found that sensed limb position changed following the observation of an actor learning to reach in a force field. The direction of the perceptual shift depended on the direction of the observed force. These changes were in the same direction as those previously described following actual motor learning (Ostry et al. 2010; Vahdat et al. 2011). Moreover, consistent with Mattar and Gribble (2005), subjects that viewed videos that were congruent with subsequent force-field learning showed greater amounts of learning and had movements that were less variable.
These effects could not be attributed to the observation of movement error alone. A control experiment showed that observing learning was important. Observing a sequence of movements that randomly varied from high to low-error trials did not produce reliable changes in sensed limb position. Random-video observation also had a reduced impact on motor learning.
A similarity in the processes underlying perceptual change following observational learning and actual motor learning is indicated by two related observations. First, the average change in perception following observational learning is in the same direction and of the same magnitude as the one for actual motor learning. Second, observational learning and real physical practice appear to tap into the same capacity for sensory change in that subjects that showed a greater change following learning by observing showed a reliably smaller change following physical motor learning and vice versa. At the same time, the sensory outcome of the two procedures is not identical. Compared with the sensory shifts described in previous investigations following physical learning, the changes reported here are characterized by greater between-subjects variability. This is in line with previous investigations showing, for the motor domain, similar performance between the physical and imagined execution of actions but with higher variability in the case of imagery (Papaxanthis et al. 2002).
The present results show that motor learning affects both motor and sensory systems regardless of whether the learning is achieved by standard physical practice or by observational learning. In the case of actual motor learning, changes to both sensory and motor function presumably ensure that the systems remain in register. Together with previous observations (Mattar and Gribble 2005), the present study provides support for a similar effect of observed motor learning on the broader sensorimotor network that is responsible for motor adaptation.
A number of studies have now shown that the somatosensory areas of the brain have mirrorlike properties, resembling those that have been previously described in premotor (Rizzolatti and Craighero 2004) and affective (Wicker et al. 2003) networks of the brain such that they are active both when an action is observed as well as when the same action is executed (Di Pellegrino et al. 1992). The observation of the action of others has been shown to evoke activation in areas BA1 and BA2 and also second somatosensory cortex (Avikainen et al. 2002; Cross et al. 2006; Gazzola and Keysers 2009; Keysers et al. 2010). BA2 activation has been reported for the observation of hands interacting with objects (Hasson et al. 2004; Pierno et al. 2009). The influence of visual information on haptic processing in BA2 presumably depends on reciprocal connections between both BA2 and second somatosensory cortex and regions of the intraparietal sulcus (e.g., the ventral intraparietal area) and the inferior parietal lobule (Lewis and Van Essen 2000; Pons and Kaas 1986; Rozzi et al. 2006). In the monkey, these parietal areas have been shown to combine visual, auditory, and somatosensory information (Lewis and Van Essen 2000; Maunsell and Van Essen 1983), which is relayed to somatosensory cortex and to circuits in premotor cortex (Keysers and Perret 2004). This pattern of connections could provide the neural substrates by which the somatosensory experience of adapting to a force field could be engaged by passive visual observation. Moreover, these areas are similar to those implicated in the perceptual changes that occur in conjunction with actual motor learning (Vahdat et al. 2011). This latter network comprises second somatosensory cortex, ventral premotor cortex, and supplementary motor cortex. It is noteworthy that the primary brain areas reported in action-observation studies, ventral premotor cortex, supplementary motor area, inferior parietal lobule, and the superior temporal sulcus (Kilner 2011), partially overlap those reported in the context of the perceptual aspects of motor learning.
A previous study has shown that motor learning is similarly influenced by watching a natural progression of learning, a scrambled sequence of high- and low-error trials, or even a sequence of high-error trials alone (Brown et al. 2010). These results are not consistent with the findings of the present control study, which shows that observing a scrambled sequence of movements has no reliable effects on perceptual function and reduced effects on motor learning. The difference in findings may lie in the fact that the previous study used videos showing eight different directions of movement, thus providing subjects with more examples of high-error movement, compared with our study in which only one direction of movement was employed. It is possible that in this previous study the amount of error information provided the basis for effective learning even in the scrambled condition. In the present study, the relatively sparse error information uncovered the importance of a coherent learning sequence for the success of observational learning. It should also be noted that this previous study (Brown et al. 2010) did not measure sensed limb position. This leaves open the possibility that their scrambled videos produced only a partial learning, one that involved the motor component but did not extend to the somatosensory system. In the present control study, there were no significant changes to estimates of sensed limb position following the observation of a scrambled CCW video. Half of the subjects tested in the control condition showed perceptual shifts in one direction and half in the other. However, the overall trend in the perceptual judgments was in a direction opposite to that obtained with the standard CCW video that shows learning and closer to that of the CW video. Indeed, it is interesting to consider the possibility that when participants are visually exposed to movements that do not involve learning but are systematically biased toward one side of the workspace, different mechanisms of cross-modal perceptual learning are engaged. An effect similar to that in the present control condition has been observed in speech-listening studies where a habituation-like phenomenon has been reported. When subjects are repeatedly exposed to a given vowel sound at one end of an auditory continuum, their ability to discriminate it from the vowels at the other end is altered. There is a shift in the perceptual boundary such that subjects are more likely to classify subsequent sounds as belonging to the other category (Cooper 1974). Similarly, in our study, participants who have been repeatedly exposed to movements deviated toward the left modify their subsequent perceptual classification reporting a greater number of deviations in the other direction (by shifting their perceptual boundary toward the left). If a similar mechanism underlies the results of the present control condition and that observed in speech-listening studies, then one would expect that presentation of a scrambled CW video would yield a symmetric effect, with somatosensory judgments biased toward the right. Whereas the present investigation was aimed at testing the effects of observing learning on somatosensory function, it would be of additional interest to assess possible habituation phenomena in experiments involving somatosensory classification and learning.
The results reported here have potential application in the field of rehabilitation, given the increasing interest in action-observation training for the rehabilitation of stroke patients (Celnik et al. 2008). Properly designed action-observation trainings could potentially be used to improve the recovery of sensory function in stroke patients. Additionally, the evaluation of sensory function could become a valuable complementary tool for assessing the outcome of action-observation training aimed at restoring motor function.
GRANTS
This research was supported by the National Institute of Child Health and Human Development (R01-HD-075740) and by Fonds québécois de la recherche sur la nature et les technologies. N. F. Bernardi was supported by the PhD program of the University of Milano-Bicocca (Italy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.F.B., M.D., E.B., and D.J.O. conception and design of research; N.F.B. performed experiments; N.F.B. and M.D. analyzed data; N.F.B., M.D., E.B., and D.J.O. interpreted results of experiments; N.F.B. prepared figures; N.F.B. and D.J.O. drafted manuscript; N.F.B., M.D., E.B., and D.J.O. edited and revised manuscript; N.F.B., M.D., E.B., and D.J.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Guillaume Houle for technical assistance.
REFERENCES
- Avikainen S, Forss N, Hari R. Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage 15: 640–646, 2002 [DOI] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Gribble PL. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cogn Neurosci 21: 1013–1022, 2009 [DOI] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Obhi SS, Gribble PL. Effect of trial order and error magnitude on motor learning by observing. J Neurophysiol 104: 1409–1416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Webster B, Glasser DM, Cohen LG. Effects of action observation on physical training after stroke. Stroke 39: 1814–1820, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WE. Adaptation of phonetic feature analyzers for place of articulation. J Acoust Soc Am 56: 617–627, 1974 [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol 102: 3505–3518, 2009 [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: observation of dance by dancers. Neuroimage 31: 1257–1267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res 91: 176–180, 1992 [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex 19: 1239–1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith A, Jackson C, Miall R, Vijayakumar S. Unifying the sensory and motor components of sensorimotor adaptation. Adv Neural Inf Process Syst 21: 593–600, 2008 [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science 303: 1634–1640, 2004 [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci 11: 417–428, 2010 [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci 8: 501–507, 2004 [DOI] [PubMed] [Google Scholar]
- Kilner JM. More than one pathway to action understanding. Trends Cogn Sci 15: 352–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137, 2000 [DOI] [PubMed] [Google Scholar]
- Mattar AA, Gribble PL. Motor learning by observing. Neuron 46: 153–160, 2005 [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 3: 2563–2586, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaxanthis C, Pozzo T, Skoura X, Schieppati M. Does order and timing in performance of imagined and actual movements affect the motor imagery process? The duration of walking and writing task. Behav Brain Res 134: 209–215, 2002 [DOI] [PubMed] [Google Scholar]
- Pierno AC, Tubaldi F, Turella L, Grossi P, Barachino L, Gallo P, Castiello U. Neurofunctional modulation of brain regions by the observation of pointing and grasping actions. Cereb Cortex 19: 367–374, 2009 [DOI] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: a correlative anatomical and electrophysiological study. J Comp Neurol 248: 313–335, 1986 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci 27: 169–192, 2004 [DOI] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex 16: 1389–1417, 2006 [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci 25: 9339–9346, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MM, Creelman CD. PEST: efficient estimates on probability functions. J Acoust Soc Am 41: 782–787, 1967 [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci 31: 16907–16915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 40: 655–664, 2003 [DOI] [PubMed] [Google Scholar]
- Wong JD, Wilson ET, Gribble PL. Spatially selective enhancement of proprioceptive acuity following motor learning. J Neurophysiol 105: 2512–2521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]