Abstract
High-frequency oscillations (HFO; gamma: 40–100 Hz, ripples: 100–200 Hz, and fast ripples: 250–500 Hz) have been widely studied in health and disease. These phenomena may serve as biomarkers for epileptic brain; however, a means of differentiating between pathological and normal physiological HFO is essential. We categorized task-induced physiological HFO during periods of HFO induced by a visual or motor task by measuring frequency, duration, and spectral amplitude of each event in single trial time-frequency spectra and compared them to pathological HFO similarly measured. Pathological HFO had higher mean spectral amplitude, longer mean duration, and lower mean frequency than physiological-induced HFO. In individual patients, support vector machine analysis correctly classified pathological HFO with sensitivities ranging from 70–98% and specificities >90% in all but one patient. In this patient, infrequent high-amplitude HFO were observed in the motor cortex just before movement onset in the motor task. This finding raises the possibility that in epileptic brain physiological-induced gamma can assume higher spectral amplitudes similar to those seen in pathologic HFO. This method if automated and validated could provide a step towards differentiating physiological HFO from pathological HFO and improving localization of epileptogenic brain.
Keywords: high-frequency oscillations, epilepsy, gamma oscillations
high-frequency oscillations (HFO) have been widely studied in animals and humans and linked to brain function in health and disease (Buzsaki and Silva 2012). Physiological high-frequency gamma oscillations (gamma: ∼40–100 Hz) are believed to coordinate cortical processing during vision (Gray and Singer 1989), motor, and language functions (Crone et al. 2011). Physiological hippocampal high-frequency oscillations (ripples: 100–200 Hz) are thought to play an important role in memory functions (Buzsaki et al. 1992).
Pathological HFO (pHFO) were initially observed in hippocampal recordings from epileptic rats and thought to be a specific electrophysiological biomarker of epileptic tissue (Bragin et al. 1999b). The pHFO observed in epileptic rats included fast ripples (250–500 Hz) that colocalize to the epileptic hippocampus-generating seizures in rats (Bragin 1999b, 2002) and humans (Bragin 1999a). In addition, ripple HFO were observed in the hippocampal dentate gyrus of epileptic rats but not in control rats and were therefore considered pHFO (Bragin 2002). The cellular correlates of normal physiological hippocampal ripples were shown to be inhibitory postsynaptic potentials (Ylinen et. al. 1995) and pathological fast ripples were shown to be synchronous population firing of large groups of pyramidal cells and decreased inhibitory interneuron firing (Bragin et al. 2007, 2011).
These early seminal studies supported the hypothesis that fast ripples were distinctly pathological oscillations associated with epileptic brain (Bragin et al. 1999a,b). Later studies, however, reported physiological somatosensory evoked HFO >250 Hz (Baker et al. 2003), likely reflecting multiunit cortical neuronal responses (Telenczuk et al. 2011). These findings were inconsistent with the notion that fast ripples were specific markers of epileptic tissue. Furthermore, subsequent studies from humans showed that both gamma and ripple frequency oscillations were increased in human epileptogenic hippocampus (Crepon et al. 2010; Jacobs and Kahana 2010; Worrell et al. 2008) and neocortex (Blanco et al. 2011; Jacobs and Kahana 2010; Schevon et al. 2009; Worrell et al. 2004). Thus the distinction between physiological and pathological HFO has remained unclear (Bragin et al. 2010; Engel et al. 2009; Worrell and Gotman 2011).
The goal of our study is to compare gamma and ripple HFO induced by a physiological task with spontaneous pHFO. We hypothesized that the HFO induced by a physiological task, here induced by presentation of a visual image and motor movement, would be distinguishable from pHFO. Physiological gamma and ripple activities induced by a visual presentation and motor movement task have traditionally been analyzed in the averaged time-frequency domain (Tallon-Baudry et al. 1997). The candidate pathological HFO were identified using an automated line-length detector and then verified by expert visual review in the time domain. We generated comparable measures for physiological-induced HFO (nHFO) and pHFO by analyzing both in the single trial time-frequency domain. More clearly defining the distinction between these two forms of HFO is important for future efforts using pHFO as biomarkers of epilepsy.
MATERIALS AND METHODS
Subjects
The Mayo Institutional Review Board approved this study. Five patients (3 females) with intractable epilepsy were admitted for chronic intracranial EEG monitoring as part of their evaluation for drug resistant focal epilepsy. Each patient participated in either a visual scanning task or a finger movement task. The duration of clinical monitoring, location and number of implanted electrodes were determined by clinical considerations. Patient characteristics and the electrodes used in this study are recorded in Table 1.
Table 1.
Characteristics of patients and electrodes
| nHFO |
pHFO |
|||||
|---|---|---|---|---|---|---|
| Patient | Sex/Age | Task | Electrode location | Cortex class | Electrode location | Cortex class |
| 1 | F/25 | Motor | Motor cortex | Normal | Frontal cortex | SOZ |
| Motor cortex | Normal | |||||
| Motor cortex | Normal | |||||
| 2 | F/22 | Visual | Occipital cortex | Normal | Hippocampus | SOZ |
| 3 | M/44 | Visual | Hippocampus | Normal | Parahippocampal | SOZ |
| Parahippocampal | SOZ | |||||
| 4 | F/21 | Visual | Hippocampus | SOZ | Parahippocampal | SOZ |
| Parahippocampal | Seizure prop | |||||
| 5 | M/45 | Motor | Motor cortex | Seizure prop | Temporal cortex | SOZ |
| Motor cortex | Seizure prop | Temporal cortex | SOZ | |||
| Motor cortex | Normal | |||||
nHFO, physiological high-frequency oscillation; pHFO, pathological high-frequency oscillation; F, female; M, male; SOZ, seizure onset zone; Seizure prop, electrode involved in early propagation from SOZ.
Intracranial EEG Recording
Intracranial EEG was recorded with depth electrodes or implanted grids and sampled at 32,500 Hz through a filtered bandwidth of DC to 9 kHz (Neurolynx, Bozeman, MT) (Brinkmann et al. 2009). The depth electrode (AD-Tech Medical Instrument, Racine, WI) consisted of a 1.3-mm diameter polyurethane shaft with platinum/iridium clinical macroelectrode contacts; each clinical contact is 2.3-mm long with either a 5- or 10-mm center-to-center spacing. Subdural electrodes are 4.0-mm diameter platinum discs with a center-to-center electrode distance of 10 mm.
Tasks
Visual scanning task.
Patients viewed images from the International Affective Picture Set on a portable computer (Lang et al. 1997). A series of 80 images were shown for a 6-s viewing period. Patients then rated the image on a five-point scale from “very pleasant” to “very unpleasant.” After a 6-s rest period, the next image was presented. Patients were aware that they might be asked to recall the images 24 h later. Details of the protocol have been described previously (Matsumoto et al. 2013).
Motor task.
Patients watched a bedside computer for commands to move a digit. Details of the task have been described in detail previously (Whitmer et al. 2010). Briefly, the computer screen displayed an image of a digit or the name of a digit. The patient was instructed to press the button on a five digit key press indicated by the screen command. A stimulus was displayed every 1.57 s. Each digit received 25 commands to move.
Electrode Localization
For depth electrode localization, images of the patient's preimplantation MRI scan were fused with the postimplantation high-resolution computed tomography scan in Analyze (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). The images were fitted to the Talairach Brain Atlas, and Talairach coordinates were read from the Mayo Brain Atlas in Analyze. Brain structures were named using the Talairach Daemon (www.talairach.org). For cortical grid electrodes, intraoperative photographs of the grids on the brain surface combined with stimulation records localized the premotor gyrus.
Data Analysis
Raw data was filtered between 0.1 and 1,000 Hz and downsampled to 5,000 Hz. The downsampled files were converted to common averaged reference datasets by using either all depth or all grid electrodes to compute the average. Custom programs written in MATLAB (Mathworks, Natick, MA) were used to extract data epochs surrounding picture presentation or finger press events by 2 s. Data epochs containing electrode artifact or excessive muscle or interictal spike activity were rejected from this analysis. Selected data epochs were transformed into the frequency domain using established methods (Tallon-Baudry et al. 1997; Canolty et al. 2006). Briefly, the time domain waveforms were filtered in 1-Hz steps from 1 to 800 and the filtered time series were normalized. The normalized time series were transformed into an analytic signal with the Hilbert function in MATLAB. The absolute value of this signal represents the normalized spectral amplitude over time at each frequency. The spectral amplitude so computed is in arbitrary units and represented as a Z-score. We discarded 1,000 ms of data from the beginning and end of the 4-s time series to eliminate edge effect. The time series at all frequencies were used to construct time-frequency spectra.
For each electrode recording a task, we averaged these spectra over all trials to detect induced gamma in the visual and motor tasks. We visually inspected averaged spectra (84 electrodes in patient 1, 50 electrodes in patient 2, 52 electrodes in patient 3, 84 electrodes from patient 4, and 96 electrodes in patient 5) and we selected the 10-electrode spectra that displayed task-induced gamma and ripple activity. This was defined as averaged spectra demonstrating confluent areas in the 40- to 1,000-Hz frequency range spanning 100 ms or more where the averaged spectral amplitude Z-score was >1.5. (Fig. 1C). Six of these electrodes were in normal cortex and four were in cortex involved in the generation or early propagation of recorded seizures (Table 1).
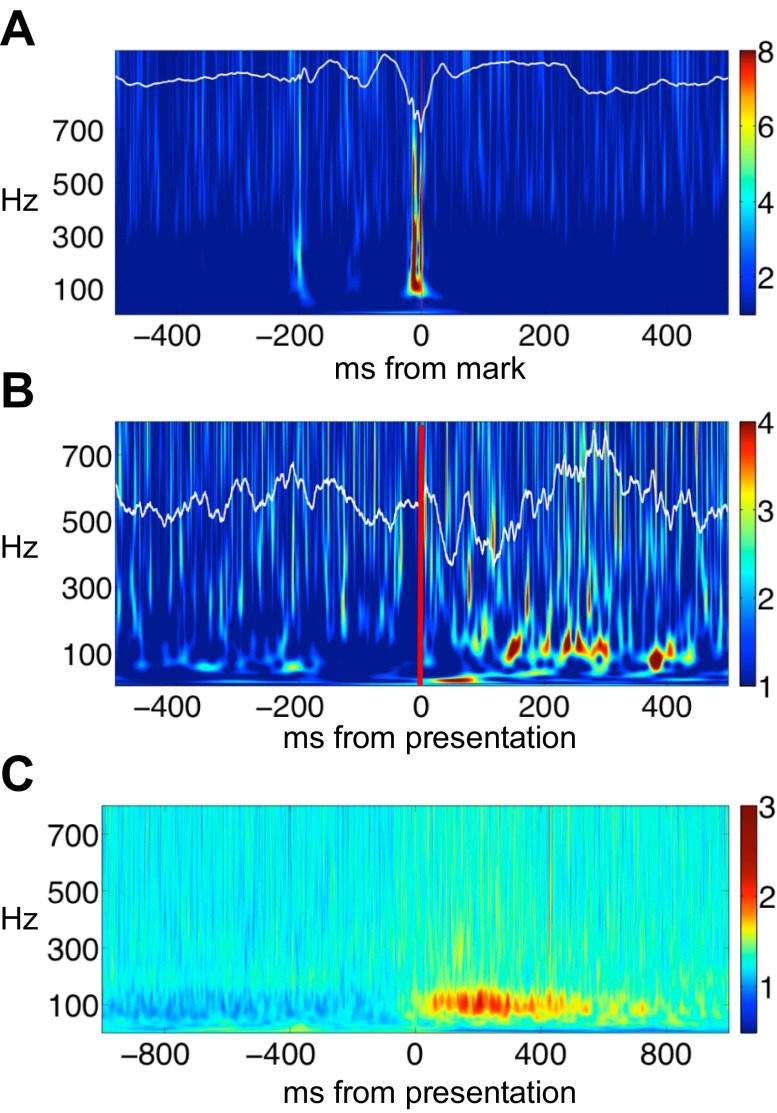
Fig. 1.
Pathologic high-frequency oscillations (pHFO) and physiologic high-frequency oscillations (nHFO) and task-induced gamma. A: pHFO identified in the time domain (white line tracing) superimposed on the corresponding Hilbert time-frequency spectrum. Time zero is the point marked on the raw record by the expert reviewer. Colorbar indicates Z-score of spectral amplitude in arbitrary units. Raw tracing and spectrogram demonstrate high-amplitude burst of HFO superimposed on a sharp wave. B: physiologic gamma oscillations induced by visual image presentation in a parahippocampal electrode from patient 4. Raw data in time domain (white line tracing) are superimposed on a single trial Hilbert time-frequency spectrum. Red line indicates onset of image presentation. Colorbar indicates Z-score of spectral amplitude in arbitrary units. Gamma oscillations appear as low amplitude, discrete modulations of the background frequencies during picture viewing. C: induced gamma oscillations appear on the averaged Hilbert time-frequency spectra between 100 and 400 ms after image presentation. Colorbar indicates Z-score of spectral amplitude in arbitrary units.
Pathologic HFO were collected from the same patients in electrodes from the seizure onset zone (SOZ). The SOZ was defined by the macroelectrodes with the earliest clear electroencephalographic change in activity. The time of the earliest intracranial electroencephalographic change and the associated electrode(s) were identified as the seizure onset time and SOZ location, respectively. Seizure onset times and location were determined by independent visual identification of a clear electrographic seizure discharge in electrode recordings and then looking back in the record for the earliest definite intracranial electroencephalographic change contiguously associated with the seizure. The electrodes involved within the initial 5 s of electrographic seizure onset were identified as the early propagation zone. These electrodes were different than the ones used for the evaluation of nHFO (Table 1). The raw data were filtered (30–700 Hz), and then a line-length detector was applied to a sliding 200-ms window, with 50-ms overlap. A statistical window of 10 s was used to identify line-length segments that were +3.5 SD above the mean line-length values within each 10-s window. Subsequently, a duration threshold was added as a cascaded feature to select events that were at least four oscillations. With the use of this method, the majority of pHFO were found associated with epileptiform sharp wave transients (Fig. 1A), but this was not a requirement (Worrell et al. 2008, 2012).
Data Marking
Both pHFO and nHFO were measured in an identical fashion. For nHFO, time-frequency spectra were generated for each individual task trial and HFO were marked during the period of induced gamma visibly evident on the previously computed averaged time-frequency spectra (Fig. 2). By marking HFO only during this period we felt we were identifying HFO with the highest likelihood of being task- or stimulus-induced and truly nHFO. For pHFO, time-frequency spectra were generated for a data segment centered on the marked peak of the pHFO. (Axmacher et al. 2008; Le Van Quyen et al. 2010) We set the colorbar intensity of the displayed time-frequency spectra such that the Z-score of normalized spectral amplitude from 3.5 to 4 was red. This display algorithm identified HFO as discrete areas of high spectral amplitude containing red on this colorscale (Fig. 2). For each HFO, we visually marked the onset and offset latency and the center frequency of the region of increased spectral amplitude (Fig. 2). An area between the latencies and surrounding the center frequency by 10 Hz was searched by computer algorithm to ascertain the maximum spectral amplitude. We also recorded the duration and center frequency of each HFO.
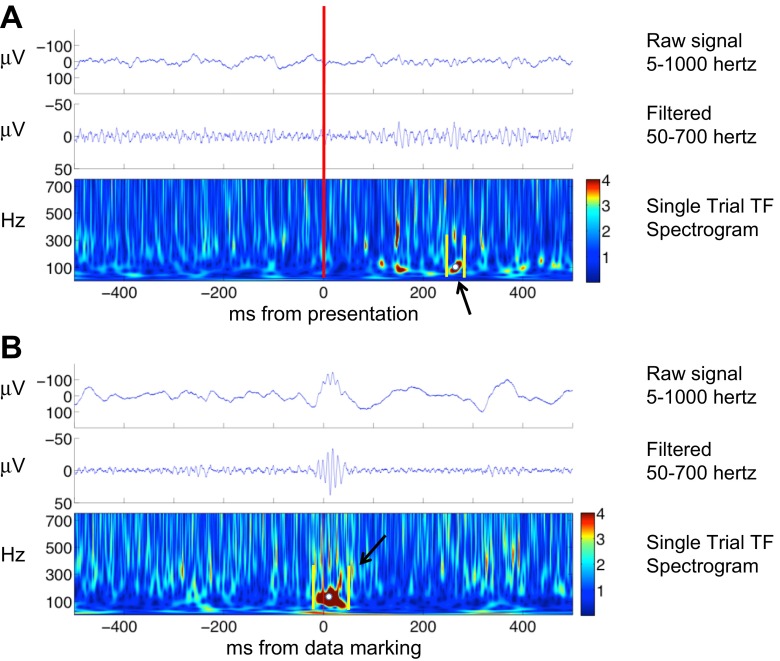
Fig. 2.
Data marking of nHFO and pHFO. A: marking nHFO during the period of visually induced gamma during a trial from the electrode represented in Fig. 1B. Red line indicates image presentation. Colorbar indicates Z-score of spectral amplitude in arbitrary units. These events are not clearly seen in the raw data and seen as low amplitude modulations in the filtered time domain tracings. They do appear as discrete events on the time-frequency spectrum where a representative marking is demonstrated. Yellow lines indicate the method of marking the HFO onset and offset latencies. White dot indicates the method of marking the center frequency. A computer algorithm found the maximal spectral amplitude between the latencies and within 10 Hz of the center frequency. B: marking of a pHFO selected from patient 4. Time 0 represents the point of data marking. Colorbar indicates Z-score of spectral amplitude in arbitrary units. The high-amplitude event marked in the raw tracing becomes more discrete in the filtered time domain tracing. It appears as a discrete, high spectral amplitude event in the time-frequency spectra and marking is demonstrated using the same parameters described above.
Statistical Analysis
We tested for differences in spectral amplitude, frequency, and duration between pHFO and nHFO by unpaired Student's t-tests. As a second level of analysis, differences in these parameters were tested between nHFO in normal and SOZ cortex. A corrected significance of 0.008 was specified for the six comparisons. A support vector machine (SVM) classification was performed on the pHFO and nHFO using a rotating cross-validation approach. For the five patients for whom pHFO and nHFO were available, a SVM classifier implemented in MATLAB (Mathworks) using a linear kernel was cross-validated by training on the data for four patients and then classifying the data for the remaining patient.
RESULTS
During the periods of induced gamma, distinct regions of high spectral amplitude appeared in the single trial time-frequency spectrograms that were discrete in frequency and time (Fig. 2). We identified 979 such events from 10 electrodes. These events could not be clearly identified in the time domain and correlated with a slight modulation of amplitude in the raw or filtered data (Fig. 2). In practice these events represented the areas of peak spectral amplitude 3.5 SD or more above the mean spectral amplitude. Maximal spectral amplitude appeared well regulated in nHFO from normal cortex with a mean tightly clustered ∼4 SD above average (M = 4.1, SD = 0.65). Plotting nHFO from normal cortex demonstrated a very tight distribution in the feature space of spectral amplitude, frequency, and duration (Fig. 3). The nHFO spanned a broadband from 50 to 700 Hz without apparent clusters of frequencies. This peak spectral amplitude corridor included observations across patients, task, and anatomical location of the electrode (Fig. 3).
Fig. 3.
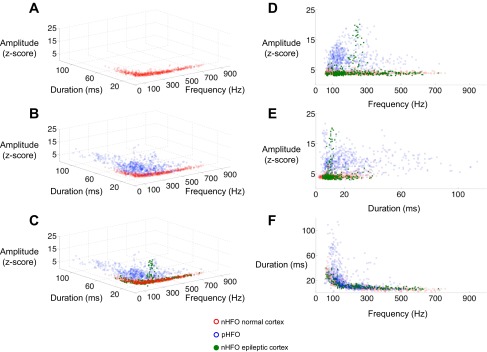
nHFO and pHFO in frequency-duration-spectral amplitude feature space. A: nHFO from normal cortex from all patients harvested from regions of induced gamma during visual or motor task. The nHFO represent events within the recording that have the highest peak normalized spectral amplitude and cluster in a tight distribution within the feature space. This distribution contains nHFO from all patients, tasks, and anatomic regions. B: plot of all nHFO from normal cortex (red) and pHFO (blue.) pHFO form a separate distribution in feature space with a clustering around 75–150 Hz and having higher spectral amplitude and durations than nHFO. C: nHFO from epileptic cortex (green) are superimposed on the distributions for nHFO from normal cortex (red) and pHFO (blue.) Most of the nHFO from epileptic cortex overlap with those from normal cortex. A small number demonstrate high spectral amplitude and map in the pHFO range. D: frequency vs. amplitude plot for all HFO. E: duration vs. amplitude plot for all HFO. F: frequency vs. duration plot for all HFO.
The nHFO from normal cortex were compared with 604 pHFO identified by expert review in the time domain (Figs. 1 and 2). In the frequency domain, pHFO appeared as high spectral amplitude events (M = 9.07, SD = 3.49), clearly different than the spectral amplitude of nHFO from normal cortex [t(1193) = 33.9698, P < 1.0 × 10−10]. pHFO had lower mean frequencies (M = 188.4 Hz, SD = 104.7) than nHFO from normal cortex [M = 264.2 Hz, SD = 162.1, t(1193) = −9.63, P < 1.0 × 10−10]. The duration of pHFO was also significantly longer (M = 23.1 ms, SD = 19.5) than nHFO from normal cortex [M = 12.1 ms, SD = 8.3, t(1193) = 12.6, P < 1.0 × 10−10]. The longer duration of pHFO was not entirely due to their higher amplitudes. Comparing a subgroup nHFO and pHFO with amplitudes between 3.5 and 5.0 revealed that the durations of pHFO were persistently longer [M = 14.9 vs. 11.6 ms, t(533) = 2.59, P = 0.01]. The longer duration of pHFO might also be due to threshold effects if nHFO amplitude peaked more gradually than pHFO. To evaluate the possibility that the difference in durations might reflect an idiosyncrasy of our chosen amplitude threshold, we recalculated durations for 20 nHFO and 20 pHFO from each of the patients at two different thresholds of 2 and 3.5 SD of amplitude. For the nHFO, the markings at the 3.5 SD threshold produced HFO of shorter durations than the 2 SD threshold [mean difference = −21.0 ms, t(99) = −4.93, P = 3.2 × 10−06]. The higher threshold also resulted in shorter durations of pHFO [mean difference = −31.1, t(99) = −4.00, P = 1.2 × 10−04]. However, there was no significant difference in the magnitude of this effect comparing nHFO to pHFO and pHFO continued to demonstrate longer durations then nHFO [M = 90.1 vs. 49.2 ms, t(205) = 3.81, P = 1.8 × 10−04] when measured at the lower threshold. pHFO spanned the entire frequency range from 50 to 700 Hz with a tendency to group between 70 and 150 Hz. While nHFO from normal cortex displayed tight peak spectral amplitude grouping, pHFO were widely dispersed in feature space (Fig. 3). SVM cross-validation classification produced a mean sensitivity of 83.8% (SD = 13.1) and a specificity of 84.6% (SD = 29.1). Full results are reported in Table 2.
Table 2.
Support vector machine classification of pathological versus physiological HFO: percent sensitivity and specificity
| Patient | Sensitivity, % | Specificity, % |
|---|---|---|
| 1 | 75.47 | 96.14 |
| 2 | 97.65 | 97.70 |
| 3 | 77.57 | 99.38 |
| 4 | 98.0 | 97.64 |
| 5 | 70.45 | 32.61 |
Sensitivity is likelihood of correct classification of pathological HFO by support vector machine. Specificity is likelihood of physiological HFO being correctly rejected by support vector machine.
Analyzing nHFO from electrodes in the seizure active areas (SOZ or early propagation from SOZ) revealed mixed results. The nHFO from seizure active zones had higher maximal spectral amplitude (M = 4.5, SD = 3.4) than nHFO from normal cortex [t(977) = 2.91, P = 0.0004] but no significant difference in frequency or duration. In feature space, most nHFO from seizure active cortex fell along the main peak spectral amplitude corridor seen in normal cortex (Fig. 3). A few nHFO from seizure active cortex (41/219) displayed spectral amplitude values >6 (Fig. 3). All of these high spectral amplitude nHFO were recorded in two electrodes from patient 5 that localized to the motor cortex (Fig. 4). These high spectral amplitude nHFO were of shorter mean duration than pHFO [M =11.5 ms, SD = 4.6, t(607)= −4.65, P = 3.3 × 10−06] and had a mean frequency near the fast ripple range (M = 221 Hz, SD = 49.0). In one of these electrodes, nHFO could be seen in time domain tracings, occurring in a nonphase locked fashion around movement onset (Fig. 4). These HFO produced intense induced gamma signal on the averaged time-frequency spectra (Fig. 4). For this patient we extended nHFO detection through periods extending from 1,000 ms before and after movement. Plotting these detections revealed that nHFO with normalized spectral amplitude Z-scores ∼4 appeared just before and after movement. However, ∼100 ms before movement there was a discrete increase in normalized spectral amplitude to values >15 SD (Fig. 4).
Fig. 4.
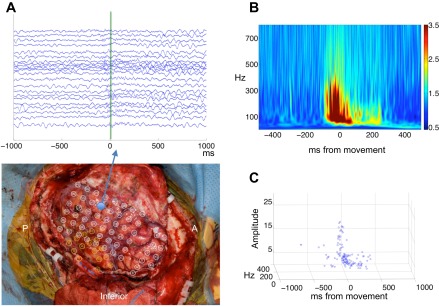
nHFO from motor cortex in an electrode with high-amplitude HFO. A, top: raster plot of all trials during the motor task from this electrode in patient 5 during index finger movement. Nonphase locked HFO are seen in the raw tracings surrounding movement onset (marked with green line). Bottom: location of this electrode is in the premotor gyrus in the “hand knob” region. A, anterior; P, posterior. Inferior aspect of surgical field is labeled. B: averaged time-frequency spectra of all trials demonstrates high-amplitude-induced gamma surrounding movement onset. Time 0 indicates keypad activation. Colorbar indicates Z-score of spectral amplitude in arbitrary units. C: plot of nHFO over time demonstrate that nHFO appear ∼300 ms before and after movement onset. At ∼100 ms before movement there is a brief burst of high spectral amplitude nHFO.
DISCUSSION
HFO are physiological phenomenon of critical importance in both normal function and epilepsy (Buzsaki and Silva 2012). Pathological HFO correlate with hippocampal and neocortical regions of seizure generation and might serve as biomarkers of epilepsy, guiding the surgical approach to the resection of epileptic tissue (Bragin et al. 1999b, 2002, 2010; Engel et al. 2009; Le Van Quyen et al. 2010; Staba et al. 2007; Worrell and Gotman 2011; Worrell et al. 2004). A method to securely differentiate nHFO from pHFO would be an essential step for such an application.
HFO have been generally described in terms of frequency. Classification of pathological vs. physiological HFO as ripples (100–200 Hz) and fast ripples (250–500 Hz) has remained a barrier to clinical applications. We sought to extend the description of HFO into a larger feature space including spectral amplitude, frequency, and duration and then classify nHFO to pHFO using these parameters with a SVM.
We defined nHFO in the frequency domain by marking peak spectral amplitude with an arbitrary lower limit cutoff during periods of induced gamma activity. By looking at single trials, HFO events likely include some that reflect “noise” and there is no certainty that nHFO in all frequency bands are functionally relevant. This method does, however, identify a population of events enriched in functionally significant nHFO. Caution is also needed in the interpretation of events marked at very high frequencies, as frequency spread is a feature of the Hilbert method (van Vugt et al. 2007). Even with these limitations, we found that nHFO parameters were consistent and highly regulated across a broadband of frequencies forming what appears to be a tight corridor of peak spectral amplitude within the defined feature space.
Previous investigators have separated pHFO morphologically and reported that those associated with sharp waves or interictal epileptiform spikes are more likely to be pathological (Crepon et al. 2010; Urrestarazu et al. 2007; Wang et al. 2013). We chose to include all pHFO in our analysis to avoid a priori judgments as to their significance. The method of visual pHFO selection is likely biased towards selection of events of the highest amplitude. However, the comparison of peak spectral amplitude pHFO to peak spectral amplitude nHFO proved valid and informative. We found that pHFO identified from the SOZ were highly distinguishable from nHFO in the defined feature space. The pHFO are of higher spectral amplitude and longer duration compared with nHFO throughout the frequency range and cluster at lower frequencies. The longer duration of pHFO does not appear to be entirely due to the confounding factors of amplitude or threshold effects. The distribution of pHFO in feature space is widely dispersed in contrast to the tight distribution of nHFO. Using a SVM classifier we were able to separate nHFO from pHFO with sensitivities ranging from 68- to 99% and specificities >90% in all but one patient. One previous study also demonstrated higher amplitude HFO from the SOZ compared with HFO induced by visual stimulation although statistical evaluation was not reported (Nagasawa et al. 2012).
The automatic separation of nHFO and pHFO in our data is not categorical. High spectral amplitude, short duration nHFO occurred infrequently in two motor cortex electrodes from the early seizure propagation zone of patient 5. The pHFO and nHFO clusters for this patient proved particularly difficult for the classifier to separate as a result of these events (Table 2). These high spectral amplitude nHFO also reduced the classifier's performance on the other patients' data sets by biasing the training set. It is possible that these high spectral amplitude values reflect the proximity of the electrode to the nHFO generator in the motor cortex. However, if electrode proximity were a major variable, one would expect to observe a range of peak nHFO spectral amplitude values with a wider variance rather than the tight cluster of spectral amplitude values observed. It is also unlikely that pHFO randomly contaminated the tracings, as the high spectral amplitude nHFO were time locked to the movement onset. In the primate motor cortex, interneurons and pyramidal cells display a burst of firing just before and after arm movements (Merchant et al. 2008). We hypothesize that this intense, synchronous cell firing creates a class of nHFO that have parameters with similarities to pHFO, which also arise from the synchronous firing of neurons (Bragin and Benassi et al. 2011). This also raises the intriguing possibility that pHFO and nHFO are linked such that pHFO may reflect pathologic neuronal synchronization in epileptic tissue induced by normal physiologic mechanisms of gamma HFO generation. Further study of these rare records of induced gamma will be needed to estimate the frequency of high-amplitude nHFO and to determine if they occur outside the motor cortex or in normal cortex.
It is important to note that our data only reflect nHFO during awake, task-induced records. Other putative physiological HFO have been described to occur spontaneously during sleep in the hippocampus, visual cortex, and sensorimotor cortex (Axmacher et al. 2008; Nagasawa et al. 2012; Wang et al. 2013). Future studies will be needed to see if these events are separable from pHFO on the basis of the parameters used here or other parameters.
The goal of separating all physiological and pathological HFO presents a myriad of complexities. Although preliminary, this method offers one step towards identifying nHFO in the waking records of studies investigating the use of pHFO as pathological biomarkers of epilepsy. While the SVM data presented here strongly support the existence of two separate groups of HFO, they do not assist in the classification of an individual HFO. Automating this process using rapid detection of HFO by a detector followed by classification using algorithms incorporating the feature differences described here would be a logical next step. Applying this process to large datasets will be necessary to further investigate its validity.
GRANTS
This work was supported by Mayo Clinic and National Institute of Neurological Disorders and Stroke Grants R01-NS-63039 (to G. Worrell) and R01-NS-078136 (to S. M. Stead).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.M., B.H.B., S.M.S., J.M., W.R.M., F.M., and G.W. conception and design of research; A.M., B.H.B., S.M.S., J.M., W.R.M., F.M., and G.W. performed experiments; A.M., B.H.B., S.M.S., J.M., M.K., and G.W. analyzed data; A.M., B.H.B., S.M.S., J.M., M.K., F.M., and G.W. interpreted results of experiments; A.M., B.H.B., S.M.S., J.M., M.K., and G.W. prepared figures; A.M., B.H.B., S.M.S., J.M., and G.W. drafted manuscript; A.M., B.H.B., S.M.S., J.M., M.K., W.R.M., F.M., and G.W. edited and revised manuscript; A.M., B.H.B., S.M.S., J.M., M.K., W.R.M., F.M., and G.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cindy Nelson, Karla Crockett, and Vincent Vasoli for technical support.
REFERENCES
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131: 1806–1817, 2008 [DOI] [PubMed] [Google Scholar]
- Baker SN, Curio G, Lemon RN. EEG oscillations at 600 Hz are macroscopic markers for cortical spike bursts. J Physiol 550: 529–534, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JA, Stead M, Krieger A, Stacey W, Maus D, Marsh E, Viventi J, Lee KH, Marsh R, Litt B, Worrell GA. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain 134: 2948–2959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J., Jr Further evidence that pathological high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia 52: 45–52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol 23: 151–156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus 9: 137–142, 1999a [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid–treated rats with chronic seizures. Epilepsia 40: 127–137, 1999b [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci 22: 2012–2021, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia 48, Suppl 5: 35–40, 2007 [DOI] [PubMed] [Google Scholar]
- Brinkmann BH, Bower MR, Stengel KA, Worrell GA, Stead M. Large-scale electrophysiology: acquisition, compression, encryption, and storage of big data. J Neurosci Methods 180: 185–192, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science 256: 1025–1027, 1992 [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Silva FL. High frequency oscillations in the intact brain. Prog Neurobiol 98: 241–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313: 1626–1628, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain 133: 33–45, 2010 [DOI] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. Int J Psychophysiol 79: 9–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia 50: 598–604, 2009 [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86: 1698–1702, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn Sci 14: 162–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FA: NIMH Center for the Study of Emotion and Attention, Univ. of Florida, 1997 [Google Scholar]
- Le Van Quyen M, Staba R, Bragin A, Dickson C, Valderrama M, Fried I, Engel J. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci 30: 7770–7782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Stead SM, Kucewicz M, Matsumoto A, Peters P, Brinkmann B, Danstrom J, Goerss S, Marsh WR, Meyer FB, Worrell G. Network oscillations modulate interictal epileptiform spike rate during human memory. Brain 136: 2444–2456, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Naselaris T, Georgopoulos AP. Dynamic sculpting of directional tuning in the primate motor cortex during three-dimensional reaching. J Neurosci 28: 9164–9172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Juhasz C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp 33: 569–583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain 132: 3047–3059, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia 48: 2130–2138, 2007 [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci 17: 722–734, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenczuk B, Baker SN, Herz AV, Curio G. High-frequency EEG covaries with spike burst patterns detected in cortical neurons. J Neurophysiol 105: 2951–2959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain 130: 2354–2366, 2007 [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. J Neurosci Methods 162: 49–63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez-Martinez J, Alexopoulos AV, Najm IM, So NK. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia 54: 370–376, 2013 [DOI] [PubMed] [Google Scholar]
- Whitmer D, Worrell G, Stead M, Lee IK, Makeig S. Utility of independent component analysis for interpretation of intracranial EEG. Front Hum Neurosci 4: 184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell G, Gotman J. High-frequency oscillations and other electrophysiologicalal biomarkers of epilepsy: clinical studies. Biomark Med 5: 557–566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain 131: 928–937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 127: 1496–1506, 2004 [DOI] [PubMed] [Google Scholar]
- Worrell GA, Jerbi K, Kobayashi K, Lina JM, Zelmann R, Le Van Quyen M. Recording and analysis techniques for high-frequency oscillations. Prog Neurobiol 98: 265–278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15: 30–46, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]