Abstract
Developmental plasticity in spiral ganglion neurons (SGNs) ensues from profound alterations in the functional properties of the developing hair cell (HC). For example, prehearing HCs are spontaneously active. However, at the posthearing stage, HC membrane properties transition to graded receptor potentials. The dendrotoxin (DTX)-sensitive Kv1 channel subunits (Kv1.1, 1.2, and 1.6) shape the firing properties and membrane potential of SGNs, and the expression of the channel undergoes developmental changes. Because of the stochastic nature of Kv subunit heteromultimerization, it has been difficult to determine physiologically relevant subunit-specific interactions and their functions in the underlying mechanisms of Kv1 channel plasticity in SGNs. Using Kcna2 null mutant mice, we demonstrate a surprising paradox in changes in the membrane properties of SGNs. The resting membrane potential of Kcna2−/− SGNs was significantly hyperpolarized compared with that of age-matched wild-type (WT) SGNs. Analyses of outward currents in the mutant SGNs suggest an apparent approximately twofold increase in outward K+ currents. We show that in vivo and in vitro heteromultimerization of Kv1.2 and Kv1.4 α-subunits underlies the striking and unexpected alterations in the properties of SGNs. The results suggest that heteromeric interactions of Kv1.2 and Kv1.4 dominate the defining features of Kv1 channels in SGNs.
Keywords: hearing, development, Kv1 channels, currents, spiral ganglion neurons, auditory information coding
voltage-dependent potassium channels (Kv) sculpt neuronal excitability by regulating resting membrane potentials, spontaneous firing rates, neurotransmitter release, and synaptic integration (Ishikawa et al. 2003; Pongs 1999; Schrader et al. 2002). Indeed, Kv are the most diverse channels in the mammalian genome (Gutman et al. 2005). The functional assortment of Kv is further abound by the heteromultimeric combination of pore forming (α) and auxiliary (β) subunits, demonstrated extensively in heterologous cells (Lai and Jan 2006; Maffie and Rudy 2008). However, in native neurons, because of the promiscuous features of Kv assembly, it is difficult to determine the precise contribution of each α-subunit to specific physiological processes in vivo or to predict the compensatory mechanisms that occur in response to perturbations in the activity of a given α- and/or β-subunit. To acquire this information, which is essential for a detailed understanding of multiple functions of Kv, in vivo analyses of gene deletion models are extremely powerful strategies (Brew et al. 2007).
The spiral ganglion of the cochlear nerve is the site where auditory information coding is initiated, transmitting precise and reliable information about the amplitude, duration, and frequency of sound waves from hair cells (HCs) to the cochlear nucleus. Ninety-five percent of the neurons (type I) innervate inner HCs, whereas the remaining 5% (type II) innervate outer HCs (Morrison et al. 1975; Spoendlin 1981). One of the Kv currents, the dendrotoxin (DTX)-sensitive K+ current (Mo et al. 2002; Schrader et al. 2002), serves as a key determinant of the resting membrane potential (RMP; Ishikawa et al. 2003), rate of accommodation (Pongs 1999), and adaptation of firing in spiral ganglion neuron (SGN) information coding. The DTX-sensitive current contains Kv1.1, 1.2, and 1.6 subunits (Clark et al. 2008; Maffie and Rudy 2008; Maffie et al. 2009). Analysis of the voltage-dependent properties of DTX-sensitive currents in murine SGNs describes currents with at least two distinct half-activation voltages at −63 and 12 mV (Rusznak and Szucs 2009; Schrader et al. 2002), raising the possibility that the current consists of heterotetrameric channels of the three Kv1 α-subunits at different combinations. Meanwhile, among the Kv subunits tested for expression profiles in SGNs (Kv1.1, 1.2, and 1.6), only Kv1.2 has distinct differential expression in type I and II neurons (Amarillo et al. 2008), suggesting cell type-specific functions. To understand the roles of Kv1.2 subunits in SGNs, we examined the functional features of Kcna2 null mutant (Kcna2−/−) neurons (Brew et al. 2007).
We report that null deletion of Kcna2 produced an unexpected result, which showed profound membrane hyperpolarization of SGNs. This effect resulted in increased membrane excitability. In accord with enhanced membrane hyperpolarization, further analyses of K+ currents of Kcna2−/− SGNs revealed an apparent approximately twofold increase in transient outward K+ currents. We have demonstrated that in vivo and in vitro association of Kv1.2 and Kv1.4 α-subunits is the mechanism underlying the unexpected results, providing insights into the complex interaction of K+ channel subunits in SGNs.
MATERIALS AND METHODS
Isolation of spiral ganglion neurons.
We conducted these experiments in agreement with the guidelines of the Institutional Animal Care and Use Committee of the University of California, Davis, which reviewed and approved the protocol. SGNs were isolated from the inner ear of postnatal day 10–12 (P10–12) mice using a combination of enzymatic and mechanical procedures as described in detail previously (Lv et al. 2010). We restricted our studies to P10–12 because Kcna2 null mutants (Kcna2−/−, hereafter referred to as Kv1.2−/−) do not survive past ∼14 days due to seizures (Brew et al. 2007). Male and female mice were euthanized, and the temporal bones were removed in a solution containing minimum essential medium with Hanks' salt (Invitrogen), kynurenic acid (0.2 g/l), 10 mM MgCl2, 2% fetal bovine serum (FBS; vol/vol), and glucose (6 g/l). Additionally, we used Kcna1 null mutants (Kcna1−/−, hereafter referred to as Kv1.1−/−) and their age-matched controls at P10–12 to conduct a set of experiments. The central spiral ganglion tissue was dissected out and split into three equal segments (apical, middle, and basal) across the modiolar axis. We pooled three mice into each SGN culture. Because Kv1.2−/− mice do not live to be more than 2 wk old, chances of obtaining mutant litters were less than the expected Mendelian ratio of 25%. Thus, for pragmatic reasons and paucity of null mutants, isolated SGNs were divided not by their location along the cochlear contour (Lv et al. 2010, 2012) but by their extent of excitability (see results).
The inner ear tissues were digested separately in an enzyme mixture containing collagenase type I (1 mg/ml) and DNase (1 mg/ml) at 37°C for 20 min. After a series of gentle triturations and centrifugation in 0.45 M sucrose, the cell pellets were reconstituted in 900 ml of culture medium [Neuralbasal-A; supplemented with 2% B27 (vol/vol), 0.5 mM l-glutamine, and 100 U/ml penicillin; Invitrogen], and filtered through a 40-μm cell strainer for cell culture and electrophysiological experiments. For adequate voltage-clamp and satisfactory electrophysiological experiments, we cultured SGNs for ∼24–48 h to allow for the detachment of Schwann cells from neuronal membrane surfaces. Additionally, by recording from neurons ∼24–48 h after dissociation, we avoided neurons with extensive neurite outgrowth to reduce space-clamp problems. Moreover, to minimize space-clamp artifacts, we targeted spherical neurons with reduced neurite outgrowth for voltage-clamp experiments (Bar-Yehuda and Korngreen 2008). All electrophysiological experiments were performed at room temperature (21–22°C). Reagents were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise specified.
Electrophysiology.
Action potentials were amplified (100×), filtered (bandpass 2–10 kHz), and digitized at 5–500 kHz. Extracellular solution for action potential recording experiments contained (in mM) 130 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 d-glucose, and 10 HEPES, adjusted to pH 7.4 with NaOH. The recording electrodes contained (in mM) 112 KCl, 2 MgCl2, 0.1 CaCl2, 10 HEPES, 1 EGTA, 5 K2ATP, and 0.5 Na2GTP, adjusted to pH 7.3 with KOH. The Ca2+ concentrations in solutions were measured with a Ca2+-sensitive electrode as described previously (Yamoah et al. 1998).
Whole cell voltage-clamp recordings of K+ currents were performed on SGNs using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Current traces were amplified, filtered (bandpass 2 kHz), and digitized at 5–500 kHz using an analog-to-digital converter (Digidata 1200; Molecular Devices) as described previously (Levic et al. 2007; Rodriguez-Contreras and Yamoah 2001). Fire-polished electrodes (2–3 MΩ) were pulled from borosilicate glass. Outward K+ current traces were generated with depolarizing voltage steps from a holding potential of −90/−70 mV and stepped to varying positive potentials (ΔV = 2.5–10 mV). The cell capacitance was measured by fitting the current response elicited from the holding potential of −80 mV and stepped to −100 mV. The capacitative transients were used to estimate the capacitance of the cell as an indirect measure of cell size. The seal resistance was typically 10–20 GΩ. Currents were measured with capacitance and series resistance compensation (>60–90%). The series resistance was monitored during the course of the experiments. The liquid junction potentials were measured (<3 mV) and corrected. In addition to these standard requirements for acceptance of data, several basic criteria were set to ensure optimum quality of recordings and acceptance of data. These include 1) stabilization of seals for at least 5 min before recordings, 2) elimination of cells with current traces that exhibit signs of voltage inhomogeneities, and 3) exclusion of cells with more than 20% change in the series resistance before and during recordings.
Whole cell K+ current amplitudes at varying test potentials were measured at the peak and steady-state levels using a peak and steady-state detection routine. The current magnitude was divided by the cell capacitance (pF) to generate the current density-voltage relationship. Dendrotoxin-K (DTX-K) and α-dendrotoxin (α-DTX) were purchased from Alomone Labs (Israel). CP 339818 hydrochloride was purchased from Tocris Bioscience (Minneapolis, MN). Curve fits and data analyses were performed using Origin software (MicroCal, Northampton, MA). Where appropriate, we present data as means ± SD. The mean values (n) listed represent data for each experimental group. Significant differences between groups were tested using paired/unpaired Student's t-test.
Heterologous expression of Kv channels.
Kv1.2 (gene ID: NM_008417, Mammalian Gene Collection ID: 170276) and Kv1.4 (gene ID: NM_021275, Mammalian Gene Collection ID:124445) cDNA clones were purchased from Open Biosystems, and the open reading frames were subcloned into the pIRES2-EGFP expression vector for patch-clamp recordings. Chinese hamster ovarian (CHO) cells were obtained from American Type Culture Collection (Manassas, VA). CHO cells were maintained in Dulbecco's modified Eagle's medium containing 10% FBS (Invitrogen). Cell cultures were kept at 37°C in a 5% CO2 incubator. The cells were trypsinized, plated at a concentration of 1.5 × 105 cells/ml in 2 ml of culture medium in 35-mm dishes, and transfected with 1 μg of total DNA per dish. Transfections were performed using Lipofectamine, following the manufacturer's protocol (Invitrogen). The cells were rinsed in fresh culture medium and incubated for 24 h before patch-clamp recordings were performed. Transfected cells were identified for recording by visualization of the enhanced green fluorescent protein (EGFP) coexpression (BD Bioscience, Clontech).
For the study of subcellular and membrane localization of Kv subunits, two different epitopes, modified c-Myc and hemagglutinin (HA) tags, were inserted into pCMV-Kv1.2/1.4 constructs in which EGFP genes were eliminated. Modified HA and c-Myc epitopes were inserted at the end of the S1–S2 loop of Kv, as described previously for Kv7.4 channels (Kim et al. 2011). The S1–S2 loop amino acid sequences were changed to ETLPIFRDENEDMHGGEQKLISEEDLGVTFHTYSNSTIGYQQSTSFTDP for c-Myc-tagged Kv1.2 and ETLPEFRDDRDLIMALSAGYPYDVPDYAGHSRLLNDTSAPHLENSGHTIFNDP for HA-tagged Kv1.4 constructs; whole S1–S2 loop sequences are shown with epitopes in bold. Epitope-tagged constructs were generated by recombination polymerase chain reaction and verified by automated sequencing.
Immunocytochemistry.
For histological cryosection experiments, sedated [Avertin (2,2,2-tribromoethanol); 300 μg/g body wt ip] mice were transcardially perfused with 10 ml of PBS, followed by 10 ml of 4% paraformaldehyde in 0.1 M PBS. The temporal bones were removed, and the cochleae were perfused via the oval and round windows. The temporal bones were then immersed in fixative for 60 min. After fixation, the cochleae were decalcified (120 mM EDTA, pH 7.0; 24 h; ∼21°C). The cochleae were processed sequentially with 10% and 30% sucrose at 4°C overnight and then embedded in OTC compound for cryosection. Sections were washed in PBS, permeabilized in 0.1% Triton X-100 for 25 min, and then incubated for 30 min in a blocking solution containing 1% bovine serum albumin and 1% goat serum. The 10-μm sections were incubated with K+ channel antibodies against Kv1.2, residues 463–480, and Kv1.4, residues 14–35 (NeuroMab Facility, Davis, CA), at 1:100 to 1:500 dilutions. To identify neurons, samples were counterstained with an antibody against the neuronal marker TUJ1 as described previously (Lv et al. 2010). Cells/tissues were then incubated with appropriate secondary antibodies for 2 h, washed and mounted using antifade mounting medium, and viewed with a Zeiss LSM 510 confocal microscope.
RESULTS
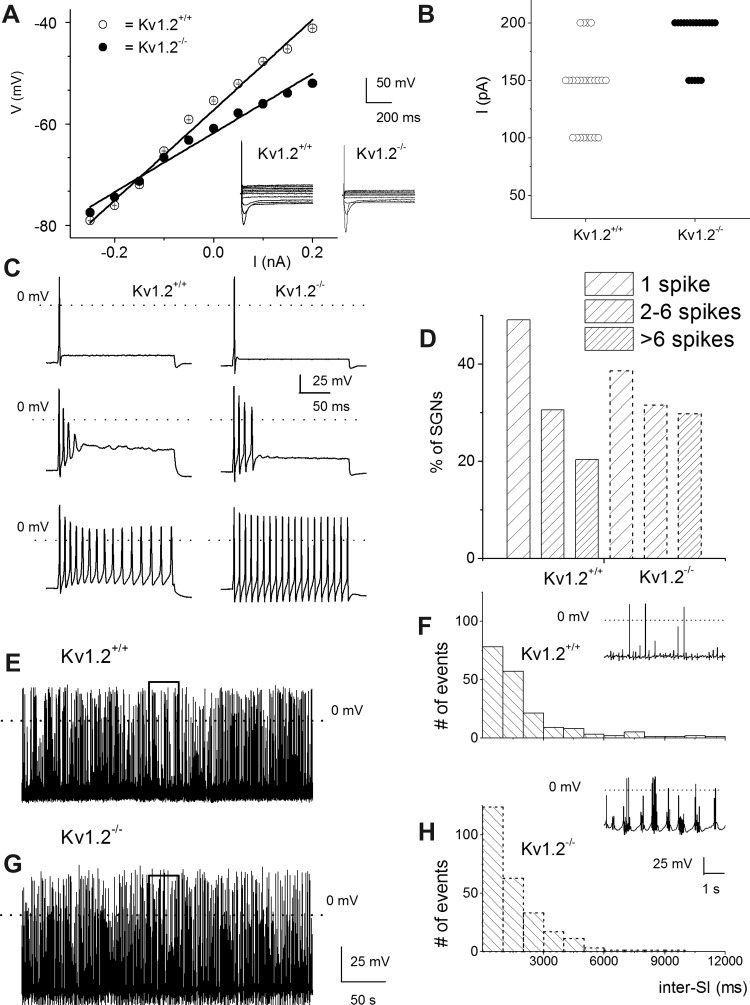
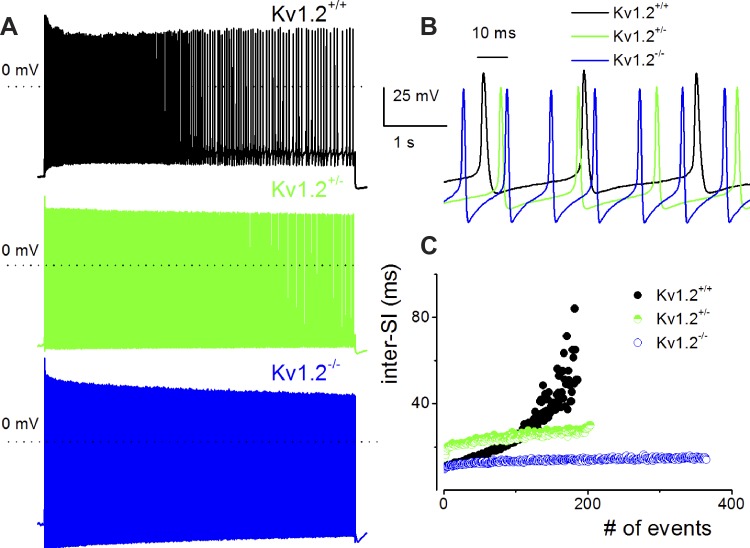
The voltage-dependent K+ channel Kv1.2 is recognized as a component of the DTX-sensitive K+ currents in SGNs, clamping the cells close to the K+ reversal potential (EK) and controlling adaptation of spike activity (Adamson et al. 2002; Mo et al. 2002). To examine the specific roles of Kv1.2 in SGN membrane properties, we determined the resting input resistance of Kv1.2−/− compared with their age-matched control wild types (WT; Kv1.2+/+). Despite removal of a prominent K+ current in SGNs, the input resistance of Kv1.2−/− neurons was reduced significantly from 89.9 ± 3.2 to 58.2 ± 3.0 MΩ (P < 0.05, n = 15; Fig. 1A). Consequently, the current required to elicit spike activity was raised (Fig. 1B). Isolated SGNs were divided not by their location along the cochlear contour (Lv et al. 2010, 2012) but by their extent of excitability. SGNs were divided into four classes according to the number of spikes per stimulus: 1) one spike, 2) two to six spikes, 3) more than six spikes, and 4) spontaneously active neurons (Fig. 1, C–E). Further examination of the RMP showed that not only were the Kv1.2−/− neurons more hyperpolarized than the Kv1.2+/+ neurons, the mutants were paradoxically more excitable (Figs. 1 and 2; Table 1). In spontaneously active neurons, the null mutants portrayed characteristic burst activity punctuated by shorter interspike intervals with robust afterhyperpolarization that were less prominent in Kv1.2+/+ neurons (Fig. 1, G and H). The distribution of the membrane properties of neurons favored an increased number of excitable cells in the null mutants (Fig. 1H). Figure 2 and Table 1 show the summary data of the spike properties of Kv1.2+/+ and Kv1.2−/− SGNs.
Fig. 1.
Null deletion of voltage-dependent potassium (Kv) channel subunit Kv1.2 results in altered membrane properties of spiral ganglion neurons (SGNs). A: SGNs from postnatal day 12 (P12) Kv1.2+/+ and Kv1.2−/− mice were isolated and cultured for 48 h. The steady-state input resistance was measured from membrane potentials (V) as a function of injected currents (I). Inset shows of a family of traces obtained from Kv1.2+/+ (left) and Kv1.2−/− neurons (right). Null deletion of Kv1.2 decreased the steady-state input resistance from 88.9 ± 3.2 MΩ in the wild-type neurons to 58.2 ± 3.0 MΩ (n = 15, P < 0.05). Data consist of neurons with 1 spike and 2–6 spikes per stimuli (see C). B: summary data of current thresholds required to generate action potentials in Kv1.2+/+ (○) and Kv1.2−/− neurons (●). C: representative action potentials were recorded by injecting a 0.2-nA current for a 200-ms duration. Quiescent neurons at rest were classified into 3 groups: neurons that generate 1 (top), 2–6 (middle), and >6 spikes per stimulus (bottom). For Kv1.2+/+, the number of neurons used for data analyses are as follows: 1 spike, n = 29; 2–6 spikes, n = 18; and >6 spikes, n = 12; and for Kv1.2−/−: 1 spike, n = 22; 2–6 spikes, n = 18; and >6 spikes, n = 17. D: histogram depicts proportions of SGNs in Kv1.2+/+ (solid outline bars) and Kv1.2−/− neurons (dashed outline bars) representing the different firing patterns. For SGNs, which display spontaneous activity, differences in the firing patterns were not discernible between Kv1.2+/+ (E) and Kv1.2−/− neurons (G). We found ∼10% of Kv1.2+/+ and 18% of Kv1.2−/− SGNs (total of 62 cells) to be spontaneously active. However, analyses of the interspike intervals (inter-SI) showed that spike activity in Kv1.2+/+ was less than that of Kv1.2−/− neurons (H). Insets in F and H portray the differences in the spike burst patterns between Kv1.2+/+ and Kv1.2−/− neurons.
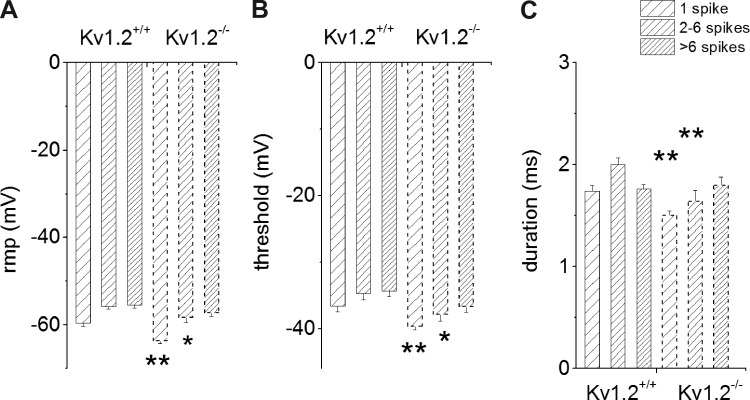
Fig. 2.
Properties of action potentials in SGNs from Kv1.2+/+ and Kv1.2−/− mice. Action potentials were recorded by injecting a 0.2-nA current for a 200-ms duration. We used SGNs from P12 Kv1.2+/+ and Kv1.2−/− mice. A: summary data describe alterations in the resting membrane potential (RMP) in Kv1.2+/+ and Kv1.2−/− SGNs. B: changes in the afterhyperpolarization potential (AHP). C: changes in duration of the action potentials. For Kv1.2+/+, the number of neurons used for analyses are as follows: 1 spike, n = 29; 2–6 spikes, n = 18; and >6 spikes, n = 12; and for Kv1.2−/−: 1 spike, n = 22; 2–6 spikes, n = 18; and >6 spikes, n = 17. *P < 0.05; **P < 0.01. Other parameters of action potential properties that remained statistically unchanged are presented in Tables 1 and 2.
Table 1.
Properties of action potentials in spiral ganglion neurons in wild-type and knockout mice
| 1 Spike |
2–6 Spikes |
>6 Spikes |
||||
|---|---|---|---|---|---|---|
| WT, n = 29 | Kv1.2 KO, n = 22 | WT, n = 18 | Kv1.2 KO, n = 18 | WT, n = 12 | Kv1.2 KO, n = 17 | |
| RMP, mV | −59.59 ± 3.52 | −63.61 ± 2.79† | −55.72 ± 2.43 | −58.33 ± 3.64* | −55.48 ± 2.15 | −57.19 ± 2.93 |
| Threshold, mV | −36.61 ± 4.15 | −39.6 ± 2.78† | −34.71 ± 3.52 | −37.83 ± 3.18* | −34.29 ± 3.04 | −36.65 ± 2.79 |
| Latency, ms | 2.27 ± 0.89 | 2.82 ± 1.01 | 1.67 ± 0.36 | 1.8 ± 0.53 | 2.21 ± 0.81 | 2.18 ± 0.47 |
| Duration, ms | 1.74 ± 0.29 | 1.5 ± 0.22† | 2 ± 0.26 | 1.64 ± 0.33† | 1.77 ± 0.16 | 1.79 ± 0.27 |
| Amplitude, mV | 90.22 ± 8.92 | 91.91 ± 9.53 | 91.43 ± 15.66 | 96.58 ± 11.1 | 100.54 ± 13.83 | 96.61 ± 9.83 |
| AHP, mV | −29.3 ± 5.95 | −32.21 ± 3.95 | −25.6 ± 5.42 | −29.8 ± 4.26 | −29.25 ± 6.14 | −32.68 ± 6.25 |
| Rise slope 10–90%, mV/ms | 166.59 ± 48.81 | 235.21 ± 48.23† | 127.89 ± 45.25 | 173.82 ± 59.44* | 157.01 ± 63.81 | 147 ± 43.06 |
| Decay slope 10–90%, mV/ms | −117.26 ± 26.43 | −181.14 ± 29.81† | −109.05 ± 14.35 | −148.25 ± 37.42† | −124.55 ± 24.28 | −148.83 ± 45.95 |
| First inter-SI, ms | 9.64 ± 1.58 | 9.8 ± 1.93 | 11.55 ± 5.19 | 10.12 ± 3.03 | ||
| ΔV to threshold, mV | 22.99 ± 3.16 | 24.01 ± 3.37 | 21.01 ± 2.65 | 20.5 ± 2.19 | 21.18 ± 2.87 | 20.54 ± 3.4 |
Values are means ± SD for neurons from wild-type (WT) and Kv1.2 knockout (KO) mice; neuron groups generate 1, 2–6, or >6 spikes per stimulus. RMP, resting membrane potential; inter-SI, interspike interval; ΔV, change in potential
P < 0.05;
P < 0.01.
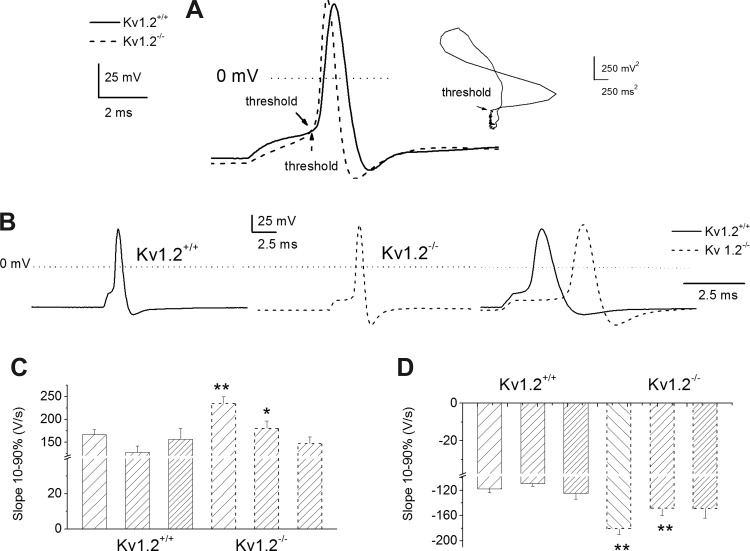
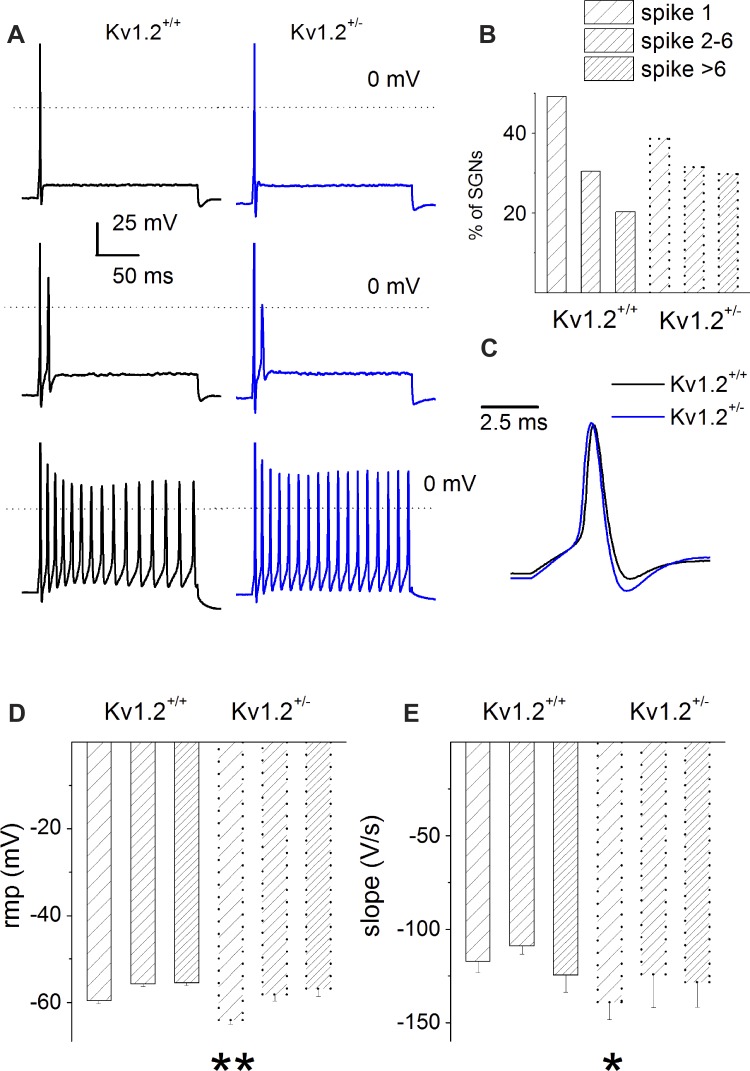
Next, we examined the features of individual spikes in the Kv1.2+/+ and Kv1.2−/− SGNs. The threshold of activation of action potentials was derived from the voltage at which the second derivative turned positive, as shown in the inset with an arrow in Fig. 3A. To avoid the masking effects of input stimulus artifacts, action potentials were elicited using very brief pulses (0.05 ms; Fig. 3C). There were significant differences in these properties of action potentials between the Kv1.2+/+ and Kv1.2−/− SGNs: RMP, threshold of activation, duration, and the rise-and-fall phases of action potentials recorded from one-spike and two-to-six-spike SGNs (Fig. 3, B–D; Table 1). For neurons that elicited more than six spikes per stimulus, action potential properties remained statistically unchanged between the Kv1.2+/+ and Kv1.2−/− SGNs (Fig. 3, D and E; Table 1). The results raise the possibility that fast-adapting SGNs may express larger Kv1.2 currents than their slowly adapting counterparts. Additionally, it can be inferred from alterations in action potential properties that after null deletion of Kv1.2, outward currents were enhanced. To identify subtle differences that may explain the functions of Kv1.2 channels, we recorded from SGNs isolated from Kv1.2+/−, which carries only one copy of the gene (heterozygous), and compared their spike features with those of the Kv1.2+/+ neurons (Fig. 4, A–E; Table 2). Deletion of one copy of Kv1.2 was sufficient to produce significant changes in the RMP and the negative slope of action potentials, but not in the threshold of activation, duration, and positive slope of spike waveforms (Table 2).
Fig. 3.
Alterations in spike properties in SGNs between Kv1.2+/+ and Kv1.2−/−. A: representative action potentials were elicited by a brief (<0.05 ms) current injection into P12 SGNs from Kv1.2+/+ (solid line) and Kv1.2−/− (dashed line). The voltage at which the first and second differential turned positive was considered the threshold voltage (inset: arrow). B: injection of a 0.25-nA current for a 0.05-ms duration produced a single spike in SGNs in Kv1.2+/+ (left) and Kv1.2−/− neurons (middle), showing similar changes in spike properties. At right, the wild-type and mutant action potentials are superimposed. C and D: summary data show alteration in rise slope 10–90% (C) and decay slope 10–90% (D) in SGNs between Kv1.2+/+ and Kv1.2−/− mice. For Kv1.2+/+, the number of neurons used for analyses are as follows: 1 spike, n = 29; 2–6 spikes, n = 18; and >6 spikes, n = 12; and for Kv1.2−/−: 1 spike, n = 22, 2–6 spikes, n = 18; and >6 spikes, n = 17. *P < 0.05; **P < 0.01.
Fig. 4.
Characteristics of action potentials in P12 SGNs. A: representative action potentials were recorded by injecting a 0.2-nA current for a 200-ms duration in SGNs isolated from Kv1.2+/+ (black) and Kv1.2+/− mice (blue). Examples of neurons with 1 (top), 2–6 (middle), and >6 spikes per stimulus (bottom) are shown. B: histogram depicts the percentages of SGNs with different firing patterns. C: single action potentials from Kv1.2+/+ (black) and Kv1.2+/− SGNs (blue) are superimposed for comparison. Data are from neurons with 1 spike per stimulus. D: changes in RMP for Kv1.2+/+ (solid outline) and Kv1.2+/− (dotted outline). E: summary data of the action potential decay slope 10–90%. For Kv1.2+/+, the number of neurons used for analyses are as follows: 1 spike, n = 29, 2–6 spikes, n = 18; and >6 spikes, n = 12; and for Kv1.2+/−: 1 spike, n = 13; 2–6 spikes, n = 11; and >6 spikes, n = 10. *P < 0.05; **P < 0.01.
Table 2.
Properties of action potentials in spiral ganglion neurons in wild-type and mutant mice
| 1 Spike |
2–6 Spikes |
>6 Spikes |
||||
|---|---|---|---|---|---|---|
| WT, n = 29 | Kv1.2 Het, n = 13 | WT, n = 18 | Kv1.2 Het, n = 11 | WT, n = 12 | Kv1.2 Het, n = 10 | |
| RMP, mV | −59.59 ± 3.52 | −64.12 ± 3.06† | −55.72 ± 2.43 | −58.18 ± 3.57* | −55.48 ± 2.15 | −56.83 ± 4.64 |
| Threshold, mV | −36.61 ± 4.15 | −39.54 ± 2.46* | −34.71 ± 3.52 | −35.83 ± 5.01 | −34.29 ± 3.04 | −38.15 ± 4.87 |
| Rise slope 10–90%, mV/ms | 166.59 ± 48.81 | 180.51 ± 41.88 | 127.89 ± 45.25 | 156.5 ± 64.61 | 157.01 ± 63.81 | 160.26 ± 48.24 |
| Decay slope 10–90%, mV/ms | −117.26 ± 26.43 | −139.11 ± 30.36* | −109.05 ± 14.35 | −124.04 ± 43.88 | −124.55 ± 24.28 | −128.4 ± 35 |
Values are means ± SD for neurons from WT and Kv1.2 heterozygous (Het) mice; neuron groups generate 1, 2–6, or >6 spikes per stimulus
P < 0.05;
P < 0.01.
By virtue of their sensitivity to DTX, one of the ascribed functions of Kv1.2 channels is to regulate the rate of accommodation and spike timing in SGNs, and thus regulating information coding to the cochlear nucleus (Mo et al. 2002; Pongs 1999). In Fig. 5, we illustrate examples of spike activity in slowly adapting SGNs from Kv1.2+/+, Kv1.2+/−, and Kv1.2−/− mice. On deletion of one copy of Kv1.2, the number of spikes increased, which was enhanced further in the null mutant SGNs (Fig. 5B). Moreover, spike activity and timing, represented as interspike intervals, were altered (Fig. 5C). Whereas the WT neurons produced trains of spikes that were irregular, showing a wide range of interstimulus intervals (∼1–80 ms), removal of Kv1.2 channel currents resulted in firing patterns with regular interspike intervals (∼15 ms). The interspike interval of the heterozygous mutants was fairly regular (∼15–30 ms).
Fig. 5.
Alterations in firing pattern in SGNs after null and partial deletion of Kv1.2. A: action potentials were recorded from SGNs isolated from Kv1.2+/+, Kv1.2+/−, and Kv1.2−/− mice by injecting a 0.2-nA current for a 5-s duration. Dashed lines indicate 0 mV. Trains of spikes from Kv1.2+/+ (black), Kv1.2+/− (green), and Kv1.2−/− (blue) are shown. B: magnified segment of the action potential profiles in Kv1.2+/+ (black), Kv1.2+/− (green), and Kv1.2−/− (blue). Note the prominent AHP after null deletion of Kv1.2. C: plot of inter-SIs reveals unambiguous transformation of irregular to regular inter-SI between Kv1.2+/+, Kv1.2+/−, and Kv1.2−/− SGNs.
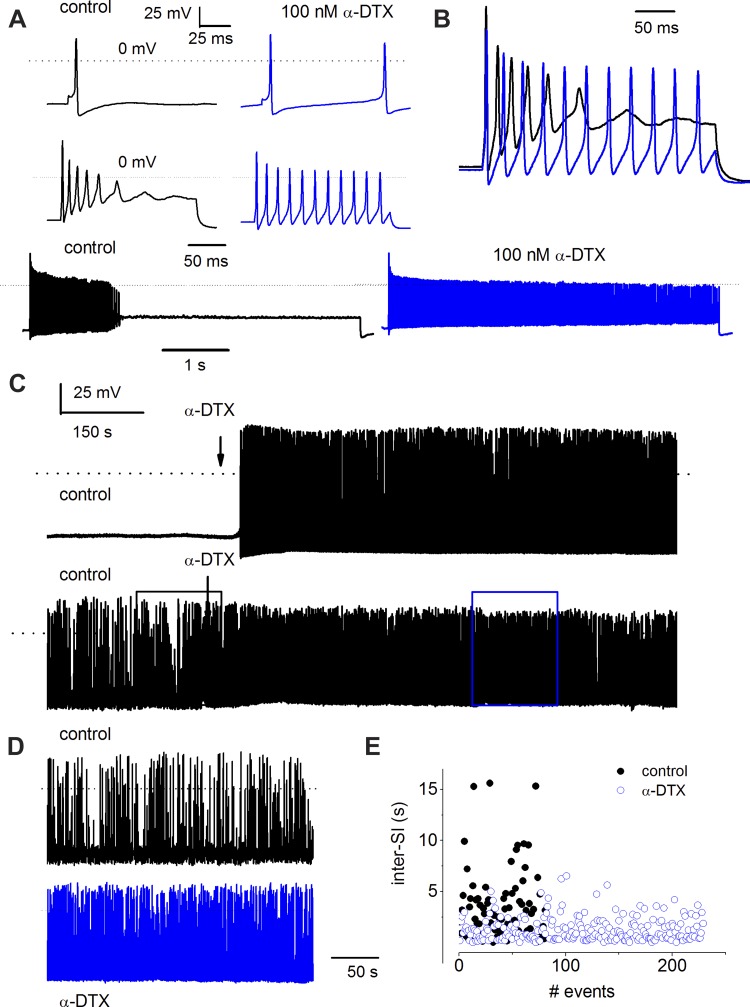
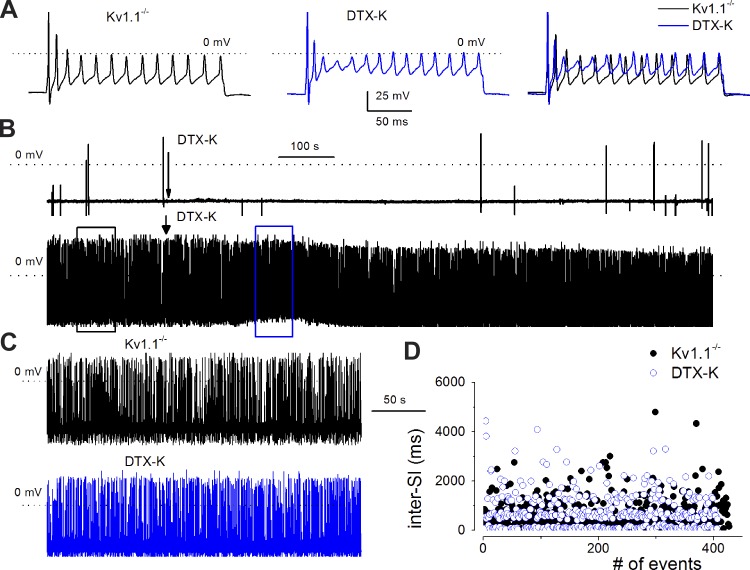
At least three Kv1 channels, namely, Kv1.1, 1.2, and 1.6 subtypes and currents, have been identified biochemically and pharmacologically (sensitivity toward α-DTX and DTX-K) in SGNs (Mo et al. 2002; Rusznak and Szucs 2009). Thus it is expected that in the Kv1.2 null mutant neurons, Kv1.1 and 1.6 are fully operational. To determine the roles of Kv1 channel subtypes in SGNs, we examined the effects of α-DTX and DTX-K on Kv1.2+/+ and Kv1.1−/− neurons (Fig. 6 and 7). The application of α-DTX resulted in an increase in spike frequency in Kv1.2+/+ neurons. For fast-adapting SGNs that elicit a single action potential on stimulation, increasing the magnitude of injected current was not sufficient to evoke subsequent spikes. However, application of 100 nM α-DTX was sufficient to render fast to slowly adapting neurons (Fig. 6, A and B). Indeed, a similar concentration of the toxin transformed quiescent SGNs to spontaneously active cells, and for neurons that were spontaneously active to begin with, α-DTX further enhanced the spike frequency (Fig. 6C). Moreover, analyses of spike timing revealed a transformation of neurons with irregular interspike intervals to those with a virtually uniform spiking pattern (Fig. 6, D and E), consistent with data described in Fig. 5 for the Kv1.2−/− SGNs. Because DTX-K blocks both Kv1.1 and 1.2, we surmised that by applying the toxin on SGNs isolated from the Kv1.1−/− mice, the distinct function of Kv1.2 could be unmasked. DTX-K did not produce substantial or significant changes in evoked action potential (Fig. 7A), and it altered neither the spike frequency nor timing in spontaneously active SGNs in Kv1.1−/− mice (Fig. 7, C and D). It can be inferred from these results that upon null deletion of Kv1.1, Kv1.2 association with other subtypes of Kv1 channels renders the heteromeric channels insensitive to DTX-K. Although not mutually exclusive, the other alternative is that the purported association of Kv1.2 and 1.6 (Rusznak and Szucs 2009) may not be prominent in SGNs.
Fig. 6.
α-Dendrotoxin (α-DTX) increases the excitability of P12 SGNs in Kv1.2+/+ mice. A: action potentials were evoked by a brief pulse (0.05 ms) to elicit spikes in fast (top)- and slow-adapting SGNs (middle and bottom). Bath application of external solution containing 100 nM α-DTX resulted in increased spike activity. B: 2 superimposed traces recorded before (black) and after (blue) application of α-DTX. C: effects of α-DTX on 2 different types of SGNs: quiescent neurons at baseline (top) and spontaneously active neurons at baseline (bottom). Arrows indicate the time at which the external solution containing 100-nM α-DTX was perfused. α-DTX transformed quiescent neurons into spontaneously active neurons and accentuated the spike frequency of neurons that were spontaneously active at baseline. D: comparison of the spike activity in the spontaneously active neuron (top) and after application of α-DTX (bottom). E: analyses of the inter-SIs with respect to the number of events. α-DTX modified the irregular activity to a quasi-regular activity.
Fig. 7.
Dendrotoxin-K (DTX-K) does not produce alterations in membrane excitability in SGNs from Kv1.1−/− mice. A: DTX-K is known to block Kv1.1 and 1.2 currents. By using DTX-K on Kv1.1−/− SGNs, we surmised that the bona fide effects of Kv1.2 on membrane properties of SGNs could be unraveled pharmacologically. Action potentials were evoked by injecting a 0.2-nA depolarizing current for 200 ms in Kv1.1−/− SGNs. Bath perfusion of 100 nM DTX-K produced no visible effects on the membrane properties. Left, evoked spikes from Kv1.1−/− SGNs (black); middle, evoked spikes after bath perfusion of external solution containing 100 nM DTX-K (blue); right, superimposition of the left and middle traces for comparison. B: membrane properties of 2 Kv1.1−/− SGNs are shown for a fairly quiescent neuron (top) and a spontaneously active neuron (bottom). Dashed lines indicate 0 mV. Arrows show the time of application of DTX-K (100 nM). There were no noticeable effects on the membrane properties after application of DTX-K. Similar data were obtained from 19 different neurons. C: segments of traces in a spontaneously active SGN from Kv1.1−/− for control (black) and after application of DTX-K (blue). D: analysis of inter-SIs in a spontaneously active Kv1.1−/− SGN (●) and after application of DTX-K (○). DTX-K had no measurable effects on Kv1.1−/− SGNs (n = 17).
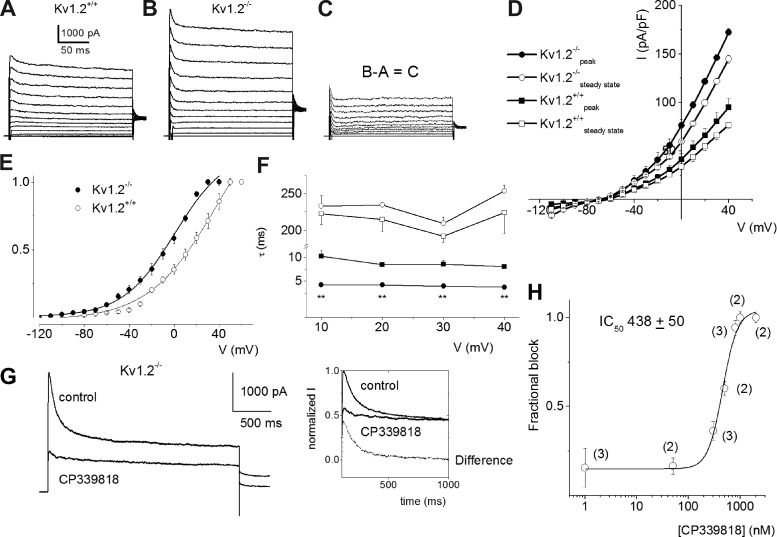
The functional paradox of apparent increase in the magnitude of outward currents after deletion of Kv1.2 in SGNs remains unresolved. To address the mechanism, we performed a voltage-clamp experiment on isolated SGNs to examine outward K+ currents in Kv1.2−/− and their age-matched Kv1.2+/+ controls. Not surprisingly, the whole cell outward current density was enhanced in Kv1.2−/− compared with the Kv1.2+/+ controls. Figure 8 describes the whole cell outward current profile in Kv1.2−/− SGNs and Kv1.2+/+ SGNs, which suggests that there is an increase in the magnitude of a transient K+ current following null deletion of Kv1.2. For example, at 0-mV step voltage, the magnitude of the whole cell K+ current density was ∼2-fold greater in the Kv1.2−/− than in the Kv1.2+/+ controls (0 mV: 77.1 ± 6.5 pA/pF in Kv1.2+/+, 41.0 ± 6.2 pA/pF in Kv1.2−/−, P < 0.05, n = 18; Fig. 8, A and B). The difference between the WT and null mutant currents revealed a transient current with a faster activation profile (Fig. 8, C and D). Analyses of the voltage dependence of the tail current indicated that the apparent increase in the whole cell current amplitude can be seen as a leftward shift in the sensitivity of the whole cell K+ current by ∼20 mV in Kv1.2−/− neurons (Fig. 8E). The kinetics of the voltage-dependent activation were also altered, because the mutant neurons expressed K+ currents that had ∼2-fold faster time constant (τ) of activation than the Kv1.2+/+ neurons (Fig. 8F). To identify the nature of the current that was upregulated in the null mutant SGNs, we tested the sensitivity of the whole cell current in the presence of low concentrations of 4-aminopyridine (4-AP; 100 μM). The difference current was virtually abolished in the presence of low concentrations of 4-AP (data not shown). Next, we streamlined on the potential current that was upregulated by testing the sensitivity of the current to CP 339818 hydrochloride, a nonpeptide specific blocker of Kv1.3 and 1.4 channels. As shown in Fig. 8G, the drug inhibited a sizable portion of the transient current, which is further depicted in the form of normalized current in the inset. The fractional block versus the concentration of CP 339818 yielded a 50% inhibitory concentration (IC50) of ∼440 nM (Fig. 8H). Since the CP 339818-sensitive current was a transient current, it could be inferred that it was derived from Kv1.4 channel activity.
Fig. 8.
Whole cell K+ currents recorded from SGNs from Kv1.2+/+ and Kv1.2−/− mice. Before the switch to voltage-clamp configuration, the membrane properties of the SGN were determined and classified as 1, 2–6, or >6 spikes per stimulus. A: representative K+ current traces were recorded from SGNs from the 1-spike group of Kv1.2+/+ mice (n = 18). A family of current traces obtained from a holding potential of −90 mV and stepped from −110 to + 40 mV using ΔV of 10 mV. B: SGNs from the 1-spike group of Kv1.2−/− mice (n = 18) showed similar results. C: difference K+ current traces between Kv1.2+/+ and Kv1.2−/− SGNs. D: current density-voltage curves obtained from Kv1.2+/+ (n = 15) and Kv1.2−/− SGNs (n = 14). The peak and steady-state current densities are shown as means ± SD. E: steady-state activation curves were generated from tail currents following a 50-ms family of voltage steps. The activation curves were fitted to the Boltzmann function in the form I/Imax = {1 + exp[(V1/2 − V)/k]}−1, where V1/2 is the half-activation voltage and k is a slope factor. V1/2 for the K+ current recorded from Kv1.2+/+ neurons (○) was 13.6 ± 2.1 mV and k = 23 ± 1 mV (n = 11). For Kv1.2−/− neurons (●), V1/2 was −1.1 ± 1.2 mV and k = 19.8 ± 3.2 mV (n = 9). F: time constant (τ) of inactivation of currents in Kv1.2+/+ (squares) and Kv1.2−/− (circles). Two τ constants, fast and slow, were obtained from the inactivation time courses. **P < 0.01 (n = 12). G: representative traces showing the effect of CP 339818 hydrochloride (1 μM) on outward currents recorded from Kv1.2−/− SGNs. To determine the drug-sensitive component, we normalized the current against the control and examined the difference current plotted with a dotted line (inset). H: the dose-response relation yielded a 50% inhibitory concentration (IC50) of 438 ± 50 nM. The numbers of cells tested are indicated in parentheses.
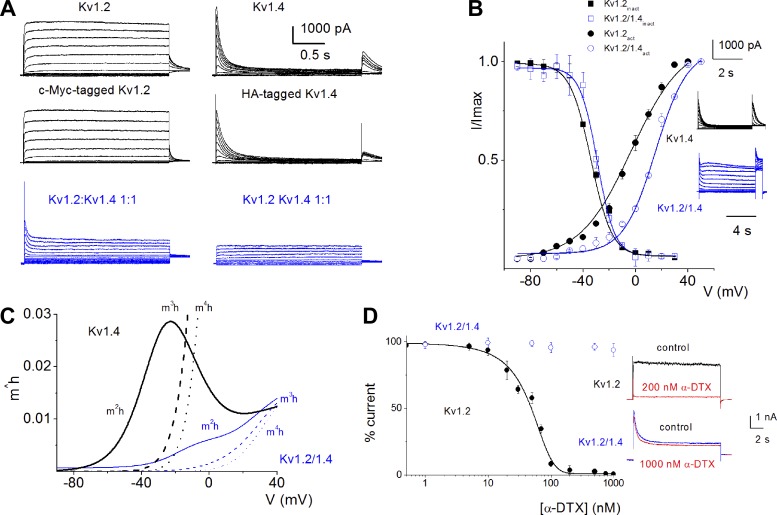
To further ascertain the identity of the difference current, we invoked the reductionist assertion that Kv1 channel subtypes form heteromeric complexes with members of the same family of channels. Since the resultant outward current after null deletion of Kv1.2 showed prominent inactivation and the difference current had a rapid onset and decay, we further surmised that the current may be derived from the activity of the Kv1.4 channel subtype. To test whether the findings from our analyses could be explained, we expressed mouse Kv1.2 and 1.4 singly or in combination at different ratios in CHO cell lines. In Fig. 9, we show exemplary current profiles recorded from CHO cells 24 h after transfection of Kv1.2 or Kv1.4 alone and in combination (Fig. 9A). We examined the voltage dependence of the steady-state inactivation as depicted with representative current traces when Kv1.4 was expressed alone and in combination with Kv1.2 at a 1:1 ratio (Fig. 9B). The voltage dependence of the resulting currents revealed several marked differences: 1) When expressed alone, Kv1.4 currents activate at a more negative potential than in combination with Kv1.2 such that the activation voltage for Kv1.4 alone was approximately −75 mV and V1/2(act) was −7.1 ± 0.6 mV (n = 14). Meanwhile, the combined (Kv1.2/1.4) current activation voltage was approximately −40 mV, and the estimated V1/2(act) was 13.3 ± 1.8 mV (n = 15). 2) The steady-state inactivation curves for Kv1.4 current alone had V1/2(inact) = −33.7 ± 1.2 mV (n = 17) compared with the combined (Kv1.2/1.4) current, which had V1/2(inact) = −29.1 ± 2.2 mV (n = 17). 3) Consequently, a marked window current ensued between approximately −75 and −40 mV for the homomeric Kv1.4 current in contrast to the heteromeric Kv1.2/1.4 current (Fig. 9C). The kinetics of activation and inactivation also revealed fast onset and decay for the Kv1.4 currents compared with the Kv1.2/1.4 combined currents. 4) Lastly, the magnitude of the homomeric Kv1.4 current was comparable to the heteromeric Kv1.2/1.4 current. At a step potential of 40 mV the current density for Kv1.4 and Kv1.2/1.4 currents were 75.9 ± 10.3 and 77.1 ± 15.8 pA/pF (n = 13, P = 0.82). Recent studies have demonstrated that functional heteromeric channels can express distinct pharmacology compared with the homomeric channels (Chen et al. 2010). We examined the sensitivity of homomeric Kv1.2 and heteromeric Kv1.2/1.4 to α-DTX. Whereas homomeric Kv1.2 channel currents showed marked sensitivity toward α-DTX with an IC50 of ∼50 nM (49.3 ± 1.8 nM, n = 8), currents derived from heteromeric Kv1.2/1.4 were impervious to α-DTX (Fig. 9D).
Fig. 9.
Properties of mouse (m)Kv1.2 and mKv1.4 channel currents. Whole cell K+ currents were recorded upon expressing the mKv1.2 and mKv1.4 channels singly and after coexpression of mKv1.2 and mKv1.4 (mKv1.2/1.4) at a 1:1 ratio. A: representative current traces for a family of K+ currents obtained from a holding potential of −90 mV and stepped from −90 to 30 mV using ΔV = 10 mV (top). c-Myc-tagged mKv1.2 and hemagglutinin (HA)-tagged mKv1.4 show similar current profiles (middle), suggesting that membrane expression and functions of the K+ channels remained intact despite introduction of HA and c-Myc tags. Chinese hamster ovary (CHO) cells were transfected with mKv1.2/1.4 at a ratio of 1:1. Cells were held at a holding potential of −90 mV and stepped from −90 to 30 mV using ΔV = 10 mV (bottom left, blue). The same cell was held at a holding potential of −30 mV and stepped to similar varying voltages (bottom right, blue). At a holding potential of −30 mV, the transient current was inactivated. B: activation curves of normalized peak tail current of mKv1.2/1.4 (blue ○; n = 15) and mKv1.4 (black ●; n = 14) are shown for comparison. Using the Boltzmann function (see Fig. 8 legend), the V1/2 of activation [V1/2(act)] for mKv1.4 alone was −7.1 ± 0.6 mV and k = 22.5 ± 3.8 mV (n = 14). Moreover, for mKv1.2/1.4 (1:1), the V1/2(act) was 13.3 ± 1.8 mV and k = 19.6 ± 4.3 mV (n = 15). A standard steady-state inactivation protocol was used by holding the cells at −90 mV and stepped to varying prepulses from −90 to 30 mV (ΔV = 10 mV). After a brief (2 ms) gap at −90 mV to allow for deactivation, the steady-state inactivation was tested at 10 mV. Insets are the representative traces (Kv1.4 in black, Kv1.2/1.4 1:1 in blue). Graph shows voltage dependence of inactivation of mKv1.4 (black ■) and mKv1.2/1.4 currents (blue □). C: using the activation (m) and inactivation (h) variables determined from the fits derived from the Boltzmann functions, we employed the HH formalism to determine the “window” current. Solid black solid line (m2h) represents currents derived from mKv1.4 alone and shows a window current that is active at the RMP of SGNs. Dashed black line, m3h; dotted black line, m4h. The fits for mKv1.2/1.4 (1:1) showed a current that only turns on at voltages positive to −40 mV, using m2h (blue solid line). Dashed blue line (m3h) and dotted blue line (m4h) turn on at voltages positive to −10 mV. Thus, for extrapolation purposes using m2h as the product of the state variables, the Kv1.4 homomeric channels are more likely to be active at the RMP of SGNs than the Kv1.2/1.4 heteromeric channels. D: dose-response curve generated using different concentrations of α-DTX on CHO cells expressing homomeric Kv1.2 channels (●). The IC50 was 49.3 ± 1.8 nM (n = 8). The heteromeric Kv1.2/1.4 channel currents were insensitive to α-DTX (○). Shown in insets are representative traces (control, black solid lines) and the effects of α-DTX (red lines) in homomeric Kv1.2 currents (top) and heteromeric Kv1.2/1.4 currents (bottom).
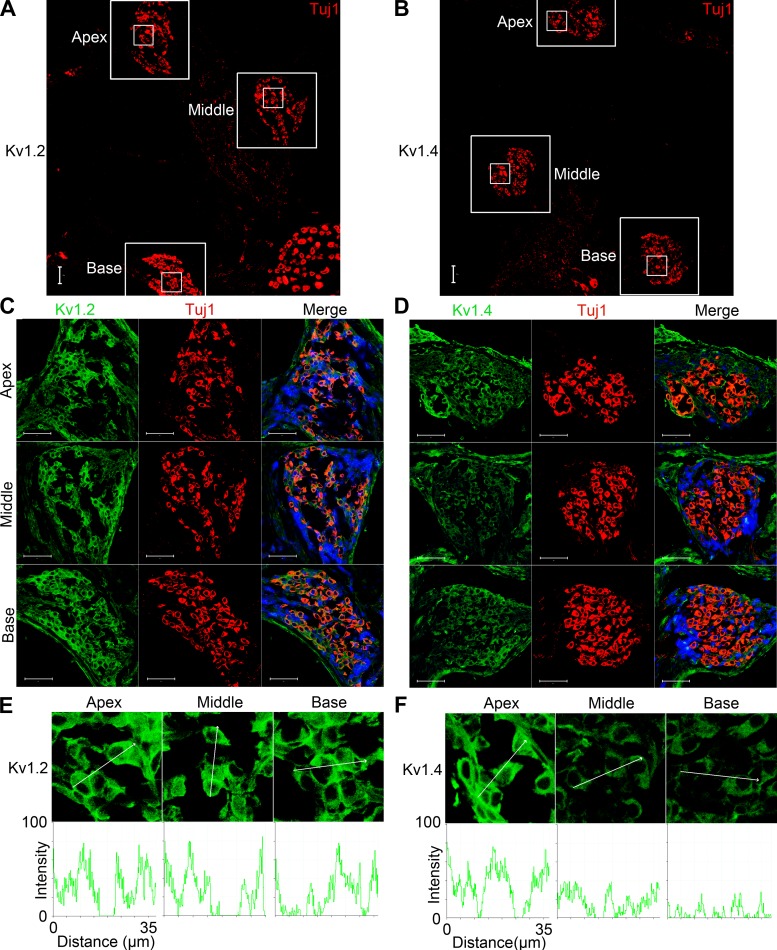
Using epitope-tagged Kv1.2 and Kv1.4 in nonpermeabilized conditions, we demonstrate in Fig. 10 that the two channel subtypes colocalized in the plasma membrane. Finally, we analyzed the expression profiles of Kv1.2 and 1.4 in SGNs. Since variations in the densities of different subtypes of K+ channels along the cochlear contour have been demonstrated, we examined the expression levels of the two Kv1 channels at the apical, middle, and basal turns (Fig. 11). Whereas positive immunoreactivity of Kv1.2 was uniform across the three turns of the cochlea, the intensity of Kv1.4 labeling was consistently (in 7 different preparations) higher at the apical and middle turns than at the base (Fig. 11).
Fig. 10.
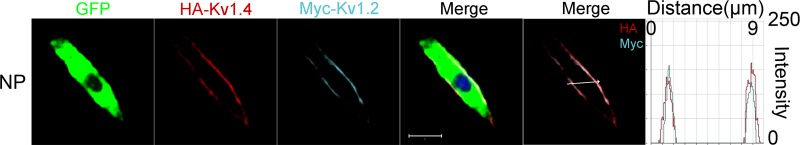
Detection of epitope-tagged wild-type mKv1.2 and mKv1.4 channels in CHO cells. c-Myc and HA epitopes were inserted into wild-type mKv1.2 and mKv1.4 channel extracellular domains, respectively. Farnesylated green fluorescent protein (GFP) was used as a reporter that binds to the plasma membrane. Expression of mKv1.2 and mKv1.4 was detected using anti-HA antibody (HA-Kv1.4) and anti-c-Myc antibody (Myc-Kv1.2) in nonpermeabilized (NP) conditions. Anti-c-Myc and anti-HA antibody stained only the channels expressed on the cell surface in NP cells. Detection of cell surface expression of c-Myc-tagged mKv1.2 and HA-tagged mKv1.4 channels shows coexpression. The overlap of cyan and red signals in the histogram in NP cells indicates their colocalization on the cell surface. Scale bar, 10 μm.
Fig. 11.
Expression of Kv1.2 and Kv1.4 in mouse cochlear sections. A and B: immunolabeling sections of the cochlea from P12 mice. SGNs (apical, middle, and basal) were labeled with the neuronal marker TUJ1 (red). C and D: photomicrographs of a magnified view of the apical, middle, and basal regions in the samples (large boxes in A and B, respectively). SGNs were labeled with antibodies against mKv1.2 and mKv1.4 (green). The nuclei (blue) were stained with DAPI, and images were merged (right). E and F: magnified view of SGNs (small boxes in A and B, respectively) shows expression pattern of Kv1.2 (E) and Kv1.4 channel (F) in apical, middle, and basal neurons (top). Fluorescence intensity is plotted with respect to distance across the neurons (bottom). Scale bars: for A and B, 100 μm; for C and D, 50 μm.
DISCUSSION
The mechanisms that regulate K+ channel subunit-specific heteromultimerization in native neurons remain unresolved. Because of the promiscuous association of Kv channel subtype families in neurons, it has been exceedingly difficult to predict and resolve the exact subunit assemblies that confer the native currents. Yet, Kv channel subtypes from the same family can have distinct current phenotypes, and a cocktail of the channels assemble to yield more diverse current properties and functions (Jenkins et al. 2011; Vacher et al. 2008). For example, Kv1.1, 1.2, and 1.6 produce a depolarization-activated K+ current with a slow onset and sustained time course (Mo et al. 2002; Smart et al. 1997). Moreover, Kv1.4 current is characterized by a rapid onset and reliably fast inactivation (Patel and Campbell 2005). As demonstrated in this study, coexpression of Kv1.2 and 1.4 and the resulting stochastic interaction between the two channels resulted in whole cell current that was not only kinetically different from currents derived from the individual channels but also pharmacologically distinct. Whereas successful identification of Kv subtype-specific blockers has been a powerful pharmacological strategy to disentangle channel-specific functions and properties, the approach loses its robustness in native cells, where Kv channel assembly is predictively and demonstrably heteromultimeric (Dodson et al. 2002; Harvey 2001; Hopkins 1998; Pongs 1999; Rhodes et al. 1997; Scott et al. 1994).
Selective null deletion of Kv subtypes may be the prevailing scheme to determine the in vivo function of a channel, but the use of this approach in understanding the underlying cellular mechanism of Kv channel has not been exploited extensively. Previously, multiple studies have used heterologous cell lines to demonstrate that surface expression of Kv1 channel subunits occurs among members of the same family (Hopkins et al. 1994; Isacoff et al. 1990). In addition, some of the latent confines for channel trafficking resides in the endoplasmic reticulum (ER; Li et al. 2000), which is dependent on the channels' ER retention signals, assembly, and folding as well as their state of phosphorylation (Isacoff et al. 1990; Yang et al. 2007).
In the present study we investigated the role of Kv1.2 channels in regulating the functions of SGNs by identifying the physiologically relevant in vivo interaction partners that define the channel as one of the determinants in information coding of auditory neurons (Davis and Liu 2011). The findings revealed a surprising puzzle that could not have been possible using only pharmacological schemes. First, genetic ablation of Kv1.2 in SGNs resulted in unanticipated increases in membrane hyperpolarization and apparent enhancement of transient outward current. Although it was tempting to invoke potential upregulation of the transient outward current as the mechanism for the results, our analyses suggest that the voltage-dependent activation of the remaining transient outward current after null deletion of Kv1.2 shifted by ∼20 mV in the negative direction, generating a window current at potentials around the RMP of SGNs (−50–60 mV). Consequently, the RMP moved toward EK. The resulting ∼5-mV change in the RMP (Table 1) is significant, since Na+ currents in SGNs have been estimated to have a gating charge (z) of ∼4.6 (Santos-Sacchi 1993), and given one elementary charge to be ∼24 mV, the Na+ channel open probability is expected to change e-fold for a ∼5.2 change in voltage. The low-voltage-activated transient Ca2+ current in SGNs (Lv et al. 2012) is expected to become readily available for activation as the RMP shifts to hyperpolarized potentials. Increased availability of inward Na+ and Ca2+ channels ready for activation at the set RMP in the Kv1.2−/− SGNs may explain not only the increased excitability but also the significant increase in the rate of rise of the action potentials. Conceivably, the apparent shift in the activation of the transient K+ current is likely to shorten the width of the action potentials and induce a rapid decline in the falling phase of the spikes.
Second, a potential cause for the apparent increase in transient outward K+ current in Kv1.2−/− SGNs derived from Kv1.4 channels may rely on the findings that suggest Kv1 α-subunits, e.g., Kv1.1 and Kv1.2, contain ER retention signals that may be transferable to heteromeric Kv1 complexes (Manganas and Trimmer 2000). Thus ablation of Kv1.2 is likely to increase surface expression of Kv1.4 channels. Additionally, in contrast to Kv1.1, which is invariably destined for ER retention, Kv1.2 is more plastic, and its translocation to the plasma membrane is not constrained by a predetermined ER localization sequence. In agreement with this assertion, we found that 1) coexpression of Kv1.2 and 1.4 did not alter the surface expression of Kv1.4; 2) there is no evidence to indicate that Kv1.2 promotes ER retention of Kv1.4; and finally, 3) there were no differences in the estimated current density when Kv1.2 and Kv1.4 were expressed singly or when combined. Data from estimates of the number of functional channels also rule out the possibility that surface expression of Kv1.2 impacted Kv1.4 expression (not shown).
Moreover, the third most significant finding was that the combined expression of Kv1.2 and Kv1.4 yielded whole cell K+ currents with steady-state activation properties that were shifted rightward by ∼15 mV compared with current derived from Kv1.4 alone. In contrast, currents derived from homomeric Kv1.2 channels had half-activation parameters that were ∼5 mV more positive than those of the heteromeric Kv1.2/1.4 channels. Additionally, the time constants of activation were starkly different between the homomeric and heteromeric channels. Heteromeric association of Kv1.2 and 1.4 resulted in currents with distinct insensitivity to α-DTX, a drug that is used to define Kv1.1, 1.2, and 1.6 currents in SGNs (Adamson et al. 2002; Rusznak and Szucs 2009). These findings are in line with a recent report that demonstrated heteromeric Kv1.x complexes of different configurations produce distinct sensitivity toward the Conus snail venom, κM-conotoxin (Chen et al. 2010). The implications of altered sensitivity of heteromeric channels to specific drugs compared with their effects on homomeric channels underpin the need to exercise caution in assigning specific pharmacology to any Kv1 channel subunit in native cells. Conversely, they raise the possibility that pharmacological agents can be designed to target specific heteromeric channel blends. Of further relevance to neuronal function is the finding that a specific Kv1 subunit, e.g., Kv1.2, can control the localization of heteromeric channels of a specific combination at axonal compartments and synaptic sites that are not seen at the cell body (Jenkins et al. 2011). Another cautionary note from the present conclusions is that any number of other transient K+ currents could be operational in the Kv1.2−/− mouse model. Only one specific blocker was used in this study, and more exhaustive testing could be done in future work. Finally, P12 is the onset of hearing in the mouse, and at P11–12 BK channels are strikingly upregulated in inner HCs within ∼1 day. The transition from spontaneous activity to graded receptor potential in inner HCs may undoubtedly alter the response properties of SGNs. However, in this study we did not examine the properties of SGNs between pre- and posthearing stages.
Heterogeneity of auditory and vestibular afferent neurons responses to rectangular pulse and synthetic excitatory postsynaptic current injection yields a broad range of response dynamics and spike timing in information coding (Eatock and Songer 2011; Iwasaki et al. 2008; Kalluri et al. 2010). In broad strokes, these afferent neurons can be classified according to their regularity of firing, namely, regular and irregular neurons (Fernandez et al. 1990; Goldberg et al. 1990a, 1990b). Whereas the firing patterns of vestibular afferents have been well studied and demarcated into morphologically and functionally distinct regular and irregular neurons (Goldberg 1991; Lysakowski et al. 1995; Lysakowski and Goldberg 2008), prehearing auditory afferents have been assumed to consist of mainly regular neurons. However, the striking resemblance between fast- and slow-adapting auditory, irregular, and regular vestibular afferents, respectively, is apparent (Adamson et al. 2002; Kalluri et al. 2010; Mo et al. 2002; Risner and Holt 2006). The presence of intrinsically generated spontaneously active SGNs may add further complexity to the dynamic range of auditory afferents (Lin and Chen 2000; Lv et al. 2012). Nonetheless, among the ionic conductances underlying the firing pattern of afferent neurons in the inner ear are the low-voltage-activated K+ currents, of which Kv1 (DTX-sensitive) and Kv7 channels are prominent components (Adamson et al. 2002; Iwasaki et al. 2008). Indeed, Kalluri et al. (2010) have demonstrated that fast-adapting vestibular afferent neurons could be transformed into slowly adapting ones, by blocking DTX-sensitive and Kv7 conductances. Thus the intricacies of dynamic range of auditory neurons further abound, given that Kv1.2 and Kv1.4 channels can undergo heteromultimers with distinct pharmacological and electrophysiological properties.
Plasticity in hippocampal neurons can be expressed in the form of changes in dendritic excitability by activity-dependent trafficking of the K+ channel subunit Kv4.2 (Kim et al. 2007). Because changes in Kv subunit composition alter functional channel properties, the effect of selective association of Kv1.2 and 1.4 in SGNs can have a robust impact on their functions. Indeed, Kv1.2 subunits can act in a dominant fashion to control membrane localization of synaptic proteins (Inda et al. 2006; Poliak et al. 1999). Thus, depending on the subcellular localization of Kv1.2 and other Kv channel subunits in SGNs, it is conceivable that Kv1.2 can be used as a bait to determine other components of SGN functions. Nonetheless, the present findings have revealed several previously unknowns. In SGNs, Kv1.2 associates with Kv1.4 to confer the functional properties as a conduit for coding auditory information. The high-voltage-dependent activation of Kv1.2 appears to dominate such that heteromeric Kv1.2/Kv1.4 channels in SGNs set the RMP to a relatively depolarized voltage compared with Kv1.4-dominant neurons. Last, the sensitivity of Kv1.2 to α-DTX may not be a reliable indicator for their roles in SGNs, in which heteromeric association of other DTX-insensitive subunits is most certain.
GRANTS
This work was supported by National Institute for Deafness and Other Communications Disorders Grants DC010386 (to E. N. Yamoah) and DC002739 (to B. Tempel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.W., H.J.K., and E.N.Y. performed experiments; W.W., H.J.K., and E.N.Y. analyzed data; W.W. and H.J.K. drafted manuscript; H.J.K., B.L.T., and E.N.Y. conception and design of research; H.J.K. prepared figures; H.J.K. edited and revised manuscript; E.N.Y. interpreted results of experiments; E.N.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of our laboratory for comments on the manuscript.
REFERENCES
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol 447: 331–350, 2002 [DOI] [PubMed] [Google Scholar]
- Amarillo Y, De Santiago-Castillo JA, Dougherty K, Maffie J, Kwon E, Covarrubias M, Rudy B. Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons. J Physiol 586: 2093–2106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Yehuda D, Korngreen A. Space-clamp problems when voltage clamping neurons expressing voltage-gated conductances. J Neurophysiol 99: 1127–1136, 2008 [DOI] [PubMed] [Google Scholar]
- Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, Robbins CA, McKee-Johnson J, Chiu SY, Messing A, Tempel BL. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol 98: 1501–1525, 2007 [DOI] [PubMed] [Google Scholar]
- Chen P, Dendorfer A, Finol-Urdaneta RK, Terlau H, Olivera BM. Biochemical characterization of kappaM-RIIIJ, a Kv1.2 channel blocker: evaluation of cardioprotective effects of kappaM-conotoxins. J Biol Chem 285: 14882–14889, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, Rudy B. DPP6 localization in brain supports function as a Kv4 channel associated protein. Front Mol Neurosci 1: 8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Liu Q. Complex primary afferents: what the distribution of electrophysiologically-relevant phenotypes within the spiral ganglion tells us about peripheral neural coding. Hear Res 276: 34–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci 22: 6953–6961, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011 [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 767–780, 1990 [DOI] [PubMed] [Google Scholar]
- Goldberg JM. The vestibular end organs: morphological, and physiological diversity of afferents. Curr Opin Neurobiol 1: 229–235, 1991 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63: 781–790, 1990a [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 791–804, 1990b [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005 [DOI] [PubMed] [Google Scholar]
- Harvey AL. Twenty years of dendrotoxins. Toxicon 39: 15–26, 2001 [DOI] [PubMed] [Google Scholar]
- Hopkins WF. Toxin and subunit specificity of blocking affinity of three peptide toxins for heteromultimeric, voltage-gated potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther 285: 1051–1060, 1998 [PubMed] [Google Scholar]
- Hopkins WF, Allen ML, Houamed KM, Tempel BL. Properties of voltage-gated K+ currents expressed in Xenopus oocytes by mKv1.1, mKv1.2 and their heteromultimers as revealed by mutagenesis of the dendrotoxin-binding site in mKv1.1. Pflügers Arch 428: 382–390, 1994 [DOI] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci USA 103: 2920–2925, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff EY, Jan YN, Jan LY. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature 345: 530–534, 1990 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nakamura Y, Saitoh N, Li WB, Iwasaki S, Takahashi T. Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. J Neurosci 23: 10445–10453, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Chihara Y, Komuta Y, Ito K, Sahara Y. Low-voltage-activated potassium channels underlie the regulation of intrinsic firing properties of rat vestibular ganglion cells. J Neurophysiol 100: 2192–2204, 2008 [DOI] [PubMed] [Google Scholar]
- Jenkins PM, McIntyre JC, Zhang L, Anantharam A, Vesely ED, Arendt KL, Carruthers CJ, Kerppola TK, Iniguez-Lluhi JA, Holz RW, Sutton MA, Martens JR. Subunit-dependent axonal trafficking of distinct alpha heteromeric potassium channel complexes. J Neurosci 31: 13224–13235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Xue J, Eatock RA. Ion channels set spike timing regularity of mammalian vestibular afferent neurons. J Neurophysiol 104: 2034–2051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lv P, Sihn CR, Yamoah EN. Cellular and molecular mechanisms of autosomal dominant form of progressive hearing loss, DFNA2. J Biol Chem 286: 1517–1527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54: 933–947, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7: 548–562, 2006 [DOI] [PubMed] [Google Scholar]
- Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BH, Yamoah EN. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci USA 104: 19108–19113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Takimoto K, Levitan ES. Surface expression of Kv1 channels is governed by a C-terminal motif. J Biol Chem 275: 11597–11602, 2000 [DOI] [PubMed] [Google Scholar]
- Lin X, Chen S. Endogenously generated spontaneous spiking activities recorded from postnatal spiral ganglion neurons in vitro. Brain Res Dev Brain Res 119: 297–305, 2000 [DOI] [PubMed] [Google Scholar]
- Lv P, Wei D, Yamoah EN. Kv7-type channel currents in spiral ganglion neurons: involvement in sensorineural hearing loss. J Biol Chem 285: 34699–34707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv P, Sihn CR, Wang W, Shen H, Kim HJ, Rocha-Sanchez SM, Yamoah EN. Posthearing Ca2+ currents and their roles in shaping the different modes of firing of spiral ganglion neurons. J Neurosci 32: 16314–16330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J Comp Neurol 511: 47–64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernandez C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol 73: 1270–1281, 1995 [DOI] [PubMed] [Google Scholar]
- Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol 586: 5609–5623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffie J, Blenkinsop T, Rudy B. A novel DPP6 isoform (DPP6-E) can account for differences between neuronal and reconstituted A-type K+ channels. Neurosci Lett 449: 189–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem 275: 29685–29693, 2000 [DOI] [PubMed] [Google Scholar]
- Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K+ currents contribute to accommodation in murine spiral ganglion neurons. J Physiol 542: 763–778, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D, Schindler RA, Wersall J. A quantitative analysis of the afferent innervation of the organ of corti in guinea pig. Acta Otolaryngol (Stockh) 79: 11–23, 1975 [DOI] [PubMed] [Google Scholar]
- Patel SP, Campbell DL. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: underlying molecular, cellular, and biophysical mechanisms. J Physiol 569: 7–39, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 24: 1037–1047, 1999 [DOI] [PubMed] [Google Scholar]
- Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett 452: 31–35, 1999 [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci 17: 8246–8258, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner JR, Holt JR. Heterogeneous potassium conductances contribute to the diverse firing properties of postnatal mouse vestibular ganglion neurons. J Neurophysiol 96: 2364–2376, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Yamoah EN. Direct measurement of single-channel Ca2+ currents in bullfrog hair cells reveals two distinct channel subtypes. J Physiol 534: 669–689, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusznak Z, Szucs G. Spiral ganglion neurones: an overview of morphology, firing behaviour, ionic channels, and function. Pflügers Arch 457: 1303–1325, 2009 [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner ear. J Neurosci 13: 3599–3611, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LA, Anderson AE, Varga AW, Levy M, Sweatt JD. The other half of Hebb: K+ channels and the regulation of neuronal excitability in the hippocampus. Mol Neurobiol 25: 51–66, 2002 [DOI] [PubMed] [Google Scholar]
- Scott VE, Muniz ZM, Sewing S, Lichtinghagen R, Parcej DN, Pongs O, Dolly JO. Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain. Biochemistry 33: 1617–1623, 1994 [DOI] [PubMed] [Google Scholar]
- Smart SL, Bosma MM, Tempel BL. Identification of the delayed rectifier potassium channel, Kv1.6, in cultured astrocytes. Glia 20: 127–134, 1997 [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Differentiation of cochlear afferent neurons. Acta Otolaryngol (Stockh) 91: 451–456, 1981 [DOI] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev 88: 1407–1447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci 18: 610–624, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci USA 104: 20055–20060, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]