Abstract
Humans and animals value the opportunity to choose by preferring alternatives that offer more rather than fewer choices. This preference for choice may arise not only from an increased probability of obtaining preferred outcomes but also from the freedom it provides. We used human neuroimaging to investigate the neural basis of the preference for choice as well as for the items that could be chosen. In each trial, participants chose between two options, a monetary amount option and a “choice option.” The latter consisted of a number that corresponded to the number of everyday items participants would subsequently be able to choose from. We found that the opportunity to choose from a larger number of items was equivalent to greater amounts of money, indicating that participants valued having more choice; moreover, participants varied in the degree to which they valued having the opportunity to choose, with some valuing it more than the increased probability of obtaining preferred items. Neural activations in the mid striatum increased with the value of the opportunity to choose. The same region also coded the value of the items. Conversely, activation in the dorsolateral striatum was not related to the value of the items but was elevated when participants were offered more choices, particularly in those participants who overvalued the opportunity to choose. These data suggest a functional dissociation of value representations within the striatum, with general representations in mid striatum and specific representations of the value of freedom provided by the opportunity to choose in dorsolateral striatum.
Keywords: consumer psychology, democracy, neuroeconomics, reward, value-based decision making
psychology, economics, and political theory all agree that the opportunity to choose is a valued good. Indeed, humans even risk their lives for political freedom and democracy. However, they also value choice in more mundane domains such as their daily shopping. Having a choice provides access to more than one outcome or course of action, and by extension, the number of available outcomes or courses of action increases with the number of choices available. Standard economic theory suggests that choice is valued because it increases the probability of obtaining better outcomes or because decision makers may be uncertain or not (yet) know about their (future) preferences (if one does not know one's preferences now, one may nevertheless know them at a later point in time and therefore should aim to preserve the opportunity to choose until then; for a review, see Pattanaik and Xu 1998). Different theories propose that choice is also valued for other reasons, for example, as part of a meaningful or autonomous life (Jones and Sugden 1982). According to these theories, choice is a good that is valued in and of itself because it enables a life consistent with one's own, chosen, plans (Nozick 1974) and the exertion of autonomy. Of course, there also are psychological reasons for why choice might be valued in and of itself, for example, because it allows us to exert control over the environment and to thereby experience ourselves as active beings (e.g., Leotti et al. 2010 and references therein).
In agreement with the theoretical notion that people value the opportunity to choose, it has been shown that people prefer to have a choice over no choice and, at least normally, to choose from more than fewer choices, for example, with respect to the foods they eat, the consumer products they buy, and the careers they follow. In addition, rats, pigeons, and monkeys also have been found to prefer having more rather than fewer choices (Catania 1975; Suzuki 1999; Voss and Homzie 1970), even when having more choices does not increase the likelihood of obtaining a more highly valued item. Moreover, having more choices can result in many positive psychological and behavioral effects, such as higher levels of intrinsic motivation, task performance, individual happiness, and consumer welfare (Brynjolfsson et al. 2003; Frey and Stutzer 2000; Perlmuter et al. 1980; Zuckerman et al. 1978). However, despite the high value of having the opportunity to choose, little is known about its neurobiological underpinnings.
We used behavioral methods and functional magnetic resonance imaging (fMRI) to investigate the notion, underpinned by economic theory, that choice is a valued good. In particular, we sought to test the hypothesis put forth by standard economic theory that the value of choice is driven by an increased probability of obtaining better outcomes. In addition to this instrumental function of choice, it is conceivable that individuals differ in the degree to which they value choice in and of itself, independent of the potential value gains from better outcomes. To dissociate the value of choice for the freedom it provides from the value of choice for the material items that can be obtained through it, we compared the neural processes involved in valuing choice in and of itself (i.e., having more choices) with those involved in the valuation of the items available to choose from. Specifically, we asked whether regions reflecting the value of choice would also process the value of consumer goods. Although this possibility is suggested from the point of view that the value of choice is based on the value of the items one chooses from, regions that reflect the degree to which individuals value choice in and of itself should be distinct from regions that reflect the value of the items available to choose from.
The striatum is a prime region of interest (ROI) in studies of the processing of the value of choice because it encodes the value of a large variety of rewards, including concrete primary rewards (e.g., drinks, food, sex), concrete secondary rewards (e.g., money, pictures of high-calorie food), and social rewards (e.g., beautiful faces, being evaluated positively by others) (e.g., Aharon et al. 2001; Goldstone et al. 2009; Gregorios-Pippas et al. 2009; Izuma et al. 2008; Kable and Glimcher 2007; McClure et al. 2003; Oyama et al. 2010; Small et al. 2001; Tobler et al. 2009). Moreover, activation in the ventral striatum increases with the desirability of concrete consumer products available for purchase (Knutson et al. 2007) and with the affective experience of control (Leotti and Delgado 2011). However, it is unclear whether the striatum encodes the reward value of having more choices and the value of material choice alternatives separately. Our present data indeed reveal a graded representation of choice value in the striatum and a regional dissociation of common and distinct processing of the value of having more choices and that of material choice items.
METHODS
Participants
Eighteen right-handed adults (13 men and 5 women, ages 19–27 yr, mean 22 yr) with no history of neurological, psychiatric, or auditory symptoms participated in the imaging study. Written informed consent was obtained from each participant. The experiment was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Nihon University School of Medicine.
Experimental Design and Task
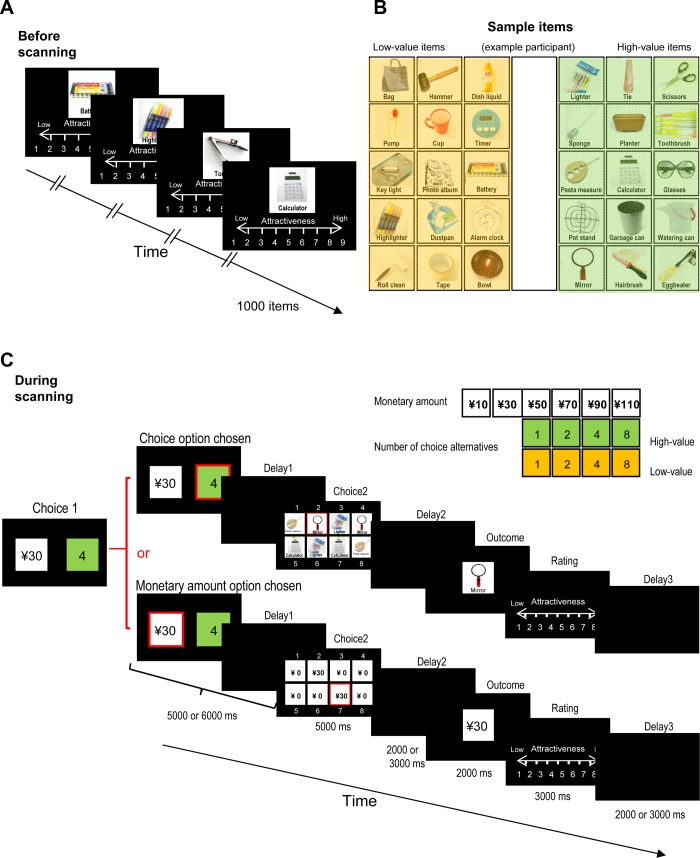
One to two days before coming to the laboratory, participants rated the attractiveness of 1,000 everyday items on a scale of 1 (very unattractive) to 9 (very attractive) (Fig. 1A). Pictures of items (Fig. 1B) were obtained from a 100 Yen Shop on the Internet. We adjusted the size of each picture so that all pictures presented on the screen were of similar size, thereby ensuring similar visual stimulation across items. The items were displayed in a white square (5.5-cm side length, which corresponded to 14.3° visual angle) on a black background with the name of each item displayed below the picture inside the white square and the rating scale presented below the white square. The participants were informed that the objective market value of each item was 100 yen (about $1). Based on the individual ratings, we selected 450 low-value and 450 high-value items for each participant. We thereby made sure that we had enough items to avoid presenting any item more than once during the experiment.
Fig. 1.
Experimental design. A: prestudy rating. One or two days before coming to the laboratory, participants rated the attractiveness of 1,000 everyday items on a scale of 1 (very unattractive) to 9 (very attractive). Based on the individual ratings, we selected 450 low-value and 450 high-value items for each participant, which were used in the low- and high-value conditions, respectively (see C). B: sample items, as classified for 1 of the participants. All of the items had the same objective market value (100 yen); in the actual experiment, the Japanese rather than English word for each item was displayed, and images were comparable to those shown here but obtained from the Internet. C: sequential choice task used during scanning. In each trial, for choice 1, 2 numbers were presented for up to 4 s on the left and right side of the monitor, and participants chose 1 of the 2 sides. One number corresponded to the number of choices, that is, the number of items (1, 2, 4, or 8) available from which they would be able to choose in choice 2 should they choose the choice option in choice 1. The color on which this number was displayed indicated whether the items were of low or high subjective value (here: green for high and orange for low value; color scheme holds for all figures). The other number corresponded to a monetary amount (10, 30, 50, 70, 90, or 110 yen) that participants would receive in choice 2 if they chose the monetary amount option instead of the choice option in choice 1. All brain activations reported (Figs. 3–6) are from choice 1. The option chosen in choice 1 was highlighted for 1 s, followed by a delay of 5–6 s. Choice 2 followed, for which 8 items were always displayed for 5 s, regardless of the option chosen in choice 1 and the number of choice alternatives. This allowed us to hold overall visual stimulation constant for all trial types. If participants had chosen the choice option in choice 1, they would now select either a low- or high-value item, depending on condition; otherwise, they would select a monetary item. The number of items available to choose from was equal to the number of items displayed in choice 1. To match difficulty of choosing the final outcome in the choice and the monetary amount options, we showed as many monetary amount duplicates as we would have shown in the corresponding choice option (illustrated is the case of 2 instances per outcome). Moreover, the proportion of duplicate monetary amounts shown in choice 2 was inversely related to the number of items offered in choice 1. After a delay of 2–3 s, the chosen item or monetary amount was displayed for 2 s. The participants then had 3 s to rate the attractiveness of the chosen item. After an intertrial interval of 2–3 s, the next trial started. ¥, yen.
In the laboratory, participants made two choices in each trial (Fig. 1C). Choice 1 (presented for up to 4 s) was between a monetary amount (10, 30, 50, 70, 90, or 110 yen) and a number (1, 2, 4, or 8). The number represented the number of low- or high-value items from which participants would choose in choice 2, should they choose the choice (as opposed to the monetary amount) option in choice 1. Whether the items would be of low or high value was randomly determined from trial to trial and was indicated to participants by the color (green or orange, counterbalanced across participants) of the square on which the choice option was presented in choice 1. Participants were told that of the 1,000 items they had rated previously, green or orange would represent the 450 items with the lowest or highest rating, respectively. The monetary amount offered in choice 1 was determined independently of the properties of any items that might be shown in choice 2 (value and number of items). The participants conveyed their choice verbally (“left” or “right”). The chosen option was highlighted with a red frame for 1 s, followed by a variable delay (until 5–6 s had elapsed) during which the screen was blank. If a participant chose the choice option in choice 1, the corresponding number of everyday items was displayed in choice 2; if a participant chose the monetary amount option in choice 1, the corresponding amount of money was displayed in choice 2 (5 s). To control for overall visual stimulation and difficulty of choice 2, we always showed eight squares and repeated items or monetary amounts, respectively, such that the proportion of duplicates shown in choice 2 was inversely related to the number of choice alternatives offered in choice 1 (for example, with 4 alternatives, each item would be shown twice, and with 2 alternatives, each item would be shown 4 times). After participants made choice 2, there was a delay of 2–3 s, and then the chosen item or monetary amount was presented for 2 s, and participants rated its attractiveness again (3 s). Finally, there was an intertrial interval of 2–3 s before the next trial started.
In total, the participants completed 240 trials (82 min) in 4 sessions. Each session started with a blank screen (6 s), followed by a text that was to be read (20 s) and a ready phase (30 s). In the text-reading phase, participants read out the numbers “1” to “9” to allow for speaker volume adjustment in the scanner control room. Trials in which participants did not give a response or a rating were rare (mean: 4 trials) and were excluded from further analysis. Thus the reported results are from completed trials. On average, participants received earnings of 11,679 yen (about $130) and 42 items, all of which were delivered to the participants ∼3 mo after the experiment. Participants reported that they did not try to add up their earnings during the experiment.
Behavioral Analysis
The subjective value of the items was determined by means of attractiveness ratings made 1–2 days before the experiment. For each participant, the 450 most attractive items were categorized as high-value items and the 450 least attractive items as low-value items. The 100 remaining, medium-attractive items were not used in the experiment. The subjective value of the opportunity to choose was ascertained by the choices made by the participants during the experiment. We used a sigmoid function to fit the frequency with which participants chose the choice option as a function of the amount offered in the monetary amount option. The monetary amount equivalents of having the opportunity to choose from 1, 2, 4, and 8 items were estimated for each participant individually as the monetary value at which the rate of choosing the choice or the monetary amount option was 50%. The monetary amount equivalents can be conceived of as “indifference values” or “points of subjective equivalence” between choice and money.
Individual differences in choice (over)valuation.
To assess individual differences in the (over)valuation of choice, for each participant, we compared the monetary amount equivalents as well as the monetary amounts rejected for the 1/high-value choice alternative and the 8/low-value choice alternative (higher monetary amount equivalents and higher amounts rejected correspond to higher valuation of the choice option). Because the subjective value of the low-value items was always below that of the high-value items, increasing the number of choices in the case of low-value items should not normally convey more subjective value than that assigned to a single high-value item in the no-choice condition. By contrast, choice overvaluation should result in a higher indifference value for the opportunity to choose from several low-value items (8/low value) compared with obtaining a high-value item without having a choice (1/high value).
To capture choice overvaluation more formally, we also determined the maximum increase in value that the opportunity to choose provided for each subject. More specifically, for each subject, we determined the theoretically possible maximum increase in subjective value that resulted from increasing numbers of choice alternatives and compared this with the actually observed increase in subjective value. First, we took 500,000 samples of 2, 4, or 8 items from the possible ratings and noted the mean and standard deviation of these samples, which we then entered into Gumbel distributions to plot the theoretical distribution of the maximum ratings when the choice option comprised 2, 4, or 8 items (see Supplemental Fig. S1, available in the data supplement online at the Journal of Neurophysiology web site). The modes of the Gumbel distributions increased with the number of items, but this increase varied between subjects. On the basis of these distributions, we determined the slope in the relationship between theoretically possible maximum ratings as a function of the number of choice items and compared this slope with the corresponding slope derived from the observed behavioral data.
Moreover, we quantified choice overvaluation with deviations from a pattern of choice behavior that maximizes expected utility. For each participant, we calculated the expected utility of each of the alternatives (1/low, 2/low, …, 4/high, 8/high) on the basis of the participants' own ratings, converted to yen (without replacement). We then compared this value with the utility of the monetary amount option presented in a given trial and determined whether participants had chosen the option with the higher utility. If they had not, two possibilities were conceivable: they had chosen the monetary amount option even though the expected utility of the choice option was higher (“money-favoring mistake”), or they had chosen the choice option even though the expected utility of the monetary amount option was higher (“choice-favoring mistake”). Either of these mistake types might be due to noise. However, a differential increase in the frequency of the mistake types could indicate choice overvaluation. In particular, choice overvaluation would be expressed as a higher proportion of choice-favoring mistakes as the number of items increased. Thus choice-overvaluing subjects consistently make more mistakes (select the option with lower expected utility) to favor the choice option with increases in the size of the choice set. This implies that mistakes are not random noise and are instead due to an actual preference that is not captured in the expected utility model. We assessed individual differences in the tendency to overvalue choice by computing the slope of the line representing the increase in choice- vs. money-favoring mistakes as a function of the number of items. We related this slope to activation increases elicited by more choice. Moreover, in a control analysis, we tested for a relationship between this brain activity and an overall tendency to make mistakes, which is not directly indicative of choice overvaluation.
Image Acquisition
All images were acquired using a 1.5-T Siemens Symphony MRI scanner (Siemens, Erlangen, Germany) at Nihon University. Gradient-recalled echo planar imaging (EPI) was used for the fMRI sequence to obtain blood oxygen level-dependent (BOLD) contrast functional images. A total of 2,460 functional scans per participant were acquired using a gradient echo EPI sequence [20 axial slices in the anterior commissure-posterior commissure plane; repetition time (TR) = 2,000 ms, echo time (TE) = 50 ms, flip angle = 90°, field of view (FOV) = 192 mm, 64 × 64 matrix, voxel size = 3 × 3 × 5 mm, slice gap = 1 mm]. A T1 anatomic scan of each participant was obtained (192 sagittal slices; TR = 2,000 ms, TE = 3.93 ms, flip angle = 15°, FOV = 256 × 224 mm, in-plane resolution = 1 × 1 mm, slice thickness = 1 mm).
Functional MRI Analysis
Preprocessing and data analysis were performed using SPM5 software. The first 28 functional scans (noise cancellation and ready phases) of each session were discarded to allow for magnetic saturation. The functional images of each participant were realigned with reference to the first image to correct for head motion. The anatomic images were coregistered with the mean functional images and normalized to the MNI (Montreal Neurological Institute) brain template. Functional data were then normalized using the same transformation parameters and smoothed in the spatial domain (isotropic Gaussian kernel of 8 mm full width at half-maximum).
Functional data were analyzed in an event-related general linear model. Regressors were formed by convolving stimulus functions with a canonical hemodynamic response function and its temporal derivative. Participant-specific motion parameters were modeled as covariates of no interest. A high-pass filter with a cutoff period of 128 s was used to remove low-frequency drifts. Condition-specific parameter estimates reflecting the strength of activation for each condition and each voxel for each participant were obtained with a general linear model. These parameter estimates were then passed to a second-level (between subject) analysis for random effects inference on value-related responses.
The stimulus functions, encoding condition-specific effects, modeled the onset of neural responses at choice 1 with a duration of 0 s. They captured four conditions, corresponding to 1, 2, 4, or 8 choice alternatives. Item value (high or low) and the amount of the monetary amount option at choice 1 were entered as binary and parametric modulators. Subsequent model events included choice 2, outcome, and rating phases. These events were of no interest but were included in the model because they potentially explained some of the variance in brain activation.
The effects of interest were calculated relative to an implicit baseline in the event-related design. With the use of random-effects analysis, the relevant contrasts of parameter estimates were entered into a series of one-way t-tests. In the first contrast, we compared trials with high- vs. low-value items to identify value-related regions. Correction for multiple comparisons was applied separately within two different ROIs but with the same result. On one hand, we corrected within the anatomically defined striatum (caudate plus putamen, including the ventral striatum), and on the other, we corrected within an 8-mm sphere around a previously described striatal region (x/y/z coordinates: 12/13/−2) coding for the desirability of consumer products available for purchase (Knutson et al. 2007). We then formed a (mean centered) contrast that used the individually determined point of subjective equivalue of 1, 2, 4, and 8 choice alternatives as contrast weights on the single-subject level. Thus, instead of testing for a constant relationship (e.g., as would be given by the mean points of subjective equivalence) between number of choice alternatives and ensuing activation, each condition (i.e., 1, 2, 4, or 8 choice alternatives) was weighed according to the subjective value it had for a given participant as determined by his/her individual point of subjective equivalence. These contrasts, capturing the subjective value of the opportunity to choose on an individual basis, were then taken up to the second, group level in a simple t-test to identify regions that code the subjective value of the opportunity to choose in the whole group of tested participants. We also formed a contrast in which we subtracted, at the single-subject level, the objective number of choice alternatives from the subjective value weights to test for activation that correlated more strongly with the value of the opportunity to choose than simply with the number of choice alternatives.
To investigate how striatal connectivity is modulated depending on the value type, we performed a whole brain psychophysiological interaction (PPI) analysis (Friston et al. 1997; Kahnt et al. 2009; Park et al. 2010). Those striatal voxels in which activity significantly covaried with both 1) the value of the opportunity to choose and 2) the value of items were selected as the seed region (see Fig. 4E). PPI methods are described in more detail and the results reported (Supplemental Fig. S2) and discussed in the data supplement.
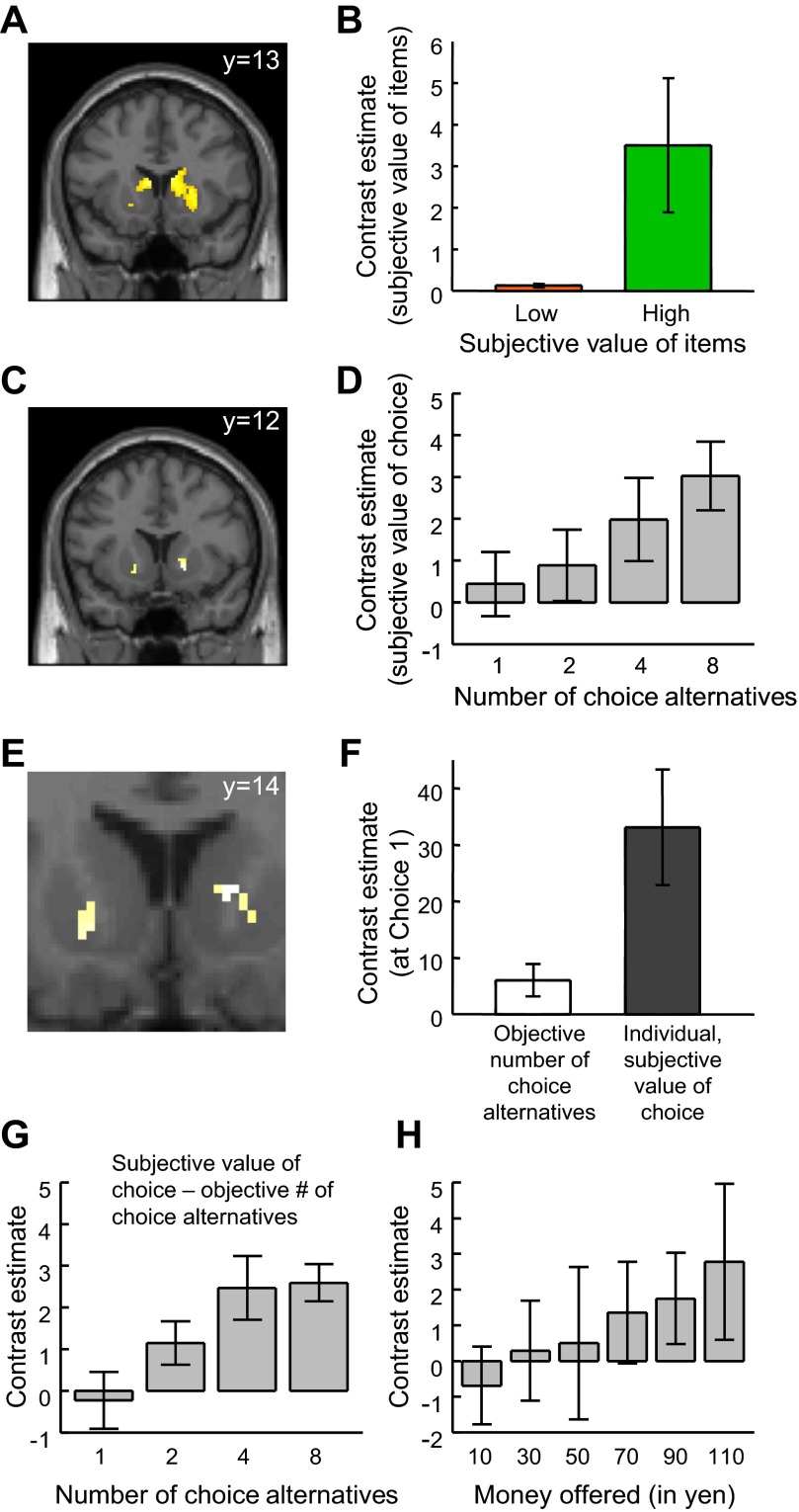
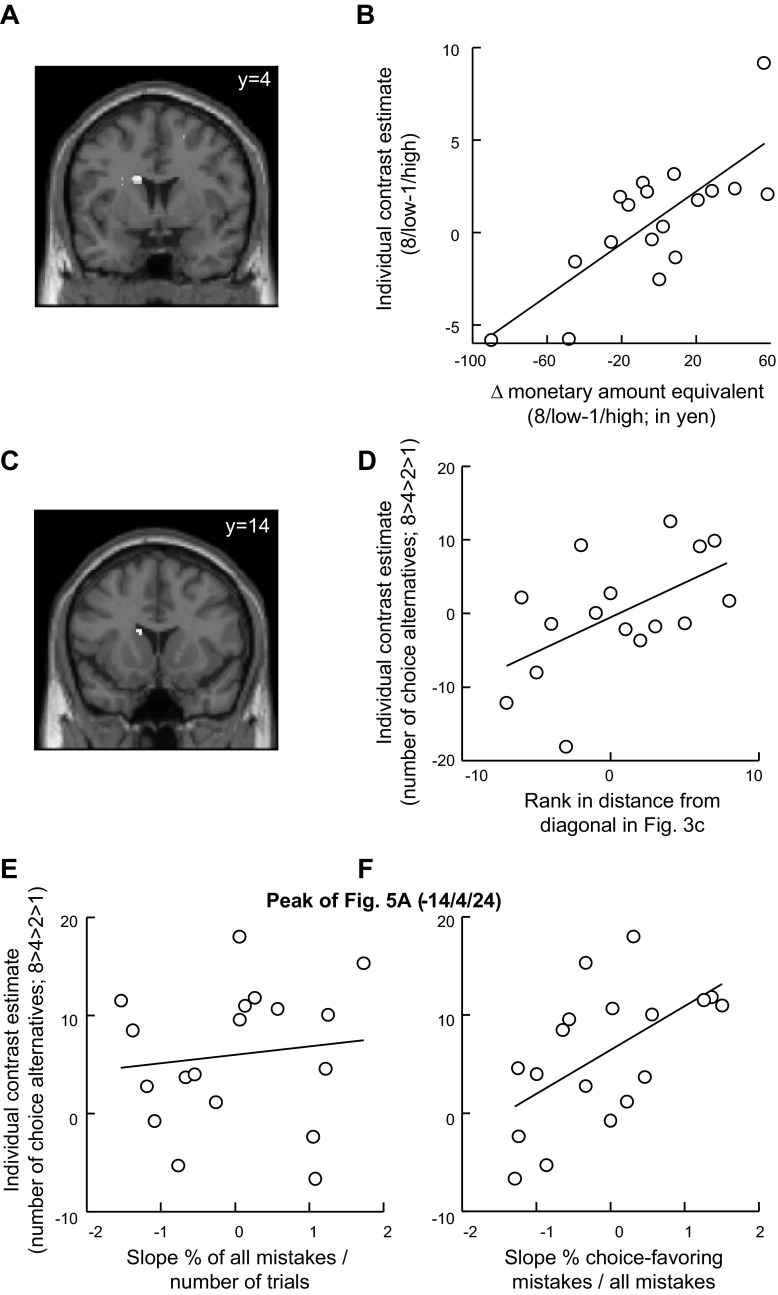
Fig. 4.
Regions coding subjective value of items and of choice at choice 1. A: location in striatum with stronger activation for trials with high- vs. low-value items at time of stimulus presentation in choice 1 (x/y/z: 12/13/−2). Item value was based on attractiveness ratings given by participants before the experiment. [Activation survived small-volume correction irrespective of whether the region of interest (ROI) was defined anatomically or functionally; thus results were the same for the 2 types of ROIs. To show the full extent of the activations, they are displayed at P = 0.005.] B: average effect size in striatal region identified in A. Activation in high-value trials was significantly stronger than in low-value trials (P < 0.05, small-volume correction). C: location in striatum with activation reflecting individual-specific subjective value of choice (x/y/z: 16/12/−2). Subjective value of choice was specified on the basis of monetary amount equivalents determined at choice 1. D: average effect size in striatal region shown in C. Activation increased with subjective value of choice (P < 0.05, small-volume correction). The bar graphs in B and D illustrate the effects of the maps shown in A and C, respectively. E: overlap of activations from A and C, indicating common coding of value of items and value of choice. F: relationship of activation to individualized subjective value of choice was stronger than to objective number of choice alternatives. G: relationship of activation to the number of choice alternatives in striatal voxels, showing better fit with individualized subjective value of choice than with the objective number of choice alternatives (x/y/z: 16/12/18). H: the subset of voxels with common activation to both the value of items and subjective value of choice in E also showed increasing activation with increasing amount of money offered (P < 0.05; x/y/z: 12/16/2). Values are means; error bars represent SE.
To assess individual differences in the overvaluation of choice, we measured the relationship between activation differences for 8/low value vs. 1/high value and corresponding behavioral differences. Moreover, we also assessed the degree to which the subjective value could maximally increase as a result of having more choices and thereby captured individual differences across the whole range of choice alternatives (see Behavioral Analysis above). We plotted actual value increases as a function of theoretically possible value increases (see Fig. 3C). The larger the distance of the monetary amount equivalent slopes from the identity line toward the top left, the more participants valued choice. A possible interpretation of the two slopes is that those based on monetary amount equivalents reflect all of the factors that increase the value of choice, whereas those based on behavioral ratings only reflect the extent to which participants could maximally expect to do better, given their ratings, when they were presented with more choice alternatives.
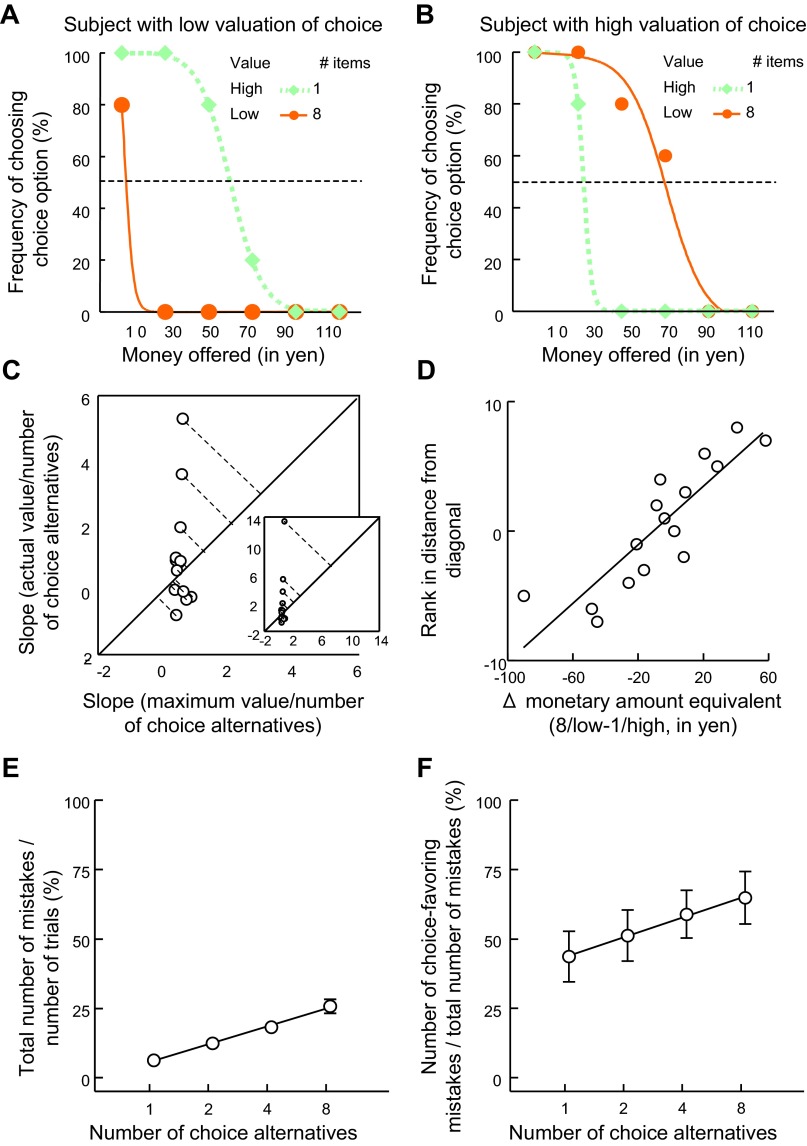
Fig. 3.
Individual differences in preference for choice. A and B: individual preference for choice was measured as subject-specific difference between the monetary amount equivalents in the 8/low-value and the 1/high-value conditions. Positive differences correspond to higher valuation of choice. A: example subject with a low valuation of choice (monetary amount equivalent of 8/low value < 1/high value). B: example subject with a high valuation of choice (monetary amount equivalent of 8/low value > 1/high value). To further support the finding of individual differences in the valuation of choice with respect to 8/low-value vs. 1/high-value conditions, we compared the monetary amounts rejected in the 2 conditions for each subject separately, with higher amounts rejected corresponding to a greater valuation of choice. According to this measure, the example participant shown in B valued the 8/low-value alternative significantly more than the 1/high-value alternative, whereas the example participant shown in A had a significant preference for the 1/high-value alternative. C: individual differences in choice valuation in relation to theoretically possible maximum increase in subjective value from increasing numbers of choice. The slopes of choice-driven monetary amount equivalents as function of the number of choice alternatives are plotted against the slopes of choice-independent maximum preratings as a function of the number of alternatives. One outlier (>9 times second-largest value) is not shown (Grubb's test identified this as an outlier: z = 2.6, P < 0.05). To still include this subject while also preventing the outlier from unduly influencing the correlations, we used ranks of the distance from the diagonal for subsequent analyses (Figs. 3D, 5C, and 5D). The data from 2 other participants are not shown because their indifference value for 1 item was equal to 0 yen, which precluded conversion into rating equivalents. Finally, in the main graph in C, 1 participant is not shown to better illustrate the participants clustering at the bottom of the graph; this participant is shown in the inset. D: relationship between the 2 measures of individual differences. Measures of choice valuation according to A and B are correlated with measures of choice valuation according to C. E: percentage of the number of mistakes relative to the total number of trials as a function of the number of choice alternatives. F: percentage of choice-favoring mistakes relative to the total number of mistakes as a function of the number of choice alternatives. For each subject, choice overvaluation was captured as slope and is related to brain activation in Fig. 5F.
To reveal individual differences in brain activation, we assessed the relationship between the ranks of the distance of the slopes from the identity line and the contrast of 8 > 4 > 2 > 1 choice alternatives. We used rank information to prevent the correlations from being unduly affected by outliers while still using all of the data points. Moreover, we assessed the relationship between the difference in the slope of mistakes favoring choice minus the slope of mistakes favoring money and the contrast of 8 > 4 > 2 > 1 choice alternatives. All correlations were performed in SPM independently of other analyses and with z-transformed measures, and thus reveal relationships with brain activation directly and uncontaminated by mean differences. For each contrast, statistical parametric maps of the t-statistic were generated on a voxel-by-voxel basis, and these t values were transformed into z scores of the standard normal distribution. Unless stated otherwise, we report activations surviving correction for multiple comparisons within the ROIs (P < 0.05, family-wise error rate). For descriptive purposes only, we report in Tables 2–6 activations at P < 0.005 (uncorrected) outside ROIs. Reported voxels conform to MNI coordinate space, with the right side of the image corresponding to the right side of the brain.
Table 2.
Brain regions with stronger activation to high-value than low-value consumer items at choice1
| Brain Region | L/R | x | y | z | z Score | Voxels |
|---|---|---|---|---|---|---|
| Lateral prefrontal cortex | L | −30 | 30 | 34 | 3.39 | 23 |
| R | 30 | 40 | 4 | 3.33 | 26 | |
| Anterior cingulate cortex | R | 16 | 32 | 28 | 3.28 | 15 |
| Posterior cingulate cortex | L | −16 | −28 | 32 | 3.78 | 96 |
| R | 18 | −26 | 30 | 4.40 | 337 | |
| 10 | −26 | 62 | 3.02 | 50 | ||
| Caudate | L | −14 | 24 | 10 | 3.98 | 554 |
| R | 12 | 13 | −2 | 4.09 | 647 | |
| Superior temporal gyrus | L | −60 | −44 | 8 | 3.25 | 39 |
| −46 | 6 | −10 | 2.98 | 18 | ||
| R | 66 | −40 | 12 | 3.33 | 38 | |
| 46 | −42 | 0 | 3.28 | 81 | ||
| Middle temporal gyrus | L | −46 | −60 | 12 | 2.81 | 10 |
| R | 48 | −4 | −20 | 3.41 | 20 | |
| 62 | −24 | −8 | 4.06 | 218 | ||
| Supramarginal gyrus | R | 56 | −38 | 42 | 3.13 | 52 |
| Superior parietal lobule | L | −12 | −50 | 68 | 2.92 | 18 |
| Inferior parietal lobule | L | −42 | −52 | 52 | 3.39 | 235 |
| −58 | −46 | 38 | 3.03 | 32 | ||
| R | 36 | −46 | 50 | 3.10 | 40 |
L/R, left/right. P < 0.005, uncorrected, >5 voxels.
Table 6.
Brain regions showing a correlation between utility-based overvaluation and activation increases with number of items available from which to choose
| Brain Region | L/R | x | y | z | z Score | Voxels |
|---|---|---|---|---|---|---|
| Dorsal striatum | L | −24 | 0 | 24 | 3.27 | 11 |
| Insula | L | −26 | −16 | 24 | 2.63 | 22 |
| R | 32 | −16 | 6 | 3.21 | 79 | |
| 34 | 10 | 4 | 3.11 | 48 | ||
| Middle cingulate gyrus | R | 12 | −28 | 48 | 3.06 | 18 |
| Posterior cingulate gyrus | L | −14 | −44 | 8 | 3.55 | 231 |
| Inferior parietal lobule | L | −36 | −44 | 46 | 3.41 | 35 |
| −44 | −36 | 26 | 3.02 | 16 |
Choice overvaluation was measured by the difference in slope relating choice-favoring mistakes and money-favoring mistakes to the logarithm of the number of items available from which to choose (results are very similar to the actual number of items available from which to choose rather than the log). P < 0.005, uncorrected, >5 voxels.
RESULTS
Behavioral Data
We used a novel consecutive choice task together with fMRI to investigate the neural foundation of the value of having more or fewer choices. Before the experiment, we asked each participant separately to evaluate 1,000 everyday consumer items with the same objective market value (100 yen; Fig. 1, A and B). By grouping items with respect to individual value (high vs. low), we were able to locate brain activations coding the subject-specific value of concrete items. To independently assess brain activations that code the value of the opportunity to choose, in choice 1 of each trial, participants chose between a monetary amount and a number (1, 2, 4, or 8) that represented the number of alternatives and thus the number of different items they would be able to choose from in choice 2 (Fig. 1C). The participants received the items and money they chose during the experiment about 3 mo later.
First, we assessed whether participants took advantage of being offered more choices and whether they continued to value a high-value item they had chosen more than a low-value item they had chosen. We compared the average ratings given to items that participants had chosen in choice 2 and found that participants rated high-value items they had chosen higher than low-value items they had chosen [t(33) = 5.8, P < 0.0001; Fig. 2A]. Moreover, items chosen from larger choice sets at choice 2 (4 or 8 choice alternatives) were subsequently rated more positively than items chosen from smaller choice sets [1 or 2 choice alternatives; t(34) = 2.3, P < 0.05; Fig. 2B]. The correlation between number of choice alternatives and average rating was significant [r(3) = 0.95, P = 0.05]. Thus, in agreement with theoretical notions about why people value choice, participants took advantage of having more choices by choosing more highly valued items.
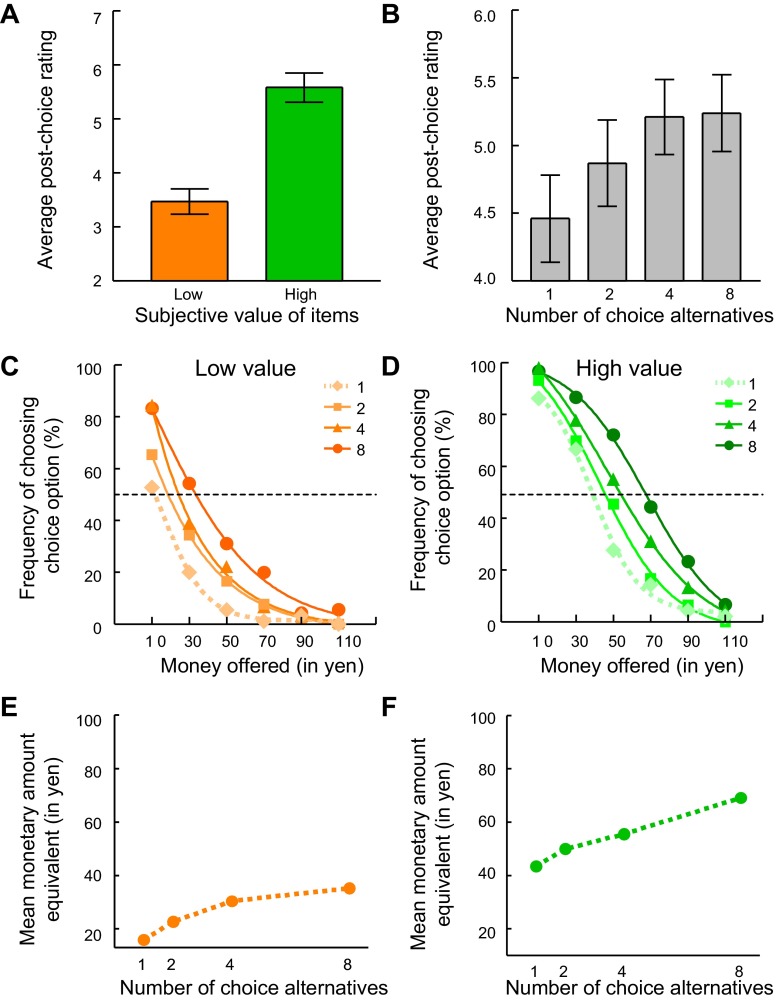
Fig. 2.
Behavior. A: postchoice rating of the low- and high-value items selected in choice 2. B: postchoice rating of the items selected in choice 2 as a function of the number of choice alternatives. For A and B, the data were averaged over all 18 participants. C and D: frequency with which participants chose the choice option as a function of the amount of money offered in the monetary amount option in choice 1. These data were averaged over all 18 participants and are displayed separately for low-value (C) and high-value trials (D). In both trial types, participants' preference for the choice option in choice 1 increased with the number of items that would be available to choose from in choice 2. Intersection of dotted horizontal lines and data fitted with a sigmoid function reflects monetary amount equivalents used in further behavioral analysis and for regression against brain activity. Monetary amount equivalents correspond to the estimated monetary amount at which choice and money would each be chosen 50% of the time. E and F: monetary amount equivalents as a function of the number of items that would be available to choose from in choice 2. The monetary amount equivalents were averaged over all 18 participants and are displayed separately for low-value (E) and high-value trials (F). In both trial types, monetary amount equivalents increased with the number of choice alternatives.
In choice 1, we independently varied the monetary amount and the number of choice alternatives that would be offered in choice 2, so we were able to measure the value of different numbers of choice alternatives. For trials with low- and high-value items, we made separate plots of the frequency with which participants chose the choice option as a function of the monetary amount option (Fig. 2, C and D). Two effects became apparent: participants chose the monetary amount option more often when higher monetary amounts were offered (and the number of choice alternatives was held constant), and they chose the choice option more often when more choice alternatives were offered (and the monetary amount was held constant). The latter effect is evidenced by the rightward shift of fitted curves for 1, 2, 4, and 8 choice alternatives in Fig. 2, C and D (for a more detailed analysis, see below). Thus, all else being equal, participants preferred the choice option more strongly when they were offered more choice alternatives.
For further behavioral analysis, we quantified the value of the opportunity to choose with greater precision. Specifically, we fitted a sigmoid function to the relationship of choosing the choice option as a function of the amount of money offered and took the estimated monetary amount indifference value at the choice frequency of 50%, that is, the monetary amount equivalent for each condition (1, 2, 4, and 8 items available from which to choose). Monetary amount equivalents were higher in trials with high- than in those with low-value items [high value: 54.5 yen, SE = 3.5; low value: 27.4 yen, SE = 4.8; t(34) = 4.4, P < 0.0001]. More importantly, monetary amount equivalents increased with increasing opportunity to choose, for both low- and high-value items [Fig. 2, E and F; ANOVA, low value: F(3,68) = 3.0, P = 0.04; high value: F(3,68) = 5.5, P = 0.02; the conditions with the highest monetary amount equivalent differed from those with the lowest monetary amount equivalent, P < 0.05 in both cases, Bonferroni-corrected for multiple comparisons]. On average, adding one additional choice alternative increased the value of the choice option by 3.6 yen [low value: 3.5 yen; high value: 3.7 yen; t(34) = 0.18, P = 0.86]. Taken together, the data suggest that the opportunity to choose was valuable to participants and that this value increased monotonically with the number of choices offered.
As the number of items offered increases, the probability of obtaining the item with the highest value approaches 1, so the increase in value from an additional item from which one could choose decreases to 0 (decreasing marginal utility). These economic considerations predict nonlinear, plateauing functions like those shown in Fig. 2, B, E, and F. Thus both the averaged monetary amount equivalents and the averaged postchoice ratings supported this basic notion.
Individual differences in choice (over)valuation.
We also used monetary amount equivalents to assess individual differences in the (over)valuation of choice (Fig. 3). To assess individual differences in an intuitive way, we first compared monetary amount equivalents for the 1/high-value choice alternative with those of the 8/low-value choice alternative. Given that the subjective value of the low-value items was always below that of the high-value items, we reasoned that offering more low-value items to choose from would not normally convey more subjective value than that assigned to a single high-value item in the no-choice condition. This is because no matter how many low-value items there are to choose from, the best one will always be valued less than the least-valued item in the high-value group. For 44% of our participants (8/18; single-subject example: Fig. 3B), the value of choosing in the 8/low-value condition went beyond that of the 1/high value condition.
However, the simple difference in monetary amount equivalents does not exclude the possibility that choices are noisy. We therefore aimed to determine which participants valued the 8/low-value alternative significantly more than the 1/high value alternative by comparing the monetary amounts rejected in the two cases for each participant. A participant who rejects higher monetary amounts is considered to value the choice option more highly. We found significant differences in five participants (Mann-Whitney U-test). Of these, three valued the 8/low-value alternative significantly more than the 1/high value alternative (U = 191.0, P < 0.0001; U = 315.5, P < 0.05; U = 312.5, P < 0.05), whereas the other two participants preferred the 1/high value alternative significantly more than the 8/low-value alternative (U = 108.5, P < 0.0001; U = 265.0, P < 0.005). The frequency of participants with a significant difference in preference was higher than would be expected by chance (χ2 = 6.88, P < 0.05).
Because the analyses mentioned so far employed only two data points, they are not very sensitive. We next assessed individual differences in choice valuation with respect to what participants could maximally gain from having more alternatives to choose from across the whole range of choice alternatives. Specifically, for each individual participant, we determined the theoretically possible maximum increase in value resulting from different amounts of additional choice and compared this with the actually observed increase in subjective value (see methods). We plotted the slopes reflecting how much participants actually gained from having more choice (based on indifference values) as a function of the slopes reflecting how much participants could theoretically gain from having more choices (Fig. 3C). We reasoned that the slopes based on indifference values should not be steeper than those based on ratings. In other words, the greater the distance from the 45° line toward the top left of the graph (Fig. 3C), the more a participant values choice. A significantly larger proportion of our sample lay above rather than below the 45° line (χ2 = 4.6, P < 0.05). Importantly, using this measure, we also found substantial differences between participants, and the distance from the 45° line correlated significantly with the difference in the monetary amount equivalent of 8/low value–1/high value (r = 0.86, P < 0.0001; Fig. 3D). Taken together, these data suggest that the participants differed with respect to the degree to which they value choice and that some participants value choice more than the value provided by the increased probability of obtaining better items. However, although the majority showed choice overvaluation (see also next paragraph), one should keep in mind that this effect does not occur uniformly on the level of the whole group, but only in some participants. For the analysis of the neuroimaging data presented below, we took advantage of this individual variation in behavior and tested whether the degree to which the overvaluation effect occurs is reflected in the individual degree of striatal activation.
Finally, we assessed choice overvaluation on the basis of deviation from utility-maximizing choice. In this study, we define choice as a function of the number of choice options such that more choice equals having more items in the choice set, and there is a parametric relation between the amount of choice and the number of items in the choice set. Accordingly, the rational valuation of choice is incorporated directly into the expected utility of the choice option and increases with the number of choice items, simply because expected utility increases with the size of the choice set. Choice overvaluation is the preference for a choice option in excess of the increase in expected utility due the size of the choice set. We calculated the expected utility of the monetary amount option and the choice option (based on initial ratings) and measured how often participants failed to choose the option with the higher expected utility. We reasoned that choice overvaluation should express itself in an increasing tendency to mistakenly choose the choice option when more items were offered. In contrast, if choice is not overvalued, the number of choice- and money-favoring mistakes should not be differentially influenced by the number of choice alternatives. We found that the number of mistakes increased with the number of choice alternatives (Fig. 3E), which may be due simply to increased complexity. However, this tendency was qualified as would be required for choice overvaluation (rather than noise or complexity of choice). In particular, choice-favoring mistakes increased more than money-favoring mistakes with the number of choice alternatives. In other words, there is an additional value for choice not captured by expected utility, i.e., choice overvaluation [Fig. 3F; t(17) = 2.8, P < 0.05; moreover, a planned comparison showed that the difference in choice-favoring mistakes for 8 alternatives vs. 1 alternative was significantly larger than the difference in money-favoring mistakes for 8 alternatives vs. 1 alternative: t(17) = 3.4, P < 0.01]. Choice overvaluation was also confirmed by regression analysis: there was a significant effect of the number of items on selecting the choice option, even when controlling for differences in the expected utility of options and only using trials in which the subject selected the option with lower expected value (Supplemental Table S1). Finally, when all trials were analyzed, the frequency with which the choice option was chosen increased with the number of choice items, and this effect again held when we controlled for the differences in expected utility between the options (Table 1 and Supplemental Table S2). Thus several distinct analyses provide converging evidence for choice overvaluation. This expressed itself in participants choosing the choice option more frequently when a higher number of choice alternatives was offered even though the expected utility of the choice option was lower than that of the monetary amount option. In other words, participants gave up more utility for the opportunity to choose from more items in choice 2 than they could expect to gain from such a choice. Note that this overvaluation effect was present on average, i.e., in the subject pool as a whole.
Table 1.
Choice overvaluation
| Probit | Logit | LPM | |
|---|---|---|---|
| EU difference | 0.028 (7.24)† | 0.052 (7.10)† | 0.006 (13.27)† |
| Two items | 0.188 (2.09)* | 0.303 (1.80) | 0.038 (2.28)* |
| Four items | 0.285 (2.02)* | 0.483 (1.79) | 0.060 (2.24)* |
| Eight items | 0.513 (2.19)* | 1.003 (2.29)* | 0.111 (2.44)* |
| Constant | −0.310 (4.24)† | −0.473 (4.02)† | 0.427 (20.40)† |
| N | 4,235 | 4,235 | 4,235 |
| R2 | 0.44 |
Dependent variable is option selected, equal to 0 (1) when money (choice) option is selected. This analysis used all choice trials. Pairwise comparisons of 2, 4, or 8 vs. 1 item are shown. The effects increase with the number of items (see also Supplemental Tables S1 and S2). Thus adding more choice results in consistently stronger effects compared with no choice. For example, participants show a choice overvaluation effect of about 4% (11%) when we compare 2 (8) items with no choice. In other words, with larger choice sets, participants give up gradually more utility to choose the choice option. Note that this holds in the whole group of subjects and after controlling for differences in expected utility (EU) between the choice and money options. Robust standard errors are clustered by subject; t-statistics are reported in parentheses. LPM, linear probability model.
P < 0.05;
P < 0.01.
Neuroimaging Data
We analyzed brain activation at the time of choice 1, that is, when the monetary amount and the number of choice alternatives were presented. We used a general linear model with four separate regressors for 1, 2, 4, and 8 choice alternatives, while low- and high-value conditions and monetary amount offered served as parametric modulators. To investigate the notion that the value of choice and that of items one might choose from are related, we tested whether the subjective value of choice is coded in the same striatal regions that code the subjective value of everyday items. Moreover, we assessed whether neural substrates reflected the subjective value of having more or fewer choice alternatives rather than simply coding the numbers that were displayed. To do so, we specifically searched for activations that were more strongly correlated with the behaviorally determined subjective value of choice (monetary amount equivalent; i.e., the monetary amount at which a given participant chose the monetary amount option and the choice option equally often) than with the objective number of choice alternatives.
In a first step of identifying regions that jointly code the subjective value of the items one can choose from and the number of choice alternatives, we tested for higher activation in trials with high- compared to those with low-value items, pooling over different numbers of choice alternatives. The analysis revealed significantly stronger activation in the striatum in trials with high- than in those with low-value item trials (Fig. 4, A and B, and Table 2). The results were the same for both types of ROIs (P < 0.05, small-volume correction for multiple comparisons, irrespective of whether ROIs were defined anatomically: striatum, i.e., caudate plus putamen, or functionally: 8-mm sphere around a previously reported ventral striatal peak activation reflecting the desirability of consumer products; Knutson et al. 2007). These data suggest that the subjective value of concrete items that one will be able to choose from is processed in the striatum.
We then assessed whether the subjective value of the opportunity to choose is coded within the same striatal region that was found to process the subjective value of items one can choose from. To this end, we used the individually determined monetary amount equivalents for 1, 2, 4, and 8 choice alternatives as contrast weights, pooling over low- and high-value items. These individualized contrasts then entered a simple t-test on the group (random effects) level. We found that striatal activation reflected the subjective value of having more or fewer choices within a subset of voxels identified with the initial contrast of high- vs. low-value items (P < 0.05, small-volume corrected; Fig. 4, C and D, and Table 3). Accordingly, activations from the item-related and the opportunity-to-choose-related subjective value contrasts not only independently activated the striatum but overlapped (Fig. 4E and Table 4) such that striatal activation increased with subjective value of both items and the opportunity to choose (this finding also held with a conjunction null analysis; P < 0.05, small-volume corrected). These data suggest that striatal activation reflects not only the value of concrete rewards, such as consumer products, but also the value of having more or fewer choices, which is in line with the view that choice valuation arises from the value of the items one can choose from.
Table 3.
Brain regions with activation reflecting the subjective value of increasing opportunities to choose at choice 1
| Brain Region | L/R | x | y | z | z Score | Voxels |
|---|---|---|---|---|---|---|
| Superior frontal gyrus | R | 20 | −20 | 56 | 2.86 | 6 |
| Insula | L | −28 | 2 | 14 | 3.52 | 25 |
| R | 32 | 8 | 14 | 3.31 | 30 | |
| Putamen | L | −24 | 14 | −2 | 2.74 | 5 |
| −14 | 12 | −4 | 2.67 | 5 | ||
| R | 16 | 12 | −2 | 2.82 | 14 | |
| Middle temporal gyrus | R | 40 | −58 | 8 | 2.80 | 19 |
| Supramarginal gyrus | R | 60 | −32 | 18 | 3.06 | 56 |
| Calcarine sulcus | L | −24 | −66 | 12 | 2.87 | 22 |
| Inferior parietal lobule | L | −44 | −32 | 28 | 3.25 | 32 |
| R | 66 | −16 | 32 | 2.95 | 10 |
P < 0.005, uncorrected, >5 voxels.
Table 4.
Brain regions showing common coding of item value and the value of the opportunity to choose
| Brain Region | L/R | x | y | z | z Score | Voxels |
|---|---|---|---|---|---|---|
| Postcentral gyrus | R | 28 | −38 | 40 | 2.97 | 7 |
| Insula | R | 34 | 12 | 8 | 2.85 | 5 |
| Posterior cingulate cortex | L | −18 | −26 | 32 | 2.83 | 6 |
| R | 18 | −34 | 34 | 3.06 | 5 | |
| Caudate | L | −12 | 22 | 8 | 3.24 | 19 |
| −4 | 10 | 12 | 2.95 | 23 | ||
| R | 16 | 14 | 4 | 2.98 | 40 | |
| 16 | 16 | 16 | 2.89 | 41 | ||
| Putamen | L | −16 | 14 | −4 | 2.76 | 10 |
P < 0.005, uncorrected, >5 voxels.
To determine the specificity of the effects, we investigated whether the activation related to the opportunity to choose particularly reflected the subjective value of having more or fewer choices as opposed to other, more general but related variables such as the objective number of choice alternatives. Although striatal activation was correlated with the objective number of choice alternatives, we found a significantly better fit with the individual-specific subjective value of the opportunity to choose than with the objective number of choice alternatives in a smaller subset of voxels [P < 0.05 (Fig. 4F); plotting activation in the identified voxels revealed a plateauing relationship between activation and number of choice alternatives (Fig. 4G) similar to the behavior shown in Fig. 2]. Taking the mean subjective value of having more or fewer choices as a contrast weight for all participants instead of the individual-specific subjective value of having more or fewer choices resulted in intermediate fits (for testing the relationship to mean subjective value, all individual monetary amount equivalents were averaged separately for each condition, and these averages were used as contrast weights for each participant). Thus the more individual and subjective information about the value of choice one takes into account, the better one captures striatal activation.
Given that the identified striatal regions (Fig. 4E) process the value of both items and choice, the question arises whether their activation also increases with the monetary amount offered. A subset of voxels showed such a relationship (Fig. 4H, peak at 12/16/12). This finding further supports the notion of generalized value coding in the mid striatum.
We then asked whether some brain regions would specifically encode choice value over and above item value, and vice versa. No regions survived striatal small-volume correction or whole brain correction. At an exploratory threshold (P < 0.001), we found a region in the temporal cortex that coded item value more strongly than choice value (48/−26/2) and a region in anterior cingulate cortex that showed preferential activation for choice value rather than item value (16/20/26). Given that these regions were previously shown to be related to the processing of items and choice, respectively, we report these results for descriptive purposes.
Individual differences in choice (over)valuation.
We obtained the neuroimaging results reported so far (Fig. 4) by using the subjective value of the opportunity to choose to reveal main effects in the neural correlates of choice valuation. To investigate the neural basis of individual differences in choice overvaluation, we took advantage of the individual variation in the value of having more or fewer choices (Fig. 3). We studied the relationship between the individual differences in monetary amount equivalents for 8/low-value vs. 1/high-value items and the activation differences elicited by the same two conditions. Moreover, we compared the activation differences induced in the 8/low-value vs. 1/high-value conditions across three groups of participants: those who valued 8/low value significantly more than 1/high value, those who showed a significant effect in the opposite direction, and those who showed no significant difference between the two conditions. In both analyses, we found that the larger the individual differences between 8/low value vs. 1/high value were, the more strongly was the dorsolateral striatal activation in the 8/low-value vs. 1/high-value conditions (P < 0.05, small-volume corrected in striatum; Fig. 5, A and B, and Table 5: note that the 2 analyses were performed independently of each other and of the analyses reported above).
Fig. 5.
Individual differences in brain activation reflecting differences in valuation of choice. A: location in dorsolateral striatum showing correlation between the valuation of 8/low value vs. 1/high value (as determined by monetary amount equivalents) and corresponding brain activation (x/y/z: −14/4/24). B: plot illustrating effect shown in A. C: location in dorsolateral striatum (x/y/z: −12/14/18) at which correlation was found between choice overvaluation (rank data from Fig. 3C) and activation related to number of choice alternatives (8 > 4 > 2 > 1). D: plot illustrating effect shown in C. E: absence of relationship between dorsolateral striatal activation and individual tendency to make mistakes overall. At the peak location identified in A, plot shows activation related to the number of choice alternatives (8 > 4 > 2 > 1) as a function of individual slopes in mistakes from different numbers of choices. F: relationship between dorsolateral striatal activation and tendency to make choice-favoring mistakes as the number of choice alternatives increases. At the same location that was plotted in E, activation related to number of choice alternatives (8 > 4 > 2 > 1) increased with the tendency to make choice-favoring mistakes. Thus the more often subjects mistakenly chose the choice option, the more dorsolateral striatal activation increased with the number of choice alternatives.
Table 5.
Brain regions showing group differences in activation to 8/low value vs. 1/high value
| Brain Region | L/R | x | y | z | z Score | Voxels |
|---|---|---|---|---|---|---|
| Superior frontal gyrus | R | 24 | 46 | 34 | 3.3 | 21 |
| Middle frontal gyrus | L | −24 | 36 | 4 | 3.06 | 10 |
| R | 32 | 16 | 42 | 2.93 | 14 | |
| Inferior frontal gyrus | R | 54 | 12 | 6 | 3.02 | 75 |
| Postcentral gyrus | L | −50 | −18 | 20 | 3.05 | 25 |
| Insula | R | 38 | −24 | 4 | 3.01 | 18 |
| Caudate | L | −14 | 4 | 24 | 3.7 | 29 |
| Superior temporal gyrus | L | −48 | −32 | 20 | 3.31 | 93 |
| Temporoparietal cortex | R | 50 | −38 | 34 | 4.04 | 97 |
| 58 | −38 | 26 | 3.05 | 25 | ||
| Supramarginal gyrus | L | −52 | −60 | 32 | 2.83 | 9 |
| R | 42 | −54 | 28 | 3.23 | 26 | |
| Cuneus | L | −14 | −84 | 22 | 3.15 | 131 |
| R | 18 | −80 | 24 | 3.13 | 28 |
Data represent group differences (8/low value >1/high value) > (8/low value ≈ 1/high value) > (8/low value <1/high value) in activation to 8/low value vs. 1/high value. P < 0.005, uncorrected, >5 voxels.
Similar results were obtained when we assessed individual differences in choice valuation with respect to the maximally possible value increases across the whole range of choice alternatives. More specifically, we assessed the relationship between the degree to which choice- and rating-based slopes deviated from the identity line (Fig. 3C), on one hand, and brain activation arising in the contrast of 8 > 4 > 2 > 1 choice alternative conditions, on the other. The choice/rating deviations from identity reflect relative value enhancements from choice. This analysis revealed a similar cluster in the left dorsal striatum (Fig. 5C). The more participants valued choice, the more strongly dorsal striatum activation increased with the number of choice alternatives (Fig. 5D). Note that although the two measures of individual differences in choice valuation were correlated, the contrasts used for the two analyses were different. Taken together, individual differences in the valuation of choice were reflected by corresponding activation differences in the dorsolateral striatum.
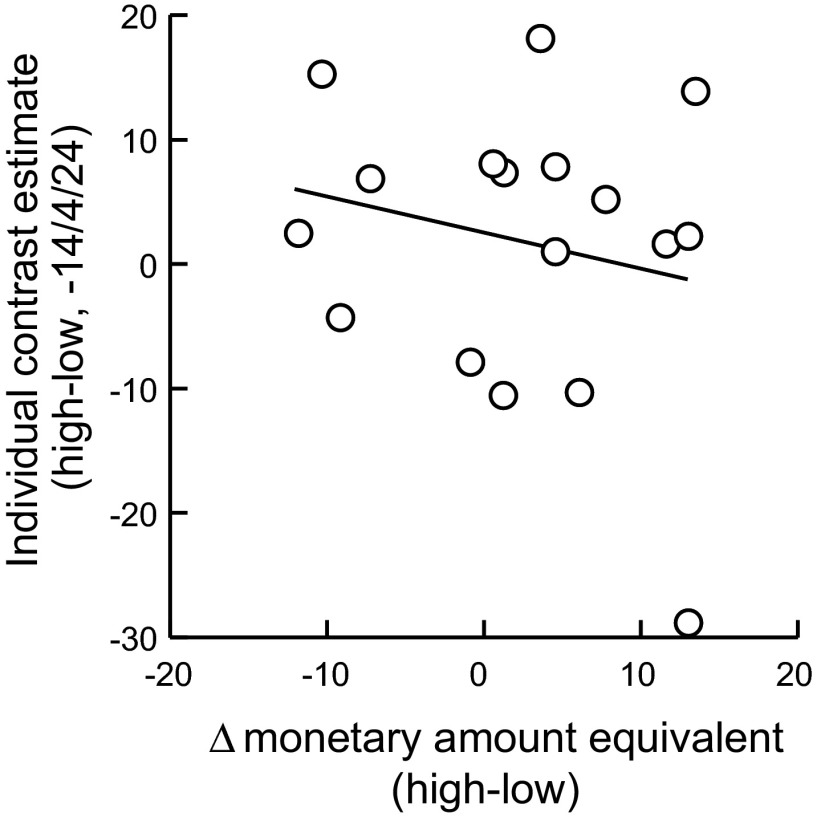
We then considered alternative explanations. First, we assessed whether the dorsolateral striatal activations that we had identified were specific to individual differences in the valuation of choice. If so, there should be little or no relationship between the activation induced by high- vs. low-value items and individual differences in the value of high- vs. low-value items. This was indeed the case. Neither the peak voxel showing a relationship to individual differences in choice valuation (−14/4/24; Fig. 6, r = −0.2, P = 0.4, resulting in a significant difference compared with correlations with individual choice differences, P < 0.05) nor voxels in the vicinity (20-mm sphere, all voxels P > 0.05, uncorrected) showed a significant relation with individual differences in item valuation. Also, when we averaged over all voxels within the 20-mm sphere, there was no significant correlation with individual differences in item valuation (P > 0.2). Thus the observed activation differences in dorsolateral striatal activation were specific for individual differences in the value of choice.
Fig. 6.
Absence of a relationship between dorsolateral striatal activation and individual differences in item valuation. Activation to high- vs. low-value items is plotted as a function of individual differences in monetary amount equivalent between high- and low-value items. Relationship illustrated at the peak voxel shows coding of individual differences in choice valuation from Fig. 5A.
We then assessed whether the individual differences in choice valuation reflect current or predicted choice difficulty or action selection. It is harder to select from a choice set of 8 items than from only 1 item, and this should be reflected in longer response times for more choices. We therefore assessed the relationship between the differences in response time of 8/low value vs. 1/high value and differences in the valuation of 8/low value vs. 1/high value at both choice 1 and choice 2. There was no significant relationship at either time point (choice 1: r = 0.07, P = 0.8; choice 2: r = 0.02, P = 0.9). Moreover, neither the peak voxel nor voxels in the vicinity (20-mm sphere, single voxels or average) showed a significant relation with individual differences at either time point (all voxels P > 0.05). A comparison of the correlation coefficients between dorsolateral striatal activation and the various behavioral correlates revealed significantly stronger effects of individual differences in choice valuation compared with all three alternative explanations [1) vs. differences in high-low value of the item; 2) vs. response times at choice 1: both z = 2.2, P = 0.003; and 3) vs. response times at choice 2: z = 3.3, P = 0.0001, Steiger's z-test].
Finally, we investigated the relationship between brain activation and the utility-based measure of choice overvaluation (Fig. 3, E and F). The more participants mistakenly chose the choice option instead of the monetary amount option as the number of choice alternatives increased, the more they overvalued choice. This, and not the number of mistakes overall, should be reflected in a region sensitive to choice overvaluation. To increase the independence of the analysis, we used a different behavioral measure and contrast to test the relationship with brain activation at the location identified (i.e., those of Fig. 5A). There was no relationship between increase in activation with increasing number of choice alternatives and the overall tendency to make mistakes in these conditions (Fig. 5E). By contrast, and in agreement with the findings reported above, the dorsolateral striatal activation arising in the contrast of 8 > 4 > 2 > 1 choice alternatives increased with the frequency with which participants mistakenly chose the choice option at the same location (Fig. 5F and Table 6). Moreover, when tested directly, a cluster in dorsolateral striatum showed a correlation between activation related to the number of choice alternatives and the propensity to choose the choice option mistakenly (Supplemental Fig. S3, A and B). Thus the more individuals overvalued choice, the more dorsolateral striatal activation increased with the number of choice alternatives.
DISCUSSION
The present study shows that more ventral striatal signals related to the value of items available from which to choose also reflect the value of having more choices, or degrees of freedom, which is in line with standard accounts of choice valuation. In contrast, dorsal striatal activation increases as a function of the degree to which individuals value choice. The latter finding was specific for choice rather than item value, which is in accordance with the notion of people giving up more to have more choices than they gain from the choices and with the notion of the opportunity to choose being valued in and of itself rather than as a way of obtaining more highly valued items. Indeed, these activations can be interpreted in terms of an individual tendency to overvalue choice. Note that the whole striatum served as the primary ROI in our study. Given that the ventral vs. dorsal dissociation was not predicted a priori, it may merit further investigation in future studies. Nevertheless, our data suggest that, overall, the striatum provides a neural underpinning to a pervasive motive found in humans and other species, namely, the value of freedom in the form of more choices.
It is well known that, at least up to a certain point, choice is beneficial and people attach importance to it (Brynjolfsson et al. 2003; Frey and Stutzer 2000; Ogden et al. 2009; Perlmuter et al. 1980; Zuckerman et al. 1978). For example, consumers often expect more choice, and producers are eager to oblige. By extension, choice provides a criterion for competition among vendors (Koelemeijer and Oppewal 1999). Moreover, not only humans but also animals prefer to have more than fewer choices (Catania 1975; Suzuki 1999; Voss and Homzie 1970). It is therefore unlikely that the phenomenon is a recent one in our ontogeny. Instead, it may have provided evolutionary benefits. It is conceivable that a preference for having more choices may have conferred an evolutionary advantage because having choices provides an opportunity to discover and explore novel situations and stimuli, such as tastier foods or safer environments. The behavioral and neural findings of the present study add further evidence to the notion that humans value the opportunity to choose.
Although traditional economic theory has employed choice to measure the value of the items available from which to choose (revealed preferences; Samuelson 1938), it also recognizes that the opportunity to choose is a valued good. In particular, economic theory suggests that having more choices is better than having fewer choices because it increases the probability that the most preferred item will be included in the choice set and thus that the needs and tastes of the decision maker will be met or that preference uncertainty will be accommodated (Pattanaik and Xu 1998). The prime contribution of the present study was to identify the neural correlates of such rational economic valuation of having more choices. Moreover, in the present experiment, at least some participants overvalued choice. This observation further shows how powerful, at least for some of us, the preference for having more over fewer choices can be and may contribute to the sometimes detrimental consequences of having too many choices (Botti and Iyengar 2006; Schwartz 2004). More generally, there appears to be variation in the degree to which individuals value choice in and of itself.
If, at least in some individuals, the instrumental property of providing access to better outcomes is not the only factor that gives choice value, what other factors make choice valuable? Choice allows us to exert control over our environment and thereby to experience ourselves as active and able to produce desired effects (e.g., see Leotti et al. 2010 and references therein). Moreover, having the opportunity to choose allows us to express ourselves and our preferences, which can enhance our confidence in our performance (Tafarodi et al. 2002). Thus, because of their positive consequences, having the opportunity to choose and executing choice may confer intrinsic reward value to having a choice over not having a choice. Although further research is needed to assess these possibilities, our data support the notion that choice can be valuable in and of itself and extend it by suggesting that this effect is a function of the number of choices available.
Previous neuroimaging studies showed that the striatum codes the value of many different goods (e.g., Aharon et al. 2001; Goldstone et al. 2009; Gregorios-Pippas et al. 2009; Izuma et al. 2008; Kable and Glimcher 2007; Knutson et al. 2007; McClure et al. 2003; Oyama et al. 2010; Small et al. 2001; Tobler et al. 2009). Interestingly, this processing occurs primarily in mid and ventral parts of the striatum, where we obtained main effects of both choice and item value. By contrast, more dorsolateral striatal regions were correlated with individual differences in the valuation of choice. Thus our findings revealed fMRI-based heterogeneity of value processes in the striatum. This possibility awaited testing because previous studies typically used only one valued good at a time.
Central striatal regions that code the value of choice overlapped with those that code the value of the items. This overlap finds a natural explanation in formal accounts of why choice is valuable proposed by standard economic theory (Pattanaik and Xu 1998). Larger choice sets increase the probability that a subjectively better item will be contained in the choice set. Accordingly, the value of choice arises from the value of items, and if the former derives from the latter, one expects common coding. Mechanistically speaking, the value of choice conceived in this way could be learned through repeated experience of better outcomes following exposure to larger choice sets (higher-order conditioning). This interpretation is in agreement with the finding that striatal activation underpins higher-order pain conditioning (Seymour et al. 2004), but direct experiments are necessary to test it. It should also be noted that the evidence for common coding is limited by the spatial resolution that can be achieved with fMRI. In principle, it is conceivable that distinct but intermingled neurons represent different value types.
In contrast, dorsolateral striatal regions reflected individual differences in choice but not item value. Given that the dorsal striatum has been associated with action selection and updating the value of chosen actions (e.g., Balleine et al. 2007; Samejima et al. 2005), a follow-up hypothesis potentially suggested by the present results would be that the value of choice may partly arise from valuing action selection and exerting control (see above). Moreover, the dorsolateral striatum may play a preferential role in processing the value of choice as captured by philosophical notions of choice being valued in and of itself (Jones and Sugden 1982) rather than the more standard notion of choice being valued for instrumental reasons because it increases access to a better outcome. One potential implication of this notion of the value of choice could be that the more someone values choice this way, the more he/she should prefer additional choice alternatives resulting in the same outcome (e.g., buttons on a drink vending machine delivering the same drink; note, however, that some theoreticians distinguish between significant and indistinguishable choice alternatives, e.g., Jones and Sugden 1982). Interestingly, this possibility is supported by a recent brain imaging study (Leotti and Delgado 2011) that reported activation in ventral parts of caudate and putamen for the comparison of having a binary choice vs. having no choice resulting in the same outcome.
The involvement of the dorsal striatum found in the present study when the value of choice was being processed converges with a preferential role of the dorsal striatum in processing rewards that result from one's own actions as opposed to those that are simply delivered (e.g., O'Doherty et al. 2004; Tricomi et al. 2004; Zink et al. 2004). Moreover, having chosen a given choice alternative enhances the subsequently rated value of that alternative and activates the dorsal striatum, whereas having previously rejected a choice alternative does not (Izuma et al. 2010; Sharot et al. 2009). Finally, a cue that informs participants that they will be choosing between two potentially rewarding alternatives is more highly valued and activates ventral parts of the dorsal striatum more than a cue informing them that the computer will make such a choice (Leotti and Delgado 2011). Thus the dorsal striatum appears to play a particular role in processing the contingencies between reward and action, a notion that is also borne out by animal studies (e.g., Balleine et al. 2007; Samejima et al. 2005).
Our data inform and extend the previous findings in several ways. An upcoming opportunity to choose activates striatal regions more than the absence of that opportunity, and this effect has been interpreted as reflecting the subjective value of having control rather no control (Leotti and Delgado 2011). If this interpretation is true, then one would expect subjective value and striatal activations to also be a function of the degree of freedom provided by different amounts of choice rather than an all-or-nothing phenomenon. Our data suggest that this is indeed the case: striatal activations increased monotonically as a function of the degree of choice. The use of more than one type of reward (items and freedom) in the present design allowed us not only to assess commonalities and differences (described above) but also to provide within-subject confirmation of the subjective value-related nature of choice activations without having to resort to previous research on more commonly used types of reward, such as food or money. Moreover, the precise monetary quantification of the value of choice in the present study allowed us to extend previous findings with an analysis of individual differences in choice valuation. Whether the participants who are particularly sensitive to the value of choice are also particularly sensitive to action-reward contingencies is an issue that merits future investigation.
The neural overlap between choice and item value implicates parts of the central striatum in generalized value processing independent of reward identity. However, alternative, reward identity-specific hypotheses are conceivable, particularly with respect to the frontal cortex. The opportunity to choose may require making a willed choice by selecting a response and overcoming response conflict. The willed choice of a response activates lateral prefrontal cortex more than instructed stimulus-response mapping (Frith et al. 1991), whereas response selection and response conflict activate regions such as the (pre)supplementary motor area and the anterior cingulate cortex (Lau et al. 2006; Krieghoff et al. 2009). On the basis of these findings and a potential role of the associated functions in coding the opportunity to choose, it could be argued that frontal regions should also process the value of having more choice. However, with the presently employed statistical thresholds, we found very little evidence supporting this view.
In conclusion, we have extended the notion of reward value processing by the striatum. Widespread cortical and subcortical inputs enable the striatum to process a variety of inputs, including reward information (Haber et al. 2006). Our results provide evidence for the existence of both common and distinct value processing regions in the striatum. Rational notions of the value of choice as described by economic theory (Pattanaik and Xu 1998) are underpinned by a common coding of the value of items and choice in more ventral striatal regions. However, we also observed individual variation with respect to the degree to which individuals overvalued choice. Such variation was reflected in more dorsal striatal activation and corresponds to the notion that this activation is a function of the degree to which choice is valued in and of itself.
GRANTS
This research was supported by Swiss National Science Foundation Grant PP00P1_128574; the Swiss National Centre of Competence in Research in Affective Sciences; Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grants-in-Aid for Scientific Research (KAKENHI) 17680027 and 19673002; MEXT Grants-in-Aid for Scientific Research on Priority Areas 17022009, 18020005, and 20019005; an Academic Frontier Project for Private Universities at Nihon University; and a matching fund subsidy from the MEXT. J. Fujiwara was supported by a Global Center of Excellence Program at Tohoku University from the MEXT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F., K.-I.T., and P.N.T. conception and design of research; J.F., N.U., and K.-I.T. performed experiments; J.F., S.Q.P., and T.W. analyzed data; J.F., S.Q.P., T.W., K.-I.T., and P.N.T. interpreted results of experiments; J.F. prepared figures; J.F., K.-I.T., and P.N.T. drafted manuscript; J.F., T.W., T.I., M.T., K.-I.T., and P.N.T. edited and revised manuscript; J.F., K.-I.T., and P.N.T. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Thorsten Kahnt, Stefan Kaiser, and Ian Krajbich for helpful discussions.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI, and behavioral evidence. Neuron 32: 537–551, 2001 [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci 27: 8161–8165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti S, Iyengar SS. The dark side of choice: when choice impairs social welfare. J Publ Policy Market 25: 24–38, 2006 [Google Scholar]
- Brynjolfsson E, Hu Y, Smith MD. Consumer surplus in the digital economy: estimating the value of increased product variety at online booksellers. Manage Sci 49: 1580–1596, 2003 [Google Scholar]
- Catania AC. Freedom and knowledge: an experimental analysis of preference in pigeons. J Exp Anal Behav 24: 89–106, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BS, Stutzer A. Happiness prospers in democracy. J Happiness Stud 1: 79–102, 2000 [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229, 1997 [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci 244: 241–246, 1991 [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, Bell JD. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci 30: 1625–1635, 2009 [DOI] [PubMed] [Google Scholar]
- Gregorios-Pippas L, Tobler PN, Schultz W. Short-term temporal discounting of reward value in human ventral striatum. J Neurophysiol 101: 1507–1523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci 26: 8368–8376, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Matsumoto M, Murayama K, Samejima K, Sadato N, Matsumoto K. Neural correlates of cognitive dissonance, and choice-induced preference change. Proc Natl Acad Sci USA 107: 22014–22019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron 58: 284–294, 2008 [DOI] [PubMed] [Google Scholar]
- Jones P, Sugden R. Evaluating choice. Int Rev Law Econ 2: 47–65, 1982 [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10: 1625–1633, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci 21: 1332–1345, 2009 [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron 53: 147–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelemeijer K, Oppewal H. Assessing the effects of assortment and ambience: a choice experimental approach. J Retail 75: 319–345, 1999 [Google Scholar]
- Krieghoff V, Brass M, Prinz W, Waszak F. Dissociating what and when of intentional actions. Front Hum Neurosci 3: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H, Rogers RD, Passingham RE. Dissociating response selection and conflict in the medial frontal surface. Neuroimage 29: 446–451, 2006 [DOI] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The inherent reward of choice. Psychol Sci 22: 1310–1318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Iyengar SS, Ochsner KN. Born to choose: the origins and value of the need for control. Trends Cogn Sci 14: 457–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron 38: 339–346, 2003 [DOI] [PubMed] [Google Scholar]
- Nozick R. Anarchy, State, and Utopia. Oxford: Blackwell, 1974 [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304: 452–454, 2004 [DOI] [PubMed] [Google Scholar]
- Ogden J, Daniells E, Barnett J. When is choice a good thing? An experimental study of the impact of choice on patient outcomes. Psychol Health Med 14: 34–47, 2009 [DOI] [PubMed] [Google Scholar]
- Oyama K, Hernádi I, Iijima T, Tsutsui K. Reward prediction error coding in dorsal striatal neurons. J Neurosci 30: 11447–11457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci 30: 7749–7753, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanaik PK, Xu Y. On preference and freedom. Theory Decis 44: 173–198, 1998 [Google Scholar]
- Perlmuter LC, Scharff K, Karsh R, Monty RA. Perceived control: a generalized state of motivation. Motiv Emot 4: 35–45, 1980 [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science 310: 1337–1340, 2005 [DOI] [PubMed] [Google Scholar]
- Samuelson P. A note on the pure theory of consumers' behaviour. Economica 5: 61–71, 1938 [Google Scholar]
- Schwartz B. The Paradox of Choice: Why More is Less. New York: Harper Collins, 2004 [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature 429: 664–667, 2004 [DOI] [PubMed] [Google Scholar]
- Sharot T, De Martino B, Dolan RJ. How choice reveals and shapes expected hedonic outcome. J Neurosci 29: 3760–3765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124: 1720–1733, 2001 [DOI] [PubMed] [Google Scholar]
- Suzuki S. Selection of forced- and free-choice by monkeys (Macaca fascicularis). Percept Mot Skills 88: 242–250, 1999 [Google Scholar]
- Tafarodi RW, Mehranvar S, Panton RL, Milne AB. Putting oneself in the task: choice, personalization, and confidence. Pers Soc Psychol Bull 28: 648–658, 2002 [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Risk-dependent reward value signal in human prefrontal cortex. Proc Natl Acad Sci USA 106: 7185–7190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron 41: 281–292, 2004 [DOI] [PubMed] [Google Scholar]
- Voss SC, Homzie MJ. Choice as a value. Psychol Rep 26: 912–914, 1970 [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron 42: 509–517, 2004 [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Porac JF, Lathin D, Smith R, Deci EL. On the importance of self-determination for intrinsically motivated behavior. Pers Soc Psychol Bull 4: 443–446, 1978 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.