Abstract
The principal inhibitory neurotransmitter in the mammalian cochlear nucleus (CN) is glycine. During age-related hearing loss (AHL), glycinergic inhibition becomes weaker in CN. However, it is unclear what aspects of glycinergic transmission are responsible for weaker inhibition with AHL. We examined glycinergic transmission onto bushy cells of the anteroventral CN in normal-hearing CBA/CaJ mice and in DBA/2J mice, a strain that exhibits an early onset AHL. Glycinergic synaptic transmission was examined in brain slices of mice at 10–15 postnatal days old, 20–35 days old, and at 6–7 mo old. Spontaneous inhibitory postsynaptic current (sIPSC) event frequency and amplitude were the same among all three ages in both strains of mice. However, the amplitudes of IPSCs evoked (eIPSC) from stimulating the dorsal CN were smaller, and the failure rate was higher, with increasing age due to decreased quantal content in both mouse strains, independent of hearing status. The coefficient of variation of the eIPSC amplitude also increased with age. The decay time constant (τ) of sIPSCs and eIPSCs were constant in CBA/CaJ mice at all ages, but were significantly slower in DBA/2J mice at postnatal days 20–35, following the onset of AHL, and not at earlier or later ages. Our results suggest that glycinergic inhibition at the synapses onto bushy cells becomes weaker and less reliable with age through changes in release. However, the hearing loss in DBA/2J mice is accompanied by a transiently enhanced inhibition, which could disrupt the balance of excitation and inhibition.
Keywords: glycinergic inhibition, presbycusis, quantal content, ventral cochlear nucleus, bushy cells
glycine is the primary inhibitory neurotransmitter in the mammalian cochlear nucleus (CN). Glycinergic inhibition has been shown to shape the neural processing of sound in CN neurons (Gai and Carney 2008), and to decrease in strength in aging models with hearing loss (Caspary et al. 2005; Wang et al. 2009, 2011). In the dorsal CN (DCN), the decrease in the strength of glycinergic inhibition, possibly in response to a loss of excitatory sensory input, has been proposed to be a consequence of both age and environmentally induced hearing impairments (Caspary et al. 2005, 2008; Frisina and Walton 2006). However, how glycinergic transmission changes at the synaptic level during aging and in response to changes in sensory input remains unclear.
As one of the principal cells in the anterior ventral CN (AVCN), bushy cells receive direct input from the auditory nerve via endbulb synapses (reviewed in Manis et al. 2011). Bushy cells play roles in encoding the fine temporal structure of sound, which is used in the discrimination of vowels and amplitude modulated sounds (Blackburn and Sachs 1990; Rhode and Greenberg 1994). While the excitatory input to bushy cells from the auditory nerve has been well studied (Cao and Oertel 2010; Manis et al. 2011; Oleskevich and Walmsley 2002; Wang and Manis 2008; Yang and Xu-Friedman 2008), less is known about glycinergic inhibition. These glycinergic synapses arise from D-stellate cells within AVCN (Arnott et al. 2004; Smith and Rhode 1989), as well as tuberculoventral (TBV) cells in the DCN (Saint Marie et al. 1991; Wickesberg and Oertel 1990). Functionally, the glycinergic synapses are poised to provide tonic inhibition that can increase the temporal precision of spikes in bushy cells (Kuenzel et al. 2011; Xie and Manis 2013). The ability to make distinctions among sounds on the basis of temporal cues can be compromised in people with age-related hearing loss (AHL), especially in noisy acoustic environments (Anderson et al. 2012; Cobb et al. 1993; Lorenzi et al. 2006; Tremblay et al. 2003). In addition to the loss of auditory information associated with damage and an age-related decline in peripheral hearing capabilities, age-related changes in central synaptic transmission and excitability can also affect signal processing and ultimately auditory perception. Since bushy cells play critical roles in central auditory processing related to temporal cues, their response to hearing loss and aging is of special interest in understanding the mechanisms of perceptual decline in the aging auditory system.
Widespread anatomical and neurochemical changes in the CN are known to accompany AHL. Both excitatory and inhibitory synapses show decreases in terminal size with age (Helfert et al. 2003), tissue glutamate and glycine levels decline with age (Banay-Schwartz et al. 1989a, 1989b), and glycine receptor mRNA for α1- and β-subunits falls, while mRNA for α2-subunits increases (Krenning et al. 1998). However, much less is known about the effects of AHL on synaptic transmission in the CN. We previously assessed the changes of excitatory synaptic transmission onto bushy cells, comparing CBA/CaJ and DBA/2J mice (Wang and Manis 2005). CBA/CaJ mice have normal and stable hearing thresholds throughout most of their life (Henry and Chole 1980). DBA/2J mice carry at least three recessive alleles that contribute to the progression of an early onset high-frequency AHL (Erway et al. 1993; Zheng et al. 1999) that starts at about 3 wk of age. Excitatory synaptic transmission from the auditory nerve onto bushy cells is normal in CBA/CaJ mice up to at least 65 days of age (Wang and Manis 2005). In contrast, although excitatory transmission at the endbulb synapses in DBA/2J mice is normal at 3–4 wk of age, the spontaneous excitatory postsynaptic current (sEPSC) frequency is lower, the EPSC decay τ is slower, and both the peak synaptic conductance and evoked release probability decrease by 6–9 wk of age. To maintain a balance between excitation and inhibition (Sun et al. 2010), the decreases in excitation in DBA/2J mice, as assessed by sEPSC frequency and amplitude, might be predicted to be accompanied by parallel decreases in inhibition.
In the present study, we compared glycinergic transmission onto bushy cells in normal-hearing CBA/CaJ mice and age-matched cohorts of DBA/2J mice with AHL, from postnatal day 10 (P10) to 7 mo old. We observed no change in the spontaneous inhibitory postsynaptic current (sIPSC) frequency or amplitude. However, the amplitude of evoked IPSCs (eIPSCs) decreased, and the release failure rate increased with age, indicating a reduction in quantal content. The coefficient of variation (CV) of the eIPSC amplitude also increased with age in both mouse strains. Surprisingly, in DBA/2J mice at P20–35, immediately following the onset of early AHL, glycinergic inhibition is enhanced as a result of a slower IPSC decay and an increased charge transfer during sustained activity.
MATERIALS AND METHODS
All experiments were performed under the guidelines of protocols approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Animals.
Two strains of mice, CBA/CaJ and DBA/2J, were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained in colonies at the University of North Carolina. Mice of either sex were used for recordings. The mice were studied in three age groups: P10–15, P20–35, and 6–7 mo.
Auditory brain stem response.
For auditory brain stem response (ABR) testing, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Tone pips with cosine2 rise-fall times of 2.5 ms, generated in MATLAB (version 2007–2012, Natick, MA), were attenuated with Tucker-Davis Technologies (Alachua, FL) PA5 programmable attenuators and presented to the ear through an EC-1 speaker in a closed system. Tone pips [4–48 kHz, 10–70 dB sound pressure level (SPL)] were repeated 800 times at a repetition rate of 40 Hz. Tone-evoked potentials were recorded differentially between the vertex (negative) and mastoid (positive), referenced to the rump, amplified 10,000×, and filtered between 100 and 3,000 Hz with a Grass-Telefactor (West Warwick, RI) P511-J amplifier, digitized with a Tucker-Davis Technologies RP2.1 real-time processor, and averaged in MATLAB. ABR threshold was determined as the lowest tone intensity that evoked a visually detectable ABR response. Thresholds were averaged from determinations by three independent, blinded, observers.
Brain slice preparation.
Brain slices were prepared as previously described (Wang and Manis 2008; Xie and Manis 2013). Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), and decapitated, and the brain stem was removed and immersed into artificial cerebrospinal fluid (ACSF) at 34°C and gassed with 5% CO2 and 95% O2. The ACSF contained the following (in mM): 122 NaCl, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 20 glucose, 3 myo-inositol, 2 sodium pyruvate, 0.4 ascorbic acid, 2.5 CaCl2, and 1.5 MgSO4. Parasagittal brain slices containing CN (350 μm thick) were cut to include all three regions of CN (AVCN, PVCN, and DCN). Slices were then bathed in ACSF at 34°C for at least 30 min before being transferred to the recording chamber. During recording, slices were submerged in a continuous flow of ACSF (∼3 ml/min) at 34°C. Cells were visualized with ×40 water immersion objective on a fixed-stage microscope (FS2, Zeiss, Germany). Cell images were acquired with a charge-coupled device camera (Retiga 2000DC, QImaging, Vancouver, BC).
Electrophysiological recordings.
Whole cell patch-clamp techniques were used to record synaptic currents. Recording pipettes were pulled using borosilicate glass (KG-33; King Precision Glass, Claremont, CA) on a Sutter P2000 puller (Sutter Instruments, San Francisco, CA). The pipettes were then backfilled with an internal solution containing the following (in mM): 105 CsMetSO3, 35 CsCl, 5 EGTA, 10 HEPES, 4 MgATP, 0.3 Tris-GTP, 10 Tris-phosphocreatine, and 3 QX-314 (chloride salt), pH adjusted to 7.2 with CsOH. Voltage and current were measured using a Multiclamp 700B amplifier, with series resistance compensated over 75% online. The amplifier and data-acquisition sequences were controlled by custom-written program in MATLAB. With an estimated Cl equilibrium potential of −31 mV, cells were voltage-clamped at −70 mV to increase the amplitude and signal-to-noise ratio of eIPSCs and sIPSCs. Voltages in this paper are not corrected for the liquid junction potential, which is calculated to be −7 mV. Fifty micromolar d-2-amino-5-phosphonopentanoic acid (d-APV) and 5 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were used to block all excitatory transmission. Ten micromolar SR95531 were also used in 23 out of 50 cells in CBA mice and 19 out of 57 cells in DBA mice to block GABAergic transmission, including all of the cells in the P10–15 age group to minimize the possible influence of GABAergic inhibition during development. No statistically significant difference was found in IPSC weighted decay τ, eIPSC PPR, eIPSC failure rate, or amplitude between cells recorded without or with SR95531 in the bath, consistent with our previous observations that GABAergic inhibition is weak or absent when stimulating glycinergic pathways onto bushy cells in the CN (Xie and Manis 2013). All chemicals were purchased from Sigma-Aldrich, except for CNQX, d-APV, and SR95531, which were purchased from Tocris Bioscience (Bristol, UK).
Glycinergic synaptic currents were elicited by electrical stimulation of the deep layer of the DCN with a 75-μm-diameter concentric electrode (FHC, Bowdoin, ME). The distance between the stimulating electrode and the recording site at the AVCN was 400 to 500 μm and was kept consistent between different brain slices, although it slightly varied depend on the physical size of the brain slice. Slices from younger animals had smaller CN, and therefore the distance was 300–400 μm. This stimulation activates the TBV cells that provide glycinergic inputs to the AVCN, but may also antidromically activate D-stellate cells whose local collaterals in the AVCN may also contribute to synaptic currents. The electrical stimulation consisted of a 100-μs current pulse, adjusted to 1.1–1.4 times the threshold to produce a synaptic response. In some experiments, trains of stimuli were also used. This stimulus intensity was chosen because it generated stable IPSCs, but was not so high as to potentially damage the slice during long stimulus trains. The amplitude of current used for stimulation was 59 ± 5 μA in P10–15 mice (n = 18), 86 ± 8 μA in P20–35 mice (n = 36), and 102 ± 11 μA in 6- to 7-mo-old mice (n = 24). The stimulus current level used increased with age (Kruskal-Wallis test, P = 0.017). Posttests revealed a significant difference (P < 0.05) between P10–15 and 6- to 7-mo-old groups, but no difference between P10–15 or the 6- to 7-mo-old group and the P20–35 group.
Cell identification.
All recordings reported in this paper were from bushy cells in the high-frequency area of the AVCN. A fluorescent dye, 0.1% Alexa Fluor 488 (Molecular Probes, Eugene, OR), was included in the internal solution to visualize cell morphology. Bushy cells were identified by having one or two short primary dendrites with heavily branched distal tufts (Cant and Morest 1979; Rouiller and Ryugo 1984; Tolbert et al. 1982; Webster and Trune 1982; Wu and Oertel 1984). Stellate cells were also found in the same regions of the AVCN, but have thin and long dendrites that are less profusely branched. Stellate cells were excluded from this study.
Data analysis.
sIPSCs were detected and analyzed using the scaled-template method (Clements and Bekkers 1997). The decay τ values were determined from fits of the average sIPSC to a double exponential function in Igor Pro (version 6.02; Wavemetrics, Portland, OR). The weighted decay τ of the IPSC was estimated as: τweighted = τfast × Afast + τslow × Aslow, where Afast and Aslow are the normalized amplitudes of the fast and slow components, and Afast + Aslow = 1.
All eIPSC measurements were made with custom-written functions in Igor Pro. Quantal content (m) of the glycinergic synapse was estimated using two different methods as described by Del Castillo and Katz (1954). 1) In the amplitude method, m can be estimated from the average amplitude of eIPSCs divided by the mean amplitude of sIPSCs in individual cells: m = mean amplitude of eIPSCs/mean amplitude of sIPSCs. 2) In the method of failures, m can be estimated by counting the number of stimulus trials that failed to evoke an eIPSC response, assuming that the release of quanta follows a Poisson distribution and that m is small. In this case, quantal content was estimated as m = ln (total number of stimulus trials/number of failed eIPSCs). In this study, eIPSCs occurred in an all-or-none manner. Minimal eIPSCs were easily detected because they were >20 pA in peak amplitude, which was well above the typical recording noise level of 10 pA or less. Release failures could be easily identified (see Fig. 4F, inset) and were determined by visual inspection. The CV of the eIPSCs was calculated as the standard deviation of the eIPSC amplitude (including failures) divided by the mean.
Fig. 4.
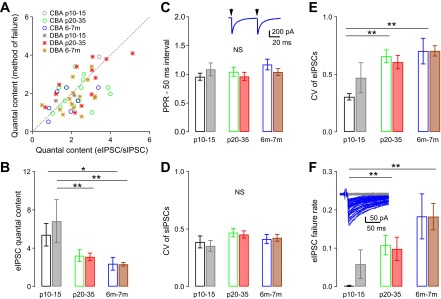
Quantal content decreases and release failure rate increases with age at the glycinergic synapse onto bushy cells. A: comparison of the estimated quantal content using the ratio of average eIPSC to sIPSC amplitudes and using the method of failures (see materials and methods), for individual cells. Dashed line: prediction of equal quantal content for the two methods. Cells with no failures in eIPSCs are not plotted in A. B: the average quantal content of eIPSC is reduced with age in both CBA/CaJ and DBA/2J mice. Quantal content was estimated using the amplitude method. See Table 1 for details in statistics. *Dunn's posttest after Kruskal-Wallis test: P < 0.05; **Tukey posttest after one-way ANOVA: P < 0.01. C: the paired-pulse ratio (PPR) at a 50-ms interval did not change with age in either mouse strain, suggesting that release probability does not change with age. Inset: example trace of PPR at 50-ms interval. Arrow: stimulus onset; stimulus artifacts are removed for clarity. D: coefficient of variation (CV) of sIPSC peak amplitude does not change with age in both CBA/CaJ and DBA/2J mice. E: CV of eIPSC peak amplitude increases with age in both CBA/CaJ and DBA/2J mice. **Tukey posttest after one-way ANOVA: P < 0.01. F: eIPSC failure rate increases with age in both CBA/CaJ and DBA/2J mice. Inset: example evoked IPSCs in a bushy cell from a 7-mo-old DBA/2J mouse. Notice that the responses are all or none, and the trials with failures of release (gray) can be easily separated from trials with successful release (blue). **Dunn's posttest after Kruskal-Wallis test: P < 0.01. See Table 1 for detailed statistics.
Statistical analysis.
Statistical analyses were performed using Prism (version 5.01; GraphPad Software, San Diego, CA). First, measurements were compared among three age groups within either CBA/CaJ or DBA/2J mice to assess the effects of age on inhibitory transmission in each strain. When data were normally distributed, a parametric one-way ANOVA followed by Tukey multiple-comparison posttests was used to detect differences between the age groups. When data were not normally distributed, a nonparametric ANOVA (Kruskal-Wallis test) was used and followed by Dunn's multiple-comparison posttest. Second, data were compared between CBA/CaJ and DBA/2J strains with two-way ANOVAs to analyze the contribution of strain, age, and the interaction between strain and age on inhibitory transmission. Bonferroni-corrected posttests were also performed. Data were presented as mean ± standard error of the mean (SEM) throughout the paper.
RESULTS
CBA/CaJ mice have normal ABR thresholds through 7 mo of age, while DBA/2J mice develop early onset AHL.
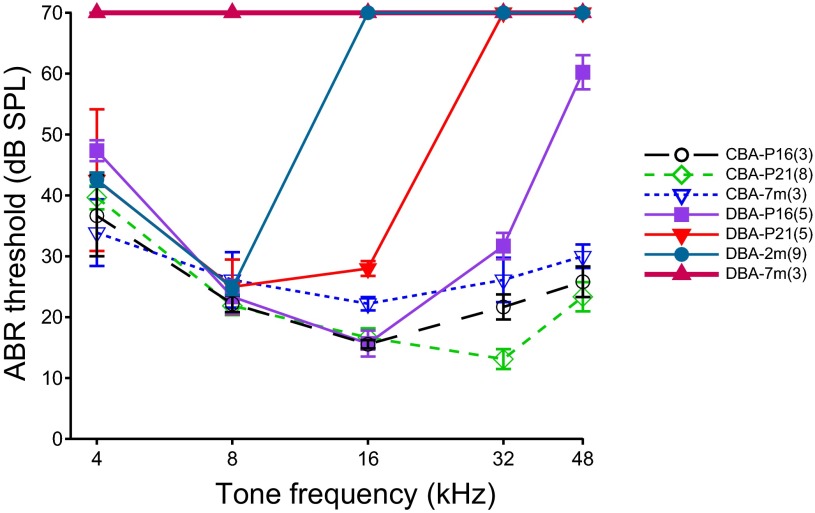
We evaluated the hearing status of both CBA/CaJ and DBA/2J mouse strains by measuring ABR thresholds to tones at different frequencies. As shown in Fig. 1, the ABR tone thresholds in CBA/CaJ mice did not change with age from 2 days after the onset of hearing (the onset of hearing is at ∼P14 in mice; see Ehret 1976), up to 7 mo of age. A two-way ANOVA showed no significant effect of age [F(2,54) = 2.43, P = 0.099] or interaction between age and frequency [F(8,54) = 1.14, P = 0.355] on ABR thresholds. ABR thresholds varied significantly as a function of frequency [F(4,54) = 10.39, P < 0.0001]. In DBA/2J mice, ABR thresholds revealed an early onset hearing loss, which began at high frequencies and progressively included lower frequencies as the animals grew older. By 7 mo of age, the ABR thresholds of DBA/2J mice for frequencies from 4 to 48 kHz were greater than 70 dB SPL. Thus, in contrast to CBA mice, a two-way ANOVA of ABR thresholds in DBA/2J mice showed significant effect of age [F(3,77) = 239.3, P < 0.0001], frequency [F(4,77) = 77.92, P < 0.0001], and an interaction between age and frequency [F(12,77) = 38.20, P < 0.0001]. A comparison between CBA/CaJ and DBA/2J mice showed significant differences in the ABR thresholds as early as P16, the earliest time that ABR thresholds were measured, and at that age DBA/2J mice started to show elevated ABR thresholds at the highest frequencies (Fig. 1). At this age, a two-way ANOVA between CBA/CaJ and DBA/2J mice revealed significant effect of strain [F(1,27) = 16.21, P = 0.0004], frequency [F(4,27) = 14.38, P < 0.0001] and the interaction between strain and frequency [F(4,27) = 4.39, P = 0.0073]. Specifically, Bonferroni-corrected posttests showed that the ABR thresholds of P16 DBA/2J mice were significantly higher than that of P16 CBA/CaJ mice at 48 kHz (P < 0.001). There were no differences in other frequencies (P > 0.05). These results are consistent with other studies showing that CBA/CaJ mice have normal ABR thresholds throughout most of their life, while DBA/2J mice develop an early onset AHL (Zheng et al. 1999).
Fig. 1.
Auditory brain stem response (ABR) thresholds in CBA/CaJ and DBA/2J mice at 3 ages. While CBA/CaJ mice have little threshold shift up to an age of 7 mo, DBA/2J mice show a hearing loss that starts at high frequencies and progresses to lower frequencies as the mice age. Thresholds >70 dB sound pressure level (SPL) are indicated as 70 dB SPL due to limitations of the speaker used to deliver the test sounds. The number of mice tested in each group is indicated in parentheses in the legend. P16 and P21, postnatal days 16 and 21, respectively; 7m and 2m, 7 and 2 mo of age, respectively.
Age-related changes in sIPSCs in bushy cells of CBA/CaJ and DBA/2J mice.
To separate the effect of the genetic hearing loss from that of age, we compared both sIPSCs and eIPSCs in CBA/CaJ mice with those in DBA/2J mice. Since the effects of the hearing loss in DBA/2J mice are progressive and start at the higher frequencies, we focused on recording from bushy cells in the dorsal, high-frequency region of the AVCN. Details of the data and statistical analysis are presented in Table 1.
Table 1.
Changes of glycinergic inhibition onto bushy cells in CBA/CaJ and DBA/2J mice
| Age Group |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P10–15 |
P20–35 |
6–7 mo |
||||||||
| Average | SEM | n | Average | SEM | n | Average | SEM | n | ANOVA P Value | |
| CBA/CaJ | ||||||||||
| sIPSCs | ||||||||||
| Fast decay τ, ms | 3.70 | 0.33 | 13 | 3.18 | 0.22 | 25 | 3.21 | 0.51 | 12 | 0.276B |
| Fast decay amplitude, % | 51.6 | 4.2 | 13 | 48.6 | 2.2 | 25 | 50.3 | 4.6 | 12 | 0.782A |
| Slow decay τ, ms | 16.26 | 1.47 | 13 | 14.33 | 1.00 | 25 | 13.39 | 2.21 | 12 | 0.260B |
| Slow decay amplitude, % | 48.4 | 4.2 | 13 | 51.4 | 2.2 | 25 | 49.7 | 4.6 | 12 | 0.782A |
| Weighted decay τ, ms | 9.93 | 1.08 | 13 | 8.81 | 0.58 | 25 | 8.42 | 1.57 | 12 | 0.390B |
| Half-width, ms | 5.12 | 0.58 | 13 | 4.42 | 0.40 | 25 | 4.78 | 0.82 | 12 | 0.640B |
| 20–80% rise time, ms | 0.17 | 0.01 | 13 | 0.15 | 0.01 | 25 | 0.2 | 0.02 | 12 | 0.269B |
| Frequency | 0.97 | 0.18 | 13 | 1.23 | 0.20 | 25 | 0.85 | 0.19 | 12 | 0.483B |
| Amplitude, pA | −94.9 | 6.8 | 13 | −119.2 | 9.7 | 25 | −107.7 | 11.0 | 12 | 0.233B |
| CV of sIPSC amplitude | 0.39 | 0.05 | 13 | 0.47 | 0.04 | 25 | 0.41 | 0.04 | 12 | 0.176B |
| eIPSCs | ||||||||||
| Fast decay τ, ms | 5.49 | 0.67 | 12 | 3.80 | 0.21 | 21 | 4.57 | 0.58 | 10 | 0.024A* |
| Fast decay amplitude, % | 55.8 | 3.4 | 12 | 50.6 | 2.1 | 21 | 53.7 | 3.1 | 10 | 0.361A |
| Slow decay τ, ms | 21.78 | 1.83 | 12 | 16.93 | 0.94 | 21 | 16.90 | 2.41 | 10 | 0.058A |
| Slow decay amplitude, % | 44.2 | 3.4 | 12 | 49.4 | 2.1 | 21 | 46.3 | 3.1 | 10 | 0.361A |
| Weighted decay τ, ms | 12.84 | 1.23 | 12 | 10.42 | 0.69 | 21 | 10.57 | 1.64 | 10 | 0.233A |
| Amplitude, pA | −409.0 | 74.1 | 12 | −251.9 | 39.7 | 21 | −181.9 | 45.9 | 10 | 0.017B* |
| Quantal content | 5.4 | 1.2 | 12 | 3.2 | 0.6 | 21 | 2.4 | 0.7 | 10 | 0.025B* |
| CV of eIPSC amplitude | 0.30 | 0.03 | 12 | 0.66 | 0.06 | 21 | 0.70 | 0.11 | 10 | 0.0005A‡ |
| eIPSC failure rate | 0.002 | 0.002 | 12 | 0.108 | 0.025 | 21 | 0.183 | 0.059 | 10 | 0.0008B‡ |
| Paired-pulse ratio (50 ms) | 0.96 | 0.06 | 12 | 1.05 | 0.08 | 21 | 1.17 | 0.09 | 10 | 0.252B |
| DBA/2J | ||||||||||
| sIPSCs | ||||||||||
| Fast decay τ, ms | 4.68 | 0.41 | 12 | 5.21 | 0.50 | 24 | 3.58 | 0.34 | 21 | 0.011B* |
| Fast decay amplitude, % | 47.6 | 3.0 | 12 | 39.2 | 2.4 | 24 | 40.2 | 2.4 | 21 | 0.096A |
| Slow decay τ, ms | 18.74 | 1.25 | 12 | 22.68 | 1.72 | 24 | 15.76 | 1.12 | 21 | 0.0041A† |
| Slow decay amplitude, % | 52.4 | 3.0 | 12 | 60.8 | 2.4 | 24 | 59.8 | 2.4 | 21 | 0.096A |
| Weighted decay τ, ms | 12.17 | 0.99 | 12 | 16.18 | 1.46 | 24 | 11.07 | 0.90 | 21 | 0.0092A† |
| Half-width, ms | 6.56 | 0.53 | 12 | 9.03 | 0.90 | 24 | 6.45 | 0.53 | 21 | 0.024A* |
| 20–80% rise time, ms | 0.17 | 0.02 | 12 | 0.21 | 0.02 | 24 | 0.17 | 0.01 | 21 | 0.217B |
| Frequency | 0.73 | 0.18 | 12 | 1.11 | 0.20 | 24 | 1.19 | 0.24 | 21 | 0.238B |
| Amplitude, pA | −101.3 | 7.6 | 12 | −123.8 | 10.7 | 24 | −97.4 | 8.1 | 21 | 0.054B |
| CV of sIPSC amplitude | 0.35 | 0.05 | 12 | 0.45 | 0.03 | 24 | 0.42 | 0.03 | 21 | 0.076B |
| eIPSCs | ||||||||||
| Fast decay τ, ms | 8.29 | 0.85 | 6 | 9.57 | 1.08 | 15 | 5.44 | 0.58 | 16 | 0.0084B† |
| Fast decay amplitude, % | 56.3 | 7.3 | 6 | 46.0 | 3.8 | 15 | 36.7 | 3.3 | 16 | 0.016B* |
| Slow decay τ, ms | 28.62 | 1.98 | 6 | 35.45 | 3.41 | 15 | 23.05 | 1.94 | 16 | 0.019B* |
| Slow decay amplitude, % | 43.7 | 7.3 | 6 | 54.0 | 3.8 | 15 | 63.3 | 3.3 | 16 | 0.016B* |
| Weighted decay τ, ms | 16.81 | 1.99 | 6 | 24.30 | 2.93 | 15 | 16.68 | 1.42 | 16 | 0.030A* |
| Amplitude, pA | −639.6 | 214.9 | 6 | −381.9 | 66.4 | 15 | −185.5 | 25.3 | 16 | 0.0040A† |
| Quantal content | 6.8 | 2.2 | 6 | 3.1 | 0.4 | 15 | 2.3 | 0.2 | 16 | 0.0015A† |
| CV of eIPSC amplitude | 0.47 | 0.13 | 6 | 0.61 | 0.06 | 15 | 0.70 | 0.05 | 16 | 0.078B |
| eIPSC failure rate | 0.058 | 0.037 | 6 | 0.098 | 0.030 | 15 | 0.182 | 0.035 | 16 | 0.044B* |
| Paired-pulse ratio (50 ms) | 1.09 | 0.11 | 6 | 0.96 | 0.07 | 15 | 1.04 | 0.06 | 16 | 0.450B |
n, No. of mice.
P, postnatal day; sIPSCs, spontaneous inhibitory postsynaptic current; τ, time constant; CV, coefficient of variation; eIPSCs, evoked inhibitory postsynaptic current.
ANOVA statistics: one-way ANOVA with Tukey multiple-comparison post test was used when data were normally distributed.
Nonparametric ANOVA (Kruskal-Wallis test) with Dunn's multiple-comparison post test was used when data were not normally distributed.
P < 0.05;
P < 0.01;
P < 0.001.
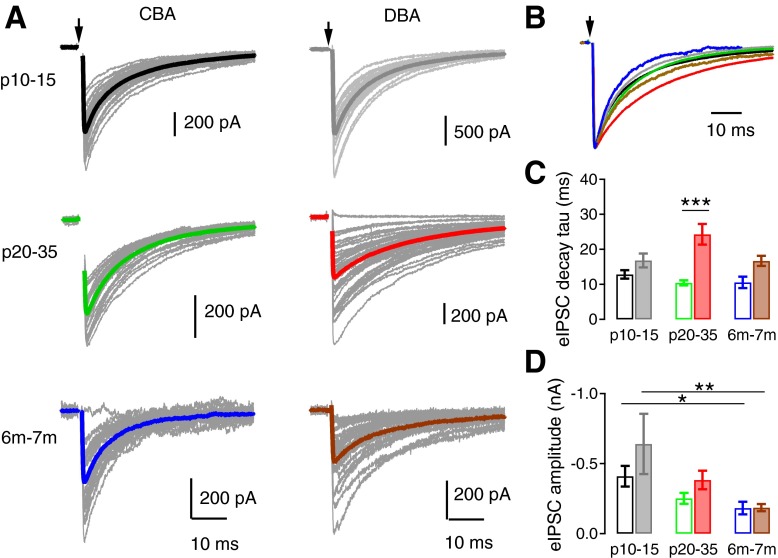
In normal hearing CBA/CaJ mice, spontaneously occurring glycinergic IPSCs onto bushy cells did not change amplitude or shape with age (Table 1, Fig. 2). The decay phase of the averaged sIPSC for each cell was best fit with a double-exponential function (Xie and Manis 2013). Among the three age groups of CBA/CaJ mice, there was no significant difference in the fast or slow component of the decay τ of sIPSCs, or in the calculated weighted decay τ of sIPSCs (Fig. 2C) (see Table 1 for details; one-way ANOVA: P > 0.05 for all comparisons). We also did not find any significant age-related change in sIPSC half-width (Fig. 2D), 20–80% rise time (Fig. 2E), frequency (Fig. 2F), or average sIPSC amplitude (Fig. 2G) (Table 1; one-way ANOVA: P > 0.05 for all comparisons). These results suggest that inhibition at the glycinergic synapses onto bushy cells is essentially adult-like by the onset of hearing (∼P14) in CBA/CaJ mice.
Fig. 2.
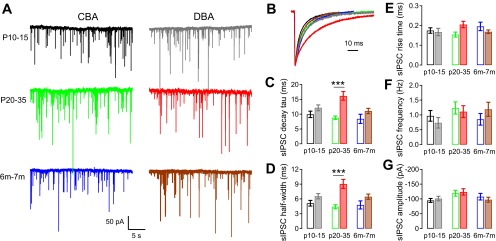
Spontaneous inhibitory postsynaptic currents (sIPSCs) in bushy cells from CBA/CaJ and DBA/2J mice. A: example traces of sIPSCs in bushy cells from CBA/CaJ and DBA/2J mice at different age groups. The color code indicates strain and age (CBA: black P10–15, green P20–35, and blue 6–7 mo; DBA: gray P10–15, red P20–35, and brown 6–7 mo), and also applies to panels B–G, as well as to Figs. 3, 4, and 6. B: kinetics of the averaged sIPSCs from cells in A. Traces were normalized to the peak to allow comparison of the time course. Note the slow decay of sIPSCs in P20–35 DBA/2J mice compared with other ages and to CBA/CaJ mice. C–G: summaries of weighted decay time constant (τ) for averaged sIPSCs (C), sIPSC half-width (D), sIPSC 20–80% rise time (E), sIPSC frequency (F), and sIPSC average amplitude (G) for all bushy cells in each group. Values and number of cells are also summarized in Table 1. Open bars: CBA/CaJ mice; filled bars: DBA/2J mice. ***Bonferroni-corrected posttest after two-way ANOVA: P < 0.001.
The DBA/2J mice, however, showed an unexpected age-dependent change in sIPSC time course, even though the sIPSC frequency and peak amplitude did not change. As shown in Fig. 2B, sIPSCs recorded from bushy cells in DBA/2J mice at P20–35 decay much more slowly than sIPSCs from either the younger or older age groups. The weighted decay τ of sIPSCs showed a significant difference among three age groups [one-way ANOVA: F(2,54) = 5.13, P = 0.0092], with the slowest decay at P20–35 (Tukey posttest showed P < 0.01 between P20–35 and 6- to 7-mo age groups, while P > 0.05 between P10–15 and P20–35, as well as between P10–15 and 6- to 7-mo groups). The fast and slow components of the decay τ were also significantly different among three age groups (Table 1; one-way ANOVA: P < 0.05 for the fast-decay and P < 0.01 for the slow-decay component; posttests showed P < 0.05 for the fast-decay component and P < 0.01 for the slow-decay component between P20–35 group and 6- to 7-mo group, while P > 0.05 for all other comparisons among three DBA/2J groups). There was no significant age-dependent difference in DBA/2J mice in the amplitude of either fast or slow decay component of the sIPSCs (Table 1), suggesting that the change in weighted sIPSC decay τ is not due to changes in the relative strength of the fast vs. slow component, i.e., both the fast and slow components of the sIPSCs in P20–35 group are slower than in other age groups. Not surprisingly, the half-width of the sIPSC events was also significantly changed [Fig. 2D, Table 1; one-way ANOVA: F(2,54) = 4.00, P = 0.024; Tukey posttest revealed a significant difference (P < 0.05) between DBA/2J P20–35 and 6- to 7-mo groups, P > 0.05 for all other comparisons]. sIPSC rise time (Fig. 2E), event frequency (Fig. 2F), and amplitude (Fig. 2G) did not show an age-dependent change in DBA/2J mice (Table 1; one-way ANOVA: P > 0.05 for all comparisons).
The age-dependent changes in sIPSC kinetics are markedly different between CBA/CaJ and DBA/2J mice. To compare the kinetics directly, a two-way ANOVA analysis on both CBA/CaJ and DBA/2J mice with all three age groups showed a significant effect of strain [F(1,101) = 16.9, P < 0.0001] on sIPSC-weighted decay τ (Fig. 2C), with slower sIPSC decay τ values in DBA/2J mice. The effect of age was not significant [F(2,101) = 2.99, P = 0.055], but there was a significant interactive effect between strain and age [F(2,101) = 3.38, P = 0.038]. As can be predicted from the analysis above, DBA/2J mice showed significantly slower sIPSC decay than CBA/CaJ mice at P20–35 (Bonferroni-corrected posttest: P < 0.001), but not at P10–15 or 6–7 mo (Bonferroni-corrected posttest: P > 0.05). A similar pattern was seen in the sIPSC half-widths as shown in Fig. 2D. A two-way ANOVA revealed a significant effect of strain [F(1,101) = 17.43, P < 0.0001] and an interaction between strain and age [F(2,101) = 3.14, P = 0.048], but no effect of age [F(2,101) = 1.405, P = 0.251]. Bonferroni-corrected posttests supported a significant difference (P < 0.001) between half-widths in CBA/CaJ and DBA/2J mice at P20–35, but not at other ages. The onset of hearing loss in DBA/2J mice starts before the 3rd wk of life (Fig. 1) and immediately precedes the age at which sIPSCs with slow kinetics appear. This result suggests that one consequence of peripheral hearing loss may be a compensatory increase in the strength (here, indicated by total charge due to the longer time course) of inhibitory transmission. It is not clear, however, why this change occurs, nor why it does not persist in the older DBA/2J mice that have experienced continued peripheral hearing loss.
Age-related decline in eIPSCs in bushy cells of CBA/CaJ and DBA/2J mice.
Although the sIPSCs appeared to be essentially normal in the oldest group of DBA/2J mice, it is possible that eIPSCs may have changed release probability or size. Therefore, we next studied the eIPSCs in the bushy cells of both CBA/CaJ and DBA/2J mice. For these experiments, a stimulating electrode was placed in the deep layer of the DCN to drive the TBV cells, which provide a purely glycinergic inhibition to the AVCN bushy cells. A knife cut was made between PVCN and DCN to minimize antidromic activation of auditory nerve fibers (Xie and Manis 2013). Excitatory transmission was blocked with d-APV and CNQX, and cells were voltage-clamped at −70 mV to increase the amplitude of eIPSCs.
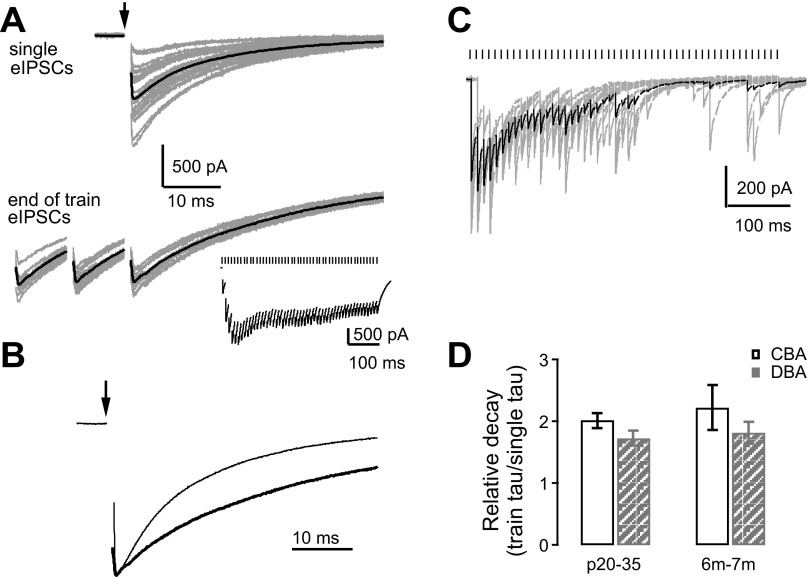
The time course of the eIPSC decay paralleled that of the sIPSCs in each mouse strain. Similar to sIPSCs, the averaged eIPSCs (Fig. 3A) in all bushy cells were best fit with a double exponential function. In CBA/CaJ mice, the weighted decay τ of eIPSCs did not change with age [Fig. 3C, Table 1; one-way ANOVA: F(2,40) = 1.51, P = 0.233]. There was also no change in the eIPSC slow decay τ, or the normalized fast or slow decay amplitudes (Table 1; one-way ANOVA: P > 0.05 for all comparisons). However, a significant age-dependent difference in the fast decay τ was detected [Table 1; one-way ANOVA: F(2,40) = 4.09, P = 0.024; Tukey posttest showed P < 0.05 between P10–15 and P20–35 age groups of CBA/CaJ mice, while P > 0.05 between P10–15 and 6–7 mo, as well as between P20–35 and 6- to 7-mo groups]. In DBA/2J mice, the weighted decay τ of eIPSCs were significantly different among the three age groups, with the slowest decay again occurring at P20–35 [Fig. 3C; Table 1; one-way ANOVA: F(2,34) = 3.90, P = 0.030; Tukey posttest showed P < 0.05 between P20–35 group and 6- to 7-mo group, while P > 0.05 between P10–15 and P20–35, or between P10–15 and 6–7 mo]. The fast and slow decay of the eIPSCs were significantly different among all three DBA/2J age groups (Table 1; Kruskal-Wallis test: P < 0.01 for fast decay and P < 0.05 for slow decay components; Dunn's posttest showed P < 0.01 for fast decay between P20–35 group and 6- to 7-mo group and P < 0.05 for slow decay between P20–35 and 6–7 mo, while P > 0.05 for all other comparisons). The normalized amplitude of the fast and slow decay components were also significantly different and showed an age-dependent switch. The amplitude of the fast τ was smaller (and that of the slow τ correspondingly larger) in the oldest DBA/2J mice (Table 1; Kruskal-Wallis test: P < 0.05 for both fast and slow amplitudes; Dunn's posttest showed P < 0.05 for both normalized fast and slow decay amplitude between P10–15 and 6- to 7-mo groups; and P > 0.05 for all other comparisons).
Fig. 3.
Evoked spontaneous inhibitory postsynaptic currents (eIPSCs) in bushy cells from CBA/CaJ and DBA/2J mice. A: example traces of eIPSCs in bushy cells from CBA/CaJ and DBA/2J mice at different age groups. The color coding is the same as in Fig. 2 and applies to panels B–D. Arrows: stimulus onset. The stimulus artifact is removed for clarity. Thin gray lines: individual eIPSCs; thick color lines: average eIPSCs. B: kinetics of the averaged eIPSCs from cells in A. Traces were normalized to the peak to allow comparison of the time course. Note the slower decay of eIPSCs in P20–35 DBA/2J mice compared with other ages and to CBA/CaJ mice. C: summary of weighted decay τ for averaged eIPSCs. ***Bonferroni-corrected posttest after two-way ANOVA: P < 0.001. D: average eIPSC amplitude decreases with age in both CBA/CaJ and DBA/2J mice. *Dunn's posttest after Kruskal-Wallis test: P < 0.05; **Tukey posttest after one-way ANOVA: P < 0.01. See Table 1 and text for values and detailed statistics.
We next compared the τ between the two mouse strains. Two-way ANOVA revealed a significant effect of strain [F(1,74) = 26.0, P < 0.001] on eIPSC-weighted decay τ (Fig. 3C), with slower eIPSC decay τ in DBA/2J mice. The effect of age was not quite significant [F(2,74) = 2.73, P = 0.071], but there was a significant interaction between strain and age [F(2,74) = 4.40, P = 0.016]. In particular, DBA/2J mice showed significantly slower eIPSC decay than CBA/CaJ mice at age group P20–35 (Bonferroni-corrected posttest: P < 0.001), but not at age of P10–15 or 6–7 mo (Bonferroni-corrected posttest: P > 0.05). The pattern of changes in eIPSC kinetics mimics those of sIPSCs in both strains (compare Fig. 3C with Fig. 2C).
The average amplitude of eIPSCs decreased with age in both CBA/CaJ and DBA/2J mice (Fig. 3D). DCN eIPSCs had the largest peak amplitudes in bushy cells at P10–15 and declined thereafter (Table 1; one-way ANOVA: P < 0.05 in CBA/CaJ mice and P < 0.01 in DBA/2J mice; posttests: P < 0.05 between P10–15 and 6–7 mo in CBA/CaJ mice, and P < 0.01 between P10–15 and 6–7 mo in DBA/2J mice). A two-way ANOVA comparing both strains revealed a significant effect of age [F(2,74) = 10.3, P < 0.001] on average eIPSC amplitude. The effect of strain was also significant [F(1,74) = 4.61, P = 0.035], reflecting the smaller eIPSC peak amplitudes in CBA/CaJ mice before 6–7 mo of age (Fig. 3D). There was no interaction between strain and age [F(2,74) = 1.20, P = 0.31]. The results suggest that, regardless of the peripheral hearing status, the strength of inhibition onto bushy cells declines with age in both CBA/CaJ and DBA/2J mice.
The decline in amplitude could be attributed to a decrease in quantal size, quantal content, or release probability. The results so far suggest that, since quantal size does not decrease with age (Fig. 2G), either quantal content or the release probability decreases. We therefore next estimated the quantal content of the glycinergic synapses onto bushy cells, using both the amplitude method and the method of failures (see materials and methods). Quantal content estimated with these two different methods compared within cells agreed reasonably well with each other (Fig. 4A). Since the method of failures cannot be used when there are few or no failures observed, we used the amplitude method in the following analysis. As shown in Fig. 4B, the quantal content was highest, at about 6, in both CBA/CaJ and DBA/2J mice at P10–15, and decreased to about 2 by 6–7 mo (Table 1; one-way ANOVA: P < 0.05 in CBA/CaJ mice and P < 0.01 in DBA/2J mice; posttests: P < 0.05 between P10–15 and 6–7 mo in CBA/CaJ mice; P < 0.01 between P10–15 and P20–35, as well as between P10–15 and 6–7 mo in DBA/2J mice). A two-way ANOVA comparing both mouse strains showed a significant effect of age [F(2,74) = 9.57, P < 0.001] on quantal content. There was no significant effect of strain [F(1,74) = 0.390, P = 0.535], or interaction between strain and age [F(2,74) = 0.486, P = 0.617]. These results suggest that the decreased strength of inhibition onto bushy cells during aging is associated with reduced quantal content in response to presynaptic action potentials at the terminals. This could result from a reduction in the number of functional terminals, or a decrease in release probability, with or without a reduction in terminal number. Therefore, we next measured the paired-pulse ratio (PPR) of the eIPSCs at 50 ms interpulse interval to test whether the decline in eIPSC amplitude is also associated with changes in release probability. As shown in Fig. 4C, we found no change in PPR among the different age groups or between the two mouse strains {Table 1; one-way ANOVA: P > 0.05 in both CBA/CaJ and DBA/2J mice groups; two-way ANOVA between two mouse strains: no significant effect of strain [F(1,74) = 0.174, P = 0.678], age [F(2,74) = 1.06, P = 0.353], or interaction between strain and age [F(2,74) = 1.36, P = 0.265]}. Although PPR is an indirect measure of release probability, our results are consistent with the hypothesis that an age-dependent decrease in the release probability is not the primary cause of the decreased eIPSCs, but rather that the quantal content is decreasing.
If the quantal content is decreasing, we should see an increased number of failures to evoke IPSCs, and this would be associated with a high CV. There was no significant difference in the CV of sIPSCs among CBA/CaJ or DBA/2J mice as a function of age {Fig. 4D and Table 1; one-way ANOVA: P > 0.05 in both CBA/CaJ and DBA/2J mice; two-way ANOVA analysis between the two strains: no significant effect of strain [F(1,74) = 0.164, P = 0.686], age [F(2,74) = 2.59, P = 0.079] or interaction between strain and age [F(2,74) = 0.138, P = 0.872]}. However, as shown in Fig. 4E, the CV of the eIPSCs significantly increased with age in CBA/CaJ mice [Table 1; one-way ANOVA: F(2,40) = 9.33, P = 0.0005; Tukey posttest: P < 0.01 between P10–15 and P20–35 as well as between P10–15 and 6–7 mo in CBA/CaJ mice]. There was also a trend toward an increased CV with age in the DBA/2J mice (Table 1; Kruskal-Wallis test: P = 0.078). Two-way ANOVA comparing both CBA/CaJ and DBA/2J mice revealed a significant effect of age [F(2,74) = 8.87, P < 0.001], but no effect of strain [F(1,74) = 0.458, P = 0.501] or interaction between age and strain [F(2,74) = 1.12, P = 0.331]. These results indicate that, while the quantal size remains constant during aging in both CBA/CaJ and DBA/2J mice, the evoked synaptic release becomes more variable with age. We then investigated the failure rate, as an index of quantal content. The failure rate of the eIPSCs increased with age in both CBA/CaJ and DBA/2J mice (Fig. 4F, Table 1; Kruskal-Wallis test: P < 0.001 for CBA/CaJ mice; P < 0.05 for DBA/2J mice; Dunn's posttest: P < 0.01 between P10–15 and P20–35 as well as between P10–15 and 6–7 mo in CBA/CaJ mice; P > 0.05 for all comparisons among DBA/2J mice). A two-way ANOVA between the strains revealed significant effect of age on eIPSC failure rates [F(2,74) = 7.71, P < 0.001], but no effect of strain [F(1,74) = 0.278, P = 0.600] or interaction between age and strain [F(2,74) = 0.417, P = 0.661]. Therefore, in addition to decreasing synaptic strength with age, the reliability of the glycinergic synaptic transmission onto bushy cells, as measured by increased variability of eIPSCs and increased failure rates, is reduced in older mice.
eIPSCs become slower during repetitive activity than single eIPSCs.
Bushy cells can be driven at high rates at both their excitatory synaptic inputs from the auditory nerve (Taberner and Liberman 2005), as well as from their inhibitory sources, including TBV cells, which can fire at rates of hundreds of Hertz (Kuo et al. 2012; Rhode 1999). To assay how inhibitory synapses followed high rates of presynaptic activity as a function of both age and hearing loss, we analyzed the IPSC kinetics during repetitive stimulation of the TBV inputs with 50 pulses at 100 Hz. Only mice in the P20–25 and 6- to 7-mo age groups were included in the analysis (Figs. 5 and 6), as IPSC responses of bushy cells in the P10–15 group were labile and could not follow 100-Hz stimulus trains (Fig. 5C). The decay τ of the last IPSC in the train was fit to a double-exponential function (Fig. 5A). Compared with the decay τ of singly eIPSCs in the same cell, the decay τ of the last eIPSC was much slower (Fig. 5B). Across a population of cells (Fig. 5D), the decay τ of the last eIPSC in the train was 2.0 ± 0.1 times (n = 19) slower than the decay of singly eIPSCs in P20–35 CBA/CaJ mice (paired t-test: t18 = 8.33, P < 0.0001). The τ was also 2.2 ± 0.4 (n = 6) times slower in 6- to 7-mo CBA/CaJ mice (paired t-test: t5 = 3.37, P = 0.0199), 1.7 ± 0.1 (n = 10) times slower in P20–35 DBA/2J mice (paired t-test: t9 = 5.97, P = 0.0002), and 1.8 ± 0.2 (n = 9) times slower in 6- to 7-mo DBA/2J mice (paired t-test: t8 = 4.61, P = 0.0017). However, the extent to which the eIPSCs slowed during a train did not differ between age or strain [Fig. 5D; one-way ANOVA: F(3,40) = 1.24, P = 0.308].
Fig. 5.
eIPSC kinetics become slower during repeated stimulation in both CBA/CaJ and DBA/2J mice. A: example traces of single eIPSCs (top) and last three eIPSCs of 50 pulse trains at 100 Hz (bottom) obtained from a bushy cell of a P22 DBA/2J mouse. Inset in the bottom panel shows an averaged trace for the 100-Hz train. Ticks on top mark the time of the 50 stimuli during the train. Stimulus artifacts are removed. B: comparison of IPSC traces between single eIPSC (thin trace) and last eIPSC of the 100-Hz 50 pulse train (thick trace). Traces are normalized to the peak of the IPSCs. C: example IPSCs in a bushy cell from a CBA mouse at P10. In the majority of the trials, excitatory postsynaptic currents could not be elicited during the second half of the stimulus train. Black trace: the average IPSC of all 12 trials. D: ratio of eIPSC decay τ (last eIPSC/single eIPSC) for 100-Hz trains. Ratios were computed for individual cells.
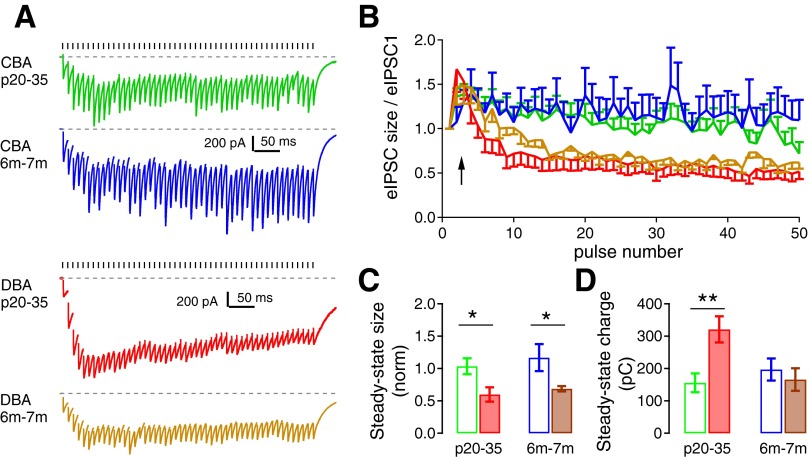
Fig. 6.
Short-term synaptic dynamics during repeated stimulation differ between CBA/CaJ and DBA/2J mice. A: eIPSCs during a 100-Hz stimulus train (50 pulses) from example bushy cells of CBA/CaJ and DBA/2J mice at two different ages. Ticks above the traces mark stimulus timing, and the dashed line is the resting current. Stimulus artifacts are removed for clarity. B: normalized eIPSC size relative to the first eIPSC during the 100-Hz train (as in A) from all bushy cells. CBA/CaJ mice show slight facilitation during the train, while DBA/2J mice show initial facilitation followed by depression. C: summary of the average steady-state eIPSCs, shown as the normalized mean of the last 40 eIPSCs during the train relative to the first eIPSC, from all bushy cells in each group. *Bonferroni-corrected posttest after two-way ANOVA: P < 0.05. D: summary of steady-state charges, calculated as the cumulative charge from the last 40 eIPSCs of the train, from all bushy cells. **Bonferroni-corrected posttest following two-way ANOVA: P < 0.01.
To gain insight into whether the release probability changes during repetitive activity, we also assessed the short-term synaptic plasticity of the TBV inputs. The eIPSCs showed a slight facilitation during 50 pulse trains at 100 Hz (Fig. 6A) in CBA/CaJ mice, and the facilitation was similar at both P20–35 and 6–7 mo (Fig. 6B). In comparison, eIPSCs in DBA/2J mice at both P20–35 and 6–7 mo showed an initial facilitation followed by depression after about the 10th stimulus. To quantify the steady-state facilitation or depression, we calculated the eIPSC amplitude by averaging the eIPSCs during the last 40 stimuli and normalized this to the first eIPSC. In CBA/CaJ mice, the normalized steady-state IPSC size was 1.03 ± 0.12 (n = 12) at P20–35 and was 1.17 ± 0.21 (n = 6) at 6–7 mo (Fig. 6C; unpaired t-test: t16 = 0.58, P = 0.57). In comparison, the normalized steady-state eIPSC size was 0.60 ± 0.11 (n = 10) and 0.69 ± 0.04 (n = 9) in DBA/2J mice, respectively (unpaired t-test: t17 = 0.74, P = 0.47). A two-way ANOVA comparing the two strains revealed a significant effect of strain on the normalized steady-state size of the eIPSC strain [F(1,33) = 12.59, P = 0.0011; Bonferroni-corrected posttests: P < 0.05 between CBA/CaJ and DBA/2J at P20–35, as well as between CBA/CaJ and DBA/2J at 6–7 mo]. There was no significant effect of age [F(1,33) = 0.74, P = 0.40] and no interaction between age and strain [F(1,33) = 0.027, P = 0.87]. These results suggest that the short-term synaptic plasticity of the glycinergic synapse onto bushy cells does not change with age after hearing onset in either CBA/CaJ and DBA/2J mice. However, there is a difference between the strains, in that DBA/2J mice have impaired release during repetitive activity at high rates. While glycine receptor desensitization (Harty and Manis 1998) could potentially play a role in the decline of the IPSC responses in DBA/2J mice, it seems to be unlikely in the CBA/CaJ mice as there is little decline in the IPSC size through the trains (Fig. 6B).
The influence of inhibitory transmission onto postsynaptic cells depends in part on the total charge transfer associated with the inhibitory conductance. The charge is determined both by the kinetics of eIPSCs and by short-term synaptic plasticity during repetitive activity. For example, the enhanced inhibitory transmission due to slower eIPSCs in DBA/2J mice at P20–35 could be offset by an increased short-term depression during eIPSC trains. Therefore, we calculated the total charge transfer of the last 40 eIPSCs during the 100-Hz train to quantify the influence of steady-state inhibition in both CBA/CaJ and DBA/2J mice. As shown in Fig. 6D, the steady-state charge transfer was 155 ± 29 pC (n = 12) in P20–35 and 197 ± 34 pC (n = 6) in 6- to 7-mo group of the CBA/CaJ mice (unpaired t-test: t16 = 0.86, P = 0.40); and it was 321 ± 40 pC (n = 10) in P20–35 group and 166 ± 35 (n = 9) in 6- to 7-mo group of the DBA/2J mice (unpaired t-test: t17 = 2.93, P = 0.0104). A two-way ANOVA analysis did not show any significant effect of either strain [F(1,33) = 3.55, P = 0.069] or age [F(1,33) = 2.54, P = 0.12], but revealed a significant interaction between strain and age [F(1,33) = 7.57, P = 0.0098]. Specifically, a Bonferroni-corrected posttest showed a significant difference between CBA/CaJ and DBA/2J mice at P20–35 (P < 0.01). Even though the eIPSCs become depressed during the train in P20–35 DBA/2J mice, the slow decay of the eIPSC (Fig. 3) results in a significantly higher total charge transfer than in comparably-aged CBA/CaJ mice or older DBA/2J mice. This indicates that glycinergic transmission is stronger in DBA/2J mice at P20–35. Previous experiments showed that auditory nerve evoked EPSCs (eEPSCs) in bushy cells of the DBA/2J mice are comparable to those in normal hearing CBA/CaJ mice at this age range (3–4 wk) (Wang and Manis 2005). The comparison of these results strongly suggests that the relative strength of excitation and inhibition is disrupted immediately following the onset of AHL in DBA/2J mice.
DISCUSSION
AHL is complicated by interactions between mechanisms affected by aging in general as well as by the consequences of hearing loss. These two processes are often difficult to disentangle when studying the effects of AHL in the central auditory system. In the present study, we found that CBA/CaJ mice, with “normal hearing”, and DBA/2J mice with early onset AHL shared changes in inhibitory synaptic transmission that are most likely intrinsic to aging, and distinct changes that may be associated with hearing loss in the DBA/2J mice. In both strains, the eIPSC amplitude decreased from P16 to 6–7 mo of age, independent of peripheral hearing status. Quantal analysis suggests that decreased quantal content, rather than quantal size, is responsible for the age-related decrease. Surprisingly, the kinetics of individual sIPSCs and eIPSCs were markedly different in DBA/2J mice shortly after the onset of their high-frequency hearing loss at P20–35. Taken together, these changes in the glycinergic synaptic transmission suggest that inhibitory synaptic transmission in the CN decreases with age, and that additional changes can appear with hearing loss.
Age-related decreases in inhibition in the CN.
The decrease in glycinergic eIPSC amplitude with age in both CBA/CaJ and DBA/2J mice is consistent with prior observations that inhibition in the auditory pathways generally becomes weaker with age (Burianova et al. 2009; Caspary et al. 2008). This could occur through a reduction in the number of inhibitory synapses. However, Helfert et al. (2003) did not observe age-related changes in the density (number) of anatomically defined inhibitory synaptic terminals in the VCN of aged Fischer 344 rats, and our results are consistent with these observations. If we assume that the frequency of sIPSCs is proportional to the number of functional synaptic sites on a cell, our results suggest that there is no change in the number of presynaptic terminals on a postsynaptic cell with age. Furthermore, we did not see changes in sIPSC amplitude with age, so it is likely that both quantal size and the number of postsynaptic glycine receptors are stable over time. These latter observations are also consistent with the lack of an age-dependent decrease in glycine receptor (strychnine binding) in the AVCN of CBA/CaJ mice and C57BL/6J mice (Willott et al. 1997). A reduction in strychnine binding was reported in 26-mo-old Fischer 344 rats (Milbrandt and Caspary 1995), although this is significantly older than the oldest mice we studied.
There are two hypotheses that could explain our results. First, a subset of synaptic terminals from a single cell might become unable to support action potential initiated transmitter release with increasing age. The evidence for lower evoked quantal content and the apparent absence of a reduction of the number of synapses supports this idea. To be consistent with our observations, including the lack of an effect on resting release probability as assessed by the PPR, such an effect would have to occur in an all-or-none fashion for each synaptic terminal. Potential mechanisms are that the ability of the presynaptic action potential to invade specific terminal branches (Debanne 2004) decreases with age, perhaps due to changes in axonal excitability (Hinman et al. 2006), or that there exists a presynaptic silencing of the release mechanism in individual synapses (Charpier et al. 1995) that might result from reduced overall activity levels in the presynaptic neurons with age. Scenarios in which either of these mechanisms occurs probabilistically across all synapses arising from a single axon could also account for our observations. A second hypothesis is that pruning of the TBV inhibitory synaptic terminals does occur, possibly as an extension of the well-documented examples of developmental pruning of inhibitory synapses in the olivary complex (Kandler and Gillespie 2005), of GABAergic synapses in neocortex (De Felipe et al. 1997; Wu et al. 2012), or the activity-dependent refinement of inhibitory synaptic connections in adult visual cortex (van Versendaal et al. 2012). However, to be consistent with other observations (Helfert et al. 2003), there would need to be a compensatory increase in the number of terminals of non-TBV origin. These two hypotheses could be differentiated by examining the number of terminals of individual turberculoventral neurons onto bushy cells as a function of age.
Hearing loss-related changes in inhibition.
Inhibitory transmission has been well documented to respond to the decreases in activity that follow hearing loss (Pradhan and Ahn 2011; Sanes and Kotak 2011; Takesian et al. 2012; Wang et al. 2011). Such changes can occur at presynaptic sites, including a decreased concentration of presynaptic glycine (Asako et al. 2005), or changes in the refinement of inhibitory synaptic terminals (Kapfer et al. 2002). They can also involve postsynaptic changes in neurotransmitter receptors (Potashner et al. 2000; Suneja et al. 1998; Xu et al. 2010). While it is possible that the kinetic changes in IPSCs in P20–35 DBA/2J mice were driven by the peripheral hearing loss, there is no easy way to directly test this idea. However, similar observations have been made in other mouse models of genetic hearing loss. Glycinergic IPSC kinetics were reported to be slower in the cells of the medial nucleus of the trapezoid body in congenitally deaf dn/dn mice (at age of P12–14, Leao et al. 2004) and in the lateral superior olive cells in the deaf Vglut3−/− mice (at age of P1–2, Noh et al. 2010). In both of these cases, the neurons never experienced normal hearing (they were deaf from birth), while the change in IPSC kinetics in DBA/2J mice occurred after a period of nearly normal hearing and development. These results suggest that hearing loss may lead to changes in expression favoring receptor populations with slower kinetics.
Interestingly, in normal-hearing animals, glycinergic inhibition onto bushy cells is established and essentially adult-like in terms of kinetics by about P10 (Milenkovic et al. 2007, also see Fig. 2C for the IPSC kinetics in CBA/CaJ mice), so the change in kinetics is not simply a reversion to an earlier developmental expression pattern. The mechanism underlying the kinetic changes in DBA/2J mice is likely to be postsynaptic, since both sIPSCs and eIPSCs were slower. Nonstationary mean-variance analysis of sIPSCs is consistent with the expression of α2-containing glycine receptors by bushy cells in juvenile and young adult mice (Xie and Manis 2013). It is possible that hearing loss causes an upregulation of a different subunit, such as the α4-subunit containing receptors. These receptors have much slower IPSC kinetics (Wassle et al. 2009) and are expressed in the CN of adult mice (Lein et al. 2007). Another possibility is that α2-homomeric receptors are substituted for α2/β-heteromeric receptors in DBA/2J mice. In contrast to synaptic α2/β-heteromeric glycine receptors (Haverkamp et al. 2004; Wassle et al. 2009), homomeric α2-receptors tend to be extrasynaptically localized and will respond to spillover of glycine from the synapse, resulting in IPSCs with slower kinetics (Mangin et al. 2003). This scenario could also explain the further slowing of the eIPSCs during high-frequency stimulus trains (Fig. 5), since the trains presumably increase the concentration of transmitter in the extrasynaptic space. However, it is less clear how spillover would explain the change in the time course of isolated sIPSCs. Other mechanisms that can regulate the kinetics of glycinergic IPSCs may be also involved, including phosphorylation or dephosphorylation of receptors (Gentet and Clements 2002), or changes in the internal concentration of chloride in the postsynaptic cell (Pitt et al. 2008). Whether or how these mechanisms contribute to changes in glycinergic inhibition in DBA/2J mice remains unknown. The mechanism by which the IPSC time course becomes shorter again in the DBA/2J mice at 6–7 mo is also not obvious, since at this age there is severe hearing loss at all frequencies (Fig. 1).
Functional considerations.
It has been proposed that an imbalance between excitatory and inhibitory synaptic drive can contribute to abnormal neural processing (Kehrer et al. 2008; Yizhar et al. 2011). In AHL, inhibition has often been reported to be weakened (Caspary et al. 2005, 2008; Frisina and Walton 2006), which could suggest an excitatory-inhibitory imbalance. However, interpretation of balance based on sIPSC/sEPSC or eIPSC/eEPSC measurements is complicated by a lack of knowledge about the patterns of activity in presynaptic neurons. In a previous study (Wang and Manis 2005), we showed that at similar ages, the excitatory transmission onto bushy cells of the CBA/CaJ and DBA/2J mice was essentially normal. Based solely on the relative synaptic strength, we can suggest that there is the potential for an age-related imbalance, favoring inhibition, which occurs in the DBA/2J mice at P20–35. On the other hand, we also observed an age-related decrease in inhibitory transmission, associated with a decreased quantal content, in both DBA/2J and CBA/CaJ mice at 6–7 mo of age. In the CBA/CaJ mice, whose auditory thresholds remain relatively constant across the ages studied, this observation would suggest that there is an imbalance in favor of excitation with age, assuming that there is not an age-dependent decline in the afferent firing rates onto the endbulb synapses. Since the glycinergic inhibition onto bushy cells plays an important role in enhancing the temporal coding (Xie and Manis 2013), it could be predicted that an age-related decrease in functional glycinergic transmission in the CN could significantly influence the ability to perform complex tasks, such as pitch detection and speech recognition in noisy environments (Lorenzi et al. 2006).
GRANTS
This work was supported by research grants from Deafness Research Foundation (now Hearing Health Foundation) (R. Xie) and US National Institute of Deafness and other Communications Disorders grant R01DC-004551 (P. B. Manis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.X. and P.B.M. conception and design of research; R.X. performed experiments; R.X. analyzed data; R.X. and P.B.M. interpreted results of experiments; R.X. prepared figures; R.X. drafted manuscript; R.X. and P.B.M. edited and revised manuscript; R.X. and P.B.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank H. O'Donohue for experimental and organizational support.
REFERENCES
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci 32: 14156–14164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott RH, Wallace MN, Shackleton TM, Palmer AR. Onset neurones in the anteroventral cochlear nucleus project to the dorsal cochlear nucleus. J Assoc Res Otolaryngol 5: 153–170, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asako M, Holt AG, Griffith RD, Buras ED, Altschuler RA. Deafness-related decreases in glycine-immunoreactive labeling in the rat cochlear nucleus. J Neurosci Res 81: 102–109, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. I. Glutamate and related amino acids. Neurochem Res 14: 555–562, 1989a [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. II. Taurine and small neutral amino acids. Neurochem Res 14: 563–570, 1989b [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Sachs MB. The representations of the steady-state vowel sound /e/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. J Neurophysiol 63: 1191–1212, 1990 [DOI] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44: 161–169, 2009 [DOI] [PubMed] [Google Scholar]
- Cant NB, Morest DK. Organization of the neurons in the anterior division of the anteroventral cochlear nucleus of the cat. Light-microscopic observations. Neuroscience 4: 1909–1923, 1979 [DOI] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Auditory nerve fibers excite targets through synapses that vary in convergence, strength, and short-term plasticity. J Neurophysiol 104: 2308–2320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211: 1781–1791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci 25: 10952–10959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Behrends JC, Triller A, Faber DS, Korn H. “Latent” inhibitory connections become functional during activity-dependent plasticity. Proc Natl Acad Sci U S A 92: 117–120, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J 73: 220–229, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb FE, Jacobson GP, Newman CW, Kretschmer LW, Donnelly KA. Age-associated degeneration of backward masking task performance: evidence of declining temporal resolution abilities in normal listeners. Audiology 32: 260–271, 1993 [DOI] [PubMed] [Google Scholar]
- De Felipe J, Marco P, Fairen A, Jones EG. Inhibitory synaptogenesis in mouse somatosensory cortex. Cereb Cortex 7: 619–634, 1997 [DOI] [PubMed] [Google Scholar]
- Debanne D. Information processing in the axon. Nat Rev Neurosci 5: 304–316, 2004 [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol 124: 560–573, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus). J Am Audiol Soc 1: 179–184, 1976 [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice. I. Inbred and F1 hybrid strains. Hear Res 65: 125–132, 1993 [DOI] [PubMed] [Google Scholar]
- Frisina RD, Walton JP. Age-related structural and functional changes in the cochlear nucleus. Hear Res 216–217: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- Gai Y, Carney LH. Influence of inhibitory inputs on rate and timing of responses in the anteroventral cochlear nucleus. J Neurophysiol 99: 1077–1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Clements JD. Binding site stoichiometry and the effects of phosphorylation on human alpha1 homomeric glycine receptors. J Physiol 544: 97–106, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty TP, Manis PB. Kinetic analysis of glycine receptor currents in ventral cochlear nucleus. J Neurophysiol 79: 1891–1901, 1998 [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Muller U, Zeilhofer HU, Harvey RJ, Wassle H. Diversity of glycine receptors in the mouse retina: localization of the alpha2 subunit. J Comp Neurol 477: 399–411, 2004 [DOI] [PubMed] [Google Scholar]
- Helfert RH, Krenning J, Wilson TS, Hughes LF. Age-related synaptic changes in the anteroventral cochlear nucleus of Fischer-344 rats. Hear Res 183: 18–28, 2003 [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse. Audiology 19: 369–383, 1980 [DOI] [PubMed] [Google Scholar]
- Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR. Age-related molecular reorganization at the node of Ranvier. J Comp Neurol 495: 351–362, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci 28: 290–296, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Seidl AH, Schweizer H, Grothe B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat Neurosci 5: 247–253, 2002 [DOI] [PubMed] [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci 1: 6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenning J, Hughes LF, Caspary DM, Helfert RH. Age-related glycine receptor subunit changes in the cochlear nucleus of Fischer-344 rats. Laryngoscope 108: 26–31, 1998 [DOI] [PubMed] [Google Scholar]
- Kuenzel T, Borst JG, van der Heijden M. Factors controlling the input-output relationship of spherical bushy cells in the gerbil cochlear nucleus. J Neurosci 31: 4260–4273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Lu HW, Trussell LO. Intrinsic and synaptic properties of vertical cells of the mouse dorsal cochlear nucleus. J Neurophysiol 108: 1186–1198, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Oleskevich S, Sun H, Bautista M, Fyffe RE, Walmsley B. Differences in glycinergic mIPSCs in the auditory brain stem of normal and congenitally deaf neonatal mice. J Neurophysiol 91: 1006–1012, 2004 [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BC. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc Natl Acad Sci U S A 103: 18866–18869, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JM, Baloul M, Prado De Carvalho L, Rogister B, Rigo JM, Legendre P. Kinetic properties of the alpha2 homo-oligomeric glycine receptor impairs a proper synaptic functioning. J Physiol 553: 369–386, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Xie R, Wang Y, Marrs GS, Spirou GA. The endbulbs of Held. In: Synaptic Mechanisms in the Auditory System. New York: Springer, 2011, p. 61–93 [Google Scholar]
- Milbrandt JC, Caspary DM. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the Fischer 344 rat. Neuroscience 67: 713–719, 1995 [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Witte M, Turecek R, Heinrich M, Reinert T, Rubsamen R. Development of chloride-mediated inhibition in neurons of the anteroventral cochlear nucleus of gerbil (Meriones unguiculatus). J Neurophysiol 98: 1634–1644, 2007 [DOI] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci 13: 232–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol 540: 447–455, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt SJ, Sivilotti LG, Beato M. High intracellular chloride slows the decay of glycinergic currents. J Neurosci 28: 11454–11467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res 147: 125–136, 2000 [DOI] [PubMed] [Google Scholar]
- Pradhan J, Ahn SC. Glycine-induced currents are insensitive to the glycine receptor alpha1 subunit-specific blocker, cyanotriphenylborate, in older circling mice. Biochem Biophys Res Commun 405: 157–161, 2011 [DOI] [PubMed] [Google Scholar]
- Rhode WS. Vertical cell responses to sound in cat dorsal cochlear nucleus. J Neurophysiol 82: 1019–1032, 1999 [DOI] [PubMed] [Google Scholar]
- Rhode WS, Greenberg S. Encoding of amplitude modulation in the cochlear nucleus of the cat. J Neurophysiol 71: 1797–1825, 1994 [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Ryugo DK. Intracellular marking of physiologically characterized cells in the ventral cochlear nucleus of the cat. J Comp Neurol 225: 167–186, 1984 [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Benson CG, Ostapoff EM, Morest DK. Glycine immunoreactive projections from the dorsal to the anteroventral cochlear nucleus. Hear Res 51: 11–28, 1991 [DOI] [PubMed] [Google Scholar]
- Sanes DH, Kotak VC. Developmental plasticity of auditory cortical inhibitory synapses. Hear Res 279: 140–148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Rhode WS. Structural and functional properties distinguish two types of multipolar cells in the ventral cochlear nucleus. J Comp Neurol 282: 595–616, 1989 [DOI] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature 465: 927–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol 154: 473–488, 1998 [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93: 557–569, 2005 [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. J Neurophysiol 107: 937–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert LP, Morest DK, Yurgelun-Todd DA. The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: horseradish peroxidase labelling of identified cell types. Neuroscience 7: 3031–3052, 1982 [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol 114: 1332–1343, 2003 [DOI] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer JP, De Zeeuw CI, Hofer SB, Heimel JA, Levelt CN. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74: 374–383, 2012 [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res 279: 111–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience 160: 227–239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol 100: 1255–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol 94: 1814–1824, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the mammalian retina. Front Mol Neurosci 2: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB, Trune DR. Cochlear nuclear complex of mice. Am J Anat 163: 103–130, 1982 [DOI] [PubMed] [Google Scholar]
- Wickesberg RE, Oertel D. Delayed, frequency-specific inhibition in the cochlear nuclei of mice: a mechanism for monaural echo suppression. J Neurosci 10: 1762–1768, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Milbrandt JC, Bross LS, Caspary DM. Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: effects of cochlear impairment and aging. J Comp Neurol 385: 405–414, 1997 [PubMed] [Google Scholar]
- Wu SH, Oertel D. Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci 4: 1577–1588, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang ZJ. GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci 32: 331–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Manis PB. Target-specific IPSC kinetics promote temporal processing in auditory parallel pathways. J Neurosci 33: 1598–1614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex. J Neurosci 30: 331–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Relative roles of different mechanisms of depression at the mouse endbulb of Held. J Neurophysiol 99: 2510–2521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477: 171–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 130: 94–107, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]