Abstract
Previously, we showed that neurons in the supraoculomotor area (SOA), known to encode vergence angle in normal monkeys, encode the horizontal eye misalignment in strabismic monkeys. The SOA receives afferent projections from the caudal fastigial nucleus (cFN) and the posterior interposed nucleus (PIN) in the cerebellum. The objectives of the present study were to investigate the potential roles of the cFN and PIN in 1) conjugate eye movements and 2) binocular eye alignment in strabismic monkeys. We used unilateral injections of the GABAA agonist muscimol to reversibly inactivate the cFN (4 injections in exotropic monkey S1 with ∼4° of exotropia; 5 injections in esotropic monkey S2 with ∼34° of esotropia) and the PIN (3 injections in monkey S1). cFN inactivation induced horizontal saccade dysmetria in all experiments (mean 39% increase in ipsilesional saccade gain and 26% decrease in contralesional gain). Also, mean contralesional smooth-pursuit gain was decreased by 31%. cFN inactivation induced a divergent change in eye alignment in both monkeys, with exotropia increasing by an average of 9.8° in monkey S1 and esotropia decreasing by an average of 11.2° in monkey S2 (P < 0.001). Unilateral PIN inactivation in monkey S1 resulted in a mean increase in the gain of upward saccades by 13% and also induced a convergent change in eye alignment, reducing exotropia by an average of 2.7° (P < 0.001). We conclude that cFN/PIN influences on conjugate eye movements in strabismic monkeys are similar to those postulated in normal monkeys and cFN/PIN play important and complementary roles in maintaining the steady-state misalignment in strabismus.
Keywords: strabismus, caudal fastigial nucleus, posterior interposed nucleus, muscimol, nonhuman primates
strabismus is a condition in which the visual axes of the two eyes are not aligned (von Noorden and Campos 2002). Exotropia is a form of strabismus in which the visual axes diverge, and esotropia refers to strabismus in which the visual axes converge. In developmental strabismus, a breakdown in binocular vision during the critical period for visual development likely leads to a cascade of neural events eventually resulting in misaligned eyes (Boothe et al. 1985; Helveston 2000). It is estimated that as many as 4% of all children in the US have strabismus, yet there is a lack of understanding of the mechanisms leading to strabismus (Govindan et al. 2005; Lorenz 2002; von Noorden and Campos 2002). Specifically, it is not clear which areas of the oculomotor system play a role in the generation and maintenance of the state of strabismus.
Although horizontal eye misalignment is the most prominent feature of strabismus, there are many associated features such as A/V-patterns and dissociated deviations that might have an oculomotor basis (von Noorden and Campos 2002). Pattern strabismus is a feature in which horizontal strabismus angle changes with vertical gaze position. Thus an A-pattern refers to a reduction of exotropia or an increase of esotropia in up-gaze compared to down-gaze (Guyton and Weingarten 1994; Urrets-Zavalia 1955). A V-pattern is an increase in exotropia or a reduction in esotropia in up-gaze compared to down-gaze. A dissociated vertical deviation (DVD) refers to a vertical deviation in which the strabismic eye is always elevated compared with the fixating eye (von Noorden and Campos 2002). In previous studies we showed that we are able to reproduce characteristic properties of strabismus including horizontal misalignment, alternating fixation, DVD, dissociated horizontal deviation (DHD—a difference in horizontal misalignment based upon the viewing eye), and A/V-patterns of strabismus in monkeys reared under specialized viewing conditions for the first 4 mo of their life (Das 2007, 2009; Das et al. 2005; Das and Mustari 2007). We also showed that there is a direct correlation between the neural activity in the horizontal and vertical motoneurons and the state of horizontal and vertical misalignment (Das and Mustari 2007; Joshi and Das 2011). These studies presented the first direct evidence that the brain is involved in maintaining the strabismic state.
Motoneurons receive information from central structures, including those areas particularly involved in binocular control. The supraoculomotor area (SOA) is a region immediately adjacent to the oculomotor nucleus. The cells in this region project monosynaptically to the medial rectus motoneurons (MRMNs) and have been shown to encode vergence angle or vergence velocity in normal nonhuman primates (Mays 1984; Mays et al. 1986; Zhang et al. 1991, 1992). We showed, in strabismic monkeys, that cells in this area encode the strabismus angle, viz. the difference in horizontal position between the two eyes (Das 2012). Thus, in animals with exotropia (divergent form of strabismus), “near response” cells in the SOA showed increased neuronal activity when the angle of exotropia was small and reduced neuronal activity for larger angle of exotropia, whereas the “far response” cells in the SOA showed the opposite effect. Similar to results from normal monkeys, neither type of cell in the strabismic monkeys encoded a conjugate eye position signal.
The objectives of the present study were twofold. One objective was to further examine the potential neural substrates involved in the maintenance of the strabismic state by examining structures that project to the SOA and also play a role in binocular control. The SOA receives direct projections that are primarily contralateral from the cerebellar nuclei—the caudal fastigial nucleus (cFN) and the posterior interposed nucleus (PIN) (May et al. 1992; Zhang and Gamlin 1998). Neurophysiological and clinical studies also show that the cFN/PIN complex plays a role in vergence eye movement control and control of eye accommodation (Gamlin 1999, 2002; Gamlin et al. 1996; Robinson and Fuchs 2001; Versino et al. 1996). The cFN could play a role in convergence, while the PIN could play a role in divergence (Gamlin and Zhang 1996; Zhang and Gamlin 1998). Given the purported role of the cFN and PIN in binocular control in normal monkeys and that the SOA is implicated in strabismus, the goal of this study was to examine the possible role of the cFN and PIN in the maintenance of strabismus in strabismic monkeys. Muscimol inactivation of the cFN in normal monkeys produces a significant horizontal saccade dysmetria and smooth-pursuit deficits, and inactivation of the PIN produces a vertical saccade dysmetria (Goffart et al. 2004; Robinson 2000; Robinson et al. 1993, 1997). Therefore another objective of the study was to investigate the effects of muscimol inactivation of the cFN/PIN on saccade and smooth-pursuit eye movements (conjugate eye movements) in strabismic monkeys. Some of these data have appeared previously in abstract form (Joshi et al. 2012; Joshi and Das 2012).
METHODS
Subjects and Surgical Procedures
The subjects for this experiment were two strabismic juvenile rhesus monkeys (Macaca mulatta), one with exotropia (monkey S1) and the other with esotropia (monkey S2). Strabismus was induced with a daily alternating monocular occlusion (AMO) method that disrupted binocular vision during the developmental critical period (Tusa et al. 2002). Briefly, within 24 h of birth, an occluding contact lens is placed in one eye. On the following day, the occluding lens is transferred to the fellow eye. Thereafter the occluded eye is alternated on a daily basis for the first 4 mo of life. After the special rearing, the monkeys are allowed to grow under normal viewing conditions until they are ∼4 yr old before commencing neurophysiological and behavior experiments. [Refer to our previous publications for properties of strabismus in AMO monkeys (Das 2009; Das et al. 2005).]
Sterile surgical procedures performed under aseptic conditions with isoflurane anesthesia (1.25–2.5%) were used to stereotaxically implant a head stabilization post (Adams et al. 2007). In a second surgery, we implanted a recording chamber that was a 19-mm-inner diameter titanium cylinder implanted at a stereotaxic location centered 8 mm posterior and 0 mm mediolateral with respect to the interaural axis and tilted 20° dorsolateral to ventromedial in the coronal plane. This chamber placement allowed full access to both cFN as well as the PIN that is located ∼4 mm lateral to the cFN (Robinson 2000). During the same surgical procedure, a scleral search coil was implanted in one eye according to procedures laid out by Judge et al. (1980). Later, in a separate surgery, a second scleral search coil was implanted in the fellow eye. All procedures were performed in strict compliance with National Institutes of Health and Association for Research in Vision and Ophthalmology guidelines, and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Houston.
Experimental Paradigms, Data Acquisition, and Analysis
Binocular eye, target, and neural data were collected as the monkeys performed fixation and horizontal or vertical sinusoidal smooth pursuit (0.2 Hz, ±10–15°) and saccade (±10° and ±15°) tasks under monocular viewing conditions. Binocular eye position was measured with the scleral search coil technique (Primelec Industries, Regensdorf, Switzerland). Eye coil signals were calibrated by rewarding the monkey for looking within a ±3° window around a 1° target rear-projected on a tangent screen 60 cm in front of the monkey. Each eye was calibrated separately during monocular viewing. Visual stimuli were generated with the ViSaGe visual stimulus generator (Cambridge Research Systems, Cambridge, UK) operated under computer control. Monocular viewing was forced by occluding one or the other eye with liquid crystal shutter goggles (Micron Technology, Boise, ID) that were under computer control.
Eye and target position signals were processed with antialiasing filters (Krohn-Hite, Brockton, MA) at 400 Hz before digitization at 1 kHz with 12-bit precision (Alpha-Lab System; Alpha-Omega Engineering, Nazareth, Israel). Data analysis was performed with custom software routines (MATLAB; MathWorks, Natick, MA). Velocity arrays were generated from the position arrays with a central difference algorithm. Eye and target position and velocity data were further filtered with an 80-point finite impulse response software digital filter with a pass band of 0–80 Hz prior to analysis. For saccade trials, saccade onset and offset were determined with an acceleration criterion and saccade gain was calculated as the ratio of saccade amplitude to target amplitude (Fu et al. 2007). Strabismus angle (difference in positions of the left and right eyes) was calculated during fixation of a straight-ahead target (100 ms before a centrifugal saccade or 150 ms after a centripetal saccade). In our convention, rightward and upward positions were positive, and therefore esotropia results in a positive value for strabismus angle and exotropia results in a negative value for strabismus angle. All values are reported as means ± SD. Statistical analysis was performed in SigmaPlot 11.0 with a parametric paired t-test (before and after muscimol injection) and a significance value of 0.05 unless data were nonnormal, in which case a nonparametric Wilcoxon signed-rank test was used.
Muscimol Injections
Prior to commencing the muscimol injection experiments, we identified the location of the cFN by standard neurophysiological methods. First we ran neural recording tracks using epoxy-coated tungsten electrodes (1–5 MΩ; Frederik Haer, Brunswick, ME) into the area of the cFN to identify saccade-related neurons. The neurons were identified by the characteristic burst of neural spikes during saccadic eye movements. cFN locations were also confirmed by electrical microstimulation methods in which a train of low-current cathodal pulses (10–30 μA, 600 Hz, 300–500 ms) delivered via the recording electrode elicited saccades. Stimulation-induced saccades were mostly horizontal, but on some electrode tracks in the area of the cFN stimulation-induced saccades also had significant vertical components. Another identifying factor was the presence of vestibular-sensitive cells over a region 1–2 mm rostral to the cFN (tested during horizontal sinusoidal rotation of the monkey on a vestibular turntable). The PIN region located ∼4 mm lateral and ∼2 mm ventral to the cFN was similarly identified by the saccade-related bursts of its neurons and mostly vertical saccadic and frequently divergent eye movements elicited by electrical microstimulation using the same stimulation parameters as for cFN stimulation.
After identifying the eye movement-related area of the FN and PIN, we injected muscimol with a custom micropipette assembly. For some experiments, the micropipette consisted of a 30-gauge stainless steel hypodermic tube (12 cm in length) with a beveled 35-gauge stainless steel hypodermic tube (∼8 mm in length) glued on one end to form the tip of the injection pipette. For other injections, the micropipette consisted of a 33-gauge stainless steel hypodermic tube with a beveled glass tip (tip diameter of ∼75 μm) glued on one end to form the tip of the injection pipette. A polyethylene tube was connected at the other end to complete the injection pipette assembly. A small volume of muscimol (concn 2 mg/ml; Sigma-Aldrich) was delivered at the desired depth with a picoliter pump (WPI-PV830) connected to the micropipette, to provide timed air pressure pulses allowing for a gradual delivery of muscimol over several (5–10) min (refer to Table 1 for details). Pre- and postinactivation eye movement data were collected for each injection experiment, and injection experiments were separated by at least 1 wk to allow the animal to fully recover prior to the next injection.
Table 1.
Summary of muscimol injections and effect on strabismus angle in monkeys S1 and S2
| Strabismus Angle,° (right eye view) |
Strabismus Angle, ° (left eye view) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Injection No. | Subject (injection type) | Location | Volume, μl | Pre | Post | Post − Pre | Pre | Post | Post − Pre |
| 1 | S1 (cFN, muscimol) | R | ∼4 | −5.6 | −12.6 | −7.0 | −5.0 | −8.9 | −4.9 |
| 2 | L | 1 | −3.9 | −12.2 | −8.3 | −1.1 | −13.6 | −12.5 | |
| 3 | R | 1 | −3.1 | −13.4 | −10.3 | −3.2 | −13.5 | −10.3 | |
| 4 | R | 0.5 | −2.0 | −14.6 | −12.6 | −1.6 | −14.1 | −12.5 | |
| 5 | S2 (cFN, muscimol) | R | 0.5 | 28.8 | 19.9 | −8.9 | 36.9 | 27.9 | −9.0 |
| 6 | R | 0.5 | 29.9 | 20.1 | −9.8 | 34.8 | 24.7 | −10.1 | |
| 7 | R | 1.5 | 31.8 | 24.1 | −7.7 | 34.5 | 27.1 | −7.4 | |
| 8 | L | 0.5 | 30.7 | 14.3 | −16.4 | 35.5 | 16.6 | −18.9 | |
| 9 | L | 0.5 | 31.5 | 20.0 | −11.5 | 42.5 | 30.1 | −12.4 | |
| 10 | S1 (PIN, muscimol) | R | 0.5 | −3.0 | −1.2 | 1.8 | −3.0 | −0.7 | 2.3 |
| 11 | L | 0.5 | −3.8 | −2.3 | 1.5 | −4.4 | −1.5 | 2.9 | |
| 12 | R | 0.5 | −4.4 | −0.6 | 3.8 | −4.2 | −0.2 | 4.0 | |
| 13 | S1 (control, saline) | R | 1 | −3.7 | −2.8 | −0.9 | −3.4 | −2.0 | −1.4 |
| 14 | S2 (control, muscimol) | 4 mm anterior to cFN | 1 | 34.6 | 34.8 | 0.2 | 39.4 | 39.7 | 0.3 |
cFN, caudal fastigial nucleus; PIN, posterior interposed nucleus; R, right; L, left; Pre, before injection; Post, after injection.
Histology
Histological evaluation was performed on the esotrope, monkey S2. A few days prior to euthanasia of this monkey, marking electrolytic lesions were placed at the injection sites by passing 30 μA of anodal direct current for 30 s. A few days later, an overdose of pentobarbital sodium (100 mg/kg) was administered intravenously to induce a deep level of anesthesia and the animal was killed. The animal was perfused through the heart first with saline, then with 4% paraformaldehyde fixative solution, and finally with 4% paraformaldehyde + 10% sucrose solution. After perfusion fixation, the brain was immediately removed for histology. For histological evaluation of the recording sites, the brain was blocked and 50-μm-thick coronal plane frozen sections were cut and stained for Nissl substance.
RESULTS
Horizontal eye misalignment in monkey S1 (exotropia—divergent strabismus) was approximately −4° with either eye viewing. Horizontal misalignment in monkey S2 (esotropia—convergent strabismus) was ∼30° while viewing with the right eye and ∼37° while viewing with the left eye. Both monkeys exhibited alternating fixation and A/V-patterns, while DVD was observed only in the esotropic monkey. We performed a total of 11 unilateral injections (9 muscimol, 2 control) in the cFN in the two monkeys and 3 unilateral injections in the PIN of the exotropic monkey (S1). Injection details are in Table 1. The objective of the study was 1) to investigate the effect of cFN/PIN inactivation on saccade and smooth-pursuit performance in strabismic monkeys and 2) to understand the role played by these structures in maintaining the state of strabismus. We examined the effect of muscimol inactivation on the strabismus by pairwise comparison of pre- and postinjection angles of esotropia or exotropia, A/V-patterns, and DVD.
Effect of Muscimol Inactivation of cFN on Saccade and Smooth-Pursuit Behavior
Horizontal and vertical saccades.
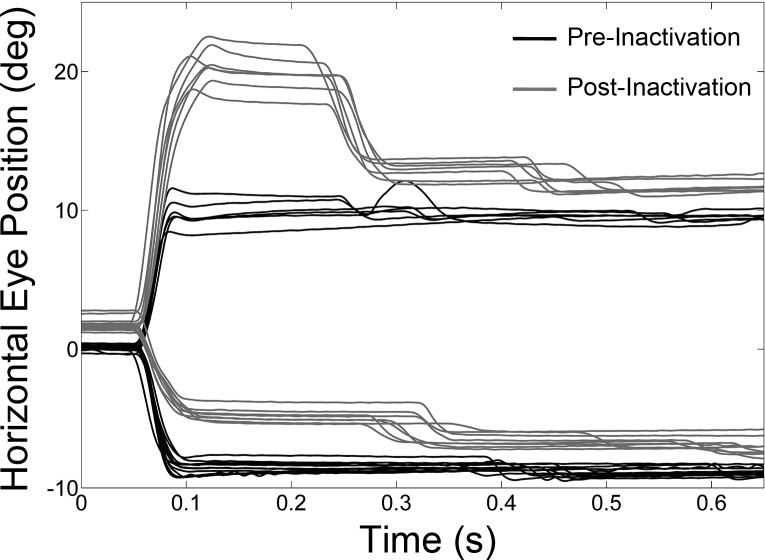
Muscimol inactivation of the cFN unilaterally in the strabismic monkeys produced a horizontal saccade dysmetria. Figure 1 shows several superimposed trials of right and left 10° saccades made by esotropic monkey S2 in a single experiment (injection 5) in which we injected muscimol into the right cFN. Comparison of pre- and postinactivation data shows that right cFN inactivation resulted in ipsilesional hypermetric saccades (i.e., rightward saccades overshoot the target) and contralesional hypometric saccades (i.e., leftward saccades undershoot the target). Saccade dysmetria was a consistent finding in all cFN muscimol inactivation experiments in both monkeys and is also consistent with published studies on normal monkeys. Another consistent finding, also described in normal monkey studies and illustrated in Fig. 1, was a small ipsilesional eye position offset during center fixation (before saccade onset). The offset was always ipsilesional irrespective of whether the ipsilateral or the contralateral (with respect to injection site) eye was viewing the target. Across all injections and viewing conditions, the magnitude of the offset was 1.1 ± 0.6°.
Fig. 1.
Effect of muscimol inactivation of right caudal fastigial nucleus (cFN) on 10° saccades in monkey S2 (injection 5). Positive values in this and other figures indicate rightward or upward positions. Note the overshoot of ipsilesional saccades and undershoot of contralesional saccades in postinactivation traces.
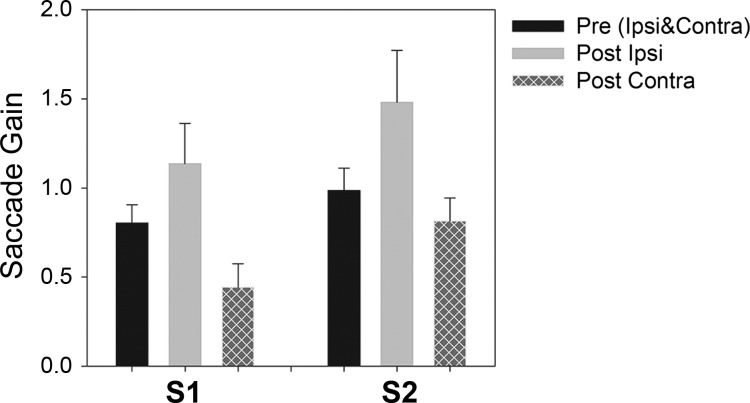
Figure 2 shows summary data of saccade gain values before and after muscimol inactivation. Both centrifugal and centripetal 10° and 15° saccade trials were used to estimate saccade gain. We found no difference between preinactivation ipsilesional and contralesional horizontal saccade gain with either eye viewing, and so these data were pooled for further statistical comparison. In both monkeys, the mean preinactivation horizontal saccade gain was significantly different from the mean postinactivation ipsilesional saccade gain (S1: P = 0.008; S2: P < 0.001) as well as the mean postinactivation contralesional saccade gain (S1: P < 0.001; S2: P < 0.001). Across all cFN injections, monkey S1 showed an average 30% increase and monkey S2 showed an average 48% increase in ipsilesional saccade gain (ranges: S1 6–46%; S2 28–67%). Also, monkey S1 showed an average 37% decrease and monkey S2 showed an average 18% decrease in contralesional saccade gain (ranges: S1 4–53%; S2 7–27%).
Fig. 2.
Summary data showing pre- and postinactivation gain (mean ± SD across all cFN muscimol injections) of ipsilesional and contralesional saccades. Muscimol inactivation results in significant increase in ipsilesional gain and decrease in contralesional gain in both monkeys.
Neither monkey showed any significant changes in vertical saccade gain due to muscimol inactivation of cFN (upward saccades gain pre vs. post comparison: S1 P = 0.27, S2 P = 0.89; downward saccades gain pre vs. post comparison: S1 P = 0.12 Wilcoxon rank sum test, S2 P = 0.34). However, we observed in both animals that saccades to vertical targets showed consistent ipsilesional horizontal deflections.
Horizontal smooth pursuit.
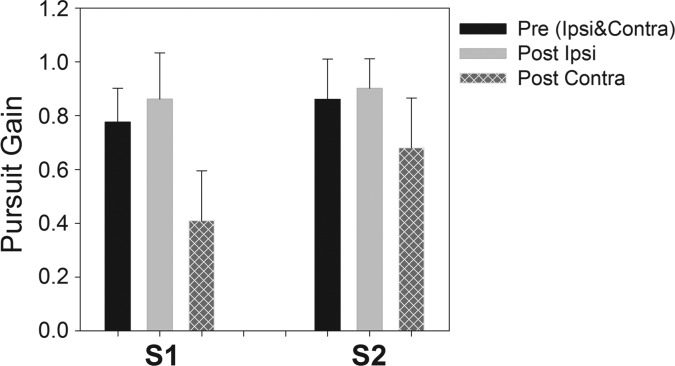
Eye velocity data during smooth-pursuit tracking were analyzed by separately averaging several leftward or rightward half-cycles in order to obtain ipsilesional and contralesional smooth-pursuit gain values. A sinusoidal fit was applied to the averaged data, and half-cycle smooth-pursuit gain was calculated as the ratio of the peak eye velocity of the fitted data to the peak target velocity. No difference was observed between the preinactivation ipsilesional and contralesional smooth-pursuit gain in either monkey with either eye viewing, and so these data were pooled to obtain a single estimate for preinactivation tracking gain. Similarly, there was no significant difference between right eye and left eye viewing conditions for postinactivation smooth-pursuit gain in either monkey, and so data for either eye viewing were pooled for analysis. Figure 3 shows the summary data for pre- and postinactivation average smooth-pursuit gain for both animals. Compared with preinactivation smooth-pursuit gain, postinactivation contralesional smooth-pursuit gain showed significant decrease in both monkeys (S1: P < 0.001; S2: P = 0.007). No statistical difference was observed for postinactivation ipsilesional gain values for either monkey (S1: P = 0.2; S2: P = 0.51).
Fig. 3.
Summary data showing pre- and postinactivation horizontal gain (mean ± SD across all cFN muscimol injections) of ipsilesional and contralesional smooth pursuit. Muscimol inactivation results in a significant decrease in contralesional gain and a small but not statistically significant increase in ipsilesional gain in both monkeys.
Effect of Muscimol Inactivation of cFN on Properties of Strabismus
Strabismus angle.
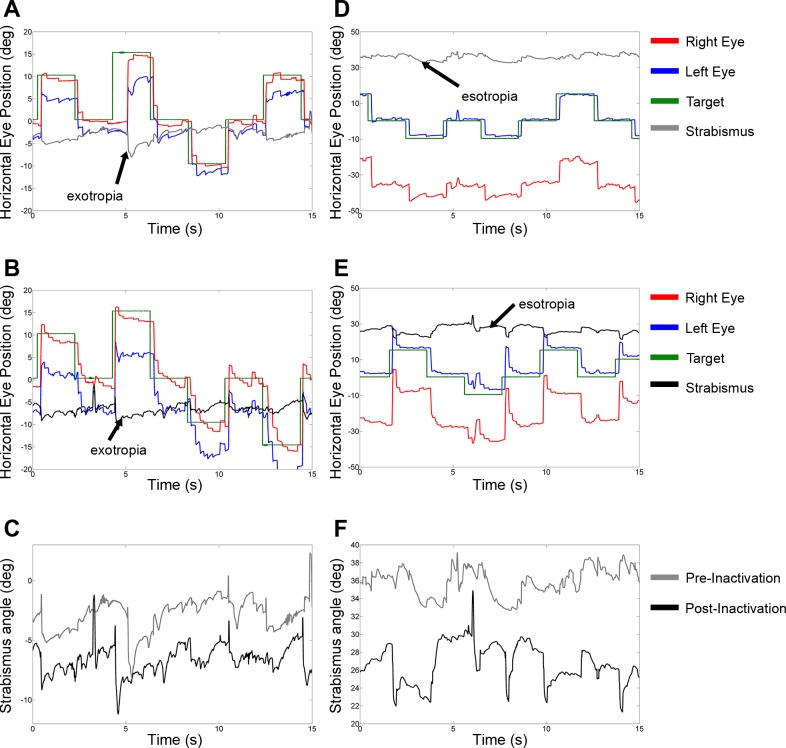
The next objective was to identify the effect of cFN inactivation on esotropia/exotropia, DVD, and A/V-patterns. Figure 4 shows sections of pre- and postinactivation raw eye movement data as the monkeys performed horizontal saccades. Prior to injection of muscimol into the right cFN, monkey S1 showed an exotropia of approximately −3° (Fig. 4A). Approximately 15 min after injection of muscimol, exotropia in this experiment increased to approximately −7° (Fig. 4B). Prior to injection of muscimol into the right cFN, monkey S2 had an esotropia of ∼37° (Fig. 4D). The esotropia decreased to ∼25° after injection of muscimol (Fig. 4E). To illustrate the change in exotropia or esotropia more clearly, Fig. 4, C and F, plot pre- and postinactivation strabismus angles for the same data sections as shown in Fig. 4, A, B, D, and E. Figure 4, C and F, show that the effect of cFN inactivation was to increase angle of exotropia in monkey S1 (strabismus angle is more negative in Fig. 4C) and to decrease angle of esotropia in monkey S2 (strabismus angle is less positive in Fig. 4F). This amounts to a divergent change in strabismus angle in both monkeys (postinactivation data are below preinactivation data in Fig. 4, C and F, reflecting an increase in divergence). Note also in Fig. 4, B and E, that ipsilesional saccades are hypermetric and contralesional saccades are hypometric as summarized in the previous section.
Fig. 4.
Example raw eye movement data showing effect of cFN inactivation on strabismus angle. Data in A–C are from the exotropic monkey (monkey S1, injection 3), and data in D–F are from the esotropic monkey (monkey S2, injection 5). A and D show preinactivation data; B and E show data after inactivation of the right cFN; C and F show a direct comparison of pre- and postinactivation strabismus angle (same data as in A and D and B and E). cFN inactivation induces an increase in exotropia (C; more negative strabismus angle—divergent change) and a decrease in esotropia (F; less positive strabismus angle—divergent change).
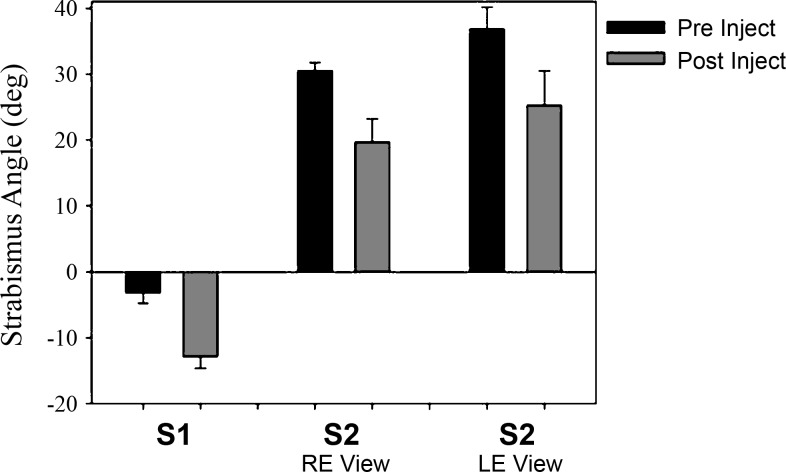
Changes in esotropia or exotropia due to each muscimol injection experiment are summarized in Table 1. Figure 5 shows mean data from all nine muscimol injection experiments, showing overall effect of cFN inactivation on strabismus angle. Right eye viewing and left eye viewing data were pooled in monkey S1 since there was no significant difference in exotropia with either eye viewing the target. In monkey S2, there was a significant difference in the esotropia depending upon which eye was viewing the target (right eye view: ∼30°; left eye view: ∼37°), indicating the presence of a DHD, and therefore these data were analyzed separately. In summary, after cFN inactivation, exotropia for monkey S1 increased from −3.2 ± 1.6° to −12.9 ± 1.8° (P < 0.001). Esotropia for monkey S2 decreased from 30.5 ± 1.2° to 19.7 ± 3.5° while viewing with the right eye and decreased from 36.9 ± 3.3° to 25.3 ± 5.2° while viewing with the left eye (both P < 0.001). In every experiment, the effect of inactivating the cFN was to induce a divergent change in the strabismus angle.
Fig. 5.
Summary data showing strabismus angle (mean ± SD across all cFN muscimol injections) before and after cFN inactivation. Exotropia is negative, and esotropia is positive. cFN inactivation results in an increase in exotropia and a decrease in esotropia. RE, right eye; LE, left eye.
We also examined whether cFN inactivation affected strabismus angle differently when monkeys viewed with either the ipsilesional or contralesional (with respect to injection location) eye but found no statistical differences. In monkey S1, the mean change in exotropia while viewing with the ipsilesional eye was −10.6° ± 2.6° and while viewing with the contralesional eye was −8.7° ± 3.7° (P = 0.43). In monkey S2, the mean change in esotropia while viewing with the ipsilesional eye was −11.6° ± 4.4° and while viewing with the contralesional eye was −10.8° ± 3.4° (P = 0.76).
In both pre- and postinactivation data, we often observed transient changes in strabismus angle that occurred during each saccade, which is an indication of saccade disconjugacy (Fu et al. 2007, Fig. 4). To examine whether cFN inactivation changed the magnitude of saccade disconjugacy, we first calculated a saccade gain ratio between viewing and nonviewing eyes and then compared this gain ratio between pre- and postinactivation conditions. Comparison of pre- and postinactivation ipsilesional and contralesional saccade gain ratios across all injections yielded no statistically significant differences (ipsilesional saccade gain ratio: preinactivation 1.1 ± 0.1, postinactivation 1.1 ± 0.2, P = 0.28; contralesional saccade gain ratio: preinactivation 1.1 ± 0.2, postinactivation 1.0 ± 0.2, P = 0.47).
A/V-patterns and DVD.
In this study, we observed a small A-pattern in the exotrope (monkey S1), a small V-pattern in the esotrope (monkey S2), and a DVD only in the esotrope (monkey S2). Figure 6 shows pre- and postinactivation data obtained during right eye viewing horizontal and vertical smooth pursuit in monkey S2. These data are plotted as vertical position vs. horizontal position for each eye so as to illustrate the vertical gaze position-dependent changes in horizontal eye misalignment that are indicative of pattern strabismus. The esotropia is reflected in the adducted position of the nonviewing left eye relative to the right eye. Preinactivation data show that this monkey has a V-pattern strabismus because there is a decrease in esotropia in up-gaze compared with down-gaze (esotropia at down-gaze ∼30°; esotropia at center fixation ∼29°; esotropia at up-gaze ∼27°). The preinactivation data also show a vertical deviation or DVD of ∼5° in the nonfixating left eye (measured at central gaze). Note that the magnitude of DVD also changes with horizontal gaze. Postinactivation data show that there is a decrease in the angle of esotropia (left eye is closer to primary position), which implies an increased divergence after inactivation of the cFN. However, the V-pattern and the DVD in the pre- and postinactivation data are similar.
Fig. 6.
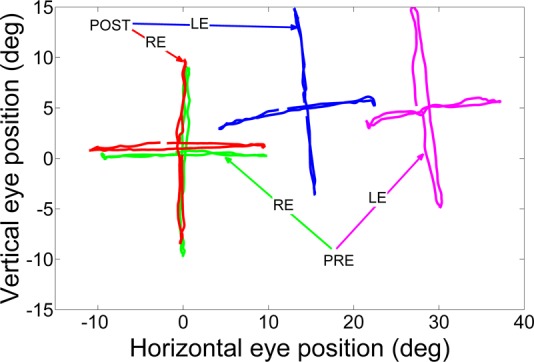
Effect of cFN inactivation on pattern strabismus and dissociated vertical deviation (DVD) in monkey S2 (injection 8). Traces show spatial position of viewing (right eye) and nonviewing (left eye) eyes as the esotropic monkey performed horizontal or vertical smooth pursuit at 0.2 Hz. The nonviewing left eye is adducted relative to the viewing right eye, indicating overconvergence or esotropia. After cFN inactivation, the horizontal position of the left eye during vertical pursuit is closer to that of the right eye, indicating a decrease in the angle of esotropia. Change in esotropia with vertical gaze position is similar in both pre- and postinactivation vertical pursuit, indicating no change in V-pattern. Also, vertical (upward) deviation of the left eye relative to the right eye is similar in pre- and postinactivation horizontal pursuit, indicating no change in DVD.
To quantify the effect of cFN inactivation on A/V-patterns we calculated the strabismus angle at five different vertical fixation positions (obtained in a vertical saccade task; −15°, −10°, 0°, 10°, and 15°) and applied a linear fit to the data. The slope of the fit provides a quantitative measure of the pattern strabismus in the esotrope and exotrope. Slopes for pre- and postinjection data from each injection experiment were calculated (monkey S1 mean preinject slope: −5.5 ± 1.3 and mean postinject slope: −5.6 ± 2.1; monkey S2 mean preinject slope: −5.5 ± 0.9 and mean postinject slope: −4.9 ± 1.7), and pairwise comparison of slope for each monkey showed no significant difference between the pre- and postinactivation conditions, indicating that there was no change in the A/V-pattern as a result of cFN inactivation (P > 0.5).
DVD was measured as difference in the vertical eye position of the fixating and strabismic eyes when viewing a center target. We examined the effect of cFN inactivation on DVD for the five experiments performed on esotropic monkey S2. There was no statistical difference observed in the DVD before and after muscimol inactivation under the right eye viewing condition (right eye viewing: preinject DVD = 5.3 ± 0.2°, postinject DVD = 4.5 ± 0.9°, P = 0.15). However, during left eye viewing, a statistical difference between the pre- and postinjection DVD was observed (left eye viewing: preinject DVD = 5.6 ± 0.4°, postinject DVD = 1.8 ± 0.3°, P < 0.001).
Effect of Muscimol Inactivation of PIN on Saccade Behavior
We successfully inactivated the PIN in the exotropic monkey (S1) three times. We attempted to inject muscimol in the PIN of the esotropic monkey (S2) but were unsuccessful because of the lack of behavioral cooperation from the monkey after injection.
A previous study by Robinson (2000) showed that PIN inactivation in normal monkeys induces a small change in vertical saccade gains. We found a similar result in the strabismic monkey (Fig. 7). Across the three PIN injections in monkey S1, we observed a mean 13% increase (range: 9–22%) in postinactivation upward saccade gain (P < 0.001). The postinjection gain for downward saccades decreased slightly compared with preinjection gain but did not reach statistical significance (mean 4% decrease; range −5% to 13%; P = 0.07). Comparison of the mean horizontal saccade gain before and after PIN inactivation showed no significant difference for contralesional saccade gain (preinject: 0.9 ± 0.1, postinject: 0.8 ± 0.1, P = 0.74), but the gain of ipsilesional saccades was higher (preinject: 0.9 ± 0.2, postinject: 1.0 ± 0.2, P = 0.003).
Fig. 7.
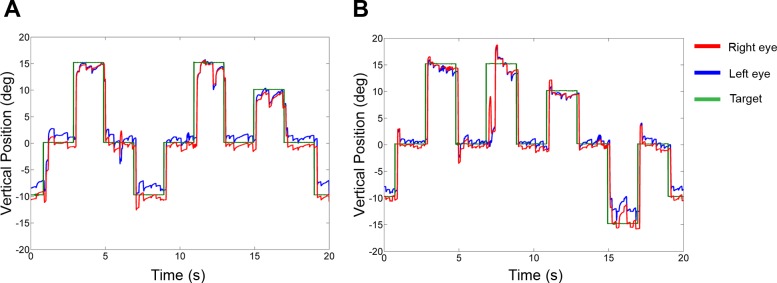
Effect of muscimol inactivation of posterior interposed nucleus (PIN) on vertical saccades in the exotropic monkey (monkey S1, injection 10). A: preinactivation data. B: postinactivation data. Note upward overshoot following PIN inactivation. Positive values indicate upward positions of the eye.
Effect of Muscimol Inactivation of PIN on Exotropia
Inactivating the PIN induced a reduction in exotropia in monkey S1. Figure 8 shows a section of raw data recorded while the monkey made horizontal saccades. Figure 8A shows the preinactivation data, and the angle of exotropia is approximately −5°. After PIN inactivation, eye misalignment is close to 0° (Fig. 8B).
Fig. 8.
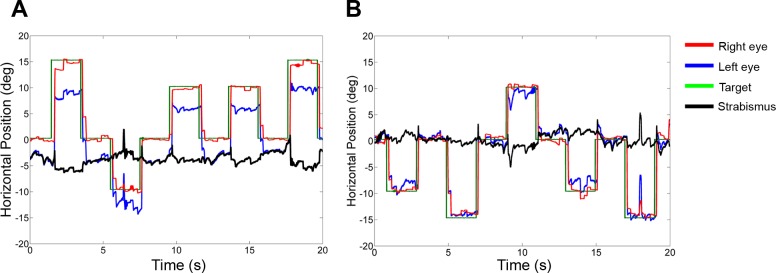
Effect of PIN inactivation on strabismus angle in monkey S1 (injection 10): data obtained during a horizontal saccade task while monocularly viewing with the right eye (preinactivation, A; postinactivation, B). Note the significant decrease in exotropia following PIN inactivation.
Table 1 shows the summary data for the mean exotropia after the three unilateral PIN injections (right eye and left eye viewing conditions pooled). Statistical comparison of the pre- and postinactivation data for all three muscimol injections showed a significant decrease in exotropia (P = 0.001), confirming a convergent effect in all injections.
Control Injections
We made one control injection into each monkey. In one control experiment, saline was injected at the site of the cFN in the exotropic monkey. There were no significant changes in horizontal (preinject gain: 0.9 ± 0.2, postinject gain: 0.9 ± 0.1; P = 0.70) or vertical (preinject gain: 0.9 ± 0.1, postinject gain: 1.0 ± 0.1; P = 0.06) saccade gains. Change in the exotropia was only around 1° (Table 1). In the second control experiment, muscimol was injected at a site 4 mm rostral to the identified cFN location in the esotropic monkey and there was no significant horizontal saccade dsymetria (rightward saccade gain: preinject = 1.0 ± 0.03, postinject = 0.9 ± 0.1, P = 0.49 Mann-Whitney rank test; leftward saccade gain: preinject = 1.2 ± 0.1, postinject = 1.2 ± 0.2, P = 0.97). Change in esotropia in this control experiment was <0.5°.
Nonoculomotor Deficits After cFN and PIN Injections
Both monkeys showed postural instability after they were returned to their cages 2–3 h after injection of muscimol into the cFN. After unilateral cFN inactivation we clearly observed the monkeys falling on the side of the lesion. For example, after muscimol injection in the left cFN, the monkey would tend to fall on his left side and was seen holding himself steady on the left side of the cage. Unlike the cFN injections, PIN injection did not induce significant postural instability but appeared to induce some small errors in reaching. Normally, the monkey was very quick and accurate in grasping the food treat held in front of him. After PIN injection, we observed that the monkey overreached with his hand ipsilesionally before correcting and picking up the treat. For example, when we injected into the right PIN, the monkey reached out farther than the target location with his right hand before correcting and picking up the treat. No such effect was observed on the left hand reaching task. Compared with the readily apparent postural instability following cFN lesion, the reaching errors following PIN lesion were much more subtle. All nonoculomotor deficits were resolved by the following day after the injection.
Histology
We were able to verify the anatomical location of the cFN injection sites via histological reconstruction of electrode tracks and electrolytic lesions placed in monkey S2 at the site of injections (Fig. 9). Comparison of the coronal section with the existing rhesus monkey atlas as well as examining anatomical sections 1 mm rostral and caudal (not shown) to the lesion confirmed that the injections were placed in the cFN.
Fig. 9.
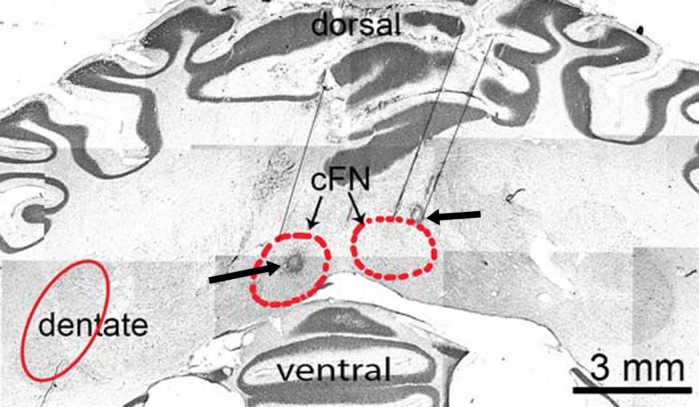
Histological section at the level of cFN (red circled areas marked with thin arrows). Thin black lines show penetrations of the electrode or injection pipette toward the cFN. The electrolytic lesions (circular black spots marked with thick arrows) confirm the placement of injection in the cFN.
DISCUSSION
The main findings of this study were that 1) cFN and PIN inactivation induced saccade and smooth-pursuit dysmetria similar to that described in normal monkey studies and 2) cFN and PIN inactivation exert significant and opposite effects on steady-state strabismus angle, with cFN inactivation producing a divergent change in both monkeys and PIN inactivation producing a convergent change in the exotropic monkey. These structures probably influence strabismus angle via their projections to the SOA. Here we discuss our findings and suggest a potential circuit that is responsible for the maintenance of the strabismic state.
Effect of cFN and PIN Inactivation on Saccadic and Smooth-Pursuit Eye Movements
Lesions of the cFN produce a characteristic hypermetria of ipsilesional saccades and hypometria of contralesional saccades in normal monkeys (Goffart et al. 2004; Robinson et al. 1993, 1997) as well as in human patients (Kheradmand and Zee 2011; Lewis and Zee 1993; Sander et al. 2009). cFN lesions in monkeys also produce an increase in gain of ipsilesional pursuit and a decrease in gain of contralesional pursuit (Robinson et al. 1997). An experimental lesion of the ventrolateral PIN in normal monkeys induces vertical deficits (hypermetria of upward saccades and hypometria of downward saccades), pointing to perhaps complementary roles of the cFN and PIN in some aspects of conjugate eye movement control (Robinson 2000; Robinson and Fuchs 2001).
In this study, we found that muscimol inactivation of cFN and PIN in strabismic monkeys produced saccade and smooth-pursuit deficits similar to those observed in normal monkey studies. After cFN inactivation, Robinson and colleagues (Robinson et al. 1993) report ipsilesional saccade gain of 1.4 ± 0.3 and contralesional saccade gain of 0.6 ± 0.2, gains that are very similar to those measured in our study (Fig. 2). Vertical saccade dysmetria following PIN inactivation is smaller in the normal monkey (increase in gain of upward saccades of only ∼12%). We found the same in the strabismic monkey (Fig. 7). Also reproducible in the strabismic monkey was an ipsilesional fixation offset following cFN inactivation that has been proposed as being due to influence of the cFN on the rostral superior colliculus (Guerrasio et al. 2010). These findings indicate that sensory strabismus does not strongly disrupt the neural circuitry through which the cerebellar nuclei influence saccades and smooth pursuit.
Effect of cFN and PIN Inactivation on Ocular Alignment
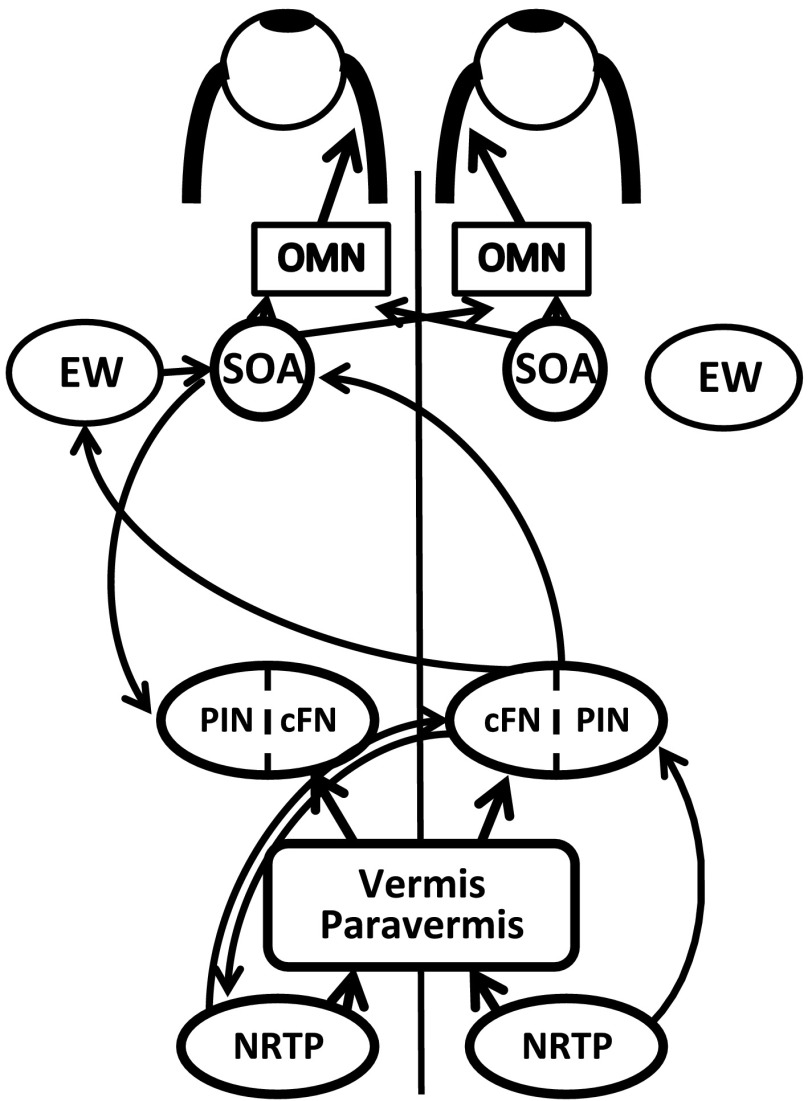
Previous anatomical, neurophysiological, and clinical studies indicate that the cFN and PIN play a role in binocular control (Kheradmand and Zee 2011; Noda et al. 1990; Yamada and Noda 1987; Zee et al. 2002). The main input to the cFN is the oculomotor vermis, vermal lobules VIc-VII (Noda and Fujikado 1987), while the PIN receives input from the paravermal region and also the ipsilateral dorsal paraflocculus (Kralj-Hans et al. 2007). The oculomotor part of the vermis receives a large input from the nucleus reticularis tegmenti pontis (NRTP) (Voogd and Barmack 2005), which is itself an important relay nucleus in the pons that receives inputs from a number of visual and oculomotor cortical areas known to be important in saccades, smooth pursuit, and vergence (Giolli et al. 2001; Stanton et al. 1988a, 1988b). The cFN/PIN receives a direct (smaller) input from the NRTP bilaterally (Noda et al. 1990). There is also an ipsilateral feedback projection to the cFN and PIN from the SOA (May et al. 1992). The efferent connections from the FN and PIN are also diverse. Specifically with respect to binocular control, there is a direct connection from the cFN and PIN to the contralateral SOA that in turn projects monosynaptically to the MRMNs (May et al. 1992). Together with the ipsilateral feedback connection from SOA to the cFN/PIN, this might form a motor pathway that is the substrate for normal eye alignment (Fig. 10). Maldevelopment of this circuit could lead to the motor component of eye misalignment. Note that the binocular signals from the cFN/PIN or the SOA must also make their way to the abducens nucleus so that the medial and lateral recti can act in push-pull, but this pathway is not yet known. In addition to the SOA, projections from only the cFN (not the PIN) include the nearby Edinger-Westphal nucleus (EW—important for eye accommodation; May et al. 1992) and therefore also provide a potential neural circuit for accommodation to influence strabismus angle.
Fig. 10.
Schematic showing connections between structures in brain playing a role in binocular control and potentially also in strabismus. OMN, oculomotor nucleus; SOA, supraoculomotor area; EW, Edinger-Westphal nucleus; NRTP, nucleus reticularis tegmenti pontis.
A significant number of vergence-related neurons have been reported in the cFN. These neurons are related to the near response, with increased firing during convergence eye movements and near accommodation (Zhang and Gamlin 1996). “Vergence” and “eye movement” neuronal populations may overlap significantly, as one report indicated as much as 63% of the vergence-related neurons are also saccade sensitive (Zhang and Gamlin 1996). Microstimulation in some areas of the FN induces convergence eye movements (Murakami et al. 1991). Unlike vergence neurons in the cFN, vergence neurons in the PIN are related to the far response, i.e., increase their activity during a divergence eye movement (Zhang and Gamlin 1998). Also unlike the vergence neurons in the cFN, vergence neurons in the PIN do not seem to have saccade sensitivity (Zhang and Gamlin 1998). Physiological activity in cells in the cFN and PIN therefore supports a possible role in setting the strabismic state. For example, far-response neurons in the PIN along with near-response neurons in the cFN could form a push-pull system that, via projections to the SOA and thereafter oculomotor and abducens motoneurons, contributes to the tone of eye muscles while fixating a straight-ahead far target (sometimes also referred to as vergence tone).
Humans with various types of cerebellar degeneration that potentially also compromises cerebellar nuclei activity show horizontal and vertical phoria and tropia that depends on orbital position, saccade disconjugacy, and disconjugate postsaccadic drift (Hoyt and Good 1995; Versino et al. 1996; Williams and Hoyt 1989). All these properties are also observed in our strabismic monkey model (Das et al. 2005; Fu et al. 2007). Another study described a patient with damage to the superior cerebellar peduncle (carrying output of cFN and PIN) who presented with a large exotropia and complete lack of vergence eye movements, suggesting that vergence tone could be mediated by cFN/PIN pathways (Ohtsuka et al. 1993). Experimental midline cerebellectomy (removal of vermis) in monkeys, in addition to saccade and smooth-pursuit deficits, also produces incomitant esodeviation, disconjugate eye movements, and disruption of phoria adaptation (Takagi et al. 1998, 2000, 2003). Inactivation of the cFN with muscimol reduces the size and speed of convergence (Gamlin and Zhang 1996). However, no static misalignment was found in this same study (P. Gamlin, personal communication).
In the present study, inactivation of the cFN imposed a divergent effect on the strabismus (increase in exotropia and decrease in esotropia). Conversely, inactivation of the PIN imposed a convergent effect (decrease in exotropia). We suggest that in esotropia the cFN/PIN-SOA-MRMN circuit (including feedforward and feedback connections; Fig. 10) is providing an excessive “convergent” bias signal to the medial rectus muscles, perhaps as a result of disruptions in development. The abducens nucleus must also be receiving these erroneous bias signals to allow for relaxation of the lateral rectus. Inactivating the cFN then leads to a reduction of this excessive vergence tone and a reduction in esotropia. In exotropia, this circuit is providing an insufficient “convergent” bias or perhaps excessive “divergent” bias. Inactivating the cFN leads to a further decrease in the overall “convergence” signal and therefore an increase in exotropia. On the other hand, inactivation of the PIN produces an increase in the vergence tone and a decrease in exotropia. Our observation of similar changes in strabismus angle following unilateral injections into either side of the brain is likely due to the fact that there are contralateral feedforward projections from the cFN/PIN to the SOA and ipsilateral feedback projections from the SOA to the cFN/PIN.
Changes in “A”-Pattern and DVD
Previously we have reported that SOA cells showed activity that was correlated with the static angle of exotropia but did not show modulation in activity when exotropia changes occurred due to the A-pattern. We hypothesized that the SOA contributed toward providing the signal for static misalignment but the A-patterns were generated elsewhere, perhaps via brain stem pathways responsible for conjugate eye movements (Das 2012). In the present study, we found no change in the A/V-pattern before and after cFN or PIN inactivation in both monkeys. Our data are therefore consistent with our previously proposed hypothesis that the cFN/PIN-SOA-MRMN circuit drives the horizontal strabismus but not alignment changes due to A/V-patterns of strabismus. The esotropic monkey also exhibited DVD, and in our study we found no change in the DVD before and after cFN inactivation when the monkey was viewing the target with his right eye. A statistical difference in DVD was observed when the left eye was viewing the target, but this might be partly due to reduction in the esotropia following cFN inactivation (since DVD also changes based on horizontal gaze position). Additional animals with DVD would be required to test whether inactivation of the cFN/PIN plays a role in maintenance of DVD.
GRANTS
This work was funded by National Eye Institute Grant R01 EY-015312 and University of Houston, College of Optometry Core Grant NIH P30 EY-07551.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C.J. and V.E.D. conception and design of research; A.C.J. performed experiments; A.C.J. analyzed data; A.C.J. and V.E.D. interpreted results of experiments; A.C.J. prepared figures; A.C.J. drafted manuscript; A.C.J. and V.E.D. edited and revised manuscript; A.C.J. and V.E.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Ernest Baskin for technical assistance, Dr. Guofu Shen, Dr. Xiaofeng Tao, and Dr. Yuzo Chino for perfusion/fixation of the animals, and Christy Willoughby and Dr. Linda McLoon for brain histology.
REFERENCES
- Adams DL, Economides JR, Jocson CM, Horton JC. A biocompatible titanium headpost for stabilizing behaving monkeys. J Neurophysiol 98: 993–1001, 2007 [DOI] [PubMed] [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci 8: 495–545, 1985 [DOI] [PubMed] [Google Scholar]
- Das VE. Alternating fixation and saccade behavior in nonhuman primates with alternating occlusion-induced exotropia. Invest Ophthalmol Vis Sci 50: 3703–3710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das VE. Alternating saccades in a primate model of strabismus. In: Advances in Understanding Mechanisms and Treatment of Infantile Forms of Nystagmus, edited by Leigh RJ, Devereaux MW. New York: Oxford Univ. Press, 2007, p. 47–54 [Google Scholar]
- Das VE. Responses of cells in the midbrain near-response area in monkeys with strabismus. Invest Ophthalmol Vis Sci 53: 3858–3864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das VE, Fu LN, Mustari MJ, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus 13: 33–41, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das VE, Mustari MJ. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with “A” pattern strabismus. Invest Ophthalmol Vis Sci 48: 665–674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Tusa RJ, Mustari MJ, Das VE. Horizontal saccade disconjugacy in strabismic monkeys. Invest Ophthalmol Vis Sci 48: 3107–3114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin P, Zhang H. Effects of muscimol blockade of the posterior fastigial nucleus on vergence and ocular accommodation in the primate (Abstract). Soc Neurosci Abstr 22: 2034, 1996 [Google Scholar]
- Gamlin PD. Neural mechanisms for the control of vergence eye movements. Ann NY Acad Sci 956: 264–272, 2002 [DOI] [PubMed] [Google Scholar]
- Gamlin PD. Subcortical neural circuits for ocular accommodation and vergence in primates. Ophthalmic Physiol Opt 19: 81–89, 1999 [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Yoon K, Zhang H. The role of cerebro-ponto-cerebellar pathways in the control of vergence eye movements. Eye 10: 167–171, 1996 [DOI] [PubMed] [Google Scholar]
- Giolli RA, Gregory KM, Suzuki DA, Blanks RH, Lui F, Betelak KF. Cortical and subcortical afferents to the nucleus reticularis tegmenti pontis and basal pontine nuclei in the macaque monkey. Vis Neurosci 18: 725–740, 2001 [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol 92: 3351–3367, 2004 [DOI] [PubMed] [Google Scholar]
- Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology 112: 104–108, 2005 [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Buttner U, Goffart L. Fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol 103: 1988–2001, 2010 [DOI] [PubMed] [Google Scholar]
- Guyton DL, Weingarten PE. Sensory torsion as the cause of primary oblique muscle overaction/underaction and A- and V-pattern strabismus. Binocular Vision Strabismus Q 9: 209–236, 1994 [Google Scholar]
- Helveston EM. The aetiology of essential infantile (congenital) esotropia. In: Advances in Strabismus Research: Basic and Clinical Aspects, edited by Lennerstrand G, Ygge J. London: Portland, 2000, p. 135–152 [Google Scholar]
- Hoyt CS, Good WV. Acute onset concomitant esotropia: when is it a sign of serious neurological disease? Br J Ophthalmol 79: 498–501, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AC, Baskin E, Das VE. Muscimol inactivation of the cerebellar fastigial oculomotor region in strabismic monkeys changes strabismus angle (Abstract). ARVO Meeting Abstracts 53: 6337, 2012 [Google Scholar]
- Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci 52: 6697–6705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AC, Das VE. Role of the midline deep cerebellar nuclei in maintaining the state of misalignment in non-human primates with strabismus. Neuroscience Meeting Planner 2012: 371 –06, 2012 [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Kheradmand A, Zee DS. Cerebellum and ocular motor control. Front Neurol 2: 53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj-Hans I, Baizer JS, Swales C, Glickstein M. Independent roles for the dorsal paraflocculus and vermal lobule VII of the cerebellum in visuomotor coordination. Exp Brain Res 177: 209–222, 2007 [DOI] [PubMed] [Google Scholar]
- Lewis RF, Zee DS. Ocular motor disorders associated with cerebellar lesions: pathophysiology and topical localization. Rev Neurol (Paris) 149: 665–677, 1993 [PubMed] [Google Scholar]
- Lorenz B. Genetics of isolated and syndromic strabismus: facts and perspectives. Strabismus 10: 147–156, 2002 [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD, Gamlin PD. Interconnections between the primate cerebellum and midbrain near-response regions. J Comp Neurol 315: 98–116, 1992 [DOI] [PubMed] [Google Scholar]
- Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol 51: 1091–1108, 1984 [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol 56: 1007–1021, 1986 [DOI] [PubMed] [Google Scholar]
- Murakami S, Noda H, Warabi T. Converging eye movements evoked by microstimulation of the fastigial nucleus of macaque monkeys. Neurosci Res 10: 106–117, 1991 [DOI] [PubMed] [Google Scholar]
- Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol 58: 359–378, 1987 [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol 302: 330–348, 1990 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Maekawa H, Sawa M. Convergence paralysis after lesions of the cerebellar peduncles. Ophthalmologica 206: 143–148, 1993 [DOI] [PubMed] [Google Scholar]
- Robinson FR. Role of the cerebellar posterior interpositus nucleus in saccades. I. Effect of temporary lesions. J Neurophysiol 84: 1289–1302, 2000 [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24: 981–1004, 2001 [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements. II. Effects of muscimol inactivation. J Neurophysiol 78: 848–859, 1997 [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol 70: 1741–1758, 1993 [DOI] [PubMed] [Google Scholar]
- Sander T, Sprenger A, Neumann G, Machner B, Gottschalk S, Rambold H, Helmchen C. Vergence deficits in patients with cerebellar lesions. Brain 132: 103–115, 2009 [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey. I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol 271: 473–492, 1988a [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. J Comp Neurol 271: 493–506, 1988b [DOI] [PubMed] [Google Scholar]
- Takagi M, Tamargo R, Zee DS. Effects of lesions of the cerebellar oculomotor vermis on eye movements in primate: binocular control. Prog Brain Res 142: 19–33, 2003 [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol 83: 2047–2062, 2000 [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998 [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann NY Acad Sci 956: 346–360, 2002 [DOI] [PubMed] [Google Scholar]
- Urrets-Zavalia A. Significance of congenital cyclo-vertical motor defects of the eyes. Br J Ophthalmol 39: 11–20, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versino M, Hurko O, Zee DS. Disorders of binocular control of eye movements in patients with cerebellar dysfunction. Brain 119: 1933–1950, 1996 [DOI] [PubMed] [Google Scholar]
- von Noorden GK, Campos EC. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. St.Louis, MO: Mosby, 2002 [Google Scholar]
- Voogd J, Barmack NH. Oculomotor cerebellum. Prog Brain Res 151: 231–268, 2005 [DOI] [PubMed] [Google Scholar]
- Williams AS, Hoyt CS. Acute comitant esotropia in children with brain tumors. Arch Ophthalmol 107: 376–378, 1989 [DOI] [PubMed] [Google Scholar]
- Yamada J, Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol 265: 224–241, 1987 [DOI] [PubMed] [Google Scholar]
- Zee DS, Walker MF, Ramat S. The cerebellar contribution to eye movements based upon lesions: binocular three-axis control and the translational vestibulo-ocular reflex. Ann NY Acad Sci 956: 178–189, 2002 [DOI] [PubMed] [Google Scholar]
- Zhang H, Gamlin P. Single unit activity within the posterior fastigial nucleus during vergence and accommodation in the alert primate (Abstract). Soc Neurosci Abstr 22: 2034, 1996 [Google Scholar]
- Zhang H, Gamlin PD. Neurons in the posterior interposed nucleus of the cerebellum related to vergence and accommodation. I. Steady-state characteristics. J Neurophysiol 79: 1255–1269, 1998 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gamlin PD, Mays LE. Antidromic identification of midbrain near response cells projecting to the oculomotor nucleus. Exp Brain Res 84: 525–528, 1991 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mays LE, Gamlin PD. Characteristics of near response cells projecting to the oculomotor nucleus. J Neurophysiol 67: 944–960, 1992 [DOI] [PubMed] [Google Scholar]