Abstract
Background:
Immune cell infiltrates are important determinants of colorectal cancer (CRC) outcome. Their presence may be driven by tumour or host-specific factors. From previous studies in mice, senescence, a state of cell cycle arrest, may moderate tumour progression through upregulation of antitumour immune responses. The relationships between senescence and immune infiltrates have not previously been studied in humans. We explore whether a marker of senescence (p16ink4a) in combination with low level expression of a proliferation marker (ki-67) relate to T cell infiltrates in CRC, and whether p16ink4a, Ki-67 and immune infiltrates have similar prognostic value.
Methods:
Immunostaining of p16inka and Ki-67 was performed within a CRC tissue microarray. Nuclear p16inka and Ki-67 were categorised as high/low. T-cell markers, CD3, CD45RO, CD8 and FOXP3 were scored separately as high/low grade in three areas of the tumour: the invasive margin (IM), tumour stroma and cancer cell nests (CCNs).
Results:
Two hundred and thirty stage I–III cancers were studied. High nuclear p16ink4a was expressed in 63% and high proliferation (Ki-67 >15%) in 61%. p16ink4a expression was associated with reduced CD45RO+ cells at the IM (P<0.05) and within the stroma (P<0.05) and reduced CD8+ cells at the IM (P<0.01). A low Ki-67 proliferative index was associated with reduced density of CD3+ cells in CCNs (P<0.01), reduced CD45RO+ cells at the IM (P<0.05) and within the CCNs (P<0.001), reduced FOXP3+ cells at the IM (P<0.001), within the stroma (P=0.001) and within CCNs (P<0.001) and reduced CD8+ cells at the IM (P<0.05) and within the CCNs (P<0.05). Tumours with both a low proliferative index and expression of p16ink4a demonstrated similar consistent relationships with reduced densities of T-cell infiltrates. On multivariate analysis, TNM stage (P<0.001), low CD3 cells at the IM (P=0.014), low CD8 cells at the IM (P=0.037), low proliferation (Ki-67; P=0.013) and low senescence (p16ink4a; P=0.002) were independently associated with poorer cancer survival.
Conclusion:
Senescence, proliferation and immune cell infiltrates are independent prognostic factors in CRC. Although related to survival, p16ink4a-associated senescence is not associated with an upregulation of antitumour T-cell responses.
Keywords: immune cells, senescence, proliferation, colorectal cancer, p16ink4a, CDKN2A
The role of immune cell infiltrates in determining outcome in colorectal cancer (CRC) is increasingly appreciated. Over 100 published studies report consistent relationships between improved cancer-specific survival and an increasing number, or density, of immune cells in and around colorectal tumours (Roxburgh and McMillan, 2012). The evidence is strongest for a generalised lymphocytic/inflammatory cell infiltrate at the invasive margin (IM), based on over 40 studies (Jass, 1987; Kilntrup et al, 2005; Roxburgh et al, 2009a, 2009b; Richards et al, 2012a, 2012b; Roxburgh and McMillan, 2012). Recently, several groups have attempted to characterise these immune reactions, most work focusing on T lymphocytes and their subsets (CD3+, CD4+, CD8+, CD45RO+ and FOXP3+) and macrophages (CD68+ Naito et al, 1998; Pages et al, 2005; Galon et al, 2006; Roxburgh and McMillan, 2012). With the appreciation that density, type and location of intratumoural immune cells predict survival independent of stage (Galon et al, 2006), several validated scores of immune cell infiltrates have been developed including the immune score (based on C45RO+ and CD8+) and the Klintrup–Makinen score (Klintrup et al, 2005; Roxburgh et al, 2009a, 2009b; Mlecnik et al, 2011); consequently, there are increasing calls for immune scoring as part of the routine prognostication in CRC (Galon et al, 2012).
To date, it is unclear whether intratumoural immune responses represent tumour- or host-specific phenomena. High-grade infiltrates are commoner in early-stage disease (low T stage, absence of lymphovascular invasion or nodal/distant metastases) degrading as tumours enlarge and disseminate (Roxburgh et al, 2009a; Mlecknik et al, 2011). Associations with molecular tumour characteristics have also been reported, namely microsatellite instability, intratumoural HLA expression, and CpG island methylation (Mlecnik et al, 2011). Microsatellite unstable tumours have an established clinical phenotype including right-sided location, moderate-well differentiation as well as the presence of a pronounced lymphocytic infiltrate (Michael-Robinson et al, 2001a,2001b). However, immune cell infiltrates are consistently reported to offer additional prognostic information, independent of stage, pathological characteristics and mismatch repair status, potentially reflecting the complex interplay between host immune responses and the tumour (Michael-Robinson et al, 2001a; Baker et al, 2007; Laghi et al, 2009; Sinicrope et al, 2009; Deschoolmeester et al, 2010).
One potential tumour characteristic, that may in part account for immune cell infiltrates, is the presence of intratumoural cellular senescence and the ‘senescence-associated secretory phenotype' (SASP). Cellular senescence, a state of stable cell cycle arrest, is thought to be an evolutionary mechanism to mitigate tumour development. The presence of senescent cells within a tumour may be a favourable prognostic feature in cancer, as such cells would not have bypassed cellular damage checkpoints (Narita and Lowe, 2005). Senescent cells are associated with a proinflammatory secretome including interleukins (ILs) and other cytokines (IL-6, IL-8, MMP 1 and 3, CXCR, IGF1 and 2; Campisi, 2005; Cairney et al, 2012). Work in animal models has identified increased infiltrates of innate immune cells (NK cells and macrophages) and CD4+ lymphocytes drawn in by the proinflammatory secretome (Xue et al, 2007). This upregulation of intratumoural immune responses was thought to delay tumour progression. Therefore, one plausible explanation for the presence of high-grade lymphocytic infiltrates in colorectal tumours is that cellular senescence and the associated inflammatory secretome drive their presence.
To date, no single gold-standard biomarker for the detection and quantification of senescent cells in tissues has been developed. Biomarkers developed so far attempt to measure three main components related to senescence: cell cycle arrest (p16, p21, and Ki-67), chromatin (for example. HMGA1 and HMGA2) and the secretome (for example, IL-6, IL-8, MMP1 and so on; Cairney et al, 2012). Cell cycle markers of senescence include p16, p21 and Ki-67 expression. Of these markers, most work has utilised p16ink4a expression, which is thought to represent an established and reliable marker of cellular senescence in tumours as well as normal tissues (Campisi, d′Adda di, 2007; Collado et al, 2007; Schmit et al, 2007; Raabe et al, 2011). P16ink4a is a CDK inhibitor (Dimri et al, 1995; Campisi, 2005), the expression of which is upregulated in cellular senescence and ageing in normal tissues (Krishnamurthy et al, 2004; McGlynn et al, 2009). Importantly, p16ink4a expression has previously ben associated with improved survival in colorectal tumours (Esteller et al, 2001; Lyall et al, 2006; Mitomi et al, 2010) but the mechanisms through which p16ink4a expression influence cancer outcome are not yet established.
It is hypothesised that senescent tumour cells are associated with an immunogenic reaction in and around colorectal tumours. The aim of the present study was to establish whether markers of intratumoural senescence (evidence of nuclear p16ink4a expression and low Ki-67 labelling) are associated with increased immune cell infiltration representative of an immunogenic phenotype in human CRCs and to determine whether senescence, proliferation and immune cell infiltration were associated with long-term survival.
Materials and methods
Since 1997, all resectional CRC surgery performed at Glasgow Royal Infirmary (GRI) has been entered into a prospectively compiled and maintained database. For the present study, 230 patients undergoing surgery for stage I–III CRC between 1997–2005, and whose tumour biopsy was included in a CRC tissue microarray were studied. On the basis of the preoperative radiological staging and intraoperative findings, patients were considered to have undergone curative resection. Exclusion criteria were neoadjuvant treatment or death within 30 days of surgery. Local ethical committee approval was granted.
Routinely assessed pathological data were taken from reports issued by the GRI Department of Pathology at the time of surgery in accordance with the RCPath data set for colorectal cancer report (Williams et al, 2007). In addition, TNM stage (5th edition), other high-risk characteristics included tumour differentiation, serosal involvement, margin involvement, tumour perforation and venous invasion. The modified Glasgow Prognostic Score (mGPS), a measure of systemic inflammation based on serum C-reactive protein and albumin measured before surgery was also assessed (CRP<10 mg l−1, albumin >35 g dl−1=mGPS 0; CRP>10 mg l−1, allbumin >35 g dl−1=mGPS 1; CRP>10 mgl−1, albumin <35 g dl−1=mGPS 2; Roxburgh and McMillan, 2012).
Tumour necrosis was graded in this cohort, scored semiquantitatively by two observers as ‘absent' (none), ‘focal' (<10% of tumour area), ‘moderate' (10–30%) or ‘extensive' (>30%) according to published methodology (Pollheimer et al, 2010; Richards et al, 2012a, 2012b). An assessment of inflammatory cell infiltrate at the IM using H&E slides (the Klintrup–Makinen score) has also been performed in all cancers within this cohort. The methods for inflammatory infiltrate scoring are described elsewhere (Klintrup et al, 2005; Roxburgh et al, 2009a, 2009b). Briefly, the generalised inflammatory cell infiltrate at the margin is graded according to a 4-point score: 0 indicates there was no increase in inflammatory cells at the tumour's IM; 1 denotes a mild/patchy increase in inflammatory cells; 2 denotes a prominent inflammatory reaction forming a band at the IM: 3 denotes a florid cup-like inflammatory infiltrate at the invasive edge with destruction of cancer cell islands. These scores are subsequently given a binary classification as either low grade (scores 0 and 1) or high grade (scores 2 and 3).
Immunohistochemistry
Full section analysis for immune cell infiltrates
Archived paraffin-embedded blocks of the central tumour were retrieved. One block, representative of the point of deepest tumour invasion, was chosen. Consecutive 4 μm sections were cut and mounted on silanised slides before being dewaxed in xylene and rehydrated using graded alcohol washes. Heat-induced antigen retrieval was performed by microwaving under pressure using a citrate or Tris/EDTA buffer before endogenous peroxidase activity was blocked (5% normal goat serum in TRIS-buffered saline (TBS)), and the following primary antibodies were applied according to manufacturer's instructions; CD8+ (DakoCytomation, Glostrup, Denmark; code M7103, 1/100 dilution), CD3+ (Vector Laboratories, Burlingame, CA, USA; code VP-RM01, 1/100 dilution), CD45R0+ (DakoCytomation, code M0742, 1/150 dilution) and FOXP3+ (Abcam, Cambridge, UK; code 20034, 1/200 dilution). Sections were washed with TBS, incubated with Dako Envision, washed again and had 3,3′-diaminobenzidine (DAB) applied. Finally, sections were washed with water, counterstained with haemotoxylin, dehydrated and mounted.
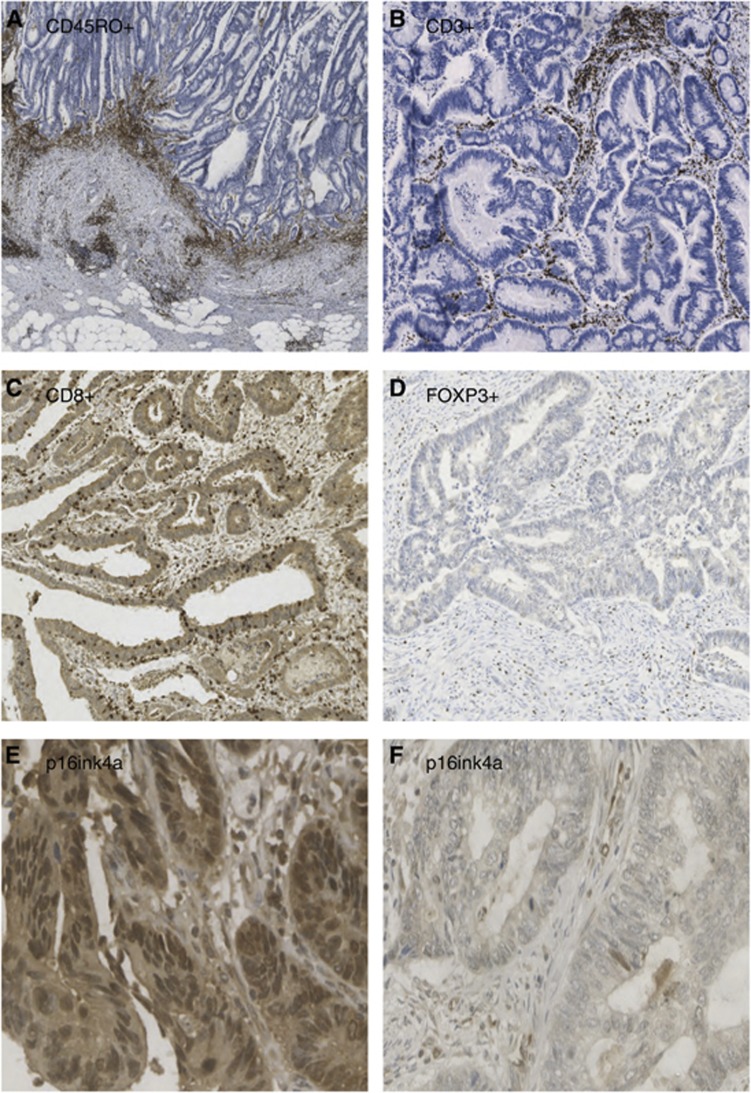
Evaluation of T-cell density was undertaken blinded to outcome. Density was graded semiquantitatively as absent, weak, moderate or strong in three separate tumour compartments: (1) IM, (2) tumour stroma (ST) and (3) cancer cell nests (CCN). Examples of immune cell staining in various tumour locations are shown in Figures 1A–D. To confirm consistency of grading, 100 cases were scored independently by two investigators (CHR and CSR).
Figure 1.
Examples of immunohistochemistry for immune cell infiltrates and p16ink4a in colorectal tumours. (A) High-density CD45RO+ staining at IM at 45 × magnification, (B) High-density CD3+ staining within ST at 40 × magnification. (C) High-density CD8+ staining within CCN at 115 × magnification. (D) Low-density FOXP3+ staining within ST at 115 × magnification. (E) High-density nuclear p16ink4a staining at 180 × magnification (F) Low-density nuclear p16ink4a staining at 200 × magnification.
In addition to assessing individual T-cell subtypes, a previously proposed ‘immune score' was applied: the Galon Immune Score, a composite immunohistochemistry-based score, which grades CD45R0+ and CD8+ infiltration in both the margin and central tumour (Mlecnik et al, 2011). The scoring groups the tumours based on high or low density of each cell type in each area HiHiHiHi to LoLoLoLo for CD45RO+ and CD8+ at the IM and tumour centre (Mlecnik et al, 2011).
TMA construction
In addition to full section analysis described above, CRC tissue microarrays (TMA) were used to assess p16ink4a and Ki-67 expression. In brief, a trained pathologist with a specialist gastrointestinal interest identified tumour-rich area within each specimen and four 0.6-mm2 tumour cores were used to construct the TMA (Tovey et al, 2005). TMA sections (2.5 μm) were cut and mounted on slides coated with aminopropyltriethoxysilane.
Immunohistochemistry for p16 ink4a and Ki-67
Before p16ink4a antibody staining of TMAs, the antibody was validated using a p16ink4a blocking peptide competitor assay on tissue sections, in the presence of p16ink4a antibody. Specificity of the p16ink4a antibody was evident by an absence of staining in the presence of the blocking peptide.
Paraffin-embedded TMAs were baked, dewaxed, and rehydrated followed by antigen retrieval in Tris/EDTA (pH 8.0 for p16ink4a and pH 6.0 for Ki-67) with boiling for 5 min in a microwave under pressure. After cooling, endogenous peroxidase was quenched by incubating sections in 3% hydrogen peroxide for 10 min. Slides were blocked for 1 h at 25 °C in 1 × casein (Vector Laboratories, SP-5020) diluted in TBS buffer. Slides were then incubated at 4 °C overnight with the primary antibodiy: 1 in 300 dilution of p16ink4a (Santa Cruz sc-468; Santa Cruz Biotechnology, Dallas, TX, USA) antibody in Dako diluent (Dako, Glostrup, Denmark, S0809), and 1 in 50 dilution of Ki-67 antibody (Monoclonal mouse anti-human, Ki-67 antigen, Clone MIB-1, CodeM7240) with a negative (no primary antibody) slide control. Slides were then washed and incubated with Dako REAL Envision HRP Rabbit/Mouse (Dako, K5007) for 1 h at 25 °C before developing with DAB (Vector Laboratories, SK-4100). Slides were washed and counterstained with haematoxylin before being dehydrated and mounted. Scoring of the nuclear p16ink4a was performed using the weighted histoscore method. Histoscoring is a widely applied and validated method of obtaining reliable and reproducible assessments of scoring immunohistochemical stains in heterogeneous tissues (Kirkegaard et al, 2006). The percentage of positive nuclei within tumour tissue was multiplied by the strength of staining: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). Only tumour tissue was scored. On the basis of the distribution of histoscores in the whole cohort, the median p16 histoscore was 19.5. Therefore, nuclear p16 expression was considered high if >20. For Ki-67 scoring, 108 specimens were co-scored by two observers blinded to outcome (AP and LM). The ICCC was 0.94. For p16ink4a scoring, 65 specimens were co-scored by two observers blinded to outcome. The ICCC was 0.72. In addition to ICCC to ensure correlation between observers, bland Altman plots were performed to ensure there was no bias. Figures 1E and F show both strong and weak nuclear staining for p16ink4a.
In order to calculate the Ki-67 proliferation index, all tumour cells within each core was scored providing the total number of positive and negative cells and allowing calculation of the percentage positive cells. The mean representative score was taken across all cores. The threshold of 15% was taken based on previously published work (Mohammed et al, 2012).
Statistical analysis
Information on date and cause of death was derived from data linkage with cancer registry data performed by the West of Scotland Cancer Surveillance Unit. Date and cause of death was cross-checked with information from clinical notes where possible.
All variables were grouped according to standard or previously published thresholds. Associations were examined using χ2-tests for linear trend as appropriate. Death records were complete until 1 December 2011, which served as the censor date. Univariate survival analyses were performed using the Kaplan–Meier survival curves with log-rank tests. Multivariate analyses were performed using Cox proportional hazards regression with a backwards method. The proportionality assumptions for age, mGPS, TNM and necrosis were explored using log–log plots and were found to be satisfactory. A P-value of <0.05 was required to enter the multivariate regression model. Statistical analyses were performed using SPSS version 19.0 (SPSS, Chicago, IL, USA).
Results
Two hundred and thirty patients were included who underwent curative resection for CRC between January 1997 and December 2005. Clinicopathological characteristics are described in Table 1. Most were ⩾65 years (67%), male (54%), and had colonic tumours (65%). Adjuvant chemotherapy was administered to 31%. On pathological analysis, most were stage I/II (54%), moderate/well differentiated (87%), with presence of venous invasion (52%), absence of serosal invasion (70%), absence of tumour perforation (96%) with uninvolved surgical margins (92%). Most had absent or weak scores for necrosis (60% Table 1).
Table 1. Clinicopathological characteristics including immune cell infiltrates, senescence and proliferation and 5-year cancer-specific survival rates in patients undergoing potentially curative resection for colorectal cancer (n=230).
| Clinicopathological characteristic | N (%) |
|---|---|
|
Age | |
| <65 | 76 (33) |
| 65–75 | 78 (34) |
| >75 |
76 (33) |
|
Sex | |
| Female | 107 (46) |
| Male |
123 (54) |
|
Site | |
| Colon | 149 (65) |
| Rectum |
81 (35) |
|
Adjuvant chemotherapy | |
| No | 159 (69) |
| Yes |
71 (31) |
|
mGlasgow Prognostic Score | |
| 0 | 128 (56) |
| 1 | 77 (33) |
| 2 |
25 (11) |
|
Stage | |
| TNM I | 17 (7) |
| TNM II | 108 (47) |
| TNM III |
105 (46) |
|
T stage | |
| I | 7 (2) |
| 2 | 18 (8) |
| 3 | 135 (59) |
| 4 |
70 (30) |
|
N stage | |
| 0 | 125 (54) |
| 1 | 80 (35) |
| 2 |
25 (11) |
|
Differentiation | |
| Mod/Well | 201 (87) |
| Poor |
29 (13) |
|
Venous invasion | |
| No | 143 (62) |
| Yes |
87 (38) |
|
Serosal involvement | |
| No | 160 (70) |
| Yes |
70 (30) |
|
Margin involvement | |
| No | 212 (92) |
| Yes |
18 (8) |
|
Tumour perforation | |
| No | 221 (96) |
| Yes |
9 (4) |
|
Tumour necrosis | |
| Absent | 15 (7) |
| Focal | 122 (53) |
| Moderate | 63 (28) |
| Extensive |
28 (12) |
|
Klintrup–Makinen infiltrate | |
| Weak | 154 (67) |
| Strong |
75 (33) |
|
CD3 infiltrate margin | |
| Weak | 126 (58) |
| Strong |
92 (42) |
|
CD45RO infiltrate margin | |
| Weak | 120 (55) |
| Strong |
99 (45) |
|
FOXP3 infiltrate margin | |
| Weak | 125 (57) |
| Strong |
93 (43) |
|
CD8 infiltrate margin | |
| Weak | 134 (61) |
| Strong |
85 (39) |
|
Tumour proliferation (Ki-67) | |
| High | 141 (61) |
| Low |
89 (39) |
|
P16ink4a expression | |
| High | 145 (63) |
| Low | 85 (37) |
Abbreviation: TNM=tumour, nodes and metastases.
Tumour immune cell infiltrates were categorised as low-grade Klintrup–Makinen inflammatory cell infiltrates (67%) and weak/absent infiltrates of IM CD3+cells (58%), CD45RO+ cells (55%), FOXP3+ cells (57%) and CD8+ cells (61% Table 1). Most were classed highly proliferative by Ki-67 staining (61%) and the most expressed p16ink4a (63% Table 1). A weak association was observed between p16ink4a expression and low tumour proliferation (P=0.087).
The relationships between intratumoural p16ink4a expression, Ki-67 labelling and clinicopathological characteristics in CRC are shown in Supplementary Table 1. p16ink4a expression was not related to any patient or tumour characteristics, including age, sex, systemic inflammatory response (mGPS), TNM stage or venous invasion. Similarly, a high Ki-67 index did not relate to any patient or tumour characteristic. A weak association was observed between low tumour proliferation and surgical margin involvement (P=0.042; Supplementary Table 1).
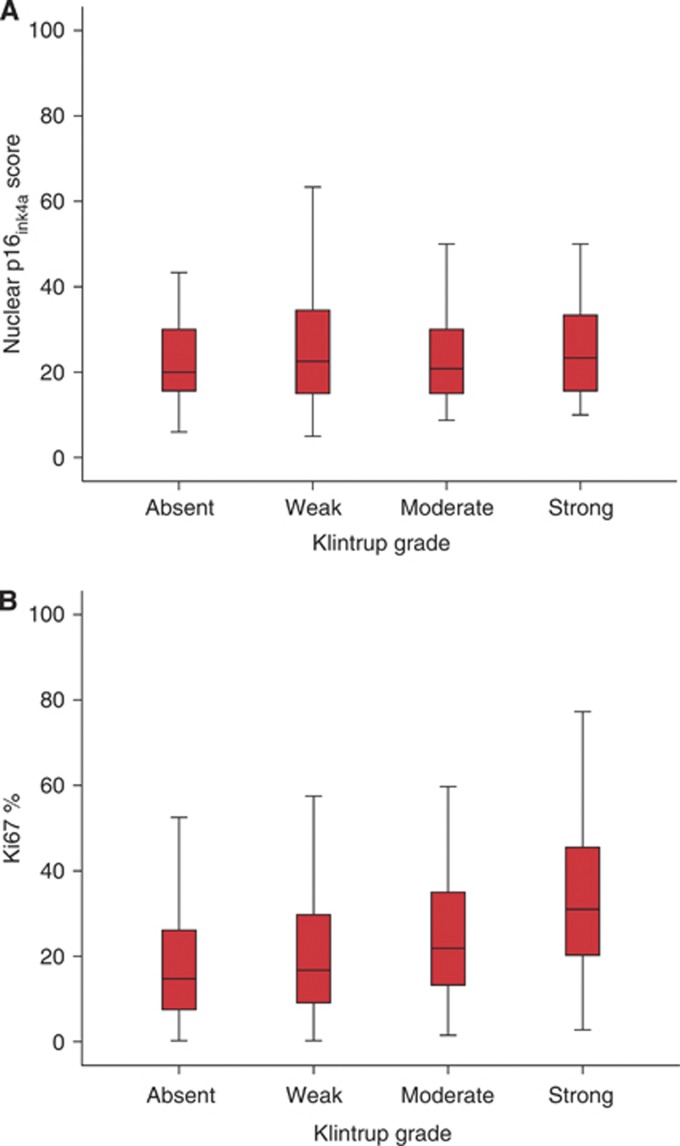
Intratumoural p16ink4a expression and a low Ki-67 proliferative index were employed as markers of senescence in this study. Relationships between intratumoural p16ink4a expression, Ki-67 proliferative index and their combination with immune cell infiltrates are shown in Table 2. High nuclear p16ink4a expression was associated with reduced CD45RO+ cells at the IM (P<0.05) and within the stroma (P<0.05) and reduced CD8+ cells at the IM (P<0.01). A low Ki-67 proliferative index was associated with reduced density of CD3+ cells in CCN (P<0.01), reduced CD45RO+ cells at the IM (P<0.05) and within the CCN (P<0.001), reduced FOXP3+ cells at the IM (P<0.001), within the stroma (P=0.001) and within CCN (P<0.001), and reduced CD8+ cells at the IM (P<0.05) and within the CCN (P<0.05). A lower proliferative index was associated with a low-grade Klintrup–Makinen inflammatory cell infiltrate (P<0.001) and a low Galon–Pages Immune Score (P<0.005; Table 2). Figure 2 shows the relationships between increasing Klintrup–Makinen category (absent/weak/moderate and strong) and p16ink4aexpresson and Ki-67 labelling.
Table 2. The relationships between intratumoural nuclear P16ink4aexpression and Ki-67 expression with type, density and location of immune cell infiltrate.
| |
P16ink4a
expression |
Ki-67 proliferation index |
P16ink4a/Ki-67 combined |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Immune cell infiltrate | Low nuclear P16ink4a exp N=85 | High nuclear P16ink4a exp N=145 | P-value | Ki-67 (high) N=141 | Ki-67 (low) N=89 | P-value | P16 low/Ki-67 high N=180 | P16 high/Ki-67 low N=50 | P-value |
|
CD3 margin | |||||||||
| Low/high |
42/37 |
84/55 |
0.297 |
71/62 |
55/30 |
0.100 |
91/79 |
35/13 |
0.017 |
|
CD3 stroma | |||||||||
| Low/high |
37/48 |
76/66 |
0.146 |
66/73 |
47/41 |
0.385 |
83/95 |
30/19 |
0.071 |
|
CD3 cancer cell nests | |||||||||
| Low/high |
53/32 |
100/42 |
0.210 |
83/56 |
70/18 |
0.002 |
111/67 |
42/7 |
0.002 |
|
CD45RO margin | |||||||||
| Low/high |
36/44 |
84/55 |
0.027 |
65/76 |
55/32 |
0.042 |
83/86 |
37/13 |
0.002 |
|
CD45RO stroma | |||||||||
| Low/high |
31/54 |
74/68 |
0.022 |
64/74 |
41/48 |
0.964 |
97/80 |
25/25 |
0.548 |
|
CD45RO cancer cell nests | |||||||||
| Low/high |
55/28 |
109/33 |
0.111 |
89/49 |
77/12 |
<0.001 |
122/55 |
45/5 |
0.003 |
|
FOXP3 margin | |||||||||
| Low/high |
40/37 |
85/56 |
0.235 |
63/69 |
62/24 |
<0.001 |
85/84 |
40/9 |
<0.001 |
|
FOXP3 stroma | |||||||||
| Absent/weak |
40/37 |
85/56 |
0.382 |
69/66 |
64/23 |
0.001 |
96/77 |
37/12 |
0.012 |
|
FOXP3 cancer cell nests | |||||||||
| Absent/weak |
43/37 |
67/75 |
0.349 |
51/84 |
59/28 |
<0.001 |
75/98 |
35/14 |
0.001 |
|
CD8 margin | |||||||||
| Low/high |
39/40 |
95/45 |
0.007 |
73/60 |
61/25 |
0.018 |
76/94 |
40/9 |
0.001 |
|
CD8 stroma | |||||||||
| Low/high |
60/22 |
107/34 |
0.653 |
98/39 |
70/16 |
0.097 |
124/50 |
44/5 |
0.008 |
|
CD8 cancer cell nests | |||||||||
| Low/high |
61/21 |
105/37 |
0.942 |
95/43 |
72/14 |
0.013 |
123/52 |
44/5 |
0.006 |
|
KM inflammatory infiltrate | |||||||||
| Weak/strong |
28/56 |
47/98 |
0.887 |
58/82 |
17/72 |
<0.001 |
110/69 |
44/6 |
<0.001 |
|
Galon–Pages immune score | |||||||||
| 0/1/2 | 42/21/14 | 88/31/16 | 0.104 | 68/36/24 | 62/16/6 | 0.002 | 90/44/21 | 40/8/1 | <0.001 |
Cells shaded in red indicate significant (P<0.05) and weakly significant (P<0.1) associations with lower density of immune cell infiltrate. The full colour version of this table is available at British Journal of Cancer online.
Figure 2.
(A) The relationship between nuclear p16ink4a expression and peritumoural inflammation measured with the Klintrup score P=0.782. (B) The relationship between tumour proliferation (Ki-67%) and increasing peritumoural inflammation measured with the Klintrup score P<0.001.
When p16ink4a and Ki-67 were combined as markers of senescence, similar and more consistent relationships were observed (Table 2). Intratumoural senescence was associated with reduced infiltrates of CD3+ cells at the IM (P<0.05) and within CCN (P<0.005), reduced CD45RO+ cells at the IM (P<0.005) and within CCN (P<0.005), reduced FOXP3+ cells at the IM (P<0.001), within the stroma (P<0.05) and within CCN (P=0.001), reduced CD8+ cells at the IM (P=0.001), within the stroma (P=0.001) and within CCN (P<0.01). Senescence was associated with a low-grade Klintrup–Makinen inflammatory cell infiltrate (P<0.001) and a low Galon–Pages Immune Score (P<0.001; Table 2).
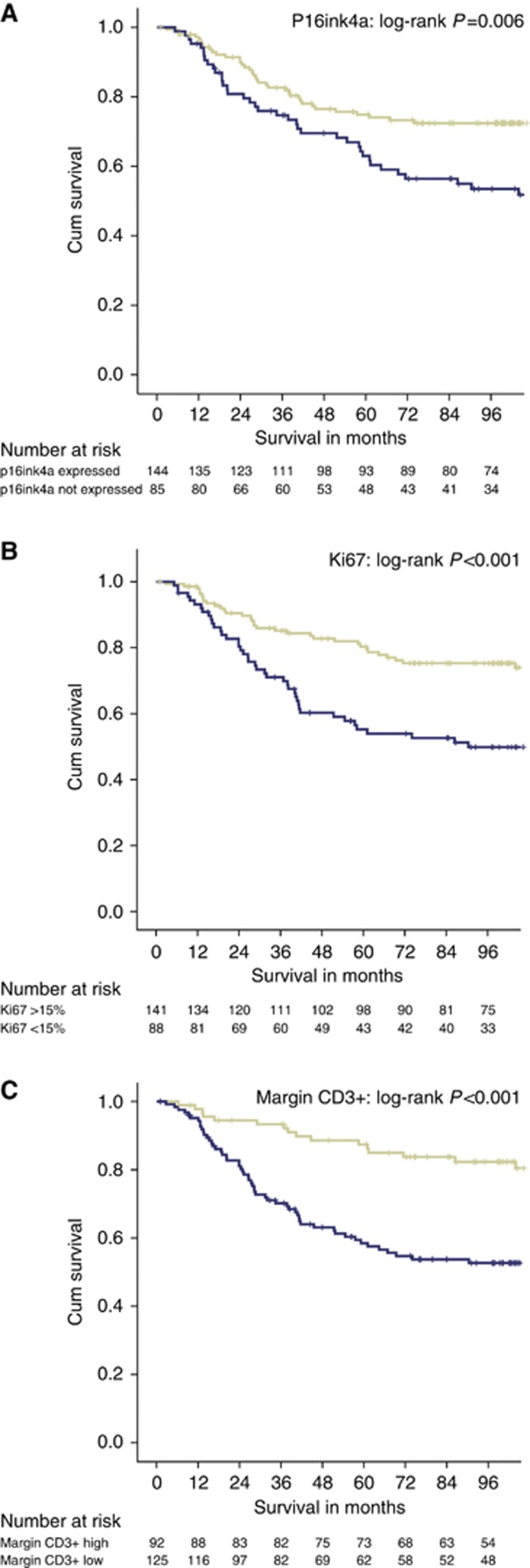
Median follow-up for survivors was 113 months (minimum 73 months), during which there were 117 deaths, of which 78 were from cancer. Univariate and multivariate analysis for cancer-specific survival is shown in Table 3. On univariate analysis, the following features were significantly associated with poorer cancer-specific survival: presence of a systemic inflammatory response (mGPS) P<0.001, increasing TNM stage (P<0.001), and tumour necrosis (P<0.05). Lower counts of immune cells at the IM also significantly related to poorer cancer-specific survival: CD3+ cells (P<0.001), FOXP3+ (P<0.01), CD8+ (P<0.001) and a lower Klintrup–Makinen Score (P<0.005). Lower Ki-67 expression (P<0.001) and reduced p16ink4a expression (P<0.01) were related to poorer cancer -specific survival (Table 3). Survival curves demonstrating relationships between margin CD3+, Ki-67 and nuclear p16ink4a expression and cancer-specific survival are shown in Figure 3.
Table 3. Univariate and multivariate analysis of clinicopathological characteristics for cancer-specific survival in stage I–III colorectal cancer (n=230): Cox regression analysis.
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|---|
|
Age | ||||
| <65/ 65–75/ >75 |
1.21 (0.92–1.60) |
0.174 |
|
|
|
Sex | ||||
| Male/female |
1.06 (0.67–1.66) |
0.809 |
|
|
|
Site | ||||
| Colon/rectum |
1.35 (0.86–2.13) |
0.198 |
|
|
|
mGlasgow Prognostic Score | ||||
| 0/1/2 |
1.74 (1.28–2.37) |
<0.001 |
1.41 (1.00–1.99) |
0.053 |
|
TNM stage | ||||
| I/II/III |
2.27 (1.50–3.44) |
<0.001 |
2.70 (1.69–4.32) |
<0.001 |
|
Necrosis | ||||
| Absent/weak/moderate/strong |
1.36 (1.03–1.81) |
0.033 |
|
0.730 |
|
CD3 margin | ||||
| High/low |
3.36 (1.93–5.87) |
<0.001 |
2.37 (1.19–4.73) |
0.014 |
|
CD45RO margin | ||||
| High/low |
2.68 (1.60–4.50) |
<0.001 |
|
0.569 |
|
CD8 margin | ||||
| High/low |
3.30 (1.87–5.83) |
<0.001 |
2.16 (1.05–4.44) |
0.037 |
|
FOXP3 margin | ||||
| High/low |
2.42 (1.25–4.72) |
0.009 |
|
0.813 |
|
Klintrup–Makinen score | ||||
| Strong/weak |
2.79 (1.53–5.06) |
0.001 |
|
0.400 |
|
Ki-67 | ||||
| Low/high |
2.32 (1.48–3.64) |
<0.001 |
1.89 (1.15–3.11) |
0.013 |
|
Nuclear P16ink4a
expression | ||||
| High/low | 1.85 (1.18–2.89) | 0.007 | 2.22 (1.33–3.72) | 0.002 |
Figure 3.
(A) The relationship between nuclear P16ink4a expression and cancer-specific survival in stage I–III CRC (n=230). P=0.006. (B) The relationship between tumour proliferative activity (Ki-67) and survival in stage I–III CRC (n=230). P<0.001. (C) The relationship between Margin CD3+ density (low/high) and cancer-specific survival in stage I–III CRC (n=230). P<0.001.
On multivariate analysis of significant variables, the following were independently associated with poorer cancer-specific survival: systemic inflammation mGPS (HR 1.41 (95% CI: 1.00–1.99) P=0.053), TNM stage (HR: 2.70 (95% CI: 1.69–4.32) P<0.001), reduced margin CD3+ count (HR 2.37 (95% CI 1.19–4.73) P=0.014), reduced margin CD8+ count (HR: 2.16 (95% CI: 1.05–4.44) P=0.037), lower tumour proliferation (Ki-67; HR: 1.89 (95% CI: 1.15–3.11) P=0.013) and reduced p16ink4a expression (HR: 2.22 (95% CI: 1.33–3.72) P=0.002; Table 4).
Table 4. The relationships between immune cell infiltrates and cancer-specific survival (log-rank P-value) depending on senescence measured with P16ink4a expression and proliferation (Ki-67) in patients undergoing curative resection for colorectal cancer (n=230).
| P16ink4a low | P16ink4a high | Ki-67 low | Ki-67 high | |
|---|---|---|---|---|
| CD3 margin Hi/Lo | 0.008 | <0.001 | 0.003 | 0.003 |
| CD3 stroma Hi/Lo | 0.005 | <0.001 | 0.058 | <0.001 |
| CD3 CCN Hi/Lo | 0.001 | 0.001 | 0.021 | 0.002 |
| CD45RO margin Hi/Lo | 0.006 | <0.001 | 0.049 | 0.003 |
| CD45RO stroma Hi/Lo | 0.067 | 0.003 | 0.013 | 0.080 |
| CD45RO CCN Hi/Lo | 0.017 | <0.001 | 0.011 | 0.046 |
| CD8 margin Hi/Lo | <0.001 | 0.001 | 0.002 | 0.013 |
| CD8 stroma Hi/Lo | 0.011 | 0.216 | 0.169 | 0.050 |
| CD8 CCN Hi/Lo | 0.004 | 0.001 | <0.001 | 0.035 |
| FOXP3 margin Hi/Lo | 0.011 | 0.112 | 0.084 | 0.370 |
| FOXP3 stroma Hi/Lo | <0.001 | 0.320 | 0.203 | 0.019 |
| FOXP3 CCN Hi/Lo | <0.001 | 0.557 | 0.623 | 0.003 |
| KM score Hi/Lo |
0.005 |
0.019 |
0.026 |
0.050 |
|
Galon–Pages immune | ||||
| Score HiHi/Hi/Lo/LoLo | 0.002 | <0.001 | 0.001 | 0.018 |
Abbreviations: CCN=cancer cell nests; HI=high; KM=Klintrup–Makinen Score; Lo=low.
Table 4 shows the relationships between tumour immune cell infiltrates and survival (log-rank P-value) in tumours with both high and low p16ink4a and according to Ki-67 expression. Increasing densities of immune cell infiltrates of CD3+, CD45RO+ and CD8+, in addition to scores such as the Klintrup–Makinen and Galon–Pages immune scores, consistently related to improved survival in tumours with high and low levels of p16ink4a and high and low Ki-67 expression. It is noteworthy that increasing levels of T-regulatory cells (FOXP3+) at the margin, ST or CCN were associated with outcome in patients whose tumours had low p16ink4a expression but not in patients with high p16ink4a expression. Similarly, survival relationships were not observed with FOXP3+ expression in low Ki-67 tumours.
Discussion
The present study reports for the first time that in a large cohort of curative CRC patients with mature follow-up, immune cell infiltrates and intratumoural senescence (measured with p16ink4a and Ki-67 labelling) are stage-independent prognostic features. It was hypothesised that based on tumour p16ink4a expression and Ki-67 proliferative index, strong relationships would be observed between markers of senescence and increased density of immune cell infiltrates, potentially drawn in by a proinflammatory SASP. Further, we hypothesised that p16ink4a expression would at least partly explain the associations between immune cell infiltrates and survival in CRC. Although p16ink4a expression was related to improved cancer-specific survival, the present study's results suggest that this is unlikely to be mediated via immune interactions involving tumour-infiltrating T lymphocytes (TILs; CD3+, CD8+, CD45RO+ and FOXP3+). If anything, higher p16ink4a expression alone and in combination with low Ki-67 labelling was associated with reduced density of several T-cell markers at the margin, within the stroma and within the CCN (Table 2). Therefore, we would conclude that the method by which the presence of intratumoural senescence slows cancer progression in CRC does not appear to relate to overt upregulation of antitumour effector T-cell immune responses.
These results provide further evidence that the in situ immune response has a key role in determining CRC outcome independent of tumour stage and makers of senescence.
The fact that no significant relationship between p16ink4a and immune cell infiltrates invalidates the present study's hypothesis. Further, tumours with high levels of senescence should demonstrate a low proliferative index. The fact that immune cell infiltrates demonstrated a strong relationship with proliferative tumours further strengthens the argument that the presence of tumour lymphocytic infiltrates exist independently of senescent cells. Data from murine models previously reported that intratumoural senescence was associated with generation of an antitumour immune response represented by the presence of CD4+ cells, macrophages and natural killer cells (Xue et al, 2007). The majority of published evidence on the prognostic value of immune cell infiltrates in human CRC has focused on T cells, particularly effector T cells (for example, CD8+ cytotoxic T cells). We chose a panel of T-cell markers representative of the most validated and studied adaptive immune cells, CD3+ (generic T-cell marker) memory T cells (CD45RO), T-regulatory cells (FOXP3+) and cytotoxic T cells (CD8+). Although we cannot say p16ink4a expression did not relate to innate (macrophages or NK cell-predominant infiltrates) or Th2-type adaptive (CD4+ infiltrates) intratumoural immune responses, we can conclude that p16ink4a expression does not appear to relate to a Th1-type response represented by higher densities of intratumoural effector CD8+ infiltrates.
P16ink4a and Ki-67 are considered part of a panel of potential biomarkers of senescence. Other biomarkers include markers of the senescence-associated secretome including the proinflammatory cytokine IL-6. Although no measure of intratumoural cytokines were available here, we included serum measures of systemic inflammation (mGPS based on C-reactive protein and albumin). Interleukin-6 is responsible for 90% of the hepatic production of C-reactive protein (Gabay and Kushner, 1999). No significant relationships were seen between p16ink4a expression, C-reactive protein and albumin. It is possible that the IL-6 produced in the presence of the SASP does not represent a systemic phenomenon and may have more subtle intratumoural effects. Further, the presence of a systemic inflammatory response is known to persist independent of local intratumoural inflammation (Roxburgh et al, 2009a, 2009b).
The present study also demonstrated significant relationships between T-cell infiltrates and highly proliferative tumours. The reasons for such observations have not been fully elucidated, but previous studies have demonstrated associations between microsatellite instability and higher proliferative index (Michael-Robinson et al, 2001). Highly proliferative tumours were associated with improved survival in this cohort; this relationship may be partly explained by increased immunogenicity of these tumours drawing in immune reactions. Unfortunately, at present no measure of microsatellite instability is available in this cohort, but the high Ki-67 labelling may be a surrogate measure for genomic instability in part explaining these relationships with immune cell infiltrates and improved survival. Alternatively, one may construct the hypothesis that an altered tumour secretome overrides any effect the SASP has. Highly proliferative tumour cells may then produce factors modulating immune activity, based on altered tumour cell metabolism and consequently the tumour cell secretome (Sebastián et al, 2012).
The role of p16ink4a expression has previously been examined in CRC using immunohistochemistry (Zhao et al, 2003; Cui et al, 2004; Gonzalez-Quevedo et al, 2004; Lyall et al, 2006; Wassermann et al, 2009; Shima et al, 2011). To date, published data on loss of p16ink4a expression is variable, reported between 8–91% in stage I–IV disease. Many studies had a low number of cancers studied (n=32–117). The largest study (n=802) reported that p16ink4a was expressed in 75%, more comparable with the current study (63%) (Shima et al, 2011). This study by Shima et al (2011) found that p16ink4a loss was associated with poorer overall survival (HR: 1.30, 95% CI: 1.03–1.63, P=0.026), also comparable with the present study's findings for poorer cancer-specific survival (univariate analysis, HR: 1.85, 95% CI: 1.18–2.90, P=0.007). However, the study by Shima et al (2011) reported that the prognostic value of p16ink4a was lost on multivariate analysis; age and tumour grade were the principal confounders. In the present study of 230 patients, p16ink4a expression was an independent prognostic factor when age and grade (differentiation) were considered.
There is an increasing literature on the role of immune scoring in CRC prognostication with calls for the introduction of these stage-independent prognostic biomarkers into routine clinical practice in addition to utilisation within clinical trials of cancer therapeutics for stratification purposes (Galon et al, 2012). The basis or driving force for the immune cell infiltrates is largely unclear. Some have suggested that this response is representative of the local in situ host immune reaction against the tumour and once lost, or evaded, the tumour can grow and metastasise or ‘escape' (Mlecnik et al, 2011; Roxburgh and McMillan, 2012). This is in keeping with previous reports that suggest the response is lost with increasing tumour size or T and N stage (Roxburgh et al, 2009a,2009b; Mlecnik et al, 2011). It is probable that intratumoural factors have a partial role in determining the specific densities of immune cell infiltrates including microsatellite instability, CpG island methylation and intratumoural HLA expression (Mlecnik et al, 2011), but at this stage these features do not yet seem to explain fully their presence.
In summary, the present study highlights the prognostic value of intratumoural senescence. Importantly, the presence of senescence determined by p16ink4a expression does not appear to mediate its effect on oncological outcome through TILs. On the other hand, high tumour proliferation is strongly associated with the presence of a local immune response. However, TILs and the in situ local inflammatory response remain strong prognostic factors independent of these intratumoural characteristics. p16ink4a expression and low tumour proliferation as markers of intratumoural senescence do not explain the presence of TILs in CRCs.
Acknowledgments
We acknowledge the assistance and expertise provided by Dr Karin Oien, Dr Jonathan Platt and Dr Clare Orange in construction of the Glasgow Royal Infirmary Colorectal Cancer tissue microarray, which was employed for assessment of p16ink4a and Ki-67 in the present study.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Baker K, Zlobec I, Tornillo L, Terracciano L, Jass JR, Lugli A. Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient versus proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer. 2007;43 (3:624–631. doi: 10.1016/j.ejca.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Cairney CJ, Bilsland AE, Evans TR, Roffey J, Bennett DC, Narita M, Torrance CJ, Keith WN. Cancer cell senescence: a new frontier in drug development. Drug Discov Today. 2012;17 (5-6:269–276. doi: 10.1016/j.drudis.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120 (4:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Campisi J, d′Adda di Fagagna. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8 (9:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130 (2:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Cui X, Shirai Y, Wakai T, Yokoyama N, Hirano S, Hatakeyama K. Aberrant expression of pRb and p16(INK4), alone or in combination, indicates poor outcome after resection in patients with colorectal carcinoma. Hum Pathol. 2004;35 (10:1189–1195. doi: 10.1016/j.humpath.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci US A. 1995;92 (20:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Gonzalez S, Risques RA, Marcuello E, Mangues R, Germa JR, Hertman JG, Capella G, Peinado MA. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19 (2:299–304. doi: 10.1200/JCO.2001.19.2.299. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340 (6:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313 (5795:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Galon J, Franck P, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O'Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D'Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10 (1:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quevedo R, Garcia-Aranda C, Moran A, De Juan C, Sánchez-Pernaute A, Torres A, Díaz-Rubio E, Balibrea JL, Benito M, Iniesta P. Differential impact of p16 inactivation by promoter methylation in non-small cell lung and colorectal cancer: clinical implications. Int J Oncol. 2004;24 (2:349–355. [PubMed] [Google Scholar]
- Jass JR. The pathological classification of colorectal cancer. Ann Acad Med Singapore. 1987;16 (3:469–473. [PubMed] [Google Scholar]
- Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JM. Observer variation in immunohistochemical analysis of protein expression, time for a change. Histopathology. 2006;48 (7:787–794. doi: 10.1111/j.1365-2559.2006.02412.x. [DOI] [PubMed] [Google Scholar]
- Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41 (17:2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114 (9:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, Mantovani A, Roncalli M, Malesci A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10 (9:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- Lyall MS, Dundas SR, Curran S, Murray GI. Profiling markers of prognosis in colorectal cancer. Clin Cancer Res. 2006;12 (4:1184–1191. doi: 10.1158/1078-0432.CCR-05-1864. [DOI] [PubMed] [Google Scholar]
- Michael-Robinson JM, Biemer-Huttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001a;48 (3:360–366. doi: 10.1136/gut.48.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Robinson JM, Reid LE, Purdie DM, Biemer-Hüttmann AE, Walsh MD, Pandeya N, Simms LA, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Proliferation, apoptosis, and survival in high-level microsatellite instability sporadic colorectal cancer. Clin Cancer Res. 2001b;7 (8:2347–2356. [PubMed] [Google Scholar]
- McGlynn LM, Stevenson K, Lamb K, Zino S, Brown M, Prina A, Kingsmore D, Shiels PG. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell. 2009;8 (1:45–51. doi: 10.1111/j.1474-9726.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- Mitomi H, Fukui N, Tanaka N, Kanazawa H, Saito T, Matsuoka T, Yao T. Aberrant p16(INK4a) methylation is a frequent event in colorectal cancers: prognostic value and relation to mRNA expression and immunoreactivity. J Cancer Res Clin Oncol. 2010;136 (2:323–331. doi: 10.1007/s00432-009-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29 (6:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- Mohammed ZM, McMillan DC, Elsberger B, Going JJ, Orange C, Mallon E, Doughty JC, Edwards J. Comparison of visual and automated assessment of Ki-67 proliferative activity and their impact on outcome in primary operable invasive ductal breast cancer. Br J Cancer. 2012;106 (2:383–388. doi: 10.1038/bjc.2011.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58 (16:3491–3494. [PubMed] [Google Scholar]
- Narita M, Lowe SW. Senescence comes of age. Nat Med. 2005;11 (9:920–922. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353 (25:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, Langner C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41 (12:1749–1757. doi: 10.1016/j.humpath.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG, Nikkhah G, Maciaczyk J, Kahlert U, Jain D, Bar E, Cohen KJ, Eberhart CG. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 2011;17 (11:3590–3599. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, McMillan DC. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. 2012a;106 (12:2010–2015. doi: 10.1038/bjc.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CH, Roxburgh CS, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012b;99 (2:287–294. doi: 10.1002/bjs.7755. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38 (5:451–466. doi: 10.1016/j.ctrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur J Cancer. 2009a;45 (12:2138–2145. doi: 10.1016/j.ejca.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009b;249 (5:788–793. doi: 10.1097/SLA.0b013e3181a3e738. [DOI] [PubMed] [Google Scholar]
- Sebastián C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151 (6:1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Nosho K, Baba Y, Cantor M, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S. Prognostic significance of CDKN2A (p16) promoter methylation and loss of expression in 902 colorectal cancers: Cohort study and literature review. Int J Cancer. 2011;128 (5:1080–1094. doi: 10.1002/ijc.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta. 2007;1775 (1:5–20. doi: 10.1016/j.bbcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137 (4:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey S, Dunne B, Witton CJ, Forsyth A, Cooke TG, Bartlett JM. Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer. Clin Cancer Res. 2005;11 (13:4835–4842. doi: 10.1158/1078-0432.CCR-05-0196. [DOI] [PubMed] [Google Scholar]
- Wassermann S, Scheel SK, Hiendlmeyer E, Palmqvist R, Horst D, Hlubek F, Haynl A, Kriegl L, Reu S, Merkel S, Brabletz T, Kirchner T, Jung A. p16INK4a is a beta-catenin target gene and indicates low survival in human colorectal tumors. Gastroenterology. 2009;136 (1:196–205 e2. doi: 10.1053/j.gastro.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Williams GT, Qurike P, Shepherd NA.2007Dataset for colorectal cancer (2nd edn) Royal College of PathologistsAvailable at www.rcpath.org (accessed 2 September 2013).
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445 (7128:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Hu YC, Talbot IC. Expressing patterns of p16 and CDK4 correlated to prognosis in colorectal carcinoma. World J Gastroenterol. 2003;9 (10:2202–2206. doi: 10.3748/wjg.v9.i10.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.