Abstract
Background:
The short-term survival following a cancer diagnosis in England is lower than that in comparable countries, with the difference in excess mortality primarily occurring in the months immediately after diagnosis. We assess the impact of emergency presentation (EP) on the excess mortality in England over the course of the year following diagnosis.
Methods:
All colorectal and cervical cancers presenting in England and all breast, lung, and prostate cancers in the East of England in 2006–2008 are included. The variation in the likelihood of EP with age, stage, sex, co-morbidity, and income deprivation is modelled. The excess mortality over 0–1, 1–3, 3–6, and 6–12 months after diagnosis and its dependence on these case-mix factors and presentation route is then examined.
Results:
More advanced stage and older age are predictive of EP, as to a lesser extent are co-morbidity, higher income deprivation, and female sex. In the first month after diagnosis, we observe case-mix-adjusted excess mortality rate ratios of 7.5 (cervical), 5.9 (colorectal), 11.7 (breast ), 4.0 (lung), and 20.8 (prostate) for EP compared with non-EP.
Conclusion:
Individuals who present as an emergency experience high short-term mortality in all cancer types examined compared with non-EPs. This is partly a case-mix effect but EP remains predictive of short-term mortality even when age, stage, and co-morbidity are accounted for.
Keywords: diagnosis, pathways, referral, survival, emergency, route
Improving cancer survival is a key challenge identified in Improving Outcomes: A Strategy for Cancer (Department of Health, 2011). Cancer survival estimates in England currently fall below those in many European countries across most cancer types (Berrino et al, 2007; Richards, 2007; Verdecchia et al, 2007). It has been estimated that if cancer survival in England was made comparable to the European average then 5000 or more deaths within 5 years of diagnosis could be avoided annually (Abdel-Rahman et al, 2009; Richards, 2009).
If analyses are restricted to include only those who survive at least 1 year from diagnosis, then the difference in 5-year survival between England and European countries is, in general, smaller (Thomson and Forman, 2009). Examination of excess mortality in the first year after diagnosis shows that, in particular, it is substantially greater in England than both Norway and Sweden in the first months after diagnosis for breast, colorectal, and lung cancer (Engholm et al, 2007; Holmberg et al, 2010; Møller et al, 2010). This would indicate that the long-term survival differences observed between England and other countries are at least partly a result of a higher number of deaths shortly after diagnosis. Understanding the mechanism that causes these excess deaths could assist in reducing their number, potentially improving long-term outcomes in England.
Recent work shows that the route a patient follows on the way to the diagnosis of their cancer is strongly predictive of 1-year survival (Elliss-Brookes et al, 2012), with those who present as an emergency faring poorly in the first year after diagnosis. In 11 of 15 cancer types examined, emergency presentations (EP) have a 1-year relative survival, which is >25% lower than those presenting via other routes. Elliss-Brookes et al (2012) shows EPs to be more likely in older patients. One can hypothesise that the lower survival is because of confounding by age and other cofactors generally detrimental to overall health.
Some literature exists on levels of EP internationally, primarily for colorectal cancer. Levels of colorectal EP reported vary: Greece 12% Australia 16% Sweden 17% Canada 24% Norway 25% (Mitchell et al, 2007; Pavlidis et al, 2008; Wong et al, 2008; Sjo et al, 2009; Gunnarsson et al, 2011). These studies tend to be based at single centres, comparatively small (<2000 patients), and based on data stretching back to the 1990s or 1980s. Case finding in secondary care may also overstate the levels of EP compared with population-based studies. Levels of EP in England, at 26% (Elliss-Brookes et al, 2012), appear comparable to or higher than internationally, at least for colorectal cancer. Emergency presentation is an informative measure with regard to death in the first year after diagnosis, and exploration of how EPs occur may help illuminate the difference in 1-year survival between England and comparable countries.
Stage at diagnosis is known to be highly predictive of cancer mortality and later stage at diagnosis is a possible explanation for the difference in cancer survival between England and Europe (Sant et al, 2003; Foot and Harrison, 2011). The completeness of staging data available varies by cancer type and by geographical region of registration. Consequently, this study is confined to cancer types where stage completeness is high enough to be analytically useful and case numbers are high enough for meaningful statistical power. For England as a whole these are colorectal and cervical cancers, and for the East of England (the former Eastern Cancer Registration and Information Centre, ECRIC, registration area) these are breast, lung, and prostate cancers. These cancers have a wide span in the proportion of observed EPs ranging from 5% for breast cancers to 39% for lung cancers; this allows the impact of EP across a range of very different cancer types to be examined.
This study explores the impact of emergency vs non-EP routes with two keys aims: to assess the sociodemographic and clinical variables associated with EP; and to test the hypothesis that these cofactors explain the association between EP and short-term mortality.
Materials and methods
All newly diagnosed invasive tumours excluding non-melanoma skin cancer (ICD-10 C00-C97 excluding C44) diagnosed between 2006 and 2008 in residents of England were extracted from the National Cancer Data Repository (NCDR), with available information on stage at diagnosis (National Cancer Intelligence Network, 2011). The NCDR contains all cases of cancer known to the English cancer registries, with a case ascertainment of 98–99% (Møller et al, 2011). Dates of death in the NCDR are derived by record level linkage with ONS mortality records and include all deaths, not just those in hospital. The NCDR data set was de-duplicated using European Network of Cancer Registries criteria (Parkin et al, 1994), removing 7% of cases. The ‘Route to Diagnosis' for each case was categorised from routinely available cancer data using a detailed algorithm explained in Elliss-Brookes et al (2012). The eight possible routes derived there were aggregated here to two categories—EPs and non-EPs. In on 131 754 cases were diagnosed with the cancer types and within the geographical regions of interest. Of these, 2161 cases (1.6%) were excluded on the basis that they were a death certificate only registration; were aged over 99 years at diagnosis; were reported as stage 0 for breast cancer; or had a misordered date of birth, date of diagnosis, and date of death.
The completeness of cancer staging within the NCDR varies strongly with cancer type. For colorectal cancer (ICD-10 codes C18-C20), 68% of cases in England diagnosed in 2006–2008 had a valid Dukes stage recorded. For cervical cancer (ICD-10 C53), 74% of cases in England in the same period had a valid FIGO stage recorded. Cases contributed to the NCDR by the ECRIC had an overall completeness of integrated TNM stage group of 93% (breast cancer: ICD-10 C50), 78% (lung cancer: ICD-10 C33–34), and 95% (prostate cancer: ICD-10 C61).
The Charlson co-morbidity index was created from diagnostic codes (ignoring cancer codes) recorded in the inpatient Hospital Episode Statistics (HES) data set for each patient in the period from 30 months before diagnosis to 3 months before diagnosis. Diagnostic codes relating to independent secondary cancers were sourced from the NCDR over the same period. A Charlson index was derived from these diagnostic codes using a lookup table (Quan et al, 2005). For purposes of generating a Charlson index, the HES records available were limited to those persons with a diagnostic code of cancer recorded at any point in any of their HES records (i.e., all records before diagnosis are available if at least one record contains a cancer code).
The income deprivation quintile was derived by linking each tumour to the Index of Multiple Deprivation 2007 (Communities and Local Government, 2007) using postcode at the time of diagnosis. Equal population quintiles were derived from the income domain score.
Multiple imputation (Nur et al, 2010) was used to generate missing values for stage and co-morbidity. The ice (Royston, 2005) and mim (Carlin et al, 2008) programs were used to perform imputation in Stata 10.1 (StataCorp, 2007). Imputation was performed on stage and co-morbidity with sex, income deprivation quintile, type of presentation, cancer registry, a binary variable indicating observed metastatic spread or not, age, and survival time included in the imputation model, with no interaction terms. Ten imputed data sets were created.
Sensitivity analysis of the impact of multiple imputation was performed by repeating regression analyses without imputation, with missing cases excluded (a ‘completer analysis'), and by assuming that missing values of stage and co-morbidity took the most extreme plausible values.
Factors predictive of EP were explored through binary logistic regression with the fact of EP as the dependent variable and the demographic and clinical factors as categorical independent variables.
Excess mortality was modelled using the strs program (Dickman et al, 2004) in Stata 10.1. Regression analysis used a generalised linear model with a Poisson error structure on collapsed data (Dickman et al, 2004), and persons aged <25 were excluded. Proportional hazards are not assumed because of different variation with time of different cofactors, instead a separate analysis is undertaken for each time period with ordinal independent variables. This ordinal assumption constrains the model to a geometrically increasing series of rate ratios with each increase in one of the independent variables. Sensitivity analyses were performed as for the first regression model.
Results
Over the 3-year period from 2006 to 2008, 131 754 newly diagnosed malignant cancers of interest were diagnosed in England or the ECRIC area. Table 1 shows the number of tumours included and excluded and the proportion of EPs broken down by age, co-morbidity, sex, income deprivation, and the stage at diagnosis for colorectal and cervical tumours at a national level and for breast, lung, and prostate tumours in the ECRIC registration area. Overall, the fraction of EPs increases with age, although for colorectal cancer in particular there is a non-monotonic relationship with a higher fraction presenting as an emergency in those aged 0–24 than those aged 25–64 (albeit with a small cohort size in the younger age group). The proportion of EPs increased with increasing co-morbidity, was higher in women compared with men, and increased with increasing income deprivation.
Table 1. Numbers of cancers and proportions diagnosed via EP for persons resident in England (cervical and colorectal) and the ECRIC registration area (breast, lung, and prostate) diagnosed in 2006–2008.
|
England |
ECRIC registration area |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Cervical |
Colorectal |
|
Breast |
Lung |
Prostate |
||||||
| Characteristic | n | %EPs | n | %EPs | n | %EPs | n | %EPs | n | %EPs | ||
|
Number | ||||||||||||
| n | 6950 | 12% | 89 484 | 26% | 12 354 | 4% | 9601 | 36% | 11 204 | 7% | ||
| Excluded |
50 |
— |
|
1932 |
— |
|
79 |
— |
71 |
— |
29 |
— |
|
Age | ||||||||||||
| 0–24 | 151 | 11% | 159 | 58% | 9 | 0% | 3 | 0% | 0 | — | ||
| 25–64 | 5264 | 8% | 24 283 | 20% | 6797 | 1% | 2370 | 29% | 2553 | 3% | ||
| 65–84 | 1251 | 22% | 54 026 | 24% | 4545 | 5% | 6087 | 36% | 7677 | 6% | ||
| 85+ |
284 |
41% |
|
11 016 |
43% |
|
1003 |
16% |
1141 |
54% |
974 |
26% |
|
Co-morbidity | ||||||||||||
| Not known | 982 | 5% | 5723 | 26% | 1744 | 4% | 1035 | 31% | 3088 | 4% | ||
| 0 | 5569 | 13% | 71 274 | 24% | 9822 | 4% | 6829 | 36% | 6894 | 7% | ||
| 1 | 243 | 21% | 6708 | 34% | 513 | 7% | 979 | 41% | 680 | 11% | ||
| 2 | 103 | 26% | 3777 | 31% | 190 | 7% | 453 | 41% | 364 | 14% | ||
| 3+ |
53 |
36% |
|
2002 |
40% |
|
85 |
9% |
305 |
43% |
178 |
13% |
|
Sex | ||||||||||||
| Male | — | — | 49 515 | 23% | — | — | 5602 | 35% | 11 204 | 7% | ||
| Female |
6950 |
12% |
|
39 969 |
29% |
|
12 354 |
4% |
3999 |
38% |
— |
— |
|
Income deprivation | ||||||||||||
| Least deprived | 1089 | 8% | 18 349 | 22% | 2962 | 3% | 1579 | 33% | 2725 | 5% | ||
| 2 | 1243 | 11% | 20 087 | 24% | 3334 | 4% | 2170 | 34% | 3081 | 6% | ||
| 3 | 1338 | 13% | 19 398 | 25% | 3058 | 4% | 2583 | 36% | 2933 | 8% | ||
| 5 | 1489 | 12% | 17 459 | 27% | 2229 | 5% | 2251 | 39% | 1859 | 8% | ||
| Most deprived |
1791 |
15% |
|
14 191 |
31% |
|
771 |
5% |
1018 |
40% |
606 |
10% |
|
Stage | ||||||||||||
| Not known | 1823 | 19% | Not Known | 28 449 | 30% | Not Known | 922 | 11% | 2154 | 41% | 527 | 18% |
| FIGO 1 | 3324 | 3% | A (Dukes) | 8718 | 8% | TNM 1 | 4754 | 1% | 1115 | 21% | 51 | 2% |
| FIGO 2 | 906 | 14% | B (Dukes) | 21 652 | 22% | TNM 2 | 5113 | 2% | 462 | 19% | 7794 | 3% |
| FIGO 3 | 490 | 24% | C (Dukes) | 21 498 | 26% | TNM 3 | 1013 | 8% | 2491 | 31% | 1270 | 4% |
| FIGO 4 |
407 |
41% |
D (Dukes) |
9167 |
36% |
TNM 4 |
552 |
29% |
3379 |
44% |
1562 |
23% |
|
Imputed stage | ||||||||||||
| FIGO 1 | 4359 | 4% | A (Dukes) | 12 651 | 11% | TNM 1 | 5118 | 1% | 1564 | 25% | 54 | 3% |
| FIGO 2 | 1278 | 16% | B (Dukes) | 31 569 | 23% | TNM 2 | 5554 | 3% | 686 | 26% | 8244 | 4% |
| FIGO 3 | 730 | 29% | C (Dukes) | 33 283 | 29% | TNM 3 | 1130 | 11% | 3969 | 36% | 1343 | 5% |
| FIGO 4 | 584 | 45% | D (Dukes) | 11 981 | 37% | TNM 4 | 552 | 29% | 3382 | 44% | 1562 | 23% |
Abbreviations: ECRIC=Eastern Cancer Registration and Information Centre; EP=emergency presentations; TNM=tumour node metastasis stage group; FIGO = International Federation of Gynecology and Obstetrics stage.
Across all tumour types, the proportion of EP increased with increasing stage. In early-stage breast, prostate, and cervical cancer, this proportion was <5%, whereas Dukes A colorectal cancers had 11% EP and TNM stage group 1 lung cancers had 25% EP. For cases without a recorded stage, the proportion of EP was broadly similar to those in cases with a later stage at presentation.
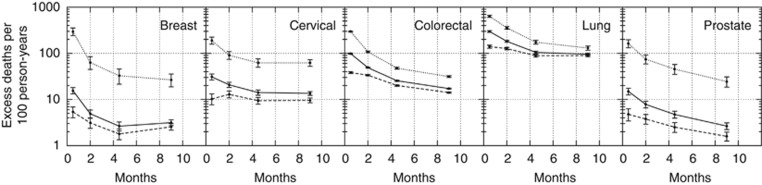
Figure 1 shows excess mortality in five cancer types in the year following diagnosis, for EP and non-EP separately and combined, in persons diagnosed between 2006 and 2008. The excess mortality is substantially higher in EPs, varying between a rate of 162 (prostate cancer); 190 (cervical cancer); 295 (breast cancer); 297 (colorectal cancer); and 635 (lung cancer) deaths per 100 person-years exposure time in the month immediately following diagnosis. In non-EPs, these rates became 5 (prostate and breast cancer); 10 (cervical cancer); 38 (colorectal cancer), and 140 (lung cancer) deaths per 100 person-years. Excess mortality remains elevated for persons presenting by EP in later periods examined and is of comparable magnitude in breast, colorectal, and prostate cancer. Unadjusted excess mortality rate ratios between EP and non-EP cases in the first month after diagnosis are 4.5 (lung cancer), 7.8 (colorectal cancer), 18.5 (cervical cancer), 34.2 (prostate cancer), and 55.1 (breast cancer).
Figure 1.
Unadjusted excess mortality rates for emergency (dotted), non-emergency (dashed), and all presentation (solid) routes in the periods 0–1, 1–3, 3–6, and 6–12 months after diagnosis, plotted semilogarithmically at the central point of those periods with 95% confidence intervals. Figures represent all persons diagnosed with selected cancer types in England (cervical and colorectal cancer) and the ECRIC registration area (breast, lung, and prostate cancer), 2006–2008. Stage and co-morbidity include imputed values.
Table 2 shows odds ratios for EP for persons diagnosed between 2006 and 2008. Emergency presentations are more common for younger ages and older ages; more common in women (the lung cancer result is statistically significant at a 95% level); more common in more income-deprived persons; and more common in later-stage cancers. These effects are broadly consistent across all cancer types, with odds ratios for increasing age and stage having the largest magnitudes.
Table 2. Odd ratios for emergency presentation and 95% CIs from a multivariate logistic model.
|
England |
ECRIC area |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Cervical |
|
Colorectal |
|
Breast |
Lung |
Prostate |
|||||
| Characteristic | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
|
Sex | ||||||||||||
| Male | — | 1.0 | — | 1.0 | — | |||||||
| Female |
— |
— |
|
1.3 |
(1.2–1.3) |
|
— |
— |
1.1 |
(1.0–1.2) |
— |
— |
|
Stage | ||||||||||||
| FIGO 1 | 1.0 | Dukes A | 1.0 | TNM 1 | 1.0 | 1.0 | 0.6 | (0.1–5.4) | ||||
| FIGO 2 | 3.7 | (2.8–4.9) | Dukes B | 2.3 | (2.2–2.5) | TNM 2 | 1.1 | (0.2–4.6) | 1.1 | (0.8–1.5) | 1.0 | |
| FIGO 3 | 7.4 | (5.3–10.3) | Dukes C | 3.3 | (3.1–3.6) | TNM 3 | 3.7 | (0.9–16.0) | 1.8 | (1.5–2.1) | 1.3 | (1.0–1.8) |
| FIGO 4 |
14.7 |
(11.1–19.6) |
Dukes D |
4.8 |
(4.5–5.2) |
TNM 4 |
13.9 |
(3.2–59.7) |
2.7 |
(2.4–3.2) |
5.9 |
(5.0–7.0) |
|
Age | ||||||||||||
| 0–24 | 1.5 | (0.9–2.7) | 5.5 | (4.0–7.6) | — | — | — | |||||
| 25–64 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||||
| 65–84 | 1.5 | (1.2–1.8) | 1.3 | (1.2–1.3) | 3.5 | (2.7–4.6) | 1.4 | (1.2–1.5) | 1.5 | (1.2–1.9) | ||
| 85+ |
2.9 |
(2.1–3.9) |
|
2.8 |
(2.6–2.9) |
|
11.1 |
(8.3–14.8) |
3.1 |
(2.7–3.6) |
7.0 |
(5.4–9.2) |
|
Co-morbidity | ||||||||||||
| Charlson 0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||||
| Charlson 1 | 1.1 | (0.8–1.6) | 1.5 | (1.4–1.6) | 1.3 | (0.8–2.0) | 1.2 | (1.0–1.4) | 1.2 | (0.9–1.5) | ||
| Charlson 2 | 1.5 | (0.9–2.5) | 1.4 | (1.3–1.5) | 1.4 | (0.7–2.8) | 1.3 | (1.1–1.6) | 1.8 | (1.2–2.7) | ||
| Charlson 3+ |
1.6 |
(0.9–3.1) |
|
2.0 |
(1.8–2.2) |
|
1.3 |
(0.6–2.8) |
1.4 |
(1.1–1.8) |
1.5 |
(0.9–2.5) |
|
Income deprivation | ||||||||||||
| Least deprived | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||||
| 2 | 1.3 | (1.0–1.8) | 1.1 | (1.0–1.1) | 0.9 | (0.7–1.3) | 1.0 | (0.9–1.2) | 1.0 | (0.8–1.3) | ||
| 3 | 1.4 | (1.1–1.9) | 1.2 | (1.1–1.2) | 1.0 | (0.8–1.4) | 1.2 | (1.0–1.3) | 1.3 | (1.0–1.7) | ||
| 4 | 1.3 | (1.0–1.8) | 1.3 | (1.2–1.3) | 1.2 | (0.9–1.6) | 1.3 | (1.1–1.5) | 1.4 | (1.1–1.7) | ||
| Most Deprived | 1.6 | (1.2–2.2) | 1.6 | (1.5–1.7) | 1.2 | (0.8–1.9) | 1.4 | (1.2–1.6) | 1.7 | (1.2–2.4) | ||
Abbreviations: CI=confidence interval; ECRIC=Eastern Cancer Registration and Information Centre; OR=odds ratio.
Persons resident in England (cervical and colorectal) or the ECRIC registration area (breast, lung, and prostate) diagnosed in 2006–2008. Stage and co-morbidity include imputed values.
Comparison of odds ratios for EP with and without the use of stage imputation shows either statistically insignificant differences or differences that, although statistically significant, do not greatly change the magnitude of the odds ratios presented (complete case analysis is included in Supplementary Information). Pairwise examination of all interaction terms shows some that are statistically significant. However, the change in the odds of EP is small compared with the magnitude of the odds in all but one case. The exception is for patients aged <25, for which substantial interactions were found.
Table 3 shows excess mortality rate ratios modelled for persons diagnosed between 2006 and 2008. There is a broad consistency across the cancer types examined. The excess mortality rate ratio for EP (compared with non-EP) and increasing age is highest for periods immediately after diagnosis; for increasing stage, the excess mortality rate ratio is highest for later periods. The excess mortality rate ratios for increasing co-morbidity and increasing income deprivation are comparatively small in magnitude compared with those of stage and age, and those for sex (for lung and colorectal cancer) are close to unity.
Table 3. Excess mortality rate ratios and 95% confidence intervals at 0–1, 1–3, 3–6, and 6–12 months after diagnosis from a multivariate model.
|
England |
ECRIC registration area |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Cervical |
Colorectal |
Breast |
Lung |
Prostate |
|||||
| Period/characteristic | EMRR | 95% CI | EMRR | 95% CI | EMRR | 95% CI | EMRR | 95% CI | EMRR | 95% CI |
|
0–1 month | ||||||||||
| Presentation route | 7.5 | (4.9–11.1) | 5.9 | (5.6–6.2) | 11.7 | (7.8–17.4) | 4.0 | (3.7–4.4) | 20.8 | (11.7–36.3) |
| Age | 2.0 | (1.6–2.5) | 2.1 | (2.0–2.2) | 1.9 | (1.5–2.4) | 1.6 | (1.4–1.7) | 3.0 | (2.1–4.2) |
| Co-morbidity | 1.3 | (1.0–1.6) | 1.2 | (1.1–1.2) | 1.4 | (1.0–1.8) | 1.1 | (1.0–1.1) | 1.4 | (1.1–1.8) |
| Sex | — | — | 1.1 | (1.0–1.1) | — | — | 0.9 | (0.8–0.9) | — | — |
| Income deprivation | 0.9 | (0.8–1.0) | 1.1 | (1.0–1.1) | 1.0 | (0.9–1.2) | 1.0 | (1.0–1.1) | 1.1 | (0.9–1.3) |
| Stage |
1.7 |
(1.4–2.1) |
1.5 |
(1.4–1.5) |
3.0 |
(2.4–3.7) |
1.2 |
(1.2–1.3) |
1.0 |
(0.8–1.3) |
|
1–3 months | ||||||||||
| Presentation route | 2.3 | (1.7–3.1) | 2.4 | (2.3–2.6) | 3.2 | (1.9–5.3) | 2.4 | (2.2–2.6) | 5.2 | (3.4–8.0) |
| Age | 2.1 | (1.7–2.6) | 1.9 | (1.8–2.0) | 2.3 | (1.6–3.2) | 1.4 | (1.3–1.5) | 2.7 | (1.9–3.9) |
| Co-morbidity | 1.2 | (1.0–1.4) | 1.2 | (1.1–1.2) | 1.1 | (0.5–1.9) | 1.1 | (1.0–1.2) | 1.3 | (1.0–1.6) |
| Sex | — | — | 1.0 | (1.0–1.1) | — | — | 1.0 | (0.9–1.1) | — | — |
| Income deprivation | 1.1 | (1.0–1.2) | 1.1 | (1.0–1.1) | 1.0 | (0.8–1.2) | 1.0 | (1.0–1.0) | 1.1 | (0.9–1.3) |
| Stage |
2.2 |
(1.8–2.5) |
2.2 |
(2.1–2.2) |
5.2 |
(3.6–7.5) |
1.6 |
(1.5–1.7) |
2.9 |
(2.2–4.0) |
|
3–6 months | ||||||||||
| Presentation route | 2.3 | (1.7–3.1) | 1.8 | (1.7–1.9) | 2.3 | (1.3–4.0) | 1.7 | (1.5–2.0) | 6.6 | (4.0–10.8) |
| Age | 2.2 | (1.8–2.7) | 1.8 | (1.7–1.9) | 2.0 | (1.4–2.9) | 1.4 | (1.3–1.5) | 1.9 | (1.2–2.8) |
| Co-morbidity | 1.2 | (0.9–1.5) | 1.3 | (1.2–1.3) | 1.8 | (1.3–2.4) | 1.1 | (1.0–1.2) | 1.9 | (1.6–2.3) |
| Sex | — | — | 1.1 | (1.0–1.2) | — | — | 0.9 | (0.8–1.0) | — | — |
| Income deprivation | 1.0 | (0.9–1.1) | 1.1 | (1.0–1.1) | 1.3 | (1.0–1.5) | 1.0 | (1.0–1.1) | 0.9 | (0.8–1.1) |
| Stage |
2.3 |
(1.9–2.7) |
2.6 |
(2.4–2.7) |
5.1 |
(3.7–7.0) |
1.8 |
(1.6–1.9) |
2.2 |
(1.7–3.0) |
|
6–12 months | ||||||||||
| Presentation route | 2.7 | (2.2–3.4) | 1.7 | (1.6–1.8) | 2.1 | (1.4–3.3) | 1.3 | (1.2–1.5) | 2.5 | (1.6–4.0) |
| Age | 1.6 | (1.3–1.9) | 1.5 | (1.4–1.6) | 1.9 | (1.5–2.4) | 1.3 | (1.2–1.5) | 1.0 | (0.7–1.5) |
| Co-morbidity | 1.2 | (1.0–1.4) | 1.3 | (1.2–1.3) | 1.7 | (1.3–2.1) | 1.0 | (0.9–1.1) | 1.4 | (1.1–1.8) |
| Sex | — | — | 1.1 | (1.0–1.2) | — | — | 0.9 | (0.9–1.0) | — | — |
| Income deprivation | 1.1 | (1.0–1.2) | 1.0 | (1.0–1.1) | 1.0 | (0.9–1.2) | 1.0 | (1.0–1.0) | 1.0 | (0.9–1.2) |
| Stage | 2.2 | (1.9–2.4) | 3.1 | (2.9–3.2) | 4.1 | (3.4–4.9) | 1.7 | (1.6–1.9) | 7.5 | (3.3–17.0) |
Abbreviations: CI=confidence interval; ECRIC=Eastern Cancer Registration and Information Centre; EMRR=excess mortality rate ratio.
Rate ratios show the increase in excess mortality in the period per step increase in presentation (non-emergency or emergency), age (25–64, 65–84, or 85+), co-morbidity (Charlson 0, 1, 2, 3+), sex (male or female), income deprivation (quintiles from least to most income deprivation), or stage (increasing FIGO, Dukes, or TNM-integrated stage on a four-point scale). The model included persons resident in England (cervical and colorectal cancer) or the ECRIC registration area (breast, lung, and prostate cancer) diagnosed in 2006–2008. Stage and co-morbidity include imputed values.
The dependence of excess mortality rate ratios on EP for the month after diagnosis are highest for prostate and breast cancer, and lower for lung and colorectal cancer. Prostate cancer shows the highest dependence on age and breast cancer on stage in this same month.
Regression coefficients recalculated after excluding stage 1–3 prostate cancer, stage 1–2 breast cancer, and stage 1 cervical cancer were not statistically significantly different from those presented in Table 3, although the rate ratio of the excess mortality for prostate cancer in the first month after diagnosis was reduced to 11.8 (95% confidence interval: 5.2–26.9). Pairwise examination of all interaction terms shows some that are statistically significant. However, the change in the excess mortality rate ratio because of EP is small compared with its magnitude in all cases. The model for cervical cancer was mildly improved by including an interaction term between stage and age. Comparison of excess mortality rate ratios with and without the use of stage imputation shows either statistically insignificant differences or differences that, although statistically significant, do not greatly change the magnitude of the rate ratios presented (complete case analysis is included in Supplementary Information).
Discussion
This study presents a two-step analysis of EP of cancer, as categorised from routinely available health service data. We show EP to be highly predictive of cancer mortality in the year following diagnosis, and especially in the month following diagnosis. That EPs were more common with older age and more advanced stage was expected: we demonstrate a case-mix effect in the frequency of EP because of stage and age at presentation across all cancer types examined with a lesser effect owing to co-morbidity, deprivation, and female sex (where relevant). Excess mortality was strongly associated with EP, independently of case-mix factors, disproving the hypothesis that it is because of these factors alone.
Strengths and weaknesses
An advantage of using routine data is the population basis of the patient cohort, thereby representing all cancers diagnosed (and without demanding additional intrusive patient contact). The ECRIC registration area has a population of around 5.5 million (similar to Scotland, Denmark, and Norway) and has overall cancer survival comparable to that of England (Quaresma et al, 2012). The fraction of EPs in the ECRIC area for breast, lung, and prostate cancer is similar to that for England as a whole (Elliss-Brookes et al, 2012). Cancer staging within the ECRIC registration area is to a consistent standard and of high completeness. Together, these observations support an argument that the results for the ECRIC area, especially once adjusted for case-mix, are robust and applicable to the broader English population.
The independent variables selected (age, sex, and income deprivation) cover major demographic causes for variation in mortality, as well as clinical causes attributed to the cancer itself (stage) and pre-existing health (co-morbidity). Performance status was not available as an independent variable, but one could anticipate that age and co-morbidity may act as a partial proxy factors for it.
Age, sex, and income deprivation are recorded with high completeness. One can criticise the assignment of the income deprivation score on the grounds of the ecological fallacy, as the lower super output areas used to assign a quintile have an average population of around 1600 persons. This may have the effect of reducing the observed impact of income deprivation compared to the actual impact. Stage and co-morbidity data are to some extent incomplete, as shown in Table 1. Reasons for missing stage data might be treatment outside NHS secondary care, death before staging was complete, or a lack of data transfer to cancer registries of key data (e.g. nodal status) needed to compute a stage. Co-morbidity data are missing because of the absence of an available HES record in the period before diagnosis. The variables in the imputation model can be expected to be sensitive to these different mechanisms and therefore make the missing at random assumption plausible.
There are several assumptions built into the assignment of a co-morbidity score. As scores are drawn from previous inpatient admissions they will not count anyone with a significant condition that is treated exclusively in outpatient or primary care, and they also only count those with an inpatient admission in the designated period. It is therefore plausible then that the influence of co-morbidity is under-represented in this study to a degree, although the higher the degree of decompensation from co-morbidity (in the years immediately before diagnosis) the more likely that this fact will be captured.
Overall, as far as can be determined, the results are methodologically robust. Sensitivity analyses show that the relationships between the likelihood of EP and the independent variables examined, and between EP and short-term mortality are not greatly affected by the imputation performed. The one substantial interaction term found in the model for the likelihood of EP – for persons aged <25 – is itself a plausible exception because of the very different referral pathways for these patients. Modelling the excess mortality rate ratios using ordinal independent variables gives good agreement with a similar, but categorical, model for colorectal cancer (Downing et al, 2013).
Variation in mortality
The overall (unadjusted) excess mortality observed in Figure 1 is consistent with other studies in magnitude and in variation over time following diagnosis for breast, colorectal, and lung cancer (Engholm et al, 2007; Holmberg et al, 2010; Møller et al, 2010). All show higher excess mortality in the first months after diagnosis, which quickly falls off towards the end of the first year after diagnosis. However, the variation in excess mortality between EPs and non-EPs in the period immediately after diagnosis (0–1 month for breast cancer and 0–3 months for lung and colorectal cancer) is greater than that between the youngest and oldest age groups in prior studies. Patients presenting as emergencies with breast, cervical, colorectal, or prostate cancer experience higher mortality in the first month after diagnosis than those presenting with lung cancer as non-emergencies.
The impact of EP on short-term mortality observed here is consistent with that in other studies. A wide literature exploring this association exists for colorectal cancer, (e.g. Faiz et al, 2010; Downing et al, 2013), but few studies exist for other cancer types. It has been shown that mortality within 30 days of a diagnosis of breast (and colorectal) cancer is associated with an emergency admission within 30 days before or after diagnosis (Brewster et al, 2011). Moreover, a case finding study of cancer patients across all tumour types in Northern Ireland who died in hospital (Blaney and Gavin, 2011) showed that 79% of deaths followed emergency admissions (this included admissions with previously known cancers as well as EP via an emergency inpatient admission).
Multivariate modelling of the excess mortality rate ratio to take into account selected case-mix factors showed that, as for the unadjusted case, the impact of EP was most marked in the first month after the diagnosis (Table 3). We saw a range in adjusted excess mortality rate ratios between 4.0 (lung) and 20.8 (prostate cancer). The impact of EP fell with time, although EPs still carried a higher risk of mortality at later periods, even when other factors were accounted for. In contrast, the impact of stage on excess mortality either stayed broadly the same within the different time intervals (cervical cancer) or increased with the time since diagnosis (breast, colorectal, lung, and prostate cancer). This difference in the dependence of excess mortality rate ratios on time implies that the causes of mortality following EP are more complex than a simple dependence on tumour stage.
For lung and colorectal cancer, case-mix had a comparatively small effect on the excess mortality rate ratio for EP compared with non-EP in the first month after diagnosis. For breast, cervical, and prostate cancer, the adjusted mortality rate ratio in the first month after diagnosis is reduced compared with the unadjusted figure, but still substantial and comparable to or greater in magnitude than the effect of stage or age. In contrast, the low dependence of the excess mortality rate ratios on sex and income deprivation suggests that patient demographics are of more importance in determining the route of presentation than the subsequent mortality within that route.
The fraction of excess mortality in the first month because of EP alone can be estimated: if the multivariate model is adjusted such that the excess mortality rate ratio for EP is 1.0, while holding other rate ratios constant, we then predict that the excess mortality in the first month falls to between 30 and 50% of the observed figure for all five cancer types. This simple approach overestimates the impact of EP as it would not account for the deaths which would then be expected to take place in the additional patient exposure time introduced, but it suggests that the contribution of EP to mortality in the first month following diagnosis is of the order of a half. A better estimate of the impact of EP requires a better understanding of their nature.
The nature of EP
Emergency presentation can be viewed as an extreme expression of the waiting time paradox (Crawford et al, 2002; Tørring et al, 2011) where the outcomes are poor but the ‘waiting time' is very short. The hypotheses to explain these poor outcomes that go beyond the influence of the independent variables already considered are of great interest. These can be broadly summarised as:
First, residual confounding by independent variables not considered (such as performance status) or imperfectly defined (co-morbidity or income deprivation) is a possibility. However, it is difficult to explain the magnitude of the excess mortality rate ratios for EP in the first month after diagnosis via this mechanism.
Second, mortality is because of other co-morbidities with a sudden onset, that is, the cancers are being diagnosed incidentally as an emergency. However, it was estimated that only 1 in 19 to 1 in 15 of calculated presentation routes are because of incidental admissions (Elliss-Brookes et al, 2012), only some of which will be emergencies. The (low) proportion of EP in early-stage breast and prostate cancer could be explained by this mechanism but sensitivity analysis, which excludes early-stage tumours from the analysis shows little impact on the observed dependence of excess mortality on EP, implying that this is not a major overall influence.
Third, EPs occur preferentially with advanced complications (Hamilton, 2012), or EP tumours are preferentially faster growing or otherwise associated with higher mortality (e.g., because of physical location in an organ). The nature of the tumour, beyond its stage, plausibly affects both route of presentation and the short-term outcome. For example, colorectal tumours that cause an obstruction or perforation of the bowel are likely to be EPs with poor outcomes. Further confounding is also possible: colorectal tumours presenting higher in the colon are likely to be more frequently EPs (because of less specific symptoms) and to have worse outcomes (because of the comparative inaccessibility).
Fourth, excess mortality is because of presentation at night or on weekends or to non-specialist centres where clinical services are less available or less capable. An emergency inpatient admission (which occurs during the majority of EPs) caused by cancer can occur for disparate reasons including metabolic emergencies, cardiovascular emergencies, infectious emergencies, and respiratory emergencies (Lewis et al, 2011). They are challenging to manage with the potential for rapidly deleterious effects (Samphao et al, 2009; Royal College of Physicians and Royal College of Radiologists, 2012). In these circumstances, excess mortality could be due to either the absence of (or delay to) specialist care or due to iatrogenic causes.
The widespread impact of EP on 1-year cancer survival (Elliss-Brookes et al, 2012), the lack of sensitivity to case-mix adjustment in lung and colorectal cancer, and the uniformly high (unadjusted) excess mortality rates in EPs suggest that a common mechanism, or mechanisms, may be at work for all cancers. Overall, this study is consistent with a mixture primarily of the third and fourth hypotheses.
Improving outcomes
The mortality associated with EP might be reduced by reducing the number of EPs – an aim closely aligned with early presentation in general. How achievable this is depends on the frequency of symptoms before those that directly lead to the presentation; in some cases EP might be inevitable, perhaps where no prior symptoms are felt before a critical event. Overall, the progression of symptoms experienced leading up to a diagnosis of cancer is not well understood. In some cases, EP can be the most clinically appropriate diagnostic route, such as for some acute haematological cancers. The frequency of EP in lung cancer can be reduced through public awareness campaigns (Calister, 2012), potentially reducing the associated mortality. It has been shown that emergency admissions with rapid mortality are more likely in those not living with a partner (Blaney and Gavin, 2011) and the likelihood of EP has been associated with several characteristics of the patient's general practice (Bottle et al, 2012). This suggests that interventions by primary and social care may be effective in reducing the number of EPs. In primary care, these interventions might be, for example, significant event audits targeting EPs and subsequent review of referral practice, perhaps similar to recent recommendations for the management of existing cases of cancer to prevent emergency admissions (Royal College of Physicians and Royal College of Radiologists, 2012).
Mortality could also be reduced by seeking to reduce the impact of EP, the extent to which this can be achieved depends on the balance between the share of the excess mortality that is due to the nature of the tumour and the extent because of care delivered in emergency settings. The latter could be affected by detailed examination of causes of death in these cases and subsequent change of practice in secondary care.
In summary, EP is a strongly predictive leading indicator of short-term mortality following cancer diagnosis. Although EPs are more frequent in older persons with advanced cancers and extensive co-morbidities, these factors do not fully explain the impact of EP on excess mortality. In particular, EP remains highly predictive of very short (<1 month) mortality. Further work to test hypotheses for this association could potentially lead to significant reductions in mortality immediately following diagnosis, in which England fares poorly in international comparisons. This might take the form of linkage to primary care data to investigate patient's interaction with a GP before an EP. The extension of the Routes to Diagnosis algorithm to international data sets may also illuminate causal factors that lead to EP.
Acknowledgments
We thank David Greenberg for his input and acknowledge those persons who supported the original ‘Routes to Diagnosis' project.
The authors declare no conflicts of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Abdel-Rahman M, Stockton D, Rachet B, Hakulinen T, Coleman MP. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable. Br J Cancer. 2009;101 (Suppl 2:S115–S124. doi: 10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrino F, De Angelis R, Sant M, Rosso S, Lasota MB, Coebergh JWW, Santaquilani M, EUROCARE Working Group Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995 –1999: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- Blaney J, Gavin A. Why cancer patients die in acute hospitals: a retrospective study by note review. N Ireland Cancer Registry. 2011.
- Bottle A, Tsang C, Parsons C, Majeed A, Soljak M, Aylin P. Association between patient and general practice characteristics and unplanned first-time admissions for cancer: observational study. Br J Cancer. 2012;107:1213–1219. doi: 10.1038/bjc.2012.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster DH, Clark DI, Stockton DL, Munro AJ, Steele RJC. Characteristics of patients dying with 30 days of diagnosis of breast or colorectal cancer in Scotland, 2003-2007. Br J Cancer. 2011;104:60–67. doi: 10.1038/sj.bjc.6606036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calister M.2012. Increasing early detection of symptomatic lung cancer—results of a combined marketing communications campaign and primary care education programme in Leeds, UK. 8th NCRI Cancer Conference, 4–7 November 2012; Leeds, UK.
- Carlin JB, Galati JC, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. The Stata J. 2008;8:49–67. [Google Scholar]
- Communities and Local Government Index of Multiple Deprivation 2007: measure of multiple deprivation at small area level made up of seven domains. Communities and Local Government. 2007.
- Crawford SC, Davis JA, Siddiqui NA, Caestecker L, Gillis CR, Hole D. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ. 2002;325:196. doi: 10.1136/bmj.325.7357.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health 2011Improving Outcomes: A Strategy for Cancer. Department of Health: London, England.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- Downing A, Aravani A, Macleod U, Oliver S, Finan PJ, Thomas JD, Quirke P, Wilkinson JR, Morris EJ. Early mortality from colorectal cancer in England: a retrospective observational study of the factors associated with death in the first year after diagnosis. Br J Cancer. 2013;108:681–685. doi: 10.1038/bjc.2012.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, Richards M. Routes to Diagnosis for cancer—determining the patient journey using multiple routine datasets. Br J Cancer. 2012;107:1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engholm G, Kejs AM, Brewster DH, Gaard M, Holmberg L, Hartley R, Iddenden R, Møller H, Sankila R, Thomson CS, Storm HH. Colorectal cancer survival in the Nordic countries and the United Kingdom: excess mortality risk analysis of 5 year relative period survival in the period 1999 to 2000. Int J Cancer. 2007;121:1115–1122. doi: 10.1002/ijc.22737. [DOI] [PubMed] [Google Scholar]
- Faiz O, Warusavitarne J, Bottle A, Tekkis PP, Clark SK, Darzi AW, Aylin P. Nonelective Excisional Colorectal Surgery in English National Health Service Trusts: a study of outcomes from hospital episode statistics data between 1996 and 2007. J Am Coll Surg. 2010;210:390–401. doi: 10.1016/j.jamcollsurg.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Foot C, Harrison T. How to improve cancer survival: Explaining England's relatively poor rates. The King's Fund. 2011.
- Gunnarsson H, Holm T, Ekholm A, Olsson LI. Emergency presentation of colon cancer is most frequent during summer. Colorectal Dis. 2011;13:663–668. doi: 10.1111/j.1463-1318.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- Hamilton W. Emergency admissions of cancer as a marker of diagnostic delay. Br J Cancer. 2012;107:1205–1206. doi: 10.1038/bjc.2012.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg L, Sandin F, Bray F, Richards M, Spicer J, Lambe M, Klint A, Peake M, Strand TE, Linklater K, Robinson D, Møller H. National comparisons of lung cancer survival in England, Norway and Sweden 2001-2004: differences occur early in follow-up. Thorax. 2010;65:436–441. doi: 10.1136/thx.2009.124222. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Hendrickson AW, Moynihan TJ. Oncologic emergencies: Pathophysiology, presentation, diagnosis, and treatment. CA Cancer J Clin. 2011;61:287–314. doi: 10.3322/caac.20124. [DOI] [PubMed] [Google Scholar]
- Mitchell AD, Inglis KM, Murdoch JM, Porter GA. Emergency room presentation of colorectal cancer: a consecutive cohort study. Ann Surg Oncol. 2007;14:1099–1104. doi: 10.1245/s10434-006-9245-z. [DOI] [PubMed] [Google Scholar]
- Møller H, Richards S, Hanchett N, Riaz SP, Lüchtenborg M, Holmberg L, Robinson D. Completeness of case ascertainment and survival time error in English cancer registries: impact on 1-year survival estimates. Br J Cancer. 2011;105 (1:170–176. doi: 10.1038/bjc.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller H, Sandin F, Bray F, Klint Å, Linklater KM, Purushotham A, Robinson D, Holmberg L. Breast cancer survival in England, Norway and Sweden a population-based comparison. Int J Cancer. 2010;127:2630–2638. doi: 10.1002/ijc.25264. [DOI] [PubMed] [Google Scholar]
- National Cancer Intelligence Network 2011. National Cancer Data Repository http://www.ncin.org.uk/collecting_and_using_data/national_cancer_data_repository/default.aspx . Accessed April 2013.
- Nur U, Shack LG, Rachet B, Carpenter JR, Coleman MP. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiol. 2010;39:118–128. doi: 10.1093/ije/dyp309. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Chen VW, Ferlay J, Galceran J, Storm HH, Whelan SL.1994Comparability And Quality Control In Cancer Registration IARC Technical Publication; 19:Lyon, France. [Google Scholar]
- Pavlidis TE, Marakis G, Ballas K, Rafailidis S, Psarras K, Pissas D, Sakantamis AK. Does emergency surgery affect resectability of colorectal cancer. Acta Chir Belg. 2008;108:219–225. doi: 10.1080/00015458.2008.11680207. [DOI] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining co-morbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Quaresma M, Whitehead S, Coleman MP, Rachet B. Combined cancer survival by primary care trusts, patients diagnosed 1996-2010, followed up to 2011. ONS. 2012.
- Richards M. EUROCARE-4 studies bring new data on cancer survival. Lancet Oncol. 2007;8:752–753. doi: 10.1016/S1470-2045(07)70247-4. [DOI] [PubMed] [Google Scholar]
- Richards M. The size of the prize for earlier diagnosis of cancer in England. Brit J Cancer. 2009;101:S125–S129. doi: 10.1038/sj.bjc.6605402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Physicians and Royal College of Radiologists . Cancer patients in crisis: responding to urgent needs. Report of a working party. RCP: London, UK; 2012. [Google Scholar]
- Royston P. Multiple imputation of missing values: Update of ice. Stata J. 2005;5:527–536. [Google Scholar]
- Samphao S, Eremin JM, Eremin O. Oncological emergencies: clinical importance and principles of management. Eur J Cancer Care. 2009;19:707–713. doi: 10.1111/j.1365-2354.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- Sant M, Allemani C, Capocaccia R, Hakulinen T, Aareleid T, Coebergh JW, Coleman MP, Grosclaude P, Martinez C, Bell J, Youngson J, Berrino F, EUROCARE Working Group Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int J Cancer. 2003;106:416–422. doi: 10.1002/ijc.11226. [DOI] [PubMed] [Google Scholar]
- Sjo OH, Larsen S, Lunde OC, Nesbakken A. Short term outcome after emergency and elective surgery for colon cancer. Colorectal Dis. 2009;11:733–739. doi: 10.1111/j.1463-1318.2008.01613.x. [DOI] [PubMed] [Google Scholar]
- StataCorp 2007. Stata Statistical Software: Release 10. College Station, Texas, USA.
- Tørring ML, Frydenberg M, Hansen RP, Olesen F, Hamilton W, Vedsted P. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer. 2011;104:934–940. doi: 10.1038/bjc.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C, Forman D. Cancer survival in England and the influence of early diagnosis: what can we learn from recent EUROCARE results. Br J Cancer. 2009;101 (Suppl 2:S102–S109. doi: 10.1038/sj.bjc.6605399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I, EUROCARE-4 Working Group Recent cancer survival in Europe: a period analysis 2000–2002 of the EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- Wong SK, Jalaludin BB, Morgan MJ, Berthelsen AS, Morgan A, Gatenby AH, Fulham SB. Tumor pathology and long-term survival in emergency colorectal cancer. Dis Colon Rectum. 2008;51:223–230. doi: 10.1007/s10350-007-9094-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.