Abstract
α-Galactosidase (EC 3.2.1.22) activity was observed in cell-free extracts of Lactobacillus fermenti, L. brevis, L. buchneri, L. cellobiosis, and L. salivarius subsp. salivarius. The cultural conditions under which the enzyme activity was detected suggest that the enzyme is constitutive and present in the soluble fraction in the cell. The enzyme preparations readily hydrolyzed melibiose and other oligosaccharides containing α(1 → 6) linked galactose. Although the cell-free extracts of L. fermenti and L. brevis are negative for β-fructofuranosidase (EC 3.2.1.26), they hydrolyzed melibiose, stachyose, and raffinose in decreasing order of activity. The β-fructofuranosidase-positive L. buchneri, L. cellobiosis, and L. salivarius preparations hydrolyzed melibiose, raffinose, and stachyose in decreasing rates of activity. The α-galactosidases from different lactobacilli showed optimum activity in pH range 5.2 to 5.9. L. fermenti and L. salivarius preparations exhibited maximum activity between 40 to 44 C and 48 to 51 C, respectively, whereas a 38 to 42 C range was observed for other lactobacilli. Cell-free extract of L. cellobiosis was studied for transgalactosylase activity. When incubated with melibiose, a new compound was detected and tentatively identified as manninotriose.
Full text
PDF
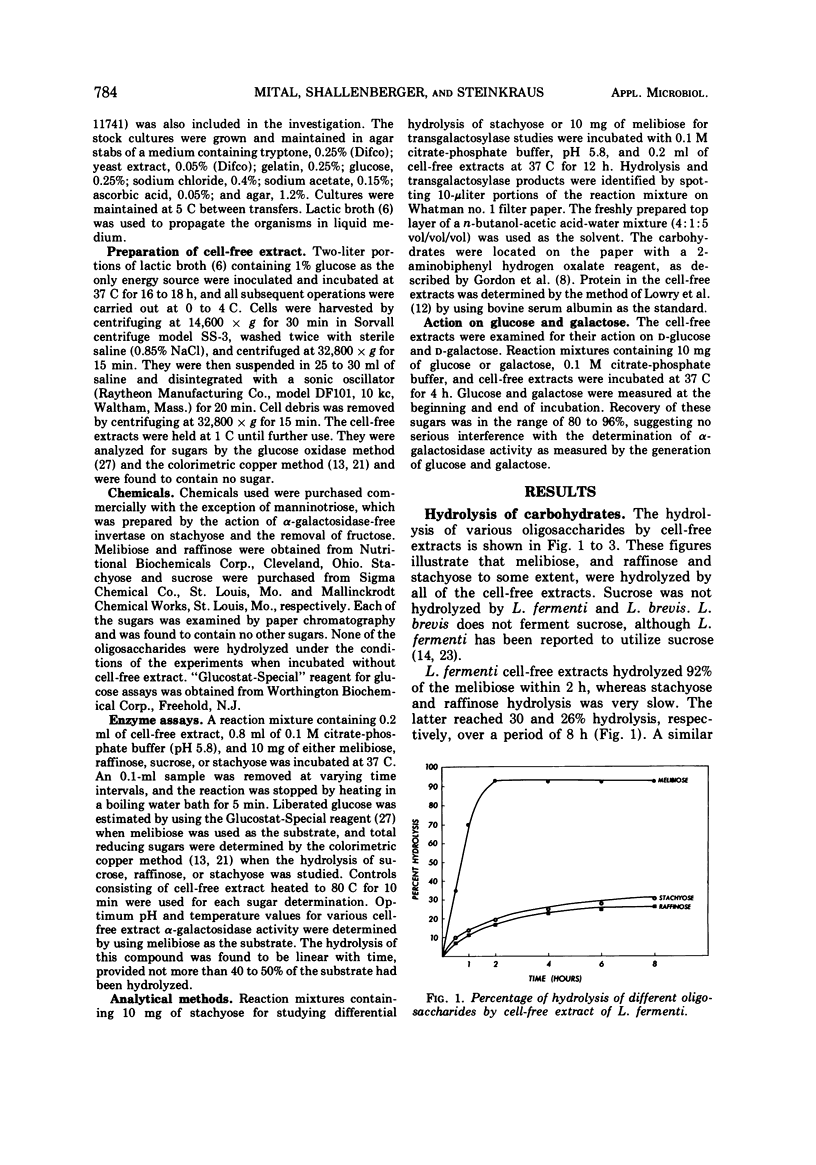
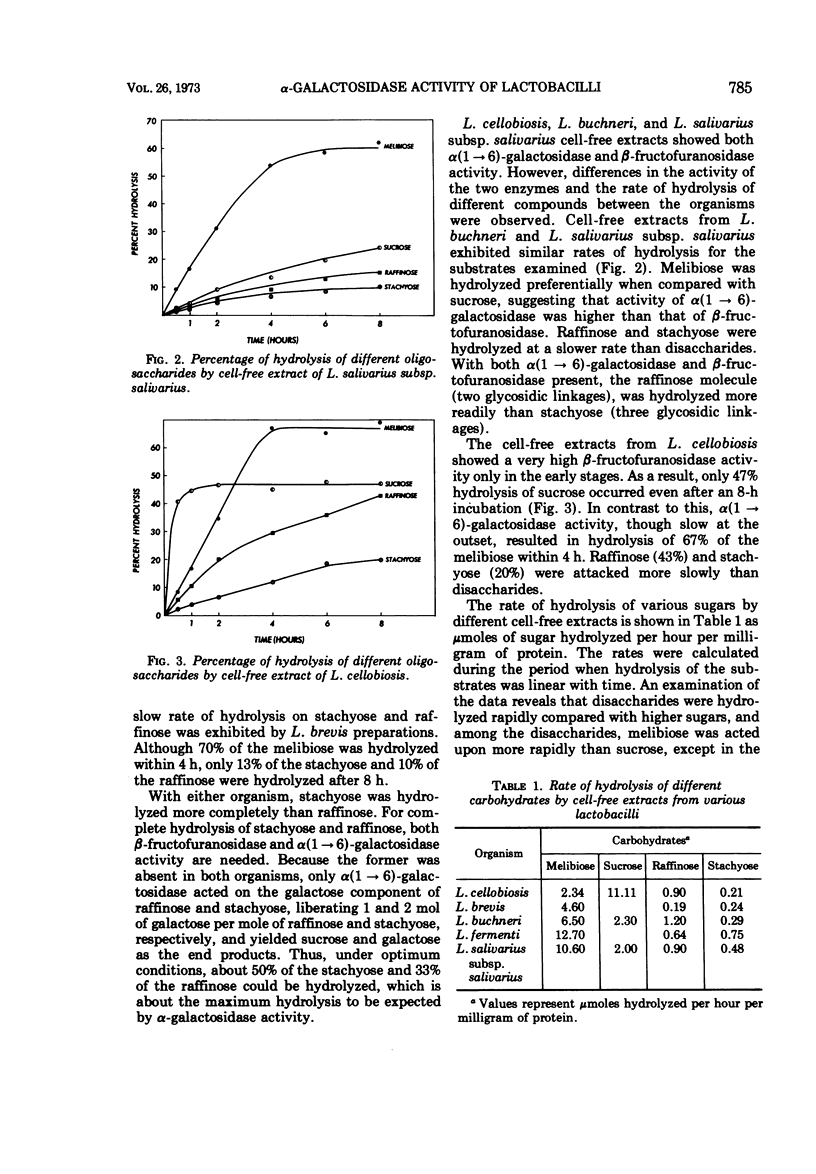
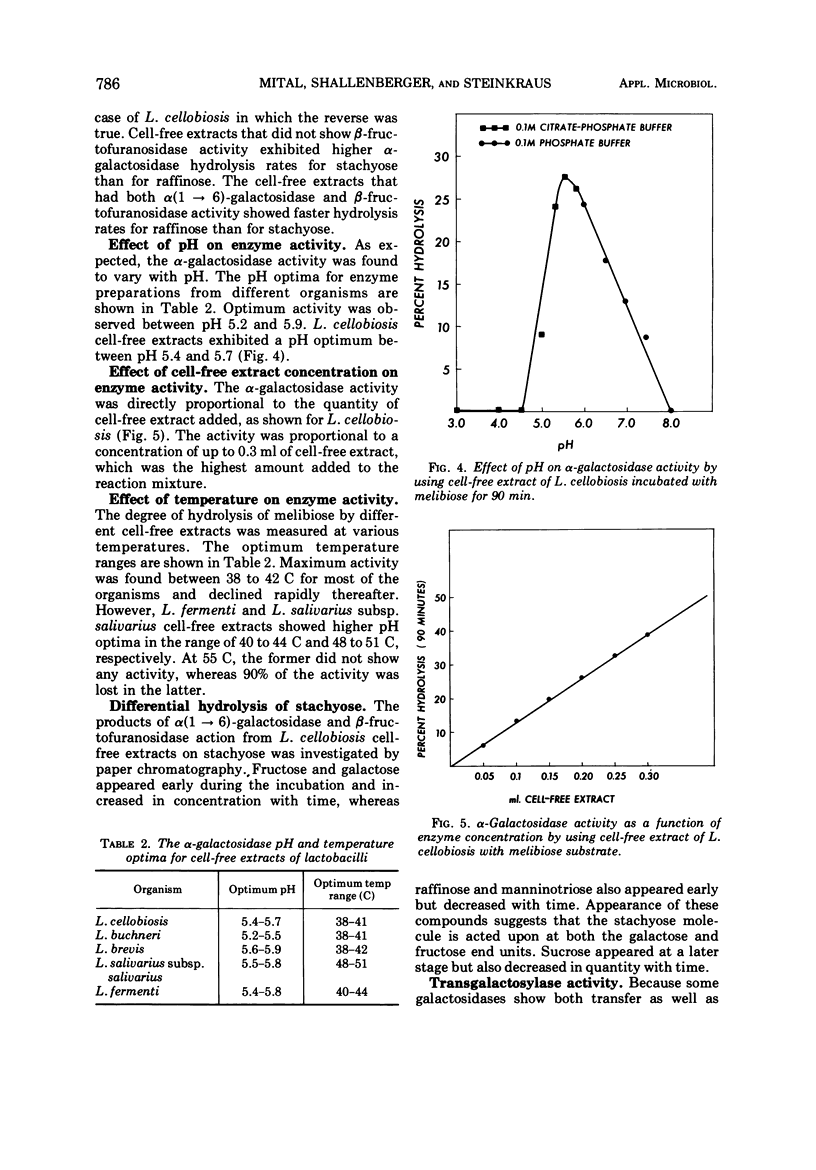


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W. The intracellular alpha-galactodidase of a rumen strain of Streptococcus bovis. Biochem J. 1963 Mar;86:509–514. doi: 10.1042/bj0860509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCHARD P. H., ALBON N. The inversion of sucrose; a complication. Arch Biochem. 1950 Nov;29(1):220–222. [PubMed] [Google Scholar]
- Burstein C., Kepes A. The alpha-galactosidase from Escherichia coli K12. Biochim Biophys Acta. 1971 Jan 26;230(1):52–63. doi: 10.1016/0304-4165(71)90053-5. [DOI] [PubMed] [Google Scholar]
- Dey P. M., Pridham J. B. Biochemistry of -galactosidases. Adv Enzymol Relat Areas Mol Biol. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- FRENCH D. The raffinose family of oligosaccharides. Adv Carbohydr Chem. 1954;9:149–184. doi: 10.1016/s0096-5332(08)60375-6. [DOI] [PubMed] [Google Scholar]
- LI Y. T., LI S. C., SHETLAR M. R. ALPHA-GALACTOSIDASE FROM DIPLOCOCCUS PNEUMONIAE. Arch Biochem Biophys. 1963 Dec;103:436–442. doi: 10.1016/0003-9861(63)90434-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SEBALD M., GASSER F., WERNER H. TENEUR GC PERCENTAGE ET CLASSIFICATION. APPLICATION AU GROUPE DES BIFIDOBACT'ERIES ET 'A QUELQUES GENRES VOISINS. Ann Inst Pasteur (Paris) 1965 Aug;109:251–269. [PubMed] [Google Scholar]
- SHEININ R., McQUILLEN K. Effect of penicillin on induced enzyme formation in normal cells and spherical forms of Escherichia coli. Biochim Biophys Acta. 1959 Jan;31(1):72–74. doi: 10.1016/0006-3002(59)90440-8. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Schmitt R., Rotman B. Alpha-galactosidase activity in cell-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1966 Mar 8;22(5):473–479. doi: 10.1016/0006-291x(66)90297-x. [DOI] [PubMed] [Google Scholar]
- Steggerda F. R., Richards E. A., Rackis J. J. Effects of various soybean products on flatulence in the adult man. Proc Soc Exp Biol Med. 1966 Apr;121(4):1235–1239. doi: 10.3181/00379727-121-31014. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., MALHOTRA O. P. Galactosidases. Adv Carbohydr Chem. 1961;16:239–298. doi: 10.1016/s0096-5332(08)60264-7. [DOI] [PubMed] [Google Scholar]
- WATKINS W. M., MORGAN W. T. Inhibition by simple sugars of enzymes which decompose the blood-group substances. Nature. 1955 Apr 16;175(4459):676–677. doi: 10.1038/175676a0. [DOI] [PubMed] [Google Scholar]



