Abstract
Objectives: Photodynamic therapy (PDT) was performed on 14 cases of tracheobronchial malignancies with variable tumor stages as an adjunct to surgery. We retrospectively reviewed these PDT cases to evaluate safety and oncologic outcome.
Methods: From June 2004 to August 2010, PDT was performed in 14 cases for lung cancer. Medical records were reviewed, including demographic data, indication of the PDT, and oncologic outcomes, during the follow-up period.
Results: There were 5 deaths, 1 loss of follow-up, and 5 patients that are still alive. There were 8 cases of complete response (CR), 4 cases of no response (NR), and 2 cases of partial response (PR). Among the recurrent tracheoendobronchial lesions in 5 patients, CR was accomplished in 4 patients and NR was observed in 1 patient. The mean survival of all patients was 23.9 months after PDT with a range of 6 – 66 months.
Conclusion: PDT demonstrates a safe and effective role as an adjunct to surgery in treating recurrent mucosal carcinoma in surgically high-risk patients. It is also effective for palliation of bronchial obstruction by cancer mass.
Introduction
Photodynamic therapy (PDT) is already in use for non-small cell lung cancer (NSCLC) and researchers are exploring the possibility of combining PDT with other forms of treatment. For example, PDT could be combined with surgery wherein surgery is used to remove cancer from the lungs and PTD therapy is used to prevent recurrence in sites such as the pleura or the lining of the lung. Currently, PDT is being explored experimentally as an option for mesothelioma patients 1–3) and researchers hope that PDT will be useful in the future as a treatment against large, solid tumors or peripheral lung cancers.4)
In clinical practice, the treatment of choice in many lung cancers is combined surgery and either concurrent chemotherapy or radiotherapy rather than PDT5–7) and therefore either recurrence or cancers newly diagnosed after surgery are frequently encountered. When these patients have an inoperable condition or the lesion is small and at an early stage, PDT often becomes the preferable treatment option.
Although PDT has been approved for reducing obstruction and palliating symptoms in patients with completely or partially obstructing endobronchial NSCLCs, radiotherapy is often preferably practiced and therefore palliative PDT has been mostly applied to recurrent or non-responding obstructive lung cancer after radiotherapy. It may be necessary to prepare PDT dosimetry separately for lesions that other treatments have failed. Understanding differences based on cytohistology would be required to design a new treatment paradigm other than conventional dosimetry. Therefore, it is desirable to assess the PDT response for lung cancer patients that received treatments such as surgery or radiotherapy.
Patients and Method
Patient Data
A retrospective review was performed of 14 cases (11 patients) of PDT performed for lung cancer in Seoul National University Bundang Hospital from June 2004 to August 2010. Ages ranged from 55 to 86 years with a mean of 68.9 years (median 69 years). All patients were male and 5 were diagnosed with synchronous multiple lung cancer and 4 had recurrent lung cancer. All lesions were histologically squamous cell carcinoma. Four patients were current smokers and 7 patients were ex-smokers. Three patients visited the hospital with blood-tinged sputum, 3 with dyspnea, 2 with hemoptysis, 2 with a cough, and 1 patient came to our hospital after an incidental finding of abnormal chest x-ray. Bronchoscopy, chest computed tomography (CT) scan, bone scintigraphy, and positron emission tomography (PET) scan were performed to decide the clinical stage. Because AFB has recently been made available at our hospital, the fibrobronchoscope was used for the detection and follow-up of lung cancers in all 12 cases. Diagnosis was made through pathological confirmation in all cases. PDT was performed with curative intention in 9 cases (6 patients) and palliative intention in 5 cases (Tables 1 and 2). Intra-cavitary PDT was carried out in 1 patient and the others received bronchoscopic PDT. The tumor locations in the patients are depicted in Fig. 1.
Table 1. Clinical Information for Patients That Received Photodynamic Therapy.
| Case Number | Sex/ Age | Chief Complain | Diagnosis, Stage | Initial Treatment | Concurrent Treatment | Combined Disease | FEV1/FVC |
|---|---|---|---|---|---|---|---|
| 1 | 73/M | Cough | Synchronous double primary lung cancer RUL, RLL. cT1N0M0, pT1N1M0 |
RULobectomy | Chemotherapy | HTN, Tbc, COPD | 1.47/2.70 (54.4%) |
| 2 | 86/M | BTS | Left main bronchus cT3N2Mx | None | Chemotherapy, RT | 1.02/1.81 (57%) | |
| 3 | 55/M | Dyspnea | Synchronous double primary lung cancer Lt main bronchus, cT2N2M0, pT1N0M0 RLL.cT1N0M0 (diagnosed after surgery) |
Lt. pneumonectomy | Chemotherapy | 2.81/4.25 (66.1%) | |
| 4 | 69/M | Chest X-ray Abnormality |
LUL lingular segment lung cancer cT2N0M0 (diagnosed during lung cancer workup) |
PCI | None 3 vessels disease | COPD | 2.05/3.55 (58%) |
| 5 | 77/M | Dyspnea | Double primary lung cancer RLL, cT2N2M0, pT3N0M0 Lt. main bronchus, cT3N0M0 (diagnosed |
RLLobectomy after surgery) | RT | HTN, DM | 1.80/2.51 (71.7%) |
| 6 | 74/M | BTS | Left lower bronchus lung cancer | PDT | None (Refused by patient) |
HTN, PAO | 2.55/3.72 (68%) |
| 7∗ | 65/M | Hemoptysis | Synchronous multiple primary lung cancer RUL, pT2N1M0 RLL, poorly differentiated NSCLC LLL basal segment, cTisN0M0 |
RULobectomy PDT | None | HTN, Tbc | 2.30/3.33 (88%) |
| 8 | 57/M | BTS | RLL lung cancer, cT3N1-2M0, pT3N1M0 recurrent lung cancer, LUL (2) and LLL (1) | RML, RLL bilobectomy Intracavitary PDT |
Chemotherapy Chemotherapy |
HTN, DM | 2.61/3.35 (77.9%) |
| 9 | 68/M | Cough | Left distal main bronchus (endobronchial) 2ndary carina cancer cT3N2M0 Recurrent lung cancer, Lt. bronchus intermedius, LUL cT3N2M0 |
PDT RT |
None None |
HTN, DM Prostate ca. |
2.11/3.89 (54.2%) 1.74 |
| 10 | 74/M | Hemoptysis | Synchronous double primary cancer RLL lung cancer cT2N0M0, pT2N0M RUL lung cancer cTisN0M0 |
Bilobectomy (RML, RLL) PDT |
None | HTN | 1.99/4.65 (43%) |
| 11∗ | 66/M | Recurrent endobronchial cancer RLL superior segment |
PDT | ||||
| 12 | 68/M | Dyspnea | Synchronous double primary lung cancer RML lung cancer cT2N0M0, pT2N1M0 LLL lung cancer pT1N1M0, cT2N0M0 Recurrent lung cancer orifice of anterior segment, LUL (small endobronchial lesion) |
Staged operation RMLobectomy, PA angioplasty LLLobectomy PDT |
None None |
HTN Esophageal cancer Laryngeal cancer |
2.29/4.33 (52.9%) |
| 13∗ | 66/M | Recurrent endobronchial cancer LLL anterior basal segment |
PDT | ||||
| 14∗ | 66/M | Recurrent endobronchial cancer LLL anterior basal segment |
PDT |
HTN: Hypertension, Tbc: Tuberculosis, COPD: Chronic obstructive pulmonary disease, BTS: Blood-tinged sputum, RT: Radiation therapy, PCI: Percutaneous coronary intervention, PAOD: Peripheral arteriosclerotic obstructive disease, NSCLC: Non-small cell lung cancer Same patient
Table 2. Characteristics of Patients That Received Photodynamic Therapy.
| Case Number | Sex/Age | Smoking History | Tumor Location | PDT indication | Purpose of PDT |
|---|---|---|---|---|---|
| 1 | 73/M 40PY | Current smoker basal segment | RLL | Poor pulmonary function | Curative |
| 2 | 86/M | Ex-smoker 65PY | Left main bronchus Left 2ndary carina |
Palliation therapy (patient want) | Palliative |
| 3 | 55/M | Ex-smoker 40PY | RLL superior segment posterior subsegment | Early endobronchial lesions | Curative |
| 4 | 69/M | Current smoker 40PY | LUL lingular segment Superior & inferior subsegment | Percutaneous coronary intervention, 4 weeks ago | Curative |
| 5 | 77/M | Ex-smoker 50PY | Left main bronchus | Poor pulmonary function | Curative |
| 6 | 74/M | Ex-smoker 20PY | Left lower bronchus | Early endobronchial lesion | Curative |
| 7∗ | 65/M | Current smoker 75PY | RLL superior segment LLL anterior basal segment | Endobronchial Carcinoma in situ lesion | Curative |
| 8 | 57/M | Current smoker 30PY | Left upper (x2) & lower (x1) lobe parenchyma | Poor pulmonary function Previous RML, RLL bilobectomy | Palliative |
| 9 | 68/M | Ex-smoker 50PY | Left distal main bronchus, 2ndary carina | Poor pulmonary function | Curative |
| 10 | 74/M | Ex-smoker 50PY | spur between apical and posterior segment of RUL | Carcinoma in situ lesion | Curative |
| 11∗ | 66/M | RLL superior segment | Recurrent, early endobronchial | Curative | |
| 12 | 68/M | Ex-smoker 3PY | Orifice of anterior segment, LUL (ask transfer) | Early endobronchial lesion | Curative |
| 13∗ | 66/M | LLL anterior basal segment | Recurrent carcinoma in situ | Curative | |
| 14∗ | 66/M | LLL anterior basal segment | Recurrent carcinoma in situ | Curative |
PY: pack-year, SqCC: Squamous cell carcinoma
Same patient
Figure 1:
Locations of tumors where photodynamic therapy was performed.
In case 1, the patient was diagnosed with synchronous double primary lung cancer at the RUL and the RLL. A mass obstructed the RUL but the RLL was an endobronchial lesion. Because a poor postoperative FEV1 (0.8) was predicted, he received gemcitabine- and cisplatin-based neoadjuvant chemotherapy. RULobectomy and PDT for the RLL lesion were performed. The patient exhibited a partial response (PR) after PDT and a second attempt at PDT was suggested, but palliative chemotherapy was used instead because of the patient's condition. He died of pneumonia.
In case 2, the patient presented with blood-tinged sputum and was diagnosed with lung cancer at the left main bronchus with clinical stage T3N2Mx. The patient received PDT 2 times because he requested PDT instead of chemotherapy but the cancerous lesion progressed and the patient died.
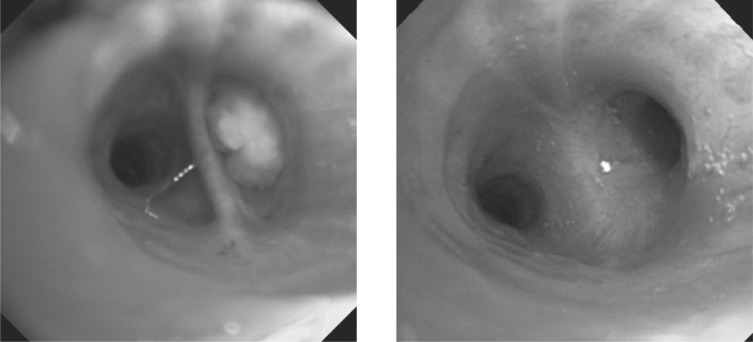
In case 3, after neoadjuvant chemotherapy and left pneumonectomy for main bronchus lung cancer, a new cancer lesion was found at the RLL superior segment. The patient received PDT and adjuvant chemotherapy and he is still alive and healthy without evidence of recurrence (Fig. 2).
Figure 2:
Bronchoscopic findings in case 3. The RLL superior segment lesion (left) was not observed after PDT (right).
In case 4, an abnormal chest x-ray led to the diagnosis of LUL lingular segment lung cancer, clinical stage T2N0M0. Because significant 3-vessel coronary artery disease was found during workup, percutaneous coronary intervention (PCI) was performed in addition to PDT for the lung cancer lesion. Tumor recurrence was not found at the PDT site but cancer cells were detected through bronchial washing cytology during the follow-up period. The patient refused further treatment and the cancer progressed to both lungs, resulting in death.
In case 5, clinical T2N2M0 RLL lung cancer was diagnosed because of a persistent cough and sputum. RLLobectomy was performed and the reported pathological stage was T3N0M0. Endobronchial cancer was found at the left main bronchus after surgery. PDT was performed and resulted in a PR but tumor re-growth was observed 1 month after PDT and the patient died of pneumonia.
In case 6, a left second carina endobronchial lesion was found because of blood-tinged sputum. This patient did not respond to PDT and the RUL small nodule size increased. The patient refused other workup and treatment including surgery and he died 6 months later, after complaining of chest pain.
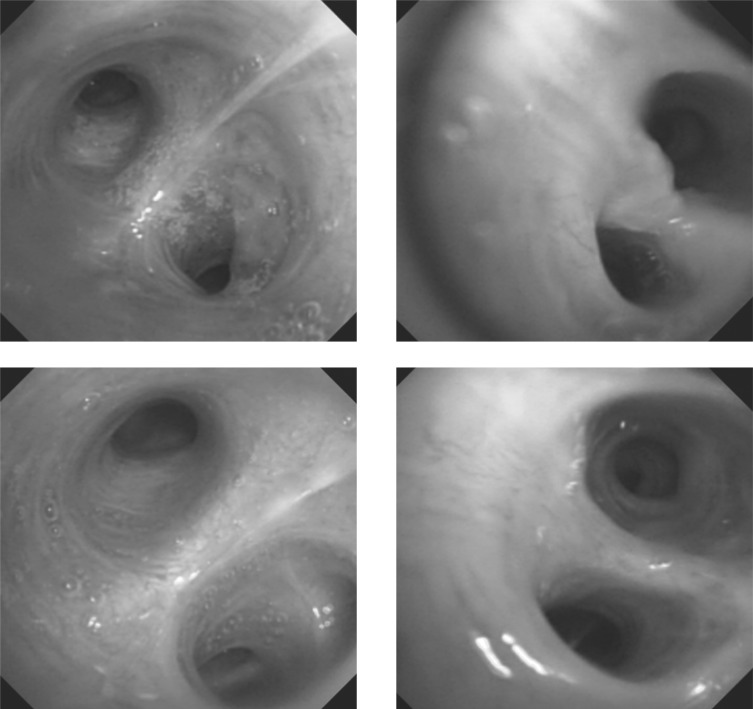
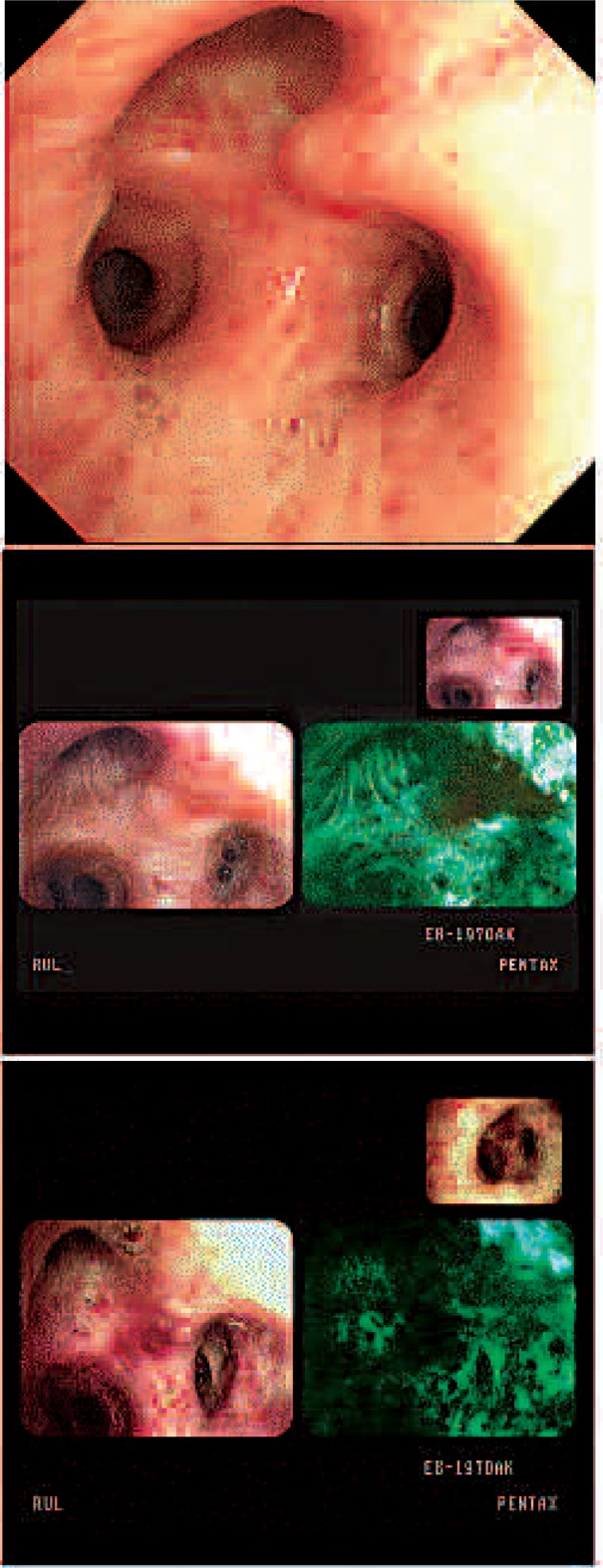
One patient received PDT 4 times and he is listed as cases 7, 11, 13 and 14. Synchronous multiple lung cancer in the RUL anterior segment squamous cell carcinoma, RLL superior segment (RB6) poorly differentiated endobronchial NSCLC, and an LLL basal segment (LB8) squamous cell carcinoma in situ lesion was diagnosed. RULobectomy was performed at another hospital with pathological stage T2N1M0 and the patient was referred to our hospital for PDT. PDT for RB6 and LB8 were performed with Photofrin® and the cancer lesions disappeared (Fig. 3-1). A recurrent lesion was confirmed through AFB and biopsy at the previous PDT
Figure 3-1:
Lesions before and after PDT was performed for the first time in case 7. The left column is RB6 and the right column is LB8 before (upper) and after (bottom) PDT.
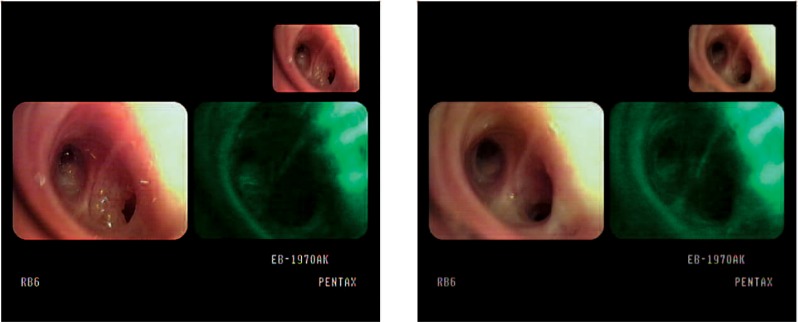
site of RB6 1 year later, PDT was performed a second time with Radachlorin®, and the lesion disappeared (Fig. 3-2). Five months later, recurrence at LB 6 was diagnosed and the patient was treated with PDT with Radachlorin® a third time. Three months later, AFB examination revealed no response (NR) a third time at the PDT site and PDT was performed a fourth time with Photofrin® and there has been no evidence of tumor in this lesion at the time of writing this manuscript (Fig. 3-3).
Figure 3-2:
AFB findings of RB6 before (left) and after (right) the second application of PDT in case 11.
Figure 3-3:
AFB findings of a LB8 lesion before (left upper) and after (right upper) the third application of PDT. Before (left bottom) and after (right bottom) the fourth application of PDT.
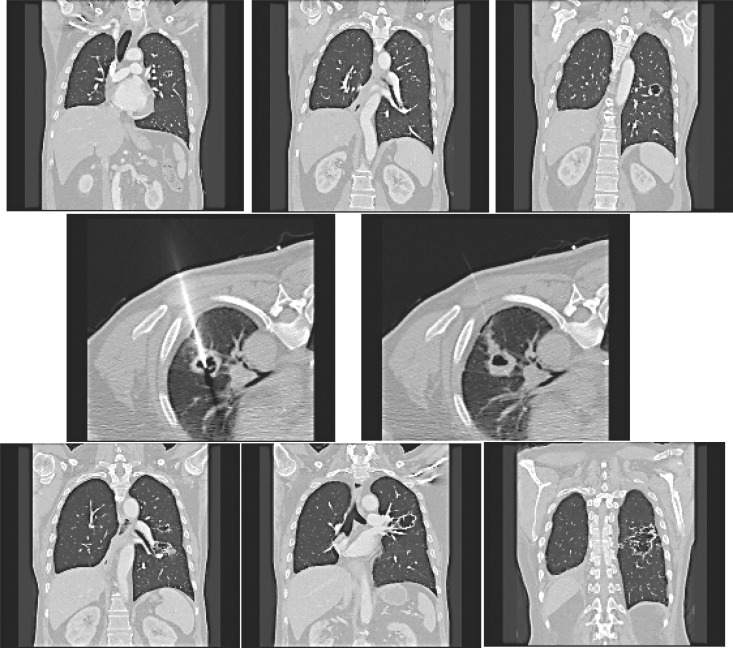
In case 8, bilobectomy (RML, RLL) was performed for RLL squamous cell carcinoma with pathological stage T3N1M0 after neoadjuvant chemotherapy and adjuvant chemotherapy. Cavitary metastases were found at LUL (2 lesions) and LLL (1 lesion) 17 months later and intra-cavitary PDT was performed with a CT-guided catheter insertion (Fig. 3). Energy was delivered to the cavitary lesion at 360 J/cm. Because of possible energy loss, 2 or 3 more energy was given. A small pneumothorax occurred but spontaneously regressed without chest tube insertion. The patient's cavitary lesions increased in size 1 year later but there was no further follow-up on this patient 17 months after PDT.
Figure 4:
Cavitary metastases in case 8. Two LUL lesions and 1 LLL lesion were observed (upper). CT guided catheter insertion (middle). After 1 year, the size of the cavitary lesions increased (bottom).
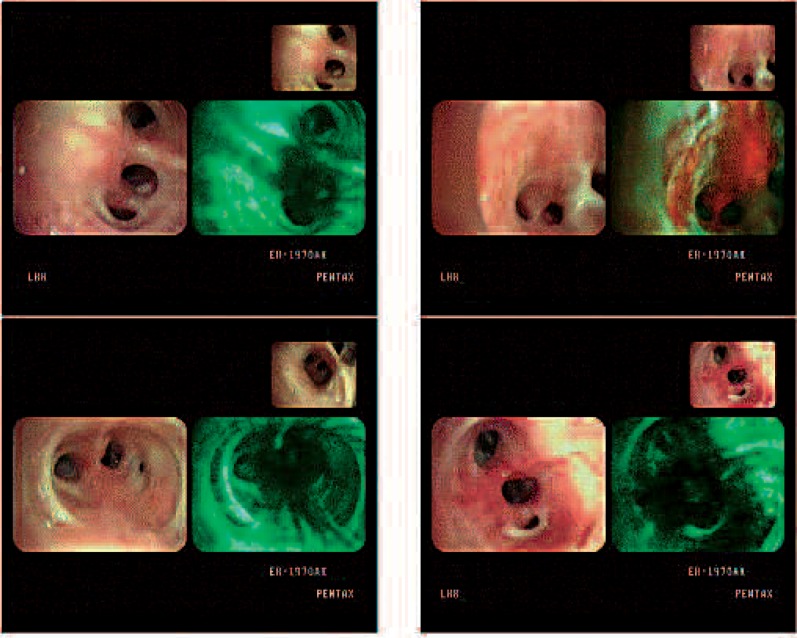
In case 9, PDT was performed for left distal main bronchus and a second carina lesion because of the patient's poor pulmonary function (Fig. 5). Cancer lesions disappeared but recurrence was found 20 months after PDT with mediastinal nodal metastases on PET. During that time, the patient was receiving robotic radical prostatectomy for prostatic cancer and he is continuing to receive radiation therapy at this time.
Figure 5.
Bronchoscopic findings in case 9. Cancer lesions were observed in the left main bronchus and second carina area (upper). The lesion was not observed after PDT (left bottom) but a low fluorescent area in the posterior wall of the second carina and the anterior wall of main bronchus (right bottom) was observed 20 months after PDT, which was revealed to be recurrent cancer on biopsy.
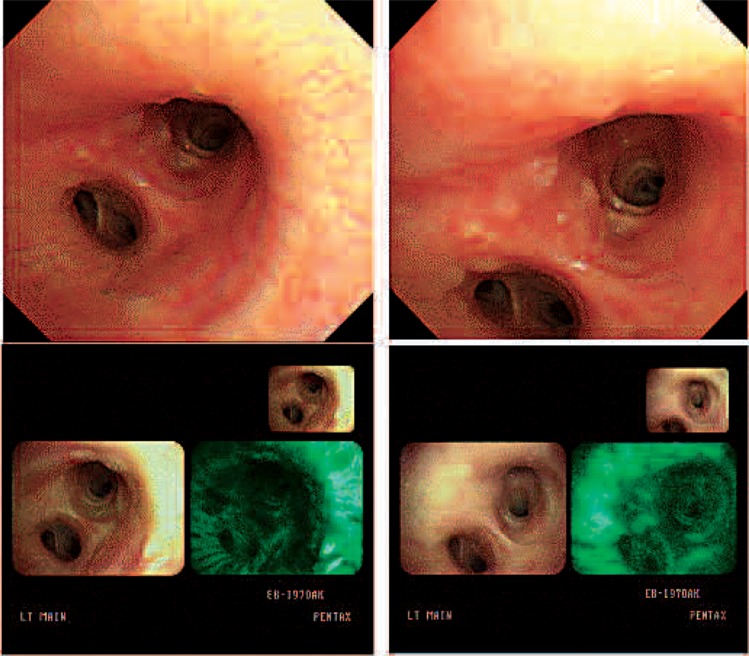
In case 10, the patient was diagnosed with synchronous double primary lung cancer at the RUL (carcinoma in situ) and the RLL with obstructing pneumonia. Bilobectomy (RML, RLL) was performed and the pathologic stage was T2N0M0. PDT for the RUL lesion was performed 2 times on 2 consecutive days (Fig. 6).
Figure 6.
The RUL cancer lesion (upper) was not observed after PDT (bottom).
Synchronous double primary lung cancer was diagnosed in case 11. Neoadjuvant chemotherapy and stage operation were performed because the patient had poor pulmonary function. RMLobectomy and pulmonary artery angioplasty were performed first and the pathological stage was T2N1M0. LLLobectomy was then performed and the pathological stage was identified as T1N1M0. These treatments were followed by adjuvant chemotherapy. One year after lung resection, the patient had esophageal resection for malignancy at the mid-thoracic level and the pathological stage was T1N0M0. A small nodular mucosal lesion was found at the orifice of the LUL anterior segment via bronchoscopy and squamous carcinoma was confirmed through biopsy 2 years after lung resection. PDT was performed and recurrence has not been detected up to the point of writing this manuscript. However, this patient was diagnosed with laryngeal cancer rather than with a metastatic lesion and he received radiation therapy at another hospital.
Methods
Photofrin® (porfimer sodium; Axcan Pharma, Canada) was used in 10 cases in 9 patients, Radachlorin®(Radapharma, Russia) was used in 3 cases in 2 patients, and Photogem® (Moscow Research Oncological Institute, Russia) was used in 1 case.
Photofrin® was injected at a dose of 2 mg/kg via the peripheral vein and the laser light was irradiated 48 hours after injection. Diomed® The light source for the activation of Photofrin® was 630 nm. At 3 days post-therapy, bronchoscopy was re-performed to evaluate the lesion and necrotic debris or secretions were removed. Patients were instructed to avoid direct sunlight for 30 days post-treatment because of adverse reactions like photosensitivity.
The major composition of Radachlorin® is chlorin e6, which can absorb at the 662 nm wavelength and may therefore penetrate the tumor to a maximum depth of 10 mm compared to 5 – 6 mm for Photofrin®. The injection dose was 0.5 – 0.6 mg/kg and laser illumination via bronchoscope was performed 3 – 5 hours after injection. The duration of the sun-shield was 6 – 7 days after injection. In the patient that received PDT 4 times, Photofrin® was used for the first application and Radachlorin® was used for the second and third applications. However, when recurrence occurred after Radachlorin®-PDT, Photofrin® was again used for the fourth PDT treatment (Table 3). Bronchoscopy was performed under general anesthesia and intubation because of poor patient endurance in most cases.
Table 3. Photodynamic Therapy and Results.
| Case | Photosensitizer | Dose (J/cm) | No of PDT | Response | Survival Months | Current status |
|---|---|---|---|---|---|---|
| 1 | Photofrin | 200 | 1 | PR | 28 | Death |
| 2 | Photofrin | 200 | 1 | NR | 8 | Death |
| 3 | Photofrin | 250 | 1 | CR | 66 | Alive |
| 4 | Photogem | 1 | CR | 49 | Death | |
| 5 | Photofrin | 200 | 1 | PR | 9 | Death |
| 6 | Photofrin | 200(LLL) 200(LUL) |
1 | NR | 6 | Death |
| 7∗ | Photofrin | 168(RLL) 119(LLL) |
1 | CR | 28 | Alive |
| 8 | Photofrin | 360(LUL, LLL) | 1 | NR | 17 | Follow-up loss |
| 9 | Photofrin | 200(LUB) 80(Lt. 2nd carina) |
1 | CR | 21 | Alive |
| 10 | Photofrin | 200 | 2 | CR | 18 | Alive |
| 11∗ | Radachlorin | 40 | 1 | CR | 15 | Alive |
| 12 | Radachlorin | 40 | 1 | CR | 13 | Alive |
| 13∗ | Radachlorin | 120 | 1 | NR | 7 | Alive |
| 14∗ | Photofrin | 200 | 1 | CR | 4 | Alive |
PR: Partial response – Decreased tumor size but larger than 50% of pre-PDT size
CR: Complete response – No bronchoscopically visible tumor with or without pathologic confirmation
NR: No response or more aggravated state
Same patient
Results
There were 8 cases with complete response (CR), 4 cases with NR, and 2 cases with PR. CR was defined as no grossly or microscopically evidence of tumor, PR was defined as decreased size but lack of complete remission, and NR was defined as no change in tumor size or a more aggravated state (Table 3).
The mean survival in all patients was 23.9 months after PDT (median 41.9, range 6 – 66 months). PDT was performed in 9 patients for early tracheo-bronchial cancer (group A), in 2 patients for local control because of recurrence (group B), and in 2 patients for palliation (group C). One patient received PDT 4 times, which included 1 treatment for an early lesion and 3 treatments for local recurrence. There were 5 deaths, 1 loss of follow-up, and 5 patients that are still alive.
In group A, the mean follow-up duration was 25.3 months and 5 patients (55.6%) survived whereas 4 patients died. The mean survival was 26.4 months (median 21 months) with a range of 6 – 66 months. The tumor progressed in 3 patients. Among the 4 patients that died, 2 patients died of pneumonia, 1 of acute myocardial infarction, and 1 for unknown reasons but he had progressed to a state of lung cancer.
In group B, 2 patients are still alive without evidence of recurrence with follow-up durations of 10 months and 15 months. In group C, follow-up was not completed on 1 patient past 17 months post-PDT and the other patient died 8 months after PDT with progression of lung cancer.
Discussion
The indications and applications of PDT for lung cancer with the exception of early stage or carcinoma in situ vary from country to country and produce various results. There is no general guideline for PDT in the treatment of lung cancer except in Japan. Although the curative PDT guidelines for early-stage lung cancer suggest target tumor size smaller than 1 cm and absence of lymph node metastasis,8) the PDT performed in this study in lung cancer patients at variable stages as an adjunct to surgery or other treatments demonstrated good therapeutic results even in cancers with lymph node involvement. CR was unexpectedly achieved in patients with tumor stages 1 – 3 and lymph node metastasis. The introduction of AFB provided a more accurate diagnosis of the tumor margin because the reduced green autofluorescence in abnormal and preneoplastic tissue allowed the abnormal area to be distinguished from the normal green area. Better delineation of the tumor margin by precise illumination is crucial to the resulting PDT efficacy and surgical resection of the tumor may increase the effectiveness of further PDT treatment by lessening the tumor burden. These 2 factors may be attributed to comparable PDT efficacy with early-stage lung cancer. Among the 5 recurrent cases that underwent PDT treatment, 80% showed CR irrespective of tumor stage or combined treatments. In 1 non-responding case, intracavitary illumination was performed with a 10 mm cylindrical optical diffuser. That may produce inappropriate light delivery at the absence of spherical diffuser in particular forwarding or backward direction during intracavitary PDT procedure where light could not be delivered with cylindrical-typed diffuser.
Photofrin-PDT was chosen for a recurrent lesion that showed NR to Radachlorin-PDT, resulting in CR. This lesion had recurred as a dysplastic nodular lesion 9 months after the second treatment with Radachlorin-PDT. This lesion did not respond to irradiation with the relatively large dose of 120 J/cm, which was presumably due to insufficient dosimetry because the tumor completely responded to Photofrin-PDT at a dose of 200 J/cm. This observation suggests an important potent efficacy for switching over photosensitizer and dosimetry in the non-responsive lesions. Hematoporphyrin derivative-type and chlorine molecules, which are localized in the membrane and mitochondria and in the membrane and lysosomes, respectively, have different PKs based on different binding chemistries with plasma proteins and intracellular distribution and thereby have potentially different mechanisms of action. In the other non-responding cases (cases 2 and 6), surgery did not accompany PDT because of either non-indication or refusal from the patient. In case 2, PDT was performed to palliate an obstructed mass on the main bronchus. Although an interstitial-typed cylindrical diffuser was preferred for the homogeneous irradiation, a conventional cylindrical diffuser was used in the absence of this diffuser and resulted in inappropriate light dose delivery to the tumor mass. In case 6, the left lower lobe endobronchial mass extended to the distal left main bronchus, accompanied by an undetermined right upper lobe 1 cm mass. Therefore, lessening the tumor burden by surgical resection was preferable but only PDT was performed on 2 lesion sites with a dose of 200 J/cm because the patient refused surgery. This patient showed PR on the LMB lesion but NR at LLL where the scattered lesion was widely spread, making it difficult to irradiate homogeneously in a single treatment by PDT.
The 5 cases of death were mostly accompanied by other conditions such as chronic obstructive pulmonary disease or cardiovascular diseases. One of these patients was diagnosed with cT3N2M0 and received chemotherapy and radiation therapy on an 80% obstructive main bronchus followed by PDT through a small passage obtained from radiation therapy that was only partially clear. Although a cylindrical diffuser was used for insertion through this indentation, the diffuser was not completely inserted through the obstructive mass in the absence of an interstitial diffuser and thereby delivered insufficient light to the entire mass region.
The advantages of PDT include no loss of pulmonary function after therapy, which enables additional treatment including lung resection, and better quality of life compared to surgery.7) The disadvantages include the need for repeated toileting or brushing bronchoscopy and the requirement that the patient stay indoors because of the risk of skin photosensitivity.
Some patients require another treatment even when they are diagnosed in the early stage of lung cancer. Examples include cases with (1) poor pulmonary function, which inhibits surgical resection including the sleeve procedure, (2) the presence of multiple endobronchial lesions, (3) a previous history of pneumonectomy, (4) the existence of co-morbidity risk factors, and (5) refusal by the patient to undergo surgery.9) In these patients, PDT could be considered a therapeutic tool. In emergent cases with an advanced stage of lung cancer, Nd-YAG laser therapy with stenting is the primary treatment of choice. In order for PDT to be equally effective and safe in relieving airway obstruction in comparison to Nd-YAG laser therapy, appropriate dosimetry with an interstitial diffuser is a prerequisite. Because PDT works differently than chemotherapy, radiation, or surgery, it can be used along with these therapies as part of a multi-modality approach in obstructive lung cancer. Another advantage of PDT as an adjunct to surgery or other treatments is its ability to trigger a tumor-directed immune response in the body,9) which attacks the treated cancer cells. No other type of therapy appears to cause this type of response.
References
- 1:Friedberg JS, Photodynamic therapy as an innovative treatment for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg (2009) 21: 177–87 [DOI] [PubMed] [Google Scholar]
- 2:Friedberg JS. et al, Photodynamic therapy for malignant pleural mesothelioma: the future of treatment?, February 2011, Expert Review of Respiratory Medicine, pp 49–63 [DOI] [PubMed]
- 3:Hahn Stephen. et al, Photodynamic therapy for mesothelioma, July 2007, Current Treatment Options in Oncology, Volume 2, No. 5, pp. 375–383 [DOI] [PubMed] [Google Scholar]
- 4:Usuda J, Kato H, Okunaka T. et al. Photodynamic Therapy (PDT) for Lung Cancers: Concise Review. J Thorac Oncol 2006; 1: 489–493 [PubMed] [Google Scholar]
- 5:Chiaki E, Akira M, Akira S, et al. Results of Longterm Follow-up of Photodynamic Therapy for Roentgenographically Occult Bronchogenic Squamous Cell Carcinoma. Chest 2009; 136(2):369–375 [DOI] [PubMed] [Google Scholar]
- 6:Timothy CK, Annette McW, Eric E, et al. Bronchial Intraepithelial Neoplasia/Early Central Airways Lung Cancer: ACCP Evidence-based Clinical Practice Guidelines (2nd edition). Chest 2007; 132: 221S–233S [DOI] [PubMed] [Google Scholar]
- 7:Moghissi K., Dixon K. BA. Update on the Current Indications, Practice and Results of Photodynamic Therapy (PDT) in Early Central Lung Cancer (ECLC): Review. Photodiag Photodyn Ther 2008; 5: 10–18 [DOI] [PubMed] [Google Scholar]
- 8:Kato H, Usuda J, Okunaka T. et al. The history of photodynamic therapy and photodynamic diagnosis in the department of surgery, Tokyo Medical University. Photodiag Photodyn Ther 2004: 1: 107–110 [DOI] [PubMed] [Google Scholar]
- 9:Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M. et al. Photodynamic Therapy: Review. J Natl Cancer Inst 1998; 90: 889–905 [DOI] [PMC free article] [PubMed] [Google Scholar]