Abstract
Background: Low level light therapy (LLLT) has attracted attention in many clinical fields with a new generation of light-emitting diodes (LEDs) which can irradiate large targets. To pain control, the first main application of LLLT, have been added LED-LLLT in the accelerated healing of wounds, both traumatic and iatrogenic, inflammatory acne and the patient-driven application of skin rejuvenation.
Rationale and Applications: The rationale behind LED-LLLT is underpinned by the reported efficacy of LED-LLLT at a cellular and subcellular level, particularly for the 633 nm and 830 nm wavelengths, and evidence for this is presented. Improved blood flow and neovascularization are associated with 830 nm. A large variety of cytokines, chemokines and macromolecules can be induced by LED phototherapy. Among the clinical applications, non-healing wounds can be healed through restoring the collagenesis/collagenase imbalance in such examples, and ‘normal’ wounds heal faster and better. Pain, including postoperative pain, postoperative edema and many types of inflammation can be significantly reduced.
Experimental and clinical evidence: Some personal examples of evidence are offered by the first author, including controlled animal models demonstrating the systemic effect of 830 nm LED-LLLT on wound healing and on induced inflammation. Human patients are presented to illustrate the efficacy of LED phototherapy on treatment-resistant inflammatory disorders.
Conclusions: Provided an LED phototherapy system has the correct wavelength for the target cells, delivers an appropriate power density and an adequate energy density, then it will be at least partly, if not significantly, effective. The use of LED-LLLT as an adjunct to conventional surgical or nonsurgical indications is an even more exciting prospect. LED-LLLT is here to stay.
Keywords: Grotthus-Draper law, nonhealing wound, photochemical cascade, photophysical reaction, irritant contact dermatitis, dissecting cellulitis, acne rosacea
INTRODUCTION
High level laser treatment (HLLT) means that high levels of incident laser power are used to deliberately destroy a specific target through a light-heat transduction process to induce photothermal damage of varying degrees. HLLT is used in many surgical fields, but probably most commonly in dermatologic, aesthetic or plastic surgery. On the other hand, when a laser or other appropriate light source is used on tissue at low incident levels of photon energy, none of that energy is lost as heat but instead the energy from the absorbed photons is transferred directly to the absorbing cell or chromophore, causing photoactivation of the target cells and some kind of change in their associated activity. In clinical applications, this was termed ‘low level laser therapy’ (LLLT) by Ohshiro and Calderhead in 1988,1) with ‘photobiomodulation’ or ‘photoactivation’ referring to the activity at a cellular and molecular level.
Genesis of LLLT
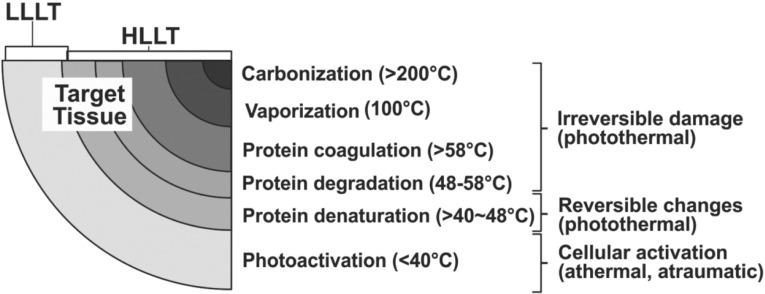
In the late 1960's, the early days of the clinical application of the laser, there was fear that laser energy could induce carcinogenesis as a side effect of the use of the laser in surgery and medicine. To assess this, in a paper published in 1968, the late Professor Endrè Mester, the recognized father of phototherapy from Semmelweis University, Budapest, applied daily doses of low incident levels of defocused ruby laser energy to the shaved dorsum of rats.2) No carcinogenetic changes were noted at all, but Mester incidentally discovered that LLLT accelerated hair regrowth in the laser-irradiated animals. Furthermore, during this period, early adopters of the surgical laser were reporting interesting and beneficial effects of using the laser as a scalpel compared with the conventional cold steel instrument, such as reduced inflammation, less postoperative pain, and better wound healing. Mester's experiments helped to show that it was the ‘L’ of laser, namely light, that was associated with these effects due to the bioactivative levels of light energy which exist simultaneously at the periphery of the photosurgical destructive zone, as illustrated in Figure 1.
Fig. 1:
Range of typical bioreactions associated with a surgical laser and their approximate temperature range. Note that some degree of photoactivation almost always occurs simultaneously with HLLT-mediated reactions. (Data adapted from Calderhead RG: Light/tissue interaction in photosurgery and phototherapy. In Calderhead RG. Photobiological Basics of Photosurgery and Phototherapy, 2011, Hanmi Medical Publishers, Seoul. pp 47–89)
In the 1970's, many clinicians, inspired by Mester's major publication in 1969 on the significantly successful use of LLLT for the treatment of nonhealing or torpid crural ulcers, started to apply LLLT clinically, particularly in France and Russia, and this spread to Japan, Korea, and other Asian countries in the early 1980's. However, it was still looked on as ‘black magic’ by the mainstream medicoscientific world in the USA. The first Food and Drug Administration (FDA) approval for laser diode phototherapy was not granted till 2002, but even then the sceptics were not silenced.
LLLT with Lasers
LLLT was first completely limited to treatment with laser sources, such as the helium neon (HeNe) laser in the visible red at 632.8 nm, various semiconductor (diode) lasers (visible red to near infrared, most notable being the GaAlAs at 830 nm) or defocused beams of a surgical laser (Nd:YAG or CO2, for example).3) There are several mechanisms which have been reported as to how LLLT can induce a biomodulative effect (Table 1). In the case of LLLT with laser sources, these effects were achieved athermally and atraumatically through the special properties associated with the ‘coherence’ of laser energy, namely monochromaticity, directionality or collimation, and the photons all in phase temporally and spatially. Another phenomenon associated only with laser energy is the so-called ‘speckle’ phenomenon. When the spot from a 670 nm laser pointer is closely examined over a period of time, for example, it appears to be composed of exceptionally brighter spots of light energy which are constantly in motion: these are laser speckles. Speckles have their own characteristics, including high energy and polarization, and these intense spots of polarized light were associated with specific reactions in the absorbing target or chromophore.
Table 1: Major mechanisms associated with photobioactivation and LLLT.
| Mild thermal (<40°C) | Biochemical | Bioelectric | Bioenergetic | |
| ↑ Nerve conduction | (Mitochondrial events) | ↑ Electromotive action on membrane bound ion transport mechanisms | ↑ Rotational & vibrational changes to membrane molecule electrons | |
| ↑ ATP production | ||||
| ↑ Release of nitric oxide (NO) | ||||
| ↑ Very low levels of reactive oxygen species (ROS) | ||||
| ↑ Capillary dilatation | ↑ Fibroblast proliferation → Collagen & elastin synthesis | ↑ Intracellular extra-cellular ion gradient changes | ↑ Stimulation of acupuncture meridian points | |
| ↑ Mast cell degranulation: cytokine, chemokine and trophic factor release | ↑ Depolarization of synaptic cleft → closure of synaptic gate | ↑ Increased biophotonic activity | ||
| ↑ Macrophage activity (chemotaxis & internalization) → release of FGF | ↑ Activation of the dorsal horn gate control mechanism → pain transmission slowed, pain control increased | |||
| ↑ Keratinocyte activity cytokine release in epidermis and dermis | ||||
| ↑ Opiate and nonopiate pain control (endorphins, dynorphins and enkephalins) | ||||
| ↑ RNA/DNA synthesis | ||||
| ↑ Enzyme production | ||||
| ↑ Superoxide dismutase (SOD) production | ||||
Up until the end of the 1990's, phototherapy was dominated by these laser sources, because although LEDs were cheap and cheerful, they were highly divergent with low and unstable output powers, and a wide waveband. With very few exceptions, old generation LEDs were incapable of producing really useful clinical reactions in tissue. It was easy to source a ‘red’ LED (output spread over approximately 600 – 700 nm) but it was more or less impossible to source LEDs at specific nominal wavelengths, for example 633 nm, similar to the HeNe laser.
LED PHOTOTHERAPY
Enter the NASA Light-Emitting Diode (LED)
All this changed in 1998 with the development of the so-called ‘NASA LED’ by Prof Harry Whelan and his group at the NASA Space Medicine Laboratory, which offered clinicians and researchers a useful phototherapy source having less divergence, much higher and more stable output powers, and quasimonochromaticity whereby nearly all of the photons were at the rated wavelength.4) This new generation of LEDs also had its own phenomenon associated with photon intensity, namely photon interference, whereby intersecting beams of LED energy from individual LEDs produced photon interference, increasing the photon intensity dramatically and thus offering much higher photon intensities than the older generation. For LEDs emitting at visible red and near IR wavelengths, the greatest photon intensity was actually seen beneath the surface of the target tissue, due to the combination of the photon interference phenomenon and the excellent tissue scattering characteristics of light at these wavebands.5) This phenomenon, together with quasimonochromaticity, meant that the new generation of LEDs was a clinically viable source for phototherapy.6) ‘Low level laser therapy’ was therefore renamed by the US photobiologist, Kendric C Smith, as ‘low level light therapy’, to encompass LED energy.7) Accordingly, useful bioreactions could then be achieved with LEDs through cellular photoactivation without heat or damage, as shown by Whelan and colleagues in their early NASA LED wound healing studies.8)
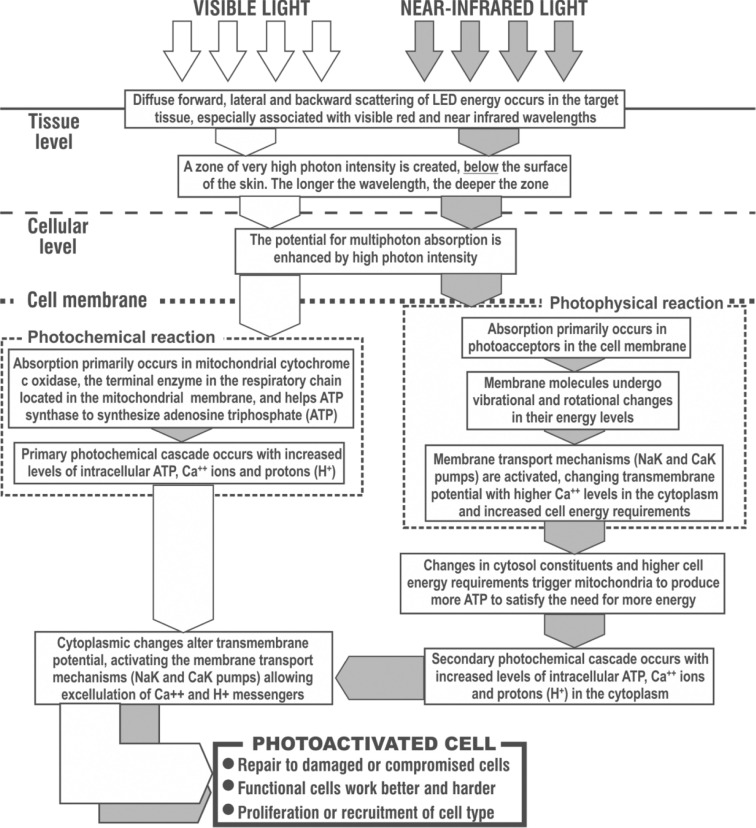
Although visible and near-infrared light energy induce the same tri-stage process in target cells, namely photon absorption, intracellular signal transduction and the final cellular photoresponse,9) it should be noted that both wavebands have different primary targets and photoreactions in target cells. Visible light is principally a photochemical reaction, acting directly and mostly on cytochrome-c oxidase, the end terminal enzyme in the cellular mitochondrial respiratory chain,10) and mainly responsible for inducing adenosine triphosphate (ATP) synthesis, the fuel of the cell and indeed the entire metabolism. Infrared light on the other hand induces a primary photophysical reaction in the cell membrane thereby kick-starting the cellular membrane transport mechanisms such as the Na++K++ pump,6) and this in turn induces as a secondary reaction the same photochemical cascade as seen with visible light, so the end result is the same even though the target is different as illustrated schematically in Figure 2.
Fig. 2:
The process of cellular photoactivation by low level light therapy (LLLT). Visible light induces a primary photochemical response particularly associated with mitochondrial cytochrome c-oxidase, whereas near IR induces a primary photophysical response in the cellular and organelle membranes. However the eventual photoresponse is the same. (Based on data from Karu & Smith, Refs 6 & 9)
LED phototherapy at appropriate wavelengths and parameters has now been well-reported in a large number of pan-speciality applications.11) How and where does LED phototherapy work? When we consider investigating how LED phototherapy or LLLT can bring about and influence the molecular mechanism for cell proliferation, we should recognize that LLLT not only has an effect on various signaling processes, but it can also significantly induce the production of cytokines, such as a number of growth factors, interleukins and various macromolecules (Table 2).12)
Table 2: Molecular level activation by LLLT with appropriate LEDs (From Ref 12).
| Classification | Molecules | LLLT-Associated Biological Effects |
| Growth factors | BNF, GDNF, FGF, bFGF, IGF-1, KGF, PDGF, TGF-β, VEGF | Proliferation |
| Differentiation | ||
| Bone nodule formation | ||
| Interleukins | IL-1α, IL-2, IL-4, IL-6, IL-8 | Proliferation |
| Migration | ||
| Immunological activation | ||
| Inflammatory cytokines | PGE2, COX2, IL1β, TNF-α | Acceleration/Inhibition of inflammation |
| Small molecules | ATP, cGMP, ROS, CA++, NO, H+ | Normalization of cell function |
| Pain relief | ||
| Wound healing | ||
| Mediation of cellular activities | ||
| Migration | ||
| Angiogenesis | ||
Journal of Biomedical Science 2009, 16:4
Phototherapy is Becoming Mainstream
The increasing number of papers on LLLT in the Photobiomodulation sessions presented at the 2010 and especially the 2011 meetings of the American Society for Lasers in Medicine and Surgery (ASLMS) bear witness to the fact that LLLT is no longer quite the bête noir it used to be in the USA, although there is still too much skepticism, and it has achieved a reliable status worldwide. LED phototherapy has now been well-proven to work, and is reported to be effective in a large variety of clinical indications such as pain attenuation, wound healing, skin rejuvenation, some viral diseases, allergic rhinitis, other allergy-related conditions and so on.
APPLICATIONS OF LLLT WITH LEDs
When we confirm in what fields LLLT phototherapy has been most used through a review of the literature, the main application is for pain control, with pain of almost all aetiologies responding well.11) For example, 830 nm LED phototherapy significantly reduced both acute and chronic pain in professional athletes.13) The first author has been using LED in the control of herpes zoster pain for some time, and also for intractable postherpetic neuralgia, corroborating previous studies with 830 nm LLLT for this indication.14,15) This and other chronic pain entities have been historically very hard to control, but the good efficacy of LED phototherapy has been well recognized. From the large body of work from Rochkind and colleagues in Israel, LED phototherapy can help nerve regeneration, so it has been used for spinal cord injuries,16) and many different types of neurogenic abnormality. In the case of the dental clinic and for the osseointegration of implants and prostheses in maxillofacial surgery it has been used for guided bone regeneration.17) At present, the research into and development of new applications for LED phototherapy, especially in the processes of inflammatory cell regulation, are being assiduously studied in the dermatology field.
Fast taking over from pain attenuation, and particularly in the dermatology field, wound healing with LED phototherapy has attracted much attention. Reports have shown that, after making uniform burn wounds with a surgical laser, LED phototherapy of experimental wounds induces faster and better organized healing than in the control unirradiated wounds. This is due to the effect of 830 nm phototherapy on raising the action potential the wound-healing cells, at all three phases of the process, particularly mast cells,18) macrophages19) and neutrophils20) in the inflammatory stage; fibroblasts in the proliferative phase (Personal Communication, Prof. Park, Seoul National University, Seoul, South Korea: unpublished data); and fibroblast-myofibroblast transformation in the remodeling phase.21) As an additional mechanism, it has also been shown that 830 nm phototherapy increased the early vascular perfusion of axial pattern flaps in a controlled speckle flowmetry Doppler trial in the rat model, with actual flap survival significantly better in the irradiated than in the unirradiated control animals.22)
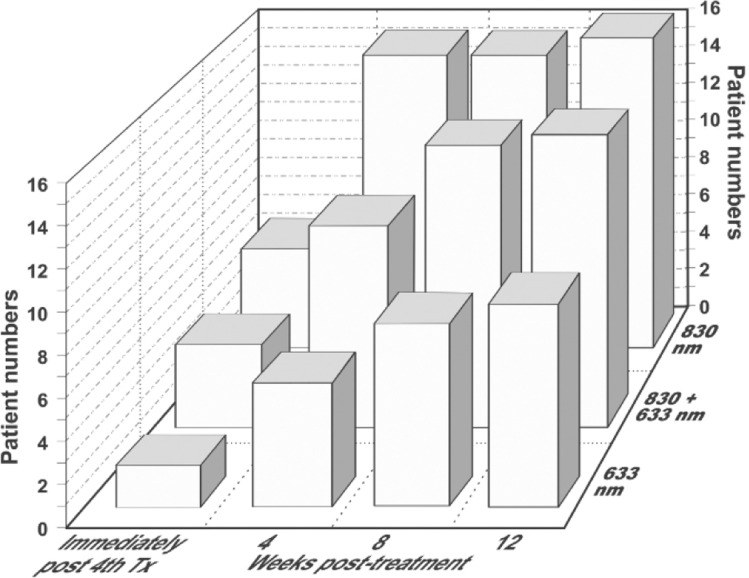
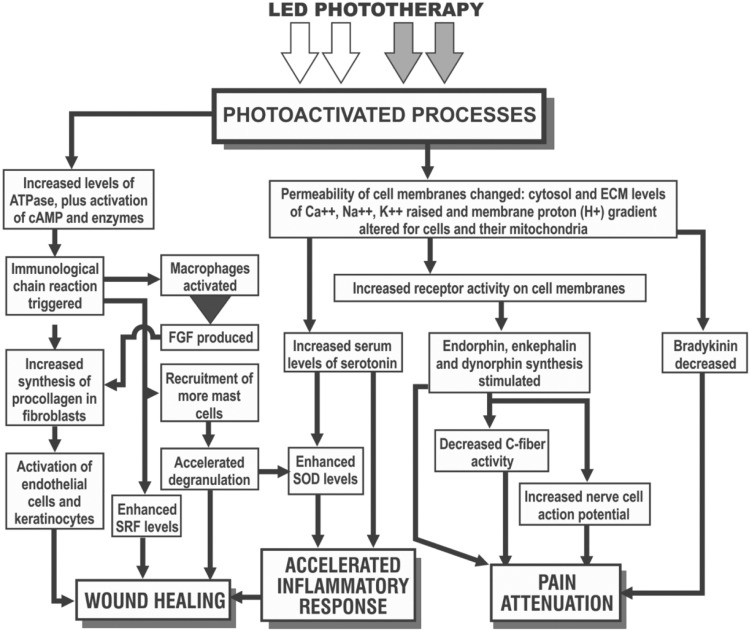
In another very popular indication, studies have reported on the use of LED phototherapy for the rejuvenation of chronologically and photodamaged skin.23,24) Lee and colleagues, in a randomized controlled study, showed that fibroblasts examined with transmission electron microscopy appeared more active, collagen and elastin synthesis was increased and tissue inhibitors of matric metalloproteinases was increased, as a result of which, effective rejuvenation could be achieved which was maintained up to 12 weeks after the final treatment session. Patient satisfaction scores bore these histopathological findings out (Figure 3).24) We must never forget that good skin rejuvenation is firmly based on the wound healing process, particularly neocollagenesis. LED phototherapy has also been reported as being very effective in the prophylaxis against scar formation, due amongst other factors to the response to photomediated interleukin-6 signaling.12) Hair loss is another field where LED phototherapy may well have real efficacy, with red and infrared being the wavelengths of choice.25–27) Figure 4 illustrates schematically the mechanisms already confirmed underlying the three main endpoints of 830 nm LLLT, namely wound healing, the anti-inflammatory response through acceleration and quenching of the post-wound inflammatory phase and pain attenuation.
Fig. 3:
Patient satisfaction curves compared for LED-mediated skin rejuvenation with 633 nm alone, 633 nm + 830 nm combined and 830 nm on its own, showing the numbers of patients who rated their improvement as excellent on a 5-scale rating. The first set of columns represents the findings immediately after the 8th of 8 weekly sessions, twice per week for 4 weeks. The 2nd, 3rd and 4th sets of columns are the findings at post-treatment weeks 4, 6 and 8 respectively. At all stages, LED phototherapy with 830 nm produced superior satisfaction. The increase over the post-treatment period is interesting, suggesting improved results through continued tissue remodeling as part of the LED-mediate wound healing process. (Data adapted from Ref 24)
Fig. 4:
Mechanisms underlying the three main LLLT endpoints, particularly associated with the wavelength of 830 nm, although 633 nm has beneficial effects as well.
SYSTEMIC EFFECTS OF LED-LLLT
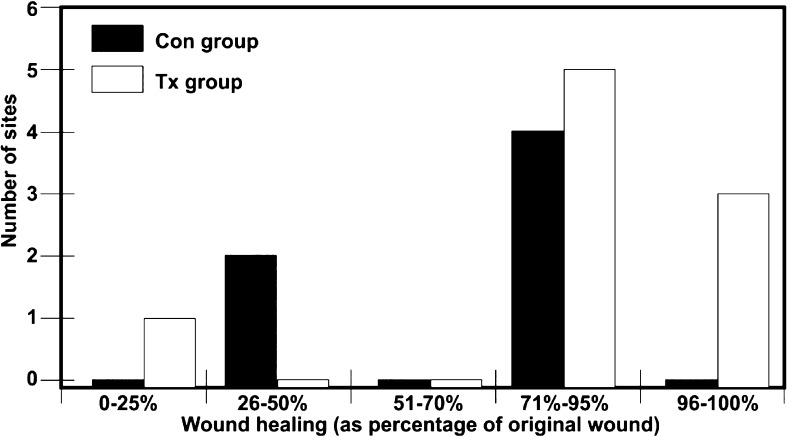
One of the advantages of LLLT with an LED system as compared with a laser source is that LED-based systems offer large planar arrays, so that they can irradiate a large area of the body in a hands-free manner, compared with the point-by-point application of a laser system. In addition, many different cell types can be simultaneously targeted. It may not even be necessary to irradiate every target area. The systemic effect of LED with an 830 nm system (HeaLite II, Lutronic Corp., Goyang, S. Korea, Figure 5) was studied by the first author.28) The systemic effect associated with LLLT has already been suggested as far back as Mester's pivotal study on non-healing ulcers in 1969, whereby irradiation of one part of the body could induce effects in another unirradiated area.29) To assess this, in the first author's study controlled wounds on the backs of rodents were created with an ablative fractional laser, and rather than irradiating the laser wounds with LED energy (HeaLite system as above), the animals' abdomens in the experimental group were irradiated, and sham irradiation was delivered to the control group. The results clearly indicated that the group which had LED treatment of the abdomen demonstrated significantly better healing than the control group (Figure 6). This means that LED phototherapy could very probably have a systemic effect on inflammatory or immune cells in nonadjacent tissues to the target area, as well as those cells in the irradiated tissues.
Fig. 5:
HeaLite II LED phototherapy system, Lutronic Corp, Goyang, South Korea.
Fig. 6:
The wound healing value compared between the group treated with 830 nm LED (Tx group) with LED and the unirradiated Con group without LED. Note that the 1 LED-irradiated animal in the 0–25% group had somehow removed the wound dressing very early in the experiment. (Adapted from Ref 28)
LED LLLT FOR SKIN INFLAMMATORY DISEASES
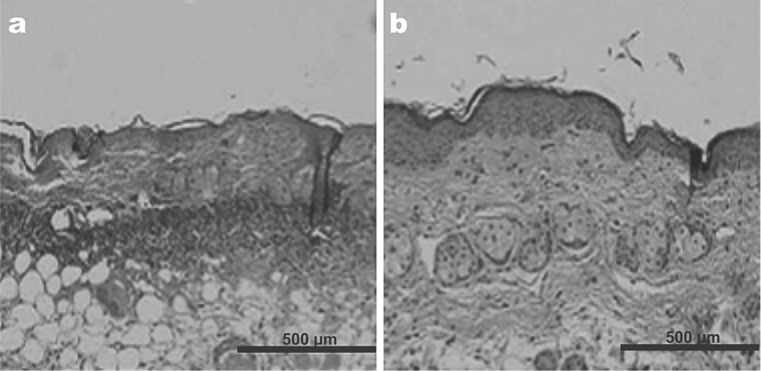
The anti-inflammatory effect of LED has been generally accepted, but up till now this has not been well shown well in inflammatory skin diseases such as allergic or irritant contact dermatitis, atopic dermatitis or rosacea, although a significant degree of success has been demonstrated and reported for inflammatory acne and recalcitrant treatment-resistant psoriasis.30,31) In an experimental animal model study the first author was able to demonstrate that when induced dermatitis in rats was treated with 830 nm LED phototherapy (HeaLite II system, Lutronic Corp, as above) at a dose of 60 J/cm2 in continuous wave, compared with an untreated control group, the histopathological findings revealed significant decreased levels of inflammatory cells (Figure 7). Based on the success of that study, treatment-resistant inflammatory contact dermatitis due to a peel compound containing alpha-hydroxy acid (AHA) in a human subject also responded very well to 3 sessions of 830 nm LED therapy, 3 days apart, irradiance of 100 mW/cm2, 10 min/session, dose of 60 J/cm2, continuous wave (Figure 8).
Fig. 7:
The changes in dermatitis-associated inflammatory cells following 830 nm LED irradiation in the rat model (A: Control specimen, B: LED irradiated specimen). A marked reduction in inflammatory infiltration is evident.
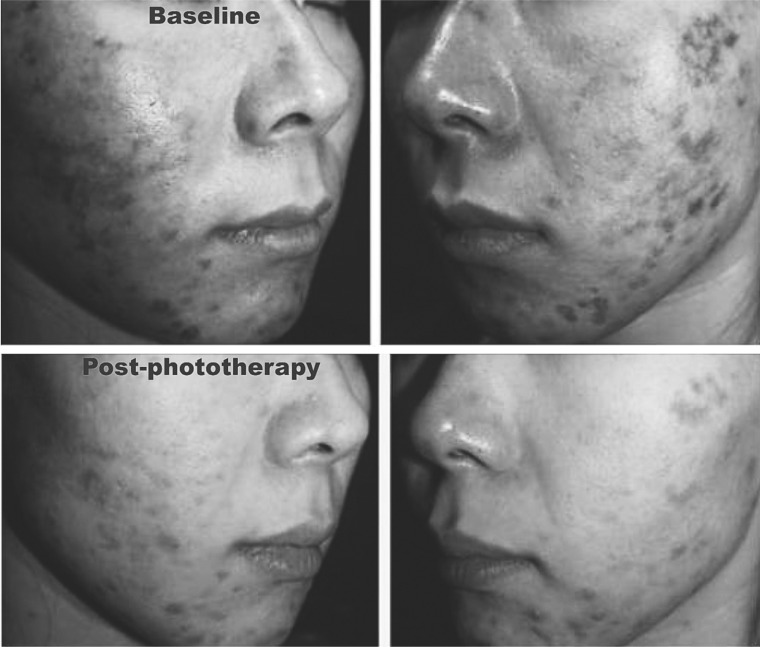
Fig. 8:
Improvement in a patient (24-year-old female) with treatment-resistant post-chemical peel irritant contact dermatitis (AHA-related ICD) seen above at baseline, and below 10 days later following 3 830 nm LED treatment sessions, 3 days apart, 20 minutes per session (60 J/cm2)
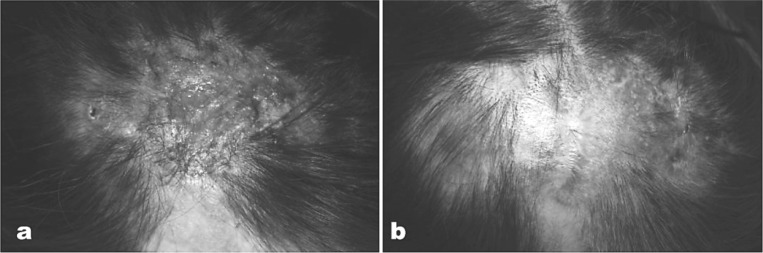
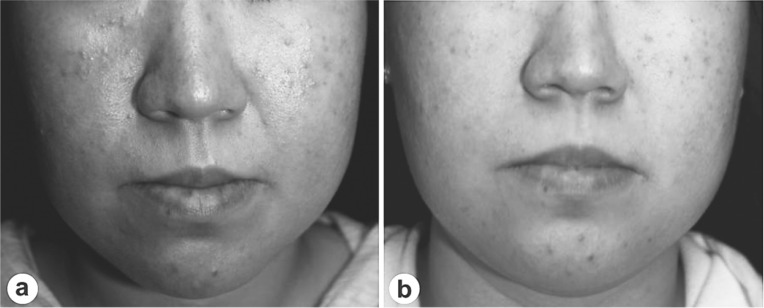
Here are another two examples of the clinical success of 830 nm LED phototherapy (continuous wave, 60 J/cm2) in difficult-to-treat conditions. Figure 9 illustrates the dramatic improvement following 830 nm LED phototherapy in a case of dissecting cellulitis of the scalp, a recalcitrant inflammatory problem, treated with 4 sessions over 2 weeks, 20 min/60 J/cm2 per session; and Figure 10 illustrates a typical result 10 weeks after 6 sessions over 6 weeks, 20 min/60 J/cm2 per session, from a clinical trial the first author has conducted on LED therapy for rosacea with neutrophilic dermatitis. This trial is as yet unreported because the full 12-week follow-up time has not yet been reached in all patients. However, preliminary results are very encouraging with no recurrence seen at 10 weeks in those patients who have reached that point.
Fig. 9:
Dramatic improvement in a case of dissecting cellulitis of the scalp (34-year-old male) (a) at baseline and (b) following 830 nm LED treatment (twice per week for 2 weeks, 20 min per session to give 60 J/cm2)
Fig. 10:
Improvement of acne rosacea (33-year-old female) at baseline (a) and following LED treatments (once per every week for 6 weeks, 20 min and 60 J/cm2 per session) (b). Although not very well noted in the grayscale illustrations, the small acneiform papules have disappeared, with a clear decrease seen in the redness on both cheeks.
CONCLUSIONS
In conclusion, based on the published data and the authors' own experience, LED phototherapy is proving to have more and more viable applications in many fields of medicine. However, it must always be remembered that not any old LED will do. In order to be effective, LED phototherapy must satisfy the following 3 criteria.
-
•
The LED system being used must have first of all, and most importantly, the correct wavelength for the target cells or chromophores. At present, the published literature strongly suggests 830 nm for all aspects of wound healing, pain, anti-inflammatory treatment and skin rejuvenation, with a combination of 415 nm and 633 nm for light-only treatment of active inflammatory acne vulgaris. If the wavelength is incorrect, optimum absorption will not occur and as the first law of photobiology states, the Grotthus-Draper law, without absorption there can be no reaction.
-
•
Secondly, the photon intensity, i.e., spectral irradiance or power density (W/cm2), must be adequate, or once again absorption of the photons will not be sufficient to achieve the desired result. If the intensity is too high, however, the photon energy will be transformed to excessive heat in the target tissue, and that is undesirable.
-
•
Finally, the dose or fluence must also be adequate (J/cm2), but if the power density is too low, then prolonging the irradiation time to achieve the ideal energy density or dose will most likely not give an adequate final result, because the Bunsen-Roscoe law of reciprocity, the 2nd law of photobiology, does not hold true for low incident power densities.
Provided these three criteria are met, LED phototherapy does indeed work, and has many useful aspects in clinical practice for practitioners in many surgical specialities. As an exciting extension of the monotherapy approach with LED-LLLT, and even more importantly, the combination of appropriate LED phototherapy as an adjunct to many other surgical or nonsurgical approaches where the architecture of the patient's skin has been altered will almost certainly provide the clinician with even better results with less patient downtime, in a shorter healing period, and with excellent prophylaxis against obtrusive scar formation.
References
- 1.Ohshiro T, Calderhead RG: Low Level Laser Therapy: a Practical Introduction. 1988. John Wiley and Sons, Chichester, UK [Google Scholar]
- 2.Mester E, Szende B, Spiry T, Scher A. (1972): Stimulation of wound healing by laser rays. Acta Chir Acad Sci Hung. Acta Chir Acad Sci Hung: 315–324 [PubMed] [Google Scholar]
- 3.Calderhead RG, Ohshiro T, Ito E, Okada T, Kato K: The Nd:YAG and GaAlAs lasers: a comparative analysis in pain therapy. In Atsumi K, Nimsakul N, Eds: Laser Tokyo ‘81. 1981, Japan Society for Laser Surgery and Medicine, Tokyo: Section 21, pp 1–4 [Google Scholar]
- 4.Whelan HT, Houle JM, Whelan NT. et al. (2000): The NASA Light-Emitting Diode Medical Program-Progress in Space Flight and Terrestrial Applications. Space Tech. & App. Int'l. Forum. Space Tech. & App. Int'l. Forum: 37–43 [Google Scholar]
- 5.Calderhead RG. (2007): The photobiological basics behind light-emitting diode (LED) phototherapy. Laser Therapy, 16: 97–108 [Google Scholar]
- 6.Smith KC. (2005): Laser (and LED) therapy is phototherapy. Photomed Laser Surg. 23: 78–80 [DOI] [PubMed] [Google Scholar]
- 7.Smith KC. (2010): Laser and LED photobiology. Laser Therapy, 19: 72–78 [Google Scholar]
- 8.Whelan HT, Smits RL, Buchmann EV. et al (2001): Effect of NASA Light-Emitting Diode (LED) Irradiation on Wound Healing. J Clin Laser Med Surg, 2001. 19: 305–314 [DOI] [PubMed] [Google Scholar]
- 9.Karu T. (1999): Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B, J Photochem Photobiol B: 1–17 [DOI] [PubMed] [Google Scholar]
- 10.Karu T: Identification of the photoreceptors. In: Karu T. Ten Lectures on Basic Science of Laser Phototherapy. 2007, Prima Books AB, Grangesberg, Sweden [Google Scholar]
- 11.Tunér J, Hode L: The New Laser Therapy Handbook. 2010, Prima Press, Grangesborg, Sweden [Google Scholar]
- 12.Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomedical Science. 2009. 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter GD, Bleakley C, Glasgow P, Calderhead RG. (2005): A near-infrared LED-based rehabilitation system: initial clinical experience. Laser Therapy, Laser Therapy: 29–36 [Google Scholar]
- 14.Moore KC, Hira N, Kumar PS, Jayakumar CS, Ohshiro T. (1988): A double blind crossover trial of low level laser therapy in the treatment of postherpetic neuralgia. Laser Therapy, Pilot Issue: 7–10
- 15.Numazawa R, Kemmotsu O, Otsuka Hi, Kakehata J, Hashimoto T, Tamagawa S. (1996): The rôle of laser therapy in intensive pain management of postherpetic neuralgia. Laser Therapy, Laser Therapy: 143–148 [Google Scholar]
- 16.Rochkind S. (2009): Review of 30-years experience: laser phototherapy in neuroscience and neurosurgery part II-nerve cells, brain and spinal cord. Laser Therapy, 18: 127–136 [Google Scholar]
- 17.Asanami S, Shiba H, Ohtaishi M, Okada Y, Ohshaka F, Tanaka Y. (1993): The activatory effect of low incident energy hene laser irradiation on hydroxyapatite implants in rabbit mandibular bone. Laser Therapy, Laser Therapy: 29–32 [Google Scholar]
- 18.Calderhead RG, Kubota J, Trelles MA, Ohshiro T. (2008): One mechanism behind LED phototherapy for wound healing and skin rejuvenation: key role of the mast cell. Laser Therapy, 17: 141–148 [Google Scholar]
- 19.Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. (1989): Macrophage responsiveness to light therapy. Lasers Surg Med, Lasers Surg Med: 497–505 [DOI] [PubMed] [Google Scholar]
- 20.Osanai T, Shiroto C, Mikami Y, Kudou E. et al. (1990): Measurement of GaAlAs diode laser action on phagocytic activity of human neutrophils as a possible therapeutic dosimetry determinant. Laser Therapy, 1990. 2: 123–134 [Google Scholar]
- 21.Enwemeka CS, Cohen-Kornberg E, Duswalt EP, Weber DM, Rodriguez IM. (1994): Biomechanical effects of three different periods of GaAs laser photostimulation on tenotomized tendons. Laser Therapy, Laser Therapy: 181–188 [Google Scholar]
- 22.Kubota J. (2002): Effects of diode laser therapy on blood flow in axial pattern flaps in the rat model. Lasers Med Sci, 17: 146–153 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg DJ, Amin S, Russell BA, Phelps R. et al: Combined 633-nm and 830-nm led treatment of photoaging skin. J Drugs Dermatol, 2006. 5: 748–753 [PubMed] [Google Scholar]
- 24.Lee SY, Park KH, Choi JW, Kwon JK, et al: A prospective, randomized, placebo-controlled, double-blinded, and split-face clinical study on LED phototherapy for skin rejuvenation: Clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settings. J Photochem Photobiol (B), 2007. 88: 51–67 (available online as Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 25.Mester E, Szende B, Gärtner P. (1968): The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl). Radiobiol Radiother (Berl): 621–626 [PubMed] [Google Scholar]
- 26.Waiz M, Saleh AZ, Hayani R, Jubory SO. (2006): Use of the infrared diode laser (904 nm) in the treatment of alopecia areata. J Cosmet Laser Ther. J Cosmet Laser Ther: 27–30 [DOI] [PubMed] [Google Scholar]
- 27.Shukla S, Sahu K, Verma Y, Rao KD, Dube A, Gupta PK. (2010): Effect of helium-neon laser irradiation on hair follicle growth cycle of Swiss albino mice. Skin Pharmacol Physiol.; Skin Pharmacol Physiol.;: 79–85 Epub Dec 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kim WS. (2011): the systemic effect of 830 nm LED phototherapy on the wound healing of burn injuries: a controlled study in mouse and rat models. J Cos Laser Ther. In press. [DOI] [PubMed]
- 29.Mester E, Mester AF, Mester A. (1985): The biomedical effects of laser application. Lasers Surg Med. Lasers Surg Med: 31–39 [DOI] [PubMed] [Google Scholar]
- 30.Lee SY, You CE, Park MY. (2007): Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med, Lasers Surg Med: 180–188 [DOI] [PubMed] [Google Scholar]
- 31.Ablon G. (2009): Combination 830-nm and 633-nm Light-Emitting Diode Phototherapy Shows Promise in the Treatment of Recalcitrant Psoriasis: Preliminary Findings. Photomed Laser Surg. 2009 Sep 21. [Epub ahead of print]. [DOI] [PubMed]