Abstract
Purpose: The aim of the present work was to study the effect of Helium-Neon (HeNe) and Gallium Arsenide (GaAs) laser upon nitric oxide (NO) plasma levels, an inflammatory biomarker associated with oxidative stress, in rats with experimental myopathy. These were evaluated through histological assessment.
Materials and Methods: The groups studied were: (A) control (intact rats that received LLLT sham exposures), (B) rats with myopathy and sacrificed at 24 h later, (C) rats with myopathy and sacrificed 8 days later, (D) rats with myopathy and treated with HeNe laser, (E) rats with myopathy and treated with GaAs laser, (F) intact rats treated with HeNe laser and (G) intact rats treated with GaAs laser. Myopathy was induced by injecting 50μl of 1% carrageenan λ (type IV) in the left gastrocnemius muscle. Low Level Laser Therapy (LLLT) was applied with 9.5 J.cm−2 daily for 10 consecutive days with each laser. The determination of the NO was made by spectrophotometry. The muscles were stained with Hematoxylin-Eosin and examined by optic microscopy. Quantitative variables were statistically analyzed by the Fisher test, and categorical by applying Pearson's Chi Squared test at p <0.05 for all cases.
Results: In groups B and C, NO was significantly increased compared to groups A, D, E, F and G (p<0.05). In group C, the percentage of area with inflammatory infiltration was significantly increased compared to the other groups (p<0.001).
Conclusions: LLLT decreased plasma levels of NO in rats with experimental myopathies and significant muscle recovery.
Keywords: Gallium Arsenide, Helium Neon, myopathy, LLLT, nitric oxide
Introduction
The term myopathy is defined as a “muscle disease” affecting the muscle's structure, morphology and biochemistry. It may arise from multiple causes and leads to changes in muscle tone and contraction with varying degrees of severity, including “idiopathic inflammatory myopathies”.1)
One of the agents that can induce inflammation is carrageenan, a polysaccharide and powerful irritant, widely used in experimental animals.2)
In inflammatory processes, nitric oxide (NO) acts as a possible modulator, being synthesized from L-arginine in an equimolar reaction with O2, NADPH, and the production of L-citrulline. High levels of NO coproduct were found in serum of patients with inflammatory diseases.3) NO plays an important role in normal physiological processes and pathological conditions;4) oxidative damage due to its overproduction and that of other reactive oxygen species (ROS) may be involved in inflammatory pathogenesis. 5. © when the increase in the intracellular content of ROS exceeds the antioxidant defenses of the cell, oxidative stress occurs, which alters the cell function and contributes to the development of inflammatory conditions.7)
To counteract the effects caused by inflammation, nonsteroid antiinflammatory drugs (NSAID) are used, which are mostly prescribed to treat inflammatory conditions, despite the tolerance problems that they cause, especially gastrointestinal and renal toxicity.8)
Since the mid ‘60s,9) the use of light energy as a therapy for inflammatory processes and cell trophism opened up a new field in the interaction of electromagnetic energy with biological tissue. Photobiostimulation is the process by which low level laser therapy (LLLT) activates cellular functions without causing significant tissue-level heating. It can stimulate and/or inhibit a biological process depending on the tissue and the dose of irradiation, and therefore a modulating action is attributed to it. It is widely used in controlling inflammatory processes, repair of skin wounds, rheumatic diseases, osteoarthromy-opathies, neuromuscular disorders, sports injuries, among others.10–14) Although LLLT is widely used in clinical rehabilitation, there are conflicting results in the literature,15–21) and we believe that investigating the cellular and molecular events that occur when lasers of different wavelengths interact in myopathies, could result in a significant contribution to optimizing its use in inflammatory muscle diseases. We set out to investigate the photobiomodulator effect of HeNe and GaAs lasers, in an experimental model of myopathy, identifying the inflammatory biomarker, nitric oxide (NO), associated with oxidative stress, and analyzing any anatomopathological changes.
Materials and Methods
Experimental group:
Seventy 5-month old Wistar strain female rats weighing 200 ± 20 g were distributed in 7 groups: (A) control (intact rats that received LLLT sham exposures) (n=10), (B) rats with myopathy and sacrificed 24 h later (n=10), (C) rats with myopathy and sacrificed 10 days later (n=10), (D) rats with myopathy and treated with HeNe laser (n=10), (E) rats with myopathy and treated with GaAs laser (n=10), (F) intact rats treated with HeNe laser (n=10), and (G) intact rats treated with GaAs laser (n=10). A larger number of animals were not used per group because of the low dispersion shown by the variables studied in previous work.16, 17, 22)
Experimental model of myopathy:
Fifty μl of 1% carrageenan λ (type IV), dissolved in distilled water, was injected in the left gastrocnemius muscle in groups B, C, D and E, with the intention of producing muscle injury and inducing an inflammatory process.2, 23) Group B was sacrificed 24 h after injection with carrageenan in order to know the degree of injury and inflammation generated, prior to starting treatment with LLL. Myopathy groups C, D and E were sacrificed 10 days after induction of myopathy.
The investigation was conducted according to the guide for the care and use of laboratory animals published by the U.S. National Institute of Health, NIH publication (No. 85-23, revised 1996).
Treatment with LLL:
This was carried out with He. Ne laser (632.8nm), continuous beam, 5 mW and one minute of irradiation per session, and with Infrared GaAs laser (λ = 904 nm), pulsed beam, 12 mW and 47 second of irradiation per session. The energy density supplied with each laser was 9.5 J.cm−2 applied daily at the points injected with carrageenan, treated for 10 days in the groups D, E, and noninjured muscle in groups F and G.
Experimental material:
a. Plasma collection
The blood was obtained by decapitation of the animals, after anesthesia by Ketamine 10 mg/kg/rat, and was centrifuged at 3000 rpm to obtain the plasma. The NO was determined by spectrophotometry as a Griess reaction.24)
b. Muscle Tissue collection
The muscles (5/10) were cut and placed in formaldehyde 10% (single-blinded), stained with Hematoxylin-Eosin and observed by optic microscopy.
Statistical Analysis:
The quantitative variable was expressed as Mean ± SEM and statistically analyzed by ANOVA and the Fisher test, and the percentage area with inflammatory infiltrates was determined in 5 optical microscopy photographs (100X) for each group and analyzed with the Axiovision 4.8 program - Carl Zeiss Imaging Solutions GmbH (Hallbengmoos—Germany), referenced to a scale of 1μm. For the quantification analysis, Pearson's Chi Squared test was applied, establishing significant difference when p < 0.05 for all cases.
Results
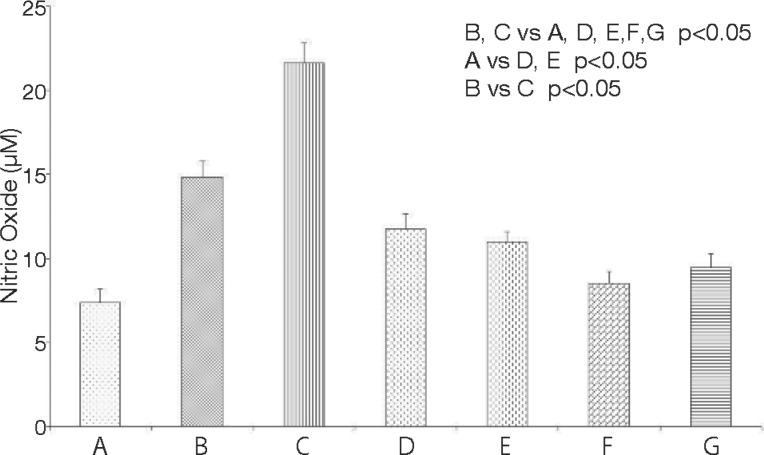
The effect of LLLT on NO (μM) levels in rats with experimental myopathy is shown in Figure 1. NO increased significantly in the group with myopathy sacrificed 24 h later (14.83 ± 0.97 μM) (B) and the group with myopathy sacrificed 10 days later (21.63 ± 1.20 μM) (C), compared with the groups: control (7.39 ± 0.77 μM) (A), with myopathy and treated with HeNe laser (11.76 ± 0.90 μM) (D), with myopathy and treated with GaAs laser (10.96 ± 0.62 μM) (E), intact rats treated with HeNe laser (8.51 ± 0.71 μM) (F) and intact rats treated with GaAs laser (9.45 ± 0.80 μM) (G) (p < 0.05). There were significant differences between groups B and C (p < 0.05). There were no significant differences between groups A, F and G.
Fig. 1:
Effect of HeNe and GaAs laser on plasma NO levels in rats with myopathy from injection of car-rageenan (n = 10). Means ± SEM are presented.
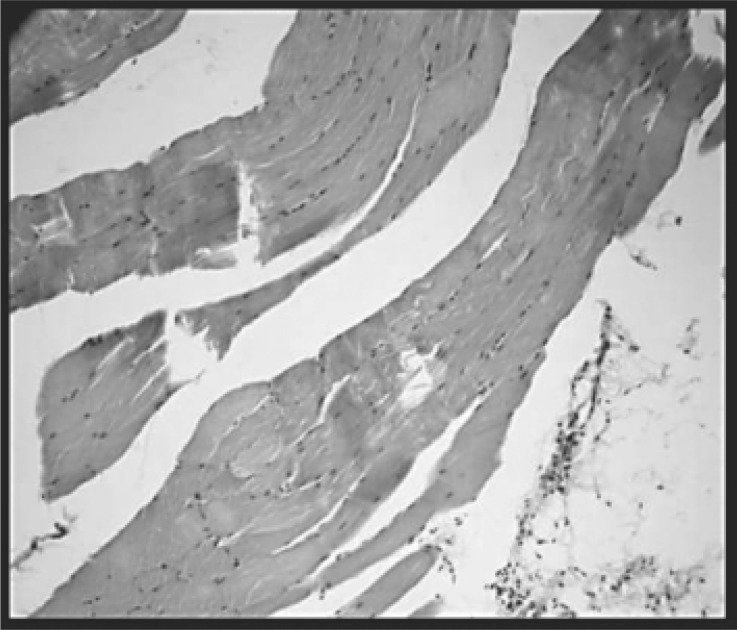
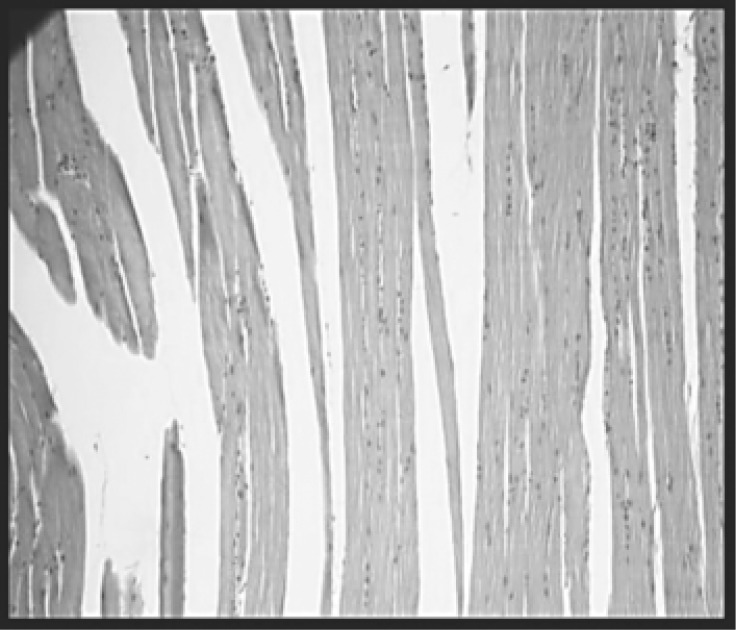
Sections of skeletal muscle of the groups: control (A), with myopathy (B and C), with myopathy and treated with HeNe laser (D) and with myopathy and treated with GaAs (E) observed by optical microscopy, are shown in Figures 2, 3, 4, 5 and 6 respectively. The percentages of area with inflammatory infiltrate in rats with induced myopathy and treated with LLLT can be seen in Figure 7.
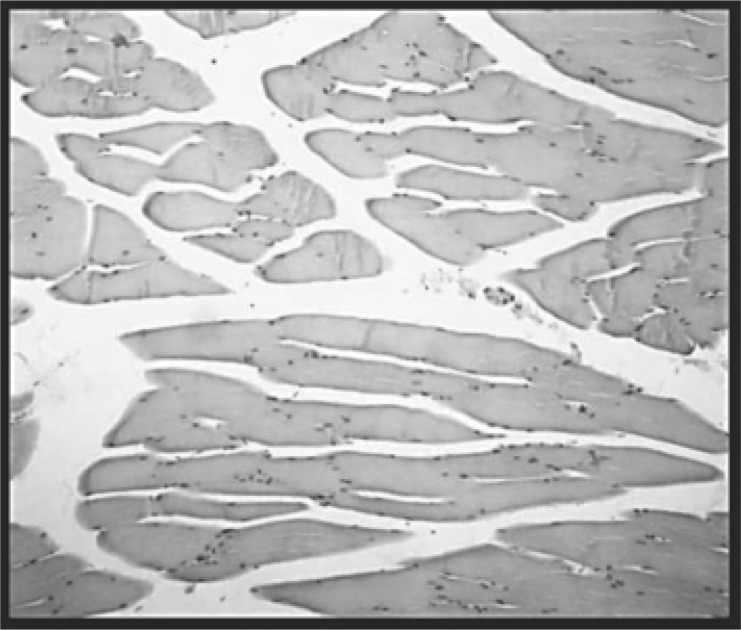
Fig. 2:
Control group (intact rats that received sham exposures) (A). Striated muscle fibers and connective tissue are preserved. No signs of myositis or fibrosis are seen. H-E (100X).
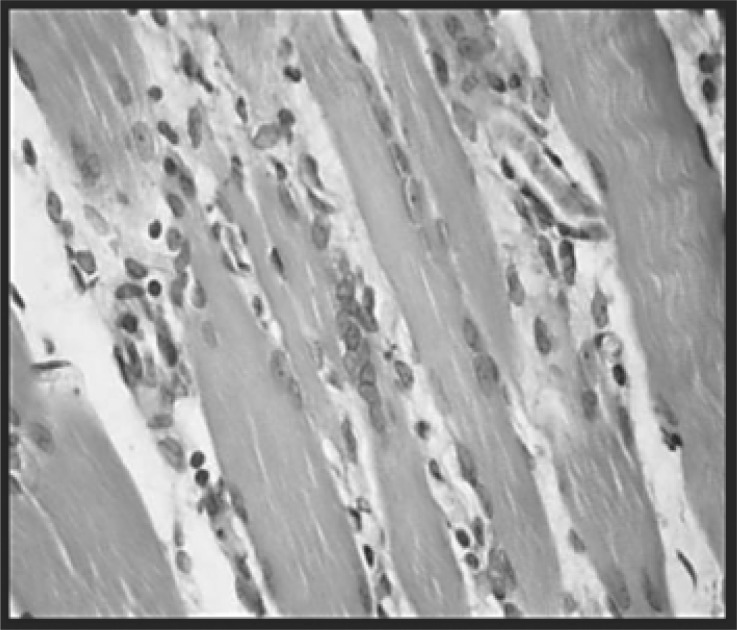
Fig. 3:
Group with myopathy and sacrificed 24 h later (B). Intense acute inflammatory infiltrate, rich in polymorphonuclear neutrophils. The inflammation and edema dissociate the fibers. H-E (100X).
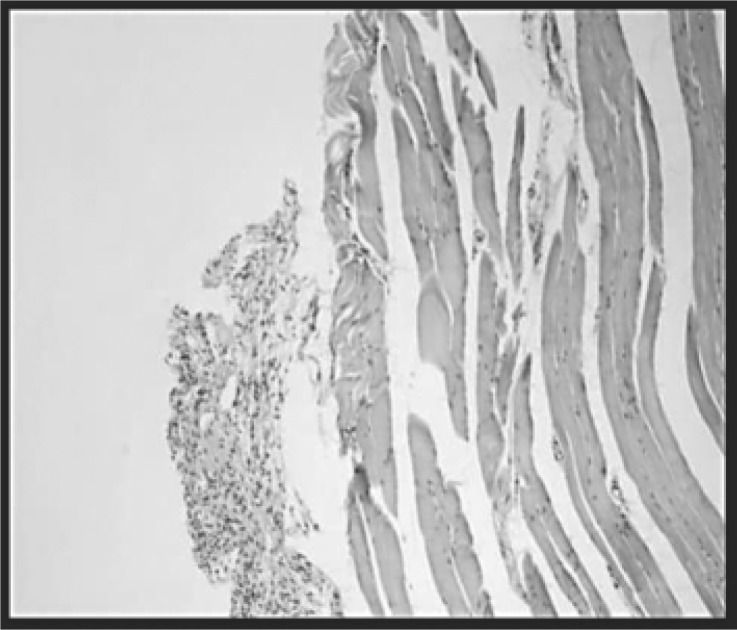
Fig. 4:
With myopathy and sacrificed 10 days later group (C): The process involves the muscle and dissociates the fibers. Chronic inflammation. H-E (400X).
Fig. 5:
With myopathy and treated with HeNe laser group (D): The muscle fibers are preserved. The inflammation was slight. H-E (100X).
Fig. 6:
With myopathy and treated with GaAs laser group (E): Striated muscle fibers and connective tissue are preserved. No signs of inflammation. H-E (100X).
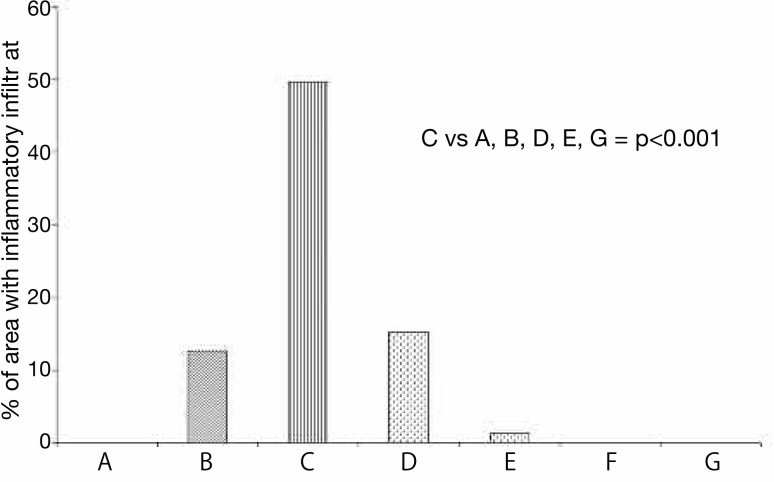
Fig. 7:
Percentage of area with inflammatory infiltrate in rats with myopathy from injections of carrageenan and treated with HeNe and GaAs laser (n=5).
The percentage of inflamed area was significantly increased in the group with myopathy sacrificed 10 days later (49.66%) (C), when compared with the groups: control (0%) (A), with myopathy sacrificed 24 h later (12.62%) (B), with myopathy and treated with HeNe laser (15.20%) (D), with myopathy and treated with GaAs laser (1.35%) (E), intact rats treated with HeNe laser (0%) (F), and intact rats treated with GaAs laser (0%) (G) (p<0.001). There were significant differences between groups D and E (p<0.001).
Discussion
Although LLLT in the clinical area is recognized for its anti-inflammatory, analgesic and trophic therapeutic properties, in the literature there are many contradictions in both clinical and experimental results.10–23) The diversity of protocols and methodologies has led to many results not being considered reliable, which motivated us to try to make a contribution through this study to consolidate criteria and knowledge.
The experimental model of myopathy was successfully reproduced. Significant edema and erythema of the limb was observed macroscopically, in addition to significant changes in concentration of biomarker and histological structures.
The results of recent work from our laboratory showed no significant plasma inflammatory indicator17) or histological structure14) changes between a group of intact rats and a group of rats injected with saline, which also confirms that the induction of the inflammatory process is attributable only to carrageenan and not to the possible stress caused by the puncture.
In the groups with myopathy, the concentration of NO (Figure 1) was significantly higher than in all other groups. These results contrast with those obtained in another experimental myopathy model performed in our laboratory,22) in which NO fell below that of the control group, so they presumably follow different routes.
When NO is found at high concentrations under conditions associated with inflammatory processes, in part it autoxidizes, generating dinitrogen trioxide (N2O3), and it partly reacts with superoxide anion (O−2) forming peroxynitrite (ONOO−). This reaction is six times faster than that of O−2 with superoxide dismutase (SOD) and leads to lower availability of NO. The significant increase of this free radical and the possible presence of ONOO− may indicate the existence of oxidative stress.7) In the groups with myopathy and treated with LLLT (D and E), NO values were significantly lower than with myopathy-untreated groups (B and C). These results may indicate that LLLT regulates the levels of ROS, possibly acting at the level of the inner mitochondrial membrane, where not only does part of the synthesis of NO occur but there is also the greatest amount of O−2.
LLLT exposed in intact rats (F and G) does not cause disruption of normal tissue at morphological or systemic levels. Concentrations of NO do not differ from control rats exposed to switched-off LLL equipment (A), nor are histological changes observed, demonstrating that the sole manipulation of the animals does not produce stress and that LLLT is a low risk treatment and does not affect cell viability at therapeutic doses.
Histological analysis of the group with myopathy and sacrificed 10 days later (C) revealed a significant percentage of area with inflammatory infiltrate (Figure 7), edema, destruction of muscle fibers and their replacement by connective tissue, with few conserved muscle fibers (Figure 4). In the group with myopathy and sacrificed 24 h later (B), there was acute inflammatory infiltrate, characteristic of the action of carrageenan (Figure 3), although the percentage of area occupied is less in this group (Figure 7). In the group of rats with myopathy and sacrificed 8 days later (C), however, the inflammation became chronic (Figure 4). The presence of neutrophils in groups B and C may explain the increased concentration of NO in the inflamed tissue. In contrast, in the groups with myopathy and treated with LLLT (D and E), there was a notable reduction in the area occupied by inflammatory infiltrate (Figure 7).
Parra Lara claims that the GaAs laser has a hypertrophic effect on rat skeletal muscle,25) since, by morphometry, it detects an increase in muscle fiber area post-irradiation without change in the density of the volume of connective tissue, results that could not be confirmed through this study.
One possible explanation for the susceptibility of skeletal muscle injured and treated with LLLT demonstrated by the reconstitution of muscle fibers (Figure 5, 6) may be related to its mitochondrial content and the presence of satellite cells. These cells would be stimulated by LLLT,26) are located over the muscle fiber but beneath the sarcolemma, are involved in skeletal muscle regeneration and participate in its growth, providing nuclei and cytoplasmic mass to the muscle fiber, 27, 28) and it is argued that LLLT promotes recovery of muscle atrophy in association with satellite cell proliferation and angiogenesis.
There were no differences between the effects generated by each of the lasers on NO levels, which behaved similarly in our experimental model of myopathy, but there were differences in the percentage of area occupied by inflammatory infiltrate between the groups D and E. In the group with myopathy and treated with GaAs laser (E), a lower percentage was found. Other authors, such as Reddy,29) argue that the HeNe laser is superior to the GaAs in promoting wound healing.
The principles of LLLT are based on photoreceptors of the mitochondrial respiratory chain, which change the membrane potential. Its effect is attributed to the formation of small amounts of ROS and antioxidants, changing the cellular redox state, reducing oxidative stress30) and leading to biological responses with therapeutic effects.31, 32) It is valued for the important benefit of having no adverse effects.
In the present work, LLLT caused great changes in inflammatory biomarkers, with decreased levels of NO in rats with experimental myopathies and significant muscle recovery.
Acknowledgements
The present work has been financed by SECyT: Universidad Nacional de La Rioja and Universidad Nacional de Córdoba and Ministerio de Ciencia y Tecnología de la Provincia de Córdoba.
References
- 1. Kumar V, Abbas AK, Fausto N. In: Trastornos de la Inmunidad. Robbins Patología humana. 2008, Elsevier España, pp 201–276 [Google Scholar]
- 2. Albertini R, Balbin Villaverde A, Aimbire F, Bjordal J, Brugnera A, Mittmann J, Silva JA, Costa M. (2008): Cytokine mRNA expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low-level laser therapy. Photom Laser Surg, 26: 19–24 [DOI] [PubMed] [Google Scholar]
- 3. Ciurtin C, Cojocaru VM, Miron IM, Preda F, Milicescu M, Bojinca M, Costan O, Nicolescu A, Deleanu C, Kovacs E, Stoica V. (2006): Correlation between different components of synovial fluid and pathogenesis of rheumatic diseases. Rom J Intern Med, 44: 171–181 [PubMed] [Google Scholar]
- 4. Pham TN, Rahman P, Tobin YM, Khraishi MM, Hamilton SF, Alderdice C, Richardson VJ. (2003): Elevated serum nitric oxide levels in patients with inflammatory arthritis associated with co-expression of inducible nitric oxide synthase and protein kinase C-eta in peripheral blood monocyte-derived macrophages. J Rheumatol, 30: 2529–2534 [PubMed] [Google Scholar]
- 5. Kanwar JR, Kanwar RK, Burrow H, Baratchi S. (2009): Recent Advances on the Roles of NO in Cancer and Chronic Inflammatory Disorders. Curr Med Chem, 16: 2373–2394 [DOI] [PubMed] [Google Scholar]
- 6. Yudoh K, van Trieu N, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. (2005): Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther, 7: 380–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sies H. (2007): Biological redox systems and oxidative stress. Cell Mol Life Sci, 64: 2181–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kandulski A, Venerito M, Malfertheiner P. (2009): Non steroidal anti-inflammatory drugs (NSAIDs) - balancing gastrointestinal complications and the cardiovascular risk. Dtsch Med Wochenschr, 134: 1635–1641 [DOI] [PubMed] [Google Scholar]
- 9. Mester E. (1966): The use of the laser beam in therapy. Orv Hetil, 29: 1012–1016 [PubMed] [Google Scholar]
- 10. Ribeiro MS, Da Silva Dde F, De Araújo CE, De Oliveira SF, Pelegrini CM, Zorn TM, Zezell DM. (2004): Effects of low-intensity polarized visible laser radiation on skin burns: a light microscopy study. J Clin Laser Med Surg, 22: 59–66 [DOI] [PubMed] [Google Scholar]
- 11. Giuliani A, Fernández M, Farinelli M. (2004): Very low level laser therapy attenuates edema and pain in experimental models. Int J Tissue React, 26: 29–37 [PubMed] [Google Scholar]
- 12. Suazo Galdames IC, Sepúlveda MCL, Cantín López MG, Zavando Matamala DA. (2007): Effect of the Low-power Laser on the Oral Mucous Injured. Int J Morphol, 25: 523–552 [Google Scholar]
- 13. Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE. (2007): Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg, 25: 436–442 [DOI] [PubMed] [Google Scholar]
- 14. Rubio C, Cremonezzi D, Moya M, Soriano F, Palma J, Campana V. (2010): Helium-Neon Laser Reduces the Inflammatory Process of Arthritis. Photomed Laser Surg, 28: 125–129 [DOI] [PubMed] [Google Scholar]
- 15. Campana V, Moya M, Gavotto A, Spitale L, Soriano F, Palma JA. (2004): Lasertherapy on artritis induced by urate crystals. Photomed Laser Surg, 22: 499–503 [DOI] [PubMed] [Google Scholar]
- 16. Soriano F, Campana V, Moya M, Gavotto A, Simes J, Soriano M, Soriano R, Spitale L, Palma J. (2006): Photomodulation of Pain and Inflammation on Microcrystalline Arthropathies Experimental and Clinical Results. Photomed Laser Surg, 24: 140–150 [DOI] [PubMed] [Google Scholar]
- 17. Rubio C, Simes JC, Moya M, Soriano F, Palma JA, Campana V. (2009): Inflammatory and oxidative stress markers in experimental crystalopathy are modified by photostimulation. Photomed Laser Surg, 27: 79–84 [DOI] [PubMed] [Google Scholar]
- 18. Reddy G. (2004): Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg, 22: 141–150 [DOI] [PubMed] [Google Scholar]
- 19. Tascioglu F, Armagan O, Tabak Y, Corapci I, Oner C. (2004): Low power laser in patients with knee osteoartritis. Swiss Med Wkly, 134: 254–258 [DOI] [PubMed] [Google Scholar]
- 20. Brosseau L, Wells G, Marchand S, Gaboury I, Stokes B, Morin M, Casimiro L, Yonge K, Tugwell P. (2005): Randomized controlled trial on low level laser therapy (LLLT) in the treatment of osteoarthritis (OA) on the hand. Laser Surg Med, 36: 210–219 [DOI] [PubMed] [Google Scholar]
- 21. Gur A, Cosut A, Sarac AJ, Cevik R, Nas K, Uyar A. (2003): Efficacy of different therapy regimes of low-power laser in painful osteoarthritis of the knee: a double-blind and randomized-controlled trial. Lasers Surg Med, 33: 330–338 [DOI] [PubMed] [Google Scholar]
- 22. Servetto N, Cremonezzi D, Simes JC, Moya M, Soriano F, Palma JA, Campana VR. (2010): Evaluation of Inflammatory Biomarkers Associated With Oxidative Stress and Histological Assessment of Low-Level Laser Therapy in Experimental Myopathy. Laser Surg Med, 42: 577–83 [DOI] [PubMed] [Google Scholar]
- 23. Bortone F, Santos HA, Albertini R, Pesquero JB, Costa MS, Silva J. (2008): Low level laser therapy modulates kinin receptors mRNA expression in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation. Int Immunopharmacol, 8: 206–210 [DOI] [PubMed] [Google Scholar]
- 24. Choi JW. (2003): Nitric production is increased in patients with rheumatoid arthritis but does not correlate with laboratory parameters of disease activity. Clin Chim Acta, 336: 83–87 [DOI] [PubMed] [Google Scholar]
- 25. Parra Lara R, Matamala Vargas F, Silva Mella H. (2007): Morphological Effect of As. Ga Laser Irradiation on Rat Skeletal Muscle. Int J Morphol, 25: 43–50 [Google Scholar]
- 26. Shefer G, Partridge T, Heslop L, Gross J, Oron U, Halevy O. (2002): Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sience, 115: 1461–1469 [DOI] [PubMed] [Google Scholar]
- 27. Goldring K, Partridge T, Watt D. (2002): Muscle stem cells. J Pathol, 197: 457–467 [DOI] [PubMed] [Google Scholar]
- 28. Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T. (2009): Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol, 94: 1005–1015 [DOI] [PubMed] [Google Scholar]
- 29. Reddy GK. (2003): Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg Med, 33: 344–351 [DOI] [PubMed] [Google Scholar]
- 30. Lindgard A, Hultén LM, Svensson L, Soussi B. (2007): Irradiation al 634 nm releases nitric oxide from human monocytes. Lasers Med Sci, 22: 30–36 [DOI] [PubMed] [Google Scholar]
- 31. Karu T, Pyatibrat L, Afanasyeva N. (2005): Cellular effects of low power therapy can be mediated by nitric oxide. Lasers Surg Med, 36: 307–314 [DOI] [PubMed] [Google Scholar]
- 32. Abdel Se, Abdel-Meguid I, Korraa S. (2007): Markers of oxidative stress and aging in Duchene muscular dystrophy patients and the possible ameliorating affect of He-Ne laser. Acta Myol, 26: 14–21 [PMC free article] [PubMed] [Google Scholar]