Abstract
Background: Rheumatoid arthritis (RA) is a systemic autoimmune disorder that involves inflammation and pain of joints. Low-level laser irradiation is being evaluated for treating RA, however, the effectiveness of linear polarized near infrared light (SuperLizer; SL) irradiation is unclear. Aim: It has been reported that interleukin 6 (IL-6) plays a key role in the progression of RA. In our previous study, using DNA microarray analysis, we examined the gene expression profiling of human rheumatoid fibroblast-like synoviocyte MH7A in response to IL-1ß administration and SL irradiation. As a result, IL-6 was listed in altered gene as increased by IL-1ß and decreased by SL irradiation.
Material and methods: The reduction of IL-6 gene expression in MH7A by SL irradiation was confirmed by reverse transcription polymerase chain reaction (RT-PCR) and real-time PCR. Effect of SL irradiation on the RA inflammation in the collagen induced arthritis (CIA) rats was also studied by measuring temperature. IL-6 production in knee joint of rats was analyzed by immunohisto-chemistry.
Results: Scatter plot analysis demonstrated that an increase in IL-6 gene expression by IL-1ß was reduced by SL irradiation. The reduction of IL-6 mRNA level by SL irradiation was successfully confirmed by RT-PCR and real-time PCR. SL irradiation treated CIA rat decreased the temperature of knee joints. The immunohistochemical analysis demonstrated a strong IL-6 staining in synovial membrane tissue of CIA rat joint, and SL irradiation significantly reduced the staining. Discussion: Since IL-6 has been identified to be an important proinflarnmatory cytokine in the pathogenesis of RA, the reduction of IL-6 expression is one of mechanisms in reduction of inflammation in RA joints by SL irradiation suggesting that SL irradiation may be useful for RA therapy.
Conclusion: SL irradiation reduced IL-6 gene expression in MH7A, and reduced inflammation and IL-6 protein expression in knee joint of CIA rats
Keywords: rheumatoid arthritis, synovial cells, rat joint, IL-6 expression
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by pain and inflammation, progressive joint destruction, significant disability, systemic manifestations and premature mortality.1) This disease is characterized by the infiltration of leukocytes into the synovial tissue and synovial fluid of joints, ultimately leading to destruction of cartilage and bone.2) A vicious cycle of altered cytokine and signal transduction pathways and inhibition of programmed cell death contribute to cartilage and bone destruction by human fibroblast-like synoviocytes and osteoclasts.3–5)
On the other hand, collagen-induced arthritis (CIA) has been widely used in the study to analyze the pathogenesis of RA, because its pathological features are similar to RA with proliferative synovitis with infiltration of polymorphonuclear and mono nuclear cells, pannus formation, cartilage degradation and erosion of bone.6) IL-6 has implicated in the pathophysiology and pathogenesis of RA.7) High levels of IL-6 can be detected in the synovial fluid and serum in RA patients. Local expression of IL-6 may in turn stimulate leukocyte recruitment to the joint, promote osteoclast maturation and activation, potentiate aggrecanase activity to increase proteoglycan breakdown, suppress chondrocytes, and stimulate synovial proliferation, eventually culminating in joint damage.8) It may also be the relevant cytokine responsible for autoimmune features in RA such as autoreactive T and B cell activation, B cell hyper reactivity and hyper-gammaglobulinemia.9) Therapeutic options for RA by reducing the activities of IL-6 have been discussed, and recently, it becomes an important strategy to block the IL-6 action using neutralizing antibody for systemic inflammation.10)
Many treatments are available to relieve RA-associated pain. Non-steroidal anti-inflammatory agents disease- modifying anti-rheumatic drugs and newer biologics are currently used, but have side effects when used over a prolonged period and are not effective in all patients. A non-invasive physiological therapy such as low-level laser irradiation (LLLI) could be important for managing pain. Since the early 1960s with different wavelength laser therapies have been used in the therapy of specific diseases, the application being the reduction in the duration of acute inflammation, the stimulation of tissue repair and the relief from pain, and bone formation.11) Different wavelength of laser including He-Ne, Ga–A1–As and Ga–As. However, there are few studies on the mechanism of anti-inflammatory effects of laser irradiation; most of the studies deal only with the analgesic properties of laser and the efficacy of LLLI as an anti-inflammatory therapy is still controversial.12)
One of LLLT instruments, the linear polarized light instrument, Super Lizer™ (SL), noninvasive properties, has been developed by Tokyo Iken Co. Ltd (http://www.tokyoiken.co.jp/). SL irradiation suppressed the Superoxide anion and hypochlorite production of human neutrophils, and suggesting an inhibitory effect against chronic pain via the reduction of reactive oxygen species production and opsonic activit.13) It has also been reported that RA-affected temporomandibular joint pain was reduced by SL Irradiation.14) Furthermore, SL irradiation was used in orthopedics and anesthesiology to treat arthralgia and neuralgia, and suggested SL irradiation is a useful tool for the treatment of alopecia areata.15) More recently, Ide16) reported that the significant analgesic effects of SL to RA patients by double blind test. We previously reported that SL irradiation may reduce IL-6 mRNA levels in the IL-1ß treated human rheumatoid fibroblast-like synoviocyte, MH7A, by DNA microarray analysis.17) However, the molecular mechanism of anti-inflammatory effect of SL on RA is still unclear.
In the present study, we confirmed the effect of SL irradiation on the reduction of IL-6 gene expression by SL irradiation, moreover, we constructed CIA rat and examined the effect of SL irradiation on the RA inflammation and IL-6 protein production in synovial membrane tissues of knee joint in vivo.
Materials and Methods
1. Synovial cell culture
MH7A human synovial cells (Cell Bank, Riken Bioresource Center, Ibaraki, Japan), which were isolated from the knee joint of a RA patient and retain the morphological and functional characteristics of primary synovial cells,18) were maintained in RPMI 1640 medium (Gibco-BRL Life Technologies Inc., Rockville, MD) supplemented with 10% fetal calf serum (FCS) and penicillin–streptomycin at 37°C under a 5% CO2 atmosphere. Cells were used at approximately 70% confluency. Prior to analysis, MH7A cells were first incubated for 16 h in medium containing 10% FCS and then in fresh medium with or without recombinant human IL-1ß (CalBiochem, EMD Bioscience Inc., Damstadt, Germany), and immediately treated SL irradiation. After 3 hrs, total RNA was recovered from MH7A cells.
The method used in SL irradiation of MH7A cells was previously described in the literature.17) Briefly, Super Lizer™ (HA2200, Tokyo Iken Co., Ltd, Tokyo, Japan) was used to irradiate MH7A cells. An output spectrum from SuperLiser™ is at wavelengths ranging from 600 nm to 1600 nm. The transmitted light irradiated to dishes thorough the polarizing plate in CO2 incubator. Irradiation occupancy was “0.5,” because the turn on/off of light was each a second. The energy density was calculated as follows: (Energy density) = (average power of density) × (exposure time). Irradiation area of laser was 45 cm2 (6.7 cm × 6.7 cm). Energy density = 0.0056 W (average power of density) × 685 s (exposure time) = 3.8 J/cm2 [Average power = 0.5 W × 0.5 (occupancy; lamp lighting time/exposure time) = 0.25 W, Average power of density = 0.25 W (average power)/45 cm2 (irradiation area) = 0.0056 W]. Cultured dishes containing IL-1ß-stimulated MH7A cells were irradiated for 685 s in the CO2 incubator (5% CO2 in 95% air at 37°C). The depth and intensity of the irradiation were regulated by, respectively, varying their distance from the laser source and the duration of the irradiation. For experimentation, we used pulsed irradiation at a frequency of 1 Hz, which produced an energy density of 3.8 J/cm2. After 3 hrs, the cells were harvested, and the RNA was extracted. In addition, the conditioned media were collected and stored at −20 °C for later use.
2. DNA microarray analysis
Total cellular RNA was isolated using Trizol (GIBCO BRL, Life Technologies, Rockville, MD, USA) by a FastPrep machine (FP120; BIO 101), and cDNA was synthesized using a Superscript II RNaseH(−) reverse transcriptase system (Invitrogen) with oligo d(T) 12–18) primer at 42°C for 1 hour. Total RNA was extracted with the Ambion RNA queous Kit (Ambion, Austin, TX) according to the manufacturer's protocol. Fluoro-dye labeled cRNA was synthesized using the Agilent Low RNA Input Fluor Linear Amp Kit (Agilent, Santa Clara, CA). For hybridization, 3.5μg of Cy3-labeled cRNA from treated and vehicle-treated cells was combined and hybridized to an Agilent 44K Whole Rat Genome Oligo Microarray (41,000 rat genes and transcripts; Agilent) according to the manufacturer's protocol. Finally, the arrays were scanned using a GeneArray Scanner (Agilent), and the scanned images were analyzed using GeneSpring 4.0 software (Silicon Genetics, Redwood City, CA). We set a cut off of > = 1.5-fold change for “induction” and “repression.”
3. Animal experiment
Female Lewis (LEW/CrlCrlj) rats, 6 weeks old, were obtained from Charles River Japan Inc. (Kanagawa, Japan), and injected with type II collagen (Sigma, Tokyo, Japan) in 250 u1 of 0.1 M acetic acid emulsified in an equal volume of complete Freund's adjuvant (Difco Labs, Michigan, USA) containing 2 mg/ml muramyl dipeptide (Wako, Tokyo, Japan) by multiple intradermal injections. Rats were anaesthetized with an intraperitoneal injection of sodium pentobarbital (Somnopentyl®, Kyoritsu Seiyaku, Tokyo, Japan) at 25 mg/kg prior to the injection. All animals were maintained and used in accordance with the guide the Care and Use of Laboratory Animals of Nihon University, School of Dentistry at Matsudo (No. 04-008). SL (SuperLizer™, HA2200, Tokyo Iken Co., Ltd, Tokyo, Japan) was used to irradiate RA cells according to the previous report. The irradiation time was 20 min, which equaled an incident energy density of 7.64 J/cm2. Each experimental group was carried out by 3 rats.
4. RT-PCR and Real time PCR Analysis
RT-PCR and real-time PCR reactions were carried out using a DNA thermal analyzer (RFN-Gene™ 6000; Corbett Life Science, Sidney, Australia). Amplification by PCR was started with an initial incubation at 95°C for 15 seconds to activate the Taq DNA polymerase, and then performed at 95°C for 5 seconds and 56°C for 15 seconds by adequate cycles. RT-PCR products were electrophoresed on 1.5% agarose gel, followed by staining with ethidium bromide to examine the size of PCR products. Real-time PCR was carried out with SYBR Premix Ex Taq™ (Perfect Real-Time PCR, Takara, Japan) and a Green PCR kit (Qiagen GmbH, Dusseldorf, Germany). To calculate gene expression fold changes, the initial template concentration was derived from the cycle number at which the fluorescent signal crossed the threshold in the exponential phase of the real-time PCR reaction. The mRNA copy unit was given by the cycle threshold value from the fluorescent signal of all the samples, including the standard curve and target genes, following the method provided by Corbett Life Science Company using RFN-Gene™ 6000 software. Details were described in an operation manual, version 1.7.40, 2006. Each assay was normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels.
The DNA primer sequences were 5′-gagccgccaggcagtggatg-3′(the forward primer for IL-6 gene): 5′-cagcgcagctgcagggttct-3′; (the reverse primer for IL-6 gene), (predicted size=399 bp); 5′-atcaccatcttccaggag-3′(the forward primer for GAPDH); and 5′-atcgactgtggtcatgag-3′(the reverse primer for GAPDH gene), (predicted size=318 bp). Values were calculated as means ± standard deviation (SD). Comparisons were made between two groups using a Student's t-test.
5. Immunohistochemistry
Formalin-fixed, paraffin-embedded specimens were subjected to antigen retrieval and endogenous peroxidase blocking (30 min), and rinsed with phosphate-buffered saline (PBS). Immunostaining was performed using Elite ABC kits (Vector, Burlingame, CA, USA) and diaminobenzidine (Kirkegaard & Perry, Gaithersburg, MD, USA) as a chromogen using anti-IL-6 polyclonal antibody (1:400; Santa Cruz Biotech, Santa Cruz, CA, USA). Peroxidase-conjugated rabbit anti-mouse immunoglobulin diluted 1:10 in PBS supplemented with 2-vol% heat-inactivated normal human serum was used. Peroxidase activity was visualised with 0.06% diaminobenzidine (Walter, Kiel, Germany) and 0.01 vol% H2O2.
Results
In this study, we searched the gene network containing IL-6 using IPA system. As a result, a network-31 was found with many network genes with Top Function; cellular assembly and organization and genetic. (Fig. 1)
Fig. 1:
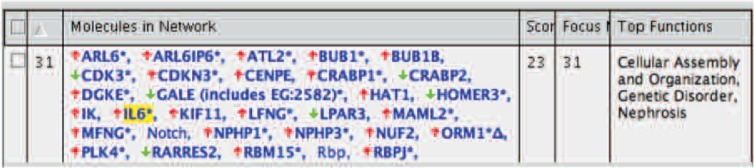
IL-6 based network search by IPA. A network-31 with up-regulated IL-6 gene expression showed 27 molecules with score 21. Arrow indicates up-regulation and down-regulation of gene expression in IL-1ß + SL compared to IL-1ß in MH7A.
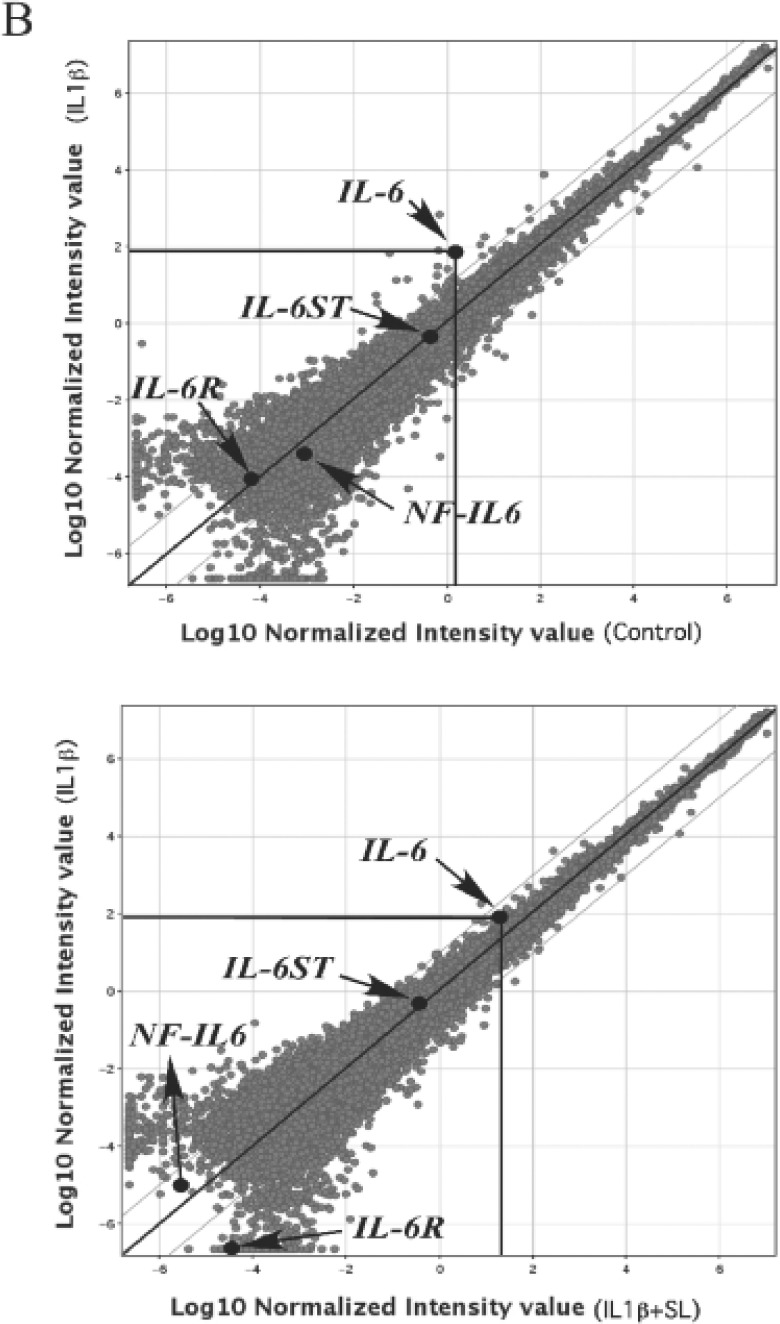
Next we analyzed candidate genes (IL-6, IL-6R, IL-6ST and NF-IL6) of IL-6 signalling pathway (Fig. 2A), and shown the scatter plot of these gene expressions in control vs IL-1ß, IL-1ß, vs IL-1ß + SL (Fig. 2B). Genes such as IL-6R, IL-6ST and NF-IL6 were marked as ‘Absent’ or less than 2-fold change by GeneChip analysis meaning did not expressed or altered those gene expressions.
Fig. 2A:
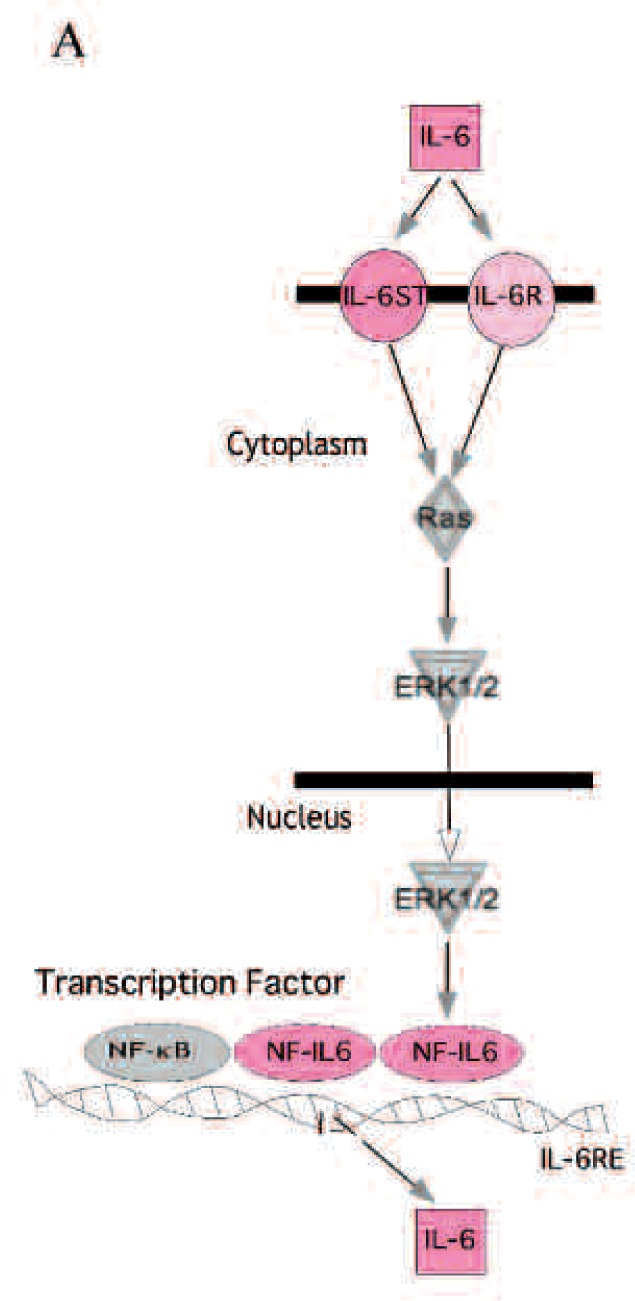
IL-6 signalling pathway and candidate genes.
Fig. 2B:
A scatter plot of mRNA levels of IL-6 signaling related genes expression in MH7A.
Since IL-6 plays a key role in the signal pathway and functions autocrine system to produce IL-6, to further investigate the elevated mRNA level of the IL-6 genes in MH7A by IL-1ß, and effect of SL irradiation. RT-PCR analysis was performed. As shown in Fig. 3, RT-PCR-amplified DNA band of IL-6 in the MH7A treated with IL-1ß was higher in density than that in control, and SL irradiation reduced the density of IL-6 DNA band in MH7A treated with IL-1ß. In contrast, mRNA levels of GAPDH, the housekeeping control, showed no differences in all these samples.
Fig. 3:
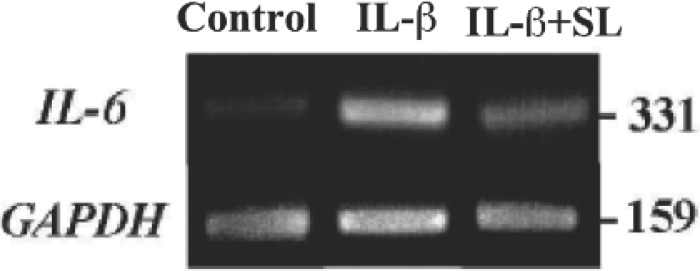
RT-PCR analysis of mRNA levels of IL-6 in MH7A. An ethidium bromide staining pattern of the amplified PCR products using agarose gel electrophoresis.
Another experiment to determine the exact rate of the enhancement of IL-6 gene expression by IL-1ß in MH7A, and the reduction of IL-6 by SL in IL-1ß treated MH7A was performed using real-time PCR. The results in real-time PCR were calculated as the mRNA copy units. As shown in Fig. 4, IL-6 gene expressions was increased by IL-1ß, and significantly decreased by SL irradiation.
Fig. 4:
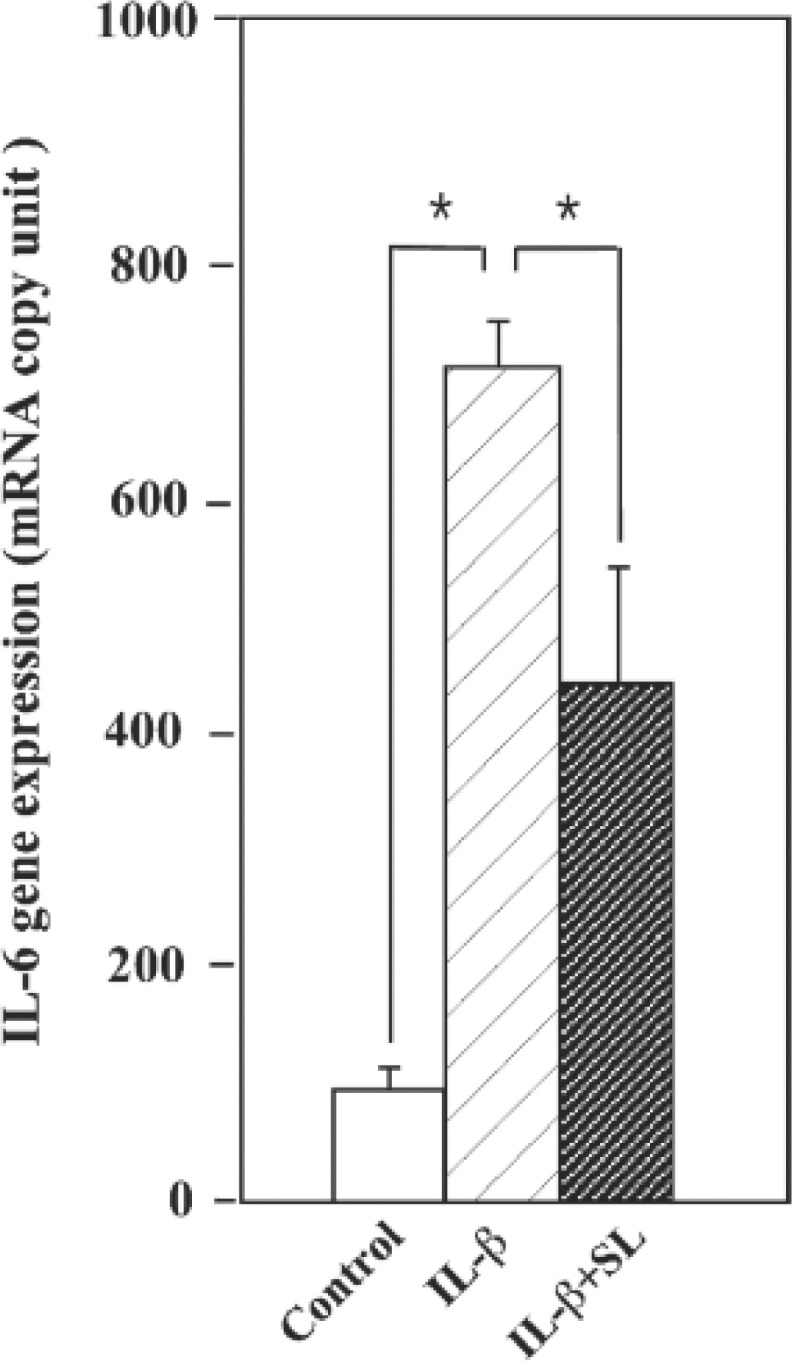
Real-time analysis of IL-6 mRNA levels in MH7A. Results were expressed as mRNA copy unit by normalization to a housekeeping gene (GAPDH). Significant differences were determined using Students t-test. * p<0.001, n=3.
Next, we tried to confirm the effect of SL irradiation on RA inflammation using rat CIA model. As shown in Fig. 5A, the hind-paw of CIA rat significantly increased temperature, and SL irradiation reduced the temperature. We examined the significant difference of the reduction rate of temperature on knee joints, SL irradiation significantly reduced the joint temperature by SL irradioation, as shown in Fig. 5. These findings suggest that SL irradiation is capable of reducing the inflammation.
Fig. 5:
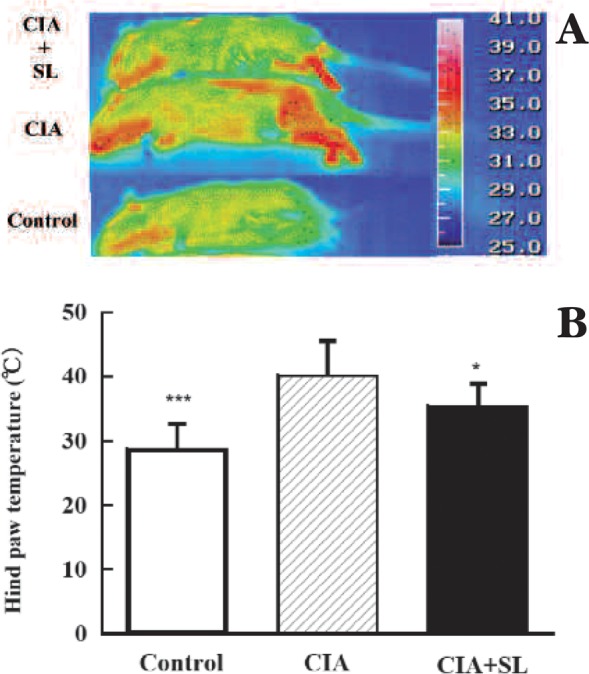
Thermographic analysis of temperature levels. A. Thermography, rat hind paws in control. CIA, and CIA + SL rats at 2 weeks. B. Analysis of significant difference, n=5, *** p < 0.001, control vs CIA; * p<0.05, CIA vs CIA+SL.
Finally, phenotypic expression of IL-6 was examined by immunohistochemistry using anti-IL-6 monoclonal antibody. Significant positive IL-6 staining was found in CIA rat when compared to control, whereas the weak staining was observed in the CIA + SL group when compared to CIA group, suggesting SL irradiation reduced IL-6 production in synovial fibroblasts in joint membrane tissue of CIA rat. (Fig. 6)
Fig. 6:
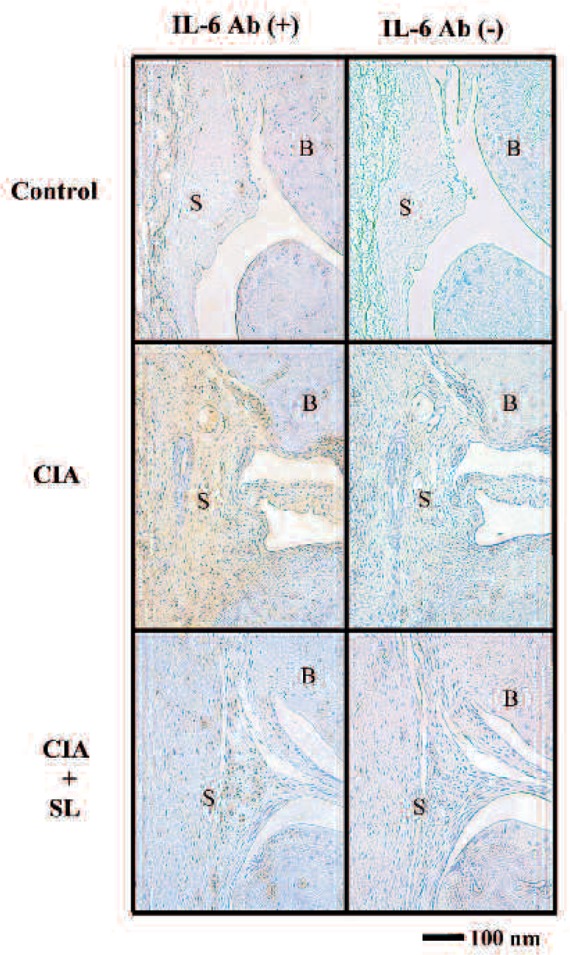
Immunostaining of IL-6. Representative photomicrographs of sections of rat knee joints stained with polyclonal anti-IL-6 antibody at 2-week.
Discussion
Transcriptional examination demonstrated that an increase in IL-6 gene expression by IL-1ß was reduced by SL irradiation. SL irradiation treated CIA rat decreased the temperature of knee joints. The immunohistochemical analysis demonstrated a strong IL-6 staining in whole synovial membrane tissue of CIA rat joint, suggesting IL-6 produced from synovial membrane fibroblasts and SL irradiation significantly reduced the staining. At this point, it is difficult to say the reduction of IL-6 production from immunoresponsed cells in synovial tissue. Such experiment needs to study. Such studies are underway in our laboratory.
Since IL-6 has been identified to be an important proinflammatory cytokine in the pathogenesis of RA, the reduction of IL-6 expression is one of mechanisms in reduction of inflammation in RA joints by SL irradiation suggesting that SL irradiation may be useful for RA therapy.
TNF-α inhibitors, and IL-1 inhibitors, have revolutionized the treatment of RA; however, a substantial number of patients fail to respond adequately to the treatment, 19–21) suggesting a role for inhibition of other cytokine signals, including IL-6. Many studies have shown that IL-6 overproduction correlates to increased RA disease activity. 22, 23) Murine models indicate that IL-6 deficiency delays the onset and reduces the severity of CIA.24) A chimeric antibody to the IL-6 receptor that blocks both soluble and membrane-bound IL-6 activity was found to inhibit CIA in monkeys, paving the way for human trials targeting this pathway. 25, 26) Overproduction of IL-6 has been shown to underlie a number of autoimmune and inflammatory diseases, including RA, and the blocking of IL-6 signaling may be therapeutic in diseases characterized by pathological IL-6 overproduction.27)
The rationale for the use of anti-cytokine therapy in inflammatory joint diseases is based on evidence from studies in vivo, which implicate major cytokines such as IL-1, tumour TNF-α and IL-6 in RA pathogenesis. In particular, IL-6 subfamily antagonists have a wide range of potential therapeutic and research applications.28)
Thus, the use of SL irradiation for RA may reduce RA inflammation, thereby alleviating joint pain, by reducing the level of IL-6. This mechanism may be more general and underlie the beneficial effects of SL irradiation on inflammatory conditions in RA.
Acknowledgements
This study was supported in part by the “Academic Frontier” Project for Private Universities: a matching fund subsidy from Ministry of Education, Culture, Sports, Science and Technology, 2007-2011 and by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (B21390497). We thank Dr. Lin Zhang for his excellent technical assistance.
References
- 1. Pincus T: Long-term outcomes in rheumatoid arthritis. Br J Rheumatol, 34: 59–73, 1995 [PubMed] [Google Scholar]
- 2. Gay S, Gay RE, Koopman WJ: Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis, 52: 39–47, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dayer JM: The saga of the discovery of IL-1 and TNF and their specific inhibitors in the pathogenesis and treatment of rheumatoid arthritis. J Bone Spine, 59: 123–132, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Inoue H, Nagata N, Nishikawa T. Oda H, Yamamoto S. and Koshihara Y: An investigation of cell proliferation and soluble mediators induced by interleukin 1ß in human synovial fibroblasts: comparative response in osteoarthritis and rheumatoid arthritis. Inflamm Res, 50: 65–72, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Firestein GS: Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol, 11: S39–S44, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Trentham DE: Collagen arthritis in rats, arthritogenic lymphokines and other aspects. Int Rev, Immunol, 4: 25–33, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Lipsky PE: Interleukin-6 and rheumatic diseases. Arth Res Therapy, 8: S4–S8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flannery C, Little C, Hughes C, Curtisa C, Caterson B, Jones S: IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Biol, 19: 549–553, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Ishihara K, Hiranoa T: IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev, 13: 357–368, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Interleukin-6: An Innovative Therapeutic Drug, Tocilizumab (Recombinant Humanized Antihuman Interleukin-6 Receptor Antibody), Unveils The Mysterious Etiology of Immune-Mediated Inflammatory Diseases. Biol Pharm Bull, 30: 2001–2006, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Mester E, Mester AF, Mester A: The biomedical effects of laser application. Lasers Surg Med, 5: 31–39, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Basford JR: Low-intensity laser therapy: controversies and new research findings. Lasers Surg Med, 9: 1–95, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Shiraishi M, Suzuki K, Nakaji S, Sugawara K, Sugita N, Suzuki KJ, Ohta S: Effect of linear polarized near-infrared ray irradiation on the chemiluminescence of human neutrophils and serum opsonic activity. Luminescence, 14: 239–243, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Yokoyama K, Oku T: Rheumatoid arthritis-affected temporomandibular joint pain analgesia by linear polarized near infrared irradiation. Can J Anaesth, 46: 683–687, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Yamazaki M, Miura Y, Tsuboi R, Ogawa H: Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol, 42: 738–740, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Ide: Phototherapy for chronic pain treatment. Masui, 58: 1401–1406, 2009 [PubMed] [Google Scholar]
- 17. Shibata Y, Araki H, Oshitani T, Imaoka A, Matsui M, Miyazawa K, Abiko Y: Effects of linear polarized infrared light irradiation on the transcriptional regulation of IL-8 expression in IL-1beta-stimulated human rheumatoid synoviocytes involves phosphorylation of the NF-kappaB RelA subunit. J Photochem Photobiol B, 94: 164–170, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Miyazawa K., Mori A., Yamamoto K. and Okudaira H.: Transcriptional roles of CCAAT/enhancer binding protein-ß, nuclear factor-KB, and C-promoter binding factor 1 in interleukin (IL)-1ß-induced IL-6 synthesis by human rheumatoid fibroblast-like synoviocytes. J Biol Chem, 273: 7620–7627, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Gomez-Reino JJ, Carmona L: Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther, 8: R29, 2006. (E-pub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ: Outcomes after switching from one antitumor necrosis factor alpha agent to a second antitumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum, 56: 13–20, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Nuki G, Bresnihan B, Bear MB, McCabe D: Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis, Rheum, 46: 2838–2846, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Sack U, Kinne RW, Marx T, Heppt P, Bender S, Emmrich F: Interleukin-6 in synovial fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatol Int, 13: 45–51, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Madhok R, Crilly A, Watson J, Capell HA: Serum interleukin-6 levels in rheumatoid arthritis: correlation with clinical and laboratory indices of disease activity. Ann Rheum Dis, 52: 232–234, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, Katada Y, Yoshizaki K, Suemura M, Kishimoto T: Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum, 42: 1635–1643, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Poli V, Maritano D: IL-6 Knockout mice. In: Fantuzzi G. (ed): Cytokine Knockouts. Totowa, New Jersey: Humana Press, pp. 213–234, 2003 [Google Scholar]
- 26. Mihara M, Kotoh M, Nishimoto N, Oda Y, Kumagai E, Takagi N, Tsunemi K, Ohsugi Y, Kishimoto T, Yoshizaki K, Takeda Y: Humanized antibody to human interleukin-6 receptor inhibits the development of collagen arthritis in cynomolgus monkeys. Clin Immunol, 98: 319–326, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Nishimoto N: Interleukin-6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Therpy, 87: 483–487, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Jazayeri JA, Carroll GJ, Vernallis AB: Interleukin-6 subfamily cytokines and rheumatoid arthritis: role of antagonists. Int Immunopharmacol, 10: 1–8 2010 [DOI] [PubMed] [Google Scholar]