Abstract
Background and aims: This study on healthy test subjects intends to show whether one-off Low-Level Laser Therapy (LLLT) has an instant effect on the perfusion or the oxygenation of the skin tissue. These possible instant effects may have an influence on the accelerated wound healing which is often observed after application of LLLT, in addition to the usual postulated effects of LLLT which occur with a time delay normally.
Study design/materials and methods: The study was carried out double-blind and placebo-controlled in two batches of testing. The test subjects received one-off LLLT on a defined area of the arch of the foot. Simultaneously a placebo treatment was carried out on the corresponding contralateral area. In the first batch of tests, the blood flow was measured immediately before and after treatment using thermography and LDI. In the second batch of tests, the blood flow and the oxygen saturation were determined immediately before and after the treatment using an O2C device.
Results: No evidence that the LLLT has a significant instant effect on the circulation or the oxygen saturation could be found.
Conclusion: No immediate effect of an LLLT on the perfusion or oxygenation situation is to be expected with physiologically normal starting conditions. An additional investigation should be carried out in which either the radiation dose is varied or the starting conditions are pathological (e.g. chronic wounds) in order to rule out immediate effects on circulation or oxygen saturation as the cause of the improved wound healing which is often observed.
Keywords: Laser-doppler flowmetry, Low-power laser therapy, Oximetry, Oxygen to see, perfusion
Introduction
Low-Level Laser Therapy (LLLT) has been used in various fields of medicine for a number of years. As a treatment principle, it is based on photobiomodulation effects which influence the components in the respiratory chain as primary photon acceptors. 1–3) Among other things, LLLT may increase cell proliferation 4–6) and have a positive impact on energy metabolism 1), as well as improving microcirculation 7) and releasing growth factors. 8)
Thanks to these postulated effects, LLLT is of particular interest in wound healing. Relevant studies address the possibility that the laser or monochromatic light may be able to influence the wound healing process. 2, 9–11) However, these results on LLLT mainly relate to in-vitro experiments, animal testing or studies of individual cases. The focus here is on the possible effects at the cellular and molecular level, which occur with a certain time delay. No investigations have been carried out as yet into whether an immediate improvement in the tissue circulation or the oxygen saturation occurs in-vivo, which would be key to wound healing. 12, 13)
The aim of this double-blind, placebo-controlled pilot study was therefore to investigate the immediate effect of one-off LLLT in-vivo on the local perfusion situation and blood oxygen content of healthy people. One interesting feature of this study was the use of a “placebo laser” which had the same structure as the LLLT device used but did not emit coherent, visible red light. An indirect influence on perfusion or oxygenation through processes which would only occur after a few hours or days was deliberately not taken into account in this study.
Materials and methods
Study protocol
Two placebo-controlled batches of tests were carried out on different healthy test subjects with no evidence of vascular disease. In the first batch of tests, the perfusion of the skin was measured using thermography and Laser Doppler Imaging (LDI). In the second batch of tests, perfusion and oxygenation were determined using an “Oxygen-to-see” device (O2C).
The LLLT and the placebo therapy were given at the same time for 15 minutes, with the actual radiation always being given on an area on the arch of the right foot and the placebo radiation always being given on an area on the arch of the left foot.
Neither the test subject nor the investigator knew which device was the placebo device and which device was the real device. The perfusion and oxygen saturation were measured immediately before and after the radiation.
Test subjects
Sixteen test subjects (ten women and six men with an average age of 35.6 years) took part in the first batch of tests in which the main circulation was recorded using a thermal camera and an LDI scanner. Nine people (three women and six men, average age 30.1 years) took part in the second batch of tests (measurement using an O2C device). Before the tests began, the foot pulse was taken from all of the test subjects, and the skin on the arch of the foot was checked. All of the participants in the study had the type and aims of the study as well as the procedures to be carried out explained to them both orally and in writing. The subjects did not receive any compensation for taking part in the study. Human studies in the article were approved by the relevant Institutional Review Board (No 4363).
Exclusion criteria
The criteria for exclusion from the study were: (1) previous LLLT; (2) known circulation disorders; (3) dermatological or inflammatory diseases in the area to be radiated.
Measurement conditions
The air humidity in the testing room was between 62 % and 84 %, and the room temperature was between 23°C and 25°C. During an individual measurement, these values changed by a maximum of 2 % or 1°C between the start of the test and the end of the test. The lighting conditions for the O2C measurements were identical for all of the test subjects and remained constant throughout the test. Before the measurements started, the participants rested with their upper body elevated for 15 minutes, with their lower ankle and foot exposed on the examination bed in order to get the circulation and the temperature to a stable level.
Measurement methods
Thermography
The recordings were made with the thermal camera “thermovision 900” made by AGEMA (measurement range: −10°C – 2000°C; resolution: 0.1 K; measurement accuracy: ± 1 K in the measurement range up to 80°C), and the distance from the measurement area on the arch of the foot was 70 cm. In order to analyse the data, the software program “Spot Explorer (Version 1.5)” was used with the Regions of Interest (ROI) defined, and the temperature changes in these areas were analysed. Both therapy and placebo areas were radiated surfaces of 5 cm × 11 cm. Square areas with comparable surfaces on the distal lower ankle were defined as reference ROIs (Fig. 1).
Fig 1.
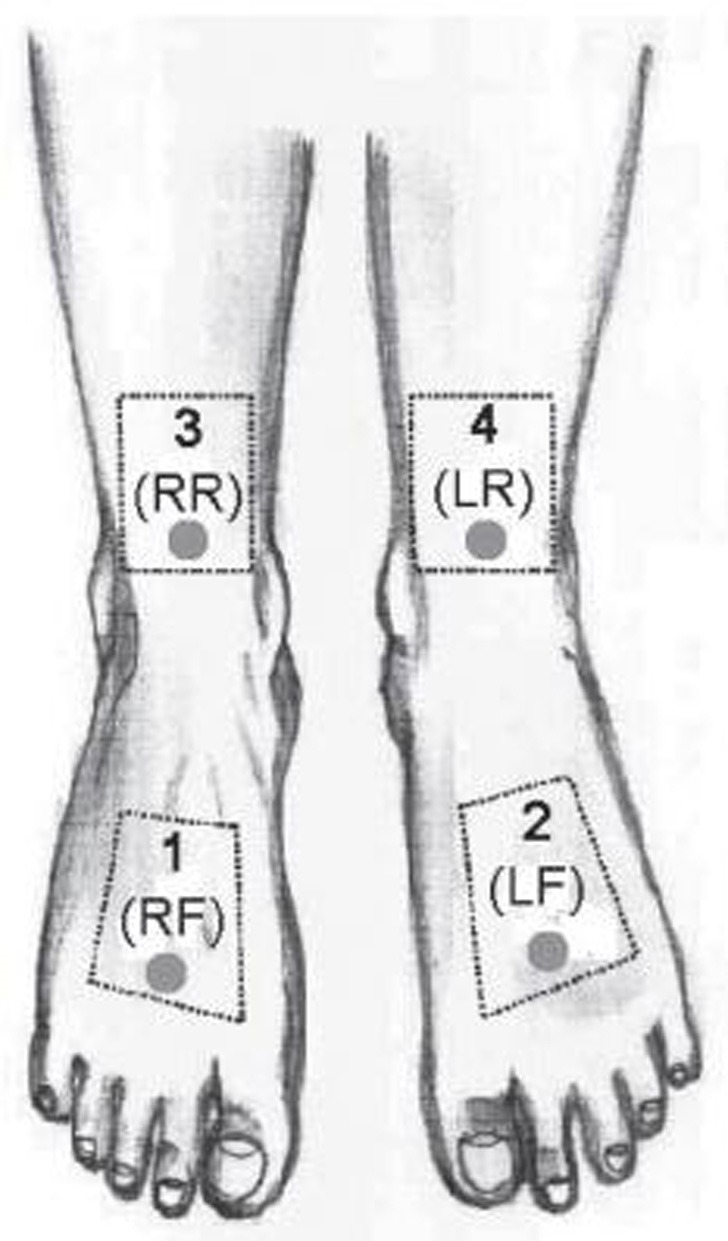
Area 1 (placebo area/RF) on the right foot and area 2 (LLLT area/LF) on the left foot are the radiation areas. Area 3 (placebo reference area/RR) and area 4 (LLLT reference area/LR) are the reference areas for the relevant radiation areas on the arch of the foot. In these areas, the O2C measurements were carried out (circulation) and the regions for the evaluation of the thermography and the LDI images were defined.
LDI scanner
The measurement was taken with the Laser Doppler Scanner “moorLDI2-VR” made by Moor Instruments (wavelength: 633 nm; measurement range: 0 – 5,000 perfusion units; resolution: 256 × 256 pixels; scan speed: 4 ms per pixel). The measurement was taken at a distance of 70 cm from the arch of the foot, and an area of 36 cm × 36 cm was assessed. The analysis of the data was carried out using the software provided by the scanner manufacturer. At the same time as this procedure was being carried out, relevant ROIs were also defined for the evaluation using the thermography images (Fig. 1).
Oxygen to see (O2C)
The measurements were taken using the “O2C type LW 1111” device made by LEA Medizintechnik GmbH. The Oxygen to see combines laser Doppler measurements (wavelength: 830 nm; power: <30 mW) with tissue spectroscopy (detection range: 450 – 850 nm; resolution: 1 nm). The flash probe LF-2 was used, which was applied parallel to the surface of the skin using an adhesive strip. After the test subjects had been resting on the examination bed for fifteen minutes, adhesive strips were attached to the relevant measurement points (Fig. 1). From a technical perspective, the measurements could only be taken one after the other using the same probe. The first measurement was therefore taken in the LLLT area, then in the placebo “laser” area, then in the LLLT reference area and finally in the placebo reference area. The time gap between the measurement in the first ROI and that in the last ROI was a maximum of two minutes. The adhesive strips were subsequently removed and the radiation was carried out. Immediately after radiation, new adhesive strips were applied to the same areas and the O2C measurements were taken once again as described above.
Radiation devices (LLLT and placebo devices)
The laser therapy device used was the FL 3500 surface laser (wavelength: 660 nm; seven semiconductor laser diodes; output power: 350 mW) made by Heltschl Medizintechnik. The placebo device was identical to the LLLT device right down to the light source. It generated an incoherent visible light using seven red LEDs with an output power of <0.3 mW per LED. The resulting heat radiation was negligible and could not be detected. Test subjects and study leaders wore laser protection goggles which filtered both the laser light and the visible light completely.
The radiation procedures were carried out under the following conditions:
Radiation duration: fifteen minutes; radiation surface: 5 cm × 11 cm (arch of the foot); equivalent dose: 5.73 J/cm2 (therapy area) or 0.03 J/cm2 (placebo area).
Analysis and evaluation
The radiation area was distal to the arch of the foot, and the associated reference area was proximal to it on the ventral lower ankle at the height of the upper ankle joint. Figure 1 shows the four different ROIs: area 1 (placebo area/RF) on the arch of the right foot and area 2 (LLLT area/LF) on the arch of the left foot correspond to the radiation areas. Area 3 (placebo reference area/RR) and area 4 (LLLT reference area/LR) on the lower ankle show the reference areas for the respective radiation areas on the arch of the foot. In these four areas, the O2C measurements were taken (circulation) and the regions for evaluating the thermography and the LDI images were defined.
The evaluation of the data is carried out using the same system for all measurement methods (in principle, calculations are the average values from the respective ROIs):
- Evaluation with no reference to the reference area
- Comparison of the four ROIs, each before and after the radiation
- Comparison of the difference between before and after the radiation (value of the relevant parameter after radiation minus its starting value)
- Relative change in the radiation area (value for the radiation area after radiation / value for the radiation area before radiation × 100 in %)
- Evaluation with reference to the reference area
- Comparison of the reference differences (value for the radiation area minus the associated reference area) at various times
- Relative change in the reference differences (value for the reference difference before the radiation / value for the reference difference after the radiation × 100 in %)
Statistics
The data was collected prospectively. In order to eliminate rogue results, only measurements which were within the range between three standard deviations from the mean were included in the analysis. All results are given as the mean ± SEM. The non-parameter Wilcoxon test for connected sampling tests was used for the statistical calculations. The evaluation was carried out using the program “Statistica (Version 7.1)” made by StatSoft, Inc. Statistical results with a value of p < 0.05 were viewed as being significant.
Results
Test subjects
None of the test subjects had to be excluded from the study — the foot pulse and the skin surface was normal in all participants. None of the participants was taking medication at the time at which the study was carried out which, to their knowledge, had any effect on the circulation or the oxygen saturation. None of the test subjects left the study early, and all of the data collected were included in the analysis.
Thermography
Figure 2 shows the temperature values of the relevant area determined for all of the test subjects. The average temperature on the test site was around 0.5°C higher than on the placebo site. Before radiation, the LLLT area differed (32.55°C ± 0.63) significantly (p < 0.03) from the placebo area (32.08°C ± 0.68). This difference increased following radiation (LLLT area: 32.61°C ± 0.67 vs. placebo area: 32.05°C ± 0.75; p < 0.006), but the different underlying temperature change between the LLLT area and the placebo area was not significant (p > 0.5).
Fig 2.
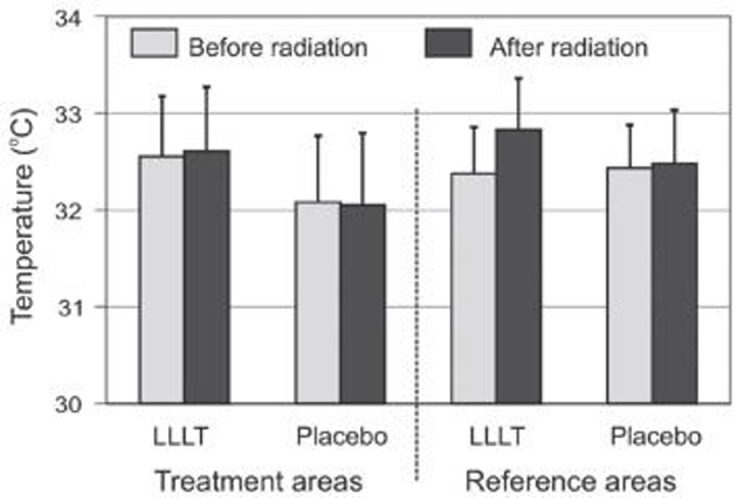
DMean temperatures of the various areas before and after radiation. Error bars represent SEM.
The temperature of the reference areas, which were not radiated, varied significantly from one another following the radiation of the test areas (0.35°C, p < 0.03). During the investigation, the temperature in the LLLT reference area increased significantly by 0.45°C (p < 0.05); the change in the placebo area, however, was not significant.
When compared to the reference areas, the radiation areas were not significantly different.
The comparison of the differences before and after radiation showed no significant difference in the radiation areas (LLLT area: 0.06 °C ± 0.30 vs. placebo area:
−0.03°C ± 0.38; p > 0.5), but there was a difference in the reference areas (LLLT reference area: 0.45 °C ± 0.19 vs. placebo reference area 0.05°C ± 0.22; p < 0.02).
Figure 3 shows the reference differences both before and after radiation. Here, the LLLT and the placebo sites only differ significantly before radiation (LLLT reference difference: 0.17°C ± 0.25 vs. placebo reference difference: −0.35 °C ± 0.34; p < 0.01), and this levelled off following the radiation (LLLT reference difference: −0.22°C ± 0.24 vs. placebo reference difference: −0.43°C ± 0.26; p > 0.07). On average, the LLLT reference difference decreased significantly by 0.39°C (p < 0.03), and the placebo reference difference increased not significantly by 0.08°C (p > 0.4). These changes differed significantly between the test leg and the placebo leg (p < 0.03).
Fig 3.
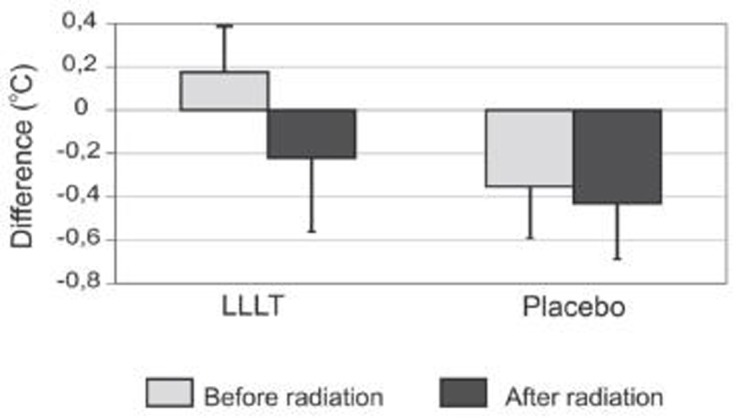
Temperature differences (radiation area minus the corresponding reference area) before and after radiation. Error bars represent SEM.
LDI scanner
Figure 4 shows the blood flow values determined for the relevant area for all test subjects. Here, there was no significant difference in the comparison between equivalent areas of the test site and the placebo site. However, the flow values in the radiation area were significantly higher than in the reference areas. This difference occurred before the radiation on both the LLLT side (LLLT area: 97.64 AU ± 9.00 vs. LLLT reference area: 71.73 AU ± 4.06; p < 0.001) and the placebo site (placebo area: 92.98 AU ± 8.55 vs. placebo reference area: 71.79 AU ± 4.64; p < 0.006). After radiation, these differences were still significant on both the LLLT site (LLLT area: 98.11 AU ± 8.87 vs. LLLT reference area: 72.57 AU ± 4.55; p < 0.0007) and on the placebo site (placebo area: 92.38 AU ± 8.00 vs. placebo reference area: 71.40 AU ± 4.40; p < 0.003) (table 2).
Fig 4.
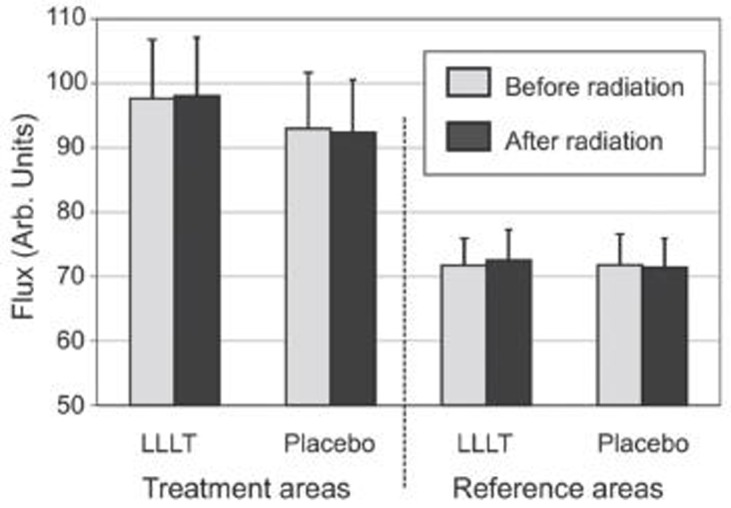
Mean blood flow as measured by Laser Doppler imaging before and after radiation. Error bars represent SEM.
Table 2.
Comparison of various parameters before and after radiation (p values)
| Thermography | ||||||
|---|---|---|---|---|---|---|
| Before radiation | After radiation | |||||
| Area compared | Value parameter 1 | Value parameter 2 | p-value before radiation | Value parameter 1 | Value parameter 2 | p-value after radiation |
| LLLT area vs. placebo area (LF & RF) | 32.55 °C ± 0.63 | 32.08 °C ± 0.68 | < 0.03* | 32.61 °C ± 0.67 | 32.05 °C ± 0.75 | < 0.006* |
| LLLT reference area vs. placebo reference area (LR & RR) | 32.38 °C ± 0.48 | 32.43 °C ± 0.45 | n. s. | 32.83 °C ± 0.53 | 32.48 °C ± 0.55 | < 0.03* |
| LLLT reference area change vs. placebo reference area change (LR afterwards - LR beforehand & RR afterwards - RR beforehand) | 0.45 °C ± 0.19 | 0.05 °C ± 0.22 | < 0.02* | |||
| LLLT area vs. corresponding reference area (LF & LR) | 32.55 °C ± 0.63 | 32.38 °C ± 0.48 | n. s. | 32.61 °C ± 0.67 | 32.83 °C ± 0.53 | n. s. |
| Placebo area vs. corresponding reference area (RF & RR) | 32.08 °C ± 0.68 | 32.43 °C ± 0.45 | n. s. | 32.05 °C ± 0.75 | 32.48 °C ± 0.55 | n. s. |
| LDI scanner | ||||||
| LLLT area vs. placebo area (LF & RF) | 97.64 AU ± 9.00 | 92.98 AU ± 8.55 | n. s. | 98.11 AU ± 8.87 | 92.38 AU ± 8.00 | n. s. |
| LLLT reference area vs. placebo reference area (LR & RR) | 71.73 AU ± 4.06 | 71.79 AU ± 4.64 | n. s. | 72.57 AU ± 4.55 | 71.40 AU ± 4.40 | n. s. |
| LLLT reference area change vs. placebo reference area change (LR afterwards - LR beforehand & RR afterwards - RR beforehand) | 0.84 AU ± 5.50 | −0.39AU ± 5.21 | n. s. | |||
| LLLT area vs. corresponding reference area (LF & LR) | 97.64 AU ± 9.00 | 71.73 AU ± 4.06 | < 0.001* | 98.11 AU ± 8.87 | 72.57 AU ± 4.55 | < 0.0007* |
| Placebo area vs. corresponding reference area (RF & RR) | 92.98 AU ± 8.55 | 71.79 AU ± 4.64 | < 0.006* | 92.38 AU ± 8.00 | 71.40 AU ± 4.40 | < 0.003* |
| O2C device | ||||||
| LLLT area vs. placebo area (LF & RF) | 21.67 AU ± 6.73 | 17.00 AU ± 6.53 | n. s. | 18.44 AU ± 3.97 | 16.22 AU ± 5.58 | n. s. |
| LLLT reference area vs. placebo reference area (LR & RR) | 19.22 AU ± 3.55 | 15.44 AU ± 2.55 | n. s. | 16.56 AU ± 2.42 | 13.67 AU ± 2.21 | n. s. |
| LLLT reference area change vs. placebo reference area change (LR afterwards - LR beforehand & RR afterwards - RR beforehand)) | −2.67 AU ± 4.04 | −1.78 AU ± 3.12 | n. s. | |||
| LLLT area vs. corresponding reference area (LF & LR) | 21.67 AU ± 6.73 | 19.22 AU ± 3.55 | n. s. | 18.44 AU ± 3.97 | 16.56 AU ± 2.42 | n. s. |
| Placebo area vs. corresponding reference area (RF & RR) | 17.00 AU ± 6.53 | 15.44 AU ± 2.55 | n. s. | 16.22 AU ± 5.58 | 13.67 AU ± 2.21 | n. s. |
If the before and after radiation difference values on the test site are compared with those on the placebo site, neither the radiation areas (LLLT area: 0.47 AU ± 8.20 vs. placebo area: −0.59 AU ± 8.22; p > 0.9) nor the reference areas (LLLT reference area: 0.84 AU ± 5.50 vs. placebo reference area: −0.39 AU ± 5.21; p > 0.5) differed significantly. There were also no significant differences in the evaluations of the reference areas.
O2C device
At no point were there any significant differences in blood flow or oxygen saturation between the test site and the placebo site. In the comparison between the radiation areas and the reference areas, no significant difference could be determined either on the test site or on the placebo site at any point. The comparison of the differences before and after radiation was also normal. No significant differences could be shown when evaluating the reference differences. An overview of the important results can be seen in tables 1 and 2.
Table 1.
Changes in individual parameters during radiation (mean values and p values)
| Thermography | ||
|---|---|---|
| Area | Mean change | P value of the change |
| LLLT reference area (LR) | + 0.45 °C ± 0.19 | < 0.05* |
| LLLT reference difference (LF — LR) | − 0.39 °C ± 0.17 | < 0.03* |
| LDI Doppler | ||
| LLLT reference area (LR) | + 0.84 AU ± 5.50 | n. s. |
| LLLT reference difference (LF — LR) | − 0.37 AU ± 5.15 | n. s. |
| O2C device | ||
| LLLT reference area (LR) | − 2.66 AU ± 4.04 | n. s. |
| LLLT reference difference (LF — LR) | − 0.55 AU ± 8.28 | n. s. |
Discussion
Study protocol and measurement methods
The problem of a possible variability of measurement between the individuals dealt with by each test subject having individual checks carried out at the same time. Since perfusion and oxygenation depend on a number of factors, the LLLT was only used once and the measurements carried out immediately afterwards, in order to achieve as isolated an observation of the effect of radiation as possible. The fifteen minute rest period before the baseline measurement appeared to be an adequate time period for the test subjects to adapt for thermography and Laser Doppler Fluxmetry, and therefore to ensure the stability of the measurement parameters. 14–16)
In terms of the dose of radiation, there are no standardised recommendations. In tests in which the main focus was on wound healing and which were carried out on people, between 1 J/cm2 and 12 J/cm2 is normally applied, rarely more. 17) This meant that the equivalent dose used in this study of 5.73 J/cm2 was around the mean of the commonly used doses.
In contrast to other studies which are based on cell cultures or animal models, this study was carried out under clinical conditions, so it is possible to make statements about the practicability of the measurement methods and effects in-vivo. The division into two batches of tests also makes it possible to compare the measurement methods used in terms of their suitability for further studies with similar hypotheses: if the microcirculation in areas of several square centimetres needs to be determined, the two dimensional Laser Doppler perfusion imaging (LDI) appears to be preferable to the one dimensional Laser Doppler Fluxmetry (LDF) of the O2C device, which only takes measurements at certain points, in terms of resolution and reproducibility of the results. 18,19) Furthermore, the measurements taken by the LDI scanner are contactless, while the fact that the measurement parameters are influenced by the necessity of fixing adhesive strips to the skin for the measurement probe should not be ruled out. Thermography alone is less suitable to show changes in microcirculation in detail, because it mainly illustrates the temperature changes in deep tissue layers. 20) However, when combined with LDI scanner measurements, as shown in this study, it is a good method of determining the peripheral circulation. In principle, combinations of measurement methods to evaluate the perfusion situation are advantageous — if the selection is made carefully then the respective disadvantages of the various methods can be balanced out. 19)
Thermography results
A study which compared the temperature values of corresponding areas on both halves of the body of healthy people showed that there are physiological differences and that these are generally not more than 0.5°C. 21) In this respect, the significant difference in temperature in the radiation areas of less than 0.5°C (Fig. 2) is not unusual. Following radiation, the difference was even more pronounced, but the underlying different temperature changes between the test site and the placebo site were not significant.
The significant difference between the reference areas after radiation is based on the increase of the temperature in the LLLT reference area during the test. This may be due to the time effect, since the skin temperature undergoes periodic fluctuations for thermoregulation reasons. 22) The change being due to the LLLT is almost completely ruled out, since the reference area was not exposed to any radiation and there was also no significant change in the relevant radiation area which may point to an effect of the LLLT. A systemic effect also appears unlikely, since the temperature did not change significantly in any of the other areas. This significant temperature increase in the LLLT area then leads to the significant change in the LLLT reference difference. In turn, this observation is the basis for the significant difference observed between the LLLT reference area change and the placebo reference area change. The significant difference between the LLLT reference difference and the placebo reference difference observed before the radiation, which anyhow only refers to temperature differences of less than half a degree, can no longer be determined due to the increase in temperature in the LLLT reference area following therapy. This means that no clear evidence can be found which would point to the temperature changes as being an effect of the LLLT.
LDI scanner results
The flux values in the distal areas (radiation areas on the arch of the foot) were significantly higher than in the proximal areas (reference areas on the distal lower ankle) at all times.
There are several studies on the physiological blood flow in the skin, which use different measurement methods and have some contradictory results. One of the oldest studies, in which the skin circulation is determined using photoplethysmography, came to the conclusion that the skin circulation on the arch of the foot is relatively low, but on the skin of the leg it was almost impossible to measure. 23) These values came from sitting test subjects, which decreased the microcirculatory blood flow in the foot significantly in comparison to the subject lying on his back due to arterial pre-capillary vasoconstriction. 24,25) However, this vasoconstriction due to the changed hydrostatic pressure should also have been found on the distal lower ankle. In this way the ratio of the values from the arch of the foot and the leg in sitting subjects would not have differed from those of lying subjects, and it would have been possible to compare the studies.
Another study using the Laser Doppler method found that the microcirculation of the arch of the foot in lying subjects was significantly higher than in a region proximal to the medial malleolus 26), a region which is directly adjacent to the reference area used here.
In contrast to this, a recent study which aimed to quantify the skin circulation in defined areas using an LDI scanner showed that the arch of the foot has the lowest perfusion rate, but the lower ankle is around the average. However, none of these differences were significant. This study, which also discussed the results of previous studies, came to the conclusion that the highest blood flow values were to be found in the face and the distal upper extremities, but there were only incongruent results for all other regions of the body. 18)
In this respect, the cause of the differences observed between the radiation and reference areas on both sides cannot be conclusively determined. It can be ruled out, however, that this is an effect of the LLLT, since the difference was already present before the radiation began. Furthermore, the change in the blood flow in the course of the test was not significant, and no difference in the change between the test site and the placebo site could be found.
O2C results
No significant different could be observed in the measurements carried out using the O2C device for any of the measurement sizes used.
This result is compatible with the first batch of tests — no evidence of an effect caused by LLLT could be found using thermography or LDI Doppler images either. The fact that the difference between the distal and proximal areas measured with the LDI scanner at the relative blood flow could not be shown with the O2C device is due to the low spatial resolution of the O2C device, which has a negative effect on the enormous local variation of the skin microcirculation. 18,27,28)
Conclusion
This study cannot make any statements about LLLT having an effect on perfusion or oxygenation only after a time delay, after repeated radiation or with pathological starting conditions. Since the postulated effects of the LLLT, such as greater cell proliferation, increased ATP availability and last but not least improved microcirculation, are based on changes in the cellular metabolism, an improvement in perfusion or oxygenation will only occur after a given latency period. 1)
The results of this study cannot identify any immediate effect of one-off LLLT on the local circulation of oxygenation in healthy tissue in-vivo. It would be sensible if further studies on the immediate effects on perfusion or oxygen saturation following LLLT would therefore take into account additional parameters, such as a variation in the radiation dose or its use with pathological starting conditions. A study including patients with chronic wounds seems to be worth recommending, in order to be able to make additional statements on the possible immediate effect of LLLT, which in addition to the postulated effects may have an influence on the accelerated wound healing which is often observed.
Editorial Comment
This paper by Heu and colleagues is extremely well-written, and the experimental protocol (apart from the laser parameters) is to be commended for its clarity and scientific structure. Given the data generated, the negative results were expected. So far so good. Negative results can be as informative as positive results, especially in the field of LLLT and photobioactivation. On the other hand, papers delivering negative results need to be scrupulously careful in design, and have an adequate range or set of parameters so that the negative results will actually mean something. In the present study the authors themselves point to the major study limitation: only one dose was used versus the sham irradiation, namely a somewhat low 5.3 J/cm2 which was delivered over a rather long 15 min.
This is possibly within the accepted range of fluences suitable for healing an open wound, if very much on the low side of the range. However, the present study did not involve an open wound, where the cellular targets are open to laser energy. To reach their vascular and associated targets, the photons had to penetrate intact skin. The skin of the foot is also quite thick, so the authors should have taken this into consideration and tried a dose-ranging study with their 5.3 J/cm2 at the low end of the range, but that gives us another problem.
Leaving the dose aside, the authors failed to mention the irradiance, which was around 6 mW/cm2, extremely low and requiring 15 min irradiation to reach the 5.8 J/cm2 fluence. The second law of photobiology, the Bunsen-Roscoe law of reciprocity, certainly allows for achieving the same biological effect when irradiance and exposure time are balanced to give the same incident fluence, but is known to fail when applied to LLLT. In vitro studies have suggested that the same fluence delivered in a shorter irradiation time with high irradiance has a greater effect on cells than with a much longer irradiation time and correspondingly lower irradiance, and I feel that is very much the case here.
There is, however, a third problem. The authors were using the contralateral foot as the sham-irradiated control. The literature has clearly shown that the systemic effect of LLLT is quite high, and I would suggest that in the 15 min irradiation period, there was more than enough time for any beneficial effect on blood flow through generation of photoproducts, cytokines or whatever, to be transferred systemically to the contralateral sham irradiated foot.
I felt, despite the recommendation to the contrary from the reviewers, that this paper should be published ‘as is’ with this editorial note, to illustrate a negative result reached with insufficient data, and perhaps inappropriate initial laser parameters. The authors need to use the same excellent study design, but with a much extended range of fluences, which do not take 15 min to deliver, and consider not using the contralateral limb model to avoid the possibility of the results being skewed by the systemic effect associated with LLLT. Finally, to get some idea of the fluences already reported, I suggest the authors use the search facility for articles in Laser Therapy on J-STAGE and look at the many papers which have shown increased blood flow, and note the very much higher fluences involved.
Acknowledgements
The authors would like to thank Heltschl GmbH (Austria, Europe) for providing the LLLT device and the placebo device free of charge.
References
- 1:Karu T. (1989): Photobiology of low-power laser effects. Health physics, 56:691 ~ 704 [DOI] [PubMed] [Google Scholar]
- 2:Lins RDAU, Dantas EM, Lucena KCR, Catão MHCV, Granville-Garcia AF, Carvalho Neto LG. (2010): Biostimulation effects of low-power laser in the repair process. Anais brasileiros de dermatologia, 85:849 ~ 855 [DOI] [PubMed] [Google Scholar]
- 3:Stadler I, Evans R, Kolb B, Naim JO, Narayan V, Buehner N, Lanzafame RJ. (2000): In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers in surgery and medicine, 27:255 ~ 261 [DOI] [PubMed] [Google Scholar]
- 4:Gao X, Xing D. (2009): Molecular mechanisms of cell proliferation induced by low power laser irradiation. Journal of biomedical science, 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5:Kreisler M, Christoffers AB, Al-Haj H, Willershausen B, d'Hoedt B. (2002): Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers in surgery and medicine, 30:365 ~ 369 [DOI] [PubMed] [Google Scholar]
- 6:Chen C, Hung H, Hsu S. (2008): Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway. Lasers in surgery and medicine, 40:46 ~ 54 [DOI] [PubMed] [Google Scholar]
- 7:Schindl A, Merwald H, Schindl L, Kaun C, Wojta J. (2003): Direct stimulatory effect of lowintensity 670 nm laser irradiation on human endothelial cell proliferation. The British journal of dermatology, 148:334 ~ 336 [DOI] [PubMed] [Google Scholar]
- 8:Peplow PV, Chung T, Ryan B, Baxter GD. (2011): Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomedicine and laser surgery, 29:285 ~ 304 [DOI] [PubMed] [Google Scholar]
- 9:da Silva JP, da Silva MA, Almeida APF, Lombardi Junior I, Matos AP. (2010): Laser therapy in the tissue repair process: a literature review. Photomedicine and laser surgery, 28:17 ~ 21 [DOI] [PubMed] [Google Scholar]
- 10:Peplow PV, Chung T, Baxter GD. (2010): Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models. Photomedicine and laser surgery, 28:291 ~ 325 [DOI] [PubMed] [Google Scholar]
- 11:Minatel DG, Frade MAC, Fran?a SC, Enwemeka CS. (2009): Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers in surgery and medicine, 41:433 ~ 441 [DOI] [PubMed] [Google Scholar]
- 12:Hopf HW, Rollins MD. (2007): Wounds: An overview of the role of oxygen. Antioxidants & redox signaling, 9:1183 ~ 1192 [DOI] [PubMed] [Google Scholar]
- 13:Leaper D. (2007): Perfusion, oxygenation and warming. International wound journal, 4 Suppl 3:4 ~ 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14:Krug A. (2007): Microcirculation and oxygen supply of tissue: method of so-called O2C. Phlebologie, 35:300 ~ 312 [Google Scholar]
- 15:Ring F. (2010): Thermal imaging today and its relevance to diabetes. Journal of diabetes science and technology, 4:857 ~ 862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16:Sun P, Jao SE, Cheng C. (2005): Assessing foot temperature using infrared thermography. Foot & ankle international / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society, 26:847 ~ 853 [DOI] [PubMed] [Google Scholar]
- 17:Sobanko JF, Alster TS. (2008): Efficacy of lowlevel laser therapy for chronic cutaneous ulceration in humans: a review and discussion. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.], 34:991 ~ 1000 [DOI] [PubMed] [Google Scholar]
- 18:St¨¹cker M, Steinberg J, Memmel U, Avermaete A, Hoffmann K, Altmeyer P. (2001): Differences in the two-dimensionally measured laser Doppler flow at different skin localisations. Skin pharmacology and applied skin physiology, 14:44 ~ 51 [DOI] [PubMed] [Google Scholar]
- 19:Wright CI, Kroner CI, Draijer R. (2006): Noninvasive methods and stimuli for evaluating the skin's microcirculation. Journal of pharmacological and toxicological methods, 54:1 ~ 25 [DOI] [PubMed] [Google Scholar]
- 20:Forster C, Greiner T, Nischik M, Schmelz M, Handwerker HO. (1995): Neurogenic flare responses are heterogeneous in superficial and deep layers of human skin. Neuroscience letters, 185:33 ~ 36 [DOI] [PubMed] [Google Scholar]
- 21:Niu HH, Lui PW, Hu JS, Ting CK, Yin YC, Lo YL, Liu L, Lee TY. (2001): Thermal symmetry of skin temperature: normative data of normal subjects in Taiwan. Zhonghua yi xue za zhi = Chinese medical journal; Free China ed, 64:459 ~ 468 [PubMed] [Google Scholar]
- 22:Kolodyazhniy V, Späti J, Frey S, Götz T, Wirz-Justice A, Kräuchi K, Cajochen C, Wilhelm FH. (2011): Estimation of human circadian phase via a multi-channel ambulatory monitoring system and a multiple regression model. Journal of biological rhythms, 26:55 ~ 67 [DOI] [PubMed] [Google Scholar]
- 23:Hertzman AB. (1938): The blood supply of various skin areas as estimated by the Photoelectric Plethysmograph. Am J Physiol, 124:328 ~ 340 [Google Scholar]
- 24:Bongard O, Fagrell B. (1990): Variations in laser Doppler flux and flow motion patterns in the dorsal skin of the human foot. Microvascular research, 39:212 ~ 222 [DOI] [PubMed] [Google Scholar]
- 25:Otah KE, Otah E, Clark LT, Salifu MO. (2005): Relationship of lower extremity skin blood flow to the ankle brachial index in patients with peripheral arterial disease and normal volunteers. International journal of cardiology, 103:41 ~ 46 [DOI] [PubMed] [Google Scholar]
- 26:Bull R, Ansell G, Stanton AW, Levick JR, Mortimer PS. (1995): Normal cutaneous microcirculation in gaiter zone (ulcer-susceptible skin) versus nearby regions in healthy young adults. International journal of microcirculation, clinical and experimental / sponsored by the European Society for Microcirculation, 15:65 ~ 74 [DOI] [PubMed] [Google Scholar]
- 27:Mack GW. (1998): Assessment of cutaneous blood flow by using topographical perfusion mapping techniques. Journal of applied physiology (Bethesda, Md. : 1985), 85:353 ~ 359 [DOI] [PubMed] [Google Scholar]
- 28:Stasinopoulos DI, Johnson MI. (2005): Effectiveness of low-level laser therapy for lateral elbow tendinopathy. Photomedicine and laser surgery, 23:425 ~ 430 [DOI] [PubMed] [Google Scholar]


