Abstract
Anecdotal reports have surfaced concerning misuse of the HIV antiretroviral medication efavirenz ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one) by HIV patients and non-infected teens who crush the pills and smoke the powder for its psychoactive effects. Molecular profiling of the receptor pharmacology of efavirenz pinpointed interactions with multiple established sites of action for other known drugs of abuse including catecholamine and indolamine transporters, and GABAA and 5-HT2A receptors. In rodents, interaction with the 5-HT2A receptor, a primary site of action of lysergic acid diethylamine (LSD), appears to dominate efavirenz's behavioral profile. Both LSD and efavirenz reduce ambulation in a novel open-field environment. Efavirenz occasions drug-lever responding in rats discriminating LSD from saline, and this effect is abolished by selective blockade of the 5-HT2A receptor. Similar to LSD, efavirenz induces head-twitch responses in wild-type, but not in 5-HT2A-knockout, mice. Despite having GABAA-potentiating effects (like benzodiazepines and barbiturates), and interactions with dopamine transporter, serotonin transporter, and vesicular monoamine transporter 2 (like cocaine and methamphetamine), efavirenz fails to maintain responding in rats that self-administer cocaine, and it fails to produce a conditioned place preference. Although its molecular pharmacology is multifarious, efavirenz's prevailing behavioral effect in rodents is consistent with LSD-like activity mediated via the 5-HT2A receptor. This finding correlates, in part, with the subjective experiences in humans who abuse efavirenz and with specific dose-dependent adverse neuropsychiatric events, such as hallucinations and night terrors, reported by HIV patients taking it as a medication.
Keywords: hallucinogen, adverse neuropsychiatric events, side effects, AIDS
INTRODUCTION
Worldwide almost 1 in 200 people are living with HIV/AIDs and every 12 s someone else becomes infected. The US Center for Disease Control reports the number of people living with HIV continues to increase due, in large part, to highly efficacious antiretroviral medicines. The Department of Health and Human Services, December 2009 guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents indicate non-nucleoside reverse transcriptase inhibitors (NNRTI) for current best practices. Efavirenz ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one; trade names: Sustiva, Stocrin) is one of the recommended NNRTIs and, because of its superior virologic efficacy, it remains the antiretroviral drug of choice (Arribas, 2003; Best and Goicoechea, 2008; Sierra-Madero et al, 2010). Efavirenz is also the key component of the most efficacious treatment cocktails, whether as a necessary add-on for widely prescribed combination drugs such as lamivudine/zidovudine (Combivir) and tenofovir/emtricitabine (Truvada) or as a one pill formulation consisting of tenofovir/emtricitabine/efavirenz (Atripla) (Gulick et al, 2004; Hammer et al, 2008).
Although highly effective, a standard dose of efavirenz is known to carry a risk of side effects that include adverse neuropsychiatric complications such as depression, anxiety, sleep disturbances, impaired concentration, aggressive behavior, night terrors, hallucinations, paranoia, psychosis, and delusions. However, the question remains as to why these side effects occur and whether recent anecdotal accounts of efavirenz abuse might somehow be connected; news reports have surfaced concerning the diversion for recreational use of efavirenz by HIV patients and non-infected teens who crush the pills and smoke the powder for its psychoactive effects (Marwaha, 2008; Sciutto, 2009). Although experiential film footage of partaking users is extremely limited, remote observation suggests a mixture of dissociative, euphoric, and relaxation effects in a couple of chronic users with intermittent aggression in the off periods. As of yet unexplored is which, if any, pharmacological properties of efavirenz might encourage illicit use.
Based on knowledge of molecular targets for other drugs of abuse, it was rationalized that profiling of receptor sites, known or suspected of mediating hallucinations, sedation, and euphoria, might provide some initial insight into efavirenz's abuse potential. Efavirenz was probed for potential hallucinogenic activity by screening for its interactions with serotonin 5-HT2A and 5-HT2C receptors, NMDA receptors (NR1aNR2a, and NR1aNR2B), the cannabinoid CB1 receptor, dopamine receptors (D1, D2, D3, and D4), CNS muscarinic receptors (M1, M4, and M5), and the Sigma1 receptor. The sedative potential of efavirenz was probed by screening for interactions with a GABAA receptor (α1β2γ2 subtype), the histamine H1 receptor, the adrenergic α2C receptor, the γ-hydroxybutyrate (GHB) receptor, and opioid receptors (μ, δ, and κ), while possible euphoric or anxiolytic effects were evaluated in the context of interactions with dopamine and serotonin transporters (DAT, SERT), and vesicular monoamine transporter 2 (VMAT), GABAA receptors, and the serotonin 5-HT1A receptor. Guided by the results of our focused receptor screen, efavirenz was tested in selected rodent behavioral assays in an effort to establish its prevailing receptor-mediated psychoactive effects in vivo.
We show here for the first time that the HIV-1 antiretroviral drug efavirenz has a pharmacological profile consistent with psychoactivity. Although efavirenz interacts with a number of molecular targets, its predominate behavioral profile in rodents is in line with lysergic acid diethylamine (LSD)-like properties mediated via the 5-HT2A receptor. While efavirenz has a dose-dependent psychoactivity and abuse potential similar to other hallucinogens, it does not appear to exert strong positive reinforcing effects based on its failure to maintain self-administration responding and inability to condition a place preference. The molecular and behavioral fingerprint for efavirenz is consistent with hallucinogen-type abuse potential and correlates with some of its medication-induced side effects described as adverse neuropsychiatric events.
MATERIALS AND METHODS
Chemicals
[3H]Mesulergine (TRK845; 80 Ci/mmol) was from GE Healthcare Life Sciences (Piscataway, NJ). Both myo-[3H]inositol (ART0261; 40 Ci/mmol) and (E,RS)-(6,7,8,9-tetrahydro-5-hydroxy-5H-benzocyclohept-6-ylidene)acetic acid ([3H]NCS-382, ART1114; 20 Ci/mmol) were from American Radiolabeled Chemicals (Saint Louis, MO). All other radiochemicals were sourced from Perkin Elmer (Saint Louis, MO): 4-(2′-methoxy)-phenyl-1-[2′-(N-2-pyridinyl)-P-fluorobenzamido]ethyl-piperazine ([3H]MPPF, NET-1109; 80 Ci/mmol); [3H]methylspiperone ([3H]MSP, NET-856; 84 Ci/mmol); [3H]-(+)-pentazocine (NET1056; 29 Ci/mmol); [3H]CP55 940 (NET1051; 180 Ci/mmol); [3H]naloxone (NET719; 60 Ci/mmol); [3H]SCH23390 (NET930;70 Ci/mmol); [3H]mepyramine (NET594; 30 Ci/mmol); [3H]quinuclidinyl benzilate (NET656; 40 Ci/mmol); [3H]rauwolscine (NET722; 79 Ci/mmol); and [3H]ketanserin (NET791; 90 Ci/mmol). Cocaine hydrochloride, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), and LSD were purchased from Sigma Aldrich (St Louis, MO). Efavirenz was purchased from Sequoia Research Products Limited (Pangbourne, UK). MDL100 907 was synthesized and kindly provided by Dr Kenner C Rice (NIDA/NIAAA). All other drugs and reagents were purchased from Sigma Aldrich or Tocris Biosciences (via R&D Systems, Minneapolis, MN).
Receptor Profiling Assays
Efavirenz was probed for its ability to interact with a number of molecular targets including serotonin receptor subtypes (5-HT1A, 5-HT2A, and 5-HT2C), dopamine receptor subtypes (D1, D2, D3, and D4), opioid receptor subtypes (μ, δ, and κ), muscarinic receptor subtypes (M1, M4, and M5), the cannabinoid CB1 receptor, the histamine H1 receptor, the GHB receptor, the adrenergic α2C receptor, and the Sigma1 receptor. Efavirenz was also tested for interactions with transporters (DAT, SERT, and VMAT2) and ion channels including a GABAA receptor (α1, β2, and γ2) and N-methyl-D-aspartate (NMDA) receptor subtypes (NR1aNR2a, and NR1aNR2B).
Preparation of Membranes for Radioligand Binding Assays
Host cell lines lacking the receptor subtypes of interest were used to stably express individual cloned receptors as described by us previously (Ericksen et al, 2009). The human embryonic kidney (HEK293) cell type was used for the expression of the following receptors: cloned rat dopamine D2 and D4 receptors, human α2C receptor, and human serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors. CHO, Att20, and CHO-K1 cells were host cell types for expressing cloned human dopamine D1, dopamine D3, and human histamine H1 receptors, respectively, and the MCF-7 cell type was the host for expressing the cloned human Sigma1 receptor. The expression level of the different receptor subtypes in individual clonal lines was determined by radioligand saturation isotherm binding utilizing rapid filtration techniques and cell membrane preparation as described previously (Ericksen et al, 2009). Permeabilized platelets from New Zealand albino rabbit was the source of VMAT2 (Cesura et al, 1990). Tissue from rat brains (with the cerebellum and brain stem removed) was the source of cannabinoid CB1, GHB, opioid (μ, δ, and κ), and muscarinic (M1, M4, and M5) receptors, and membranes were prepared as described previously for CB1 receptors (Lacivita et al, 2010).
Radioligand Binding Assays
Compounds were tested for their ability to compete with radioligands specifically bound to membranes from cells or tissue expressing one of the following molecular targets: dopamine D1, D2, D3, or D4 receptors, serotonin 5-HT1A, 5-HT2A, or 5-HT2C receptors, μ, δ, and κ opioid receptors, adrenergic α2C receptor, histamine H1 receptor, Sigma1 receptor, cannabinoid CB1 receptor, GHB receptor, or VMAT2. The equilibrium binding conditions are shown in Table 1. Plain glass tubes were used in all binding reactions, and were silanized in the case of cannabinoid receptor assays. Binding reactions were rapidly terminated by filtration through GF/C filters pretreated with 0.5% polyethyleneimine, or GF/B filter pretreated with 1% polyethyleneimine in the case of cannabinoid receptors, by washing with 3 × 3 ml of ice-cold (0–2 °C) binding buffer pHed at that temperature. Radioactivity bound to filters was quantified in by scintillation spectroscopy. Membrane protein concentrations varied from 0.01 to 0.05 mg/ml.
Table 1. Conditions for Measuring Efavirenz Displacement of Radioligands Specifically Bound to Selected Receptor Subtypes.
| Receptor | Radioligand | Drug for defining nonspecific binding | Binding buffer | Binding conditions |
|---|---|---|---|---|
| 5-HT1A | [3H]MPPF | 5 μM NAN-190 | 50 mM Tris, pH=7.4 at 25 °C | 90 min at 25 °C |
| 5-HT2A | [3H]MSP | 5 μM mianserin | 50 mM Tris, pH=7.4 at 25 °C | 90 min at 25 °C |
| 5-HT2C | [3H]mesulergine | 5 μM mianserin | 50 mM Tris, pH=7.4 at 25 °C | 90 min at 25 °C |
| D1 | [3H]SCH23390 | 5 μM (+)-butaclamol | 50 mM Tris, pH=7.4 at 25 °C | 90 min at 25 °C |
| D2, D3, D4 | [3H]MSP | 5 μM (+)-butaclamol | 50 mM Tris, pH=7.4 at 25 °C | 90 min at 25 °C |
| α2C | [3H]rauwolscine | 5 μM risperidone | 50 mM Tris, pH=7.4 at 25 °C | 90 min at 25 °C |
| H1 | [3H]mepyramine | 5 μM cetirizine | 50 mM Tris, pH=7.4 at 25 °C | 180 min at 25 °C with shaking |
| Sigma1 | [3H]-(+)-pentazocine | 5 μM BD1063 | 50 mM Tris, pH=8.0 at 37 °C | 180 min at 37 °C with shaking |
| VMAT2 | [3H]ketanserin | 10 μM tetrabenazine | 50 mM Tris, 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.5 mM EDTA pH=7.4 at 25 °C | 60 min at 25 °C |
| GHB | [3H]NCS382 | 1 mM NCS382 | 50 mM KH2PO4 pH=6.0 at 4 °C | 120 min at 4 °C |
| Opioid (μ, δ, κ) | [3H]naloxone | 10 μM naltrexone | 50 mM Tris, pH=7.4 at 25 °C | 45 min at 25 °C |
| M1, M4, M5 | [3H]Quinuclidinyl benzilate | 100 nM atropine | 50 mM phosphate, pH=7.4 at 25 °C | 60 min at 25 °C |
| CB1 | [3H]CP55,940 | 10 μM O-2050 | 50 mM Tris, pH=7.4 at 30 °C, 2.5 mM EDTA 5 mM MgCl2 5 mg/ml fatty acid-free BSA | 180 min at 30 °C with shaking |
The GHB, opioid, muscarinic, and CB1 receptor assays were performed in rat brain membranes, rather than cloned receptors. Test concentrations for all radioligand were approximately 0.5 nM, except for [3H]quinuclidinyl benzilate, which was tested at 0.15 nM; [3H]-(+)-pentazocine, [3H]naloxone, and [3H]mepyramine, which were tested at a concentration of 1 nM; and [3H]CP55 940 and [3H]ketanserin, which were tested at a concentration of 2 nM.
All data sets were performed in duplicate or triplicate and reported as the mean with associated errors.
Inositol Phosphate Formation
Inositol phosphate (IP) formation in HEK293 cells was determined by labeling cellular inositol lipids with myo-[3H]inositol. Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum at 37 °C in a 5% CO2 humidified atmosphere on 12-well plates and transfected to transiently express the human 5-HT2A receptor (pcDNA3.1-c-Myc-5-HT2A), using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions (see Gonzalez-Maeso et al, 2008). After 12 h, 1 μCi/ml myo-[3H]inositol was added. After 24 h, cells were washed three times with Hanks' balanced salt solution (HBSS) supplemented with 20 mM LiCl and 20 mM HEPES, and incubated with the desired concentrations of the drugs for 30 min at 37 °C. At the end of the incubation, the plates were placed on ice and, immediately after removal of the media by aspiration, cells were treated with 1 ml of 100 mM formic acid on ice for 1 h, and were mixed well by gentle swirling. The extracts were applied to Dowex columns (Bio-Rad AG 1-X8, 200–400 mesh) that had been pretreated with H2O and equilibration buffer (2.5 mM HEPES, 0.5 mM EDTA, pH 7.4). After the samples were loaded, the columns were washed three times with equilibration buffer, and then eluted with a buffer containing 1 M ammonium formate and 0.1 M formic acid. The radioactive eluate was quantified by scintillation spectroscopy.
[3H]Neurotransmitter Flux Assays for DAT and SERT
The interactions of efavirenz with transporters were tested by measuring intracellular accumulation of extracellularly applied [3H]neurotransmitter in cells lines expressing cloned human DAT and SERT. No significant [3H]neurotransmitter accumulation was observed in untransfected HEK293 cells. Methodology was similar to that described by Han and Gu (2006). The extent of blockade of transporter protein was determined as a relative measure of accumulated intracellular [3H]neurotransmitter in the absence and presence of test compound. All responses were normalized relative to maximal blockade of [3H]neurotransmitter uptake produced by 10 μM nomifensine for DAT and 10 μM fluoxetine for SERT.
GABAA and NMDA Receptor Electrophysiology
Whole-cell currents were obtained and analyzed from HEK293 cells stably expressing (α1β2γ2 (long isoform of the γ2-subunit) or transiently expressed NMDA receptors (NR1a with either NR2A or NR2B subunits), as described previously (Gonzalez et al, 2009; Huang et al, 2010). A minimum of three individual experiments was conducted for each paradigm. All data are presented as means±SEM.
Protein Assay
Membrane or whole-cell protein concentrations were determined by bicinchoninic acid assay (Pierce, Chicago, IL) according to the manufacturer's instructions. Purified bovine serum albumin (BSA) was utilized to construct a protein standard curve.
Behavioral Studies
All animals were housed and procedures were conducted in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003) and were approved by Institutional Animal Care and Use Committees (IACUC). Efavirenz was administered intraperitoneally or intravenously because it is known to cross the blood–brain barrier (Best et al, 2011).
Locomotor Activity Procedures
Open-field locomotor activity in a novel environment was utilized for initial dose finding studies. Male ND4 Swiss Webster mice (weight 25–35 g) were purchased from Harlan laboratories (Houston, TX) at 7 weeks of age. Upon arrival, the animals were allowed to acclimate to the UNTHSC vivarium for 1 week. All animals were housed in groups of four in polycarbonate cages (17.8 × 30.5 × 12.7 cm3) at 20±2 °C, fed Prolab RMH 1800 5LL2 (PMI Nutrition International, St Louis, MO), and received water ad libitum. All behavioral testing was completed during the light cycle of the 12 h light/dark cycle (lights on at 0700 hours).
Locomotor activity was assessed using 40 Digiscan (Model RXYZCM; Omnitech Electronics, Columbus, OH) locomotor activity-testing chambers (40.5 × 40.5 × 30.5 cm3) housed in sets of two within sound-attenuating chambers. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination. Fans provided an 80-dB ambient noise level within the chamber.
Separate groups of eight mice received intraperitoneal injections of vehicle (2% methylcellulose or saline), LSD (3 mg/kg in 0.9% saline), or efavirenz (3, 10, or 30 mg/kg in 2% methylcellulose). Horizontal activity (interruption of photocell beams) was measured for 120 min within 10-min time intervals, beginning at 0800 hours (2 h after lights on). Testing was conducted with one mouse per activity chamber.
Drug Discrimination Procedures
Male Sprague–Dawley rats were obtained from Harlan Laboratories, housed individually, and maintained on a 12 h light/dark cycle with lights on at 0700 hours. Body weights were maintained at 320–350 g by limiting food (20 g per day including food received during training sessions), whereas water was freely available in the home cages.
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC-compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in MED-PC IV (Med Associates) for the operation of the chambers and collection of data. Separate groups of rats were used that had received discrimination training for (+)-LSD (0.1 mg/kg intraperitoneally, 15 min before testing), (±)-methylenedioxymethamphetamine (MDMA) (1.5 mg/kg intraperitoneally, 15 min before testing), cocaine (10 mg/kg intraperitoneally, 10 min before testing), or carisoprodol (100 mg/kg intraperitoneally, 20 min before testing) according to the two-lever choice methodology described previously (Gatch et al, 2009). All these training drugs were dissolved in 0.9% saline. Efavirenz (3 to 30 mg/kg, i.p.) was studied in separate groups of rats discriminating (+)-LSD, (±)-MDMA, carisoprodol, or cocaine according to a repeated-measures design (Gatch et al, 2009). Subsequently, efavirenz (18 mg/kg intraperitoneally, 5 min before testing) was trained as a discriminative stimulus using doses from 3 to 30 mg/kg administered in a 2% methylcellulose solution. A dose of 0.1 mg/kg of LSD was studied in rats discriminating efavirenz.
Head-Twitch Procedures
Head twitch is a common mouse behavioral proxy of human hallucinogenic potential and this behavior appears to be centrally mediated. Previous studies have shown that a range of structurally diverse hallucinogenic 5HT2A agonists, such as LSD and psilocybin, induce head twitch in mice, while non-hallucinogenic 5HT2A agonists, such as lisuride or ergotamine, do not. Head twitch is absent in 5HT2A-KO mice, but the loss of head-twitch behavior can be rescued in 5HT2A-knockout (KO) mice by re-expression of a 5HT2A transgene only in cortical glutamatergic neurons (González-Maeso et al, 2007). Experiments were performed on adult (8–12 weeks old) male 129S6/SvEv. 5-HT2A-KO mice have been described previously (González-Maeso et al, 2007). Wild-type and 5-HT2A-KO were generated by interbreeding heterozygous mice. Animals were housed at 12 h light/dark cycle at 23 °C with food and water ad libitum.
Head-twitch behavioral response (rapid lateral movements of the head similar to the pinna reflex) was measured as previously reported with minor modifications (González-Maeso et al, 2007). Efavirenz was dissolved in a final solution containing 1 : 1000 (v/v) DMSO : 0.9% saline. Briefly, animals received an intraperitoneal injection with appropriate treatments or vehicle; they were immediately placed into the center of a Plexiglas cage (28 × 18 × 15 cm3) for 15 min, during which time they were videotaped at close range by a digital video camcorder positioned directly above the cage. Videotapes were scored for head twitches by an experienced observer blind to genotype and treatment. Testing cages were thoroughly cleaned after each animal was tested to eliminate any odor cues.
Drug Self-administration Procedures
For self-administration studies, eight male Sprague–Dawley rats (Harlan Laboratories), weighing 250–300 g on arrival, were housed individually in a temperature (24±1 °C) and humidity (50±10%) controlled room under a 12/12 h light/dark cycle. Experiments were conducted during the light period. Water was freely available in the home cage. After test sessions, rats were returned to their home cages where they had restricted access to standard laboratory rat chow (Harlan Teklad 7912) such that they maintained a body weight 85% of their age-appropriate weight, as determined by growth curves provided by the vendor. Data are presented only for the five rats that completed all phases of the study (see below).
Experimental chambers (25.5 × 29.5 × 19 cm3 high; MED Associates) used for drug self-administration studies were located inside sound-attenuating, ventilated cubicles (Model ENV-22 M; MED Associated). Each chamber was equipped with two levers (11.5 cm apart) with 2.5-cm translucent stimulus lights located above each lever. Food pellets were delivered via a hopper to a 5 × 5 cm2 opening located between the levers. MED-PC IV software and interface linked to a computer controlled the experimental devices and allowed for data acquisition.
Following at least 1 week of habituation to the laboratory, access to food was restricted to 10 g per day and rats were trained to press levers for food pellets. In daily session, rats could press the right lever (left lever was not active) to receive a food pellet; sessions ended after the delivery of 50 pellets or 60 min, whichever occurred first. The criterion for completion of lever press training was the delivery of 50 food pellets in three consecutive sessions.
Under aseptic conditions, a chronic, indwelling intravenous catheter was surgically implanted into the jugular vein of rats anesthetized with isofluorane. In instances when catheterization of the jugular vein failed, the femoral vein was catheterized instead. Specifically, each catheter (10 cm CBAS-C30 (3 French) heparin-coated polyurethane; Instech Solomon, Plymouth Meeting, Pennsylvania, USA) was implanted, secured to the vessel, and then tunneled subcutaneously from the site of insertion to the midscapular region where it exited and was connected to an access port (PMINA-CBAS-C30 (7 mm high); Instech Solomon). Rats wore a mesh jacket (RJ02; Lomir Biomedical, Malone, NY) to which the exteriorized port and catheter were attached. Self-administration sessions began 2 days after surgery. During self-administration sessions, the port was connected to PI tubing and a 30-ml plastic syringe by a 22-G Huber point needle. The syringe was mounted in and the injections controlled by a Razel syringe driver (Model A; Stamford, CT). Catheters were filled with heparinized saline immediately after surgery and after every experimental session. When a catheter failed (as evidenced by leakage or by lack of response to an intravenous infusion of methohexital), it was surgically repaired or replaced (jugular or femoral).
During self-administration sessions, the stimulus light above the right lever was illuminated and rats could press that lever to receive an intravenous infusion of cocaine (0.32 mg/kg per infusion), saline, vehicle, or a dose of efavirenz. As the ability of efavirenz to serve as a positive reinforcer was initially uncertain, the dosage was presented incrementally beginning with low doses (1.0, 0.32, 3.2, or 10.0 mg/kg per infusion), so as to avoid initial presentation of a dose that potentially had aversive effects. Daily sessions lasted 1 h or until rats received 20 infusions, whichever occurred first. Each infusion was followed by a 30-s timeout, during which the chamber was dark and lever presses had no programmed consequence. As responding for cocaine stabilized, the response requirement was increased to fixed ratio 2. Immediately before the beginning of a session, rats received a single noncontingent (priming) infusion of the same substance that was subsequently available only after pressing the lever. Initially rats were trained to self-administer cocaine in a saline vehicle until individual animals reached the following criteria for three consecutive sessions: self-administering at least 10 infusions in each session with the number of infusions per session not varying by more than ±15% of the mean of number of infusions of those sessions. Next, rats were tested with cocaine dissolved in the same 1 : 1 : 18 (v/v/v) ethanol, emulphor, and 0.9% saline (1 : 1 : 18) vehicle that was used to dissolve efavirenz. Response to cocaine dissolved in the 1 : 1 : 18 vehicle was studied before, during (ie, between the second and third dose of efavirenz), and after studies with efavirenz. Substances were examined for a minimum of three sessions and until responding was stable (ie, number of infusions did not vary by more than ±15% over 3 consecutive days). Tests with cocaine and with different doses of efavirenz were each followed by sessions in which 1 : 1 : 18 vehicle was available for self-administration.
Conditioned Place Preference Procedure
Male Sprague–Dawley rats were obtained, provided water, fed, and housed as in the drug discrimination procedures described in the previous section. Cocaine (10 mg/kg) and different doses of the antiretroviral compound efavirenz (5 and 10–20 mg/kg) were tested for their ability to condition a place preference using an unbiased procedure adapted from Cunningham et al (2006). The apparatus consisted of eight acrylic test chambers (22 × 12 × 12 in) each with four different, interchangeable, floor options. During a pre- and post-test, the floor was split into two 11 × 12 in floors, one consisting of grid bars (rods) and the other a sheet with perforations (holes). During conditioning, the entire floor of the apparatus was either rods or holes. The position of the rat within the apparatus was recorded using a photocell-based system (Model 71-CDCN; CDCX; CDFS; Accuscan). The acrylic chambers were housed separately in sound-attenuating chambers (Model 71-CDEC; Accuscan). Ambient noise within the chambers was 64 db and testing took place under dim illumination (31.8±1.5 lx).
Sprague–Dawley rats (2–4 months of age) received a 15-min pretest in which the apparatus had split floors (half-rods and half-holes) and the time spent on each floor was recorded. Conditioning sessions began on the third day following the pretest, using full floors. Drug injection (intraperitoneally) was paired with one of the floor types or vehicle with the other floor type, during each of eight, 15-min conditioning sessions conducted on separate days (alternating each day between drug and vehicle). Just before each of the four drug conditioning sessions, different groups of rats received 10 mg/kg cocaine, 5 mg/kg efavirenz, or an escalating dose of efavirenz (10 mg/kg for the first two sessions and then 20 mg/kg for the second two drug conditioning sessions). The acquisition of conditioned place preference was assessed in a 15-min post-test conducted using the split floors on the day following the last conditioning session. An increase in the time spent on the cocaine-paired floor during the post-test, relative to that on the same floor during the pretest, was considered evidence for conditioned place preference. Conversely, a decrease in time would provide evidence for conditioned place aversion.
RESULTS
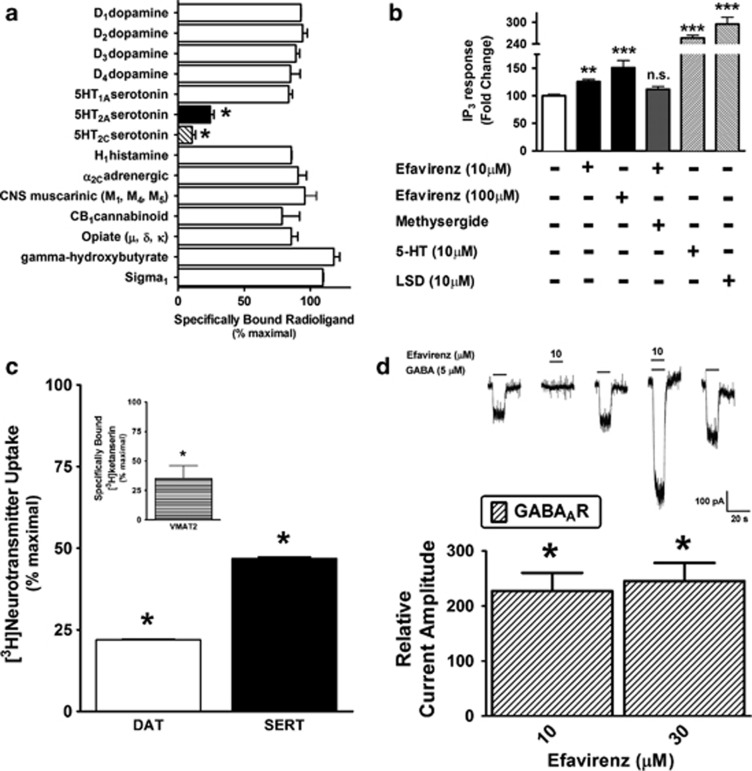
Of the over two dozen known molecular targets for psychoactive drugs selected for probing, efavirenz (10 μM) had significant interactions with serotonin 5-HT2A and 5-HT2C receptors (Figure 1a), DAT, SERT, VMAT2 (Figure 1c), and α1β2γ2 GABAA receptors (Figure 1d). When IP accumulation is used as a measure of activation, efavirenz acts as a relatively weak partial agonist of the cloned human 5-HT2A receptor as compared with saturating concentrations of the partial agonists LSD and serotonin (Figure 1b), and efavirenz's partial agonist effect was prevented by blockade of the 5-HT2A receptor with methysergide. Since efavirenz has a relatively modest affinity (Ki=2.2±0.3 μM, data not shown) for the 5-HT2A receptor, we additionally tested what is essentially a saturating dose (occupancy=[ligand]/([ligand]+KD)=100/102.2 μM=0.98=98%), which also was the highest dose of efavirenz that would stay in solution under the assays conditions, and while its apparent efficacy modestly increased at this highest achievable dose (100 μM), the partial agonist properties persisted. This discovery that efavirenz is a 5-HT2A receptor partial agonist prompted further evaluation of its possible LSD-like effects in animal models.
Figure 1.
Profiling of the receptor psychopharmacology of efavirenz demonstrates its interaction with cloned serotonin 5-HT2A receptors, catecholamine and indoleamine neurotransmitter transporters (dopamine (DAT), serotonin (SERT), and vesicular monoamine transporter 2 (VMAT2)), and γ-aminobutyric acid type A (GABAA) (α1,β2, and γ2) receptors in heterologous cellular expression systems. (a) Efavirenz (10 μM) displaces specifically bound [3H]radioligand from cloned serotonin 5-HT2A and 5-HT2C receptors expressed in HEK293 cells (n=3, *P<0.05 significant displacement compared to specific binding in the absence of efavirenz; one-way ANOVA with Bonferroni post hoc analysis), but not from CB1 cannabinoid, dopamine D1, D2, D3, and D4, serotonin 5-HT1A, opioid (μ, δ, and κ), histamine H1, adrenergic α2C, γ-hydroxybutyrate, or Sigma1 receptors. (b) Agonist-stimulated Gq-coupled phospholipase C activity was measured as inositol phosphate accumulation. Efavirenz acts as a partial agonist of cloned serotonin 2A (5-HT2A) receptors, and blockade of this receptor by pretreating with methysergide (10 μM) antagonizes its partial agonist effect (n=3–5, **P<0.01, ***P<0.001, NS means not significantly different from basal control levels; one-way analysis of variance (ANOVA) followed by a Dunnett's post hoc analysis). Lysergic acid diethylamine (LSD) (10 μM) acts as a full agonist of cloned 5-HT2A receptors relative to the full agonist reference compound 5-HT (10 μM) effect (n=3, ***P<0.001, significantly greater levels than basal control). (c) Efavirenz (10 μM) significantly blocks the transport of DAT-mediated [3H]dopamine and SERT-mediated [3H]serotonin (n=2, *P<0.05, significant reduction in [3H]serotonin uptake compared with control uptake in the absence of test compound (defined as 100% uptake) and relative to complete inhibition by 10 μM nomifensine for DAT, and 10 μM fluoxetine for SERT; one-way ANOVA followed by Dunnett's post hoc analysis). (Inset) Efavirenz (10 μM) displaces tetrabenazine-displaceable specifically bound [3H]ketanserin to isoform 2 of the VMAT2 naturally expressed at high levels in rabbit platelets. Note that VMAT1 is not expressed in rabbit platelets. (d) Efavirenz is an allosteric potentiator of cloned α1β2γ2 GABAA receptors. Whole-cell chloride currents mediated by these receptors are potentiated by efavirenz (⩾10 μM), although efavirenz alone has no activity at the GABAA receptor (*P<0.05 significantly different from control currents; Student's t-test).
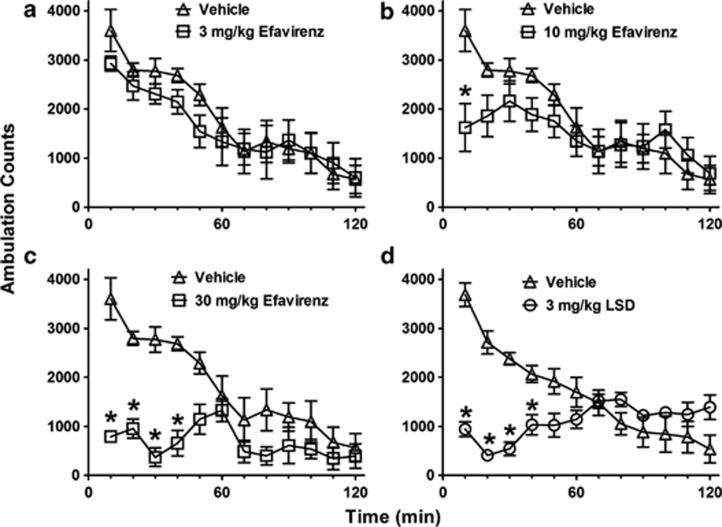
Intraperitoneal administration of efavirenz produced dose-dependent decreases in ambulation in an open-field novel environment in the range 10–30 mg/kg (Figures 2a–c). This locomotor depression was sustained for over 40 min at a dose of 30 mg/kg efavirenz and was similar in form and duration to that produced by LSD (3 mg/kg) (Figures 2c and d). At a dose of 0.3 mg/kg, LSD had no effect on ambulation in this mouse strain, and at 1 mg/kg, a significant decrease was evident only in the first 10 min followed by a significant increase at the 80–90 min time points (P<0.05 two-way ANOVA with a Bonferroni post hoc, data not shown).
Figure 2.
Efavirenz dose-dependently depresses open-field locomotor activity in a novel environment in mice similar to lysergic acid diethylamine (LSD). The ambulation data are shown for groups of eight mice as mean (±SE) counts within 10-min periods for separate groups of eight mice injected intraperitoneally with vehicle, efavirenz, or LSD. (a) At a dose of 3 mg/kg, efavirenz had no effect on ambulation. (b) At a dose of 10 mg/kg, efavirenz decreased ambulation only during the first 10 min. (c) At a dose of 30 mg/kg, efavirenz produced near-maximal suppression of ambulation that was sustained for 40 min. (d) LSD (3 mg/kg) produces a more potent sustained decrease in ambulation similar to that produced by 30 mg/kg efavirenz. The asterisks (*) indicate a significant difference from vehicle control (P<0.05).
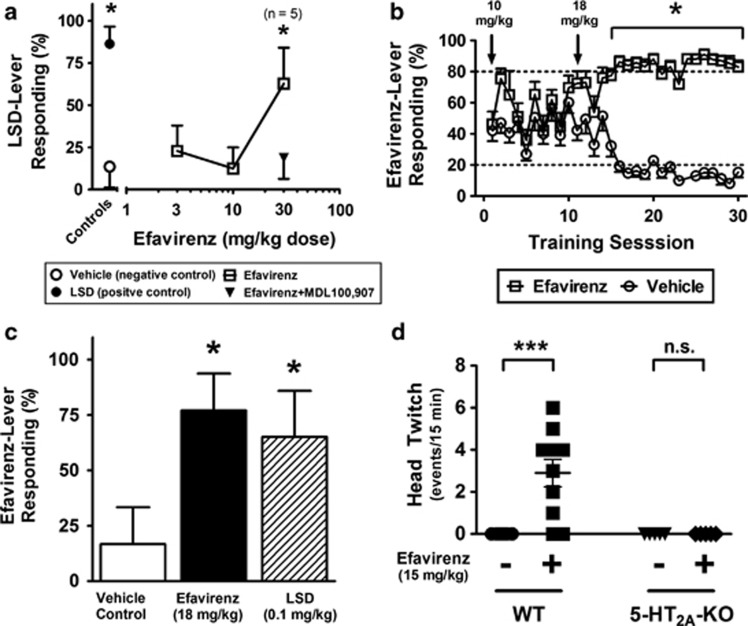
Efavirenz (30 mg/kg, intraperitoneally) yielded a maximum average 63% drug-appropriate responding in rats discriminating LSD from saline (Figure 3a and Table 2). A suppression of responding prevented testing at a larger dose. Drug-appropriate responding by efavirenz in rats discriminating LSD was completely blocked by pretreatment with a low dose of the brain-penetrating 5-HT2A-selective antagonist MDL100 907 (0.1 mg/kg, intraperitoneally; Figure 3a). Conversely, in rats discriminating efavirenz (18 mg/kg; Figure 3b) from saline, LSD produced a maximum average of 65% drug-appropriate responding at a dose of 0.1 mg/kg (Figure 3c and Table 2). Suppression of responding prevented studies with larger doses.
Figure 3.
Efavirenz has discriminative stimulus effects that are similar to lysergic acid diethylamine (LSD) and consistent with serotonin 2A (5-HT2A) receptor activity. (a) The data represent mean (±SEM) percent drug-appropriate responding (DAR) for eight rats discriminating 0.1 mg/kg LSD. Control data for vehicle and the training dose are shown to the left of the axis break, whereas substitution data for different doses of efavirenz alone and in combination with MDL100 907 are shown to the right. Drug-appropriate responding by efavirenz (30 mg/kg intraperitoneally) in rats discriminating LSD was fully blocked following pretreatment with a low dose of the 5-HT2A-selective antagonist MDL100 907 (0.1 mg/kg intraperitoneally). The value n=5 indicates that three of the eight rats tested following 30 mg/kg efavirenz failed to complete the drug discrimination test. The asterisks (*) indicate the absence of a significant difference from the LSD control at P=0.05. Although rates of responding (responses per s) significantly decreased at all doses of efavirenz tested, a 58% decrease in rate of responses occurred at the 30 mg/kg dose (0.29±0.11 compared with 0.70±0.12 for the 0.1 mg/kg intraperitoneal training dose of LSD, P<0.05). The almost undetectable rates of responding at doses of efavirenz higher than 30 mg/kg precluded interpretation of the outcome of discrimination tests. MDL100 907 alone (0.1 mg/kg intraperitoneally) did not influence the rate of responding. (b) The graph shows efavirenz-appropriate lever responding as a function of discrimination training sessions in the presence of efavirenz (open squares) vs sessions with vehicle. With an 18 mg/kg intraperitoneal training dose, rats (n=6) reliably discriminated efavirenz from vehicle (⩾80% correct responding) within 20 training sessions, whereas a training dose of 10 mg/kg (the initial training dose) failed to yield evidence of stimulus control. *P<0.05, DAR is statistically different for vehicle vs efavirenz sessions. (c) LSD (0.1 mg/kg) occasioned drug-lever responding in rats discriminating efavirenz. *P<0.05, not statistically different from the training drug. All rats completed the first fixed ratio and there were no significant differences in the rates of responding (0.34±0.05 compared with 0.37±0.06 for the 18 mg/kg intraperitoneal training dose of efavirenz, P>0.05). (d) Efavirenz (15 mg/kg intraperitoneally) induces head-twitch in wild-type 129S6/SvEv mice, but not in 5-HT2A-knockout (KO) littermates. Mice were injected with vehicle or efavirenz (15 mg/kg), and the head-twitch response was scored immediately after injection for 15 min. ***P<0.001; Bonferroni's post hoc test within two-way analysis of variance (ANOVA).
Table 2. Substitution Studies of Efavirenz in Rats Trained for Discrimination of Different Drugs of Abuse.
| Training drug | Test compound | Dose (mg/kg) | N testa | %DARb | Rate (responses/s)c |
|---|---|---|---|---|---|
| LSD | Vehicle | 0 | 8/8 | 13.6±12.4 | 0.675±0.107 |
| LSD | LSD | 0.1 | 8/8 | 86.4±10.2 | 0.703±0.122 |
| LSD | Efavirenz | 30 | 5/8 | 62.8±21.3d | 0.294±0.106e |
| Efavirenz | Vehicle | 0 | 6/6 | 16.7±16.7 | 0.447±0.071 |
| Efavirenz | Efavirenz | 18 | 6/6 | 77.1±16.6 | 0.372±0.060 |
| Efavirenz | LSD | 0.1 | 6/6 | 65.2±20.7d | 0.336±0.051 |
| MDMA | Vehicle | 0 | 9/9 | 1.0±1.0 | 0.672±0.032 |
| MDMA | MDMA | 1.5 | 9/9 | 100.0±0.0 | 0.725±0.068 |
| MDMA | Efavirenz | 30 | 5/9 | 40.0±24.5d | 0.189±0.064e |
| Cocaine | Vehicle | 0 | 6/6 | 0.0±0.0 | 0.596±0.072 |
| Cocaine | Cocaine | 10 | 6/6 | 83.3±16.7 | 0.995±0.243 |
| Cocaine | Efavirenz | 30 | 5/6 | 21.8±19.6 | 0.201±0.053e |
| Carisoprodol | Vehicle | 0 | 9/9 | 14.7±9.9 | 0.445±0.052 |
| Carisoprodol | Carisoprodol | 100 | 9/9 | 100.0±0.0 | 0.718±0.101 |
| Carisoprodol | Efavirenz | 18 | 7/9 | 25.2±13.9 | 0.262±0.059e |
Of the total rats tested, the number receiving at least one reinforcer that could be used in the assessment of substitution.
Mean percent of responses (%DAR) on the training drug lever (±SEM) at the dose yielding the greatest substitution.
Rate of responding (lever responses per second±SEM).
Values that reached the criteria for substitution (⩾40% drug-appropriate responding and not statistically different from the training drug at P<0.05).
Significantly different from vehicle control at P<0.05 (single degree of freedom F-test within one-way ANOVA).
Head-twitching induced by efavirenz (15 mg/kg) was measured in wild-type and 5-HT2A-KO littermates (Figure 3d). A significant difference was observed for the effects of efavirenz treatment and genotype (two-way ANOVA, F(1,25)=8.84; P<0.01), with efavirenz activating a significant head-twitch response in wild-type mice (Bonferroni's post hoc analysis, P<0.001), but not in 5-HT2A-KO mice (Figure 3d).
In addition to its apparent partial agonist activity at 5-HT2A receptors, efavirenz (10 μM) blocked by 75% [3H]dopamine uptake into CHO-K1 cells expressing cloned DAT and approximately 50% of the [3H]serotonin uptake into HEK293 cells expressing cloned SERT (Figure 1c). The same concentration of efavirenz also displaced over 60% of specifically bound radioligand from VMAT2 (Figure 1c, inset). Stimulants like cocaine, methamphetamine, and MDMA inhibit reuptake of dopamine and serotonin through their actions on DAT and SERT (Han and Gu, 2006). MDMA and amphetamine are also amine releasers and VMAT2 substrates while cocaine is a VMAT2 stimulator (Erickson et al, 1996; Brown et al, 2001). However, efavirenz (30 mg/kg) failed to occasion drug-appropriate responding in rats discriminating cocaine, and produced only modest levels of drug-appropriate responding (40%) in rats discriminating MDMA from saline (Table 2).
Although efavirenz (10 μM) had no direct agonist activity on GABAA receptors (Figure 1d), it potentiated GABA-mediated chloride currents in HEK293 cells stably expressing the most common type of cloned GABAA receptor (α1β2γ2 subtype) found in the brain (Figure 1d). However, up to a dose of 30 mg/kg, efavirenz did not share discriminative stimulus effects with another GABAA receptor potentiator, carisoprodol (Gonzales et al, 2009; Table 2). At the same concentrations that affected the GABAA receptors in vitro, efavirenz exerted no significant effect on NR1a/2a or NR1a/2b configurations of the cloned NMDA receptor (data not shown).
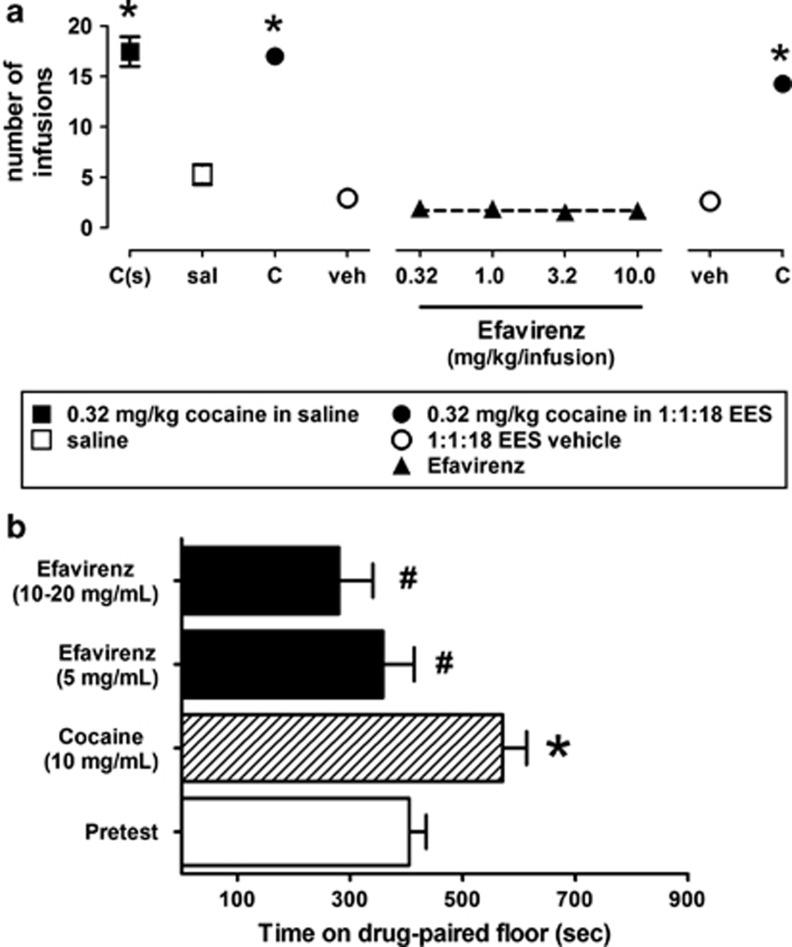
Although efavirenz's actions on catecholamine and indoleamine transporters and GABAA receptors apparently have no prominent role in its discriminative stimulus effects, the interaction of efavirenz with DAT and VMAT2 observed in receptor assays prompted an investigation into the possible reinforcing effects of efavirenz modeled by tests of self-administration and place conditioning. However, no evidence for any positive reinforcing effects of efavirenz was found in either test The eight rats that started the self-administration study completed food training (receiving 50 food pellets in three consecutive sessions) after an average of 10.1±1.0 (SEM) sessions (range=7–16) and the subset of five rats that finished all phases of this study completed food training after an average of 10.8±1.5 sessions (range=7–16). Further, the eight rats that started the study received an average of 17.2±1.3 infusions of 0.032 cocaine (in saline; range=10–20) per session; the five rats that completed all phases of this study self-administered an average of 17.5±1.5 infusions of cocaine (in saline; range=10–20) per session (leftmost data point; Figure 4a). However, when saline was substituted for cocaine, the same five rats self-administered an average of 5.3±1.0 infusions (range=1–9) per session (Figure 4a). When prepared in the 1 : 1 : 18 vehicle, cocaine maintained self-administration responding in only five of eight rats. Those five rats received an average of 17.0±0.8, 15.3±1.1, and 14.3±0.6 infusions of cocaine (in 1 : 1 : 18 vehicle) at the beginning, middle (between the first two and the last two doses of efavirenz; data not shown), and end of studies with efavirenz, respectively (closed circles; Figure 4a). When vehicle was substituted for cocaine, responding decreased markedly with rats receiving an average of 2.9±0.4 and 2.6±0.2 infusions of vehicle at the beginning and the end of studies with efavirenz, respectively (open circles; Figure 4a). Efavirenz failed to maintain self-administration responding above that obtained with vehicle: rats (n=5) received, on average, fewer than two infusions per session of 0.32, 1.0, 3.2, and 10.0 mg/kg of efavirenz (closed triangles; Figure 4a).
Figure 4.
Efavirenz fails to exert positive reinforcing effects. (a) Efavirenz fails to maintain self-administration responding in rats that readily self-administer intravenous cocaine but not saline. The five of eight total rats that completed all phases of this study received an average of over three times the infusions of cocaine (dissolved in saline) per session (solid squares) than when saline was substituted for cocaine (open squares). When prepared in the 1 : 1 : 18 vehicle, cocaine maintained self-administration responding (dissolved in 1 : 1 : 18 vehicle) at the beginning (closed circles), middle (between the first two and the last two doses of efavirenz; data not shown), and end (solid circles, far right-hand side of graph) of the studies with efavirenz, respectively. When vehicle was substituted for cocaine, responding decreased markedly at the beginning and end of studies with efavirenz, respectively (open circles). At all doses tested, efavirenz (solid triangles) failed to maintain self-administration responding above what was obtained with vehicle (open circles). C(s), cocaine dissolved in saline (solid squares); sal, saline (open squares); C, cocaine dissolved in 1 : 1 : 18 (v/v/v) ethanol, emulphor, and 0.9% saline vehicle (solid circles); veh, 1 : 1 : 18 vehicle only (open circles); different doses of efavirenz dissolved in 1 : 1 : 18 vehicle (solid triangles). (b) Rats receiving place conditioning with cocaine (10 mg/kg intraperitoneally), but not efavirenz (5–20 mg/kg intraperitoneally), acquired a significant place preference (*P<0.05 significantly different from pretest; #P<0.05 significantly different from cocaine; one-way analysis of variance (ANOVA) with Bonferroni post hoc analysis). The data represent the mean (±SE) time spent on the drug-paired floor during the pretest for all rats tested (n=24), and during the post-test for groups of eight following acquisition under cocaine or efavirenz.
In tests of place conditioning utilizing two types of flooring for the pairing, the pretest data for the group indicated a neutral floor preference before conditioning. Rats conditioned using cocaine showed an increase in time on the cocaine-paired floor during the post-test, whereas rats conditioned with efavirenz showed no effect or a decrease in time on the efavirenz-paired floor. When data were considered in a two-way ANOVA (with Drug treatment as a between and Conditioning as a within-groups factor), there was a significant effect of Drug F(2,21)=5.57, P<0.011, as well as an interaction of Drug treatment with Conditioning F(2,21)=4.49, P<0.024, reflecting the different conditioning outcomes for cocaine vs efavirenz. Planned individual comparisons of time spent on the cocaine-paired floor during the pretest vs post-test using a one-way repeated-measures ANOVA indicated a significant difference for cocaine F(1,7)=7.87, P<0.026, but not for efavirenz at 5 or 10–20 mg/kg (F-values <2.0, P-values<0.2; Figure 4b).
DISCUSSION
Because the recreational use of efavirenz has only been the subject of news reports, our objective was to evaluate in a mechanistic context the in vitro and in vivo properties and the abuse potential of efavirenz. Our molecular and behavioral studies demonstrate that efavirenz has pharmacological properties predictive of psychoactive effects in humans. Although efavirenz interacts with several receptors within a similar range of concentration, several lines of evidence suggest that the psychoactive effects of efavirenz are predominately mediated by activation of the serotonin 5-HT2A receptor similar to the psychoactive effects of LSD and other related hallucinogens (Fiorella et al, 1995; Winter, 2009). First, efavirenz has partial agonist properties at cloned serotonin 5-HT2A receptors, which are blocked by antagonists of this receptor. Although the 5-HT2A receptor-mediated in vitro measures of IP accumulation demonstrate efavirenz has much lower efficacy than LSD, the behavioral pharmacology of efavrienz is nevertheless very similar to that of LSD. Efavirenz induces head-twitching in mice like LSD and other related hallucinogens (Glennon et al, 1984; Fiorella et al, 1995; Vollenweider et al, 1998; González-Maeso et al, 2007; Fantegrossi et al, 2008). That head-twitching generated by efavirenz is mediated by the 5-HT2A receptor is supported by its inability to induce such a response in 5-HT2A-KO mice. The relatively low affinity of efavirenz for the 5-HT2A receptor is a likely explanation as to why we needed a relatively high intraperitoneal dose to elicit an effect in vivo and is consistent with receptor affinity and in vivo potency data for other hallucinogens acting via the 5-HT2A receptors, including some with low affinity and potency such as 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) (Fantegrossi et al, 2006; Fantegrossi et al, 2008). This may also help to explain the reduced duration and intensity of the head-twitch response to efavirenz compared with other hallucinogens (González-Maeso et al, 2007). Second, at higher doses both efavirenz (⩾10 mg/kg) and LSD (3 mg/kg) produce similar reductions in ambulation in mice exposed to a novel open field consistent with hallucinogen potentiation of neophobia in rats (Adams and Geyer, 1982, 1985), but different in some respects from the reported effects of hallucinogens on ambulation in other mouse strains (Halberstadt et al, 2009; Grailhe et al, 1999). Third, efavirenz produces discriminative stimulus effects in rats that appear to be similar to those of LSD: efavirenz occasions 63% responding on the LSD-associated lever and this effect is completely blocked by the 5-HT2A-selective antagonist MDL100 907, which, at the dose used, has no effect by itself. The functional effect of efavirenz on 5-HT2A receptors is of interest, because activation of 5-HT2A receptors by LSD and psilocin correlates with their ability to produce hallucinations in humans (Glennon et al, 1984; Vollenweider et al, 1998). LSD also occasioned 65% responding on the efavirenz-associated lever, providing additional evidence to support the view that efavirenz might have LSD-like subjective effects in humans. Taken together, our studies are consistent with the suggestion that efavirenz functions as a hallucinogen similar to LSD (eg, a report describing HIV patients recovering from drug abuse having experienced ‘flashbacks' on efavirenz; Vazquez, 1999). A hallucinogen-like mechanism of action is also consistent with reports of hallucinations, vivid dreaming, and psychosis as part of a range of adverse psychiatric events that have been reported as dose-dependent side effects for efavirenz when used orally as an antiretroviral medication to treat HIV-1 (Gutiérrez et al, 2005; Cespedes and Aberg, 2006). We could find no evidence of positive reinforcing effects for efavirenz in a self-administration model, and a place preference was not detected for efavirenz under conditions yielding cocaine-conditioned place preference. These outcomes do not rule out the possibility that efavirenz has reinforcing effects under other conditions; however, it seems reasonable to suggest that abuse liability of efavirenz in humans would be similar to other hallucinogens like LSD (Anthony et al, 1994; Chen et al, 2009). LSD does not have positive reinforcing effects in monkeys and the two reports indicating place preference conditioning for LSD in rats were only able to achieve these results utilizing doses (ie, 0.2 mg/kg) that activate D2 dopamine receptors (Meltzer et al, 1977, Nichols, 2004). Despite their having a low risk of physical dependence, hallucinogens are nevertheless well-established drugs of abuse leading to significant problems for the users. All the above notwithstanding, we recognize that some of the effects of efavirenz might be explained by interactions with it or one of its metabolites or by-products with the same targets explored in this study or other receptor targets not explored here.
Even though our receptor pharmacology studies indicate that efavirenz interacts with multiple other sites for drugs of abuse, the behavioral effects of efavirenz appear to be mediated predominately by the 5-HT2A receptor. Efavirenz did not share discriminative stimulus effects with cocaine and produced only very low levels of drug-appropriate responding in rats discriminating MDMA, suggesting that the subjective effects of efavirenz are neither psychostimulant-like nor are they mediated by DAT, SERT, or VMAT2. Further, efavirenz also did not share discriminative stimulus effects with the GABAA receptor potentiator carisoprodol suggesting that the subjective effects of efavirenz are likely not mediated by potentiation of the GABAA receptor.
While our focus was on identifying potential receptor targets for efavirenz, some of the receptors not found to be targets for efavirenz deserve comment. First, the opioid and muscarinic receptor assays were intentionally performed on whole brain tissue with radioligands that do not readily distinguish between the different subtypes so that all subtypes present in the brain could be surveyed simultaneously. While this approach is capable of revealing very high-affinity interactions for one or more subtypes, it has the potential drawback of possibly not being sensitive enough to reveal more moderate interactions with a single subtype when two or more other subtypes are present at similar densities. This is because partial radioligand displacement from one subtype would be essentially ‘diluted out' by a lack of displacement of the other subtypes present: the sum result being low levels of total radioligand displacement from all subtypes combined. Second, efavirenz does not act on the orthosteric site of the CB1 receptor (Figure 1a), which when stimulated mediates the psychoactive effects of cannabis (Huestis et al, 2001). This is relevant because patients taking efavirenz can falsely test positive for cannabis upon urinalysis because of cross-reactivity of the 8-glucuronide metabolite of efavirenz with anti-Δ9-tetrahydrocannabinol antibodies in some, but not all, immunoassays (Rossi et al, 2006).
Given efavirenz's outstanding virologic efficacy, it seems unlikely our discovery of the neuropharmacological mechanisms underpinning its abuse potential would result in discontinuation of its use. Rather, our findings are more likely to serve as baseline scientific studies to inform policy makers and health-care workers, and to guide future efforts aimed at developing antiretroviral abuse prevention and treatment strategies and lessening the risk of adverse psychiatric events in those at risk. One approach might be a medicinal chemistry effort aimed at developing efficacious antiretroviral drugs with combined reduced CNS penetration and side effects and reduced abuse potential.
In a global context, the recreational use of HIV antiretroviral drugs undermines charitable government emergency plans for AIDS relief (eg, President's Emergency Plan for AIDS Relief, PREFAR). There are also significant potential public health implications farther reaching than a new type of drug abuse problem. Diverting HIV medications endangers HIV patients because insufficient dosing will not effectively suppress the virus (Dybul et al, 2002; Patel and Patel, 2006; Sciutto, 2009). Improper dosing in HIV patients and in non-infected efavirenz abusers living in a high HIV-risk environment, like South Africa where HIV prevalence rates are approaching 40% of the population (Central Intelligence Agency (CIA) World Fact Book, 2009), creates near-optimal conditions for the emergence of HIV strains resistant to efavirenz and similar antiretroviral medications. Yet, regardless of whether resistance to efavirenz develops in Africa or elsewhere, it poses an eminent threat to health with a potential global impact. Our studies at the molecular, cellular, and whole animal level suggest that efavirenz has psychoactive properties similar to the hallucinogen LSD, which appears to explain not only its cited abuse potential in news reports but also its dose-dependent side-effect profile consisting of adverse neuropsychiatric events. Future directions might include controlled investigations of the subjective effects in humans as well as monitoring of how widespread the practice has become.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Dr Michael Oglesby for critical reading of early versions of the manuscript, Dr Kathyrn Cunningham for insightful discussions related to this work, and Brian Weiss, Shahnawaz Amdani, Marshayla McPhaul, Terrell Holloway and Fatima Sahyouni for technical assistance. We also thank Dr David Lynch (University of Pennsylvania) for providing the cDNAs encoding rat NR1a, NR2A, and NR2B, Dr Eldo Kuzhikandathil (UMDNJ-New Jersey Medical School) for providing an Att20 cell line stably expressing the human D3 receptor, and Dr David B Bylund (University of Nebraska Medical Center) for providing the HEK293 cell line stably expressing the human α2C adrenergic receptor. This work was supported, in part, by the National Institutes of Health (Grant R01-MH063162 (JAS); K05DA17918 (CPF); R01 MH084894 (JGM); R01-DA022370 (GHD, MJF); N01DA-7-8872 (HHSN271200700014C) (MJF)), UNTHSC Intramural Grant RI-6015 (JAS, MG, and MJF), and institutional funds (JAS, MJF, and GHD). A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism (KCR).
References
- Adams LM, Geyer MA (1982). LSD-induced alterations of locomotor patterns and exploration in rats. Psychopharmacology (Berl) 77: 179–185. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA (1985). Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry 9: 121–132. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC (1994). Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the national comorbidity survey. Exp Clin Psychopharmacol 2: 244–268. [Google Scholar]
- Arribas JR (2003). Efavirenz: enhancing the gold standard. Int J STD AIDS 14(Suppl 1): 6–14. [DOI] [PubMed] [Google Scholar]
- Best BM, Goicoechea M (2008). Efavirenz—still first-line king? Expert Opin Drug Metab Toxicol 4: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB et alCHARTER Group (2011). Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother 66: 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE (2001). Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther 296: 762–767. [PubMed] [Google Scholar]
- Central Intelligence Agency (CIA) World Fact Book (2009). Country Comparisons—HIV/AIDS—adult prevalence rate. Data from 2007 estimates Available at: https://www.cia.gov/library/publications/the-world-factbook/rankorder/2155rank.html.
- Cespedes MS, Aberg JA (2006). Neuropsychiatric complications of antiretroviral therapy. Drug Saf 29: 865–874. [DOI] [PubMed] [Google Scholar]
- Cesura AM, Bertocci B, Da Prada M (1990). Binding of [3H]dihydrotetrabenazine and [125I]azidoiodoketanserin photoaffinity labeling of the monoamine transporter of platelet 5-HT organelles. Eur J Pharmacol 1990 186: 95–104. [DOI] [PubMed] [Google Scholar]
- Chen CY, Storr CL, Anthony JC (2009). Early-onset drug use and risk for drug dependence problems. Addict Behav 34: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1: 1662–1670. [DOI] [PubMed] [Google Scholar]
- Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK, Panel on Clinical Practices for Treatment of HIV (2002). Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med 137: 381–433. [DOI] [PubMed] [Google Scholar]
- Ericksen SS, Cummings DF, Weinstein H, Schetz JA (2009). Ligand selectivity of D2 dopamine receptors is modulated by changes in local dynamics produced by sodium binding. J Pharm Exp Ther 328: 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E (1996). Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA 93: 5166–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC et al (2006). Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav 83: 122–129. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ (2008). The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC (1995). The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl) 121: 347–356. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Carbonaro T, Forster MJ (2009). Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology 204: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD (1984). Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35: 2505–2511. [DOI] [PubMed] [Google Scholar]
- Gonzalez LA, Gatch MB, Taylor CM, Bell-Horner CL, Forster MJ, Dillon GH (2009). Carisoprodol-mediated modulation of GABAA receptors: in vitro and in vivo studies. J Pharmacol Exp Ther 329: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R et al (2007). Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–452. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF et al (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA et al (2004). Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment. N Engl J Med 350: 1850–1861. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D et al (1999). Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron 22: 581–591. [DOI] [PubMed] [Google Scholar]
- Gutiérrez F, Navarro A, Padilla S, Antón R, Masiá M, Borrás J et al (2005). Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis 41: 1648–1653. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Powell SB (2009). 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice (2009). Neuropsychopharmacology 34: 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SM, Eron JJJr, Reiss P, Schooley RT, Thompson MA, Walmsley S et al (2008). International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 300: 555–570. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH (2006). Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RQ, Singh M, Dillon GH (2010). Genistein directly inhibits NMDA receptors in mouse hippocampal neurons. Neuropharmacology 58: 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET et al (2001). Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58: 322–328. [DOI] [PubMed] [Google Scholar]
- Lacivita E, Paola De Giorgio P, Lee IT, Rodeheaver SI, Weiss BA, Fracasso C et al (2010). Design, synthesis, radiolabeling and in vivo evaluation of carbon-11 labeled N-[2-[4-(3-cyanopyridin-2-yl)piperazin-1-yl]ethyl]-3-methoxybenzamide, a potential PET tracer for the dopamine D4 receptors. J Med Chem 53: 7344–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha A (2008). Getting High on HIV drugs in S Africa, BBC News, 8 December 2008 Available at: http://news.bbc.co.uk/2/hi/africa/7768059.stm.
- Meltzer HY, Fessler RG, Simonovic M, Doherty J, Fang VS (1977). Lysergic acid diethylamide: evidence for stimulation of pituitary dopamine receptors. Psychopharmacology (Berl) 54: 39–44. [DOI] [PubMed] [Google Scholar]
- National Research Council (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press: Washington, DC. [PubMed] [Google Scholar]
- Nichols DE (2004). Hallucinogens. Pharmacol Ther 101: 131–181. [DOI] [PubMed] [Google Scholar]
- Patel AK, Patel KK (2006). Future implications: compliance and failure with antiretroviral treatment. J Postgrad Med 52: 197–200. [PubMed] [Google Scholar]
- Rossi S, Yaksh T, Bentley H, van den Brande G, Grant I, Ellis R (2006). Characterization of interference with 6 commercial delta-9-tetrahydrocannabinol immunoassays by Efavirenz (glucuronide) in urine. Clin Chem 52: 896–898. [DOI] [PubMed] [Google Scholar]
- Sciutto J (2009). ABC World News Report (4 May 2009) Shorten version 6 April 2009: ‘No Turning Back': Teens Abuse HIV Drugs. Teens in South Africa Smoke Anti-Retroviral Drug Efavirenz for Cheap High portions archived at http://www.youtube.com/watch?v=hjVtthQJnyU.
- Sierra-Madero J, Villasis-Keever A, Méndez P, Mosqueda-Gómez JL, Torres-Escobar I, Gutiérrez-Escolano F et al (2010). Prospective, randomized, open label trial of Efavirenz vs Lopinavir/Ritonavir in HIV+ treatment-naive subjects with CD4+<200 cell/mm3 in Mexico. J Acquir Immune Defic Syndr 53: 582–588. [DOI] [PubMed] [Google Scholar]
- Vazquez E (1999). Sustiva flashbacks. Posit Aware 10: 17. [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–3902. [DOI] [PubMed] [Google Scholar]
- Winter JC (2009). Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology (Berl) 203: 251–263. [DOI] [PubMed] [Google Scholar]