Abstract
Mice with a mutation in the Clock gene (ClockΔ19) exhibit increased preference for stimulant rewards and sucrose. They also have an increase in dopaminergic activity in the ventral tegmental area (VTA) and a general increase in glutamatergic tone that might underlie these behaviors. However, it is unclear if their phenotype would extend to a very different class of drug (ethanol), and if so, whether these systems might be involved in their response. Continuous access voluntary ethanol intake was evaluated in ClockΔ19 mutants and wild-type (WT) mice. We found that ClockΔ19 mice exhibited significantly increased ethanol intake in a two-bottle choice paradigm. Interestingly, this effect was more robust in female mice. Moreover, chronic ethanol experience resulted in a long-lasting decrease in VTA Clock expression. To determine the importance of VTA Clock expression in ethanol intake, we knocked down Clock expression in the VTA of WT mice via RNA interference. We found that reducing Clock expression in the VTA resulted in significantly increased ethanol intake similar to the ClockΔ19 mice. Interestingly, we also discovered that ClockΔ19 mice exhibit significantly augmented responses to the sedative effects of ethanol and ketamine, but not pentobarbital. However, their drinking behavior was not affected by acamprosate, an FDA-approved drug for the treatment of alcoholism, suggesting that their increased glutamatergic tone might underlie the increased sensitivity to the sedative/hypnotic properties of ethanol but not the rewarding properties of ethanol. Taken together, we have identified a significant role for Clock in the VTA as a negative regulator of ethanol intake and implicate the VTA dopamine system in this response.
Keywords: alcohol preference or consumption, Clock gene, circadian
INTRODUCTION
The majority of living organisms display daily cycles in behavior and physiology that enable them to adapt to their environment and react to a variety of stimuli known as zeitgebers or ‘time-givers' (eg, light, food, etc). In mammals, the central circadian pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus, is entrained by light and controls activity rhythms. The SCN coordinates other oscillators in the brain and in peripheral organs (Reppert and Weaver, 2002). Thus, circadian clocks are present throughout the body and regulate numerous metabolic and behavioral rhythms. In mammals, two core components of the molecular clock are the circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein-1 (BMAL1) proteins. CLOCK and BMAL1 are transcription factors that heterodimerize and promote transcription of the Period genes (Per1, Per2, and Per3), the Cryptochrome genes (Cry1 and Cry2), as well as many other genes by binding the E-box elements in their promoters (Reppert and Weaver, 2001; Takahashi et al, 2008). Circadian genes and proteins are widely expressed throughout the brain. Further, there exist SCN-independent pacemakers that can entrain to nonphotic stimuli, like food and drugs (Iijima et al, 2002; Stephan, 1984). In addition, drugs of abuse have been suggested to act as zeitgebers, as they can entrain locomotor activity rhythms when given daily (Kosubud et al, 1998, 2007). Physiological, behavioral, and molecular rhythms are all affected by rewarding stimuli, including drugs of abuse and food. Rodent studies have shown that prolonged alcohol treatments can disrupt the circadian pattern of a variety of hormonal and behavioral rhythms (Kakihana and Moore, 1976; Kosobud etal, 2007, Madeira et al, 1997; Rajakrishnan et al, 1999; Rosenwasser et al, 2005; Spanagel et al, 2005a). However, the molecular mechanisms of these disruptions are yet to be determined.
Genetic animal models have also revealed that circadian genes are important regulators of behavioral responses to drugs of abuse. The first studies that revealed this relationship were carried out in Drosophila melanogaster, showing that flies bearing a mutation in the circadian genes Clock, Per, Cycle, or Doubletime all fail to sensitize to cocaine (Andretic et al, 1999). Following these studies, Abarca et al (2002) found that mPer1Brdm-null mutant mice also fail to sensitize to cocaine and exhibit decreased cocaine conditioned place preference (CPP), while mPer2Brdm-null mutants exhibit hypersensitization to cocaine and strong cocaine CPP. Furthermore, studies have shown that Per2Brdm mutants are hypersensitive to ethanol; they exhibit increased ethanol preference and consumption, increased sedation, and decreased hypothermia (Liu et al, 2005; Perreau-Lenz et al, 2009).
Alcoholism in human populations is associated with disruptions in circadian rhythms, which can persist during abstinence and increase risk for relapse (Brower, 2001; Fonzi et al, 1994; Kuhlwein et al, 2003; Landolt and Gillin, 2001; Sano et al, 1993). In addition, studies have reported in rodents that chronic consumption of high levels of ethanol can alter rPer2 gene expression in the SCN and activity rhythms (Chen et al, 2004; Seggio et al, 2009). Furthermore, variations (SNPs or mutations) in the Clock gene and the Per2 gene associate with increased alcohol consumption in humans (Spanagel et al, 2005b; Sjoholm et al, 2010). The role of Clock in the reinforcing and motivational aspects of ethanol intake remains unknown. McClung et al (2005) and Ozburn et al (2012) identified an important role for Clock in the regulation of cocaine reward and self-administration. Mice bearing a dominant-negative mutation in Clock (Clock Δ19 mice) exhibit increased cocaine sensitivity and preference (King et al, 1997; McClung et al, 2005; Ozburn et al, 2012; Vitaterna et al, 1994). Furthermore, these mice exhibit increased locomotor activity, reduced anxiety- and depression-like behavior, increased intracranial self-stimulation (ICSS) at a lower threshold, and increased dopaminergic cell activity in the ventral tegmental area (VTA) (McClung et al, 2005; Roybal et al, 2007). Many of these behavioral phenotypes are rescued by expressing functional CLOCK in the VTA of ClockΔ19 mutants or are recapitulated by reducing Clock expression in the VTA of wild-type (WT) mice via RNA interference (RNAi; Mukherjee et al, 2010; Roybal et al 2007). What remains unknown is if Clock in the VTA is important for the response to only stimulant drugs, or if it also has a role in ethanol consumption and ethanol-related behaviors. The aim of this study was to determine if Clock is a mediator of ethanol intake. Further examination of the role of circadian genes in ethanol-related behaviors has important translational significance for treatment, as mutations in circadian genes might increase the vulnerability for alcoholism.
MATERIALS AND METHODS
Mice
ClockΔ19 mutant mice were created by N-ethyl-N-nitrosourea mutagenesis, resulting in a dominant-negative CLOCK protein (King et al, 1997; Vitaterna et al, 1994). The ClockΔ19 mutation is maintained on a BALB/c background (>10 generations of backcrossing) by heterozygous breeding and genotyping was carried out using PCR (as reported in Ozburn et al, 2012). Female Clock mutant (ClockΔ19/ClockΔ19) and WT littermate controls were used in all experiments. In addition, as described in Supplementary Materials, continuous access two-bottle choice experiments were performed to determine ethanol or quinine preference in male mice. Blizard et al (2004) reported female BALB/c mice drink 10% ethanol with a higher preference than male mice (with females exhibiting an estimated preference score of 23% compared with 17% in males). While both male and female ClockΔ19 mice exhibit a similar behavioral phenotype, behavioral changes are more robust in female mice in their behavioral responses in measures of activity and anxiety (Easton et al, 2003). Mice were 8–12 weeks old at the beginning of experiments and were group housed on a 12 h light/12 h dark cycle with food and water ad libitum, unless otherwise specified. All experiments were in compliance with protocols approved by the IACUC at University of Texas Southwestern Medical Center and the University of Pittsburgh.
Continuous Access Two-bottle Choice
As described in Ozburn et al (2010), mice were habituated to individual housing and sipper bottles for 1 week before the start of the experiment. Mice were offered water and 3% ethanol (v/v in tap water) for 2 days. After 3% ethanol, escalating concentrations (up to 21%) were offered vs water, 2 days each. Fluid intake was measured daily. Mice were weighed every 4 days. Ethanol preference (ml ethanol solution consumed/ml total fluid consumed), ethanol consumption (g pure ethanol/kg body weight per day), and total fluid consumption were measured (n=9–11 per genotype).
Loss of Righting Reflex
Mice were injected with ethanol (3.6 g/kg) at zeitgeber 5 (ZT5; n=7–8 per genotype) or ZT11 (n=6–7 per genotype), pentobarbital (100 mg/kg; n=9 per genotype) at ZT5, or ketamine (50 mg/kg; n=9 per genotype) at ZT7-8, and when they became ataxic, they were placed in the supine position until they were able to right themselves three times within 60 s. Data for three mice were excluded from the ZT11 ethanol trial because of misplaced injection.
Gene Expression
As it has been shown that recently abstinent alcoholics have reduced Clock expression, we measured Clock expression in reward-related brain regions after the continuous access two-bottle choice and 1 week of abstinence (Huang et al, 2010). Mice were killed at two time points over the circadian cycle corresponding to ZT4 and ZT16. Our group has observed peaks and troughs in Clock gene expression in the nucleus accumbens (NAc) and VTA at approximately ZT4-8 and ZT16-20. Brains were removed and frozen on powdered dry ice. NAc and VTA punches were taken on a freezing stage from 300-μM sections taken on a cryostat. RNA isolation and cDNA synthesis were carried out as described previously (McClung et al, 2005). Real-time PCR was performed in duplicate using Power SYBR Green PCR Master Mix and the ABI 7900 Real-Time PCR system (Applied Biosystems, Carlsbad, CA) with primers specific for 18s and Clock (18s forward: 5′-ACCGCAGCTAGGAATAATGGA-3′, 18s reverse: 5′-GCCTCAGTTCCGAAAACCA-3′ Clock forward: 5′-CAGAACAGTACCCAGAGTGCT-3′, Clock reverse: 5′-CACCACCTGACCCATAAGCAT-3′) (Mukherjee et al, 2010). Relative expression levels were determined by normalizing the CTs (cycle thresholds) for Clock to corresponding 18s CTs and fold change was calculated using the 2−ΔΔCt method (n=5–10 mice per ZT per treatment).
AAV Purification
Construction, production, purification, and validation of the Clock and scramble shRNAs are described in Mukherjee et al (2010). Viral production and shRNA design were carried out using a helper-free triple transfection method in human embryonic kidney 293 cells (American Type Culture Collection, Manassas, VA). The AAV Clock and scramble shRNA vectors also express GFP. Sequences and other details are in Mukherjee et al (2010).
Stereotaxic Injection of AAV-shRNA into VTA
Stereotaxic surgery was performed as described in Mukherjee et al (2010). WT mice (littermates to the ClockΔ19 mutants) were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) in saline (0.9% NaCl). Bilateral stereotaxic injections into the VTA (from bregma: angle 7°, A/P −3.2 mm, M/L +1.0, D/V −4.6) of 1 μl AAV-Clock shRNA or AAV-scramble shRNA (1 × 1012 infectious particles per ml) were performed using a 33-G Hamilton syringe (Hamilton, Reno, NV). The virus was injected (0.1 μl/min) and the needle was kept in place for 5 min before it was withdrawn. Mice recovered for 3 weeks before behavioral testing (n=12–13 per treatment).
Immunohistochemistry and Validations of Stereotaxic Injection Placement/AAV Infection
As described in Mukherjee et al (2010), mice were deeply anesthetized (175 mg/kg sodium pentobarbital) and perfused intracardially with 0.01 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were removed, postfixed in 4% paraformaldehyde overnight at 4 °C, placed in 30% glycerol in PBS for 24 h, and then placed in PBS with 0.01% sodium azide. VTA containing sections (30 μm) were collected using a freezing microtome (Leica, Wetzlar, Germany) and immunohistochemical staining was carried out using standard procedures with the following antibodies: tyrosine hydroxylase (TH) mouse monoclonal antibody (1 : 5000; T2928; Sigma, St Louis, MO), and GFP rabbit polyclonal antibody (1 : 20 000; ab290; AbCam, Cambridge, MA). Secondary antibodies (1 : 400 anti-mouse Alexa 488 and 1 : 400 anti-rabbit rhodamine) were purchased from Invitrogen (Carlsbad, CA). Immunostained sections were mounted using Vectashield with DAPI (Vector Labs, Burlingame, CA) and observed with an epifluorescence microscope to identify the location of viral injection and levels of infection. As described in Mukherjee et al (2010), VTA cells that displayed more than 50% of GFP colocalization with TH antibody staining were included for behavioral analysis (two mice were eliminated from analysis based on this criteria; thus, the final number of mice included in the analysis is n=12–13 per treatment).
Statistical Analysis
All data are expressed as mean±SEM. Two-bottle choice data, ethanol-induced loss of righting reflex (LORR), and gene expression data were analyzed by two-way analysis of variance (ANOVA). Repeated measures were applied where appropriate. Significance for data for the ketamine- and pentobarbital-induced LORR experiments were determined by Student's t-test. In all experiments, p<0.05 is considered significant. The presence of outliers was identified (defined as greater than or less that two times the standard deviation of the mean) for ethanol two-bottle choice (for all experiments combined eight mice showed side preference, only drinking from one side regardless of solution in bottle) and LORR testing (three mice did not lose their righting reflex) and these data were removed before statistical analysis.
RESULTS
ClockΔ19 Exhibit Increased Ethanol Preference and Consumption
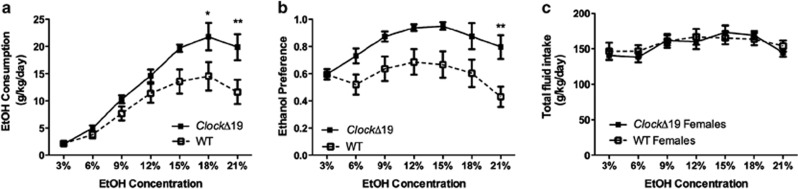
To determine if functional CLOCK is important for voluntary ethanol drinking, we measured ethanol preference and consumption in ClockΔ19 and WT littermates using the continuous access two-bottle choice paradigm. Ethanol intake in this paradigm positively correlates with operant ethanol self-administration (Greene and Grahame, 2007). ClockΔ19 female mice exhibited significantly increased ethanol consumption and preference, without any effect on total fluid consumption (Figures 1a–c; ethanol consumption: genotype × concentration interaction—F(6,108)=3.69, p<0.01, main effect of genotype—F(1,18)=5.17, p<0.05, main effect of concentration—F(6,108)=58.18, p<0.0001; ethanol preference: main effect of genotype—F(1,18)=7.11, p<0.05, main effect of concentration—F(6,108)=7.80, p<0.0001). Ethanol preference and consumption data for both genotypes displayed a typical inverted U shape. Bonferroni post hoc analysis revealed that ClockΔ19 mice consumed significantly more ethanol and showed a higher preference for high ethanol concentrations. Male Clock mutants exhibited a strong trend toward significantly increased ethanol preference (Supplementary Figure 1).
Figure 1.
ClockΔ19 mice exhibit significantly increased ethanol (EtOH) intake. (a) Ethanol consumption, (b) ethanol preference, and (c) total fluid intake. *p<0.05; **p<0.01. WT, wild type.
As preference for ethanol can be influenced by taste, we examined preference for the bitter tastant, quinine, in male and female WT and ClockΔ19 mice. We found that both male and female WT and ClockΔ19 mice exhibit a similar taste avoidance profile for quinine solutions (Supplementary Figure 2). We have previously reported a modest increase in sucrose preference for ClockΔ19 mice (Roybal et al, 2007).
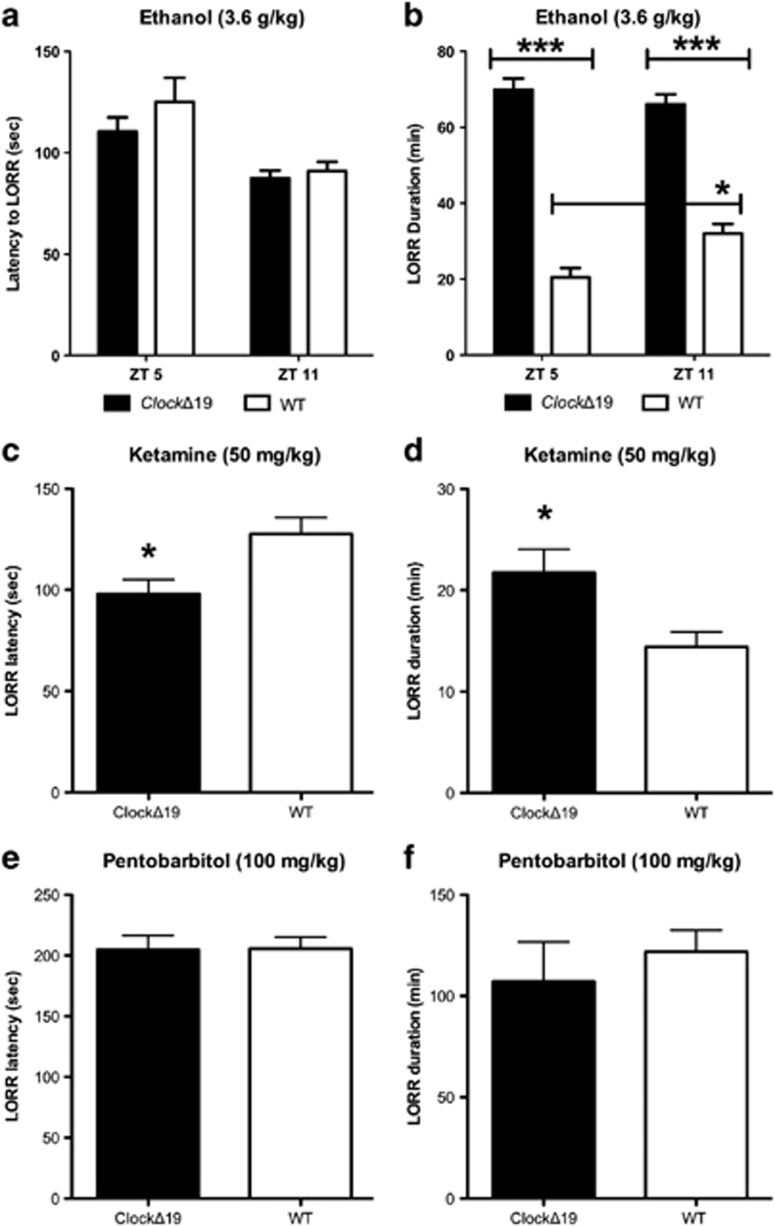
ClockΔ19 Mice Exhibit Augmented Responses to the Sedative Effects of Ethanol and Ketamine, but not Pentobarbital
Perreau-Lenz et al (2009) reported a diurnal variation in sensitivity to the sedative effects of ethanol, with greatest duration of LORR at ZT11 and shortest duration at ZT5. In this study, we found that latency to ethanol-induced LORR was similar for both genotypes; however. the duration of LORR produced by 3.6 g/kg ethanol was significantly greater for ClockΔ19 mice at ZT5 and ZT11 (Figures 2a and b; LORR duration: genotype × ZT interaction—F(1,24)=7.92, p<0.01, main effect of genotype—F(1,24)=234.6, p<0.0001). Diurnal variation in sensitivity to ethanol's sedative effects is absent in ClockΔ19 mice. It is possible that ClockΔ19 mice may be more sensitive/responsive to other sedative drugs; thus, we tested whether the LORR effect was specific to ethanol. As ethanol has been shown to act directly on GABAa and NMDA receptors, we tested pentobarbital- and ketamine-induced LORR (Harris et al, 2008). ClockΔ19 mice exhibited a significantly shorter latency to ketamine-induced LORR and greater duration of LORR (p<0.05) than WT mice (Figures 2c and d). However, both genotypes showed a similar latency to and duration of LORR to pentobarbital (Figures 2e and f). Taken together, these results suggest that the increased sensitivity to the sedative/hypnotic effects of ethanol seen in ClockΔ19 mice may be because of a hyperglutamatergic system and are likely not due to differences in GABAergic transmission. Gupta et al (2008) reported that glutamatergic signaling has a role in binge-like ethanol drinking in the drinking in the dark (DID) paradigm, where they reported that mice pre-treated with acamprosate or MPEP (an mGluR5 antagonist) exhibited decreased DID. Perhaps, the altered glutamatergic tone indirectly measured by ketamine LORR and directly measured and described by Beaulé et al (2009) in ClockΔ19 mice would lead to increased binge-like ethanol drinking in the DID paradigm. We found that ClockΔ19 and WT mice exhibit comparable ethanol intake in the DID paradigm and respond to acamprosate similarly (data not shown). This suggests that the increased sensitivity to ketamine seen in ClockΔ19 mutants (and possible altered glutamatergic tone) may not have an important role in their binge-like ethanol consumption. Furthermore, we found no other obvious predictors of their increased ethanol intake in a behavioral battery (including ethanol-induced taste aversion and acute functional tolerance) (Supplementary Figure 3). ClockΔ19 and WT mice exhibit similar behavioral and metabolic responses to ethanol.
Figure 2.
ClockΔ19 mice exhibit augmented responses to the sedative effects of ethanol and ketamine. Latency (a) and duration (b) to ethanol-induced loss of righting reflex (LORR) at ZT5 and ZT11. Latency (c) and duration (d) to ketamine-induced LORR. Latency (e) and duration (f) to pentobarbitol-induced LORR. *p<0.05; ***p<0.001. WT, wild type; ZT, zeitgeber time.
Decreased Clock Expression in Reward-Related Brain Regions after Chronic Ethanol Intake and Abstinence
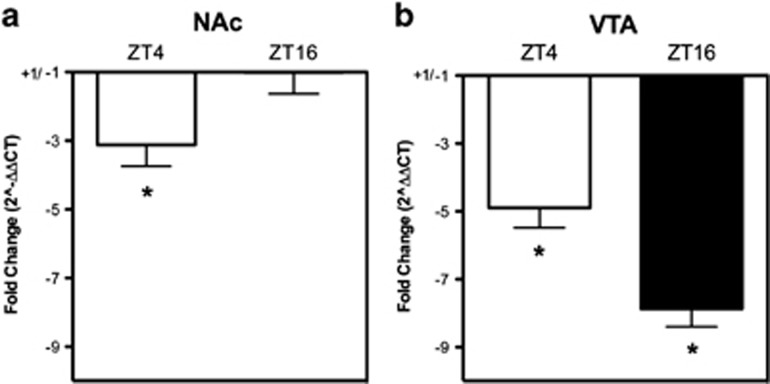
Decreased plasma Clock expression has been reported for recently abstinent alcoholics (Huang et al, 2010). We hypothesized that this phenomenon would extend to reward-related brain regions, specifically the NAc and VTA. To determine if Clock expression in the NAc and VTA was modulated by ethanol consumption, WT mice voluntarily consumed ethanol in a choice paradigm for 2 weeks, were subjected to 1 week of abstinence, and were then killed at ZT4 or ZT16. Clock mRNA expression in NAc and VTA was quantified via qPCR. Clock expression in the NAc was significantly lower in ethanol-experienced mice in a ZT-dependent manner (Figure 3a, main effect of ZT—F (1,24)=13.55, p<0.05; main effect of treatment—F (1,24)=234.6, p<0.05). Clock expression in VTA was significantly decreased in ethanol-experienced mice at both times of day tested (Figure 3b, main effect of treatment—F(1,26)=6.25, p<0.05). As chronic ethanol intake and abstinence resulted in decreased VTA Clock expression at both times of day tested, similar to what is expected when RNAi is invoked, we focused on the VTA for the next experiment.
Figure 3.
Chronic ethanol intake and abstinence results in significantly decreased nucleus accumbens (NAc) and ventral tegmental area (VTA) Clock expression. Clock expression in the NAc (a) and VTA (b) at ZT4 and ZT16 was normalized to 18s, and fold change was calculated using the ΔΔCT method (ΔCTethanol−ΔCTcontrol). *p<0.05. ZT, zeitgeber time.
Clock Knockdown in the VTA Leads to Increased Ethanol Preference and Consumption in WT Mice
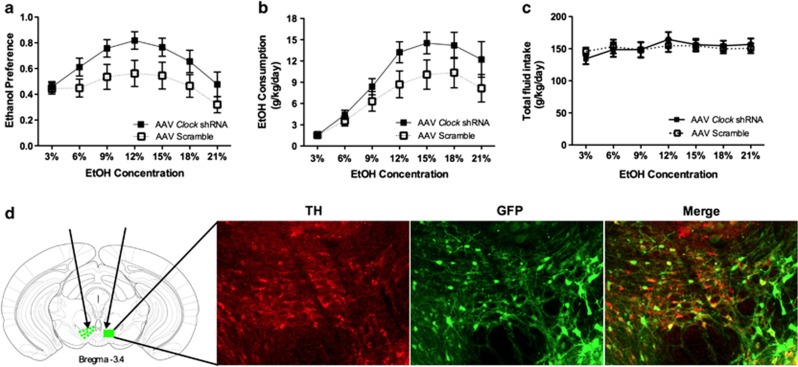
To test if decreased VTA Clock expression was sufficient to produce increased ethanol consumption and preference, we used viral-mediated gene transfer of an shRNA to knock down Clock expression in the VTA in WT mice and measured ethanol preference and consumption using the continuous access two-bottle choice paradigm. Knocking down Clock expression in the VTA led to significantly increased ethanol consumption and preference, without affecting total fluid intake (Figures 4a–c; ethanol consumption: main effect of treatment—F(1,161)=11.30, p<0.001, main effect of concentration—F(6,161)=14.40, p<0.0001; ethanol preference: main effect of treatment—F(1,161)=17.02, p<0.0001, main effect of concentration—F(6,161)=3.85, p<0.01). Representative image of VTA-specific targeting of AAV Clock shRNA is shown in Figure 4d.
Figure 4.
Reducing Clock in the ventral tegmental area (VTA) via RNA interference (RNAi) results in significantly increased ethanol intake. (a) Ethanol (EtOH) consumption, (b) EtOH preference, (c) total fluid intake, and (d) diagram of VTA containing coronal section, where green indicates VTA regions targeted by stereotaxic injection (indicated by arrows). Representative photomicrographs showing VTA-specific targeting of adeno-associated virus (AAV) Clock short hairpin RNA (shRNA). Brain sections were immunostained with anti-tyrosine hydroxylase (TH) and -green fluorescent protein (GFP) antibodies and 20x images were merged to observe colocalization.
DISCUSSION
Results of this study establish that CLOCK is involved in regulating ethanol intake. ClockΔ19 mice exhibit significantly increased ethanol intake, with female mice exhibiting a more robust phenotype. Moreover, chronic ethanol intake and abstinence results in a long-lasting decrease in NAc and VTA Clock expression in WT mice. The most compelling evidence showing VTA Clock is a negative regulator of ethanol intake is shown in the RNAi experiment, where we found that shRNA-mediated reduction of Clock results in increased ethanol intake similar to what is observed in the ClockΔ19 mice. These findings demonstrate the involvement of the circadian transcription factor, CLOCK, in regulating ethanol intake.
A growing body of evidence implicates glutamatergic systems in drug-related behaviors and drug-induced plasticity. Pharmacological approaches targeting glutamatergic systems for the treatment of alcoholism have proven utility. Acamprosate is an FDA-approved treatment for reducing craving and relapse in alcoholics (Olive et al, 2012; Yahn et al, 2013). Although the exact mechanism of action has yet to be determined, studies have shown that acamprosate can act as an NMDA modulator and is thought to restore balance to perturbations in excitatory and inhibitory neurotransmission (Olive et al, 2012). Recent studies have revealed evidence of altered glutamatergic systems in ClockΔ19 mice that may contribute to their drug-preferring phenotype (Beaulé et al, 2009; Dziraza et al, 2010). Beaulé et al (2009) showed that functional CLOCK and Per2 proteins are important for regulating glutamate levels. ClockΔ19 mice have reduced mRNA and protein levels of the glial excitatory amino-acid transporter, EAAT1, as well as reduced glutamate uptake. Further, Spanagel et al (2005a, 2005b) reported that mutation of another circadian gene, Per2, also results in reduced EAAT1 expression and increased alcohol intake. Thus, it is possible that the decreased glutamate uptake and increased NMDA receptor levels result in a hyperglutamatergic tone and this could contribute to the observed increase in neuronal excitability and increased sensitivity to ethanol (and ketamine) observed in ClockΔ19 mice. However, we found that acamprosate fails to reduce differentially ethanol intake (in a binge-drinking paradigm) in the ClockΔ19 mice, suggesting that the altered glutamatergic tone is not responsible for the increase in alcohol intake. Further, Brager et al (2011) reported that acamprosate decreases ethanol drinking in a similar manner for WT and Per2 mutants. Nevertheless, the finding that ClockΔ19 mice are more sensitive to the sedative/hypnotic effects of ketamine but not pentobarbital suggests that the altered glutamatergic tone in these mice is likely involved in the increased sedative/hypnotic properties of alcohol.
The mesolimbic dopaminergic system has long been implicated in motivational behaviors, including drug-induced behaviors and neuronal plasticity (Nestler, 2005; Schultz, 1998). Drugs of abuse increase levels of the rate-limiting enzyme in dopamine synthesis, Th (tyrosine hydroxylase), and augment firing rates of VTA dopamine neurons via direct and indirect mechanisms (Nestler, 2005; Stuber et al, 2008). Importantly, CLOCK is involved in regulating dopaminergic transmission. ClockΔ19 mice have increased tonic and phasic dopamine transmission, as well as cell volume changes (McClung et al, 2005; Coque et al, 2011). Augmented DA firing is associated with increased locomotor activity and drug reward, which is also seen in ClockΔ19 mice (Adamantidis et al, 2011; Marinelli and White, 2000; McClung et al, 2005; Ozburn et al, 2012). Knocking down Clock expression in the VTA recapitulates the increased DA cell firing seen in ClockΔ19 mice (Mukherjee et al, 2010). In addition, overexpressing an inwardly rectifying potassium channel subunit (Kir2.1) selectively in the VTA of ClockΔ19 mice reduces the firing rate of dopamine neurons in ClockΔ19 mice and results in a normalization of their locomotor- and anxiety-related behaviors (Coque et al, 2011). Taken together, these studies show that abnormalities in dopamine cell firing and associated morphology underlie alterations in locomotor, anxiety-, and drug-related behavior.
The regulation of the VTA dopamine system by CLOCK is likely achieved through its actions as a transcription factor. We have previously reported that several genes in the VTA known to control dopaminergic activity are altered in ClockΔ19 mice (McClung et al, 2005). Th expression in the VTA exhibits a circadian pattern, and striatal dopamine levels are increased during the light phase of the light/dark cycle in the ClockΔ19 mice (Coque et al, 2011). The mechanism underlying CLOCK regulation of Th is currently under investigation in our laboratory. In addition to the upregulation of Th seen in ClockΔ19 mice, we have previously reported downregulation of Cholecystokinin (Cck). Cck is a peptide neurotransmitter that is colocalized and coreleased with dopamine and acts as a negative modulator of dopaminergic transmission (Ghijsen et al, 2001; Hökfelt et al, 1980; Lança et al, 1998; Voigt et al, 1986). Arey et al (2009, 2013) have shown that CLOCK binds to an E-box in the Cck promoter, and positively regulates Cck expression. Furthermore, reducing Cck expression in the VTA (via RNAi) recapitulates the behavioral phenotype observed in ClockΔ19 mice (decreased anxiety- and depression-like behaviors). Previous studies have shown that increased Cck results in increased anxiety and depression-like behavior, whereas Cck receptor blockade is anxiolytic and antidepressant (Rotzinger et al, 2010). The downregulation of Cck in the ClockΔ19 mice is particularly interesting in the context of this study as Cck receptors have been implicated in alcohol-related behaviors in both rodent and human studies (Crespi, 1998; Miyasaka et al, 2004, 2005).
Chronic alcohol treatment leads to lasting changes in Clock gene expression, which likely contributes to the pervasive disruptions in rhythms and sleep in alcoholics. Although we did not measure Clock expression in the SCN, others have shown that the SCN is responsive to ethanol (Chen et al, 2004; Prosser and Glass, 2009; Seggio et al, 2009). Studies in different species have illustrated that circadian clock neurons are important for alcohol tolerance and that alcohol can modulate circadian phase-shifting (Brager et al, 2010; Ghezzi et al, 2013; McElroy et al, 2009; Prosser and Glass, 2009; Ruby et al, 2009; Seggio et al, 2009). In this study, animals consumed far less alcohol (and for a shorter period of time) than the study by Chen et al (2004), showing chronic ethanol liquid diet alters SCN rPer2 gene expression or the study by Seggio et al (2009), showing chronic (no choice) ethanol consumption alters circadian activity rhythms; thus, we would not have expected changes in circadian gene expression in the SCN or altered circadian rhythms with this paradigm. It will be important to pursue how SCN Clock expression is altered in future studies, although it is difficult under these conditions to do so. All experiments were completed under light/dark (12 : 12) conditions (where both WT and Clock mutants show entrainment under these conditions) and none of our experiments were performed under dark/dark conditions. Although it is possible that Clock in other brain regions may modulate ethanol intake, this study focused on the VTA (based on previous findings by Mukherjee et al, 2010), where we found that VTA Clock expression is sufficient to modulate ethanol intake.
Taken together, our results suggest that decreased CLOCK function results in increased dopaminergic tone via its actions as a transcription factor in the VTA, and this underlies the drug-preferring phenotype, which seems to generalize to multiple classes of drugs of abuse. Moreover, the increased glutamatergic tone in these mice may be important in regulating the sedative/hypnotic properties of alcohol but not the rewarding properties. In addition to Th and Cck, we are currently investigating other interesting CLOCK target genes that may influence neuronal excitability in reward- and stress-related brain regions.
FUNDING AND DISCLOSURE
Dr McClung has received research funding and honoraria from IMHRO Johnson & Johnson, GlaxoSmithKline and Pfizer on projects not related to this work. She has also received honoraria from Servier. Drs Ozburn, Falcon, Mukherjee, Gillman, Arey and Spencer have no financial conflicts to disclose.
Acknowledgments
This study was supported by NIH Grants DA-07290 and AA-020452 (to ARO) and DA-023988 (to CAM). We thank Ryan Logan for help revising the manuscript and Heather Buresch, Emily Webster, Elizabeth Gordon, and Ariel Ketcherside for technical assistance. ClockΔ19 mice were a generous gift from Dr Joseph Takahashi.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Arey RN, Enwright JF, III, Spencer SM, Falcon E, Ozburn AR, Ghose S, et al. 2013An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors Mol Psychiatry(E-pub ahead of print 12 February 2012, PMID 23568193). [DOI] [PMC free article] [PubMed]
- Arey R, Spencer S, Falcon E, Enright JF, III, McClung C.2009Regulation of the Cholecystokinin Gene by Lithium in a Mouse Model of Mania. Program No. 746.3/U20. Neuroscience Meeting Planner Society for Neuroscience: Chicago, IL, USA; 2009 (online). [Google Scholar]
- Beaulé C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One. 2009;4:e7476. doi: 10.1371/journal.pone.0007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcohol Clin Exp Res. 2011;35:1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KKJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi F. The role of cholecystokinin (Cck), Cck-A or Cck-B receptor antagonists in the spontaneous preference for drugs of abuse (alcohol or cocaine) in naive rats. Methods Find Exp Clin Pharmacol. 1998;20:679–697. doi: 10.1358/mf.1998.20.8.487502. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, et al. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, et al. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994;21:109–112. [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol. Alcohol. 2007;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Al-Hasan YM, Krishnan HR, Wang Y, Atkinson NS. Functional mapping of the neuronal substrates for drug tolerance in Drosophila. Behav Genet. 2013;43:227–240. doi: 10.1007/s10519-013-9583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghijsen WE, Leenders AG, Wiegant VM. Regulation of cholecystokinin release from central nerve terminals. Peptides. 2001;22:1213–1221. doi: 10.1016/s0196-9781(01)00444-2. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, et al. Acute effects of acamprosate and MPEP on ethanol drinking-in-the-dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. Ethanol's molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and Cck in meso-limbic neurones. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Huang MC, Ho CW, Chen CH, Liu SC, Chen CC, Leu SJ. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34:1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976;46:301–305. doi: 10.1007/BF00421118. [DOI] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AEK, Gillman AG, Leffel JK, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse can entrain circadian rhythms. Scientific World J. 2007;7:203–212. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AEK, Pecoraro NC, Rebec GV, Timberlake W. Circadian activity precedes daily methamphetamine injections in the rat. Neurosci Lett. 1998;250:99–102. doi: 10.1016/s0304-3940(98)00439-x. [DOI] [PubMed] [Google Scholar]
- Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Lança AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19:859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Wan C, Zhou W, Peng T, Liu Y, et al. The role of mPer1 in morphine dependence in mice. Neuroscience. 2005;130:383–388. doi: 10.1016/j.neuroscience.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci. 1997;17:1302–1319. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of clock in the ventral tegmental area through rna interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka K, Yoshida Y, Matsushita S, Higuchi S, Maruyama K, Niino N, et al. Association of cholecystokinin-A receptor gene polymorphism with alcohol dependence in a Japanese population. Alcohol Alcohol. 2004;39:25–28. doi: 10.1093/alcalc/agh002. [DOI] [PubMed] [Google Scholar]
- Miyasaka K, Hosoya H, Takano S, Ohta M, Sekime A, Kanai S, et al. Differences in ethanol ingestion between cholecystokinin-A receptor deficient and -B receptor deficient mice. Alcohol Alcohol. 2005;40:176–180. doi: 10.1093/alcalc/agh143. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction. Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Behavioral differences between C57BL/6J × FVB/NJ and C57BL/6J × NZB/B1NJ F1 hybrid mice: relation to control of ethanol intake. Behav Genet. 2010;40:551–563. doi: 10.1007/s10519-010-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Larson EB, Self DW, McClung CA. Cocaine self-administration behaviors in ClockΔ19 mice. Psychopharmacology. 2012;223:169–177. doi: 10.1007/s00213-012-2704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol. 2009;14:253–259. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Glass JD. The mammalian circadian clock exhibits acute tolerance to ethanol. Alcohol Clin Exp Res. 2009;33:2088–2093. doi: 10.1111/j.1530-0277.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakrishnan V, Subramanian P, Viswanathan P, Menon VP. Effect of chronic ethanol ingestion on biochemical circadian rhythms in Wistar rats. Alcohol. 1999;18:147–152. doi: 10.1016/s0741-8329(98)00077-9. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31:736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Suzuki Y, Yazaki R, Tamefusa K, Ohara K, Yokoyama T, et al. Circadian variation in plasma 5-hydroxyindoleacetic acid level during and after alcohol withdrawal: phase advances in alcoholic patients compared with normal subjects. Acta Psychiatr Scand. 1993;87:291–296. doi: 10.1111/j.1600-0447.1993.tb03374.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2009;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm L, Kovanen L, Saarikoski S, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. J Circadian Rhythms. 2010;8:1. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body's biological clock. Alcohol Clin Exp Res. 2005a;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005b;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Stephan FK. Phase-shifts of circadian rhythms in activity entrained to food access. Physiol Behav. 1984;32:663–671. doi: 10.1016/0031-9384(84)90323-8. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong H-K, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Wang RY, Westfall TC. Cholecystokinin octapeptides alter the release of endogenous dopamine from the rat nucleus accumbens in vitro. J Pharmacol Exp Ther. 1986;237:147–153. [PubMed] [Google Scholar]
- Yahn SL, Watterson LR, Olive MF. Safety and efficacy of acamprosate for the treatment of alcohol dependence. Subst Abuse. 2013;6:1–12. doi: 10.4137/SART.S9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.