Abstract
Dual orexin receptor antagonists (DORAs) induce sleep by blocking orexin 1 and orexin 2 receptor-mediated activities responsible for regulating wakefulness. DORAs represent a potential alternative mechanism to the current standard of care that includes the γ-aminobutyric acid (GABA)A receptor-positive allosteric modulators, eszopiclone and zolpidem. This work uses an innovative method to analyze electroencephalogram (EEG) spectral frequencies within sleep/wake states to differentiate the effects of GABAA modulators from DORA-22, an analog of the DORA MK-6096, in Sprague–Dawley rats. The effects of low, intermediate, and high doses of eszopiclone, zolpidem, and DORA-22 were examined after first defining each compound's ability to promote sleep during active-phase dosing. The EEG spectral frequency power within specific sleep stages was calculated in 1-Hz intervals from 1 to 100 Hz within each sleep/wake state for the first 4 h after the dose. Eszopiclone and zolpidem produced marked, dose-responsive disruptions in sleep stage-specific EEG spectral profiles compared with vehicle treatment. In marked contrast, DORA-22 exhibited marginal changes in the spectral profile, observed only during rapid eye movement sleep, and only at the highest dose tested. Moreover, while eszopiclone- and zolpidem-induced changes were evident in the inactive period, the EEG spectral responses to DORA-22 were absent during this phase. These results suggest that DORA-22 differs from eszopiclone and zolpidem whereby DORA-22 promotes somnolence without altering the neuronal network EEG activity observed during normal sleep.
Keywords: EEG, dual orexin receptor antagonist, eszopiclone, GABAA, spectral analysis, zolpidem
INTRODUCTION
Insomnia is estimated to affect 10–15% of the general population (Ohayon, 2002) and many patients are treated with the γ-aminobutyric acid (GABA)A receptor modulators eszopiclone (Sunovion Pharmaceuticals) and zolpidem (Sanofi) to help induce and maintain sleep. In recent years, orexin (hypocretin) receptor antagonism has been shown to be an effective mechanism of modulating arousal and promoting somnolence (Brisbare-Roch et al, 2007; Herring et al, 2012). Dual orexin receptor antagonists (DORAs) reversibly block both orexin 1 and orexin 2 receptors (OX1R and OX2R) to promote sleep by a mechanism consistent with suppressing wakefulness (Bettica et al, 2012a; Brisbare-Roch et al, 2007; Winrow et al, 2011, 2012). It is currently unclear whether sleep produced by GABAA-positive allosteric modulators differs quantitatively from that induced by DORAs.
Spectral electroencephalogram (EEG) frequency analysis is a useful quantitative analytical technique for measuring neuronal network activity. Clinically, zolpidem and zopiclone (a racemic mixture of eszopiclone and its inactive enantiomer) have been shown to reduce wakefulness and increase non-rapid eye movement (NREM) sleep while modifying the EEG frequency distribution in delta (slow-wave or NREM) sleep (Landolt et al, 2000; Lundahl et al, 2012; Brunner et al, 1991; Trachsel et al, 1990). GABAA modulators have also been associated with central nervous system-related adverse events (AEs) including sleep walking, sleep driving, sleep eating, amnesia, and cognitive impairment (Hindmarch et al, 2006; Hoque and Chesson, 2009; Otmani et al, 2008; Rush et al, 1998). Orexin receptor antagonism has emerged as an alternative mechanism for the potential treatment of insomnia that specifically targets neuronal orexinergic networks involved in wakefulness. It is as yet unknown whether these distinct pharmacological mechanisms can be quantitatively differentiated based on unique EEG signatures within the central nervous system (CNS).
DORA-22 is an analog of MK-6096, an orally bioavailable, potent and selective reversible antagonist of OXR1 and OXR2 currently in clinical development for insomnia (Winrow et al, 2012). To better understand the potential differences in sleep architecture between the GABAA modulators eszopiclone and zolpidem and DORA-22, we developed an analysis technique to evaluate quantitative EEG (qEEG) spectral power changes in CNS activity across rat sleep/wake states at sleep-effective doses of these compounds.
MATERIALS AND METHODS
Rat Sleep Architecture and qEEG Data Collection
All animal studies were performed in accordance with The National Research Council's Guide for the Care and Use of Laboratory Animals and were approved by the Merck Institutional Animal Care and Use Committee. All efforts were made to minimize animal use and suffering.
Rat sleep and qEEG studies were conducted in ambulatory animals via radio telemetry in a manner similar to that described previously (Renger et al, 2004; Winrow et al, 2011). Cortical EEG/electrocorticogram (ECoG), electromyogram (EMG), and generalized locomotor activity recordings were collected in male Sprague–Dawley rats (n=16 per study; age: 6–9 months; weight: 500–750 g). Telemetric physiological monitors were implanted subcutaneously (4ET; Data Sciences International, St Paul, MN), and included electrodes for EEG monitoring and electrodes implanted beneath the neck muscle for EMG recording. The animals were singly housed with food and water available ad libitum, and on a 12 : 12 light : dark cycle with lights off at 0400 hours (zeitgeber time; ZT12) and on at 1600 hours (ZT0).
Rat sleep/qEEG studies were performed on animals treated with DORA-22, eszopiclone (Myoderm USA, Norristown, PA), and zolpidem (Myoderm USA). Compounds were dosed orally in independent studies in a counterbalanced, vehicle-controlled, crossover design. In the first arm of the study, half the animals were treated with vehicle and the other half were treated with a single dose of compound daily for 3 consecutive days. After a 3-day washout period, the groups were subsequently reversed in the second arm of the experiment in which they received the alternative treatment condition for 3 consecutive days such that all animals ultimately received both vehicle and compound treatments over the course of the entire experiment. The study design included a 1-day vehicle only run-in period, a 3-day between-crossover washout, and a 2-day end-of-study washout (11 total oral dosings per animal).
To identify doses of eszopiclone, zolpidem, and DORA-22 that could achieve similar reductions in active wake, dose–response curves were generated for each compound in independent 3-day crossover studies following their daily oral administration during the active phase. Reduction of active wake over the first 4 h following dosing was used as the primary measure of sleep-promoting efficacy.
Effects of each compound were analyzed and compared with within-animal controls using 20% vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS; Eastman Chemical, Kingsport, TN) as vehicle; all dosing was performed orally (per os). Data were averaged by animal for each 3-day treatment crossover condition and subsequently analyzed for within subject comparisons of vehicle to compound treatment as described previously (Cox et al, 2010; Renger et al, 2004; Winrow et al, 2011). EEG/ECoG, EMG, and generalized activity signals were automatically classified in 10 s epochs for each 24 h recording using the Somnologica Science software (Embla, Denver, CO) for four sleep/wake states: active wake, delta sleep, REM sleep, and light sleep/quiet wake (reported here as light sleep). Active wake was classified as low amplitude, high-frequency EEG, and high amplitude EMG (muscle tone) with or without locomotor activity. Light sleep was classified as having higher delta and theta EEG frequency components and decreased EMG amplitude relative to active wake, and no locomotor activity. Scoring epochs classified as delta sleep exhibited delta power as the major EEG component, decreased EMG muscle tone, and no locomotor activity. REM sleep included high theta power with increased higher frequencies similar to active wake in the EEG, with little EMG muscle tone and no locomotor activity. Active-phase (wake) dosing studies occurred 5 h into active-phase (ZT17); inactive-phase (sleep) dosing studies occurred 1 h before lights on (ZT23). The MK-6096 analog, DORA-22, has well-characterized rodent pharmacokinetic properties (Coleman et al, 2012; Gotter et al, 2012; Winrow et al, 2012).
The effects of compounds on EEG frequencies were determined by comparing the EEG spectral frequency changes relative to vehicle within each sleep/wake state. The spectral ratio data (response to compound divided by vehicle response) indicate where responses to the compound result in an EEG frequency that increases (>1), decreases (<1), or has no effective change (=1).
Quantitative EEG Analysis
A custom-developed qEEG analysis algorithm was coded and compiled in Matlab (R2007a; MathWorks, Natick, MA) to perform artifact rejection and short-time Fourier transform (spectrogram function) on EEG signals; qEEG data were analyzed from 1 to 100 Hz in 1 Hz increments and aligned with sleep epoch data based on sleep/wake state epoch assignment. qEEG spectral powers were log-transformed and averaged by sleep/wake state by animal. Data from the first 4 h after the dose (ZT17–ZT21, active-phase dosing; ZT23–ZT03, inactive-phase dosing) for each animal following compound administration were divided by within-subject vehicle data to normalize spectral power measures (expressed as a compound/vehicle spectral ratio of treatment effect size). A locally weighted regression with a linear polynomial fit (Matlab, LOWESS) was applied and 95% confidence bounds were calculated using a bootstrap method to model the resulting spectral ratio using Matlab R2011a. Significance was determined by non-overlapping 95% confidence interval segments of the model with the threshold value 1±5% given a baseline (non-dosing)/vehicle dosing effect.
Area Over/Under Spectral Frequency Curve as a Function of Decreased Active Wake
Further analysis of the area over or under the spectral frequency curves (AUC) (outside of the ±5% dose effect) for 1–100 Hz by sleep state and dose using trapezoidal integration (Matlab R2011a) as a function of decreases in active wake reveals linear trends and correlations. Decreases in active wake for all treatments at each dose were used to normalize sleep time for the first 4 h after the dose (ZT17–ZT21; ZT23–ZT03). Standard error of the means were calculated for both the AUC (y axis) and decreases in active wake time (x axis) for each data point.
RESULTS
Sleep Promotion
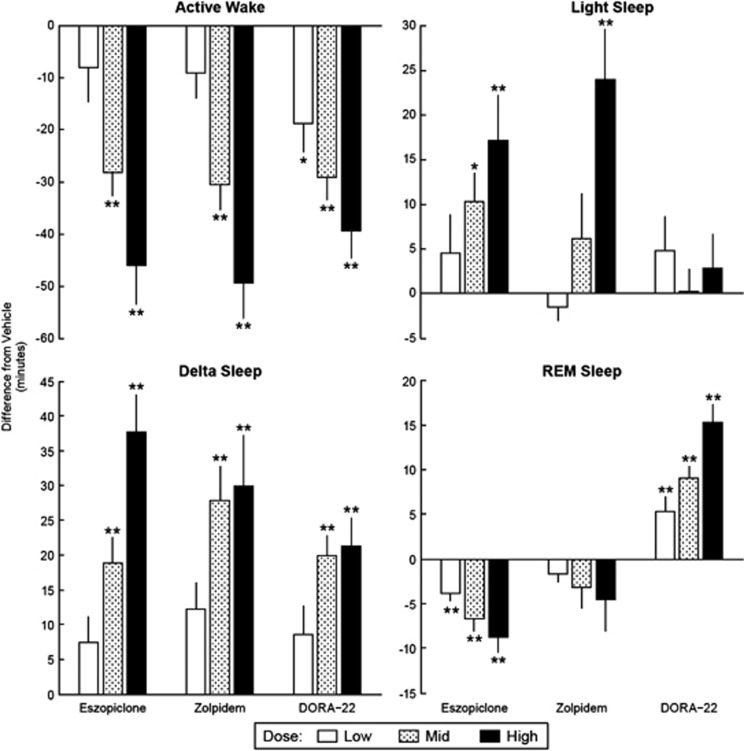
The respective low, intermediate, and high doses of eszopiclone, 3, 6, and 10 mg/kg, zolpidem, 10, 30, and 60 mg/kg, and DORA-22, 3, 10, and 30 mg/kg, promoted sleep to a similar extent as measured by compound-induced reductions in active wake (Figure 1, Active Wake panel). At 3, 6, and 10 mg/kg, eszopiclone dose-dependently decreased active wake relative to vehicle by 8.1±6.5, 28.3±4.5, and 46.0±7.5 min, respectively, with significant reductions achieved by the 6 and 10 mg/kg doses (P<0.01). Zolpidem at 10, 30, and 60 mg/kg decreased active wake by 9.1±5.0, 30.6±4.7, and 49.4±6.8 min, respectively; both the 30 and 60 mg/kg doses significantly decreased active wake (P<0.01). DORA-22 significantly reduced active wake at all three doses tested by 18.7±5.7 (P<0.05), 29.1±4.5 (P<0.01), and 39.4±5.3 min (P<0.01), respectively. Subsequent analyses relied on these previously determined pharmacologically normalized doses to conduct side-by-side comparisons of sleep-stage and qEEG spectral profile changes.
Figure 1.
γ-Aminobutyric acid (GABAA) modulators and dual orexin receptor antagonist (DORAs) dose-responsively increase sleep time in active-phase dosing (zeitgeber time (ZT)17). Average time (min±standard error of mean (SEM)) male Sprague–Dawley rats (n=16 per study) spent in sleep/wake state compared with vehicle treatment for respective low, mid, and high (eszopiclone: 3, 6, and 10 mg/kg; zolpidem: 10, 30, and 60 mg/kg; DORA-22: 3, 10, and 30 mg/kg) oral (per os) doses during 3-day crossover design studies during active phase. Each treatment dose was conducted independently and compared with within-subject vehicle. Statistics were calculated within each study using two-way analysis of variance (ANOVA) with repeated measure (factors for treatment and day); *P<0.05, **P<0.01.
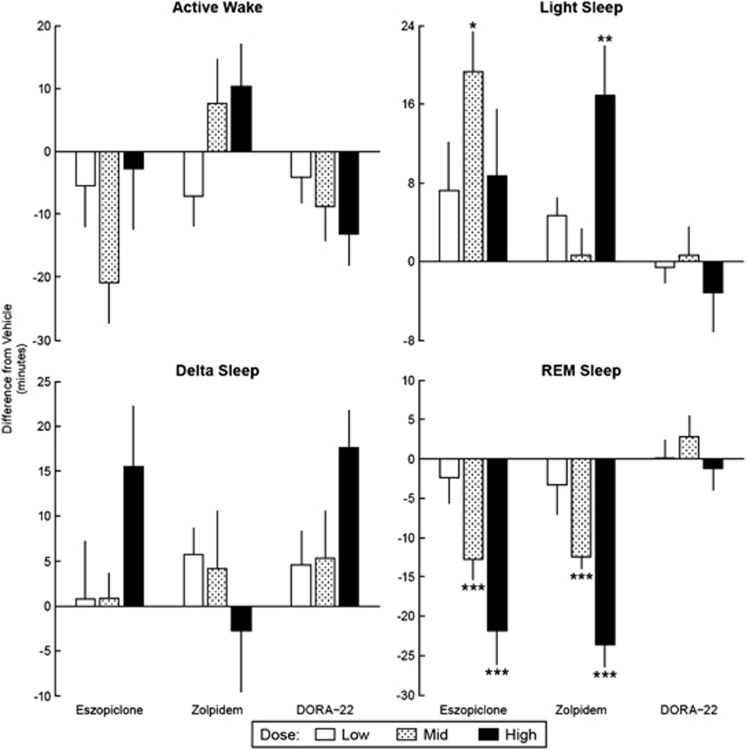
Further examination of sleep states indicated that eszopiclone and zolpidem modulated sleep states differently compared with DORA-22. Both GABAA modulators dose-dependently increased the amount of time spent in light and delta sleep during the active phase, whereas DORA-22 increased delta sleep, but had no significant effect on light sleep in the 4 h following treatment (Figure 1, Light Sleep panel Figure 1 and Delta Sleep panel). Marked differences were seen particularly in REM sleep: eszopiclone dose-dependently decreased REM sleep, zolpidem nonsignificantly trended towards a dose-responsive decrease in REM sleep, whereas DORA-22 dose-dependently increased REM sleep (Figure 1, REM Sleep panel). When similar studies were conducted during the inactive phase (by administering the study compounds 1 h before ‘lights on' (ZT23)), DORA-22 showed no significant changes in any of the sleep/wake states. Similarly, neither zolpidem nor eszopiclone significantly altered active wake or delta sleep in the inactive phase studies, both GABAA modulators did significantly decrease REM sleep (both GABAA modulators at the intermediate and high doses; P<0.001) with a concurrent increase in light sleep (eszopiclone and zolpidem intermediate and high doses, respectively; P<0.01) (Figure 2).
Figure 2.
γ-Aminobutyric acid (GABAA) modulators but not dual orexin receptor antagonist (DORAs) decrease rapid eye movement (REM) sleep in inactive-phase dosing (zeitgeber time (ZT)23). Average time (min±standard error of mean (SEM)) male Sprague–Dawley rats (n=16 per study) spent in sleep/wake state compared with vehicle treatment for respective low, mid, and high (eszopiclone: 3, 6, and 10 mg/kg; zolpidem: 10, 30, and 60 mg/kg; DORA-22: 3, 10, and 30 mg/kg) oral (per os) doses during 3-day crossover design studies during active phase. Each treatment dose was conducted independently and compared with within-subject vehicle. Statistics were calculated within each study using two-way analysis of variance (ANOVA) with repeated measure (factors for treatment and day); *P<0.05, **P<0.01, and ***P<0.001.
Alteration of Sleep Stage-Specific EEG Spectral Frequency
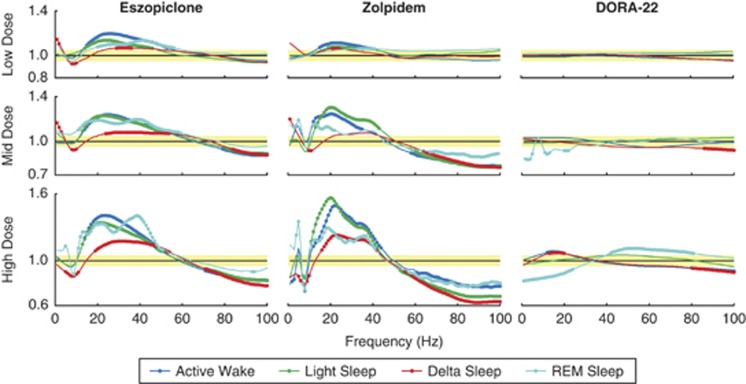
Spectral EEG records spontaneous brain activity in terms of rhythmic neural firing. When dosed during the active phase, both eszopiclone and zolpidem produced significant and dose-dependent changes in EEG spectral frequencies within sleep/wake vigilant states compared with normal vehicle controls, while DORA-22 had significantly smaller effects across all doses. Administration of eszopiclone or zolpidem was associated with increased mid (12–55 Hz) and concurrent decreased high (70–100 Hz) frequencies during active wake and light, delta, and REM sleep (Figure 3). At low and mid doses, zolpidem and eszopiclone increased the 1–3 Hz spectral power and decreased 4–12 Hz power during delta sleep. In contrast, no changes in sleep stage-dependent frequencies were observed with the lowest dose of DORA-22 (3 mg/kg), a dose exhibiting significant active wake reduction exceeding that observed in response to the lowest doses of eszopiclone and zolpidem. The small deviations in frequencies at this dose were no different in magnitude from the ±5% fluctuations associated with vehicle alone (yellow shaded area, Figure 3). Small changes in spectral frequency power during REM sleep were observed relative to vehicle at the intermediate and highest doses of DORA-22 (decreases at 1–4, 10–12, and 21–23 Hz for 10 mg/kg, and decreases at 1–25 Hz and increases at 42–82 Hz for 30 mg/kg). At 30 mg/kg, marginal decreases in spectral frequency power were observed during active wake and delta sleep (increases at 12–20 Hz and decrease at 80–100 Hz).
Figure 3.
γ-Aminobutyric acid (GABAA) modulators dose-responsively alter electroencephalogram (EEG) spectral frequency of sleep/wake states in active-phase dosing (zeitgeber time (ZT)17). EEG spectral changes (as a ratio of treatment over vehicle) from 1 to 100 Hz within each sleep/wake state for each treatment at respective doses (low, mid, and high). Yellow shaded area represents 1 (no change from vehicle)±5% as observed baseline dosing effect. 95% Confidence bounds were calculated and filled circles indicate non-overlapping areas above/below baseline dosing effect and 95% confidence intervals for each sleep/wake state. All treatments and doses were collected as independent 3-day crossover study designs in male Sprague–Dawley rats (n=16) during the active phase (ZT17).
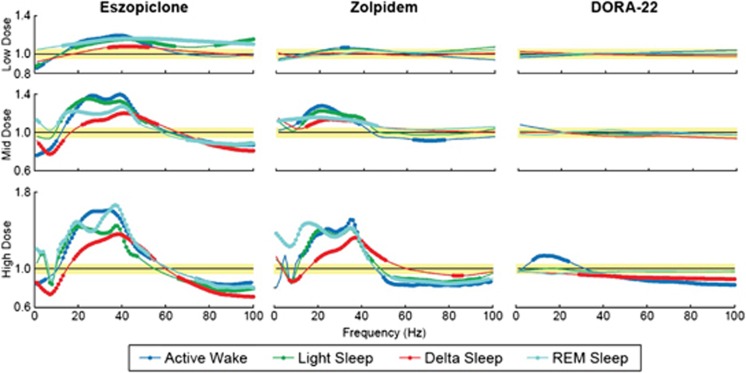
Consistent with polysomnographic data, the effects of eszopiclone and zolpidem on EEG spectral power were observed in the inactive phase, whereas DORA-22-induced changes were even further diminished relative to active-phase treatments. Dosing 1 h (ZT23) before normal sleep resulted in eszopiclone- and zolpidem-induced EEG spectral power changes reminiscent of those that occurred during the active phase and were again dose-responsive (Figure 4). Marginal changes vs vehicle in the spectral profile of delta and active wake reached significance in response to DORA-22 at the high dose (Figure 4).
Figure 4.
γ-Aminobutyric acid (GABAA) modulators dose-responsively alter electroencephalogram (EEG) spectral frequency of sleep/wake states in inactive-phase dosing (zeitgeber time (ZT)23). EEG spectral changes (as a ratio of treatment over vehicle) from 1 to 100 Hz within each sleep/wake state for each treatment at respective doses (low, mid, and high). Yellow shaded area represents 1 (no change from vehicle)±5% as observed baseline dosing effect. 95% Confidence bounds were calculated and filled circles indicate non-overlapping areas above/below baseline dosing effect and 95% confidence intervals for each sleep/wake state. All treatments and doses were collected as independent 3-day crossover study designs in male Sprague–Dawley rats (n=16) during the inactive phase (ZT23).
Effects on Normal Rat Sleep
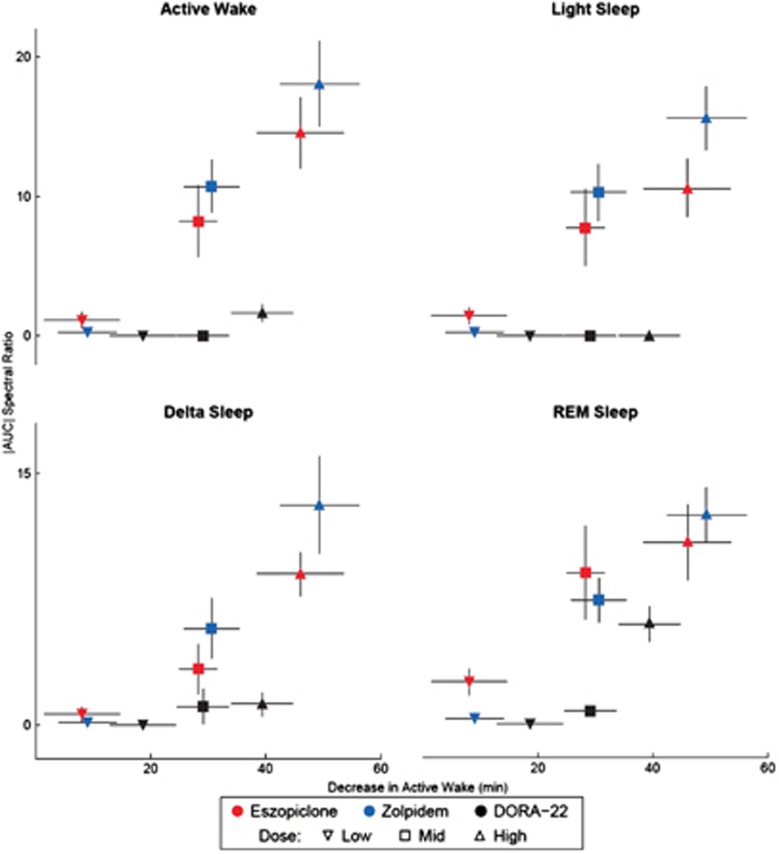
The correlation between the magnitude of compound-induced EEG spectral changes within each sleep stage and sleep-promoting efficacy (as measured by decreases in active wake) was calculated to better quantify the difference between agents. Eszopiclone and zolpidem produced a strong relationship between changes in EEG spectral frequency (y axis) and decreases in active wake time (x axis) across all sleep/wake states (Figure 5). DORA-22, however, exhibited no correlation between changes in the EEG spectral frequency and decreases in active wake time except in REM sleep, which was associated with a decrease in the lower frequencies and an increase in the higher frequencies at the high dose. When the EEG spectral frequency was analyzed separately by sleep/wake state, eszopiclone and zolpidem exhibited significant correlations between most of these measures during active wake and light, delta, and REM sleep (P<0.05 through P<0.001; Supplementary Table S1); the exception was the correlation between changes in spectral frequency and delta sleep with zolpidem, which was not significant. DORA-22 showed a significant correlation between spectral power changes and sleep efficacy only during REM sleep (R2 correlation value, 0.13; P<0.05) (Supplementary Table S1).
Figure 5.
Dual orexin receptor antagonist (DORA-22) minimally alters EEG frequency as a function of sleep. Area under/over the electroencephalogram (EEG) spectral frequency (Figure 3) as a function of decreases in active wake (Figure 1) for each sleep/wake state (different panes). Inverted triangle (▾) indicates low dose, square (▪) indicates mid dose, and triangle (▴) indicates high dose. Symbols represent average decrease in active wake (x axis)±standard error of mean (SEM), and average area under/over the EEG frequency curve outside of the±5% dosing effect area (y axis)±SEM.
DISCUSSION
To better understand the different effects on sleep architecture of the GABAA modulators eszopiclone and zolpidem and the orexin receptor antagonist DORA-22, we developed an innovative analysis technique to evaluate qEEG spectral power changes across rat sleep/wake vigilance states at dose ranges with sleep-promoting effects.
We report that at doses producing similar amounts of sleep during the active phase (as measured by decreases in active wake), eszopiclone and zolpidem substantially and dose-dependently altered EEG spectral frequencies. Generalized decreases in spectral power at frequency ranges of 1–10 and 50–100 Hz along with increases in power at frequencies of 20–40 Hz relative to within-subject vehicle control were seen across sleep/wake stages with these two GABAA modulators. These effects were even observed at the lowest doses, which also did not promote sleep. The lowest DORA-22 dose tested (3 mg/kg) significantly increased sleep during the active phase, but had no effect on EEG spectral frequency as compared across matching sleep/wake states. The intermediate dose (10 mg/kg) of DORA-22 promoted substantially more sleep than low-dose DORA-22 and proportionally as much sleep as the intermediate doses of eszopiclone (6 mg/kg) and zolpidem (30 mg/kg). Minor EEG spectral changes were observed with the intermediate dose of DORA-22 that were of a lesser magnitude compared with those observed with eszopiclone and zolpidem during active-phase dosing.
The more targeted distribution of orexin receptors, vs the broader distribution and more diverse actions of GABA receptors, is likely to have a substantial role in the distinct downstream effects of their respective modulators, including the changes in EEG spectra observed with eszopiclone and zolpidem. Distribution of target receptors may have important clinical implications in terms of their specificity to desired sleep effects with reduced concerns of off-target effects.
Bettica and co-workers (2012b) reported similar findings to those described herein with the DORA SB-649868 (10 and 30 mg doses) and zolpidem (10 mg); zolpidem and both doses of SB-649868 significantly (P<0.05) increased total sleep time in human subjects exposed to a traffic noise model of situational insomnia. However, while zolpidem significantly increased slow-wave sleep and disrupted EEG power spectra during NREM (non-REM) sleep compared with placebo, neither dose of SB-689698 was found to have these effects (Bettica et al, 2012b). This similarity between humans and rats suggests that animal models can be used as translational models of EEG spectral frequency assessment within and among sleep/wake states.
Eszopiclone and zolpidem minimally affected active wake when administered during the inactive phase; however, both compounds significantly reduced REM sleep, and moreover, substantially disrupted EEG spectral patterns during all sleep stages in this phase. In common with eszopiclone and zolpidem, DORA-22 did not alter sleep in the inactive phase when compared with within-subject vehicle control. In contrast with the GABAA modulators, however, DORA-22 did not alter the EEG spectra within sleep/wake states at the low dose and mid-dose.
As noted, DORA-22 has a different mechanism of action to that of the GABAA modulators, specifically targeting orexin signaling responsible for arousal in neuronal pathways controlling sleep/wake. Orexin peptides cycle with a circadian rhythm, reaching their nadir during prolonged natural sleep. Therefore, blocking of orexin receptor signaling by administration of DORA-22 during the inactive (sleep) phase would be expected to differ little from vehicle administration because of naturally lower endogenous orexin levels at this time (Desarnaud et al, 2004; Kiyashchenko et al, 2002; Martinez et al, 2002; Saper et al, 2005).
Alteration of EEG frequencies to a similar extent during the active and inactive phases by eszopiclone or zolpidem suggests that GABAA modulators that induce sleep have consistent effects on neuronal activity within the brain, and that brain activity in rats during compound-induced sleep differs from brain activity occurring during physiologically normal sleep. Furthermore, it appears that the compound-induced EEG spectral pattern of sleep in the active or inactive phase is similar with GABAA modulators, irrespective of the natural wake drive during the active phase, thus demonstrating a consistent effect on neuronal network activity independent of circadian phase. The consistency of this effect on EEG activity during both the active and inactive phases, and across sleep/wake states, suggests a general and widespread GABAA receptor activity enhancement throughout the brain. In contrast to eszopiclone and zolpidem, DORA-22 did not suppress REM sleep at any dose during the active or inactive phases, suggesting a potential adverse effect of GABAA modulators with respect to memory consolidation and learning (Karni et al, 1994; Mintzer et al, 1997; Smith et al, 2004). Furthermore, DORA-22 did not reduce the duration of time spent in REM sleep, nor did it substantially change the EEG spectral frequency within REM sleep compared with normal vehicle controls, again suggesting that DORA-22 may not have the same memory-related adverse effects as the GABAA modulators.
Taken together, these data suggest that DORAs promote sleep that is more similar to physiological sleep and quantitatively different from the sleep induced by GABAA modulators. There is no specific evidence that the observed spectral EEG changes with GABAA modulators are a precursor to or are direct evidence of the AEs associated with these compounds. However, it has been reported that changes in the frequencies involved in sleep spindles (or sigma power bands; bursts of oscillatory brain activity during stage 2 sleep) are related to amnesia, changes in the beta band are related to sensorimotor control and cognition, and changes in the slower delta band are related to subjective sleep complaints (Espa et al, 2000; Krystal et al, 2002; Spiegelhalder et al, 2012). In humans, the distinct typical spectral EEG change in delta sleep induced by benzodiazepine and benzodiazepine-like hypnotics, including zolpidem and zopiclone, have been well characterized (Achermann and Borbely, 1987; Aeschbach et al, 1994; Feige et al, 1999; Lundahl et al, 2012; Trachsel et al, 1990).
CONCLUSION
In summary, spectral EEG frequency analysis is a useful analytical technique for differentiating sleep/wake state changes for sleep-inducing insomnia treatments. It has been demonstrated here that DORAs promote sleep that is more similar in spectral frequency distribution characteristics to normal sleep in rats after administration of a vehicle control, and is different to the sleep induced by the GABA modulators eszopiclone and zolpidem. Further clinical studies will reveal the translatability of these preclinical findings to compound-induced changes in human frequency distributions within sleep/wake states and the underlying meaning of these EEG frequency changes.
FUNDING AND DISCLOSURE
Steven Fox, Anthony Gotter, Spencer Tye, Susan Garson, Alan Savitz, Jason Uslaner, Joseph Brunner, Pamela Tannenbaum, Terrence McDonald, Robert Hodgson, Lihang Yao, Mark Bowlby, Scott Kuduk, Paul Coleman, Richard Hargreaves, Christopher Winrow, and John Renger were full-time employees of Merck at the time of the design, execution, and reporting of this study. Except for income received from our primary employer and stock or stock options owned in the Company, no other financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service. Editorial support was provided by Gary Dever, PhD at Complete Medical Communications and was funded by Merck.
Acknowledgments
We acknowledge Drs Ellen Snyder, Vladimir Svetnik, Junshui Ma, and Shubhankar Ray for their analytic and statistical support.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Achermann P, Borbely AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum Neurobiol. 1987;6:203–210. [PubMed] [Google Scholar]
- Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbely AA. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology. 1994;11:237–244. doi: 10.1038/sj.npp.1380110. [DOI] [PubMed] [Google Scholar]
- Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012a;37:1224–1233. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012b;37:1224–1233. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Brunner DP, Dijk DJ, Munch M, Borbely AA. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 1991;104:1–5. doi: 10.1007/BF02244546. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Schreier JD, Cox CD, Breslin MJ, Whitman DB, Bogusky MJ, et al. 2012Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2-(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties Chem Med Chem 7415–424.337. [DOI] [PubMed] [Google Scholar]
- Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53:5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, et al. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espa F, Ondze B, Deglise P, Billiard M, Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–939. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- Feige B, Voderholzer U, Riemann D, Hohagen F, Berger M. Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol. 1999;110:1965–1974. doi: 10.1016/s1388-2457(99)00147-9. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J. A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. Br J Clin Pharmacol. 2006;62:538–545. doi: 10.1111/j.1365-2125.2006.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque R, Chesson AL., Jr Zolpidem-induced sleepwalking, sleep related eating disorder, and sleep-driving: fluorine-18-flourodeoxyglucose positron emission tomography analysis, and a literature review of other unexpected clinical effects of zolpidem. J Clin Sleep Med. 2009;5:471–476. [PMC free article] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–640. [PubMed] [Google Scholar]
- Landolt HP, Finelli LA, Roth C, Buck A, Achermann P, Borbely AA. Zolpidem and sleep deprivation: different effect on EEG power spectra. J Sleep Res. 2000;9:175–183. doi: 10.1046/j.1365-2869.2000.00192.x. [DOI] [PubMed] [Google Scholar]
- Lundahl J, Deacon S, Maurice D, Staner L. EEG spectral power density profiles during NREM sleep for gaboxadol and zolpidem in patients with primary insomnia. J Psychopharmacol. 2012;26:1081–1087. doi: 10.1177/0269881111424457. [DOI] [PubMed] [Google Scholar]
- Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955:1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Otmani S, Demazieres A, Staner C, Jacob N, Nir T, Zisapel N, et al. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Dunn SL, Motzel SL, Johnson C, Koblan KS. Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res. 2004;1010:45–54. doi: 10.1016/j.brainres.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol. 1998;18:154–165. doi: 10.1097/00004714-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Smith CT, Nixon MR, Nader RS. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem. 2004;11:714–719. doi: 10.1101/lm.74904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Dijk DJ, Brunner DP, Klene C, Borbely AA. Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology. 1990;3:11–18. [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, et al. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, et al. Pharmacological characterization of MK-6096—a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62:978–987. doi: 10.1016/j.neuropharm.2011.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.