Abstract
Despite the prevalent worldwide abuse of stimulants, such as amphetamines and cocaine, no medications are currently approved for treating this serious public health problem. Both preclinical and clinical studies suggest that the opioid antagonist naltrexone (NTX) is effective in reducing the abuse liability of amphetamine, raising the question of whether similar positive findings would be obtained for cocaine. The purpose of this study was to evaluate the ability of oral NTX to alter the cardiovascular and subjective effects of D-amphetamine (D-AMPH) and cocaine (COC). Non-treatment-seeking COC users (N=12) completed this 3-week inpatient, randomized, crossover study. Participants received 0, 12.5, or 50 mg oral NTX 60 min before active or placebo stimulant administration during 10 separate laboratory sessions. Oral AMPH (0, 10, and 20 mg; or all placebo) was administered in ascending order within a laboratory session using a 60-min interdose interval. Smoked COC (0, 12.5, 25, and 50 mg; or all placebo) was administered in ascending order within a laboratory session using a 14-min interdose interval. Active COC and AMPH produced dose-related increases in cardiovascular function that were of comparable magnitude. In contrast, COC, but not AMPH, produced dose-related increases in several subjective measures of positive drug effect (eg, high, liking, and willingness to pay for the drug). NTX did not alter the cardiovascular effects of AMPH or COC. NTX also did not alter positive subjective ratings after COC administration, but it did significantly reduce ratings of craving for COC and tobacco during COC sessions. These results show that (1) oral AMPH produces minimal abuse-related subjective responses in COC smokers, and (2) NTX reduces craving for COC and tobacco during COC sessions. Future studies should continue to evaluate NTX as a potential anti-craving medication for COC dependence.
Keywords: cocaine, amphetamine, naltrexone, abuse liability, laboratory
INTRODUCTION
Abuse of stimulants is a serious public health problem. Of the approximately 210 million illicit drug users worldwide, approximately 56 million abuse amphetamines (AMPHs) and approximately 17 million abuse cocaine (COC; United Nations Office on Drugs and Crime, World Drug Report, 2011). Although methamphetamine abuse in the United States appeared to be declining during the early- to mid-2000s, it has remained constant over the past several years. For example, between 2007 and 2011, the number and percentage of current methamphetamine abusers ranged between 353 000 (0.1%) and 530 000 (0.2% Substance Abuse and Mental Health Services Administration, 2012). In 2010, among the 1.9 million emergency department visits involving drug misuse or abuse, methamphetamine/AMPH was ranked fourth (55 visits per 100 000 population aged 21 or older) of the illicit drugs, behind COC (211 visits), marijuana (151 visits), and heroin (93 visits; Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2012). However, no medications are currently approved by the Food and Drug Administration for treating stimulant dependence.
Recent research has suggested that naltrexone (NTX), an antagonist at the mu, delta, and kappa subtypes of opioid receptors, may be useful for treating AMPH dependence. Research conducted in Sweden, including both laboratory studies and a randomized treatment trial, demonstrated that oral NTX reduced the positive subjective effects of AMPH, as well as AMPH use (Jayaram-Lindstrom et al, 2004, 2008a, 2008b). For example, in both AMPH-dependent patients with attention-deficit/hyperactivity disorder (ADHD; N=20; Jayaram-Lindstrom et al, 2008b) and normal, healthy volunteers with no current or history of drug abuse (N=12; Jayaram-Lindstrom et al, 2004), 50 mg oral NTX significantly decreased the positive subjective effects of 30 mg oral D-AMPH without altering the physiological effects of AMPH. Furthermore, a randomized clinical trial of oral NTX (50 mg) for treating AMPH dependence demonstrated that treatment outcomes (number of AMPH-negative urine samples, rate of continuous abstinence, craving, and self-reported consumption of AMPH) were significantly improved in NTX compared with placebo-treated patients (N=40 per group; Jayaram-Lindstrom et al, 2008a). Consistent with the clinical data, preclinical studies have demonstrated that NTX reduces the reinforcing effects of intravenous AMPH (Jimenez-Gomez et al, 2011), AMPH-induced decreases in the threshold for intracranial brain stimulation reward (Todtenkopf et al, 2009), and reinstatement of AMPH seeking (Haggkvist et al, 2009). The exact mechanism by which NTX altered AMPH-induced responses remains unclear.
NTX has also been examined as a potential treatment medication for COC dependence, with mixed results. Kosten et al (1992) showed that NTX produced small decreases in subjective ratings of the value of intravenous COC. Walsh et al (1996) investigated the ability of NTX to attenuate the effects of intravenous COC, hydromorphone, and their combination. Although NTX did succeed in antagonizing the effects of hydromorphone, it did not alter the subjective or physiological effects of COC alone. NTX partially reduced the subjective effects produced by the hydromorphone/COC combination, but this effect appeared to be due to antagonism of hydromorphone. Sofuoglu et al (2003) investigated the potential use of NTX and isradipine, a calcium channel blocker, alone and in combination for the treatment of COC abuse. NTX alone decreased intranasal COC-induced increases in ratings of good effects, but, again, this change was small.
Given that both AMPH and COC increase synaptic dopamine levels, albeit through different mechanisms, it is not clear why NTX appears to alter the effects of AMPHs more than COC. Therefore, the purpose of our study was to replicate the positive findings reported by Jayaram-Lindstrom et al (2004, 2008b) and to directly compare the ability of NTX to alter the abuse liability of D-AMPH and COC using a within-subjects crossover design in which all participants receive both AMPH and COC. NTX and D-AMPH were administered orally in order to more closely replicate the studies by Jayaram-Lindstrom et al (2004, 2008b). COC was not administered via the oral route because it is typically not used in this manner. NTX was administered acutely in this study, rather than chronically as it would be used in clinical treatment settings, because robust reductions in AMPH-induced positive subjective responses were obtained with acute NTX pre-treatment in the laboratory studies reported by Jayaram-Lindstrom et al (2004, 2008b). As these findings in the laboratory setting were predictive of treatment response as noted above (Jayaram-Lindstrom et al, 2008a), we used the same acute dosing procedure in our study. We predicted that NTX compared with placebo would produce a dose-dependent decrease in the subjective effects of AMPH, but not COC. In addition, we predicted that NTX would neither alter the physiological effects of AMPH nor COC.
MATERIALS AND METHODS
Screening
After a telephone interview, volunteers found to be eligible for the study were scheduled for additional screening. During initial screening visits, volunteers completed questionnaires on drug use, general health, and medical history, as well as a medical and psychological evaluation. Laboratory analyses included a blood chemistry panel, complete blood count, liver profile, thyroid function test, pseudocholinesterase function to examine the body's ability to metabolize COC, and HCG levels in women. A urine drug toxicology (opioids, benzodiazepines, cannabis, methadone, buprenorphine, AMPH, COC, and oxycodone) was performed at each screening visit. Participants also received an electrocardiogram, tuberculosis test, and chest X-ray.
Participants were included in the study if they smoked COC at least twice per week for the previous 6 months and spent at least $50 per week on COC. Participants were excluded from the study if they were seeking treatment for their drug use, not between the ages of 21 and 50 years, dependent on any substance other than COC, nicotine or caffeine, had a major neurological or axis I psychiatric disorder, other than substance dependence, were currently pregnant or breastfeeding, did not use an effective method of birth control, were taking any medications that would interfere with the study measures (eg, blood pressure medications, antiretroviral medications), had any unstable physical disorders (eg, AIDS, hypertension, or heart disease), or had any liver, gastrointestinal, or renal disease that would alter the pharmacokinetics of the study drugs. Any participant with current or recent risk of violence, or who was on parole/probation were also excluded from the study. Participants were required to be physically healthy and able to complete all study procedures.
Before laboratory sessions began, participants completed a practice session, where computerized questionnaires and tasks were explained to them in detail. Volunteers were paid $15 for each screening visit for up to 5 visits, $25 per inpatient day, and an additional $25 per day bonus if they completed the study. Volunteers were also paid $25 per visit at 1-month and 3-month follow-up visits after study completion. Participants signed consent forms describing the study and potential risks and benefits of all study procedures and drugs involved. At the end of the study or during the study if requested, participants were offered referrals for treatment of their drug use. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute and was conducted in accordance with the tenets of the Declaration of Helsinki.
Apparatus
During experimental sessions, participants were seated in a comfortable chair in front of a Macintosh computer and a response manipulandum (‘mouse') was used for the completion of tasks and questionnaires presented on the screen. Experimenters in an adjacent control room continually monitored all computer activities, vital signs, and behaviors. The control room contained a computer, used for data collection and control of the participants' computer, a one-way mirror, and vital signs monitors. Cardiovascular function was measured using a Sentry II vital signs monitor and an electrocardiogram.
General Procedure
Participants admitted to the study were not allowed to have visitors or to leave the facility unescorted by study staff. On admission, a 12-lead ECG was performed to ensure cardiovascular health and that no major changes in functioning had arisen after screening. The first 1 to 2 days after admission were used as drug ‘washout' days to allow the effects of any street drugs to dissipate. After this period, participants began laboratory sessions. Sessions were conducted approximately every other day, excluding weekends. The subjective and physiological effects of oral D-AMPH (0, 10, and 20 mg) and smoked COC (0, 12, 25, and 50 mg) given in combination with oral NTX (0, 12.5 or 50 mg) were compared. Dose combinations were: (1) placebo NTX+placebo COC throughout the session, (2) 50 mg NTX+placebo COC throughout the session, (3) placebo NTX+increasing doses of 0, 12, 25, and 50 mg COC, (4) 12.5 mg NTX+increasing doses of 0, 12, 25, and 50 mg COC, (5) 50 mg NTX+increasing doses of 0, 12, 25, and 50 mg COC, (6) placebo NTX+placebo AMPH throughout the session, (7) 50 mg NTX+placebo AMPH throughout the session, (8) placebo NTX+increasing doses of 0, 10, and 20 mg AMPH, (9) 12.5 mg NTX+increasing doses of 0, 10, and 20 mg AMPH, and (10) 50 mg NTX+increasing doses of 0, 10, and 20 mg AMPH. One NTX dose was administered per session under double-blind conditions. Participants were not informed that the drug they received was NTX, but were told that the medication may be one of the following: modafinil, gabapentin, NTX, or bupropion. Multiple doses of COC or AMPH were administered per session (either all placebo doses or ascending active doses). Although participants were aware whether they were receiving oral or smoked drug during laboratory sessions, they were blind to the AMPH and COC doses administered. All participants received each combination of doses in a random order, completing 10 laboratory sessions in total. Urine samples, for drug analysis, were randomly collected once a week to ensure that participants were compliant with study procedures.
Experimental Sessions
During all sessions, participants completed tasks and questionnaires on a computer. ECG was continually monitored throughout the session. During the first 30 min of each session, baseline (BL) blood pressure, heart rate, and presence of a normal ECG were determined and subjects completed a performance task battery as well as a BL assessment of subjective effects using the visual analog scale (VAS). If at any time blood pressure or heart rate deviated from normal (see Measures: Physiological effects), or the ECG was abnormal for longer than 6 min, the session was discontinued. Participants were not allowed to smoke tobacco or eat a full meal during sessions.
D-AMPH sessions
During D-AMPH sessions, NTX (0, 12.5, or 50 mg) was administered at time point 0. AMPH dosing began 1 h after NTX administration and continued on a 60-min interdose interval. AMPH doses were either three doses of placebo, or three increasing doses of AMPH (0, 10, and 20 mg) per session. The VAS was administered at 15-min intervals after the NTX, placebo AMPH, and 10 mg AMPH doses. After the 20 mg dose of AMPH, the VAS was administered every 15 min for the next hour, and every 30 min after that for another 2 h. One hour after the last dose of AMPH, the performance task battery was administered. D-AMPH sessions lasted approximately 6.5 h.
COC sessions
During COC sessions, NTX (0, 12.5, or 50 mg) was administered at time point 0. COC dosing began 1 h after NTX administration and continued on a 14-min interdose interval. COC doses were either four doses of placebo, or four increasing doses of COC (0, 12, 25, and 50 mg) per session. The VAS was administered at 15-min intervals after the NTX dose, and then 2 min after each COC dose administration. The VAS was administered 2, 10, 20, 30, and 60 min after the final dose of COC. Thirty minutes after the last dose of COC, the performance task battery was administered. COC sessions lasted approximately 3.5 h.
Measures
Subjective effects
The VAS was used to assess subjective and self-perceived physiological effects. The VAS consisted of 25 items. Participants indicated on a 100 mm line, from ‘not at all' to ‘extremely' in response to each question. The first 17 questions were labeled ‘I feel' ‘Stimulated', ‘Anxious', ‘Depressed', ‘Sedated', ‘High', ‘Focused', ‘Calm', ‘Able to Concentrate', ‘Alert', ‘Tired', ‘Talkative', ‘Self-confident', ‘Social', ‘Irritable' ‘Confused', ‘a Good Effect', and ‘a Bad Effect'. The next three questions assessed drug craving and were labeled ‘I Want…' ‘Cocaine', ‘Alcohol', and ‘Tobacco'. The next three questions asked on a scale of ‘not at all' to ‘extremely' about the quality and potency of the drug and how much they liked the drug that they had received. The last question asked the participant to mark on a scale from $0 to $25 how much they would pay for the drug received.
Physiological effects
A blood pressure cuff was attached to the participant's non-dominant arm and recorded blood pressure and heart rate every 6 min during D-AMPH sessions and every 2 min during COC sessions. If systolic/diastolic blood pressure rose above 160/100 mm Hg or heart rate rose above (220−participant's age−0.85) for >6 min, no further doses were administered. If systolic/diastolic blood pressure rose above 180/120 mm Hg or heart rate rose above (220−participant's age−0.85) for >6 min, the session was discontinued. No doses were withheld and no sessions were terminated based on these criteria.
Behavioral performance tasks
The behavioral performance task battery consisted of four tasks. A 3-min digit-symbol substitution task, a 10-min divided attention task, a 10-min rapid information processing task, and a 3-min repeated acquisition of response sequences. The results of the performance tasks will be published in a separate paper, so they will not be discussed further.
Side effects
Adverse events (AEs) were assessed each day throughout the study using a modified version of the Systematic Assessment for Treatment Emergent Effects questionnaire (SAFTEE; Guy et al, 1986; Rabkin and Markowitz, 1986). AEs were coded from a list of possible events and the severity (mild, moderate, or severe), potential causes (study drug, concurrent drug, concurrent illness, other known cause, or uncertain cause), action(s) taken (none, decreased dose, symptomatic therapy, study drug discontinued, and hospitalization), and outcome (recovered, abated with decreased dosage, ongoing, and under treatment, death) were recorded.
Drugs
Cocaine
COC HCl was purchased from Mallinckrodt (Phillipsburg, NJ) and manufactured into pellets of smokable COC (12, 25, and 50 mg) by the New York State Psychiatric Institute pharmacy. The 0 mg COC dose was an inhalation of warmed air through the pipe. A physician or nurse administered smoked COC by placing the pellet at the end of a pre-made glass tube with a wire-mesh screen and lighting the end for the participant to inhale. All visual cues were blocked immediately before smoking with a blindfold to ensure that the participants would not get any information about the drug by seeing it. Doses were administered using a 14-min interdose interval because previous studies have shown that it would result in cumulative (increasing) subjective effects (eg, Foltin et al, 2003).
D-amphetamine
D-AMPH was purchased from Cardinal Health (New York, NY) in 10 mg tablets. Doses (0, 10, and 20 mg) were administered as two identical capsules. Tablets were over-encapsulated with lactose filler and were identical in appearance in order to maintain the dosing blind. Placebo doses consisted of capsules containing only lactose powder; the 10 mg dose consisted of one capsule containing a 10 mg tablet of D-AMPH and one placebo capsule; and the 20 mg dose consisted of two capsules containing one 10 mg tablet each of D-AMPH. A nurse administered the tablets. Doses were administered using a 60-min interdose interval because based on previous studies (eg, Brauer et al, 1996), we expected that it would result in cumulative subjective effects.
Naltrexone
NTX was purchased from Cardinal Health in 50 mg tablets. Doses (0, 12.5, or 50 mg) were administered as two identical tablets. The placebo dose consisted of two capsules containing only lactose powder. The 12.5 mg dose contained a quarter of a 50 mg tablet in one capsule and a placebo capsule. The 50 mg dose contained a full 50 mg tablet in one capsule and a placebo capsule. A nurse administered NTX 60 min before the first dose of D-AMPH or COC. This pre-treatment time was chosen because previous studies have reported that the maximum NTX plasma concentration after an acute dose of 50 mg NTX is achieved in 1 h (Meyer et al, 1984) and pharmacologically relevant plasma concentrations (ie, those that are associated with robust antagonism of the effects of mu opioid agonists) are sustained for 6–12 h (Wall et al, 1981; Meyer et al, 1984).
Statistical Analysis
Repeated-measures analyses of variance (ANOVA) were used to analyze the physiological and subjective effects of AMPH and COC, and to examine any main effects of NTX. Separate ANOVAs were performed to analyze the effects of AMPH and COC according to dose and time for each drug. Planned comparisons were made among dose conditions, and both dose and dose × time interactions were examined. Planned comparisons were used to answer two specific questions: (1) How does NTX alter the effects of AMPH and COC? and (2) Do COC and AMPH produce dose-dependent effects within each session? Active NTX dose sessions were compared with placebo NTX sessions and active doses of AMPH or COC were compared with placebo doses within each condition. Physiological effects were analyzed as mean values and subjective effects were analyzed as peak scores before (BL) and after each dose. The SAFTEE was inadvertently not administered to the first four participants who completed the study, so only data from the last eight participants who completed the study were reported below. Comparisons were considered statistically significant at P<0.01.
RESULTS
Participants
Twelve participants (9 men and 3 women; 8 Black, 2 Hispanic, and 2 mixed) completed the study. On average, participants were 44±2 years of age (range: 29–50) and had used COC for 20±2 years (range: 5–33). All participants reported current use of COC and spent an average of $263±55 per week on it (range: $70–500). Two participants reported previous use of methamphetamine, one of whom used in the month before study participation. Two participants had previously used over-the-counter or prescription stimulants (Ritalin, Dexedrine, diet pills, ‘uppers' one of these participants was among the two who had used methamphetamine). Eleven of the 12 completers were current cigarette smokers, ranging from 2 cigarettes per week to 20 cigarettes per day. All 12 participants reported using alcohol, ranging from once per month to once per day (range: 1–4 12-ounce beers per day). Four of the 12 completers reported using marijuana, approximately 2–3 times per month. One participant reported using heroin 20 years previously. Five participants began the study, but did not complete it. Four were discontinued for medical reasons (diabetes, chest pain, high blood pressure before initiation of laboratory sessions, and T-wave inversion after D-AMPH administration). One was dropped because of a paranoid reaction that developed mid-way through the study; the participant had received active COC and active AMPH during separate sessions before the paranoid reaction.
Physiological Effects
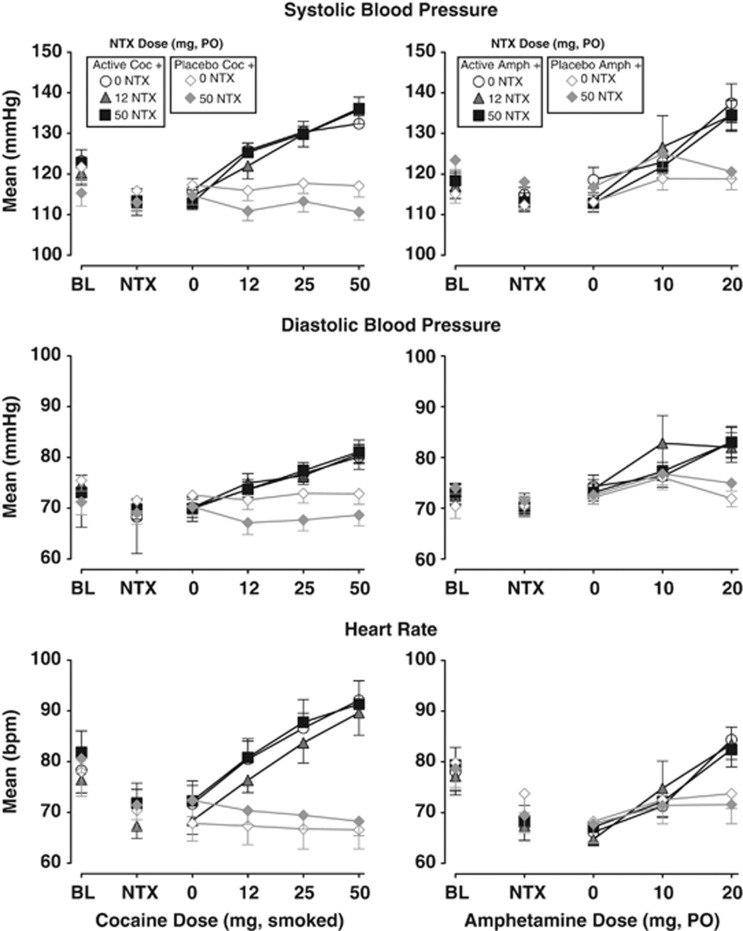
Figure 1 shows that both COC (left panels) and AMPH (right panels) produced cardiovascular effects that were of comparable magnitude. The main effects of dose were significant for both COC and AMPH for all of the physiological endpoints. COC significantly increased diastolic pressure (F(5, 50)=19.1; P<0.0001), systolic pressure (F(5, 50)=38.8; P<0.0001), and heart rate (F(5, 50)=39.7; P<0.0001). Similarly, AMPH significantly increased diastolic pressure (F(4, 44)=13.7; P<0.0001), systolic pressure (F(4, 44)=18.0; P<0.0001), and heart rate (F(4, 44)=53.5; P<0.0001). NTX had no effect on AMPH- and COC-induced physiological responses.
Figure 1.
Physiological effects of oral NTX in combination with smoked COC (left panels) and oral AMPH (right panels). The upper panels represent the mean±1 SEM systolic blood pressure (mmHg) before (BL) and after each dose administration. Data points above NTX represent effects after administration of NTX and before administration of the placebo stimulant dose. The middle panels represent the mean±1 SEM diastolic blood pressure (mmHg), and the lower panels represent the mean±1 SEM heart rate (beats per minute, b.p.m.). Data points connected by black lines represent sessions involving active COC or AMPH doses in combination with NTX (0, 12.5, and 50 mg) and data points connected by grey lines represent sessions involving placebo COC or AMPH doses in combination with NTX (0 and 50 mg).
Subjective Effects
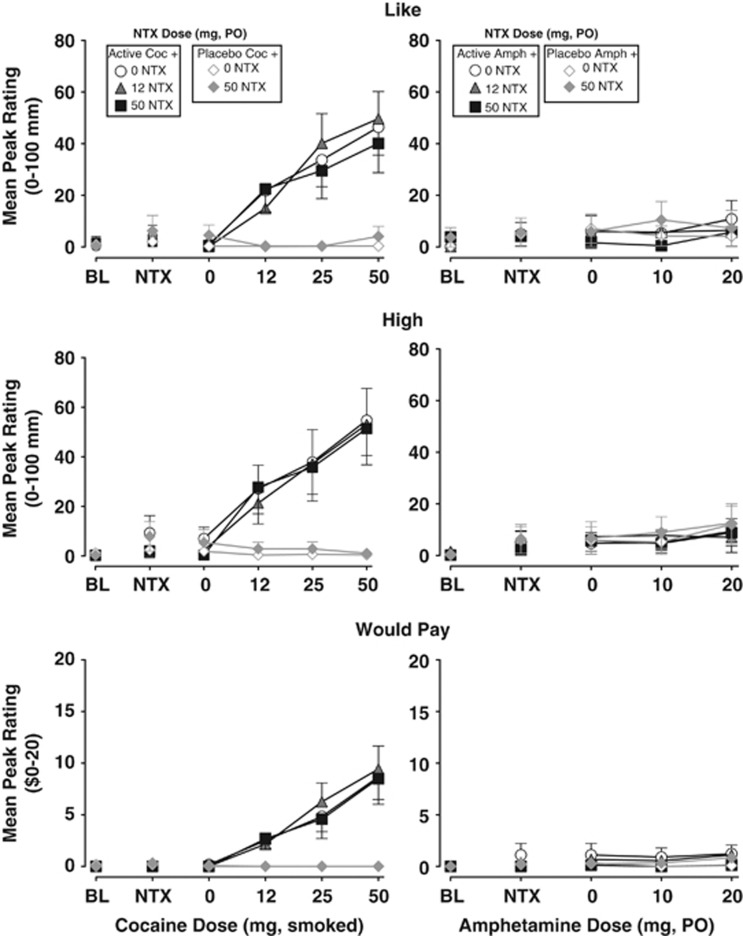
Figure 2 and Table 1 illustrate the effects of smoked COC (left panels) and oral AMPH (right panels) in combination with NTX on subjective-effect ratings. Participants reported that they ‘liked' COC (F(5, 55)=13.7; P<0.0001), felt ‘high' from it (F(5, 55)=11.3; P<0.0001), and ‘would pay' for it (F(5, 55)=15.6; P<0.0001). Active COC also significantly increased ratings of ‘anxious,' ‘calm,' ‘confused,' ‘good effect,' ‘high quality,' ‘potent,' ‘sedated,' ‘social,' ‘stimulated,' and ‘talkative,' and it decreased ratings of ‘tired' (Table 1). In contrast to the findings with COC, participants reported minimal subjective effects after administration of active AMPH (Figure 2, Table 1). AMPH did produce small, although statistically significant increases in some subjective measures, including ratings of ‘alert,' ‘anxious,' ‘calm,' ‘irritable,' ‘self-confident,' ‘social,' and ‘tired' (Table 1).
Figure 2.
Selected ratings of subjective effects. The upper panels display the mean peak±1 SEM rating of ‘I liked the choice' (mm) before (BL) and after each dose administration. Data points above NTX represent effects after administration of NTX and before administration of the placebo stimulant dose. The middle panels display the mean±1 SEM peak rating of ‘I feel high' (mm), and the lower panels display the mean±1 SEM peak rating of ‘I would pay' ($). Data points connected by black lines represent sessions involving active COC or AMPH doses in combination with NTX (0, 12.5, and 50 mg) and data points connected by grey lines represent sessions involving placebo COC or AMPH doses in combination with NTX (0 and 50 mg).
Table 1. Select Subjective Effects of Oral Naltrexone in Combination with Oral Amphetamine and Smoked Cocaine.
|
Naltrexone (0 mg) |
Naltrexone (50 mg) |
Naltrexone (0 mg) |
Naltrexone (50 mg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine dose (mg, smoked) | Amphetamine dose (mg, PO) | |||||||||||||
| VAS item | 0 | 12 | 25 | 50 | 0 | 12 | 25 | 50 | 0 | 10 | 20 | 0 | 10 | 20 |
| Alert | 62.2 (4.7) | 63.2 (6.0) | 64.7 (5.3) | 75.9 (4.4) | 64.5 (5.9) | 65.2 (5.2) | 68.0 (6.0) | 78.2 (5.2) | 71.2 (5.3) | 69.8 (5.8) | 74.8 (5.6) | 69.7 (5.7) | 76.9 (4.3) | 79.5 (5.1)a |
| Anxious | 17.2 (7.2) | 32.8 (10.8) | 33.2 (10.3) | 38.9 (9.7) | 8.9 (5.1) | 16.8 (8.4) | 19.1 (9.5) | 30.5 (10.5)a,b | 7.7 (3.3) | 5.1 (2.6) | 20.0 (7.7) | 15.4 (6.9) | 12.1 (5.8) | 34.1 (10.7)a,b |
| Calm | 60.3 (5.7) | 54.5 (7.9) | 53.2 (7.1) | 71.1 (4.4) | 56.8 (4.3) | 51.8 (7.0) | 54.9 (7.1) | 65.6 (5.5)a | 70.9 (4.9) | 72.1 (3.8) | 73.5 (5.1) | 67.2 (4.5) | 70.4 (5.0) | 75.8 (4.4)a |
| Confused | 4.2 (3.2) | 9.7 (6.6) | 8.2 (6.3) | 27.8 (10.6) | 5.5 (5.1) | 5.2 (4.9) | 11.7 (7.8) | 21.2 (9.0)a | 8.1 (5.2) | 9.8 (6.6) | 17.1 (8.8) | 5.6 (4.2) | 4.8 (3.7) | 10.4 (7.1) |
| Good effect | 1.8 (1.3) | 23.5 (8.3) | 33.1 (10.0) | 44.1 (10.8) | 0.4 (0.2) | 21.4 (8.5) | 31.3 (11.6) | 39.7 (11.5)a | 5.7 (5.0) | 4.4 (4.0) | 9.3 (5.9) | 2.7 (1.6) | 4.2 (4.0) | 6.2 (5.5) |
| High | 5.2 (3.5) | 22.9 (7.6) | 31.4 (10.4) | 43.4 (11.2) | 0.3 (0.2) | 21.3 (8.6) | 27.3 (11.0) | 40.7 (12.2)a | 4.8 (4.05 | 5.0 (4.6) | 9.3 (5.0) | 5.8 (2.2) | 4.7 (3.7) | 8.8 (5.2) |
| High quality | 1.2 (1.2) | 19.8 (7.7) | 29.5 (10.3) | 45.8 (10.4) | 0.3 (0.2) | 20.2 (7.9) | 27.7 (10.3) | 37.5 (11.5)a | 4.9 (4.2) | 4.5 (4.3) | 9.2 (5.7) | 1.9 (1.1) | 0.6 (0.5) | 4.8 (4.0)b |
| Irritable | 12.3 (6.1) | 8.1 (4.4) | 11.2 (8.0) | 14.0 (6.4) | 7.6 (5.9) | 8.2 (6.2) | 5.8 (3.4) | 12.9 (6.9) | 8.9 (6.6) | 7.0 (6.0) | 16.7 (8.8) | 23.8 (9.5) | 10.4 (6.0) | 23.3 (8.8)a |
| I would pay | 0.2 (0.2) | 2.5 (0.7) | 4.8 (1.5) | 8.7 (2.2) | 0.0 (0.0) | 2.7 (1.0) | 4.6 (1.9) | 8.5 (2.5)a | 0.8 (0.8) | 0.7 (0.7) | 1.0 (0.6) | 0.1 (0.1) | 0.0 (0.0) | 0.2 (0.1)b |
| Liking | 1.0 (1.0) | 21.8 (8.3) | 33.8 (10.4) | 46.4 (10.8) | 0.6 (0.3) | 22.5 (8.8) | 29.6 (10.8) | 40.2 (11.4)a | 6.2 (6.0) | 5.3 (5.1) | 10.8 (7.2) | 1.7 (1.2) | 0.5 (0.5) | 5.8 (5.3) |
| Potent | 0.8 (0.8) | 17.8 (6.4) | 30.3 (10.0) | 44.0 (10.7) | 0.3 (0.3) | 21.2 (8.5) | 26.8 (10.0) | 38.0 (11.3)a | 4.3 (3.8) | 3.5 (3.3) | 6.5 (4.6) | 1.3 (1.1) | 0.4 (0.4) | 4.3 (4.0) |
| Sedated | 4.8 (3.9) | 9.8 (5.0) | 8.8 (4.4) | 14.7 (4.5) | 1.0 (0.5) | 3.9 (2.52 | 9.0 (5.1) | 13.8 (5.4)a | 10.5 (6.0) | 12.0 (8.2) | 17.0 (8.3) | 11.0 (5.1) | 8.7 (5.3) | 10.8 (7.4) |
| Self-confident | 64.5 (5.1) | 65.8 (6.0) | 60.5 (5.6) | 67.2 (6.2) | 61.7 (6.7) | 59.0 (6.5) | 56.2 (8.3) | 68.0 (5.6) | 70.0 (5.8) | 69.6 (6.0) | 73.8 (6.0) | 70.5 (5.8) | 70.1 (6.2) | 76.2 (5.5)a |
| Social | 38.6 (7.7) | 31.7 (7.1) | 32.8 (7.0) | 38.8 (7.3) | 39.7 (7.8) | 32.5 (6.6) | 31.1 (6.9) | 41.1 (7.7)a | 43.2 (6.8) | 40.6 (7.8) | 44.2 (7.6) | 48.9 (5.6) | 41.7 (7.1) | 54.2 (9.0)a |
| Stimulated | 6.8 (4.6) | 20.1 (7.4) | 33.2 (10.2) | 44.2 (10.8) | 0.5 (0.3) | 19.5 (8.3) | 31.9 (10.6) | 42.2 (11.7)a | 5.8 (3.6) | 8.3 (6.2) | 10.8 (3.6) | 11.2 (4.9) | 8.2 (4.5) | 10.3 (6.1) |
| Talkative | 25.5 (7.8) | 25.0 (7.0) | 20.9 (6.6) | 34.9 (6.9) | 35.5 (7.3) | 24.8 (6.2) | 20.7 (6.0) | 32.8 (6.7)a | 34.5 (6.7) | 34.2 (7.0) | 36.8 (7.0) | 36.5 (6.0) | 37.8 (7.0) | 39.0 (7.3) |
| Tired | 23.5 (6.9) | 11.6 (5.3) | 7.3 (3.3) | 17.2 (6.9) | 18.7 (9.0) | 8.5 (3.8) | 3.8 (2.1) | 21.8 (7.2)a | 32.2 (5.0) | 31.0 (8.1) | 37.1 (8.2) | 41.1 (9.5) | 40.4 (9.6) | 37.2 (8.7)a |
| Want alcohol | 8.8 (8.3) | 10.3 (8.1) | 12.2 (8.6) | 15.1 (8.8) | 4.5 (4.0) | 6.8 (5.3) | 11.8 (8.3) | 12.5 (8.4)a,b | 11.1 (8.2) | 9.9 (8.3) | 11.4 (8.3) | 9.0 (7.9) | 8.6 (8.0) | 8.9 (8.3)b |
| Want cocaine | 36.2 (11.0) | 41.8 (12.3) | 43.1 (11.9) | 42.9 (11.0) | 16.1 (8.9) | 24.7 (10.3) | 30.3 (12.5) | 36.4 (12.5)a,b | 20.2 (10.9) | 20.4 (11.2) | 18.2 (11.1) | 16.4 (10.3) | 14.6 (9.5) | 17.3 (10.9)b |
| Want tobacco | 31.8 (10.9) | 37.0 (10.6) | 47.2 (10.3) | 60.2 (10.3) | 14.5 (7.9) | 20.3 (9.2) | 29.4 (10.6) | 54.8 (12.0)a,b | 30.9 (11.1) | 36.0 (11.4) | 53.8 (10.7) | 24.8 (10.8) | 41.0 (13.1) | 54.8 (11.4)a |
Data points represent mean peak subjective ratings (±1 SEM).
Represents significant main effects of dose for oral amphetamine and smoked cocaine.
Represents significant differences between the 0 and 50 mg doses of naltrexone in combination with the active doses of oral amphetamine or smoked cocaine.
As shown in Figure 2 and Table 1, NTX did not alter COC-induced increases in ratings of ‘liking,' ‘high,' and ‘would pay,' although compared with 0 mg NTX, both 12.5 and 50 mg NTX did reduce COC-induced ratings of ‘anxious' (F(1, 110)=27.6; P<0.0001 and F(1, 110)=22.0; P<0.0001, respectively). NTX did not alter most of the subjective effects of AMPH. However, post hoc tests revealed that 50 mg NTX did produce statistically significant decreases in ratings of ‘would pay' (F(1, 88)=10.5; P<0.01) and drug ‘quality' (F(1, 88)=8.0; P<0.01), and decreases in ‘liking' that approached statistical significance (F(1, 88)=5.7; P=0.019). The 50 mg dose of NTX also significantly increased AMPH-induced ratings of ‘anxious' (F(1, 88)=8.6; P<0.01).
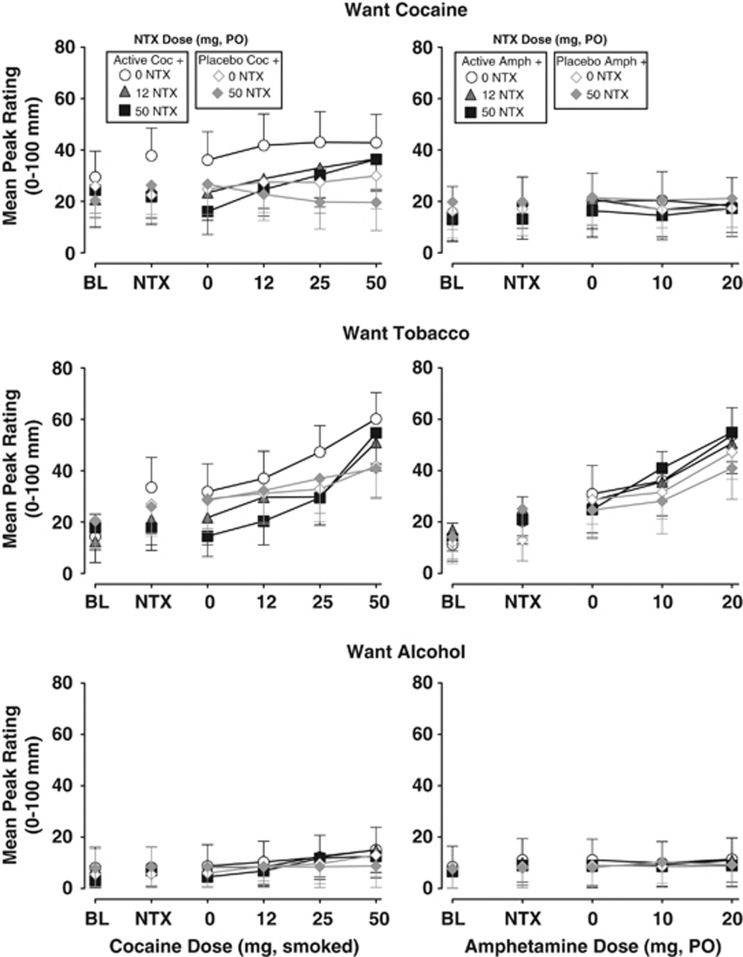
Figure 3 and Table 1 illustrate the effects of smoked COC (left panels) and oral AMPH (right panels) in combination with NTX on ratings of craving for COC, tobacco, and alcohol. During COC sessions, ratings of ‘I want cocaine' (F(5, 55)=3.9; P<0.01) and ‘I want tobacco' (F(5, 55)=15.8; P<0.0001) significantly increased across the session, but ratings of ‘I want alcohol' (F(5, 55)=2.4; P=0.04) did not. There was a modest change in craving scores in the COC placebo/NTX placebo condition (open diamonds: BL=26.0, Coc1stPbo=24.8, Coc2ndPbo=27.6, Coc3rdPbo=27.5, Coc4thPbo=30.0), but the increases were small and not statistically significant. In contrast, increases in craving scores in the COC active/NTX placebo condition (open circles: BL=29.5, CocPbo=36.2, Coc12=41.8, Coc25=43.1, Coc50=42.9) were larger and statistically significant (F(1, 110)=15.6, P<0.0001). During AMPH sessions, only ratings of ‘I want tobacco' (F(4, 44)=9.5; P<0.0001) increased across the session. Both 12.5 and 50 mg NTX significantly decreased ratings of ‘I want cocaine' (F(1, 110)=32.6; P<0.0001 and F(1, 110)=58.7; P<0.0001, respectively) and ‘I want tobacco' (F(1, 110)=21.1; P<0.0001 and F(1, 110)=35.8; P<0.0001, respectively) during active COC sessions. NTX (50 mg) also significantly decreased ratings of ‘I want cocaine' during placebo COC sessions (F(1, 55)=7.2; P<0.01). However, NTX did not alter ratings of ‘I want tobacco' during placebo COC sessions. Although 50 mg NTX produced statistically significant reductions in ratings of ‘I want cocaine' during AMPH sessions (F(1, 88)=8.2; P<0.01), the changes were small in magnitude. NTX did not significantly alter ratings of drug craving during placebo AMPH sessions.
Figure 3.
Selected ratings of drug craving. The upper panels display the mean±1 SEM peak rating of ‘I want cocaine' (mm) before (BL) and after each dose administration. Data points above NTX represent effects after administration of NTX and before administration of the placebo stimulant dose. The middle panels display the mean±1 SEM peak rating of ‘I want tobacco' (mm), and the lower panels display the mean±1 SEM peak rating of ‘I want alcohol' (mm) over dose and time. Data points connected by black lines represent sessions involving active COC or AMPH doses in combination with NTX (0, 12.5, and 50 mg) and data points connected by grey lines represent sessions involving placebo COC or AMPH doses in combination with NTX (0 and 50 mg).
Adverse Events
NTX, AMPH, and COC were generally well tolerated by the participants, although all of the eight participants from whom SAFTEE data were collected reported at least one AE. The most common treatment-related AEs were ‘general disorders' (eg, insomnia, headache, nausea, loss of appetite, and fatigue), with most having occurred in two or fewer participants. All reported AEs were generally resolved within 24–48 h, with some persisting for 5 days at most. A significant number of participants reported insomnia after active AMPH administration compared with placebo AMPH (P<0.01), although the insomnia produced by active AMPH did not significantly differ from active COC (P<0.05). Specifically, 4 out of 8 participants reported insomnia after active AMPH administration in combination with placebo NTX, whereas 1 out of 8 and 2 out of 8 participants reported insomnia after active AMPH in combination with 12.5 and 50 mg NTX, respectively. After active COC, placebo COC, or placebo AMPH sessions, either 0 or 1 participant out of 8 reported insomnia.
DISCUSSION
This study demonstrated that (1) AMPH and COC produced similar maximal cardiovascular changes, and NTX did not alter these effects, (2) COC but not AMPH significantly increased ratings of positive subjective effects, (3) NTX did not significantly alter COC-induced positive subjective ratings, (4) craving for tobacco increased during both COC and AMPH sessions, but craving for COC only increased during COC sessions, and (5) NTX decreased craving for COC and tobacco during active COC sessions, but it did not decrease craving for tobacco during AMPH sessions. Taken together, these results show that NTX was only effective in altering COC- and COC cue-induced increases in craving for COC and tobacco (ie, during active COC and placebo COC sessions). Interestingly, the doses of oral AMPH tested in this study did not elicit robust changes in positive subjective effects among COC users, which is in contrast to its demonstrated abuse liability in other populations, such as light and moderate recreational drug users (eg, Comer et al, 1996; Stoops et al, 2007; Acheson and de Wit, 2008; Sevak et al, 2010). As AMPH did not produce robust changes in positive subjective responses, it was not possible to replicate earlier findings demonstrating that NTX reduces the abuse liability of AMPH (Jayaram-Lindstrom et al, 2004, 2008b).
The finding that NTX did not alter the positive subjective effects of COC in this study is generally consistent with previous studies examining the ability of NTX to alter COC-induced responses (Kosten et al, 1992; Walsh et al, 1996; Sofuoglu et al, 2003). However, the fact that it did significantly reduce craving for COC and tobacco during COC sessions is intriguing and warrants further investigation. The present results are consistent with results from preclinical studies investigating the effects of NTX in behavioral paradigms that model craving for COC by humans. For example, both NTX and a novel mu opioid receptor selective antagonist, GSK 1 521 498, produced dose-dependent decreases in responding by rats on a COC-associated lever before COC availability (Giuliano et al, 2013). Similarly, in a COC cue-induced reinstatement paradigm in rats, NTX produced dose-related reductions in responding on a lever that had been associated previously with COC infusions (Burattini et al, 2008). When COC itself was used to reinstate responding on a lever previously reinforced by COC, NTX did not reduce responding after one NTX dose administration (Stewart, 1984; Comer et al, 1993), but it did reduce responding after multiple doses of NTX were administered (Gerrits et al, 2005). Thus, both cue- and COC priming-induced reinstatement of responding were reduced by NTX. In this study, NTX reduced craving for COC in both cue only (placebo COC) and COC plus cue (active COC) sessions. NTX was not administered chronically, however, which is a limitation of this study. Another limitation is that NTX may have differentially affected COC- and AMPH-induced responses because the time courses of smoked COC and oral AMPH are quite different. Partly mitigating this issue is the fact that 50 mg oral NTX reaches its peak plasma concentration within an hour and is sustained at relatively constant levels for several hours after ingestion (Wall et al, 1981; Meyer et al, 1984). Another limitation of the study, however, is that the half-lives of NTX and 6-beta-naltrexol have been estimated to be 4 and 12 h, respectively, after oral administration (Meyer et al, 1984). Thus, the five half-life ‘rule of thumb' used by many to determine appropriate intersession intervals is met in this study for NTX, but not its active metabolite.
The effects of NTX on craving for tobacco were less consistent, with reductions found in tobacco craving during active COC, but not placebo COC sessions. Although several preclinical studies have demonstrated an effect of NTX on responding after exposure to COC cues and priming doses, the effects of NTX on COC-induced conditioned place preference and COC self-administration in laboratory animals are more variable, with some studies showing reductions (Corrigall and Coen, 1991; Ramsey and van Ree, 1991) and others showing no effect (Ettenberg et al, 1982; Carroll et al, 1986; Winger et al, 1992; Mello et al, 1995; Giuliano et al, 2013).
Similarly, whether NTX is able to reduce COC use in clinical settings is somewhat unclear. Some studies suggest that NTX may be useful for treating COC abuse (Kosten et al, 1989; McCaul and Svikis, 1996; Oslin et al, 1999; Schmitz et al, 2001; Comer et al, 2006) and others do not (Hersh et al, 1998; Schmitz et al, 2009; DeFulio et al, 2012; Dunn et al, 2012). In humans seeking treatment for COC dependence, NTX significantly increased the percentage of COC-negative urines among patients receiving 50 mg NTX plus relapse prevention therapy relative to those receiving 0 mg NTX plus relapse prevention, and those receiving 0 mg or 50 mg NTX plus drug counseling (Schmitz et al, 2001). Although craving for COC did not significantly differ as a function of medication or therapy condition (Schmitz et al, 2001), the craving questionnaire was administered in the clinic and participants were asked to rate how much they had craved COC during the previous week. In this study, participants were asked to rate their craving for COC within close temporal proximity to the presentation of cues associated with COC smoking, as well as actual COC smoking, which may account for the different results. The positive findings reported by Schmitz et al (2001) support the use of NTX for treating COC dependence, but other studies have failed to demonstrate an effect of NTX on COC use. For example, in a study of patients with comorbid alcohol and COC dependence, rates of COC use did not differ between patients who received 100 mg/day NTX and patients who received placebo medication (Schmitz et al, 2009). Thus, the utility of NTX as a stand-alone medication for treating COC dependence is somewhat unclear.
The fact that oral D-AMPH did not produce robust positive subjective effects in COC abusers was surprising, but consistent with previous studies (Fillmore et al, 2003, 2005; Reed and Evans, 2010). Fillmore et al (2003, 2005), for example, showed that oral D-AMPH (0, 10, 20, and/or 30 mg) produced statistically significant increases in ratings of good effects and desire to take the drug again, but the effects were small in magnitude (ratings were ‘a little bit' on a scale ranging between ‘not at all' to ‘very much'). Slightly greater ratings were reported by Stoops et al (2004) after administration of 16 and 24 mg oral D-AMPH: ratings of high, drug liking, willingness to take the drug again, and willingness to pay for it that ranged between ‘a little bit' and ‘moderately'. In contrast to those who participated in the studies by Fillmore et al (2003, 2005) and Stoops et al (2004), participants in this study were older, had been using COC for a longer period of time, and spent more money each week on COC. It is thus possible that the severity of COC use contributed to the minimal subjective effects produced by AMPH in this study. In fact, Martinez et al (2007) reported that among COC abusers who had a use pattern and history of use similar to the current population, an intravenous dose of AMPH (0.3 mg/kg) produced significantly less dopamine release in the ventral striatum and putamen and less self-reported euphoria compared with a control group of individuals who did not abuse drugs. A ‘contrast effect', which is a limitation of this study, could also explain the differences in results between this study and Stoops et al (2004). That is, the effects produced by oral AMPH may have felt particularly weak when it was administered in close temporal proximity to smoked COC.
The finding that oral AMPH has low abuse liability in heavy COC users has interesting implications for agonist maintenance therapy for COC dependence. Similar to the agonist maintenance-based strategies for treating opioid and nicotine dependence, investigators have examined the possibility of using stimulant medications to treat stimulant dependence (for reviews, see Grabowski et al, 2004; Mariani and Levin, 2012). Both preclinical and clinical studies suggest that maintenance on AMPH reduces COC use (Grabowski et al, 2004). Like this study, previous research indicates that AMPH is generally safe and well tolerated by COC abusers. The finding in this study that immediate-release oral D-AMPH was not liked, did not produce a feeling of being high, and would not be purchased by heavy COC users is reassuring from a safety perspective. This is particularly true because immediate-release AMPH would be expected to have higher abuse liability than the sustained-release AMPH formulations that have most often been tested in clinical trials.
In sum, the present results could not be used to determine whether NTX is useful in reducing the abuse liability of AMPH, which was a primary aim of our study. However, it did reveal that COC- and COC cue-induced increases in craving for COC and tobacco were significantly reduced by NTX. The relationship between craving and drug use is complicated and somewhat controversial, although emerging prospective studies are beginning to demonstrate that craving does predict relapse to drug use (Paliwal et al, 2008). New technologies have also demonstrated a close relationship between craving and drug use. For example, Epstein et al (2010) asked methadone-maintained patients who smoked cigarettes and used heroin and COC to record their craving for tobacco, heroin, and COC on a hand-held device in an outpatient treatment setting. Although causality could not be determined, they found that smoking and tobacco craving were strongly associated with use of and craving for COC and heroin. Previous studies have shown that NTX reduces both non-opioid drug use and craving (eg, cigarette smoking and alcohol use), but it is not clear whether the reductions in smoking/alcohol use were caused by reductions in craving (eg, King and Meyer, 2000; Volpicelli et al, 1992). Nevertheless, these findings suggest a potential role for NTX, perhaps as an adjunct medication, in facilitating reductions in craving for both tobacco and COC, which may ultimately improve treatment outcome. The minimal abuse liability of immediate-release oral D-AMPH among COC abusers revealed by this study also has implications for its potential utility in treating COC dependence.
FUNDING AND DISCLOSURE
In the past 3 years, SDC served as a consultant to Analgesic Solutions LLC, BioDelivery Sciences International, Cephalon, Cytogel Pharma LLC, Grunenthal GmbH, Innovative Science Solutions LLC, King Pharmaceuticals, Neuromed Pharmaceuticals, Ortho-McNeil Janssen Scientific Affairs, Pfizer, Salix Pharmaceuticals, Sepracor, and Shire Pharmaceuticals. In addition, in the past 3 years, SDC has received research funding from Endo, Johnson & Johnson, Medicinova, and Reckitt-Benckiser. SKV received partial salary support from and served as a consultant to Grunenthal USA and Analgesic Solutions. In the past 3 years, JDJ has received compensation (partial salary support) from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals and Schering-Plough Corporation. The remaining authors declare no conflict of interest.
Acknowledgments
We thank Drs Elias Dakwar, Abigail Herron, Nasir Naqvi, Rajkumar Kalapatapu, Taoi Huynh, and Alicia Murray for providing medical coverage during laboratory sessions and participant screening. In addition, we thank Janet Murray, RN and Claudia Tindall, RN, NP for providing nursing support during the study. The authors gratefully acknowledge NIDA for providing funding for this study (DA022222). NIDA had no role in study design or in the decision to submit the paper for publication. NIDA also had no role in the collection, analysis and interpretation of data in the writing of this report.
AUTHOR CONTRIBUTIONS
SD Comer, RW Foltin, and M Haney designed the study. SD Comer, S Mogali, A Pines, and EL Berkower wrote sections of the paper. PA Saccone and P Askalsky collected data and provided preliminary statistical analyses of the results. S Mogali, D Martinez, E Rubin, MA Sullivan, and JM Manubay provided medical coverage and/or medical advice during the study. SD Comer, ZD Cooper, JD Jones, P Roux, EA Walker, and SK Vosburg interviewed potential participants and supervised data collection. All of the co-authors read a draft of the paper.
Footnotes
Portions of these data were presented at the 2011 and 2012 meetings of the College on Problems of Drug Dependence, as well as the 2011 meeting of the Colloque Europeen et International Toxicomanies Hepatites SIDA
References
- Acheson A, de Wit H. Buproprion improves attention but does not affect impulsive behavior in healthy young adults. Exp Clin Psychopharmacol. 2008;16:113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, Ambre J, de Wit H. Acute tolerance to subjective but not cardiovascular effects of d-amphetamine in normal, healthy men. J Clin Psychopharmacol. 1996;16:72–76. doi: 10.1097/00004714-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Burattini C, Burbassi S, Aicardi G, Cervo L. Effects of naltrexone on cocaine- and sucrose-seeking behaviour in response to associated stimuli in rats. Int J Neurospychopharmacol. 2008;11:103–109. doi: 10.1017/S1461145707007705. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Walker MJ, Kragh R, Newman T. Effects of naltrexone on intravenous cocaine self-administration in rats during food satiation and deprivation. J Pharmacol Exp Ther. 1986;238:1–7. [PubMed] [Google Scholar]
- Comer SD, Haney M, Foltin RW, Fischman MW. Amphetamine self-administration by humans: modulation by contingencies associated with task performance. Psychopharmacology. 1996;127:39–46. doi: 10.1007/BF02805973. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Curtis LK, Carroll ME. Effects of buprenorphine and naltrexone on reinstatement of cocaine-reinforced responding in rats. J Pharmacol Exp Ther. 1993;267:1470–1477. [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology. 1991;104:167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Everly JJ, Leoutsakos JMS, Umbricht A, Fingerhood M, Bigelow GE, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48–54. doi: 10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Defulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, et al. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Exp Clin Psychopharmacol. 2012;21:74–83. doi: 10.1037/a0030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addictive Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Pharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Abroms BD. D-amphetamine-induced enhancement of inhibitory mechanisms involved in visual search. Exp Clin Psychopharmacol. 2005;13:200–208. doi: 10.1037/1064-1297.13.3.200. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Ward AS, Haney M, Hart CL, Collins ED. The effects of escalating doses of smoked cocaine in humans. Drug Alcohol Depend. 2003;70:149–157. doi: 10.1016/s0376-8716(02)00343-5. [DOI] [PubMed] [Google Scholar]
- Gerrits MAFM, Kuzmin AV, van Ree JM. Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. Eur Neuropsychopharmacol. 2005;15:297–303. doi: 10.1016/j.euroneuro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Robbins TW, Wille DR, Bullmore ET, Everritt BJ. Attenuation of cocaine and heroin seeking by μ-opioid receptor antagonism. Psychopharmacology. 2013;227:137–147. doi: 10.1007/s00213-012-2949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Guy W, Wilson WH, Brooking B, Manov G, Fjetland O. Reliability and validity of SAFTEE: preliminary analyses. Psychopharmacol Bull. 1986;22:397–401. [PubMed] [Google Scholar]
- Haggkvist J, Lindholm S, Frank J. The effect of naltrexone on amphetamine-induced conditioned place preference and locomotor behaviour in the rat. Addict Biol. 2009;14:260–269. doi: 10.1111/j.1369-1600.2009.00150.x. [DOI] [PubMed] [Google Scholar]
- Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology. 1998;139:44–52. doi: 10.1007/s002130050688. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008a;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008b;33:1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to ampthetamine in healthy volunteers. J Clin Psychopharmacol. 2004;24:665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Winger G, Dean RL, Deaver DR, Woods JH. Naltrexone decreases d-amphetamine and ethanol self-administration in rhesus monkeys. Behav Pharmacol. 2011;22:87–90. doi: 10.1097/FBP.0b013e3283423d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav. 2000;66:563–572. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD, Morgan C. Role of opioid antagonists in treating intravenous cocaine abuse. Life Sci. 1989;44:887–892. doi: 10.1016/0024-3205(89)90589-4. [DOI] [PubMed] [Google Scholar]
- Kosten T, Silverman DG, Fleming J, Kosten TA, Gawin FH, Compton M, et al. Intravenous cocaine challenges during naltrexone maintenance: a preliminary study. Biol Psychiatry. 1992;32:543–548. doi: 10.1016/0006-3223(92)90223-m. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. 2012;35:425–439. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Svikis DS. Measures of service utilization. NIDA Res Monogr. 1996;166:225–241. [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze J. A primate model of polydrug abuse: cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Meyer MC, Straughn AB, Man-Wai L, Schary WL, Whitney CC. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–19. [PubMed] [Google Scholar]
- Oslin DW, Pettinati HM, Volpicello JR, Wolf AL, Kampman KM, O'Brien CP. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16:163–167. doi: 10.1016/s0740-5472(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: Further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG, Markowitz JS. Side effect assessment with SAFTEE: pilot study of the instrument. Psychopharmacol Bull. 1986;22:389–396. [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM. Intracerebroventricular naltrexone treatment attenuates acquisition of intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1991;40:807–810. doi: 10.1016/0091-3057(91)90090-o. [DOI] [PubMed] [Google Scholar]
- Reed SC, Evans SM.2010The effects of oral d-amphetamine on impulsivity, mood and performance in smoked cocaine users [abstract]In College on Problems of Drug Dependence. 2010 June 12–17. Scottsdale, Arizona. CPDD; 2010. p 136. Abstract nr 542.
- Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, Moeller FG. High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addiction. 2009;18:356–362. doi: 10.3109/10550490903077929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Glaser PEA, Hays LR, Rush CR. Reinforcing effects of d-amphetamine: influence of novel ratios on a progressive-ratio schedule. Behav Pharmacol. 2010;21:745–753. doi: 10.1097/FBP.0b013e32833fa7b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Singha A, Kosten TR, McCance-Katz FE, Petrakis I, Oliveto A. Effects of naltrexone and isradipine, alone or in combination, on cocaine responses in humans. Pharmacol Biochem Behav. 2003;75:801–808. doi: 10.1016/s0091-3057(03)00157-6. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984;20:917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PEA, Fillmore MT, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Vansickel AR, Lile JA, Rush CR. Acute d-amphetamine pretreatment does not alter stimulant self-administration in humans. Pharmacol Biochem Behav. 2007;87:20–29. doi: 10.1016/j.pbb.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality . The DAWN Report: Highlights of the 2010 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Rockville, MD, USA; 2012. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration 2012Results from the 2011 National Survey on Drug Use and Health: Summary of National FindingsNSDUH Series H-44, HHS Publication No. (SMA) 12-4713Substance Abuse and Mental Health Services Administration, 2012: Rockville, MD, USA [Google Scholar]
- Todtenkopf MS, O'Neill KS, Kriksciukaite K, Turncliff RZ, Dean RL, Ostrovsky-Day I, et al. Route of administration affects the ability of naltrexone to reduce amphetamine-potentiated brain stimulation reward in rats. Addict Biol. 2009;14:408–418. doi: 10.1111/j.1369-1600.2009.00161.x. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, World Drug Report 2011. United Nations Publication, Sales No. E. 10. XI. 13.
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wall ME, Brine DR, Perez-Reyes M. Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metab Disposition. 1981;9:369–375. [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Winger G, Skjoldager P, Woods JH. Effects of buprenorphine and other opioid agonists and antagonists on alfentanil- and cocaine-reinforced responding in rhesus monkeys. J Pharmacol Exp Ther. 1992;261:311–317. [PubMed] [Google Scholar]