Abstract
High rates of early relapse following electroconvulsive therapy (ECT) are typically reported in the literature. Current treatment guidelines offer little information to clinicians on the optimal nature of maintenance therapy following ECT. The aim of this study was to provide a systematic overview of the existing evidence regarding post-ECT relapse. A keyword search of electronic databases was performed for studies appearing in the peer-reviewed literature before January 2013 reporting on relapse rates in responders to an acute course of ECT administered for a major depressive episode. Meta-analyses were performed where appropriate. Thirty-two studies with up to 2 years' duration of follow-up were included. In modern era studies of continuation pharmacotherapy, 51.1% (95% CI=44.7–57.4%) of patients relapsed by 12 months following successful initial treatment with ECT, with the majority (37.7%, 95% CI=30.7–45.2%) relapsing within the first 6 months. The 6-month relapse rate was similar in patients treated with continuation ECT (37.2%, 95% CI=23.4–53.5%). In randomized controlled trials, antidepressant medication halved the risk of relapse compared with placebo in the first 6 months (risk ratio=0.49, 95% CI=0.39–0.62, p<0.0001, number needed to treat=3.3). Despite continuation therapy, the risk of relapse within the first year following ECT is substantial, with the period of greatest risk being the first 6 months. The largest evidence base for efficacy in post-ECT relapse prevention exists for tricyclic antidepressants. Published evidence is limited or non-existent for commonly used newer antidepressants or popular augmentation strategies. Maintenance of well-being following successful ECT needs to be improved.
Keywords: depression, relapse, electroconvulsive therapy, meta-analysis
INTRODUCTION
Electroconvulsive therapy (ECT) is a highly effective acute treatment for major depression (Eranti et al, 2007; Kellner et al, 2010; The UK ECT Review Group, 2003). Although remission rates exceed those seen with other somatic treatments, high rates of relapse, especially early relapse, are observed and acknowledged as a major clinical problem (Kellner et al, 2006; Sackeim et al, 2001). Consolidating and prolonging remission is a key clinical challenge surrounding ECT use (Kellner 2013).
Following introduction of the first effective antidepressants, continuation antidepressant monotherapy following ECT appeared to minimize the likelihood of relapse. Early research from the United Kingdom demonstrated the efficacy of antidepressants over placebo with 6-month relapse rates in tricyclic antidepressant (TCA)- or monoamine oxidase inhibitor (MAOI)-treated patients of about 20% compared with 40–70% in untreated or benzodiazepine-only-treated patients (Imlah et al, 1965; Kay et al, 1970; Seager and Bird, 1962). However, more recent studies are less favorable, with relapse rates typically about 40–50% at 6 months despite vigorous continuation therapy, such as antidepressant–lithium combination or continuation ECT (C-ECT; Kellner et al, 2006; Prudic et al, 2013; Sackeim et al, 2001). Of note, in a more recent trial where patients were randomized to TCA monotherapy, TCA–lithium combination, or placebo, TCA monotherapy was not significantly more effective than placebo in preventing relapse (Sackeim et al, 2001).
Higher rates of relapse in recent decades may be due to historical changes in ECT patient populations (Sackeim, 1994). ECT is a unique treatment in psychiatry that predates modern psychopharmacology. Once used as first-line treatment for severe depression in often medication-naive patients, its use nowadays is reserved for a minority of patients with severe, chronic, difficult-to-treat depression where several treatment steps have usually been unsuccessful. Such treatment-resistant patients are generally less likely to achieve full remission and, when they do, are prone to relapse (Fekadu et al, 2009).
The negative impact of medication resistance on ECT outcomes had been noted decades ago (Bruce et al, 1960; Hamilton, 1974) and was subsequently demonstrated by studies showing that patients with established medication resistance have worse acute (Prudic et al, 1996; Prudic et al, 1990) and longer-term (Sackeim et al, 1990) outcomes. A recent meta-analysis confirmed that acute remission rates with ECT are lower in treatment-resistant patients (48%) compared with those in whom medication resistance had not been established (65%) (Heijnen et al, 2010).
Currently, there is no agreement on what constitutes optimal post-ECT relapse prevention treatment. The American Psychiatric Association guidelines on ECT, now over a decade old, recommend continuation therapy with either pharmacotherapy or C-ECT for virtually all patients (American Psychiatric Association, 2001). However, no specific guidelines on choice of agent or duration of treatment exist. Most experimental work over the past 3 decades has focused primarily on optimizing ECT treatment parameters (eg, electrode placement, stimulus dose, and pulse width) to produce the best possible balance between clinical and neuropsychological outcomes. These studies unequivocally show that ECT is a powerful treatment option capable of producing full remission where other treatments have failed (Dunne and McLoughlin, 2012; Eranti et al, 2007; Kellner et al, 2010; Loo et al, 2012; Sackeim et al, 2009). However, given that relapse following ECT is a key clinical problem, we carried out a systematic review of all existing evidence, randomized and observational, to provide an overview of current knowledge on this important question.
MATERIALS AND METHODS
Search Strategy
An electronic literature search of PubMed, Embase, CINAHL, PsycINFO, and Cochrane Library databases was performed up to January 2013 with no time, language or other restrictions. Keywords used were (ECT OR electroconvulsive therapy OR convulsive therapy) AND (depression OR depressive OR mood disorder OR bipolar disorder OR affective disorder OR melancholi*) AND (long term OR follow up OR relapse OR prognosis OR mortality OR maintenance OR continuation). Hand-searches of reference sections of previous reviews and included studies were carried out.
Following exclusion of database duplicates and clearly ineligible reports, judging by title and abstract screening, two reviewers (AJ, EK) independently evaluated for eligibility all studies retained for full-text screening. Where studies met inclusion criteria (described below), the reviewers independently extracted data from reports. Information regarding study design, ECT treatment parameters, sample characteristics, type of continuation therapy, type of outcome measure, definition of relapse, valid sample size at each follow-up, cumulative number of relapses at each time point, and cumulative number of dropouts at each time point was extracted. Discrepancies were resolved by joint re-evaluation of reports.
When extracting relapse proportions from reports, preference was given to information in the body of texts and tables. Where the study explicitly reported relapse rates only for the study endpoint but where patients were assessed at multiple intermediate time points, survival curves were examined; where it was deemed that the number of relapses could be extracted from graphs, this was done jointly by the reviewers. Where studies met inclusion criteria but data were reported in a non-extractable format, we contacted the authors. Given the literature age span, this was not always possible as authors were sometimes untraceable or deceased.
Study Eligibility Criteria
The following inclusion criteria were applied:
prospective study reported in a peer-reviewed publication;
participant age ⩾18 years;
an acute course of ECT was administered for treating a major depressive episode (unipolar or bipolar) diagnosed by clinical judgement or formal diagnostic criteria (eg, DSM-IV);
those deemed to be ECT responders or remitters were prospectively followed-up and monitored for relapse;
relapse was operationally defined by the original investigators and reported in a categorical fashion (ie, as the percentage of the initial responder or remitter sample who relapsed);
relapse was ascertained on the basis of clinical judgement or by using formal diagnostic criteria and/or pre-specified cutoff scores on clinician-rated depression severity rating scales (eg, Hamilton Depression Rating Scale); and
clinical outcome assessment was carried out ⩾3 months following the last ECT session.
Exclusion criteria:
case studies or series with N<10;
retrospective studies;
prospective studies where relapse was not established directly via patient interview but instead on the basis of proxy measures (eg, rehospitalization rates), mailed self-report questionnaires, or information obtained from third-parties (eg, patients' relatives or treating physicians);
presence of non-affective psychosis, dementia, neurological disease, or unstable medical conditions in the sample; and
unmodified ECT.
Outcomes
Relapse rate was defined as the proportion of the original ECT responder or remitter sample that subsequently experienced a return of depressive symptoms deemed to be significant enough to merit the designation of relapse by the original investigators. Specific criteria for relapse varied between the studies; original investigators' definitions were retained. Studies using inadequate measures of relapse likely to underestimate its true prevalence (eg, rehospitalization rates only) were excluded.
The primary outcome was cumulative relapse proportion at the 6-month follow-up after last ECT for which we expected most data would be available. In all primary analyses, only samples treated with antidepressant pharmacotherapy were included, because virtually all ECT patients today receive long-term prophylactic therapy most commonly administered in the form of medication. We also carried out secondary analyses of relapse rates on C-ECT, which is used less frequently than medication. C-ECT is a form of relapse prevention where the patient continues to receive ECT after the acute course at a reduced schedule. It is indicated in patients with a past history of good ECT response where antidepressant continuation therapy was either ineffective or could not be tolerated at therapeutic doses (American Psychiatric Association, 2001). Other secondary analyses investigated relapse rates on placebo or no maintenance treatment.
Additional secondary outcomes were relapse rates at 3, 12, and 24 months after last ECT, again in patients receiving antidepressant medication. Finally, to investigate the relative efficacy of different relapse prevention strategies, we aimed to calculate relative risks (RRs) of relapse in randomized controlled trials (RCTs) of different continuation therapies at 3, 6, and 12 months where at least two studies comparing the same strategy were available.
Statistical Analyses
All analyses were based on study completers. Attrition rates for each study were recorded. Mean relapse proportions with 95% confidence intervals (CIs) were calculated by pooling samples using a random-effects model (DerSimonian and Laird, 1986), as we expected substantial differences in study designs and patient populations. Heterogeneity was assessed using the I2 statistic (Higgins et al, 2003). Where substantial heterogeneity was observed and where sufficient data were available, random-effects meta-regression analyses with unrestricted maximum likelihood estimation were carried out to explore possible sources of heterogeneity. Pre-specified covariates investigated were mean age, proportion of psychotic patients, and proportion of medication-resistant patients. Planned subgroup analyses compared study designs (trial vs observational), relapse criteria (standardized symptom rating scale vs clinical judgement), and whether concomitant pharmacotherapy was allowed during the index ECT course. To investigate the possibility of changes in relapse rates over time, a cumulative meta-analysis was carried out for the primary endpoint (6 months).
For head-to-head comparisons of different continuation therapies, RRs with 95% CIs and numbers needed to treat (NNT) were calculated.
Publication bias was assessed by visual inspection of funnel plots where >10 studies were available. All statistical analyses were carried out using Comprehensive Meta Analysis Version 2.2 software (Borenstein et al, 2011).
Results
Search Results
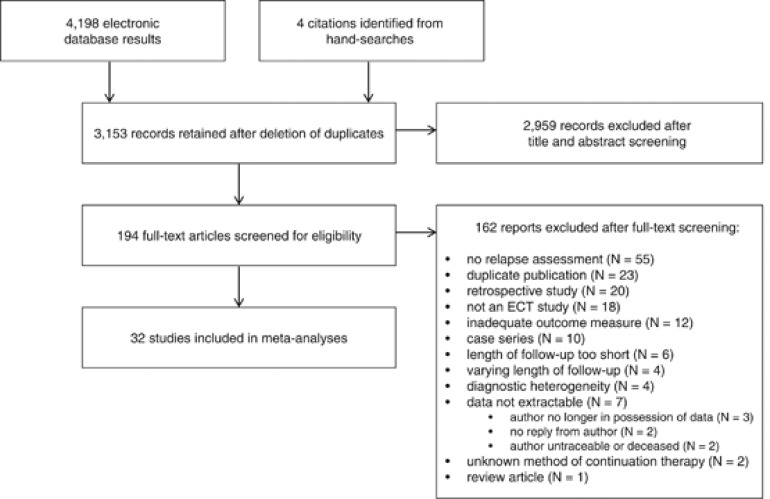
The computerized search retrieved 4198 results (Figure 1). Hand-searches identified four additional eligible studies. Following exclusion of database duplicates and initial exclusion of ineligible studies, 194 titles were retained for full-text screening. Of these, 32 studies met inclusion criteria and provided extractable data either from published reports or contact with original authors (Supplementary Table 1).
Figure 1.
Study flow diagram.
Relapse Rate at 6 Months
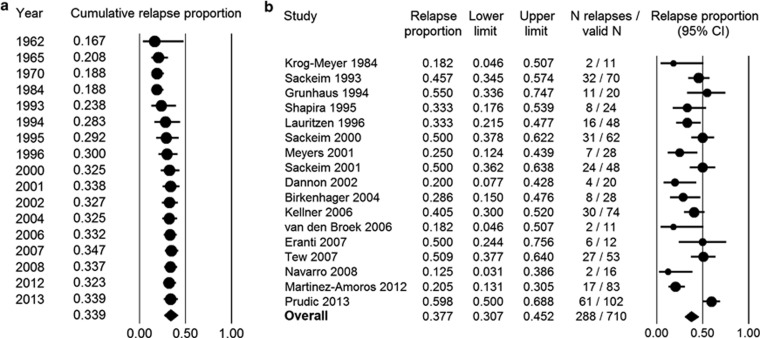
By 6 months following ECT, 34.0% (95% CI=27.2–41.5%, I2=76%) of patients (N=844) treated with continuation pharmacotherapy had relapsed. Because long-term outcomes are believed to have worsened over the many decades of ECT use, we performed a cumulative meta-analysis with each study added to the previous ones in chronological order (Figure 2a). Beginning with the first controlled studies of continuation pharmacotherapy in the 1960s, relapse rates held at around 20%. As modern studies of more treatment-resistant patients and clearer reporting of methodology began to be conducted, relapse rates rose towards present-day levels. It should be noted that following the publication of three important early trials (Imlah et al, 1965; Kay et al, 1970; Seager and Bird, 1962), with the exception of one small trial in 1984 (Krog-Meyer et al, 1984), no other prospective long-term follow-up studies of continuation pharmacotherapy meeting inclusion criteria were found between 1970 and the early 1990s, perhaps coinciding with diminishing use of ECT. Given this gap in evidence, it is unclear when precisely the shift in relapse rates might have occurred.
Figure 2.
Outcomes at 6 months following ECT. Panel (a) shows a cumulative meta-analysis of relapse rates at 6 months following ECT across all eligible studies from 1962 onwards. Panel (b) shows relapse rate at 6 months following ECT in modern-era studies.
Due to the historical trend observed in the data, we carried out a sensitivity analysis where only modern post-DSM-III studies of pharmacologically treated patients (N=710) were included in the meta-analysis. Relapse rate across these studies was 37.7% (95% CI=30.7–45.2%, I2=70%) (Figure 2b). Visual inspection of the funnel plot showed no evidence of publication bias (data not shown).
Due to remaining high heterogeneity, we performed random-effects meta-regressions to investigate the possible contribution of study characteristics on outcome. As only a small number of studies reported relevant moderators, multivariate analyses could not be conducted; hence, each moderator was modelled separately. In modern studies, there was no effect of baseline medication resistance on likelihood of relapse (p=0.429). However, there was a suggestion of lower relapse rates in samples with a greater percentage of psychotic patients (p=0.004) and a higher mean age (p=0.038).
Methodological factors appeared to influence outcome. In subgroup analyses, studies using clinical judgement to determine relapse reported lower rates (28.3%, 95% CI=17.1–43.1%) than studies using cutoff scores on depression rating scales (41.7%, 95% CI=34.8–48.9%). Studies where concomitant pharmacotherapy was permitted during the ECT course had lower relapse rates (29.2%, 95% CI=18.0–43.6%) than those where maintenance pharmacotherapy was begun after the course (41.6%, 95% CI=35.0–48.6%). Naturalistic studies (39.1%, 95% CI=29.2–50.0%) and controlled trials (36.1%, 95% CI=26.9–46.4%) of continuation pharmacotherapy did not differ in relapse rates.
Relapse Rates at 3, 12 and 24 Months
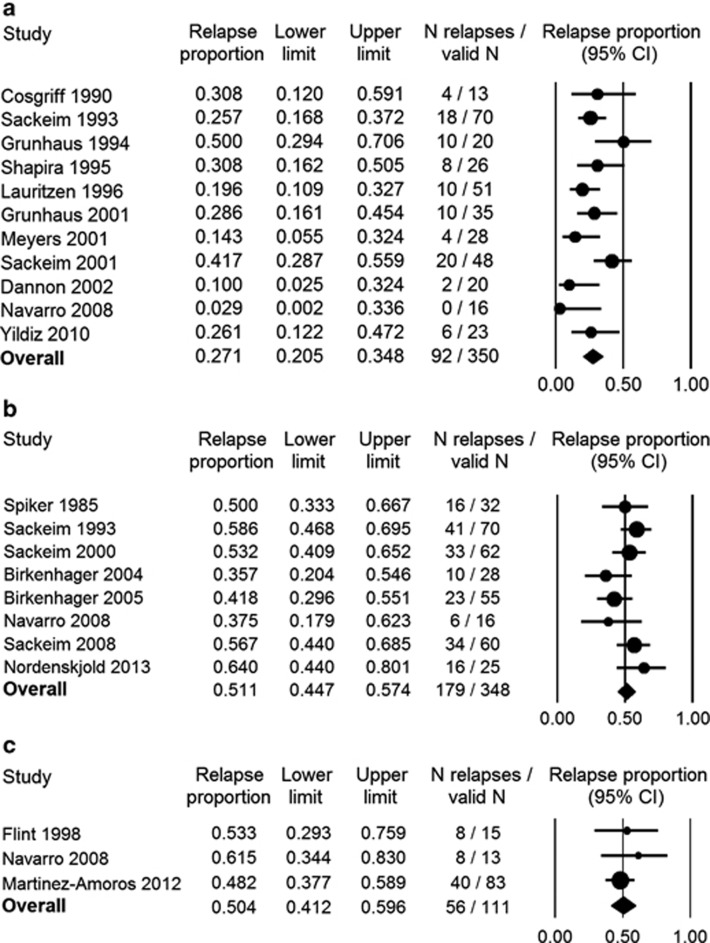
By 3 months following ECT, 27.1% of patients (N=350) on continuation pharmacotherapy had relapsed (95% CI=20.5–34.8%, I2=48%) (Figure 3a), and by 1 year (N=348) 51.1% (95% CI=44.7–57.4%, I2=27%) had relapsed (Figure 3b). Only three prospective studies with a 2-year follow-up were found: two investigating outcomes in psychotic elderly patients (N=28) treated with nortriptyline monotherapy (Flint and Rifat, 1998; Navarro et al, 2008) and one in a general adult sample (N=83) maintained on treatment-as-usual pharmacotherapy (Martinez-Amoros et al, 2012). Relapse rate at 2 years was 50.4% (95% CI=41.2–59.6%, I2=0) (Figure 3c).
Figure 3.
Outcomes at 3, 12, and 24 months following ECT. Panels a, b, and c show relapse rates at 3, 12, and 24 months following ECT, respectively.
Relapse Rates With C-ECT
At 6-month follow-up, relapse rate across the four eligible C-ECT samples (N=146) was 37.2% (95% CI=23.4–53.5%, I2=57%), a virtually identical relapse rate to the figure for modern-era pharmacologically treated patients presented above (37.7%). Given the similarity in 6-month relapse rates in medication and C-ECT samples, we also carried out a meta-analysis of all eligible modern-era studies where patients were treated with any form of recognized continuation therapy, pharmacological or C-ECT. Across 19 eligible studies (N=1001), 39.5% of patients had relapsed (95% CI=31.9–47.7%, I2=81%).
When the two studies (Kellner et al, 2006; Wijkstra et al, 2000) where patients (N=86) were treated with C-ECT only and where no concomitant medication was permitted were analyzed separately, relapse rate at 6 months rose to 45.4% and heterogeneity was eliminated (95% CI=35.2–55.9%, I2=0). For 1 and 2-year follow-ups, only two studies at each time point met inclusion criteria. Patients in these studies were treated with C-ECT and pharmacotherapy combination therapy. Relapse rate at 12 months (N=33) was 20.5% (95% CI=3.0–68.1%, I2=73%), and at 24 months (N=56) it was 30.3% (95% CI=2.9–86.4%, I2=85%). High levels of heterogeneity were present in the analyses.
Relapse Rates in Untreated Samples
To examine the long-term efficacy of a course of ECT in the absence of continuation treatment, studies reporting outcomes in unmedicated patients were meta-analyzed. Two studies published in 1973, both with a 3-month follow-up, reported relapse in ECT responders not permitted to take antidepressant medication during follow-up (Arfwidsson et al, 1973; Barton et al, 1973). By 3 months after ECT, 47.9% had relapsed (95% CI=38.1–57.9%, I2=0). No modern studies featuring entirely untreated (including no placebo) samples were found.
Next we analyzed relapse rates in placebo-treated samples where some non-specific benefit can be expected. Three RCTs (Lauritzen et al, 1996; Sackeim et al, 2001; Yildiz et al, 2010) provided extractable data at 3 months and seven (Imlah et al, 1965; Kay et al, 1970; Krog-Meyer et al, 1984; Lauritzen et al, 1996; Sackeim et al, 2001; Seager and Bird, 1962; van den Broek et al, 2006) at 6 months. Relapse rates were 62.7% (95% CI=47.6–75.8%, I2=0) at 3 months and 65.5% (95% CI=49.7–78.5%, I2=72%) at 6 months. As with active continuation therapy, relapse rates were substantially lower in earlier placebo samples. When only modern day RCTs (Krog-Meyer et al, 1984; Lauritzen et al, 1996; Sackeim et al, 2001; van den Broek et al, 2006) are considered (N=65), relapse rate on placebo reached 78.0% (95% CI=66.1–86.5%, I2=0) at 6 months.
RR of Relapse on Continuation Antidepressant Pharmacotherapy vs Placebo
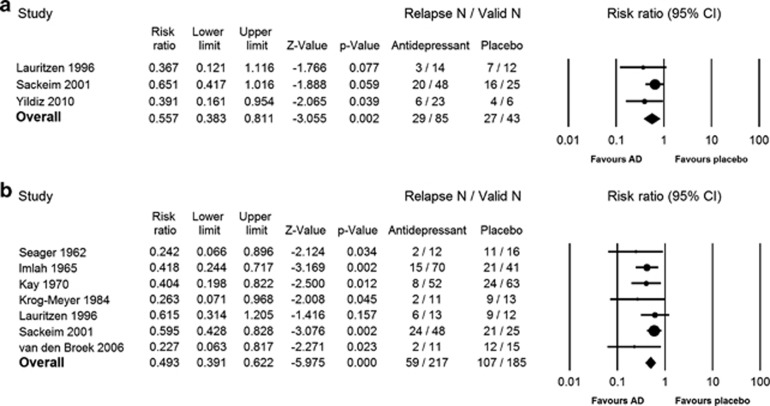
RRs of relapse in RCTs of active relapse prevention strategies vs placebo were investigated at 3 and 6 months after ECT (Figure 4a and b).
Figure 4.
Relative risk (RR) of relapse in patients treated with pharmacotherapy vs placebo at 3 and 6 months following ECT. Panels a and b, respectively, show the RR of relapse in patients maintained on active antidepressant pharmacotherapy vs placebo at 3 and 6 months following ECT.
For the 3-month follow-up, three placebo-controlled RCTs (N=128) provided extractable data: two (Lauritzen et al, 1996; Yildiz et al, 2010) evaluating selective serotonin reuptake inhibitor (SSRI) monotherapy vs placebo and the other (Sackeim et al, 2001) comparing TCA monotherapy and TCA–lithium combination to placebo. The first meta-analysis measured RR of relapse in patients treated with any antidepressant pharmacotherapy vs placebo. RR of relapse on medication was 0.56 (95% CI=0.38–0.81, p=0.002, NNT=3.5, I2=0). Next, the two studies (N=55) comparing SSRI monotherapy vs placebo were separately analyzed. Pooled analysis showed SSRI monotherapy to be significantly more effective than placebo in preventing relapse at 3 months (RR=0.38, 95% CI=0.19–0.77, p=0.007, NNT=2.7, I2=0).
At 6 months, two meta-analyses could be carried out: one featuring any antidepressant pharmacotherapy vs placebo; another featuring TCA monotherapy vs placebo. No meta-analyses of other medication classes or combination strategies vs placebo could be carried out for the 6-month time point as only one study evaluated efficacy of an MAOI vs placebo (Imlah et al, 1965), one study compared an SSRI with placebo (Lauritzen et al, 1996), while one study featured a TCA–lithium combination treatment group vs placebo (Sackeim et al, 2001). Across the seven included studies (Imlah et al, 1965; Kay et al, 1970; Krog-Meyer et al, 1984; Lauritzen et al, 1996; Sackeim et al, 2001; Seager and Bird, 1962; van den Broek et al, 2006; N=402), continuation pharmacotherapy halved the risk of relapse compared with placebo at 6 months (RR=0.49, 95% CI=0.39–0.62, p<0.0001, NNT=3.3, I2=0). Patients in these studies were predominantly treated with TCAs. When TCA monotherapy samples are considered separately, this strategy was found to reduce the RR of relapse slightly further (RR=0.44, 95% CI=0.29–0.66, p<0.0001, NNT=3.2, I2=36%). In all included studies where TCAs were used, with the exception of one trial that compared nortriptyline with placebo (Sackeim et al, 2001), TCA monotherapy was significantly more effective than placebo. Other included studies used either imipramine (Imlah et al, 1965; Seager and Bird, 1962; van den Broek et al, 2006) or amitriptyline (Kay et al, 1970; Krog-Meyer et al, 1984) monotherapy.
No placebo-controlled RCTs of continuation pharmacotherapy with a 1-year (or longer) follow-up were identified. No meta-analyses of head-to-head comparisons of different active relapse prevention strategies could be carried out as only one study contained the same comparison.
DISCUSSION
Relapse rates following ECT are disappointingly high and appear to have increased over time. In patients treated with continuation pharmacotherapy, the main focus of our investigation, relapse was highest in the first 6 months, plateauing afterwards. In present day clinical practice, nearly 40% of ECT responders can be expected to relapse in the first 6 months and roughly 50% by the end of first year.
A course of ECT, in the absence of active continuation therapy, does not appear to have much lasting effect. In early trials where no continuation therapy was permitted, half of all patients who responded to ECT relapsed within 3 months (Arfwidsson et al, 1973; Barton et al, 1973). This suggests that the natural course of depressive illness severe enough to warrant ECT is a prompt return to depression in the absence of long-term treatment. When modern placebo samples were analyzed, relapse rates were even higher, approaching 80% at 6 months. In the current ECT practice, therefore, we recommend that initial gains are consolidated with vigorous maintenance therapy.
Nonetheless, these findings need to be interpreted in the context of superior acute remission rates with ECT compared with other existing treatments for treatment-resistant depression. A meta-analysis investigating acute outcomes found ECT to be more effective than pharmacotherapy (The UK ECT Review Group, 2003). Although our systematic review did not identify any long-term studies directly comparing outcomes in ECT vs medication-treated patients, when our results are compared with the existing literature on short- and longer-term antidepressant effectiveness in refractory MDD, similar outcomes are observed. In the STAR*D study (Rush et al, 2006), relapse rates were predictably higher in patients entering follow-up after more previous failed treatment steps. During the 1-year follow-up, remitters from the third and fourth successive treatment steps relapsed at rates of 43 and 50%, respectively. These long-term outcomes in medication-treated patients with similar degree of treatment resistance to modern ECT samples are very similar to our findings of a 51% relapse rate 1 year following ECT. Acute remission rates for every treatment step in STAR*D, however, were much lower compared with those typically observed in ECT trials, hence more patients overall can be expected to benefit from ECT.
Our systematic review cannot offer clear guidance on what type of continuation therapy works best and for which patients. Many ECT patients routinely receive continuation therapy with the same medication(s) that failed to elicit a clinical response before ECT, a counterintuitive strategy (Sackeim, 1994). To our knowledge, no evidence is available to suggest this practice might be effective, although no particular evidence to the contrary exists either. Our meta-analysis suggests that continuation pharmacotherapy is significantly more effective than placebo at both 3- and 6-month follow-ups. Most available evidence consists of trials of older antidepressants, such as imipramine and amitriptyline. Our search of the published literature could not identify any placebo-controlled trials of some of the most commonly used newer-generation antidepressants, such as serotonin-norepinephrine reuptake inhibitors, mirtazepine, or popular augmentation strategies with mood stabilizers (other than lithium) or atypical antipsychotics. Even for SSRIs, published evidence is relatively sparse. ECT research has favored the use of TCAs; however, as TCAs produce many undesirable side-effects, carry an overdose risk, and cannot be tolerated at adequate doses by many patients, efficacy of newer antidepressants with more favorable side-effect profiles merits further investigation. Also requiring future study is the optimization of treatment schedules for C-ECT, which has thus far tended to be used with fixed dosing schedules in prospective studies. This may have underestimated its true efficacy when using more flexible, symptom-titrated dosing schedules currently under investigation (Lisanby et al, 2008).
When interpreting results of this meta-analysis, certain limitations should be borne in mind. Much of the available evidence comes from small, underpowered, predominantly observational studies. There was substantial variability between the included studies in design, quality, and patient selection criteria that appeared to influence outcomes. Very few RCTs of continuation therapies with long-term follow-up exist, with evidence particularly lacking for outcomes beyond 6 months. Data from prospective controlled studies are particularly lacking for certain important clinical outcomes such as suicide and indeed all-cause mortality in this severely ill and treatment-resistant patient population.
In summary, our review found that up to half of all patients who respond to ECT relapse within the first year, the period of highest risk being the first 6 months. Continuation pharmacotherapy or C-ECT significantly reduces the risk of relapse. However, many questions remain unanswered. Future studies should clarify which patient characteristics might predict relapse and what the optimal post-ECT continuation treatment or combination thereof entails. More focus is required on treatments other than TCAs, including psychotherapy and indeed optimization of treatment schedules for C-ECT, preferably in conjunction with concomitant pharmacotherapy. Such research is required to keep ECT patients in remission for as long as possible and with the fewest side-effects.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr T.K. Birkenhäger, Dr V. Navarro, and Dr H.A. Sackeim for providing us with additional information about their studies. The study was devised by AJ and DMM and data were collected by AJ and EK. All the authors were involved in interpretation of the results, drafting and revising the manuscript, and approved the final version. This work was supported by awards from the Health Research Board (TRA/2007/5 awarded to Declan McLoughlin) and the Friends of St. Patrick's Hospital. These organisations had no roles in the design of the study, collection and analysis of data or the decision to publish. The authors declare that there are no competing financial interests in relation to the work described.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- American Psychiatric Association 2001The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. A Task Force Report of the American Psychiatric Association2nd edn.American Psychiatric Association: Washington, DC, USA [Google Scholar]
- Arfwidsson L, Arn L, Beskow J. Chlorpromazine and the anti-depressive efficacy of electroconvulsive therapy. Acta Psychiatr Scand. 1973;49:580–587. doi: 10.1111/j.1600-0447.1973.tb04449.x. [DOI] [PubMed] [Google Scholar]
- Barton JL, Mehta S, Snaith RP. The prophylactic value of extra ECT in depressive illness. Acta Psychiatr Scand. 1973;49:386–392. doi: 10.1111/j.1600-0447.1973.tb04432.x. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H.2011. Comprehensive Meta Analysis Version 2.2.064, Biostat: Engelwood, NJ, USA.
- Bruce EM, Crone N, Fitzpatrick G, Frewin SJ, Gillis A, Lascelles CF, et al. A comparative trial of ECT and Tofranil. Am J Psychiatry. 1960;117:76. doi: 10.1176/ajp.117.1.76. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dunne RA, McLoughlin DM. Systematic review and meta-analysis of bifrontal electroconvulsive therapy versus bilateral and unilateral electroconvulsive therapy in depression. World J Biol Psychiatry. 2012;13:248–258. doi: 10.3109/15622975.2011.615863. [DOI] [PubMed] [Google Scholar]
- Eranti S, Mogg A, Pluck G, Landau S, Purvis R, Brown RG, et al. A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. Am J Psychiatry. 2007;164:73–81. doi: 10.1176/ajp.2007.164.1.73. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Rifat SL. Two-year outcome of psychotic depression in late life. Am J Psychiatry. 1998;155:178–183. doi: 10.1176/ajp.155.2.178. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Drug-resistant depressions: response to ECT. Pharmakopsychiatr Neuropsychopharmakol. 1974;7:205–206. [Google Scholar]
- Heijnen WT, Birkenhager TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30:616–619. doi: 10.1097/JCP.0b013e3181ee0f5f. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlah NW, Ryan E, Harrington JA. The influence of antidepressant drugs on the response to electroconvulsive therapy and on subsequent relapse rates. Neuropsychopharmacol. 1965;4:438–442. [Google Scholar]
- Kay DW, Fahy T, Garside RF. A seven-month double-blind trial of amitriptyline and diazepam in ECT-treated depressed patients. Br J Psychiatry. 1970;117:667–671. doi: 10.1192/bjp.117.541.667. [DOI] [PubMed] [Google Scholar]
- Kellner CH. Relapse after electroconvulsive therapy (ECT) J ECT. 2013;29:1–2. doi: 10.1097/YCT.0b013e31826fef01. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196:226–234. doi: 10.1192/bjp.bp.109.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE) Arch Gen Psychiatry. 2006;63:1337–1344. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krog-Meyer I, Kirkegaard C, Kijne B. Prediction of relapse with the TRH test and prophylactic amitriptyline in 39 patients with endogenous depression. Am J Psychiatry. 1984;141:945–948. doi: 10.1176/ajp.141.8.945. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Odgaard K, Clemmesen L, Lunde M. Relapse prevention by means of paroxetine in ECT-treated patients with major depression: a comparison with imipramine and placebo in medium-term continuation therapy. Acta Psychiatr Scand. 1996;94:241–251. doi: 10.1111/j.1600-0447.1996.tb09856.x. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Sampson S, Husain MM, Petrides G, Knapp RG, McCall WV, et al. Toward individualized post-electroconvulsive therapy care: piloting the Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE) intervention. J ECT. 2008;24:179–182. doi: 10.1097/YCT.0b013e318185fa6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CH, Katalinic N, Martin D, Schweitzer I. A review of ultrabrief pulse width electroconvulsive therapy. Ther Adv Chronic Dis. 2012;3:69–85. doi: 10.1177/2040622311432493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Amoros E, Cardoner N, Soria V, Galvez V, Menchon JM, Urretavizcaya M. Long-term treatment strategies in major depression: a 2-year prospective naturalistic follow-up after successful electroconvulsive therapy. J ECT. 2012;28:92–97. doi: 10.1097/YCT.0b013e31823e2705. [DOI] [PubMed] [Google Scholar]
- Navarro V, Gastobal C, Torres X, Masana G, Penades R, Guarch J, et al. Continuation/maintenance treatment with nortriptyline versus combined nortriptyline and ECT in late-life psychotic depression: a two-year randomized study. Am J Geriatr Psychiatry. 2008;16:498–505. doi: 10.1097/JGP.0b013e318170a6fa. [DOI] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, McCall WV, Isenberg K, Cooper T, Rosenquist PB, et al. Pharmacological strategies in the prevention of relapse after electroconvulsive therapy. J ECT. 2013;29:3–12. doi: 10.1097/YCT.0b013e31826ea8c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res. 1990;31:287–296. doi: 10.1016/0165-1781(90)90098-p. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. Continuation therapy following ECT: directions for future research. Psychopharmacol Bull. 1994;30:501–521. [PubMed] [Google Scholar]
- Sackeim HA, Dillingham EM, Prudic J, Cooper T, McCall WV, Rosenquist P, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry. 2009;66:729–737. doi: 10.1001/archgenpsychiatry.2009.75. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285:1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Decina P, Kerr B, Malitz S. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. J Clin Psychopharmacol. 1990;10:96–104. doi: 10.1097/00004714-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Seager CP, Bird RL. Imipramine with electrical treatment in depression: a controlled trial. J Ment Sci. 1962;108:704–707. doi: 10.1192/bjp.108.456.704. [DOI] [PubMed] [Google Scholar]
- The UK ECT Review Group Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- van den Broek WW, Birkenhager TK, Mulder PG, Bruijn JA, Moleman P. Imipramine is effective in preventing relapse in electroconvulsive therapy-responsive depressed inpatients with prior pharmacotherapy treatment failure: a randomized, placebo-controlled trial. J Clin Psychiatry. 2006;67:263–268. doi: 10.4088/jcp.v67n0213. [DOI] [PubMed] [Google Scholar]
- Wijkstra J, Nolen WA, Algra A, van Vliet IM, Kahn RS. Relapse prevention in major depressive disorder after successful ECT: a literature review and a naturalistic case series. Acta Psychiatr Scand. 2000;102:454–460. doi: 10.1034/j.1600-0447.2000.102006454.x. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Mantar A, Simsek S, Onur E, Gokmen N, Fidaner H. Combination of pharmacotherapy with electroconvulsive therapy in prevention of depressive relapse: a pilot controlled trial. J ECT. 2010;26:104–110. doi: 10.1097/YCT.0b013e3181c189f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.