Abstract
Cue-induced cocaine craving intensifies, or ‘incubates', during the first few weeks of abstinence and persists over extended periods of time. One important factor implicated in cocaine addiction is the endogenous opioid β-endorphin. In the present study, we examined the possible involvement of β-endorphin in the incubation of cocaine craving. Rats were trained to self-administer cocaine (0.75 mg/kg, 10 days, 6 h/day), followed by either a 1-day or a 30-day period of forced abstinence. Subsequent testing for cue-induced cocaine-seeking behavior (without cocaine reinforcement) was performed. Rats exposed to the drug-associated cue on day 1 of forced abstinence demonstrated minimal cue-induced cocaine-seeking behavior concurrently with a significant increase in β-endorphin release in the nucleus accumbens (NAc). Conversely, exposure to the cue on day 30 increased cocaine seeking, while β-endorphin levels remained unchanged. Intra-NAc infusion of an anti-β-endorphin antibody (4 μg) on day 1 increased cue-induced cocaine seeking, whereas infusion of a synthetic β-endorphin peptide (100 ng) on day 30 significantly decreased cue response. Both intra-NAc infusions of the δ opioid receptor antagonist naltrindole (1 μg) on day 1 and naltrindole together with β-endorphin on day 30 increased cue-induced cocaine-seeking behavior. Intra-NAc infusion of the μ opioid receptor antagonist CTAP (30 ng and 3 μg) had no behavioral effect. Altogether, these results demonstrate a novel role for β-endorphin and the δ opioid receptor in the development of the incubation of cocaine craving.
Keywords: cocaine, beta-endorphin, nucleus accumbens, incubation of craving, delta opioid receptor
INTRODUCTION
Cocaine addiction poses a serious problem to society. Countless deaths occur worldwide as a result of cocaine abuse, levels of which are on the rise (Watson, 2007). Cocaine craving, induced by drug-associated environmental cues, intensifies or ‘incubates' over the first few weeks of abstinence and persists over extended periods of time. As a result, addicts are prone to relapse even after long durations of abstinence (Gawin and Kleber, 1986). Grimm et al (2001) first demonstrated an analogous incubation phenomenon in rats, in which onset of craving is delayed and craving does not decay but rather progressively increases over a prolonged period of forced abstinence. Many studies have been conducted to elucidate the nature of this process, and the main findings have been reviewed (Lu et al, 2004b; Pickens et al, 2011). The main findings implicate the glutamatergic system as a major contributor to the incubation process as activation of the AMPA receptor, and specifically the presentation of its subunit composition on the post-synaptic membrane, is associated with this incubation process, and furthermore, blockade of GluR2-lacking AMPA receptors attenuates the incubation of cocaine craving (Conrad et al, 2008). A possible role for other neuronal systems in this process, particularly the opioid system, has, however, remained relatively obscure thus far.
The opioid system consists of three classical opioid receptors, μ, κ, and δ, which are abundantly expressed in the mesolimbic system, including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Le Merrer et al, 2009; Peng et al, 2012). Opioid receptors have a substantial role in motivational and reward processes (Pradhan et al., 2011; Shippenberg et al, 2008). A single-nucleotide polymorphism in the gene coding for the μ opioid receptor results in a reduced response to natural reward in humans (Lee et al, 2011). Moreover, μ opioid receptor occupancy, measured by PET, predicted the treatment outcome in cocaine-abusing patients (Ghitza et al, 2010). In rats it has been shown that methadone, which binds to μ receptors, blocked cocaine-induced behavioral and neural adaptations (Kreek et al, 2009; Leri et al, 2009). Nonetheless, several other studies have demonstrated that blockade of these receptors had no effect on cocaine self-administration and reward (Badiani et al, 2011; Mello and Negus, 1996; Pettit et al, 1984).
There is also evidence for an involvement of the δ opioid receptor in reward and addiction processes, yet findings are equivocal. Non-peptidic δ receptor agonists elicit reward in the conditioned place-preference paradigm (Longoni et al, 1998), but other reports have shown negligible abuse-related effects in a monkey self-administration model (Negus et al, 1998) and in a rat model of intracranial self-stimulation (Do Carmo et al, 2009). Moreover, i.p. administration of the selective δ receptor antagonist naltrindole decreases responding for cocaine in rats, regardless of the schedule of reinforcement (Reid et al, 1995). However, others (de Vries et al, 1995) have reported that only a high dose of naltrindole (i.p.), which markedly depresses locomotor activity, results in a modest (16%) reduction of cocaine self-administration. Furthermore, intra-NAc infusion of naltrindole decreases cocaine self-administration, whereas intra-VTA infusion of this antagonist increases cocaine-maintained responses (Ward and Roberts, 2007).
One of the prominent endogenous opioid receptor ligands is β-endorphin. This opioid peptide is produced, in part, in the arcuate nucleus of the hypothalamus and released in various brain regions, including reward-related areas (Roth-Deri et al, 2003; 2008). β-Endorphin was shown not only to attenuate acquisition as well as maintenance of cocaine self-administration (Roth-Deri et al, 2008) but also to reinstate cocaine seeking in rats after injections into the nucleus accumbens (Simmons and Self, 2009).
β-Endorphin binds at high affinity to both μ and δ receptors (Mansour et al, 1995). It was suggested that cocaine seeking is reinstated through β-endorphin's interaction with the μ, but not δ, receptor in the NAc (Simmons and Self, 2009).
To date, only few reports have addressed a possible role for the opioid system in the incubation of craving. It was found that heroin priming–induced reinstatement of cocaine craving incubates over time, while cocaine priming–induced reinstatement does not (Lu et al, 2004a). The authors hypothesized that the difference between the drugs' ability to induce craving after abstinence may be related to time-dependent neuroadaptations in the opioid system, which are modified by chronic cocaine exposure (Lu et al, 2004b; Turchan et al, 1999). It has also been demonstrated that naloxone, an opioid antagonist, attenuates incubation of sucrose craving in rats (Grimm et al, 2007). Bearing in mind the difference between sucrose and cocaine craving, this latter report is consistent with time-dependent changes in the opioid system following forced reward abstinence.
In the present study, we examined the role of β-endorphin in the incubation of cocaine craving. In this animal model, rats respond for the presentation of a cue that was previously associated with self-administration of cocaine. The extent of responding is considered as a measure of reward seeking and serves to evaluate craving. Thus, rats underwent 10 days of intensive (6 h/day) cocaine intake. We then examined the cue-induced cocaine-seeking behavior after short-term (1 day) and long-term (30 days) periods of forced abstinence and concurrent changes in NAc β-endorphin release. In addition, we examined the behavioral impact of β-endorphin on cocaine craving by evaluating cue-induced cocaine seeking after intra-NAc infusion of an anti-β-endorphin antibody (day 1) or exogenous β-endorphin peptide (day 30). Next, we examined which opioid receptor may mediate the observed effect of β-endorphin on craving via intra-NAc infusions of μ and δ receptor antagonists.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan, Israel) weighing 250–350 g were maintained on a 12-h light/12-h dark reversed cycle, with food and water available ad libitum. Rats were housed two per cage with a metal-perforated divider between them. Experiments were conducted during the dark cycle. All experimental procedures were approved by the University Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines.
Guide Cannula Implantation
Rats were anesthetized with xylazine and ketamine (10 and 100 mg/kg, respectively). A 20-gauge guide cannula was then unilaterally implanted either into the NAc (AP, +1.4 mm; LM, +1.2 mm to bregma and DV, −5.6 mm) or dorsal striatum (AP +1.4 mm; LM +2.4 mm to bregma and DV, −4.4) of rats with the aid of a stereotactic device (David-Kopf Instruments, CA, USA).
Jugular Vein Catheterization
Immediately after cannula implantation, rats were implanted with intravenous silastic catheters (ID 0.55 mm, OD 0.94 mm, Dow Corning, MI) into the right jugular vein. The catheter was secured to the vein with silk sutures and was passed subcutaneously to the top of the skull, where it exited into a connector (a modified 22-gauge cannula; Plastics One, VA, USA) mounted to the skull with MX-80 screws (Small Parts, FL, USA) and dental cement (Yates and Bird, IL, USA).
Self-Administration Training Sessions
The self-administration chambers (Med-Associates; St Albans, VT, USA) had two levers, one active and one inactive. An active lever press generated a cocaine infusion (0.75 mg/kg, 0.13 ml, 5 s/infusion; cocaine obtained from the National Institutes on Drug Abuse, MD, USA) through the i.v. catheter, and also activated a light located above the lever, which was lit for 5 s. Active lever presses during the next 35 s after the light cue did not result in additional cocaine infusion. Presses on the inactive lever did not activate the infusion pump and light. The number of active lever responses, infusions, and inactive lever responses were recorded. Rats were returned to their home cages at the end of the daily session.
Forced Abstinence
Immediately after the 10-day period of self-administration training sessions, rats were subjected to either a 1-day or a 30-day period of forced abstinence. During abstinence, rats were left in their home cages and handled three times a week.
Cue-Induced Cocaine-Seeking Behavioral Test
On day 1 or day 30 of forced abstinence, rats were again placed in the self-administration chambers, connected to the infusion line, and drug-seeking behavior was tested for 60 min. Upon active lever presses, only the contingent light cue appeared, without delivery of the drug.
Microdialysis Procedure
Twelve hours before the test, a microdialysis probe (2 mm length, 20 kDa cutoff value, CMA/10; Carnegie Medicine, Sweden) was inserted into the guide cannula. Artificial cerebrospinal fluid (aCSF; consisting of 126 mM NaCl, 2.4 mM CaCl2, 1.2 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, and NaHCO; pH 7.4) was continuously pumped (l.0 μl/min) through the dialysis probe. Dialysates were collected at 20-min intervals, before the cue test (for baseline measurement, in home cages) and during the cue test (in the self-administration cages). Dialysates were immediately frozen on dry ice until assay of β-endorphin content.
Quantification of β-Endorphin Content
Microdialysate β-endorphin content was determined using an ELISA assay kit (Peninsula; CA, USA), according to the manufacturer's instructions. The linear portion of the standard curve was between 0.04 and 5 ng/ml of β-endorphin, and experimental levels of β-endorphin were within the linear portion. Intra-assay variation was 4%, and inter-assay variation was 13%.
Drugs and Intracranial Infusions
β-Endorphin-(1–17) (C157H254N42O44S, 100 ng, 28 nM, Sigma), anti-β-endorphin antibody (SC-18264, 4 μg, 28 nM, Santa Cruz Biotechnology, CA, USA), the selective δ receptor antagonist (naltrindole,1 μg; Sigma, MO, USA), and the selective μ receptor antagonist (CTAP, 3 μg and 30 ng, Sigma, St Louis, MO, USA) were dissolved in aCSF. Drugs were infused (5 min, 0.2 μl/min) into the NAc or dorsal striatum via the guide cannula, using an electronical syringe pump (CMA 400, CMA/Microdialysis). Control rats received similar infusions of aCSF only (5 min, 0.2 μl/min) into the same brain regions as treated rats from the respective groups. Infusions were performed immediately (<5 min) before testing of cue-induced cocaine-seeking behavior. The internal cannula remained in place for 5 min after infusion, to avoid reflux. Doses of β-endorphin, CTAP, naltrindole, and anti-β-endorphin were selected based on previous studies (Do Carmo et al, 2009; Roth-Deri et al, 2004).
Verification of Cannula Placement
On completion of the experiments, rats (n=7–10 from each group) were anesthetized and transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were removed and immersed in 4% paraformaldehyde for 24 h, then in phosphate buffer with 30% sucrose for 48 h. Brains were then frozen on dry ice and sliced (40-μm sections) with a cryostat. Sections were mounted on glass slides coated with 2% gelatin, and cannula placement was verified under a microscope. Average hit rate was 85%. Data were analyzed only after verifying that the guide cannula was accurately implanted (a total of eight rats were excluded from the experiment due to technical reasons, such as cannula placement, weakness of the animal, and so on. See Figure 1c and Supplementary Figure 1A of cannula placement in the NAc).
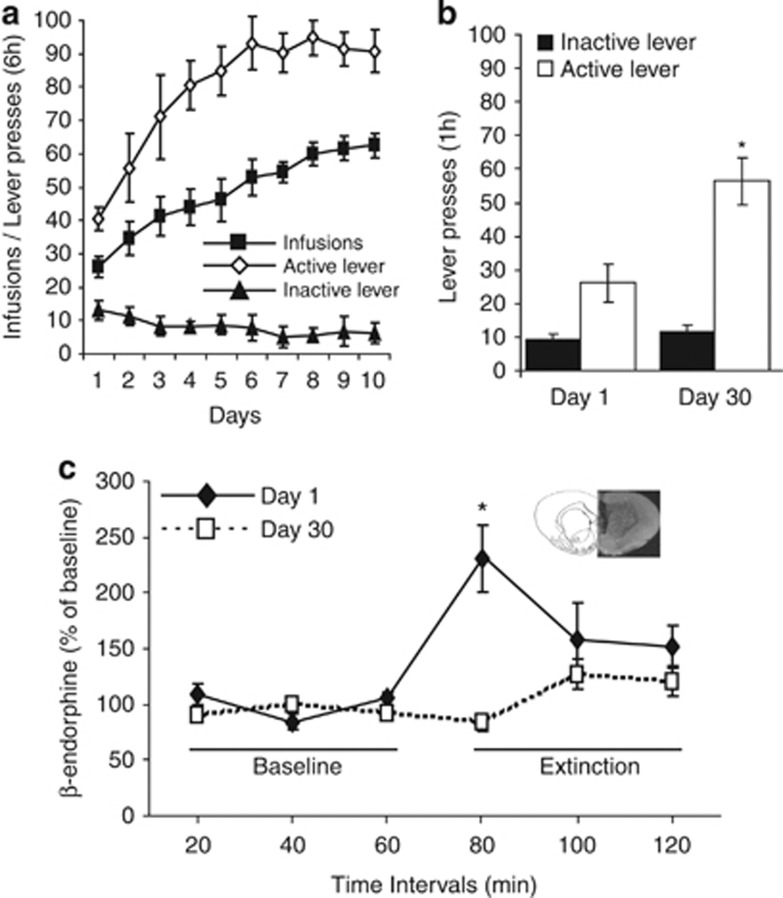
Figure 1.
Incubation of cocaine craving and β-endorphin extracellular levels. (a) Self-administration training: Number of active and inactive lever presses and cocaine infusions over the 10-day, 6 h/day self-administration sessions. Cocaine (0.75 mg/kg per infusion) induced intensive pressing on the active lever, whereas the amount of presses on the inactive lever was minimal. (b) Cue-induced drug-seeking behavior. The number of active lever presses in cocaine-trained rats tested on day 30 of abstinence was significantly higher than on day 1. Values are expressed as mean±SEM; n=9 rats per group. *p<0.001 vs day 1. (c) Cue-evoked β-endorphin levels in the NAc were significantly higher after 1 day of abstinence from cocaine than after 30 days. Values are expressed as mean±SEM; n=9 rats per group.*p<0.001 vs baseline and day 30. Top right corner: representative image for cannula placement in the NAc.
Statistical Analysis
One-way ANOVA was used to examine differences between the groups in active lever presses during cue tests, one-way ANOVA with repeated measures was used for analysis of β-endorphin levels during the cue test (day of abstinence as the between-subjects factor and time intervals as the within-subjects factor). Student–Newman–Keuls was used as post-hoc analysis. p<0.05 was considered significant. Results are presented as mean±SEM.
RESULTS
For all the trials, rats were implanted with a guide cannula into the NAc or dorsal striatum (according to the specific experiment). After recovery, experiments consisted of three phases: cocaine self-administration training (10 days, 6 h/day), a period of forced abstinence (1 or 30 days), and then testing for cue-induced cocaine seeking (without cocaine reinforcement).
Extracellular β-Endorphin Levels During Cue-Induced Cocaine Seeking After Abstinence
Rats demonstrated reliable cocaine self-administration behavior during the 10-day, 6-h/day training sessions (Figure 1a). Similar results were also obtained during training sessions for subsequent experiments.
After training, rats were subjected either to a 1-day or a 30-day period of forced abstinence. Cue-induced cocaine seeking was then tested, and NAc β-endorphin levels were concurrently measured using the microdialysis technique.
For the cue test, one-way ANOVA showed a main effect of group (F(1,32)=28, p<0.0001). Post-hoc comparisons showed a significantly higher number of cue-elicited active lever presses on day 30 of abstinence, as compared with day 1 (Student–Newman–Keuls, p<0.001). The number of inactive lever presses was similarly low on both days (p>0.05; Figure 1b; n=9 per group).
Repeated measures ANOVA for NAc β-endorphin levels during cocaine seeking revealed a main effect of group (F(1,80)=42.06; p<0.0001), main effect of fractions (F(5,80)=23.85; p<0.0001), and an interaction between group and fractions (F(5,80)=30.43; p<0.0001). NAc β-endorphin levels were inversely related to active lever responding, demonstrating a significant twofold increase during cocaine seeking on day 1, relative to day 30 (Student–Newman–Keuls post hoc; p<0.0001; Figure 1c). Exposure to the cue transiently increased extracellular levels of β-endorphin, which returned to baseline within 20 min. There was no significant differences between baseline and all extinction time points (A one-way repeated measures ANOVA, followed by Dunnet's post-hoc test, (F (3, 7)=3.51; p>0.05). There was no statistical difference between basal levels of β-endorphin on day 1 and day 30 of abstinence (0.483±0.041 mg/μl vs 0.466±0.1 mg/μl, respectively, p>0.05, Student's t-test).
Intra-NAc β-Endorphin After Long-Term Abstinence Attenuates Cue-Induced Cocaine Seeking
In this experiment, we infused a synthetic β-endorphin peptide into the NAc or dorsal striatum on day 30 of abstinence and examined response to cocaine cues (n=9/group). A two-way ANOVA revealed a main effect of treatment (F(1,31)=9.19, p<0.005), an interaction between brain region and treatment (F(1,31)=4.71, p<0.05), but no main effect of brain region (F(1,31)=0.36, p>0.05). Cue-elicited cocaine-seeking was significantly decreased in intra-NAc β-endorphin-infused rats, as compared with aCSF-infused control rats (Student–Newman–Keuls post-hoc test, p<0.01; Figure 2a). For rats which received dorsal striatum infusions, there were no differences in active lever pressing between β-endorphin-treated rats and controls (Student–Newman–Keuls post-hoc test, p>0.05; Figure 2b). There were no group differences in inactive lever pressing for either intra-NAc- or dorsal striatum-infused rats (p>0.05).
Figure 2.
Effect of intra-NAc infusion of β-endorphin on cue-induced cocaine-seeking behavior on day 30. β-Endorphin microinjections into the NAc (a) but not into the dorsal striatum (b) significantly decreased cocaine-seeking behavior after 30 days of abstinence. Values are expressed as mean±SEM; n=10 rats per group. *p<0.002 vs aCSF-infused rats.
Intra-NAc Anti-β-Endorphin Antibody After Short-Term Abstinence Increases Cue-Induced Cocaine Seeking
Here, we infused an anti-β-endorphin antibody into the NAc or dorsal striatum on day 1 and then examined cocaine-seeking behavior (n=7–8/group). A two-way ANOVA revealed a main effect of brain region (F(1,27)=18.93; p<0.0005), an interaction between brain region and treatment (F(1,27)=4.61; p<0.05), but no main effect of treatment (F(1,27)=3.09; p>0.05). Responding on the active lever was significantly higher for rats that received intra-NAc anti-β-endorphin infusion, as compared with aCSF-infused controls (Student–Newman–Keuls post-hoc test, p<0.01; Figure 3a). For rats which received a dorsal striatum infusion, there were no differences in active lever presses between the anti-β-endorphin-treated and aCSF-treated groups (Student–Newman–Keuls post-hoc, p>0.05; Figure 3b). There were no group differences in inactive lever pressing for either intra-NAc- or dorsal striatum-infused rats (p>0.05).
Figure 3.
Effect of intra-NAc infusion of anti-β-endorphin antibody on cue-induced cocaine-seeking behavior on day 1. β-Endorphin antibody infusion into the NAc (a) but not dorsal striatum (b) significantly increased cocaine-seeking behavior after 1 day of abstinence. Values are expressed as mean±SEM, n=7-8 rats per group. *p<0.01 vs aCSF-infused rats.
The Effect of Intra-NAc μ or δ Receptor Antagonists on Cue-Induced Cocaine Seeking After Abstinence
Here, we infused either naltrindole (a δ receptor antagonist) or CTAP (a μ receptor antagonist) into the NAc on day 1. On day 30, rats received the same intra-NAc antagonist treatments, preceded by infusion of a synthetic β-endorphin peptide. Controls received intra-NAc aCSF infusion. Additionally, treatment groups on day 1 were compared with results obtained for intra-NAc anti-β-endorphin, and treatment groups on day 30 were compared with results obtained for intra-NAc synthetic β-endorphin injection.
For day 1, one-way ANOVA revealed a significant difference between treatments (F(3,32)=4.799, p<0.01). Treatment with naltrindole, similar to anti-β-endorphin, resulted in significantly increased cocaine-seeking behavior, as compared with CTAP (3 μg) or aCSF (Student–Newman–Keuls post-hoc, p<0.05; n=9 per group; Figure 4a). Injection of CTAP at a lower dose (30 ng) showed results similar to that of 3 μg CTAP (active lever presses: 21±5 vs 28±6, respectively; p>0.05).
Figure 4.

Effect of intra-NAc infusion of δ or μ antagonist cue-induced cocaine-seeking behavior after 1 day or 30 days of forced abstinence. (a) Day 1: Number of active lever presses after infusion of naltrindole (δ antagonist) is significantly higher than after infusion of CTAP (μ antagonist; 3 μg) and showed the same effect as anti-β-endorphin antibody infusion (anti β-endorphin). *p<0.05 vs aCSF-infused rats. (b) Day 30: Antagonism of the δ receptor reversed the effect of exogenous β-endorphin on cue-induced cocaine-seeking behavior. The number of active lever presses after infusion of naltrindole together with β-endorphin is significantly higher than after infusion of CTAP (3 μg) with β-endorphin or infusion of a β-endorphin alone. *p<0.01 vs aCSF-infused rats. Values are expressed as mean±SEM; n=9 rats per group.
For day 30, a two-way ANOVA revealed a main effect of group (F(1, 38)=12.97; p<0.001) but no main effect of treatment (F(2,38)=0.33; p>0.05) and no interaction (F(2,38)=1.65; p>0.05). Subsequent post-hoc tests showed that CTAP (3 μg) co-injected with β-endorphin, similar to injection of β-endorphin alone, significantly decreased active lever responding compared with aCSF (Student–Newman–Keuls, p<0.01; Figure 4b). Co-injection of CTAP at a lower dose (30 ng) showed results similar to those of co-injected 3 μg CTAP (active lever presses: 24±6 vs 34±7, respectively; p>0.05). However, infusion of naltrindole together with β-endorphin reversed the effect of β-endorphin alone and of co-injected CTAP, by significantly elevating the amount of active lever presses to control levels (Student–Newman–Keuls, p<0.05; n=9 per group; Figure 4b).
Infusion of either CTAP (3 μg) or naltrindole (1 μg) without co-injection of β-endorphin showed no significant effect on cocaine-seeking behavior, as compared with aCSF (one-way ANOVA; F(3,29)=6.456, followed by Student–Newman–Keuls post-hoc test, p>0.0.5).
DISCUSSION
β-Endorphin levels in the NAc are associated with cocaine self-administration and craving (Roth-Deri et al, 2003, 2008). In the present study, we demonstrate novel evidence supporting the involvement of this opioid peptide in the incubation of cocaine craving via its interaction with the δ receptor.
In addition to its involvement in cocaine self-administration (Roth-Deri et al, 2003, 2008), β-endorphin also possesses rewarding and reinforcing properties of its own (Amalric et al, 1987). Moreover, it can moderate behavioral responses to stressful stimuli (Merenlender-Wagner et al, 2009; Young et al, 1990). Thus, we suggest that the increase in extracellular β-endorphin levels on day 1 may facilitate coping both with the stress caused by the sudden lack of cocaine supply and with the resultant heightened drug craving. This lowering of craving can account for the reduction in cue-induced cocaine seeking on day 1, demonstrated herein and in previous reports (Grimm et al, 2001; Lu et al, 2004b).
We additionally showed that intra-NAc infusion of anti-β-endorphin antibody on day 1 enhanced cue-induced cocaine seeking, whereas infusion of exogenous β-endorphin on day 30 significantly attenuated cocaine seeking. This further suggests that release of β-endorphin in the NAc may function as a mechanism for lowering of cue-induced craving. However, this mechanism appears to be short-lived, as during cue exposure on day 30 extracellular β-endorphin levels remained unchanged, when cocaine craving was high. Interestingly, basal levels of β-endorphin did not change after cocaine abstinence, indicating that tonic release remained unchanged yet phasic release was altered following the exposure to the cue on day 30. Incubation is usually interpreted as an increase in the expression of an existing effect or mechanism. Herein we postulate that during abstinence, an endogenous braking mechanism, ie, the endogenous release of NAc β-endorphin in response to the cue, is apparently lost.
Contrary to our findings, a recent study demonstrated that intra-NAc infusion of β-endorphin reinstates previously extinguished cocaine seeking (Simmons and Self, 2009). These different effects of β-endorphin may be due to the differences in methodological factors, such as daily dosage of cocaine and intensity of exposure and the between-session analysis applied. Yet most significant, in our opinion, is the different paradigms applied: the frequently used extinction training by daily exposure to a cue, employed in the study by Simmons and Self (2009), as opposed to a prolonged forced abstinence period followed by a single exposure to the cue, employed herein.
Several recent findings suggest involvement of β-endorphin and the δ receptor, in cocaine seeking (reviewed in (Yadid et al, 2012)). It was shown that cocaine-induced reward is reduced in β-endorphin-deficient but not μ receptor knockout mice. This implies that β-endorphin is essential for the rewarding action of cocaine while the μ receptor may not mediate this regulatory action (Nguyen et al, 2012).
Our findings, showing reduced β-endorphin release concurrently with heightened drug craving after long-term abstinence, are consistent with previous studies demonstrating attenuated β-endorphin response during ethanol or nicotine relapse. One study examined the extent to which β-endorphin response to stress is associated with early smoking relapse. The authors found that smokers who relapsed exhibited an attenuated β-endorphin response to stressors, compared with those who maintained abstinence over the same period (Shaw and al'Absi, 2008). Moreover, smokers who underwent weekly exercise sessions had higher β-endorphin plasma levels and demonstrated a reduced smoking rate (Leelarungrayub et al, 2010). In another study, a 10-day withdrawal from ethanol consumption led to decreased β-endorphin plasma levels. Chronic treatment with acamprosate, which increases β-endorphin plasma concentrations, caused a significant reduction in ethanol intake. The authors concluded that restoring alcohol-induced deficits in β-endorphin levels may be an important factor in preventing craving and maintaining abstinence (Zalewska-Kaszubska et al, 2008).
Altogether, our results reveal a novel role for β-endorphin and the δ opioid receptor in the incubation of cocaine craving (Roth-Deri et al, 2008). To our knowledge, this study provides the first evidence for modulation of cue-induced incubation of cocaine craving by intra-NAc application of exogenous β-endorphin. Future research can focus on β-endorphin-releasing agents and δ opioid agonists for alternative treatment of enhanced cocaine craving that incubates during extended abstinence periods.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Dr T. Green-Sadan for assistance in editing.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries TJ, Babovic-Vuksanovic D, Elmer G, Shippenberg TS. Lack of involvement of delta-opioid receptors in mediating the rewarding effects of cocaine. Psychopharmacology (Berl) 1995;120:442–448. doi: 10.1007/BF02245816. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin F, Kleber H. Pharmacologic treatments of cocaine abuse. Psychiatr Clin North Am. 1986;9:573–583. [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B, et al. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biol Psychiatry. 2010;68:697–703. doi: 10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Manaois M, Osincup D, Wells B, Buse C. Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology (Berl) 2007;194:537–544. doi: 10.1007/s00213-007-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to the bench. Curr Opin Pharmacol. 2009;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Gallen CL, Zhang X, Hodgkinson CA, Goldman D, Stein EA, et al. Functional polymorphism of the mu-opioid receptor gene (OPRM1) influences reinforcement learning in humans. PLoS One. 2011;6:e24203. doi: 10.1371/journal.pone.0024203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelarungrayub D, Pratanaphon S, Pothongsunun P, Sriboonreung T, Yankai A, Bloomer RJ. Vernonia cinerea Less. supplementation and strenuous exercise reduce smoking rate: relation to oxidative stress status and beta-endorphin release in active smokers. J Int Soc Sports Nutr. 2010;7:21. doi: 10.1186/1550-2783-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Zhou Y, Goddard B, Levy A, Jacklin D, Kreek MJ. Steady-state methadone blocks cocaine seeking and cocaine-induced gene expression alterations in the rat brain. Eur Neuropsychopharmacol. 2009;19:238–249. doi: 10.1016/j.euroneuro.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni R, Cadoni C, Mulas A, Di Chiara G, Spina L. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 2. Place-preference and brain microdialysis studies in rats. Behav Pharmacol. 1998;9:9–14. [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004a;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47 (Suppl 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995;18:69–70. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Merenlender-Wagner A, Dikshtein Y, Yadid G. The beta-endorphin role in stress-related psychiatric disorders. Curr Drug Targets. 2009;10:1096–1108. doi: 10.2174/138945009789735147. [DOI] [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Nguyen AT, Marquez P, Hamid A, Kieffer B, Friedman TC, Lutfy K. The rewarding action of acute cocaine is reduced in beta-endorphin deficient but not in mu opioid receptor knockout mice. Eur J Pharmacol. 2012;686:50–54. doi: 10.1016/j.ejphar.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124:223–228. doi: 10.1016/j.drugalcdep.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Glick SD, Menkens KA, French ED, Bilsky EJ, Porreca F. Cocaine self-administration and naltrindole, a delta-selective opioid antagonist. Neuroreport. 1995;6:1409–1412. doi: 10.1097/00001756-199507100-00012. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiol. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Schindler CJ, Yadid G. A critical role for beta-endorphin in cocaine-seeking behavior. Neuroreport. 2004;15:519–521. doi: 10.1097/00001756-200403010-00027. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, et al. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Shaw D, al'Absi M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacol Biochem Behav. 2008;90:357–362. doi: 10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Roberts DC. Microinjection of the delta-opioid receptor selective antagonist naltrindole 5'-isothiocyanate site specifically affects cocaine self-administration in rats responding under a progressive ratio schedule of reinforcement. Behav Brain Res. 2007;182:140–144. doi: 10.1016/j.bbr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. Cocaine use rises in Europe while overall drug use levels out. BMJ. 2007;335:1117. doi: 10.1136/bmj.39412.365718.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Redlus L, Barnea R, Doron R. Modulation of mood states as a major factor in relapse to substance use. Front Mol Neurosci. 2012;5:81. doi: 10.3389/fnmol.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Spencer RL, McEwen BS. Changes at multiple levels of the hypothalamo-pituitary adrenal axis following repeated electrically induced seizures. Psychoneuroendocrinology. 1990;15:165–172. doi: 10.1016/0306-4530(90)90027-7. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Gorska D, Dyr W, Czarnecka E. Effect of chronic acamprosate treatment on voluntary alcohol intake and beta-endorphin plasma levels in rats selectively bred for high alcohol preference. Neurosci Lett. 2008;431:221–225. doi: 10.1016/j.neulet.2007.11.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.