Abstract
Hepatocellular carcinoma (HCC) is a hypervascular highly angiogenic tumor usually associated with liver cirrhosis. Vascular endothelial growth factor plays a critical role in vascular development in HCC. In contrast to the treatment of early-stage HCC, the treatment options for advanced HCC are limited and prognosis is often poor, which contributes to this tumor type being the third leading cause of cancer-related deaths worldwide. Metronomic chemotherapy, which was originally designed to inhibit angiogenesis, involves low-dose chemotherapeutic agents administered in a frequent regular schedule with no prolonged breaks and minimizes severe toxicities. We reviewed the potential effects and impact of metronomic chemotherapy in preclinical studies with HCC models and in patients with advanced HCC, especially when combined with a molecular targeted agent. Metronomic chemotherapy involves multiple mechanisms that include antiangiogenesis and antivasculogenesis, immune stimulation by reducing regulatory T cells and inducing dendritic cell maturation, and possibly some direct tumor cell targeting effects, including the cancer stem cell subpopulation. The total number of preclinical studies with HCC models shows impressive results using metronomic chemotherapy-based protocols, especially in conjunction with molecular targeted agents. Four clinical trials and two case reports evaluating metronomic chemotherapy for HCC indicate it to be a safe and potentially useful treatment for HCC. Several preclinical and clinical HCC studies suggest that metronomic chemotherapy may become an alternative type of chemotherapy for advanced unresectable HCC and postsurgical adjuvant treatment of HCC.
Introduction
Systemic chemotherapy with cytotoxic agents remains the most common systemic therapy to treat patients with metastatic disease. Most anticancer agents are designed to inhibit growth or kill rapidly dividing tumor cells. These drugs are usually administered at the highest doses possible to induce the maximum therapeutic effect; this is referred to as maximum tolerated dose (MTD) therapy [1,2]. However, administration of anticancer agents at MTD requires prolonged breaks between cycles of the therapy to allow recovery from the induced adverse side effects in different tissues and organs. These gaps in chemotherapy can allow or facilitate tumor regrowth including growth of clones resistant to the therapy. The regrowth of tumor or drug resistance clones during such gaps can prevent or compromise improvement of overall survival of patients with advanced cancer even when the first cycle of MTD therapy is effective [1,3–6].
A new concept of anticancer treatment that targets the tumor vasculature was first proposed by Folkman in 1971 [7]. This treatment concept is based on the indispensable role of the vasculature in tumor growth [8,9]. Antiangiogenic therapy has been investigated extensively in both preclinical and clinical studies [10,11]. In 1991, Kerbel [12] suggested that some conventional cytotoxic anticancer agents can suppress vascular development in tumors based on the immature and proliferative nature of endothelial cells present in the neovasculature. Klement et al. [13] and Browder et al. [14] reported that frequent repetitive low doses of chemotherapy drugs such as cyclophosphamide or vinblastine could markedly suppress tumor growth. Hanahan et al. coined the term metronomic therapy to describe this type of therapeutic schedule [15]. Metronomic therapy generally consists of the continuous administration of low-dose chemotherapeutic agents without extended intervals [2]. It was originally designed with the intention to inhibit tumor growth by antiangiogenic mechanisms, though other mechanisms can contribute to its antitumor efficacy as described below, and is usually associated with much less severe acute toxicities compared to conventional MTD chemotherapy [16]. So, recently, metronomic chemotherapy has been investigated in pediatric oncology [17]. Most new cancer cases and deaths now occur in low-income and middle-income countries [18]. As metronomic chemotherapy is a low-cost, well-tolerated, and easy-to-access treatment, it will be an attractive therapeutic option in resource-limited countries [19].
Hepatocellular carcinoma (HCC) is the sixth most common solid tumor and the third leading cause of cancer-related death globally [20,21]. Although the major blood supply to HCC is the portal veins at the early stage of hepatocarcinogenesis, the main supply ultimately is provided by neoarteries that develop in parallel with tumor growth [22–24]. For advanced HCC, such as Barcelona Clinic Liver Cancer (BCLC) stage C, classical chemotherapy is sometimes selected [25]. However, HCC is usually associated with liver cirrhosis, and thus aggressive chemotherapy can cause severe side effects [26]. Unfortunately, the prognosis of patients with advanced HCC is usually poor even in those treated with sorafenib [27,28]. To improve the therapeutic efficacy and prognosis of patients with advanced HCC, new strategies are clearly needed.
In this review, we evaluate the potential effects and impact of metronomic chemotherapy in patients with advanced HCC, especially when combined with a molecular targeted agent such as sorafenib.
Treatment for Advanced HCC
The development of sophisticated diagnostic modalities, such as computed tomography, magnetic resonance imaging, and abdominal ultrasonography, has allowed early diagnosis of HCC [29–32]. Patients with small HCCs are usually treated by surgical resection, liver transplantation, percutaneous ethanol injection therapy, microwave coagulation therapy, or percutaneous radiofrequency ablation [33]. The prognosis of patients with small HCCs has improved following the application of these therapeutic modalities [33].
Treatment of advanced HCC includes transhepatic arterial chemoembolization, transhepatic arterial infusion chemotherapy, systemic chemotherapy, hormonal therapy, and immunotherapy [32,34–37]. However, only transhepatic arterial chemoembolization has been confirmed to improve long-term survival in BCLC stage B [38–41].
In large randomized trials, themedian survival time (MST) of patients treated with doxorubicin were 6.8 and 7.4 months, respectively [42,43]. The MST of patients treated with PIAF regimen (cisplatin, interferon, doxorubicin, and fluorouracil) and FOLFOX4 regimen (oxaliplatin and fluorouracil) was 8.7 and 6.4 months, respectively. In three doubleblinded, placebo-controlled trials, no survival benefit of tamoxifen was confirmed [44–46]. In several small studies, the MST of patients with HCC treated with capecitabine and gemcitabine was 10.1 and 6.9 months, respectively [47,48]. Other drugs such as cisplatin, 5-fluorouracil (5-FU), mitoxantrone, etoposide, paclitaxel, irinotecan, and fludarabine have also failed to demonstrate meaningful activity [49–55]. Despite maximum effort by many investigators, any definitive evidences that systemic chemotherapy is effective for advanced HCC have not been provided [56]. Sorafenib is an orally active multi-kinase inhibitor that targets vascular endothelial growth factor receptor 2 (VEGFR-2) and PDGF receptors, among others, and also blocks tumor cell proliferation by targeting the Raf/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinases (ERKs) signaling pathway by virtue of its targeting the intracellular threonine kinase Raf [57–59]. The efficacy of sorafenib for advanced HCC was confirmed for the first time in the phase III SHARP trial (MST; 10.7months) and theAsian-Pacific phase III region trial (MST; 6.5 months) [27,28]. For advanced unresectableHCC with vascular invasion or extrahepatic metastasis (BCLC stage C), administration of sorafenib is now recommended worldwide [1,60,61]. Several trials with molecular target agents are underway. In the phase III trial, the MST of brivanib was 9.5 months and that of sorafenib was 9.9 months. In another phase III trial, the MST of linifanib and sorafenib was 9.1 and 9.8 months, respectively. In combination therapy, sorafenib and erlotinib (MST; 9.5 months) failed to prove the survival benefit comparing with sorafenib alone (MST; 8.5 months). Any other molecular target agents fail to surpass the efficacy of sorafenib so far. Due to the associated liver cirrhosis, patients with HCC sometimes develop severe side effects during conventional MTD chemotherapy, as noted above. Since metronomic chemotherapy is less toxic and, moreover, inhibits tumor growth through antiangiogenic mechanisms, this new therapeutic strategy using certain conventional chemotherapeutic drugs could be suitable for the treatment of advanced HCC.
Metronomic Chemotherapy
Preclinical Studies
The first preclinical studies of metronomic chemotherapy came from the laboratories of Folkman and Kerbel [14]. To date, there are more than 300 papers published on the preclinical effects of metronomic chemotherapy, as listed in PubMed. These reports describe the therapeutic efficacy of metronomic chemotherapy against at least 18 different types of cancers in the gastrointestinal tract, respiratory system, blood, brain, skin, and genitourinary systems. The most frequently selected anticancer drug for preclinical metronomic chemotherapy studies is cyclophosphamide. One interesting aspect of some of these studies is the potent antitumor efficacy of metronomic chemotherapy regimens in models of advanced metastatic cancer especially when combined with a targeted antiangiogenic drug which itself has minimal activity in this setting [62,63].
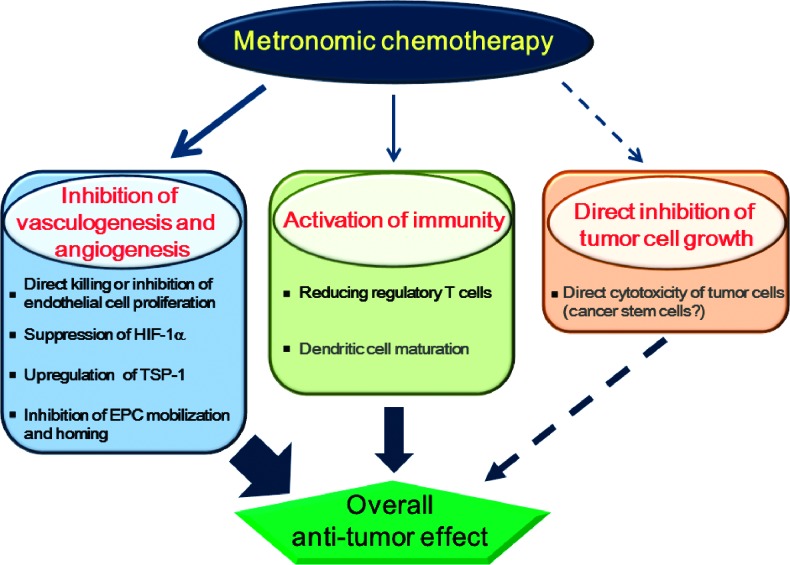
The main antitumor effects caused by metronomic chemotherapy are thought to be inhibition of tumor-associated vascular development and stimulation of immunity rather than direct cytotoxic effects on tumor cells (Figure 1) [12,64–66]. However, intriguingly, some recent reports have implicated direct targeting of cancer stem cells as a possible mechanism of metronomic cyclophosphamide [67], in contrast to MTD cyclophosphamide that does not target this subpopulation [68]. In the following section, we discuss recent information regarding mechanisms of metronomic chemotherapy, especially inhibition of vascular development and stimulation of immunity mediated by metronomic chemotherapy.
Figure 1.
Mechanisms of action of metronomic chemotherapy. The beneficial effects of metronomic chemotherapy are mediated through inhibition of vasculogenesis and angiogenesis, activation of immunity, and probably direct inhibition of tumor cell proliferation. Inhibition of vasculogenesis and angiogenesis plays a critical role in metronomic chemotherapy. Inhibition of vasculogenesis and angiogenesis includes direct inhibition of endothelial cell proliferation, up-regulation of endogenous angiogenic inhibitor such as TSP-1, suppression of HIF-1?, and inhibition of EPC homing in tumor tissues. Metronomic chemotherapy also stimulates antitumor immunity by reducing the number of Treg cells and possibly inducing dendritic cell maturation.
Inhibition of tumor angiogenesis/vascular development.
Direct cytotoxicity or inhibition of endothelial cell proliferation. Many conventional cytotoxic chemotherapeutic agents, such as cyclophosphamide, vinblastine, paclitaxel, docetaxel, tegafur/uracil (UFT), and tegafur/gimeracil/oteracil potassium (S-1), have antiangiogenic effects [69–75]. S-1 is composed of three compounds, namely, tegafur, gimeracil, and oteracil. UFT and S-1 decrease thymidine phosphorylase that is also called platelet-derived endothelial growth factor. The effect of UFT and S-1 seems to induce the antiangiogenic effect. The activated endothelial cells of newly formed blood capillaries are highly and selectively sensitive in vitro to very low concentrations of many conventional cytotoxic anticancer agents [76–81]. The antiangiogenic effects of conventional cytotoxic anticancer drugs seem to be optimized by administration of smaller doses without long breaks for prolonged periods [12].
Up-regulation of endogenous antiangiogenic factors and downregulation of endogenous angiogenic factors. Angiogenesis is thought to be switched off or downregulated when levels of endogenous antiangiogenic factors such as thrombospondin-1 (TSP-1) and angiostatin exceed those of angiogenic factors such as VEGF and basic fibroblast growth factor (bFGF) [82]. Bocci et al. [83,84] reported that protracted exposure of endothelial cells in vitro to low concentrations of various anticancer chemotherapeutic agents and ceramide analog caused marked induction of gene and protein expression of TSP-1. A number of other groups have reported up-regulation of circulating levels of TSP-1 in mice or patients exposed to metronomic chemotherapy [85]. TSP-1, a component of the extracellular matrix produced by endothelial cells, tumor cells, and infiltrating stromal cells, seems to act by binding to CD36 expressed on the cell membrane of endothelial cells [86–88]. TSP-1 also binds to VEGF and sequesters its angiogenic activity [89]. Hypoxia-inducible factor 1 (HIF-1) regulates the expression of angiogenic factors such as VEGF, bFGF, and stromal cell-derived factor 1 (SDF-1). Continuous administration of low-doseα topotecan was reported to decrease the expression of HIF-1 [34], VEGF, and SDF-1 [90]. Administration of low-dose anthracycline chemotherapeutic agents also inhibited HIF-1 transcription and the expression of VEGF and SDF-1 [91]. VEGF is the major factor in angiogenesis/vascular development in many tumors [92,93]. A decrease in serum VEGF levels was observed in patients with advanced breast cancer treated with metronomic cyclophosphamide [94]. In addition, metronomic chemotherapy with weekly platinum and daily etoposide administration in patients with non-small cell lung cancer resulted in a decrease in VEGF level during treatment [95].
Inhibition of vasculogenesis by reducing the number and viability of circulating endothelial progenitor cells. Vascular development in tumor tissues consists of angiogenesis and vasculogenesis. Vasculogenesis is generally defined as the contribution to the formation of new blood vessels by circulating bone marrow-derived cells, possibly including endothelial progenitor cells (EPCs) [96,97]. Accumulating evidence suggests that circulating bone marrow-derived EPCs migrate into tumor tissues to support vascular formation and tumor growth [98,99]. In addition, local release of VEGF and SDF-1 induce the migration of EPCs to tumor tissues through VEGFRs and CXCR4 on the cell surfaces of EPCs [100].
Bertolini et al. [101] reported that the administration of MTD cyclophosphamide induced a robust EPC mobilization a few days after the end of treatment in tumor-bearing mice bearing human lymphoma cells. In marked contrast, metronomic chemotherapy of cyclophosphamide, using lower doses given daily, was associated with consistent decreases in the numbers and viability of EPCs, with a much more durable and marked inhibition of tumor growth [101].
Stimulation of Immunity
Metronomic chemotherapy with certain chemotherapeutic agents can stimulate the immune response by reducing regulatory T (Treg) cells and inducing dendritic cell maturation [65,66,102]. Treg cells are CD4+CD25+ lymphocytes known to accumulate in variety of cancers [103]. Increased frequency of Treg cells correlate with tumor progression and lack of treatment response [103]. Metronomic chemotherapy with cyclophosphamide and temozolomide was shown to increase the antitumor immune responses by suppressing the number and activity of Treg cells and also by increasing lymphocyte proliferation and memory T cells [65,66,104–106]. The reduction in Treg cell number was specific, and the treatment had no effects on other types of lymphocytes [106]. This effect was specific for metronomic chemotherapy. However, conventional MTD or high-dose chemotherapy can result in depletion of all types of lymphocytes. Reduction of Treg cells by metronomic chemotherapy restored the antitumor immune response by recovering the activity of both tumor-specific (cytotoxic T lymphocytes and helper T cells) and tumor-nonspecific effect cells (natural killer and natural killer T cells) [106]. Other immunostimulatory effects of metronomic chemotherapy have been proposed recently. As an example, Tanaka et al. [102] reported that vinblastine, paclitaxel, and etoposide promoted dendritic cell maturation at nontoxic concentrations. They also found that local injection of low-dose vinblastine induced the maturation of tumor-infiltrating dendritic cells and stimulated antitumor immune responses in vivo [107]. However, the involvement of dendritic cell maturation bymetronomic chemotherapy needs to be further investigated and confirmed. Preclinical studies using immunodeficient mice have shown that metronomic chemotherapy can result in marked tumor growth suppression. Such results indicate that the involvement of the immune system in metronomic chemotherapy is not necessarily critical [108,109]. Nevertheless, it is interesting to consider the potential benefits of combining metronomic chemotherapy with immunotherapeutic treatments, e.g., tumor vaccines [110].
Metronomic Chemotherapy: Studies Using HCC Models
The potential efficacy of various metronomic chemotherapy protocols using cyclophosphamide, UFT, cisplatin, and doxorubicin have been investigated in animal models of HCC [111,112], as summarized in Table 1. Park et al. [113] reported that metronomic chemotherapy with cyclophosphamide inhibited HCC growth and prolonged survival without inducing major toxicities using a rat HCC model with accompanying liver cirrhosis. Tang et al. [111] reported that single or doublet metronomic chemotherapy using cyclophosphamide, UFT, and/or doxorubicin without any added antiangiogenic agents did not have survival benefits. In contrast, they reported a significant improvement of overall survival in animals that received various combinations of metronomic chemotherapeutic regimens with DC101, an anti-VEGFR-2 targeting antibody that potently inhibits angiogenesis. They also reported that metronomic chemotherapy with metronomic UFT and sorafenib delayed the onset of tumor progression (i.e., delayed development of resistance to chemotherapy) [114]. Zhou et al. [115] also reported that metronomic doxorubicin in combination with bevacizumab had a profound effect on tumor growth inhibition and survival of HCC xenograft model. The appearance of resistance to molecular targeted agents, such as sorafenib, is an inevitable problem in the treatment of advanced unresectable HCC. Thus, this report may be hopeful with respect to the clinical application of metronomic chemotherapy with sorafenib for advanced HCC. Iwamoto et al. [116] demonstrated that metronomic chemotherapy with S-1 inhibited tumor growth and prolonged survival of hepatoma tumor-bearing mice and that these effects were enhanced by the addition of vandetanib, an oral inhibitor of both the epidermal growth factor receptor and VEGFR-2. The antitumor effects of metronomic chemotherapy with S-1 alone were shown to be mediated mainly through inhibition of angiogenesis by upregulation of TSP-1 expression and direct inhibition of endothelial cell proliferation in tumor tissues. With regard to the toxic effects of such therapies, the use of MTD S-1 caused body weight loss and myelosuppression, whereas S-1 metronomic chemotherapy or S-1 metronomic chemotherapy with vandetanib did not cause any severe toxicity. Metronomic chemotherapy with a single agent did not cause an antitumor effect in one study by Tang et al. [111]. However, not only S-1 metronomic chemotherapy with vandetanib but also metronomic S-1 monotherapy caused significant antitumor effects in the study by Iwamoto et al. Perhaps these differences might be due to greater antitumor effects caused by S-1 compared with UFT [116], although the different models could be another explanation.
Table 1.
Preclinical Studies Evaluating Metronomic Chemotherapy Regimens in Rodent Models of HCC.
| Animal Model | Drug Used | Reference |
| Human HCC cell line orthotopic xenografts in SCID mice | Oral UFT + cyclophosphamide plus sorafenib or DC101 | Tang et al. [111] |
| Human HCC cell line orthotopic xenografts in SCID mice | Oral UFT + sorafenib | Tang et al. [114] |
| Human HCC cell line orthotopic xenografts in nude mice | Intravenous doxorubicin plus bevacizumab | Zhou et al. [115] |
| Chemically induced HCC in rats | Oral cyclophosphamide | Park et al. [113] |
| Chemically induced HCC in rats | Cyclophosphamide | Jang et al. [117] |
| Human HCC cell line subcutaneous xenografts in nude mice | Oral S-1 + vandetanib | Iwamoto et al. [116] |
| Human HCC cell line xenografts and primary HCC cells from patients in Nonobese diabetic/SCID/interleukin-2 (IL-2) receptor γ null mice | Oral cyclophosphamide | Martin-Padura et al. [67] |
SCID indicates severe combined immunodeficiency.
S-1 is an oral 5-FU prodrug; UFT is an oral 5-FU prodrug; vandetanib is an oral tyrosine kinase inhibitor that targets VEGFRs and epidermal growth factor receptors; DC101 is an anti-mouse VEGFR-2 neutralizing monoclonal antibody.
Jang et al. [117] used a chemically induced model of HCC in rats and compared an MTD versus metronomic chemotherapy protocol using cyclophosphamide. The metronomic protocol was more effective in prolonging survival than the MTD method and also suppressed metastasis formation, not just intrahepatic tumor growth. Among the mechanisms implicated for the results included suppression of HIF-1α levels and matrix metalloproteinases (MMPs), including MMP-2 and MMP-9, and also of the MMP-2 activator, tissue inhibitor of metalloproteinase-2 (TIMP-2). In a previous study by the same group using the rat HCC model, suppression of VEGFR-2 caused by metronomic cyclophosphamide was also reported [113].
Metronomic Chemotherapy: Clinical Studies
To date, more than 50 clinical trials, mostly phase II trials, of metronomic chemotherapy have been reported in adult patients with breast cancer, lung cancer, prostate cancer, malignant brain tumor, colon cancer, multiple melanoma, malignant lymphoma, HCC, and other types of tumors [118–120]. Many of those clinical trials included both chemotherapeutic and antiangiogenic agents. About 80% of the trials have reported positive efficacy of metronomic chemotherapy. In addition to the improvement in therapeutic response rate (complete response + partial response) and/or clinical benefit (complete response + partial response + stable disease), Orlando et al. [121] showed that 27% of patients with advanced breast cancer who were already resistant to trastuzumab responded to treatment using doublet metronomic cyclophosphamide and methotrexate, in combination with trastuzumab. Furthermore, Kato et al. [122] and Watanabe et al. [123] reported that continuous daily administration of nontoxic doses of UFT was safe and effective as postoperative adjuvant treatment in randomized phase III adjuvant trials undertaken in patients with non-small cell lung cancer and breast cancer, respectively. UFT was administered daily with no breaks for 2 years and can be viewed as a metronomic chemotherapy-like trial. In contrast, a few other clinical trials of metronomic chemotherapy reported negative outcomes. In particular, malignant brain tumors seem to be resistant to metronomic chemotherapy [124–127]. With regard to adverse effects, metronomic chemotherapy was associated with minimal toxicity and severe adverse events are rare. The most common mild side effects were nausea, vomiting, fatigue, and bone marrow suppression [128,129]. In view of the encouraging preclinical and clinical findings evaluating metronomic chemotherapy or metronomic chemotherapy combined with targeted agents—especially antiangiogenic drugs—a number of randomized phase III trials have been initiated, four in breast cancer and two in colorectal cancer (www.clinicaltrials.gov) [130]. Two are adjuvant trials. The chemotherapy drugs involved include cyclophosphamide, methotrexate, and capecitabine, and the antiangiogenic drug, when used, is bevacizumab (Avastin), the monoclonal anti-VEGF antibody.
Metronomic Chemotherapy: Clinical Setting of HCC
To date, there are only four clinical trials evaluating metronomic chemotherapy for HCC (Table 2). One reported negative result, whereas others reported positive natures. Treiber et al. [131] randomly classified 38 patients with advanced HCC into the following four treatment groups: patients of group 1 received 30mg of octreotide on day 1, group 2 received octreotide on day 1 and 400 mg of imatinib daily, group 3 received oxaliplatin (60–90 mg/m2) on day 1, and group 4 received oxaliplatin (20–30 mg/m2) on days 1, 8, and 15 combined with 30 mg of octreotide on day 1 and 400 mg of imatinib daily. The time to progression and overall survival were not different among the groups in this phase I/II trial. Hsu et al. [132,133] conducted another phase II study of the combination of sorafenib (400 mg twice daily) with metronomic UFT (125 mg/m2 based on tegafur twice daily) for advanced HCC. They evaluated the efficacy and safety in 53 patients with Child-Pugh class A. The median progressionfree survival was 3.7 months, and median survival was 7.4 months. Four patients showed partial response and 26 had stable disease. Treatment was associated with some severe toxicity including fatigue (15%), abnormal liver function (13%), elevated serum lipase (10%), hand-foot skin reaction (9%), and bleeding (8%). The authors concluded that metronomic chemotherapy with UFT could be safely combined with sorafenib and that such combination could improve the efficacy of sorafenib in patients with advanced HCC when compared to previous reports in similar patient cohorts treated with sorafenib alone [28,134]. The concurrent use of metronomic chemotherapy and sorafenib might augment antitumor efficacy but without a high incidence of severe side effects. Woo et al. [135] reported the results of a phase II trial involving infusion of metronomic epirubicin with cisplatin and 5-FU and found it to be a safe and potentially useful treatment for HCC patients with portal vein thrombosis (MST; 162 days). In addition, Shao et al. [136–138] undertook a metronomic UFT plus thalidomide, sorafenib, or bevacizumab trial in patients with advanced HCC and observed it to be safe, demonstrating modest activity (MST; 4.8 months). There are also two case reports reporting encouraging results in individual HCC patients treated with metronomic capecitabine [139,140]. In addition, Allegrini et al. [141] reported that metronomic UFT and cyclophosphamide plus celecoxib in heavily pretreated gastrointestinal patients including two patients with HCC were well tolerated and associated with interesting activity. To confirm the therapeutic efficacy and safety of metronomic chemotherapy in patients with advanced HCC, more (randomized) phase II trials with other anticancer agents and molecular targeted agents, including randomized controlled trials in larger populations, will be required.
Table 2.
Clinical Studies Evaluating the Therapeutic Efficacy of Metronomic Chemotherapy in Patients with HCC.
| Drugs Used | Results/Comments | References |
| Octreotide, imatinib, oxaliplatin | Phase I/II study. Metronomic chemotherapy with oxaliplatin in combination with antiangiogenic drugs suppressed the increase of serum E-selectin, VEGF-A, PDGF-BB, and α-fetoprotein levels. | Treiber et al. [131] |
| UFT, sorafenib | Phase II study. Metronomic chemotherapy with UFT was safely combined with sorafenib and showed activity to improve the efficacy of sorafenib. | Hsu et al. [132] |
| 5-FU, sorafenib, bevacizumab, thalidomide | Phase II study. An early α-fetoprotein response was a useful surrogate marker to predict treatment efficacy and prognosis of metronomic chemotherapy with 5-FU in combination with antiangiogenic agents. | Shao et al. [136] |
| Capecitabine | Case report. Metronomic chemotherapy with capecitabine induced complete remission with minimal toxicity. | Brandi et al. [140] |
| Capecitabine | Case report. Metronomic chemotherapy with capecitabine for HCC patient with Child-Pugh class B was effective and well tolerated. | Ballardini et al. [139] |
| UFT, sorafenib | Phase II study. Vascular response measured by dynamic contrast-enhanced MRI predicted tumor response and survival by metronomic UFT therapy with sorafenib. | Hsu et al. [133] |
| UFT, sorafenib | Phase II study. High baseline circulating EPC levels were associated with poor prognosis by sorafenib and metronomic chemotherapy with UFT. | Shao et al. [138] |
| Epirubicin, cisplatin, 5-FU | Prospective study. Metronomic chemotherapy might be a safe and useful palliative treatment for HCC patients with major portal vein tumor thrombosis. | Woo et al. [135] |
| UFT, thalidomide | Phase II study. High baseline IL-6 and IL-8 levels were associated with poor prognosis. Metronomic chemotherapy with UFT and thalidomide was safe and demonstrated modest activity. | Shao et al. [137] |
MRI indicates magnetic resonance imaging.
UFT is an oral 5-FU prodrug.
Conclusions
In this review, we have attempted to outline the many reasons why we feel metronomic chemotherapy, especially when used in conjunction with an antiangiogenic drug such as sorafenib, is a potentially promising strategy to consider for the treatment of patients with advanced HCC. In summary, these reasons are given as follows:
HCCis a highly angiogenic tumor, driven by such proangiogenic growth factors such as VEGF and bFGF.
Sorafenib is already approved for treatment of patients with HCC.
Antiangiogenic drugs can augment the efficacy of metronomic chemotherapy and vice versa, as shown in a very large number of diverse preclinical studies—especially those involving treatment of mice with advanced metastatic disease—and also as suggested, or shown, in a number of phase II clinical trial results of other types of cancer.
Metronomic chemotherapy, which functions more as a biologic therapy, is now known to involve multiple mechanisms that include antiangiogenesis and antivasculogenesis, immune stimulation, and possibly some direct tumor cell targeting effects, including of the cancer stem cell subpopulation.
There is no effective standard chemotherapy for HCC when using conventionalMTDtreatment protocols, and in part, this is related to the toxicity of such treatments in patients with HCC who have the underlying comorbidity of liver cirrhosis; in contrast, the less toxic regimens associated with metronomic chemotherapy and the different cellular targets and mechanisms of action involved may make this an attractive and alternative type of chemotherapy to consider, especially for treatment of advanced HCC, but perhaps also for postsurgical adjuvant treatment of early-stage HCC, given the successes of metronomic-like protocol of UFT reported in adjuvant phase III breast and lung cancer trials.
The total number of preclinical studies showing impressive results using metronomic chemotherapy-based protocols, especially in conjunction with antiangiogenic drugs (even in models of advanced metastatic disease) along with the number of promising clinical study and trial results that have been published to date, argues strongly for giving more consideration to testing more extensively this type of treatment strategy for advanced HCC.
Some limited preliminary results of several preclinical HCC studies using metronomic chemotherapy in conjunction with antiangiogenic drugs indeed suggest that this treatment strategy can be highly active and, as such, should be given proactive clinical consideration.
Perspective for Future Directions
In unresectable advanced HCC (BCLC stage C), sorafenib is recommended as the standard treatment. As HCC is usually accompanied with liver cirrhosis, a combination treatment with less adverse events will be required to improve the survival benefit of sorafenib. Metronomic chemotherapy will be a candidate treatment that meets these criteria. To confirm the synergy of metronomic chemotherapy, prospective trials of metronomic chemotherapy with sorafenib compared with sorafenib alone as the control arm will be necessary as soon as possible.
Footnotes
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities. The authors have declared no conflicts of interest.
References
- 1.Bruix J. Liver cancer: an evolving challenge reaching research maturity. Hepatology. 2008;47:1103–1104. doi: 10.1002/hep.22259. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Nieto Y. The verdict is not in yet. Analysis of the randomized trials of high-dose chemotherapy for breast cancer. Haematologica. 2003;88:201–211. [PubMed] [Google Scholar]
- 5.Roche H, Viens P, Biron P, Lotz JP, Asselain B. High-dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control. 2003;10:42–47. doi: 10.1177/107327480301000105. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 10.Pahernik S, Harris AG, Schmitt-Sody M, Krasnici S, Goetz AE, Dellian M, Messmer K. Orthogonal polarisation spectral imaging as a new tool for the assessment of antivascular tumour treatment in vivo: a validation study. Br J Cancer. 2002;86:1622–1627. doi: 10.1038/sj.bjc.6600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judy BF, Aliperti LA, Predina JD, Levine D, Kapoor V, Thorpe PE, Albelda SM, Singhal S. Vascular endothelial-targeted therapy combined with cytotoxic chemotherapy induces inflammatory intratumoral infiltrates and inhibits tumor relapses after surgery. Neoplasia. 2012;14:352–359. doi: 10.1593/neo.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 13.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 15.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquier E, Kieran MW, Sterba J, Shaked Y, Baruchel S, Oberlin O, Kivivuori MS, Peyrl A, Diawarra M, Casanova M, et al. Moving forward with metronomic chemotherapy: meeting report of the 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology. Trans Oncol. 2011;4:203–211. doi: 10.1593/tlo.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swaminathan R, Lucas E, Sankaranarayanan R. Cancer survival in Africa, Asia, the Caribbean and Central America: database and attributes. IARC Sci Publ. 2011;162:23–31. [PubMed] [Google Scholar]
- 19.André N, Banavali S, Snihur Y, Pasquier E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013;14:e239–e248. doi: 10.1016/S1470-2045(13)70056-1. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 21.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 22.Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493–497. doi: 10.1148/radiology.178.2.1846240. [DOI] [PubMed] [Google Scholar]
- 23.Honda H, Tajima T, Kajiyama K, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Vascular changes in hepatocellular carcinoma: correlation of radiologic and pathologic findings. AJR Am J Roentgenol. 1999;173:1213–1217. doi: 10.2214/ajr.173.5.10541091. [DOI] [PubMed] [Google Scholar]
- 24.Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) 2008;291:721–734. doi: 10.1002/ar.20668. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 26.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, El-Serag HB. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182.e1–1188.e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 28.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, doubleblind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 29.Nakano M, Ando E, Kuromatsu R, Torimura T, Sumie S, Takata A, Fukushima N, Kurogi J, Niizeki T, Iwamoto H, et al. Recent progress in the management of hepatocellular carcinoma detected during a surveillance program in Japan. Hepatol Res. 2010;40:989–996. doi: 10.1111/j.1872-034X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- 30.Yu DQ, Lin SG, Chen JY, Xue L, Li G, Dong HJ, Zhou YL. Effect of atorvastatin therapy on borderline vulnerable lesions in patients with acute coronary syndrome. Arch Med Sci. 2011;7:433–439. doi: 10.5114/aoms.2011.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe RP, Lewis D, Kane PA, Portmann BC, O'Grady JG, Karani JB, Rela M, Heaton ND. Manganese-enhanced MRI predicts the histological grade of hepatocellular carcinoma in potential surgical candidates. Clin Radiol. 2011;66:237–243. doi: 10.1016/j.crad.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Lencioni R. Surveillance and early diagnosis of hepatocellular carcinoma. Dig Liver Dis. 2010;42(suppl 3):S223–S227. doi: 10.1016/S1590-8658(10)60509-9. [DOI] [PubMed] [Google Scholar]
- 33.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 34.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 35.Nagamatsu H, Hiraki M, Mizukami N, Yoshida H, Iwamoto H, Sumie S, Torimura T, Sata M. Intra-arterial therapy with cisplatin suspension in lipiodol and 5-fluorouracil for hepatocellular carcinoma with portal vein tumour thrombosis. Aliment Pharmacol Ther. 2010;32:543–550. doi: 10.1111/j.1365-2036.2010.04379.x. [DOI] [PubMed] [Google Scholar]
- 36.Nerenstone S, Friedman M. Medical treatment of hepatocellular carcinoma. Gastroenterol Clin North Am. 1987;16:603–612. [PubMed] [Google Scholar]
- 37.Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31–39. doi: 10.2174/157488708783330549. [DOI] [PubMed] [Google Scholar]
- 38.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 39.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 40.O'Neil BH, Venook AP. Hepatocellular carcinoma: the role of the North American GI Steering Committee Hepatobiliary Task Force and the advent of effective drug therapy. Oncologist. 2007;12:1425–1432. doi: 10.1634/theoncologist.12-12-1425. [DOI] [PubMed] [Google Scholar]
- 41.Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–1681. [PubMed] [Google Scholar]
- 42.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 43.Gish RG, Porta C, Lazar L, Ruff P, Feld R, Croitoru A, Feun L, Jeziorski K, Leighton J, Gallo J, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol. 2007;25:3069–3075. doi: 10.1200/JCO.2006.08.4046. [DOI] [PubMed] [Google Scholar]
- 44.Castells A, Bruix J, Brú C, Ayuso C, Roca M, Boix L, Vilana R, Rodés J. Treatment of hepatocellular carcinoma with tamoxifen: a double-blind placebo-controlled trial in 120 patients. Gastroenterology. 1995;109:917–922. doi: 10.1016/0016-5085(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 45.Manesis EK, Giannoulis G, Zoumboulis P, Vafiadou I, Hadziyannis SJ. Treatment of hepatocellular carcinoma with combined suppression and inhibition of sex hormones: a randomized, controlled trial. Hepatology. 1995;21:1535–1542. [PubMed] [Google Scholar]
- 46.Riestra S, Rodriguez M, Delgado M, Suárez A, Gonzalez N, de la Mata M, Diaz G, Miño-Fugarolas G, Rodrigo L. Tamoxifen does not improve survival of patients with advanced hepatocellular carcinoma. J Clin Gastroenterol. 1998;26:200–203. doi: 10.1097/00004836-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, Vauthey JN, Ellis LM, Schnirer II, Wolff RA, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101:578–586. doi: 10.1002/cncr.20368. [DOI] [PubMed] [Google Scholar]
- 48.Fuchs CS, Clark JW, Ryan DP, Kulke MH, Kim H, Earle CC, Vincitore M, Mayer RJ, Stuart KE. A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2002;94:3186–3191. doi: 10.1002/cncr.10607. [DOI] [PubMed] [Google Scholar]
- 49.Okada S, Okazaki N, Nose H, Shimada Y, Yoshimori M, Aoki K. A phase 2 study of cisplatin in patients with hepatocellular carcinoma. Oncology. 1993;50:22–26. doi: 10.1159/000227142. [DOI] [PubMed] [Google Scholar]
- 50.Stuart K, Tessitore J, Huberman M. 5-Fluorouracil and alpha-interferonin hepatocellular carcinoma. Am J Clin Oncol. 1996;19:136–139. doi: 10.1097/00000421-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Tetef M, Doroshow J, Akman S, Coluzzi P, Leong L, Margolin K, Morgan RJ, Jr, Raschko J, Shibata S, Somlo G, et al. 5-Fluorouracil and high-dose calcium leucovorin for hepatocellular carcinoma: a phase II trial. Cancer Invest. 1995;13:460–463. doi: 10.3109/07357909509024907. [DOI] [PubMed] [Google Scholar]
- 52.Davis RB, Van Echo DA, Leone LA, Henderson ES. Phase II trial of mitoxantrone in advanced primary liver cancer: a Cancer and Leukemia Group B Study. Cancer Treat Rep. 1986;70:1125–1126. [PubMed] [Google Scholar]
- 53.Shiu W, Mok SD, Leung N, Li M, Zacharia A, Li A, Martin C. Phase 2 study of high dose etoposide (VP16-213) in hepatocellular carcinoma. Jpn J Clin Oncol. 1987;17:113–115. [PubMed] [Google Scholar]
- 54.Yoshino M, Okazaki N, Yoshida T, Kanda Y, Miki M, Oda H, Sasagawa Y, Hayashi S, Hashimoto N. A phase II study of etoposide in patients with hepatocellular carcinoma by the Tokyo Liver Cancer Chemotherapy Study Group. Jpn J Clin Oncol. 1989;19:120–122. [PubMed] [Google Scholar]
- 55.Harvey WH, Fleming TR, Von Hoff DD, Katterhagen JG, Coltman CA., Jr Phase II study of fludarabine phosphate in previously untreated patients with colorectal carcinoma: a Southwest Oncology Group Study. Cancer Treat Rep. 1987;71:1319–1320. [PubMed] [Google Scholar]
- 56.Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004;5:409–418. doi: 10.1016/S1470-2045(04)01508-6. [DOI] [PubMed] [Google Scholar]
- 57.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 58.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 59.Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 60.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 61.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, Kerbel RS. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto K, Man S, Xu P, Cruz-Munoz W, Tang T, Kumar R, Kerbel RS. Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Mol Cancer Ther. 2010;9:996–1006. doi: 10.1158/1535-7163.MCT-09-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghiringhelli F, Manckoundia P, Pfitzenmeyer P. Acute tubulointerstitial nephritis due to Salmonella typhimurium infection. Eur J Intern Med. 2004;15:401. doi: 10.1016/j.ejim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Loeffler M, Krüger JA, Reisfeld RA. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 2005;65:5027–5030. doi: 10.1158/0008-5472.CAN-05-0646. [DOI] [PubMed] [Google Scholar]
- 66.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Padura I, Marighetti P, Agliano A, Colombo F, Larzabal L, Redrado M, Bleau AM, Prior C, Bertolini F, Calvo A. Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab Invest. 2012;92:952–966. doi: 10.1038/labinvest.2012.65. [DOI] [PubMed] [Google Scholar]
- 68.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 69.Ma J, Chen CS, Blute T, Waxman DJ. Antiangiogenesis enhances intratumoral drug retention. Cancer Res. 2011;71:2675–2685. doi: 10.1158/0008-5472.CAN-10-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vacca A, Ribatti D, Iurlaro M, Merchionne F, Nico B, Ria R, Dammacco F. Docetaxel versus paclitaxel for antiangiogenesis. J Hematother Stem Cell Res. 2002;11:103–118. doi: 10.1089/152581602753448577. [DOI] [PubMed] [Google Scholar]
- 71.Pasquier E, Honore S, Pourroy B, Jordan MA, Lehmann M, Briand C, Braguer D. Antiangiogenic concentrations of paclitaxel induce an increase in microtubule dynamics in endothelial cells but not in cancer cells. Cancer Res. 2005;65:2433–2440. doi: 10.1158/0008-5472.CAN-04-2624. [DOI] [PubMed] [Google Scholar]
- 72.Chen YM, Fan WC, Tsai CM, Liu SH, Shih JF, Chou TY, Wu CH, Chou KT, Lee YC, Perng RP, et al. A phase II randomized trial of gefitinib alone or with tegafur/uracil treatment in patients with pulmonary adenocarcinoma who had failed previous chemotherapy. J Thorac Oncol. 2011;6:1110–1116. doi: 10.1097/JTO.0b013e3182121c09. [DOI] [PubMed] [Google Scholar]
- 73.Ooyama A, Oka T, Zhao HY, Yamamoto M, Akiyama S, Fukushima M. Anti-angiogenic effect of 5-fluorouracil-based drugs against human colon cancer xenografts. Cancer Lett. 2008;267:26–36. doi: 10.1016/j.canlet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Kubisch R, Meissner L, Krebs S, Blum H, Gunther M, Roidl A, Wagner E. A comprehensive gene expression analysis of resistance formation upon metronomic cyclophosphamide therapy. Trans Oncol. 2013;6:1–9. doi: 10.1593/tlo.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emmenegger U, Francia G, Chow A, Shaked Y, Kouri A, Man S, Kerbel RS. Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia. 2011;13:40–48. doi: 10.1593/neo.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- 77.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- 78.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 79.Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, Pellegrino A, Dammacco F. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood. 1999;94:4143–4155. [PubMed] [Google Scholar]
- 80.Wang J, Lou P, Lesniewski R, Henkin J. Paclitaxel at ultra low concentrations inhibits angiogenesis without affecting cellular microtubule assembly. Anticancer Drugs. 2003;14:13–19. doi: 10.1097/00001813-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Grant DS, Williams TL, Zahaczewsky M, Dicker AP. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere) Int J Cancer. 2003;104:121–129. doi: 10.1002/ijc.10907. [DOI] [PubMed] [Google Scholar]
- 82.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 83.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bocci G, Fioravanti A, Orlandi P, Di Desidero T, Natale G, Fanelli G, Viacava P, Naccarato AG, Francia G, Danesi R. Metronomic ceramide analogs inhibit angiogenesis in pancreatic cancer through up-regulation of caveolin-1 and thrombospondin-1 and down-regulation of cyclin D1. Neoplasia. 2012;14:833–845. doi: 10.1593/neo.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, Sudhakar A, Kalluri R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–1574. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 86.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- 88.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 89.Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- 90.Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ, Parchment R, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8:1867–1877. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumorinduced mobilization of circulating angiogenic cells. Proc Natl Acad Sci USA. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 93.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 94.Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, Ghisini R, Sandri MT, Zorzino L, Nolè F, et al. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol. 2006;17:232–238. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 95.Correale P, Cerretani D, Remondo C, Martellucci I, Marsili S, La Placa M, Sciandivasci A, Paolelli L, Pascucci A, Rossi M, et al. A novel metronomic chemotherapy regimen of weekly platinum and daily oral etoposide in high-risk non-small cell lung cancer patients. Oncol Rep. 2006;16:133–140. doi: 10.3892/or.16.1.133. [DOI] [PubMed] [Google Scholar]
- 96.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 97.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, Varner J. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–662. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741–9750. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- 100.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 101.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 102.Tanaka H, Matsushima H, Mizumoto N, Takashima A. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009;69:6978–6986. doi: 10.1158/0008-5472.CAN-09-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 105.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69:6987–6994. doi: 10.1158/0008-5472.CAN-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 109.Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, Hicklin D, Kerbel RS. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res. 2002;8:221–232. [PubMed] [Google Scholar]
- 110.Chen CA, Ho CM, Chang MC, Sun WZ, Chen YL, Chiang YC, Syu MH, Hsieh CY, Cheng WF. Metronomic chemotherapy enhances antitumor effects of cancer vaccine by depleting regulatory T lymphocytes and inhibiting tumor angiogenesis. Mol Ther. 2010;18:1233–1243. doi: 10.1038/mt.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang TC, Man S, Lee CR, Xu P, Kerbel RS. Impact of metronomic UFT/cyclophosphamide chemotherapy and antiangiogenic drug assessed in a new preclinical model of locally advanced orthotopic hepatocellular carcinoma. Neoplasia. 2010;12:264–274. doi: 10.1593/neo.91872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen FZ, Wang J, Liang J, Mu K, Hou JY, Wang YT. Low-dose metronomic chemotherapy with cisplatin: can it suppress angiogenesis in H22 hepatocarcinoma cells? Int J Exp Pathol. 2010;91:10–16. doi: 10.1111/j.1365-2613.2009.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park ST, Jang JW, Kim GD, Park JA, Hur W, Woo HY, Kim JD, Kwon JH, Yoo CR, Bae SH, et al. Beneficial effect of metronomic chemotherapy on tumor suppression and survival in a rat model of hepatocellular carcinoma with liver cirrhosis. Cancer Chemother Pharmacol. 2010;65:1029–1037. doi: 10.1007/s00280-009-1108-4. [DOI] [PubMed] [Google Scholar]
- 114.Tang TC, Man S, Xu P, Francia G, Hashimoto K, Emmenegger U, Kerbel RS. Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia. 2010;12:928–940. doi: 10.1593/neo.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou F, Hu J, Shao JH, Zou SB, Shen SL, Luo ZQ. Metronomic chemotherapy in combination with antiangiogenic treatment induces mosaic vascular reduction and tumor growth inhibition in hepatocellular carcinoma xenografts. J Cancer Res Clin Oncol. 2012;138:1879–1890. doi: 10.1007/s00432-012-1270-7. [DOI] [PubMed] [Google Scholar]
- 116.Iwamoto H, Torimura T, Nakamura T, Hashimoto O, Inoue K, Kurogi J, Niizeki T, Kuwahara R, Abe M, Koga H, et al. Metronomic S-1 chemotherapy and vandetanib: an efficacious and nontoxic treatment for hepatocellular carcinoma. Neoplasia. 2011;13:187–197. doi: 10.1593/neo.101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jang JW, Park ST, Kwon JH, You CR, Choi JY, Jung CK, Bae SH, Yoon SK. Suppression of hepatic tumor growth and metastasis by metronomic therapy in a rat model of hepatocellular carcinoma. Exp Mol Med. 2011;43:305–312. doi: 10.3858/emm.2011.43.5.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 119.Romiti A, Cox MC, Sarcina I, Di Rocco R, D'Antonio C, Barucca V, Marchetti P. Metronomic chemotherapy for cancer treatment: a decade of clinical studies. Cancer Chemother Pharmacol. 2013;72:13–33. doi: 10.1007/s00280-013-2125-x. [DOI] [PubMed] [Google Scholar]
- 120.Ribatti D, Nico B, Ranieri G, Specchia G, Vacca A. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia. 2013;15:231–238. doi: 10.1593/neo.121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Orlando L, Cardillo A, Ghisini R, Rocca A, Balduzzi A, Torrisi R, Peruzzotti G, Goldhirsch A, Pietri E, Colleoni M. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 123.Watanabe T, Sano M, Takashima S, Kitaya T, Tokuda Y, Yoshimoto M, Kohno N, Nakagami K, Iwata H, Shimozuma K, et al. Oral uracil and tegafur compared with classic cyclophosphamide, methotrexate, fluorouracil as postoperative chemotherapy in patients with node-negative, high-risk breast cancer: National Surgical Adjuvant Study for Breast Cancer 01 Trial. J Clin Oncol. 2009;27:1368–1374. doi: 10.1200/JCO.2008.18.3939. [DOI] [PubMed] [Google Scholar]
- 124.Herrlinger U, Rieger J, Steinbach JP, Nägele T, Dichgans J, Weller M. UKT-04 trial of continuous metronomic low-dose chemotherapy with methotrexate and cyclophosphamide for recurrent glioblastoma. J Neurooncol. 2005;71:295–299. doi: 10.1007/s11060-004-1726-y. [DOI] [PubMed] [Google Scholar]
- 125.Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, O'Neill A, Drappatz J, Chen-Plotkin AS, Ramakrishna N, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9:354–363. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, Sathornsumetee S, McLendon RE, Herndon JE, II, Marcello JE, Norfleet J, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101:1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clarke JL, Iwamoto FM, Sul J, Panageas K, Lassman AB, DeAngelis LM, Hormigo A, Nolan CP, Gavrilovic I, Karimi S, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861–3867. doi: 10.1200/JCO.2008.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jurado JM, Sánchez A, Pajares B, Pérez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–586. doi: 10.1007/s12094-008-0254-7. [DOI] [PubMed] [Google Scholar]
- 129.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 130.Kerbel RS. Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast cancer. J Mammary Gland Biol Neoplasia. 2012;17:229–239. doi: 10.1007/s10911-012-9266-0. [DOI] [PubMed] [Google Scholar]
- 131.Treiber G, Wex T, Malfertheiner P. Impact of different anticancer regimens on biomarkers of angiogenesis in patients with advanced hepatocellular cancer. J Cancer Res Clin Oncol. 2009;135:271–281. doi: 10.1007/s00432-008-0443-x. [DOI] [PubMed] [Google Scholar]
- 132.Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, Hsu C, Cheng AL. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126–131. doi: 10.1016/j.jhep.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 133.Hsu CY, Shen YC, Yu CW, Hsu C, Hu FC, Hsu CH, Chen BB, Wei SY, Cheng AL, Shih TT. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011;55:858–865. doi: 10.1016/j.jhep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 134.Yau T, Chan P, Ng KK, Chok SH, Cheung TT, Fan ST, Poon RT. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer. 2009;115:428–436. doi: 10.1002/cncr.24029. [DOI] [PubMed] [Google Scholar]
- 135.Woo HY, Youn JM, Bae SH, Jang JW, Cha JH, Kim HL, Chun HJ, Choi BG, Choi JY, Yoon SK. Efficacy and safety of metronomic chemotherapy for patients with advanced primary hepatocellular carcinoma with major portal vein tumor thrombosis. Korean J Hepatol. 2012;18:32–40. doi: 10.3350/kjhep.2012.18.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alphafetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 137.Shao YY, Lin ZZ, Hsu C, Lee KD, Hsiao CH, Lu YS, Huang CC, Shen YC, Hsu CH, Cheng AL. Efficacy, safety, and potential biomarkers of thalidomide plus metronomic chemotherapy for advanced hepatocellular carcinoma. Oncology. 2012;82:59–66. doi: 10.1159/000336126. [DOI] [PubMed] [Google Scholar]
- 138.Shao YY, Lin ZZ, Chen TJ, Hsu C, Shen YC, Hsu CH, Cheng AL. High circulating endothelial progenitor levels associated with poor survival of advanced hepatocellular carcinoma patients receiving sorafenib combined with metronomic chemotherapy. Oncology. 2011;81:98–103. doi: 10.1159/000331684. [DOI] [PubMed] [Google Scholar]
- 139.Ballardini P, Marri I, Margutti G, Aliberti C, Benea G, Manfredini R. Long-lasting response with metronomic capecitabine in advanced hepatocellular carcinoma. Tumori. 2010;96:768–770. doi: 10.1177/030089161009600521. [DOI] [PubMed] [Google Scholar]
- 140.Brandi G, de Rosa F, Bolondi L, Agostini V, Di Girolamo S, Nobili E, Biasco G. Durable complete response of hepatocellular carcinoma after metronomic capecitabine. Tumori. 2010;96:1028–1030. [PubMed] [Google Scholar]
- 141.Allegrini G, Di Desidero T, Barletta MT, Fioravanti A, Orlandi P, Canu B, Chericoni S, Loupakis F, Di Paolo A, Masi G, et al. Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers. Angiogenesis. 2012;15:275–286. doi: 10.1007/s10456-012-9260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]