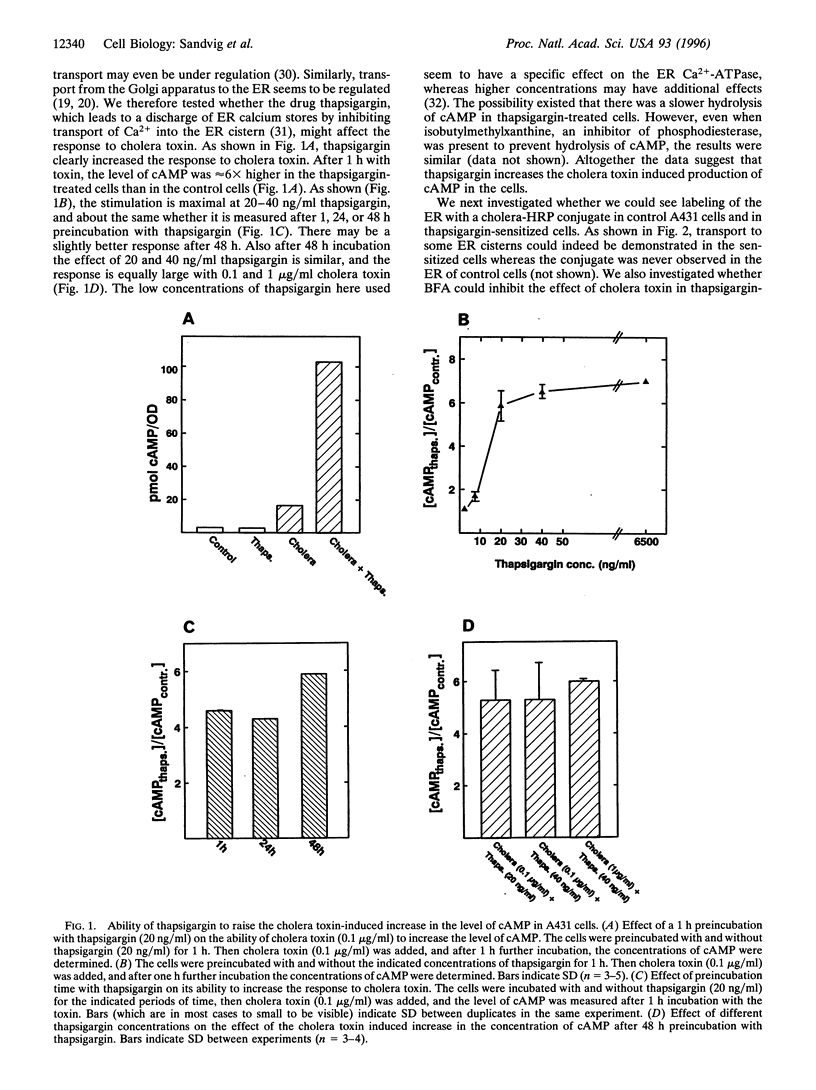
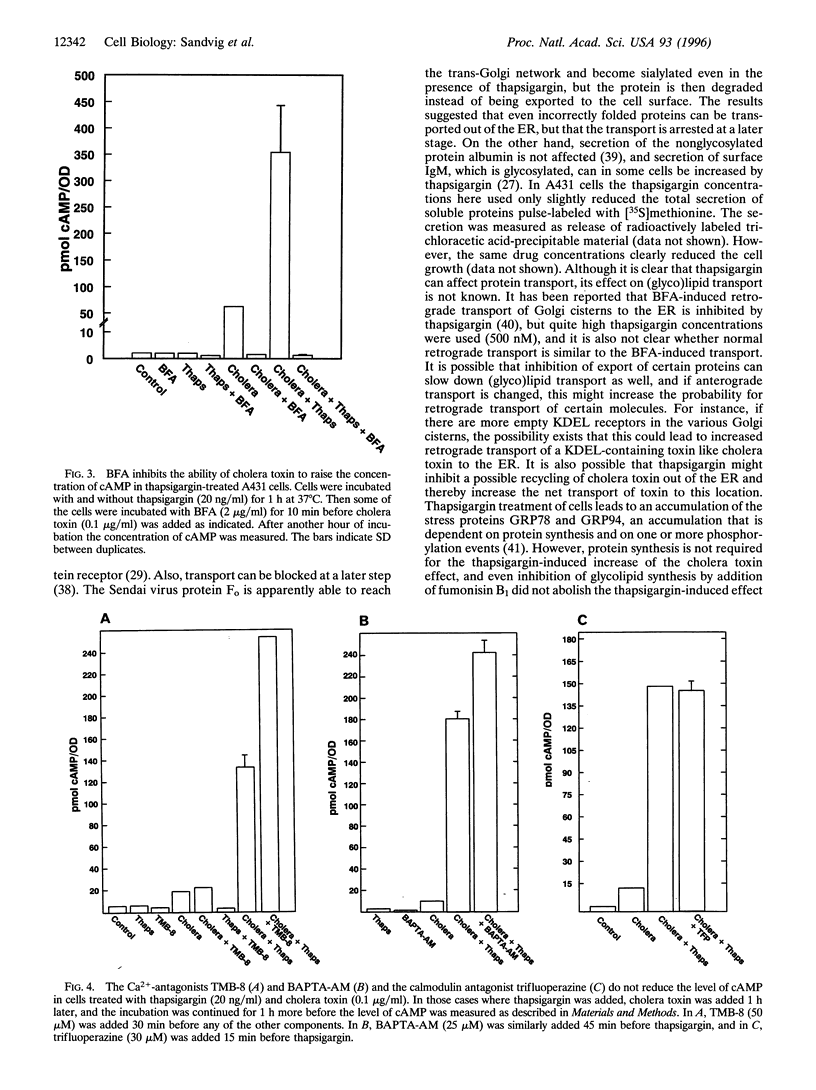
Abstract
Cholera toxin is normally observed only in the Golgi apparatus and not in the endoplasmic reticulum (ER) although the enzymatically active A subunit of cholera toxin has a KDEL sequence. Here we demonstrate transport of horseradish peroxidase-labeled cholera toxin to the ER by electron microscopy in thapsigargin-treated A431 cells. Thapsigargin treatment strongly increased cholera toxin-induced cAMP production, and the formation of the catalytically active A1 fragment was somewhat increased. Binding of cholera toxin to the cell surface and transport of toxin to the Golgi apparatus were not changed in thapsigargin-treated cells, suggesting increased retrograde transport of cholera toxin from the Golgi apparatus to the ER. The data demonstrate that retrograde transport of cholera toxin can take place and that the transport is under regulation. The results are consistent with the idea that retrograde transport can be important for the action of cholera toxin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brostrom M. A., Brostrom C. O., Huang S. C., Wolff D. J. Cholera toxin-stimulated cyclic AMP accumulation in glial tumor cells: modulation by Ca2+. Mol Pharmacol. 1981 Jul;20(1):59–67. [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplak W., Jr, Messer R. J., Konkel M. E., Grant C. C. Role of a potential endoplasmic reticulum retention sequence (RDEL) and the Golgi complex in the cytotonic activity of Escherichia coli heat-labile enterotoxin. Mol Microbiol. 1995 May;16(4):789–800. doi: 10.1111/j.1365-2958.1995.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Cusolito S., Sharp G. W. Effects of calcium antagonist TMB-8 on active Na and Cl transport in rabbit ileum. Am J Physiol. 1986 May;250(5 Pt 1):G691–G697. doi: 10.1152/ajpgi.1986.250.5.G691. [DOI] [PubMed] [Google Scholar]
- Fabbri M., Bannykh S., Balch W. E. Export of protein from the endoplasmic reticulum is regulated by a diacylglycerol/phorbol ester binding protein. J Biol Chem. 1994 Oct 28;269(43):26848–26857. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Garred O., Dubinina E., Holm P. K., Olsnes S., van Deurs B., Kozlov J. V., Sandvig K. Role of processing and intracellular transport for optimal toxicity of Shiga toxin and toxin mutants. Exp Cell Res. 1995 May;218(1):39–49. doi: 10.1006/excr.1995.1128. [DOI] [PubMed] [Google Scholar]
- Geiszt M., Káldi K., Szeberényi J. B., Ligeti E. Thapsigargin inhibits Ca2+ entry into human neutrophil granulocytes. Biochem J. 1995 Jan 15;305(Pt 2):525–528. doi: 10.1042/bj3050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Ericsson M., Krijnse-Locker J., Nilsson T., Goud B., Söling H. D., Tang B. L., Wong S. H., Hong W. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994 Dec;127(6 Pt 1):1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa N. E., De Lemos-Chiarandini C., Gravotta D., Sabatini D. D., Kreibich G. The Brefeldin A-induced retrograde transport from the Golgi apparatus to the endoplasmic reticulum depends on calcium sequestered to intracellular stores. J Biol Chem. 1995 Oct 27;270(43):25960–25967. doi: 10.1074/jbc.270.43.25960. [DOI] [PubMed] [Google Scholar]
- Janicot M., Fouque F., Desbuquois B. Activation of rat liver adenylate cyclase by cholera toxin requires toxin internalization and processing in endosomes. J Biol Chem. 1991 Jul 15;266(20):12858–12865. [PubMed] [Google Scholar]
- Joseph K. C., Kim S. U., Stieber A., Gonatas N. K. Endocytosis of cholera toxin into neuronal GERL. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2815–2819. doi: 10.1073/pnas.75.6.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khine A. A., Lingwood C. A. Capping and receptor-mediated endocytosis of cell-bound verotoxin (Shiga-like toxin). 1: Chemical identification of an amino acid in the B subunit necessary for efficient receptor glycolipid binding and cellular internalization. J Cell Physiol. 1994 Nov;161(2):319–332. doi: 10.1002/jcp.1041610217. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Arvan P. Calnexin and BiP act as sequential molecular chaperones during thyroglobulin folding in the endoplasmic reticulum. J Cell Biol. 1995 Jan;128(1-2):29–38. doi: 10.1083/jcb.128.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb F. I., Roberts L. M., Lord J. M. Nucleotide sequence of cloned cDNA coding for preproricin. Eur J Biochem. 1985 Apr 15;148(2):265–270. doi: 10.1111/j.1432-1033.1985.tb08834.x. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Constable C., Moe S., Jobling M. G., Webb H. M., Ruston S., Madara J. L., Hirst T. R., Holmes R. K. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995 Nov;131(4):951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W. I., de Almeida J. B., Moe S., Stow J. L., Ausiello D. A., Madara J. L. Entry of cholera toxin into polarized human intestinal epithelial cells. Identification of an early brefeldin A sensitive event required for A1-peptide generation. J Clin Invest. 1993 Dec;92(6):2941–2951. doi: 10.1172/JCI116917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990 Jul 5;265(19):10893–10899. [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):12753–12760. [PubMed] [Google Scholar]
- Majoul I. V., Bastiaens P. I., Söling H. D. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J Cell Biol. 1996 May;133(4):777–789. doi: 10.1083/jcb.133.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G., Rothman J. E. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995 Apr;129(2):309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of cholera toxin and Escherichia coli heat-labile enterotoxins by ADP-ribosylation factors, a family of 20 kDa guanine nucleotide-binding proteins. Mol Microbiol. 1991 Nov;5(11):2621–2627. doi: 10.1111/j.1365-2958.1991.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nambiar M. P., Oda T., Chen C., Kuwazuru Y., Wu H. C. Involvement of the Golgi region in the intracellular trafficking of cholera toxin. J Cell Physiol. 1993 Feb;154(2):222–228. doi: 10.1002/jcp.1041540203. [DOI] [PubMed] [Google Scholar]
- Ono A., Kawakita M. Transport of envelope proteins of Sendai virus, HN and F0, is blocked at different steps by thapsigargin and other perturbants to intracellular Ca2+. J Biochem. 1994 Sep;116(3):649–656. doi: 10.1093/oxfordjournals.jbchem.a124575. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Curran P. K., Fishman P. H. Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action. J Biol Chem. 1993 Jun 5;268(16):12010–12016. [PubMed] [Google Scholar]
- Pelham H. R., Roberts L. M., Lord J. M. Toxin entry: how reversible is the secretory pathway? Trends Cell Biol. 1992 Jul;2(7):183–185. doi: 10.1016/0962-8924(92)90230-k. [DOI] [PubMed] [Google Scholar]
- Price B. D., Mannheim-Rodman L. A., Calderwood S. K. Brefeldin A, thapsigargin, and AIF4- stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J Cell Physiol. 1992 Sep;152(3):545–552. doi: 10.1002/jcp.1041520314. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Garred O., Prydz K., Kozlov J. V., Hansen S. H., van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992 Aug 6;358(6386):510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Ryd M., Garred O., Schweda E., Holm P. K., van Deurs B. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J Cell Biol. 1994 Jul;126(1):53–64. doi: 10.1083/jcb.126.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachar I., Rabinovich E., Kerem A., Bar-Nun S. Thiol-reducing agents and calcium perturbants alter intracellular sorting of immunoglobulin M. J Biol Chem. 1994 Nov 4;269(44):27344–27350. [PubMed] [Google Scholar]
- Simpson J. C., Dascher C., Roberts L. M., Lord J. M., Balch W. E. Ricin cytotoxicity is sensitive to recycling between the endoplasmic reticulum and the Golgi complex. J Biol Chem. 1995 Aug 25;270(34):20078–20083. doi: 10.1074/jbc.270.34.20078. [DOI] [PubMed] [Google Scholar]
- Spangler B. D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992 Dec;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuer C. P., Buchner J., FitzGerald D., Pastan I. The N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertion. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7774–7778. doi: 10.1073/pnas.90.16.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales R., Chaddock J. A., Roberts L. M., Lord J. M. Addition of an ER retention signal to the ricin A chain increases the cytotoxicity of the holotoxin. Exp Cell Res. 1992 Nov;203(1):1–4. doi: 10.1016/0014-4827(92)90032-4. [DOI] [PubMed] [Google Scholar]
- Wong W. L., Brostrom M. A., Kuznetsov G., Gmitter-Yellen D., Brostrom C. O. Inhibition of protein synthesis and early protein processing by thapsigargin in cultured cells. Biochem J. 1993 Jan 1;289(Pt 1):71–79. doi: 10.1042/bj2890071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Ueda T., Takauji R., Liu Y. P., Fukushima T., Inuzuka M., Nakamura T. Role of calcium ion in induction of apoptosis by etoposide in human leukemia HL-60 cells. Biochem Biophys Res Commun. 1993 Oct 29;196(2):927–934. doi: 10.1006/bbrc.1993.2338. [DOI] [PubMed] [Google Scholar]
- Zhang R. G., Scott D. L., Westbrook M. L., Nance S., Spangler B. D., Shipley G. G., Westbrook E. M. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995 Aug 25;251(4):563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Holm P. K., Kayser L., Sandvig K., Hansen S. H. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993 Aug;61(2):208–224. [PubMed] [Google Scholar]
- van Deurs B., Tønnessen T. I., Petersen O. W., Sandvig K., Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986 Jan;102(1):37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]