Abstract
Intestinal commensal bacteria have recently been shown to trigger macrophages to produce diffusible clastogens (or chromosome-breaking factors) through a bystander effect (BSE) that mediates DNA damage and induces chromosomal instability in neighboring cells. Colon macrophages appear central to colon carcinogenesis and BSE through the expression of tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-2). The former induces netrin-1, a regulator of intestinal epithelial cell apoptosis, and the latter generates trans-4-hydroxy-2-nonenal (4-HNE), an endogenous mutagen. To test whether colon macrophages are key effectors for BSE, we depleted these cells in interleukin-10 knockout mice colonized with Enterococcus faecalis using encapsulated liposomal clodronate (ELC), a bisphosphonate that causes macrophage apoptosis. We observed that E. faecalis polarizes colon macrophages to an M1 phenotype. In addition, depleting these cells suppressed COX-2 and TNF-α, blocked the formation of 4-HNE protein adducts, and inhibited up-regulation of netrin-1—all markers for BSE. Finally, treatment with ELC prevented colitis, β-catenin activation, and cancer formation. These results show that selected human commensals can polarize colon macrophages to the M1 phenotype and, when activated, serve as the key effector for bacterial-induced BSE. Our findings suggest that depleting M1-polarized macro-phages is a mechanism for the chemopreventive activity of bisphosphonates and that it represents a new strategy for preventing colon cancer induced by intestinal commensals.
Introduction
Inflammatory tissue microenvironments and immune cells controlling inflammatory responses are widely appreciated as key drivers for colon carcinogenesis [1,2]. These cells include macrophages, mast cells, dendritic cells, T cells, and natural killer cells among others [3–10]. The pro-inflammatory and anti-inflammatory properties of macrophages in particular have been characterized with evidence that they can enhance invasion, suppress antitumor immunity, and promote carcinogenesis through vascular endothelial, platelet-derived, and fibroblast growth factors, and transforming growth factor-β [1,2]. Finally, Wnt/β-catenin signaling in epithelial cells—a hallmark of colon carcinogenesis—is also important to the regulation of inflammatory responses by macrophages [11,12].
Tissue macrophages are polarized by local environmental cues into distinct M1 or M2 phenotypes [13]. Classically, the M1 state is induced by bacterial antigens, interferon-γ, and tumor necrosis factor-α (TNF-α) and renders macrophages microbicidal and tumoricidal. M1-polarized cells release nitric oxide and inflammatory cytokines that promote differentiation of Th1 and Th17 T helper cells. In contrast, M2-polarized (or regulatory) macrophages are generated by anti-inflammatory factors such as interleukin-10 (IL-10), IL-13, IL-4, IL-1ra, and transforming growth factor-β. These cells are considered alternatively activated and metabolize arginine to polyamines, release anti-inflammatory cytokines that contribute to immune tolerance, angiogenesis, and tissue remodeling, and, in association with solid tumors, promote malignancy.
In the colon, intestinal macrophages are most commonly considered innate immune cells that sample the commensal microbiota to assure tolerance and prevent infection by exogenous pathogens [14]. Interactions between macrophages and the intestinal microbiota, however, have become ever more important to understand because commensal bacteria are increasingly recognized as important cofactors in colon carcinogenesis [15]. We recently reported that intestinal commensal bacteria can trigger macrophages to produce diffusible clastogens (or chromosome-breaking factors) through a bystander effect (BSE) that mediates DNA damage and induces chromosomal instability in neighboring cells [16,17]. Cyclooxygenase-2 (COX-2), a target for drugs that can prevent colon cancer [18,19], is closely linked to BSE [16,20]. Although downstream products from COX-2 such as prostaglandin E2 have pro-proliferative and anti-apoptotic properties that contribute to tumor growth [21], this enzyme also generates diffusible mediators for BSE [22,23]. One such product, trans-4-hydroxy-2-nonenal (4-HNE), is a mutagen that can modify proteins, phospholipids, and DNA to form damaging adducts [17,22,24,25]. In addition, 4-HNE can induce COX-2 to maintain enzymatic production through positive feedback [26]. Of note, macrophages in pre-neoplastic colon adenomas commonly express COX-2 [27]. These same cells express inducible nitric oxide synthase (iNOS), which implies an M1 phenotype [28].
TNF-α is expressed by colon macrophages and represents another key mediator for BSE. This inflammatory cytokine has been associated with chromosomal instability and the up-regulation of netrin-1, an anti-apoptotic ligand [29,30]. Netrin-1 is secreted by intestinal epithelial cells and binds dependence receptors (e.g., Deleted in Colorectal Cancer and UNC5 homologs) that serve to regulate epithelial cell apoptosis [31]. When overexpressed, or when cognate receptors are lost, the ligand promotes colon cancer [32,33]. In the IL-10 knockout model of colitis and cancer [34], the human intestinal commensal Enterococcus faecalis activates colon macrophages to express COX-2 and TNF-α that mediate BSE and promote carcinogenesis [16,17,24,30]. We hypothesized that if colon macrophages were solely responsible for BSE, then selective depletion of these cells should eliminate biologic markers for BSE and thereby protect against cancer.
To test this hypothesis, we colonized IL-10 knockout mice with E. faecalis and depleted colon macrophages using encapsulated liposomal clodronate (ELC). Clodronate is a non-nitrogenous bisphosphonate that selectively targets macrophages and has been used to prevent colitis and cancer in murine models [35,36]. ELC, when administered per rectum, is phagocytosed by macrophages with clodronate released into the cytosol where it is enzymatically converted to a nonhydrolyzable ATP analog—adenosine 5′-(β,γ-dichloromethylene) triphosphate—that enters mitochondria to depolarize inner membranes and induce apoptosis [37]. Of interest, multiple large cohort and case-control studies show that bisphosphonate use in humans is associated with a significant reduction in the risk for colon cancer [38,39].
To further establish the role of colon macrophages in E. faecalis-induced BSE and carcinogenesis, we found that these cells were polarized in Il10-/- mice to an M1 phenotype. Depleting colon macrophages using ELC suppressed TNF-α and COX-2, blocked the formation of 4-HNE protein adducts, and inhibited up-regulation of netrin-1—all markers for BSE—and prevented cancer formation. These findings show that selected human commensals can chronically polarize colon macrophages to an M1 state where the phenotype generates bacterial-induced BSE. Our findings suggest that depleting M1-polarized macrophages is a mechanism for the chemopreventive activity of bisphosphonates and that it represents a new strategy for preventing colon cancer induced by intestinal commensals.
Materials and Methods
Bacteria and Mice
E. faecalis strain OG1RFSS is a spontaneously derived isolate of OG1RF [40], a human oral commensal [41]. This strain is resistant to streptomycin and spectinomycin and was grown as previously described [16]. Conventionally housed and specific pathogen-free C57BL/6 Il10+/+ (i.e., wild-type) and Il10-/- mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed at Oklahoma City Veterans Affairs Medical Center Animal Research Facility. Protocols were approved by the University of Oklahoma Health Sciences Center and Oklahoma City Department of Veterans Affairs animal studies committees.
ELC and Control Liposomes
ELC and sham liposomes (SL) were purchased from Encapsula NanoSciences (Nashville, TN). To limit the systemic effects of clodronate on the immune system during a long-term experiment, ELC was administered topically to the colonic mucosa through rectal enema. To determine the optimal dose of ELC for depleting colon macrophages, 0, 50, 100, and 200 µl were administered weekly per rectum to Il10-/- mice (three mice per dose) using inhaled isoflurane for sedation. All doses were well tolerated. After 6 weeks, colons were removed at necropsy, fixed, and immunohistochemically stained for macrophages using anti-F4/80 antibody (see below). Both 100- and 200-µl doses depleted colon macrophages by >90% (data not shown). As a consequence, 100 µl was selected as the dose for enemas in subsequent experiments. Enemas using ELC or SL were begun 2 weeks before colonization with OG1RFSS, or phosphate-buffered saline (PBS) sham, and continued weekly thereafter for 9 months at which time necropsies were performed.
The experiment was designed considering genotype, colonization, and enemas as independent variables. We sought the minimal number of control groups that would allow monitoring of the effect of genotype (i.e., wild type vs IL-10 knockout), colonization (E. faecalis-colonized vs sham-colonized), and enema (ELC vs SL) on induction of colitis and cancer. Mice were divided into four groups: 1) OG1RFSS-colonized Il10+/+ mice that were administered ELC (n = 9); 2) sham-colonized Il10-/- mice that were administered ELC (n = 9); 3) OG1RFSS-colonized Il10-/- mice that were administered SL (n = 12); 4) OG1RFSS-colonized Il10-/- mice that were administered ELC (n = 12).
Colonization with E. faecalis or Sham
Colonization with E. faecalis or PBS sham was performed as previously described [30]. In a single long-term experiment, 10-week-old male mice were orogastrically administered with 1 x 109 colony forming units of E. faecalis strain OG1RFSS or PBS as control. ForOG1RFSS-colonized mice, high-density fecal colonization at an average of 109 colony forming units per gram developed within 1 week. Colonization was maintained by providing water containing streptomycin (500 mg/l) and spectinomycin (500 mg/l) throughout the 9-month experiment. OG1RFSS was never recovered from fecal pellets of mice given PBS sham.
Immunohistochemistry
At necropsy, colon biopsies were fixed with 10% PBS-buffered formalin, stored in 70% ethanol, and embedded in paraffin. Biopsies were obtained using a x10 magnifying glass to identify areas of potential pathology in the rectum and distal, middle, and proximal colons. Four samples were obtained from each mouse with each sample from approximately the same area of the colon. Cross sections were stained with hematoxylin and eosin or mounted unstained for immunohistochemical study. Immunohistochemical staining was performed as previously described [17,24]. Epitope retrieval was done by incubating sections in 10 mM sodium citrate buffer (pH 6.0) for 20 minutes at 95°C. Primary antibodies for murine macrophages (F4/80), TNF-α, and mouse dendritic cell marker (33D1) antibodies were purchased from eBioscience (San Diego, CA). COX-2 and netrin-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Other reagents included antibodies to mast cell tryptase (Thermo Scientific, Rockford, IL), 4-HNE protein adducts (Alpha Diagnostic International, San Antonio, TX), and active β-catenin (Millipore, Temecula, CA). HRP goat anti-rabbit IgG conjugate (Invitrogen, Camarillo, CA), HRP rabbit anti-goat IgG conjugate (Santa Cruz Biotechnology), and HRP goat anti-rat IgG conjugate (Millipore) were used as secondary antibodies. All antibodies were diluted as per the manufacturers' recommendations. Chromogenic development was performed using DAB-enhanced liquid substrate with nuclei counterstained by Mayer's hematoxylin solution (Sigma, St Louis, MO). The number of macrophages (F4/80), dendritic cells (33D1), and mast cells (mast cell tryptase) was determined from photomicrographs of five randomly selected x20 fields for each colon biopsy, and average numbers were calculated.
Scoring Colon Inflammation
Colon sections were scored from 0 to 4 based on the number of inflammatory cells, decrease in goblet cells, mucosal thickening, submucosal infiltration, ulceration, reactive atypia, and dysplasia as previously described [24]. Inflammation scores were the summation of all scores with the presence of cancer noted separately.
Statistics
The mean ± SD was calculated for all parameters determined. Statistical significance for inflammatory scores for each group and numbers of positive staining cells per x20 field were evaluated using analysis of variance. Comparisons among groups were performed using one-way analysis of variance with nonparametric pairwise comparisons calculated using the Wilcoxon method (JMP version 9.0.0; SAS Institute, Cary, NC). Comparisons between groups having colon cancer were performed using a two-sided Fisher exact test. P values < .05 were considered statistically significant.
Results
Depleting Macrophages Prevents E. faecalis-Induced Inflammation and Colon Cancer in Il10-/- Mice
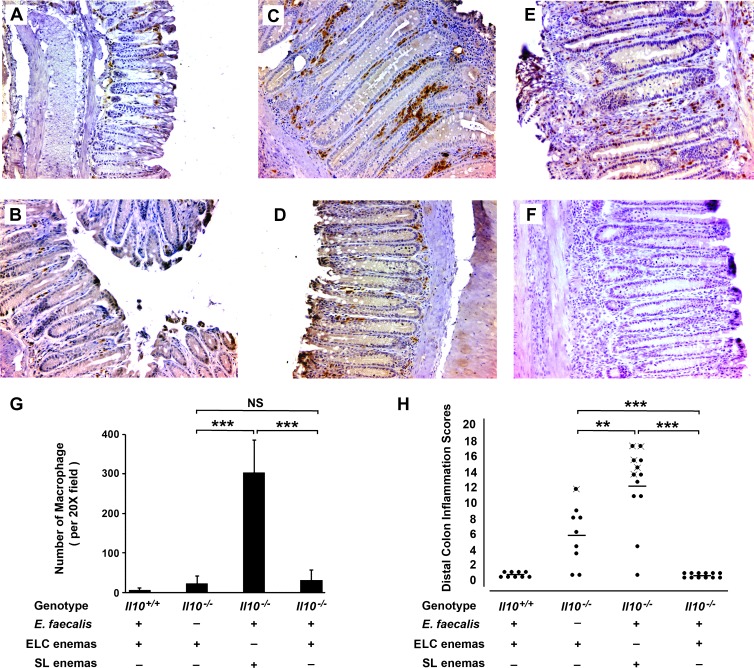
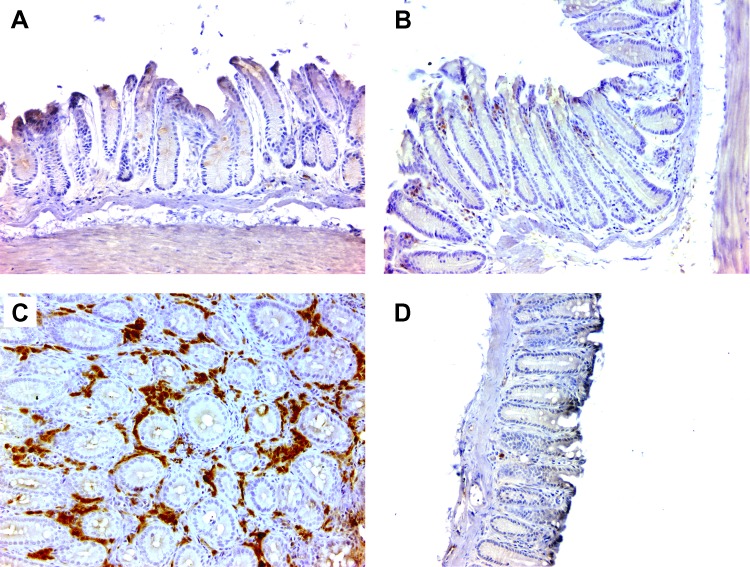
Because the activation of macrophages by E. faecalis initiates inflammation and colon carcinogenesis through BSE [16,23,24,30], we sought to directly test the role of these cells in carcinogenesis in vivo. Il10-/- mice were administered ELC or SL weekly per rectum. After 9 months, immunohistochemical staining showed an approximately 15-fold decrease in colon macrophages by ELC compared to SL-treated mice (Figure 1, A–D and G). Macrophages in the lamina propria from Il10-/- mice that were colonized with E. faecalis expressed iNOS (Figures 1E and W1) but not arginase (Figures 1F and W2), suggesting an M1 phenotype.
Figure 1.
Depleting colon macrophages with ELC suppresses inflammation and prevents cancer in Il10-/- mice colonized with E. faecalis. (A) Immunohistochemical staining of colon biopsies using anti-F4/80 antibody (brown) shows few cells in the lamina propria of wild-type mice (i.e., Il10+/+) colonized with E. faecalis and administered ELC (n = 9). (B) Staining of sham-colonized Il10-/- mice administered ELC also show few macrophages (n = 9). (C) Numerous macrophages are present in colon biopsies from E. faecalis-colonized Il10-/- mice administered SL (n = 12). (D) Macrophage staining is infrequent in E. faecalis-colonized Il10-/- mice administered ELC (n = 12). (E) Macro-phages in the lamina propria of colon biopsies from E. faecalis-colonized Il10-/- mice administered SL are positive for iNOS, suggesting that E. faecalis polarized these cells to an M1 phenotype. (F) Staining of biopsies in E for arginase is negative and consistent with the M1 polarization of macrophages. (G) Colon macrophages for mice administered ELC are significantly lower than for mice given SL (***P < .001). (H) Inflammatory scores are significantly lower for colon biopsies from E. faecalis-colonized and sham-colonized Il10-/- mice administered ELC compared to SL-treated mice (***P < .001 and **P < .01, respectively); among ELC-treated mice, inflammation scores are higher for sham-colonized Il10-/- mice compared to E. faecalis-colonized mice (***P < .001); more colon cancers are seen in SL-treated and E. faecalis-colonized mice than similarly colonized and ELC-treated mice (mice with cancer indicated by crossed closed circles; P <.05). All photomicrographs are x20.
Colons from E. faecalis-colonized Il10-/- mice administered SL showed severe inflammation compared to E. faecalis-colonized and ELC-treated mice (11.5 ± 6.3 vs 0.0 ± 0.0; P = .0001; Figure 1, C, D, and H). In the SL-treated group, colon cancer was observed in 5 of 12 (42%) biopsies. This frequency was similar to previous observations using this model [24,30]. As expected, E. faecalis-colonized Il10+/+ mice treated with ELC showed few macrophages and had no inflammation or pathology (Figure 1, A, E, and H). Surprisingly, Il10-/- mice that were sham-colonized and administered ELC showed significantly higher inflammation scores than E. faecalis-colonized Il10-/- mice similarly treated with ELC (5.6 ± 4.1 vs 0.0 ± 0.0; P = .0004; Figure 1, H), with one mouse having a high inflammation score developing cancer. This observation suggested the presence of an uncharacterized antibiotic-resistant intestinal commensal that could promote bacterial-induced BSE but was suppressed or excluded by E. faecalis. Characterization of the intestinal microbiota in this control group, however, was not further investigated.
Effect of ELC on Dendritic and Mast Cells
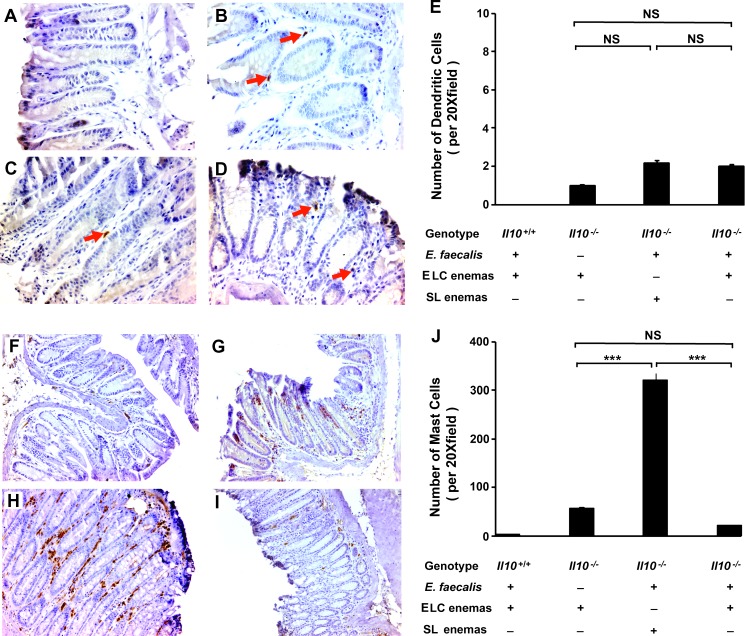
Dendritic and mast cells contribute to inflammation, adenoma formation, and carcinogenesis in colon cancer [4,7,42–44]. To determine whether ELC altered these immune cells in colon biopsies, we stained for them by immunohistochemistry. No dendritic cells were observed in E. faecalis-colonized wild-type mice (Figure 2A) and only one to two dendritic cells per x20 field across all Il10-/- groups (Figure 2, B–E). The lack of differences in numbers of dendritic cells did not appear to support a role for these cells in promoting colon cancer in this model.
Figure 2.
Effect of ELC treatment on colonic dendritic cells and mast cells. (A) Immunohistochemical staining of colon biopsies for mouse dendritic cell marker (33D1) is negative for wild-type mice colonized with E. faecalis and administered ELC (n = 9). (B–D) Few 33D1-positive cells are seen in colon biopsies for Il10-/- mice (red arrows). (E) Average number of 33D1-positive cells per x20 field across Il10-/- groups (NS, not significant). (F) Immunohistochemical staining of colon biopsies for mast cell tryptase shows few cells from biopsies of wild-type mice colonized with E. faecalis and administered ELC. (G) Increased mast cell staining is seen in biopsies for sham-colonized Il10-/- mice administered ELC (n = 9). (H) Larger increase in mast cell staining is noted in biopsies for E. faecalis-colonized Il10-/- mice administered SL (n = 12). (I) Depleting macrophages with ELC decreases mast cell staining in biopsies for E. faecalis-colonized Il10-/- mice (n = 12). (J) Average number of mast cells per x20 field across groups (NS, not significant; ***P < .001). All photomicrographs are x20.
We next stained for mast cells and, similar to dendritic cells, observed few cells in Il10+/+ mice administered ELC (Figure 2, F and J). However, significantly increased numbers of mast cells were noted among Il10-/- mice with the greatest increase observed in E. faecalis-colonized mice administered SL (Figure 2, G–J). Treatment of E. faecalis- and sham-colonized Il10-/- mice with ELC reduced the number of mast cells by 17- and 6.5-fold, respectively, compared to SL-treated mice (P < .001; Figure 2J). As suggested by others [4,45], these findings suggest that macrophages help attract mast cells in this model.
COX-2 and 4-HNE Protein Adducts Are Decreased after Macrophage Depletion
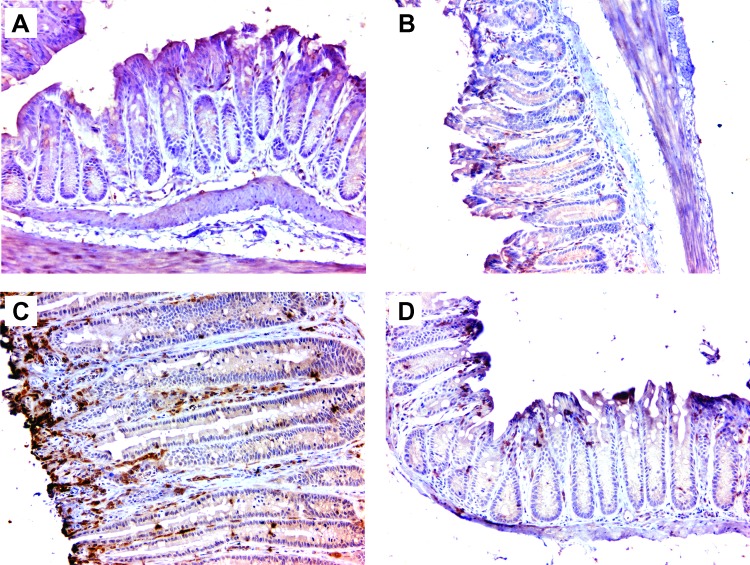
We have previously shown that E. faecalis-infected macrophages produce 4-HNE, a mutagenic breakdown product of arachidonic acid that causes DNA damage and chromosomal instability in a COX-2-dependent manner [23,24]. To confirm that macrophages produce this mutagen in vivo as a mediator for BSE, we stained colon biopsies for COX-2 and 4-HNE protein adducts. Few macrophages were positive for COX-2 in colon biopsies from Il10+/+ mice treated with ELC (Figure 3A). COX-2-positive cells were abundant in colon biopsies from sham-colonized Il10-/- mice administered ELC (Figure 3B). Finally, COX-2-positive cells were significantly increased in colon biopsies from E. faecalis-colonized Il10-/- mice administered SL compared to mice colonized with E. faecalis and treated with ELC (Figure 3, C and D).
Figure 3.
Depleting colon macrophages is associated with reduced COX-2 expression. (A) Immunohistochemical staining shows no COX-2 in colon biopsies in wild-type mice colonized with E. faecalis and administered ELC. (B) COX-2 expression is noted for sham-colonized Il10-/- mice administered ELC. (C) Increased COX-2 expression is seen for Il10-/- mice colonized with E. faecalis and administered SL. (D) Depleting macrophages with ELC significantly decreases COX-2 expression for E. faecalis-colonized Il10-/- mice. All photomicrographs are x20.
Similarly, staining was sparse for 4-HNE protein adducts in colon biopsies for wild-type mice colonized with E. faecalis and treated with ELC (Figure 4A). Slight staining for 4-HNE protein adducts was evident in colon biopsies from Il10-/- mice treated with ELC (Figure 4B). However, colon crypts and macrophages stained strongly for 4-HNE protein adducts in biopsies from E. faecalis-colonized Il10-/- mice administered SL (Figure 4C). In comparison, staining for 4-HNE protein adducts in colon biopsies from E. faecalis-colonized Il10-/- mice treated with ELC was negative (Figure 4D), implying that depleting macrophages negated COX-2 expression and resulted in a loss of 4-HNE as a mediator for BSE.
Figure 4.
Depleting colon macrophages decreases 4-HNE protein adducts. (A) Immunohistochemical staining for 4-HNE protein adducts was negative in colon biopsies from wild-type mice colonized with E. faecalis and administered ELC. (B) Slight staining is noted for 4-HNE protein adducts in the lamina propria and epithelium for sham-colonized Il10-/- mice administered ELC. (C) Colon crypts (red arrow) and lamina propria (green arrow) stain strongly for 4-HNE protein adducts for Il10-/- mice colonized with E. faecalis and administered SL. (D) Staining for 4-HNE protein adducts is reduced for E. faecalis-colonized Il10-/- mice administered ELC. All photomicrographs are x20.
TNF-α and Netrin-1 Are Decreased after Macrophage Depletion
In addition to generating 4-HNE in the Il10-/- model, E. faecalis-infected macrophages also express TNF-α that serves to upregulate netrin-1 expression in colon crypts [30]. This extracellular ligand acts to delay apoptosis as epithelial cells migrate up the crypt. To determine whether macrophage depletion also resulted in the loss of TNF-α-induced netrin-1 expression, we stained colon biopsies for TNF-α and netrin-1. Again, few cells were positive for TNF-α in wild-type mice colonized with E. faecalis and treated with ELC or in sham-colonized Il10-/- mice administered ELC (Figure 5, A and B). In contrast, macrophages in E. faecalis-colonized Il10-/- mice administered SL stained strongly for TNF-α (Figure 5C). Finally, no TNF-α-positive cells were seen in E. faecalis-colonized Il10-/- mice administered ELC (Figure 5D).
Figure 5.
Depleting colon macrophages inhibits TNF-α production. (A) Immunohistochemical staining for TNF-α-positive cells is not evident in colon biopsies from wild-type mice colonized with E. faecalis and administered ELC. (B) A few TNF-α-positive cells are noted in the lamina propria of biopsies from sham-colonized Il10-/- mice administered ELC. (C) In contrast, numerous cells stain strongly for TNF-α for E. faecalis-colonized Il10-/- mice administered SL. (D) Few TNF-α-positive cells are evident in the lamina propria of colons from E. faecalis-colonized Il10-/- mice administered ELC. All photomicrographs are x20.
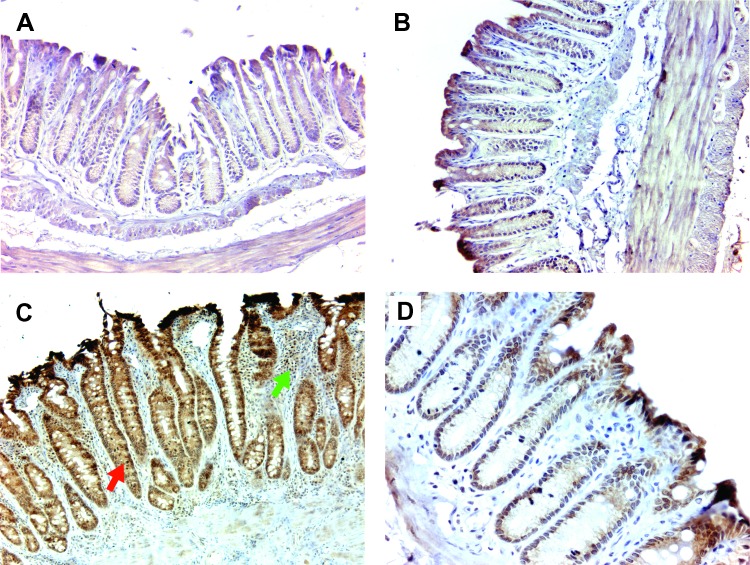
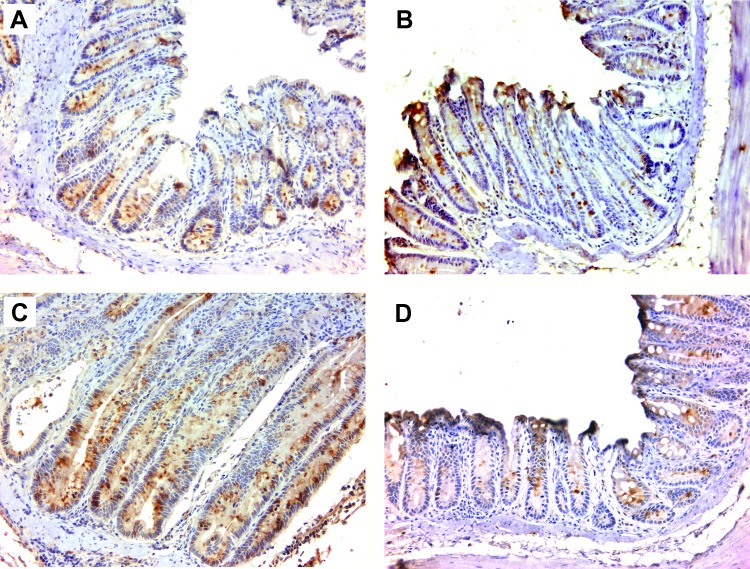
We next investigated the effect of macrophage depletion on netrin-1 expression since TNF-α promotes crypt hyperplasia by inhibiting epithelial cell apoptosis through netrin-1. Consistent with previous findings in this model [30], netrin-1 expression was observed in the colon crypts of wild-type mice colonized with E. faecalis and administered ELC (Figure 6A) and in sham-colonized Il10-/- mice treated with ELC (Figure 6B). For E. faecalis-colonized Il10-/- mice administered SL that developed severe inflammation and elongated crypts, netrin-1 expression was markedly increased (Figure 6C). Depleting macrophages using ELC decreased netrin-1 production in Il10-/- mice colonized with E. faecalis to levels that were similar to wild-type mice or sham-colonized mice (Figure 6D). These observations show that by depleting colon macrophages netrin-1 expression is left unchanged.
Figure 6.
Depleting colon macrophages blocks TNF-α-induced netrin-1 expression. (A) Typical expression of netrin-1 (brown) in epithelial cells is observed at the bottom of colon crypts for wild-type mice colonized with E. faecalis and administered ELC. (B) Similar staining is noted for netrin-1 staining for sham-colonized Il10-/- mice administered ELC. (C) Colonic epithelial cells stain more strongly for netrin-1 in biopsies from E. faecalis-colonized Il10-/- mice administered SL. (D) ELC decreases netrin-1 staining in biopsies from E. faecalis-colonized Il10-/- mice to levels similar to that found in wild-type mice colonized with E. faecalis. All photomicrographs at x20.
Macrophage Depletion Blocks Wnt/β-Catenin Signaling
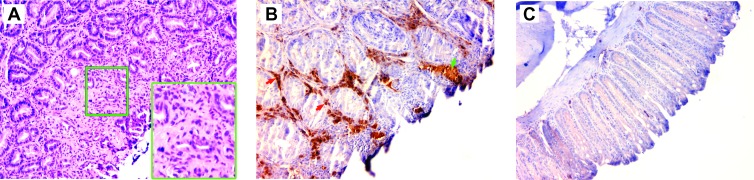
Colon cancer was noted in five E. faecalis-colonized Il10-/- mice administered SL and one sham-colonized mouse given ELC (Figures 1F and 7A). Because Wnt/β-catenin signaling is a hallmark of colon cancer and important regulator of inflammation [11,12], we stained biopsies from SL-treated mice that had colon cancer for active (i.e., unphosphorylated) β-catenin and compared these findings to E. faecalis-colonized Il10-/- mice administered ELC. Strong staining for active β-catenin was noted in neoplastic epithelial cells and macrophages in the SL-treated group (Figure 7B) but not in mice administered ELC (Figure 7C).
Figure 7.
Depleting colon macrophages inhibits Wnt/β-catenin signaling. (A) Hematoxylin and eosin staining shows typical cancer in distal colon of an E. faecalis-colonized Il10-/- mouse administered SL at x20 and x40 (inset). (B) Immunohistochemical staining for unphosphorylated (active) β-catenin shows strong signaling in neoplastic epithelial cells (green arrow) and inflammatory cells in the lamina propria (red arrows) for Il10-/- mice colonized with E. faecalis and administered SL (x20 magnification). (C) No active β-catenin is evident in biopsies from E. faecalis-colonized Il10-/- mice administered ELC (x20).
Discussion
BSE is characterized by genomic damage arising in target cells due to diffusible clastogens that are generated by activated cells. Classically, this phenomenon occurs after irradiating myeloid or mesenchymal cells [46], although it can also occur, as we have shown [16,17], when macrophages are chronically infected. Activation of macro-phages by intestinal commensals like E. faecalis leads to chromosomal instability in epithelial cells, crypt hyperplasia, decreased apoptosis, and transformation [23,24,30,47]. In this study, we found that depleting colon macrophages in Il10-/- mice colonized with E. faecalis prevented inflammation and carcinogenesis. In addition, we found that these macrophages were polarized to the M1 phenotype. When these macrophages were depleted, the molecular markers for BSE, namely, 4-HNE protein adducts, COX-2, TNF-α, and netrin-1, were also reduced. These findings suggest that M1 colon macro-phages in this model are the primary effector cells for bacterial-induced BSE and carcinogenesis [24,30]. In contrast to the role of M2-polarized (or tumor-associated) macrophages in promoting tumor progression [13], our findings suggest that M1-polarized macrophages—phagocytes that ordinarily help resolve acute infections—can initiate cellular transformation through BSE when chronically activated. This concept provides a mechanism for how bacterial infections that promote chronic inflammation are associated with an increased risk for cancer [1].
Our findings are consistent with prior reports showing that Helicobacter spp. can trigger macrophages in Il10-/- mice to express interferon-γ [48]. This, along with the knowledge that Escherichia coli can also cause colitis and colon cancer in Il10-/- mice [49], suggests that induction of an M1 phenotype in these mice is unlikely to be specific for E. faecalis. It also remains unclear whether Il10-/- mice are able to induce macrophages with an M2 phenotype. This is an area of ongoing investigation in our laboratory. Finally, iNOS expression by M1-polarized colon macrophages suggests nitric oxide as an additional mediator of BSE along with COX-2 and TNF-α. This radical could promote carcinogenesis by inducing pro-inflammatory cytokines, including COX-2, and inactivating p53-mediated caspases [50]. Any role for nitric oxide in tumor development, however, is likely to be multifaceted, as this molecule is also associated with apoptosis and tumor regression.
Additional evidence for the role of macrophages in colon carcinogenesis can be found in studies that show bisphosphonates, like clodronate, block inflammation and prevent dysplasia and cancer in dextran sulfate-induced models of colitis [36,51]. Similarly, depleting colon macrophages with dichloromethylene-containing microspheres prevents colitis and cancer in conventionally colonized IL-10 knockout mice [52]. Finally, these animal models, along with our findings, provide a plausible mechanism for the reduction in colon cancer risk due to bisphosphonates that has been shown in retrospective human studies [38,39].
Wnt/β-catenin signaling is a hallmark of colon carcinogenesis [12]. The nuclear translocation of β-catenin is an early event in human colon cancer and models of colitis-associated or Adenomatous polyposis coli-deficient neoplasia [53–57]. Moreover, activated macrophages, COX-2, and TNF-α all promote Wnt/β-catenin signaling [11,58,59]. In this study, we found that depleting colon macrophages eliminated β-catenin signaling in E. faecalis-colonized Il10-/- mice and link macrophage-induced BSE to this paradigm.
We observed a significant decrease in mast cells when colon macrophages were depleted in E. faecalis-colonized Il10-/- mice. The loss of macrophages may have resulted in decreased tissue levels of prostaglandin E2 or chemokine (C-C motif) ligand 2 (or monocyte chemoattractant protein-1) that are known to attract mast cells [45,60]. Alternately, a loss of macrophages may have perturbed mast cell proliferation through CD4+Foxp3+ T regulatory cells [9]. However, we did not further investigate these hypotheses since the depletion of mast cells in Il10-/- mice through KitW-sh/KitW-sh mutations failed to alter the incidence or severity of Helicobacter-induced colitis or cancer [61]. Thus, the reduction in mast cells seemed an unlikely explanation for a lack of colitis or cancer in E. faecalis-colonized Il10-/- mice administered ELC.
Dendritic cells, in a fashion similar to macrophages, have been implicated in inflammatory bowel disease and colon cancer [7,62]. However, in the present study, regardless of colonization status or presence or absence of colon macrophages, there was no difference in the small numbers of dendritic cells observed in colon biopsies across all groups of Il10-/- mice. In contrast to macrophages, these cells seem to play no more than a minimal role in bacterial-induced BSE in this model.
Il10-/- mice have proven to be a useful model for studying colon carcinogenesis. This is true, in part, because IL-10 induces CD4+Foxp3+ T regulatory and myeloid-derived suppressor cells that inhibit macrophage function [63]. In addition, in a model of familial adenomatous polyposis, IL-10 was necessary in order for CD4+Foxp3+ Tregulatory cells to inhibit intestinal polyps [9,10]. A loss of this potent immunosuppressive cytokine sensitizes mice to colitis and colon cancer when monoassociated or colonized by specific triggers such as E. faecalis [24,49,64]. E. faecalis, a human intestinal commensal, produces extra-cellular superoxide when deprived of heme [65]. This pro-oxidant physiology helps to activate macrophages to generate BSE in vitro [16,17] and in vivo [24,30]. Although a case-control study in an elderly cohort failed to find an association between human colonization with these bacteria and colon adenomas or cancer, the lack of data in this study on colonization density, and the discovery of marked instability in enterococcal colonization over time, limited the conclusions [66]. In contrast, E. faecalis colonization was associated with colorectal cancer when the density of colonization in feces was measured by real-time polymerase chain reaction [67]. Finally, among patients with colon cancer, greater levels of serum antibody to E. faecalis have been noted compared to Streptococcus gallolyticus subsp. gallolyticus (formerly, Streptococcus bovis biotype I [68]), an intestinal commensal bacterium that has been implicated in colon cancer-related infections [15,69].
Several pathologic features of the Il10-/- model are analogous to those found in precursor lesions for human colon cancer. For example, in human colon adenomas, macrophages commonly express TNF-α, COX-2, and iNOS [27,28,70]. Similar molecular signatures were evident in SL-treated Il10-/- mice colonized with E. faecalis. In addition, as noted in our study, human colon adenomas are rich in macro-phages and mast cells [4,27]. The IL-10 knockout model may also mimic human colon cancer through polymorphisms in the promoter region for IL10. In one case-control study, single-nucleotide polymorphism promoter genotypes associated with a reduced expression of IL10 mRNA showed a four- to five-fold increased risk for colon cancer [71]. Finally, preventing inflammation and colon cancer in Il10-/- mice by depleting macrophages with ELC, and thereby blocking BSE, is a plausible mechanism for human studies that show bisphosphonates reduce the risk for colon cancer [38,39].
Despite these similarities, the intestinal pathology in the Il10-/- model more closely mimics colitis-associated disease than human sporadic colon cancer. Most notably, villous and tubular colon adenomas do not develop as typically seen in human colon cancer [64]. In addition, unlike in humans, the use of COX-2 inhibitors in IL-10 knockout mice is not protective and paradoxically causes severe colitis and potentiates premalignant changes [72]. Nonetheless, many parallels between human colon cancer and Il10-/- mice render it a powerful model with which to study basic mechanisms of bacterial-induced carcinogenesis.
The approach to intestinal colonization used in this study relied on orally administered (and nonabsorbable) antibiotics to maintain high-density colonization with E. faecalis. Although antibiotics can affect the normal intestinal microbiota, our conclusions on the role of macrophages in bacterial-induced BSE remain sound because these antibiotics were similarly administered to ELC- and SL-treated mice. In addition, this approach relied on conventionally raised Il10-/- mice, rather than gnotobiotic mice, which could be considered an advantage since conventional mice do not show defects in intestinal mucus as found in germ-free mice [73]. The loss of this barrier is not reported for human colon adenomas and could alter bacterial-driven carcinogenesis in mice by allowing luminal bacteria to come into direct contact with epithelial cells that should otherwise be protected [74]. Although we did not evaluate gene expression, measuring cellular proteins by immunohistochemistry using validated antibodies is well accepted in clinical practice and has the advantage of allowing precise spatial and cellular localization of these proteins. Finally, although these findings are based on a single long-term experiment involving 42 mice, the incidence of colitis and colon cancer in SL-treated Il10-/- mice was similar to that previously reported [24,49,64] and included sufficient replicates in each group such that highly significant differences were found across groups.
In summary, our findings show that E. faecalis polarizes colon macrophages to an M1 phenotype in the IL-10 knockout model and that these cells are essential to the development of inflammation and cancer. The reduction or elimination of markers for BSE, namely, COX-2, TNF-α, netrin-1, and 4-HNE protein adducts, supports a key role for these innate immune cells in generating BSE. Inhibiting bacterial-induced BSE by depleting colon macrophages may provide a mechanism for the chemopreventive activity of bisphosphonates [38,39]. Interventions that block bacterial-induced M1 polarization of macrophages or inhibit mediators of BSE will provide novel strategies for preventing colon cancer.
Supplementary Material
Footnotes
This study was supported by the Oklahoma Center for the Advancement of Science and Technology (HR10-032; X.W.), grant CA127893 (M.M.H.), and funds from the Francis M. Duffy Endowment. The authors disclose no conflicts.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.transonc.com.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 3.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, Lord GM, Glickman JN, Glimcher LH. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenomalinked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 11.Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol. 2011;90:553–559. doi: 10.1016/j.ejcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macro-phages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551–561. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Allen TD, May RJ, Lightfoot S, Houchen CW, Huycke MM. Enterococcus faecalis induces aneuploidy and tetraploidy in colonic epithelial cells through a bystander effect. Cancer Res. 2008;68:9909–9917. doi: 10.1158/0008-5472.CAN-08-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speed N, Blair IA. Cyclooxygenase- and lipoxygenase-mediated DNA damage. Cancer Metastasis Rev. 2011;30:437–447. doi: 10.1007/s10555-011-9298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Allen TD, Yang Y, Moore DR, Huycke MM. Cyclooxygenase-2 generates the endogenous mutagen trans-4-hydroxy-2-nonenal in Enterococcus faecalis-infected macrophages. Cancer Prev Res (Phila) 2013;6:206–216. doi: 10.1158/1940-6207.CAPR-12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, Huycke MM. 4-Hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology. 2012;142:543–551. doi: 10.1053/j.gastro.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci USA. 2004;101:8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 27.Chapple KS, Cartwright EJ, Hawcroft G, Tisbury A, Bonifer C, Scott N, Windsor ACJ, Buillou PJ, Markham AF, Coletta PL, et al. Localization of cyclooxygenase-2 in human sporadic colorectal adenomas. Am J Pathol. 2000;156:545–553. doi: 10.1016/S0002-9440(10)64759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 29.Yan B, Wang H, Rabbani ZN, Zhao Y, Li W, Yuan Y, Li F, Dewhirst MW, Li CY. Tumor necrosis factor-α is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Wang X, Moore DR, Lightfoot SA, Huycke MM. TNF-α mediates macrophage-induced bystander effects through netrin-1. Cancer Res. 2012;72:5219–5229. doi: 10.1158/0008-5472.CAN-12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehlen P, Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol. 2010;22:46–54. doi: 10.1097/CCO.0b013e328333dcd1. [DOI] [PubMed] [Google Scholar]
- 32.Paradisi A, Maisse C, Coissieux MM, Gadot N, Lépinasse F, Delloye-Bourgeois C, Delcros JG, Svrcek M, Neufert C, Fléjou JF, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci USA. 2009;106:17146–17151. doi: 10.1073/pnas.0901767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY, Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 34.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 35.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 37.Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(β, γ-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 38.Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J Cancer. 2012;131:E717–E725. doi: 10.1002/ijc.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thosani N, Thosani SN, Kumar S, Nugent Z, Jimenez C, Singh H, Guha S. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta-analysis. J Clin Oncol. 2013;31:623–630. doi: 10.1200/JCO.2012.42.9530. [DOI] [PubMed] [Google Scholar]
- 40.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–536. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 41.Bourgogne A, Garsin DA, Qin X, Singh KV, Sillanpaa J, Yerrapragada S, Ding Y, Dugan-Rocha S, Buhay C, Shen H, et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008;9:R110. doi: 10.1186/gb-2008-9-7-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox CC, Lazenby AJ, Moore WC, Yardley JH, Bayless TM, Lichtenstein LM. Enhancement of human intestinal mast cell mediator release in active ulcerative colitis. Gastroenterology. 1990;99:119–124. doi: 10.1016/0016-5085(90)91238-2. [DOI] [PubMed] [Google Scholar]
- 43.Nolte H, Spjeldnaes N, Kruse A, Windelborg B. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut. 1990;31:791–794. doi: 10.1136/gut.31.7.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, Phillips J, Blatner NR, Hix LM, Zhang M, Dennis KL, et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCΔ468 mice. Cancer Res. 2011;71:1627–1636. doi: 10.1158/0008-5472.CAN-10-1923. [DOI] [PubMed] [Google Scholar]
- 45.Alam R, Kumar D, Anderson-Walters D, Forsythe PA. Macrophage inflammatory protein-1 alpha and monocyte chemoattractant peptide-1 elicit immediate and late cutaneous reactions and activate murine mast cells in vivo. J Immunol. 1994;152:1298–1303. [PubMed] [Google Scholar]
- 46.Lorimore SA, Wright EG. Radiation-induced genomic instability and bystander effects: related inflammatory-type responses to radiation-induced stress and injury? A review. Int J Radiat Biol. 2003;79:15–25. [PubMed] [Google Scholar]
- 47.Wang X, Huycke MM. 10th Annual AACR International Conference on Frontiers in Cancer Prevention Research. Boston, MA: American Association for Cancer Research; 2011. Long-term exposure of colonic epithelial cells to Enterococcus faecalis-infected macrophages causes cellular transformation; p. 109. [Google Scholar]
- 48.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107–119. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Sassa S, Okabe H, Nemoto N, Kikuchi H, Kudo H, Sakamoto S. Ibadronate may prevent colorectal carcinogenesis in mice with ulcerative colitis. Anticancer Res. 2009;29:4615–4619. [PubMed] [Google Scholar]
- 52.Watanabe N, Ikuta K, Okazaki K, Nakase H, Tabata Y, Matsuura M, Tamaki H, Kawanami C, Honjo T, Chiba T. Elimination of local macro-phages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci. 2003;48:408–414. doi: 10.1023/a:1021960401290. [DOI] [PubMed] [Google Scholar]
- 53.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 54.Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, Okayasu I. Decreased expression of CD44, alpha-catenin, and deleted colon carcinoma and altered expression of beta-catenin in ulcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer. 2000;89:733–740. doi: 10.1002/1097-0142(20000815)89:4<733::aid-cncr3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 55.Hasselblatt P, Gresh L, Kudo H, Guinea-Viniegra J, Wagner EF. The role of the transcription factor AP-1 in colitis-associated and β-catenindependent intestinal tumorigenesis in mice. Oncogene. 2008;27:6102–6109. doi: 10.1038/onc.2008.211. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimi K, Tanaka T, Takizawa A, Kato M, Hirabayashi M, Mashimo T, Serikawa T, Kuramoto T. Enhanced colitis-associated colon carcinogenesis in a novel Apc mutant rat. Cancer Sci. 2009;100:2022–2027. doi: 10.1111/j.1349-7006.2009.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mees ST, Mennigen R, Spieker T, Rijcken E, Senninger N, Haier J, Bruewer M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and β-catenin. Int J Colorectal Dis. 2009;24:361–368. doi: 10.1007/s00384-009-0653-y. [DOI] [PubMed] [Google Scholar]
- 58.Castellone MD, Teramoto H, Gutkind JS. Cyclooxygenase-2 and colorectal cancer chemoprevention: the β-catenin connection. Cancer Res. 2006;66:11085–11088. doi: 10.1158/0008-5472.CAN-06-2233. [DOI] [PubMed] [Google Scholar]
- 59.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-α in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallgren J, Gurish MF. Mast cell progenitor trafficking and maturation. Adv Exp Med Biol. 2011;716:14–28. doi: 10.1007/978-1-4419-9533-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chichlowski M, Westwood GS, Abraham SN, Hale LP. Role of mast cells in inflammatory bowel disease and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS One. 2010;5:e12220. doi: 10.1371/journal.pone.0012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karlis J, Penttila I, Tran TB, Jones B, Nobbs S, Zola H, Flesch IE. Characterization of colonic and mesenteric lymph node dendritic cell sub-populations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:834–847. doi: 10.1097/00054725-200411000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Tanikawa T, Wilke CM, Kryczek I, Chen GY, Kao J, Nunez G, Zou W. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huycke MM, Moore D, Joyce W, Wise P, Shepard L, Kotake Y, Gilmore MS. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol Microbiol. 2001;42:729–740. doi: 10.1046/j.1365-2958.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 66.Winters MD, Schlinke TL, Joyce WA, Glore SR, Huycke MM. Prospective case-cohort study of intestinal colonization with enterococci that produce extracellular superoxide and the risk for colorectal adenomas or cancer. Am J Gastroenterol. 1998;93:2491–2500. doi: 10.1111/j.1572-0241.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 67.Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. Real-time polymerase chain reaction quantification of specific butyrateproducing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol. 2008;23:1298–1303. doi: 10.1111/j.1440-1746.2008.05490.x. [DOI] [PubMed] [Google Scholar]
- 68.Osawa R, Sasaki E. Novel observations of genotypic and metabolic characteristics of three subspecies of Streptococcus gallolyticus. J Clin Microbiol. 2004;42:4912–4913. doi: 10.1128/JCM.42.10.4912-4913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darjee R, Gibb AP. Serological investigation into the association between Streptococcus bovis and colonic cancer. J Clin Pathol. 1993;46:1116–1119. doi: 10.1136/jcp.46.12.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardwick JCH, van den Brink G, Offerhaus GJ, van Deventer SJH, Peppelenbosch MP. NF-kappaB, p38 MAPK and JNK are highly expressed and active in the stroma of human colonic adenomatous polyps. Oncogene. 2001;20:819–827. doi: 10.1038/sj.onc.1204162. [DOI] [PubMed] [Google Scholar]
- 71.Čačev T, Radoševič S, Križanac S, Kapitanovič S. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis. 2008;29:1572–1580. doi: 10.1093/carcin/bgn164. [DOI] [PubMed] [Google Scholar]
- 72.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 73.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 74.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.