Abstract
The evidence base in support of HbA1c as a diagnostic test for diabetes mellitus is focused on predicting a clinical outcome, considered to be the pinnacle of the Stockholm Hierarchy applied to reference intervals and clinical decision limits. In the case of diabetes, the major outcome of interest is the long term microvascular complications for which a large body of data has been accumulated, leading to the endorsement of HbA1c for diagnosis in many countries worldwide, with some variations in cut-offs and testing strategies.
Introduction
HbA1c is now formally endorsed in many countries as a diagnostic test for (type 2) diabetes as well as for monitoring, although some debate still continues regarding its applicability for diagnosis.1–5 Pivotal to this discussion is the evidence base upon which these recommendations have been made. In considering the diagnosis of diabetes, we are primarily concerned with defining a disease state rather than establishing a reference interval for health. In particular, the evidence base is focused on predicting a clinical outcome, considered to be the pinnacle of the Stockholm Hierarchy applied to reference intervals and clinical decision limits.6 In the case of diabetes, the major outcome of interest is the long-term microvascular complications for which a large body of data has been accumulated, as discussed below. The debate surrounding the role of HbA1c as a diagnostic test addresses the relative merits and disadvantages of glucose versus HbA1c and brings into focus many pre-analytical, analytical and other biological considerations as well as factors such as cost and accessibility.
Background
Type 1 diabetes usually presents with symptoms and unequivocal hyperglycaemia, thus diagnosis is usually uncomplicated. The onset of type 2 diabetes, however, is slower with a more gradual increase in glucose levels over time. A continuum exists from health through to diabetes, from low risk through to high risk of complications.
Effective management of the disease has been shown in the United Kingdom Prospective Diabetes Study (UKPDS) to significantly reduce the risk of developing complications.7 Furthermore, long-term follow-up of UKPDS participants demonstrated that more effective glycaemic control from the time of diagnosis in people with type 2 diabetes conferred a long-term benefit that persisted even though glycaemic control may deteriorate over time.8 This observation implies that earlier detection of diabetes should result in improved outcomes, with major long-term health benefits.
Glucose Based Criteria for the Diagnosis of Diabetes
Conventionally, blood glucose levels measured either in the fasting state or following a standard glucose load have formed the basis for diagnosis of diabetes. Some populations with a high prevalence of diabetes, such as the Pima Indians and the Micronesian population of Nauru, demonstrate a bimodal distribution of glucose levels.9 Taking this into consideration, the intersect of these two curves has been used to provide an indication of the level at which individuals should be classified as either having or not having diabetes. In 1979, on the basis of these and other outcome data, the National Diabetes Data Group (NDDG) in the US and subsequently the World Health Organization (WHO) Expert Committee on Diabetes Mellitus published recommendations for the diagnosis of diabetes using both fasting and 2-hour plasma glucose measured during an oral glucose tolerance test.10,11 It was recommended that diabetes be diagnosed when glucose levels were ≥7.8 mmol/L in the fasting state or ≥11.1 mmol/L following a 75 g glucose load. An intermediate range, termed ‘impaired glucose tolerance (IGT)’, was defined by a post-load glucose between 7.8 mmol/L and 11.0 mmol/L.10,11 These criteria formed the basis for the diagnosis of type 2 diabetes for nearly two decades. In 1997, an expert committee shifted the emphasis away from the bimodal distribution to focus more on clinical outcomes. They updated the diagnostic criteria based on the relationship between glycaemia, measured as fasting or 2-hour plasma glucose, and prevalent retinopathy in three studies.12 These studies were conducted in Pima Indians, Egyptians and participants from the National Health and Nutrition Examination Survey (NHANES) in the United States. It was recommended that the fasting glucose cut-off be lowered to ≥7.0 mmol/L and that a new pre-diabetic category be introduced, ‘impaired fasting glycaemia (IFG)’, defined as a fasting glucose between 6.1 mmol/L and 6.9 mmol/L.12 No changes were made to the post-load glucose criteria. These criteria are still recommended by the WHO.13
The Case for HbA1c as a Diagnostic Test
Firstly, HbA1c gives an indication of chronic glycaemia rather than being a test of glycaemia at a single point in time. It gives an integrated index of glycaemia over the entire 120-day lifespan of the red blood cell, but within this period of 120 days, recent glycaemia has the largest influence on the HbA1c value, with 50% of HbA1c formed in the month prior to sampling and 25% in the month before that.14 It therefore seems logical that such a test would be appropriate in diagnosing a disease characterised by chronic hyperglycaemia and a gradual progression to complications.
Secondly, it is a relatively convenient test, not requiring the patient to fast and only using a single blood sample. This is an important consideration, in that it may enable improved uptake of testing and improved detection of diabetes, given the large proportion of diabetes cases that go undiagnosed.15,16
For an oral glucose tolerance test (OGTT), more extensive pre-test preparation is required, including an appropriate diet for 3 days before the test and a satisfactory period of overnight fasting. The OGTT is also time-consuming, taking at least 2 hours. The glucose load is poorly tolerated by a significant number of people, with nausea, vomiting, delayed gastric emptying and issues of venous access all potentially contributing to an invalid test result. The test often needs to be repeated and has poor patient compliance. A recent study from South Australia showed that only 27% of patients identified on admission to hospital as potentially having diabetes presented for a diagnostic OGTT despite consenting to undertake the test.17 HbA1c in contrast is not affected by prandial status and has no diurnal rhythm, allowing measurement at any time of day.
Unlike plasma glucose, HbA1c shows minimal pre-analytical variability. It is very stable after collection with no change in its concentration ‘in the collection tube’. Ideally when measuring plasma glucose, the venous blood sample should be spun and plasma separated within minutes of taking the sample as red blood cells continue to consume glucose at about 7% per hour in vitro, leading to a falsely low measured glucose.18 Collection of the sample into a container with a Antiglycolytic preservative (fluoride) is only partially effective.18 Ideally the sample should also be placed in iced water and processed within 30–60 minutes, although most laboratories do not fulfil these rigorous sample handling requirements. HbA1c in contrast has high pre-analytical stability (one week at 4 °C).
Within-subject biological variation of HbA1c is in the order of 3.6%, compared with 5.7% for fasting plasma glucose and 16.7% for the 2-hour post-OGTT value.19 Analytical precision for HbA1c now approaches that for glucose, with intra- and between-laboratory analytic variability in the order of 2.5%.20 Standardisation of HbA1c measurement is also better than for glucose.
Overall reproducibility of oral glucose tolerance testing is poor, in the order of 66% variability, which can result in inappropriate labels being given to patients.21 Furthermore no attempt at weight adjusting the dose of glucose is included in OGTT protocols – so a 60 kg person given 75 g glucose receives twice the dose in mg/kg compared to a 120 kg person. This in part explains the poor correlation of 2-hour post oral glucose levels with significant prevalent diabetic retinopathy in patients with previously undiagnosed diabetes,22 and it is therefore somewhat arbitrary that the 2-hour post glucose cut point for diabetes is currently set at 11.1 mmol/L.
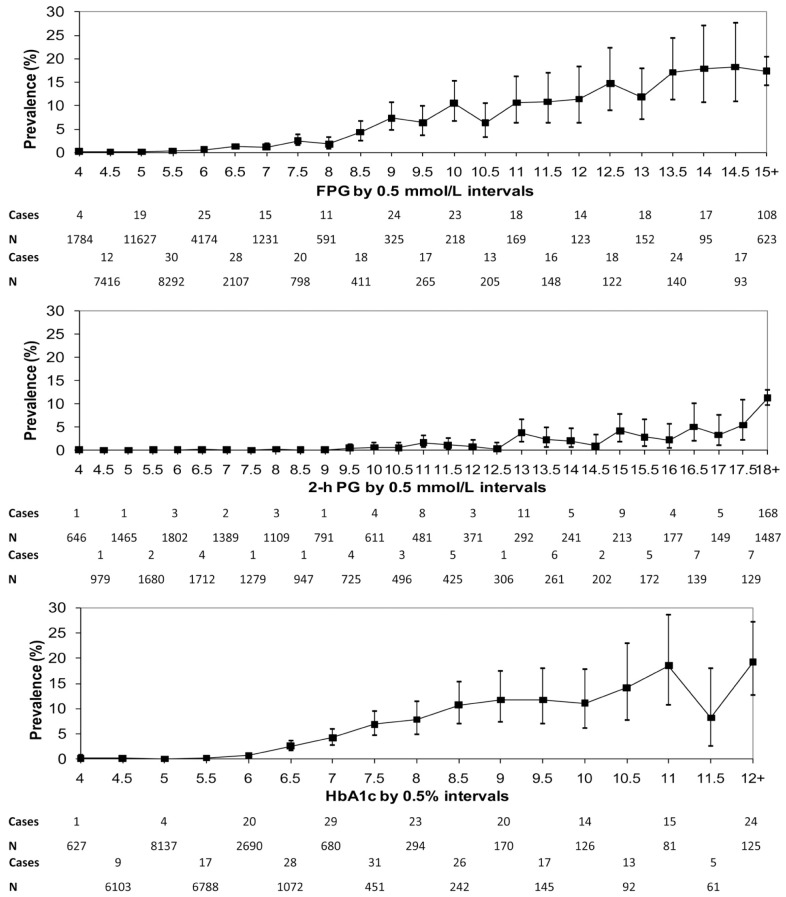
Most importantly, with respect to prediction of clinical outcomes (the central tenet of the evidence base), HbA1c has a similar relationship with prevalent diabetic retinopathy as that of both fasting and 2-hour plasma glucose, as shown in the recent DETECT-2 analysis.22
This landmark study involved data pooling of nine studies from five countries, with 44623 participants aged 20–79 y with gradable retinal photographs. The study examined the relationship between diabetes-specific retinopathy (defined as moderate or more severe retinopathy) and three glycaemic measures: fasting plasma glucose (n=41411), 2-hour post oral glucose load plasma glucose (n=21334), and glycated haemoglobin (HbA1c, n=28010).22 It was found that both fasting plasma glucose and HbA1c have narrow threshold ranges within which the prevalence of diabetes-specific retinopathy begins to increase significantly (Figure). The prevalence of retinopathy was low with HbA1c <42 mmol/mol (<6.0%) but increased above this level, with an optimal threshold of ≥46 mmol/mol (6.4%) calculated using receiver-operative characteristic curve analysis. These findings suggest that HbA1c is at least as good at predicting microvascular complications as either fasting or 2-hour plasma glucose and that the diagnostic threshold of ≥48 mmol/mol (≥6.5%) is appropriate.22 The relationship between HbA1c and prevalent retinopathy is similar to that of plasma glucose, whether glucose and HbA1c are plotted in deciles, in vigintiles or as continuous variables (Figure).22
Figure.
Prevalence of retinopathy for fasting plasma glucose (FPG), 2-h post oral glucose load plasma glucose (2-h PG) and HbA1c, for any retinopathy and diabetes-specific retinopathy (≥ moderate non-proliferative diabetic retinopathy) from DETECT-2. (Reproduced from Ref. 22 with permission.)
Aside from any consideration of the relationship between HbA1c and microvascular complications, it should also be noted that the relationship between glycaemic parameters (whether glucose or HbA1c) and cardiovascular outcomes is different from that seen for microvascular disease. This also brings into question the validity of any single chosen cut-off and whether risk prediction may be expressed in any other way. There is also a relationship with cardiovascular outcomes associated with lower levels of HbA1c. In the EPIC-Norfolk study, a prospective population study, cardiovascular disease events increased above HbA1c 31 mmol/mol (5%) in both men and women and independently of age, body mass index (BMI) and other factors.23 The relationship with HbA1c is therefore different dependent upon the outcome of interest and it could be argued that it might be better to assign a measure of ‘glycaemia-attributable risk’ rather than an ‘all or nothing’ diagnosis based upon an arbitrary cut-off.
Recommendations for HbA1c as a Diagnostic Test
The case for HbA1c for as a diagnostic test was put forward as early as the mid-1980s, but concerns regarding its availability and poor assay standardisation prevented its uptake.24 It wasn’t until 2009 that an international expert committee recommended HbA1c be introduced into diagnostic criteria at a threshold level of ≥48 mmol/mol (≥6.5%).25 This recommendation was adopted by the American Diabetes Association (ADA) the following year and more recently by the WHO.1,2
American Diabetes Association Recommendations
The ADA endorsed HbA1c as a diagnostic test for diabetes at a cut-off of ≥48 mmol/mol (≥6.5%) with the provision that this be measured in a laboratory using a NGSP-certified assay aligned to the DCCT study, and that in the absence of unequivocal hyperglycaemia the test should be repeated (Table 1).1 Individuals with an HbA1c of 39–46 mmol/mol (5.7–6.4%) are considered to be at ‘increased risk’ for diabetes as well as cardiovascular disease, and should be counselled about effective strategies, such as weight loss and physical activity, to lower their risks.1
Table 1.
Diagnostic criteria for diabetes; the American Diabetes Association (Ref. 1).
| 1. HbA1c ≥48 mmol/mol (≥6.5%). The test should be performed in a laboratory using a method that is NGSP certified and standardised to the DCCT assay.* |
| OR |
| 2. FPG ≥7.0 mmol/L (≥126 mg/dL). Fasting is defined as no caloric intake for at least 8 h.* |
| OR |
| 3. 2-h plasma glucose ≥11,1 mmol/L (≥200 mg/dL) during an OGTT. The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.* |
| OR |
| 4. In a patient with classic symptoms of hyperglycaemia or hyperglycaemic crisis, a random plasma glucose ≥11.1 mmol/L (≥200 mg/dL). |
NGSP = National Glycohemoglobin Standardization Program; DCCT = Diabetes Control and Complications Trial; FPG = fasting plasma glucose; OGTT = oral glucose tolerance test.
In the absence of unequivocal hyperglycaemia, criteria 1–3 should be confirmed by repeat testing.
In formulating the WHO recommendations (Table 2), a process of consultation included experts in diabetology, biochemistry, immunology, genetics, epidemiology and public health. The main question to be answered for the update was agreed upon by the expert group: how does HbA1c perform in the diagnosis of type 2 diabetes based on the detection and prediction of microvascular complications? Applying the principles of Evidence Based Medicine, a search for existing systematic reviews in Embase did not identify any such review. Therefore, a systematic review to answer this question was conducted by the Boden Institute of Obesity, Nutrition and Exercise, The University of Sydney, Australia. The recommendation was drafted by the expert group following the GRADE methodology, and the process outlined in the WHO Handbook for Guideline Development.26 The decision process took into account the findings of the systematic review and the other advantages and disadvantages of using HbA1c to diagnose diabetes. The recommendation, quality of evidence and strength of the recommendation were discussed and consensus was reached. All the experts agreed on the recommendation.
Table 2.
World Health Organization recommendations for HbA1c as a diagnostic test for diabetes (Ref. 2).
| HbA1c can be used as a diagnostic test for diabetes providing that stringent quality assurance tests are in place and assays are standardised to criteria aligned to the international reference values, and there are no conditions present which preclude its accurate measurement. |
| An HbA1c of 6.5% is recommended as the cut point for diagnosing diabetes. A value of less than 6.5% does not exclude diabetes diagnosed using glucose tests. |
| Quality of evidence assessed by GRADE: moderate. |
| Strength of recommendation based on GRADE criteria: conditional. |
GRADE = Grading of Recommendations Assessment, Development and Evaluation.
Australian Recommendations
The Australian Diabetes Society established an expert committee in 2011, including invited representatives of the Royal College of Pathologists of Australasia (RCPA) and the Australasian Association of Clinical Biochemists (AACB), to review the available evidence and provide a position statement concerning the role of HbA1c in the diagnostic pathway. Additionally, the committee sought to ensure that its recommendations otherwise concur with recently published National Health and Medical Research Council (NHMRC) guidelines for the detection and diagnosis of type 2 diabetes.27 A summary of the committee’s recommendations is shown in Table 3.3
Table 3.
Australian recommendations for HbA1c as a diagnostic test for diabetes (Ref. 3).
| Measurement of HbA1c level can be used as a diagnostic test for diabetes if analysis is performed in a facility producing acceptable performance in external quality assurance, assays are standardised to criteria aligned to international reference values, and if no conditions which preclude its accuracy are present. It is important to note that HbA1c testing is not currently funded by Medicare for the purpose of diagnosis of diabetes. |
| An HbA1c level of ≥48 mmol/mol (≥6.5%) is recommended as the cut-off point for diagnosing diabetes. |
| An HbA1c level of <48 mmol/mol (<6.5%) does not negate a diagnosis of diabetes based on elevated glucose parameters. The existing criteria based on fasting and random glucose levels, and on the oral glucose tolerance test, remain valid, and are the diagnostic tests of choice for gestational diabetes, type 1 diabetes and in the presence of conditions that interfere with HbA1c measurement. |
A HbA1c level of 48 mmol/mol (6.5%) is recommended as the cut-off point for diagnosing diabetes. In an asymptomatic patient with a positive test result, the test should be repeated to confirm the diagnosis. The use of HbA1c measurement will simplify the diagnostic process and may lead to earlier diagnosis of more patients with diabetes. However, HbA1c should not be used as a general screening test for diabetes; initial screening should be on the basis of the Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK) score, as recommended in the NHMRC guidelines.27 At the time of writing, the Australian recommendations, although formally endorsed, are yet to receive funding support, although an application has been made.
New Zealand Recommendations
In New Zealand, HbA1c as a diagnostic test was formally endorsed by the New Zealand Society for the Study of Diabetes (NZSSD) from 3 October 2011.4 This recommendation was coordinated with the adoption of exclusively molar units for reporting HbA1c (following a two year period of dual reporting, like the United Kingdom), with the diagnostic cut-off rounded up to ≥50 mmol/mol (≥6.7%), and with repeat testing on a second occasion in asymptomatic individuals.28 Individuals with HbA1c in the range 41–49 mmol/L are categorised as having ‘dysglycaemia’ or abnormal glucose tolerance, with the recommendation for re-testing in 6–12 months and also implementation of cardio-vascular risk management. Part of the NZSSD rationale for rounding of HbA1c was to make the molar units more memorable, although in addition, to maximise the specificity for the diagnosis of diabetes.4 It may be argued that sensitivity is being compromised by adoption of HbA1c as a diagnostic test, and especially at a still higher level, and that cases of diabetes will be missed. NZSSD would contend, however, that individuals with HbA1c close to the cut-off (41–49 mmol/mol) will be re-tested in 6–12 months and will enter a lifestyle programme where cardiovascular risk factors will be appropriately addressed. Although they will not acquire the diagnostic label of diabetes, nor enter an annual programme of microvascular complications screening at that time, they are not really being ‘missed’. In addition, although glucose-based criteria remain valid, the NZSSD recommendations (Table 4) strongly favour HbA1c as the diagnostic test in preference to OGTT testing.4
Table 4.
New Zealand recommendations for type 2 diabetes screening. (Reproduced from Ref. 4 with permission from the New Zealand Society for the Study of Diabetes.)
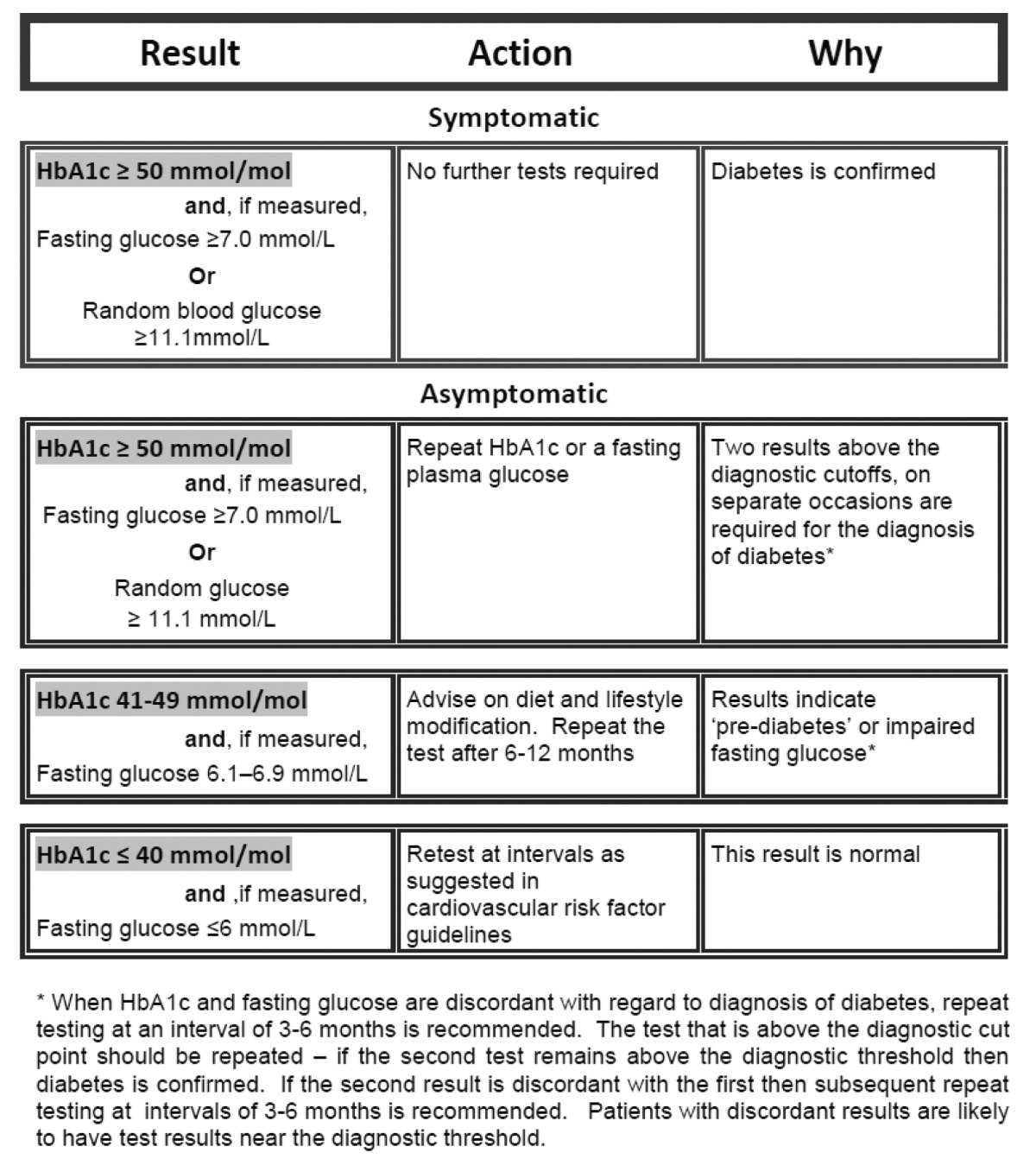
|
The New Zealand position is therefore somewhat inconsistent with approaches adopted in other countries, although the rationale and arguments in support of this variance have been well presented and the approach is pragmatic and practical.4
Cautions and Caveats Regarding HbA1c as a Diagnostic Test
There are some important caveats. If used as a diagnostic test, the HbA1c assay needs to be reliable and consistent across different centres. There have been problems in the past with HbA1c results varying considerably between laboratories. In a recent Australian study, whole blood samples were sent to more than 200 laboratories, and more than 90% of HbA1c results fell within 6% of the median.29 Further improvements in standardisation of HbA1c measurements should be achieved following the development of a national whole blood external quality control program by the RCPA Quality Assurance Programs and the AACB.29
The ADA recommended that Point-of-Care Testing (POCT) HbA1c assays are not sufficiently accurate at this time to use for diagnostic purposes,1 a view endorsed by other recommendations.4 The WHO stipulated, however, that the diagnosis should be made by the best technology available, avoiding blood glucose monitoring meters and single-use HbA1c test kits (except where this is the only option available or where there is a stringent quality assurance programme in place).2 Although not formally endorsed at the present time, it is recognised that some POCT devices do perform satisfactorily with correct usage, and may be the only practical option in remote rural settings.
When applying HbA1c testing for the diagnosis of diabetes, some medical conditions may affect the test and cause falsely high or low readings (Table 5). The test’s accuracy is affected principally by conditions that affect red blood cell survival time or non-enzymatic glycation of haemoglobin.30 A reduced red blood cell survival time will lower the HbA1c level and may lead to a false negative result. Red blood cell survival time is reduced in any haemolytic anaemia, and it can also be reduced in chronic renal failure, severe liver disease and anaemia of chronic disease. Vitamin B12 and folic acid deficiencies may also shorten red blood cell survival time. A common clinical situation that shortens red blood cell survival time occurs when patients undergo regular phlebotomy for medical indications (e.g. haemochromatosis). Iron deficiency may also have an impact on red blood cell survival and increase the HbA1c level.31 The congenital variants of the haemoglobin molecule (haemoglobinopathies), which may be relatively common in certain ethnic communities (e.g. African, Mediterranean) affect the result to a variable amount, principally due to interference with the laboratory measurement of HbA1c. Many newer laboratory methods have measures in place to reduce this problem. The NGSP provides a summary of the effect of common haemoglobinopathies on measurement of HbA1c levels using various methods.32 Any HbA1c result, however, that is not consistent with clinical expectations or the results of self-monitored capillary blood glucose readings should be regarded with suspicion and should alert the medical practitioner to a potential problem. Some methodologies for HbA1c measurement (such as boronate affinity chromatography) are less susceptible to the effects of haemoglobinopathies and such methods may be favoured for populations where a higher proportion of abnormal haemoglobins may be expected. Otherwise, it is good to have access to HbA1c measurement by an alternative method when a potential haemoglobin variant is suspected and to follow up with further investigations such as haemoglobin electrophoresis or mass spectrometry.
Table 5.
Arguments for and against the use of HbA1c as a diagnostic test.
| Advantages | Disadvantages |
|---|---|
| Indicative of chronic glycaemia and reflective of tissue glycation status. | Results can be affected by haemolysis and other conditions with increased red cell turnover (reduced HbA1c) or conditions with reduced red cell turnover e.g. iron deficiency (increased HbA1c) or in any other chronic disease state. |
| More convenient, as patient not required to fast and only one blood sample taken. | May vary with age and between different ethnic groups. |
| Validated against clinical outcomes, particularly retinopathy as a long-term microvascular complication of diabetes. | More expensive, being unaffordable in many low income country situations. |
| Less intra-individual variability compared with fasting and 2-hour glucose in an OGTT. | |
| Greater pre-analytical stability compared with plasma glucose. |
Simplistically, if a haemoglobin variant is suspected, then HbA1c is not an appropriate diagnostic test and glucose based criteria should be preferred. HbA1c should also not be regarded as the appropriate test to confirm the diagnosis of diabetes in patients with any significant chronic medical disease, any anaemia or any abnormality of red blood cell structure. If any of these conditions exist, the diagnosis of diabetes should be based on measures of blood glucose levels. It should also be recognised that HbA1c is more expensive than plasma glucose testing and this may prohibit its use in many countries worldwide. Others, however, have argued that its practical advantages may, indeed improve access to care in disadvantaged populations (Table 5).33
Ethnic Variations in HbA1c
There is also evidence which indicates that HbA1c will detect a different population as having diabetes to that identified by plasma glucose. For example, in the US, a number of reports have suggested that African Americans have higher HbA1c than both Mexican Americans and non-Hispanic Whites.34 It may be that the prevalence of conditions affecting erythrocyte turnover or genetic differences in the physiology of glycation differ with ethnicity. Alternatively, it may be that HbA1c detects real differences in chronic glycaemia that are not represented by the fasting and 2-hour plasma glucose levels of the OGTT. If the former explanation is true, then HbA1c may not be appropriate for diagnosis or else it may be necessary to consider the use of ethnic-specific cut points for HbA1c both in the management and diagnosis of diabetes. Conversely, if the latter explanation is true, it would argue in favour of using HbA1c to diagnose diabetes, as the observed elevations of HbA1c are likely to be reflective of increased complication risk at the tissue level. This point also brings into consideration the presumption that the OGTT is a gold standard for diagnosis which may not necessarily be the case, and that HbA1c is a more valid marker of tissue glycation. It remains to be determined whether or not these supposed ethnic differences in HbA1c are clinically consequential, although this is another factor that needs to be taken into further consideration as we move forward.
An Intermediate Position for HbA1c in Diagnosis
Another perspective emanates from the studies of Zhong Lu et al.35 who studied two populations: the clinical population (MP), including all those referred to Melbourne Pathology Services for an OGTT who had a concomitant HbA1c measured (n=2494) and a validation population from the population based AusDiab study who also underwent OGTT and had HbA1c measured (n=6014).16 Among those with undiagnosed diabetes (34.6%) by OGTT criteria in the MP population, HbA1c at the 2.5th percentile was 38 mmol/mol (5.6%) and at the 97.5th percentile was 52 mmol/mol (6.9%). From these data, HbA1c ≤37 mmol/mol (≤5.5%) was chosen to rule out diabetes and ≥53 mmol/mol (≥7.0%) to rule in diabetes. Applying these cut-offs to the AusDiab population, HbA1c at 37 mmol/mol (5.5%) provided high negative predictive value (99%) and at 53 mmol/mol (7.0%), 100% positive predictive value. By dropping the cut-off to 48 mmol/mol (6.5%), specificity remained at 99% with positive predictive value near 100%. Other authors, rather than accepting a single cutoff have advocated for separate rule out and rule in criteria applying the same cut-offs suggested by the Melbourne studies.36
Conclusions
The case for HbA1c as a diagnostic test for diabetes has therefore been submitted to a very rigorous examination based upon the principles of evidence based medicine. In particular, the evidence base is focused mainly on predicting clinical outcomes (particularly microvascular complications) considered to be the pinnacle of the Stockholm Hierarchy applied to reference intervals and clinical decision limits.6 In addition, many other factors need to be taken into consideration, including pre-analytical, analytical and other biological parameters as well as cost and accessibility. There are clear advantages for HbA1c over glucose (and in particular OGTT) as a diagnostic test for diabetes, although with an important series of caveats that clinicians need to be aware of. As always, there is need for educational resources to be widely available and for on-going dialogue between clinicians and the laboratory.
Footnotes
Competing Interests: None declared.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Abbreviated Report of a WHO Consultation. Geneva: WHO; 2011. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. [PubMed] [Google Scholar]
- 3.d’Emden MC, Shaw JE, Colman PG, Colagiuri S, Twigg SM, Jones GR, et al. The role of HbA1c in the diagnosis of diabetes in Australia. Med J Aust. 2012;197:220–1. doi: 10.5694/mja12.10988. [DOI] [PubMed] [Google Scholar]
- 4.Braatvedt GD, Cundy T, Crooke M, Florkowski C, Mann JI, Lunt H, et al. Understanding the new HbA1c units for the diagnosis of Type 2 diabetes. N Z Med J. 2012;125:70–80. [PubMed] [Google Scholar]
- 5.Kilpatrick ES, Winocour PH. ABCD position statement on haemoglobin A1c for the diagnosis of diabetes. Pract Diab Int. 2010;27:306–10. [Google Scholar]
- 6.Sikaris K. Application of the stockholm hierarchy to defining the quality of reference intervals and clinical decision limits. Clin Biochem Rev. 2012;33:141–8. [PMC free article] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 8.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 9.Zimmet P, Whitehouse S. Bimodality of fasting and two-hour glucose tolerance distributions in a Micronesian population. Diabetes. 1978;27:793–800. doi: 10.2337/diab.27.8.793. [DOI] [PubMed] [Google Scholar]
- 10.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Second Report WHO Technical Report Series 646. Geneva: WHO; 1980. WHO Expert Committee on Diabetes Mellitus. [PubMed] [Google Scholar]
- 12.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva: WHO; 2006. [Google Scholar]
- 14.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–7. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 15.Borch-Johnsen K, Colagiuri S. Diagnosing diabetes—time for a change? Diabetologia. 2009;52:2247–50. doi: 10.1007/s00125-009-1526-1. [DOI] [PubMed] [Google Scholar]
- 16.Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, Sicree RA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25:829–34. doi: 10.2337/diacare.25.5.829. [DOI] [PubMed] [Google Scholar]
- 17.Valentine NA, Alhawassi TM, Roberts GW, Vora PP, Stranks SN, Doogue MP. Detecting undiagnosed diabetes using glycated haemoglobin: an automated screening test in hospitalised patients. Med J Aust. 2011;194:160–4. doi: 10.5694/j.1326-5377.2011.tb02954.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruns DE, Knowler WC. Stabilization of glucose in blood samples: why it matters. Clin Chem. 2009;55:850–2. doi: 10.1373/clinchem.2009.126037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167:1545–51. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 20.RCPA Quality Assurance Programs Pty Ltd http://www.rcpaqap.com.au (Accessed 7 June 2013).
- 21.Ko GT, Chan JC, Woo J, Lau E, Yeung VT, Chow C-C, et al. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998;35:62–7. doi: 10.1177/000456329803500107. [DOI] [PubMed] [Google Scholar]
- 22.Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K, DETECT-2 Collaboration Writing Group Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care. 2011;34:145–50. doi: 10.2337/dc10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–20. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 24.Simon D, Coignet MC, Thibult N, Senan C, Eschwège E. Comparison of glycosylated hemoglobin and fasting plasma glucose with two-hour post-load plasma glucose in the detection of diabetes mellitus. Am J Epidemiol. 1985;122:589–93. doi: 10.1093/oxfordjournals.aje.a114138. [DOI] [PubMed] [Google Scholar]
- 25.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. GRADE Working Group Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colagiuri S, Davies D, Girgis S, Colagiuri R. National Evidence Based Guideline for Case Detection and Diagnosis of Type 2 Diabetes. Canberra: Diabetes Australia and National Health and Medical Research Council; 2009. [Google Scholar]
- 28.New Zealand Society for the Study of Diabetes: HbA1c http://www.nzssd.org.nz/hba1c.html (Accessed 7 June 2013). [PubMed]
- 29.RCPA Chemical Pathology Programs Program information circular: glycohaemoglobin program, whole blood samples, 22/06/09 http://www.rcpaqap.com.au/chempath/documents/uploadedfiles/455_Whole%20Blood%20Samples%20270809.pdf (Accessed 6 June 2013).
- 30.Jiao Y, Okumiya T, Saibara T, Park K, Sasaki M. Abnormally decreased HbA1c can be assessed with erythrocyte creatine in patients with a shortened erythrocyte age. Diabetes Care. 1998;21:1732–5. doi: 10.2337/diacare.21.10.1732. [DOI] [PubMed] [Google Scholar]
- 31.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112:126–8. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 32.HbA 1c Assay Interferences http://www.ngsp.org/interf.asp (Accessed 7 June 2013).
- 33.Degeling C, Rock M, Rogers WA. Testing relationships: ethical arguments for screening for type 2 diabetes mellitus with HbA1C. J Med Ethics. 2012;38:180–3. doi: 10.1136/medethics-2011-100086. [DOI] [PubMed] [Google Scholar]
- 34.Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770–7. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lu ZX, Walker KZ, O’Dea K, Sikaris KA, Shaw JE. A1C for screening and diagnosis of type 2 diabetes in routine clinical practice. Diabetes Care. 2010;33:817–9. doi: 10.2337/dc09-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen RM, Haggerty S, Herman WH. HbA1c for the diagnosis of diabetes and prediabetes: is it time for a mid-course correction? J Clin Endocrinol Metab. 2010;95:5203–6. doi: 10.1210/jc.2010-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]