Abstract
Gastric cancer is the second leading cause of cancer-related mortality in the world. Recently, serum Helicobacter pylori antibodies and pepsinogen (PG) have been used for gastric cancer screening. The incidence of gastric cancer in Bhutan is reported to be quite high compared with that in neighbouring countries. In this study, 381 subjects from three areas of Bhutan were assessed for gastric mucosal atrophy and serological parameters. Anti-H. pylori IgG, PG I, PG II and cytotoxin-associated gene A (CagA) antibodies were measured using ELISA. Subjects were classified into four groups according to H. pylori and PG seropositivity: Group A (H. pylori-negative/PG-negative), Group B (H. pylori-positive/PG-negative), Group C (H. pylori-positive/PG-positive) and Group D (H. pylori-negative/PG-positive). The prevalence of H. pylori in the 381 subjects was 71.1 % (271/381), with high infection rates found in rural areas. The PG I/II ratio was significantly inversely correlated with the atrophy score in the antrum and the corpus (P<0.001). Multivariate analysis showed that the PG status was significantly associated with the presence of atrophy in the corpus. The prevalence of the PG-positive status was significantly higher among H. pylori-positive subjects than among H. pylori-negative subjects (P<0.001). Based on the ABC method, Group B was the most dominant, followed by Group A, Group C and Group D. The high incidence of gastric cancer in Bhutan can be attributed to the high prevalence of H. pylori infection and gastric mucosal atrophy.
Introduction
Gastric cancer (GC) is the second leading cause of cancer-related mortality in the world (Ferlay et al., 2010). The risk of GC varies among countries and populations (Ferlay et al., 2010). In East Asia, high-risk countries include Korea, Japan and China, where the age-standardized incidence rate (ASR) is higher than 20 cases of GC per 100 000 inhabitants per year (Ferlay et al., 2010; also available from the International Agency for Research on Cancer, GLOBOCAN 2008; http://globocan.iarc.fr). Intermediate-risk countries include Malaysia, Singapore and Taiwan, and low-risk areas include India, Bangladesh and Thailand (Ferlay et al., 2010). Chronic gastritis is considered a preceding condition in the sequential histopathological changes that lead to cancer (Correa, 1992; Miki, 2006). Helicobacter pylori infection is accepted as the primary cause of chronic gastritis (Suerbaum & Michetti, 2002). H. pylori infection occurs mostly during childhood and H. pylori generally remains in the stomach for life (Goh et al., 2011). Furthermore, the International Agency for Research on Cancer has categorized H. pylori infection as a definite group I carcinogen (IARC Working Group, 1994). Interestingly, despite the high prevalence of H. pylori infection in Africa and South Asia, the GC incidence in these areas is much lower than in other countries – the so-called African and Asian enigmas (Malaty, 2007).
Measurement of serum pepsinogen (PG) is a reliable, non-invasive serological screening test for GC, particularly in Japan (Leung et al., 2008). PG has been identified as a marker of gastric mucosal status, especially of mucosal atrophy (Miki, 2011). There are two forms of PG, PG I and PG II, and both are produced by chief cells and mucous neck cells in the gastric fundus and corpus. PG II is also produced by the pyloric glands in the antrum and Brunner’s glands in the proximal duodenum. Although atrophy is usually diagnosed by endoscopic biopsy, a potentially significant sampling error can occur when identifying atrophy by random biopsy because gastric mucosal atrophy can be patchy. On the other hand, PG has been used as a surrogate marker for atrophy (Kim & Jung, 2010; Sipponen & Graham, 2007). Serum PG I and PG II are upregulated by H. pylori infection. However, because PG II exhibits a greater increase than PG I, the PG I/II ratio decreases in the presence of H. pylori. Over time, as the fundic gland mucosa is reduced, PG I levels gradually decrease, whereas PG II levels remain fairly constant. As a result, a stepwise reduction of the PG I/II ratio is closely correlated with the progression from normal gastric mucosa to extensive atrophic gastritis. Low PG I levels and PG I/II ratios have been associated with severe gastric atrophy and are frequently detected in GC patients (Kim & Jung, 2010; Miki, 2011).
The GC risk can be assessed by the presence of H. pylori infection and gastric atrophy (Sipponen & Graham, 2007). In particular, the combination of H. pylori serology and the measurement of PG I and PG I/II serum levels can be applied to GC screening in Japan (Leung et al., 2008; Miki, 2011). Individuals can be classified into four groups according to the presence of H. pylori infection and PG (the so-called ‘ABC method’): Group A (H. pylori-negative/PG-negative), Group B (H. pylori-positive/PG-negative), Group C (H. pylori-positive/PG-positive) and Group D (H. pylori-negative/PG-positive) (Miki, 2011). In general, Group D is considered the highest-risk group for developing GC, followed by Group C, Group B and Group A (Miki, 2011). The values of PG I ≤70 ng ml−1 and PG I/II ratio ≤3 are used as cut-off points to discriminate between these groups. A meta-analysis showed that a PG I level ≤70 ng ml−1 and a PG I/II ratio ≤3 has a sensitivity of 57 %, specificity of 80 %, positive predictive value of 15 % and negative predictive value of 83 % in screening for atrophic gastritis to detect GC (Miki, 2006).
Bhutan is a small landlocked country in South Asia, located at the eastern end of the Himalayas. It shares borders with the Republic of India and the People’s Republic of China. The ASR of GC in Bhutan is reported to be quite high (24.2 cases per 100 000 population per year) compared with that of neighbouring countries such as India, Bangladesh and Thailand (Ferlay et al., 2010; also available from the International Agency for Research on Cancer, GLOBOCAN 2008; http://globocan.iarc.fr). In our previous study, we found that the overall prevalence of H. pylori infection in Bhutan was 73.4 % when subjects were considered to be H. pylori-positive if at least one of the following tests – rapid urease test (CLO-test), culture, histology, immunohistochemistry or H. pylori serology – showed positive results (Vilaichone et al., 2013). The high incidence of GC in Bhutan can be attributed to the high prevalence of H. pylori infection; however, there have been no reports about the assessment of GC risk based on H. pylori infection and gastric atrophy in Bhutan. We hypothesized that the high prevalence of H. pylori infection and/or advanced gastric atrophy in the absence of H. pylori (e.g. host genetic background, diet) contributes to the high incidence of GC in Bhutan. In this study, we evaluate whether H. pylori infection and PG I and II are associated with gastric atrophy in a population from Bhutan.
Methods
Subjects.
We recruited 381 volunteers (222 female and 159 male) with dyspeptic symptoms and age greater than 16 years (range 16–99 years, mean ± standard deviation 39.6±14.9 years) over a period of 4 days (6–9 December 2010). None of the patients enrolled had a history of taking anti-secretory drugs, such as H2 receptor blockers or proton-pump inhibitors, or of H. pylori eradication therapy before the present study. The survey took place in the capital city of Thimphu (n = 200) and in two rural cities within a radius of 200 km from the capital, Punakha (n = 120) and Wangdue (n = 61). In 2005, there were 92 929 residents in Thimphu, 23 462 in Punakha and 31 135 in Wangdue (National Statistics Bureau in Bhutan, http://www.nsb.gov.bt).
Peripheral blood was collected from each subject after an overnight fast. Samples were collected into serum tubes and centrifuged within 1 h after collection. Separated sera were used for serological identification of H. pylori and measurement of PG levels. We also performed endoscopy on the same day as blood collection. Two gastric biopsy specimens from the antrum and corpus were used for histological examination. Written informed consent was obtained from all participants, and the protocol was approved by the Ethics Committee of Jigme Dorji Wangchuk National Referral Hospital.
Serological analysis of H. pylori infection and PG and CagA status.
Anti-H. pylori IgG levels were quantified using an ELISA kit (Eiken) according to the manufacturer’s instructions. Serum PG I and PG II levels were measured using Pepsinogen ELISA (Eiken) according to the manufacturer’s instructions. Individuals with a serum H. pylori antibody titre ≥10 U ml−1 were classified as H. pylori-positive according to the manufacturer’s instructions; those with PG I levels ≤70 ng ml−1 and a PG I/II ratio ≤3.0 were classified as PG-positive according to Japanese guidelines (Miki, 2011). Serum anti-cytotoxin-associated gene A (CagA) IgG antibodies were measured using an ELISA kit (Genesis Diagnostics); concentrations ≥6.25 U ml−1 were considered positive. According to the manufacturer, the sensitivity and specificity of this kit are 96.3 % and 96.7 %, respectively.
Histology.
Histological samples from antrum, corpus, and both were collected in 375, 372, and 371 subjects, respectively. All biopsy materials were fixed in 10 % buffered formalin for 24 h and embedded in paraffin. Serial sections were stained with haematoxylin and eosin and with May–Giemsa stain. The state of the gastric mucosa was evaluated according to the updated Sydney system (Dixon et al., 1996). The degree of atrophy was classified using four grades: 0, normal; 1, mild; 2, moderate; and 3, marked; samples of grade 1 or more were considered atrophy-positive, as according to a previous report (Bornschein et al., 2012). In addition, the stage of gastritis was assessed according to the Operative Link for Gastritis Assessment (OLGA) system (Rugge & Genta, 2005; Rugge et al., 2007).
Statistical analysis.
Data were analysed using SPSS version 19. Statistical evaluation was performed by the chi-squared test to compare discrete variables and the Mann–Whitney U-test and the t-test to compare continuous variables. Spearman rank coefficients (r) were determined to evaluate the association between PGs and gastric mucosal atrophy. Differences in prevalence in each group were analysed using the Mantel–Haenszel method. Multiple backward stepwise logistic regression analyses were performed to examine the associations of atrophy with the main predictor variables, such as age, sex, H. pylori infection and PG levels. For each variable, the odds ratio and 95 % confidence interval were calculated. A two-tailed P-value of <0.05 was considered significant.
Results
Prevalence of H. pylori infection in Bhutan
The prevalence of H. pylori infection by serology was 71.1 % (271/381). Interestingly, it significantly decreased with age (P = 0.001) (Fig. 1), with prevalences of 77.1 % (84/109) for ≤29 years, 83.0 % (83/100) for 30–39 years, 59.3 % (48/81) for 40–49 years, 67.4 % (31/46) for 50–59 years and 55.6 % (25/45) for ≥60 years. This phenomenon was significant in the Thimphu area. Because the number of subjects older than 50 years in Punakha and Wangdue was insufficient to evaluate the statistical significance, this trend was not observed in these two areas. The prevalence of H. pylori infection differed among the three cities: the highest prevalence was detected in Punakha (100/120, 83.3 %), followed by Wangdue (43/61, 70.5 %) and Thimphu (128/200, 64.0 %). These numbers were analysed by the Mantel–Haenszel method to adjust for age, but the prevalence of H. pylori infection was significantly lower in Thimphu than in Punakha even after age adjustment (P = 0.002), and although statistically insignificant, the infection rates still tended to be lower in Thimphu than in Wangdue (P = 0.12). After univariate analysis, the mean age appeared to be significantly lower for H. pylori-positive subjects than for H. pylori-negative subjects (P<0.01) (Table 1).
Fig. 1.
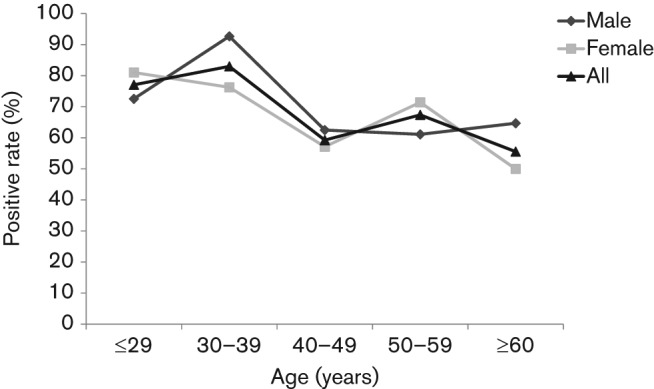
Age-related prevalence of H. pylori infection among the 381 volunteers from Bhutan. H. pylori seropositivity was determined by ELISA. Individuals with a serum H. pylori antibody titre ≥10 U ml−1 were classified as H. pylori-positive.
Table 1. Characteristics of each group.
Unless otherwise stated, values are mean±sd.
| H. pylori-negative | H. pylori-positive | P-value | |
| n | 110 | 271 | – |
| Age (years) | 43.8±15.7 | 37.9±14.3 | 0.001 |
| Male (n) | 42 (38.2 %) | 117 (43.2 %) | 0.37 |
| PG I (ng ml−1) | 65.2±77.8 | 67.6±42.6 | <0.001 |
| PG II (ng ml−1) | 11.8±10.6 | 20.9±15.7 | <0.001 |
| PG I/II ratio | 5.4±1.8 | 3.5±1.3 | <0.001 |
| PG-positive (n) | 8 (7.3 %) | 66 (24.4 %) | <0.001 |
Status of PG and mucosal atrophy in Bhutan
Histological findings showed that 34 cases were grade 0 for atrophy in the antrum; 225 patients had grade 1 atrophy, 106 had grade 2 atrophy and 10 had grade 3 atrophy. In the corpus, 232, 119, 20, and 1 cases were of grades 0, 1, 2, and 3 atrophy, respectively. The staging of gastritis was also assessed according to the OLGA system. Stage I was found in 59.0 % (219/371) of patients, and stage II in 27.5 % (102/371). Stages 0, III and IV were detected in 7.8 %, 4.9 % and 0.8 % of patients, respectively. Because stages I–IV were considered atrophy-positive, 341 patients had mucosal atrophy in the antrum and 140 patients also had mucosal atrophy in the corpus. Although the PG I level did not correlate with gastritis stages, the PG I/II ratio showed a significant inverse correlation with the atrophy score in the antrum and corpus (P<0.001). Among the 72 PG-positive patients, 70 (97.2 %) had atrophy in the antrum (Table 2). Although 271 of the 303 PG-negative patients (89.4 %) also had atrophy in the antrum, the proportion was significantly higher for the PG-positive group than for the PG-negative group (P = 0.03) (Table 2). Furthermore, the association was even stronger in the corpus (P<0.001): 59.7 % (43/72) of the PG-positive patients had atrophy, compared to 32.3 % (97/300) of the PG-negative patients (Table 2).
Table 2. Association between PG and mucosal atrophy in the antrum and corpus.
| Atrophy-positive, n (%) | Atrophy-negative, n (%) | Total, n | |
| Antrum | |||
| PG-positive | 70 (97.2 ) | 2 (2.8 ) | 72 |
| PG-negative | 271 (89.4 ) | 32 (10.6) | 303 |
| Total | 341 (90.9 ) | 34 (9.1 ) | 375 |
| Corpus | |||
| PG-positive | 43 (59.7 ) | 29 (40.3 ) | 72 |
| PG-negative | 97 (32.3 ) | 203 (67.7 ) | 300 |
| Total | 140 (37.6 ) | 232 (62.4) | 372 |
The overall prevalence of the PG-positive subjects was 19.4 % (74/381). The PG I and PG II levels were significantly higher in H. pylori-positive patients (P<0.001) (Table 1), while the PG I/II ratio was significantly lower in H. pylori-positive subjects than in H. pylori-negative subjects (P<0.001). Therefore, the prevalence of the PG-positive status was significantly higher for the H. pylori-positive patients (P<0.001). The correlation between PG levels and H. pylori infection was especially significant in the age groups of ≤29, 40–49 and 50–59 years (P = 0.009, 0.02 and 0.02, respectively) (Fig. 2). After age adjustment, the prevalence of PG-positivity showed an increase with age (P = 0.09), but while this correlation was statistically significant for H. pylori-negative subjects (P = 0.007), it was not for H. pylori-positive patients (P = 0.13).
Fig. 2.
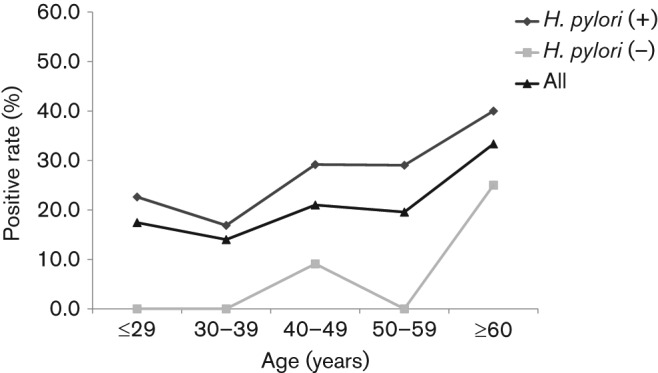
Age-related positive PG status among the 381 H. pylori-positive and -negative volunteers from Bhutan. PG I and II concentrations were determined by ELISA. Individuals with PG I levels ≤70 ng ml−1 and a PG I/II ratio ≤3 were classified as PG-positive. The PG-positive status was significantly higher in H. pylori-positive subjects than in negative subjects, especially in the age categories of ≤29, 40–49 and 50–59 years.
To evaluate predictive factors for the presence of atrophy, we performed a multivariate analysis. Results from a multiple logistic regression analysis examining the association of atrophy with various risk factors are shown in Table 3. In the antrum, older age and H. pylori infection were independent risk factors for the development of atrophy (P = 0.002 and P<0.001, respectively); the PG level was also significantly associated with atrophy in the corpus (P = 0.003).
Table 3. Multivariate analysis of the risk for the presence of atrophy in the antrum and corpus according to age, sex, H. pylori infection and PG status.
| Site | Risk factor | Adjusted odds ratio | 95 % confidence interval | P-value |
| Antrum | Age (per 1 year) | 1.04 | 1.01–1.08 | 0.002 |
| Sex (male) | 1.12 | 0.52–2.41 | 0.76 | |
| H. pylori infection | 6.31 | 2.91–13.66 | <0.001 | |
| PG-positive | 2.15 | 0.47–9.70 | 0.31 | |
| Corpus | Age (per 1 year) | 1.02 | 1.00–1.04 | 0.002 |
| Sex (male) | 1.06 | 0.67–1.67 | 0.79 | |
| H. pylori infection | 3.68 | 2.06–6.56 | <0.001 | |
| PG-positive | 2.31 | 1.33–4.01 | 0.003 |
ABC method used for GC screening
Subjects were classified into four groups according to H. pylori and PG seropositivity: Group A (H. pylori-negative/PG-negative), Group B (H. pylori-positive/PG-negative), Group C (H. pylori-positive/PG-positive) and Group D (H. pylori-negative/PG-positive). Group B (205/381, 53.8 %) was the most dominant, followed by Group A (102/381, 26.7 %), Group C (66/381, 17.3 %) and Group D (8/381, 2.0 %). The distribution of these four groups in each age category is shown in Fig. 3; the age-dependent difference appeared to be significant (P<0.001). Overall, the PG-positive rate among the H. pylori-positive subjects was 24.3 %. The proportional distribution of the PG-positive subjects in the H. pylori-positive group was 22.6 %, 16.8 %, 29.1 %, 29.0 % and 40.0 % for the age categories of ≤29, 30–39, 40–49, 50–59 and ≥60 years, respectively. It was significantly higher for patients older than 60 years than for those in the 30–39 years category (40.0 % vs 16.8 %, P = 0.01).
Fig. 3.
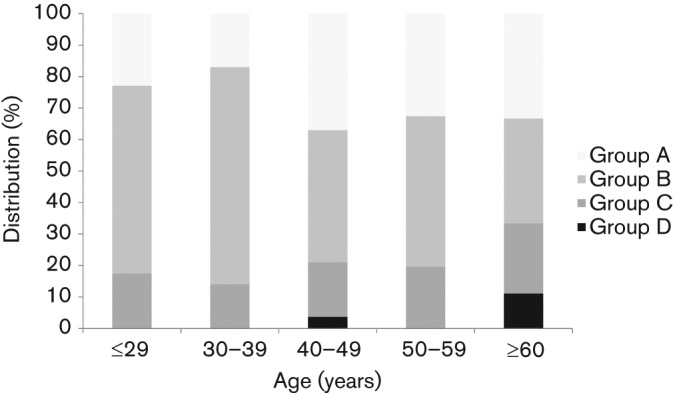
Age-related distribution of H. pylori and PG status among the 381 volunteers from Bhutan. Subjects were classified into four groups according to the results of the two serological tests for H. pylori and PG detection: Group A (H. pylori-negative/PG-negative), Group B (H. pylori-positive/PG-negative), Group C (H. pylori-positive/PG-positive) and Group D (H. pylori-negative/PG-positive). There was a significant age-related difference in the distribution of the four groups.
Status of serum CagA antibody
To confirm the results of the serum H. pylori IgG ELISA, we measured serum CagA antibody levels because patients can potentially remain positive for CagA antibody longer than for other anti-H. pylori antibodies (Shiota et al., 2010). We selected 30 samples to test the levels of CagA antibody. Five samples were found positive by H. pylori serology, as well as by the rapid urease test, culture and histology, while five samples were negative by all four tests. Ten samples were serum H. pylori antibody-positive but H. pylori-negative by the rapid urease test, culture and histology. Another 10 samples were serum H. pylori antibody-negative but H. pylori-positive by at least one of the other tests (rapid urease test, culture or histology). All five samples positive by the four tests were also positive for the serum CagA antibody. Four out of five all-negative samples were also serum CagA antibody-negative. Eight out of 10 samples positive for the serum H. pylori antibody but negative by other tests were positive for the serum CagA antibody. Among the 10 samples negative for the serum H. pylori antibody but positive by at least one other test, seven samples were negative for the serum CagA antibody.
Discussion
The ASR of GC in Bhutan is reported to be quite high (24.2 cases per 100 000 population per year) (Ferlay et al., 2010; also available from the International Agency for Research on Cancer, GLOBOCAN 2008; http://globocan.iarc.fr), although the reason for such a high incidence of GC is not known. Bhutan has no national guidelines or recommendations for GC screening. Therefore, a mass screening program for detection in this population with a high risk for GC is necessary. The risk of developing GC can be categorized by the combination of H. pylori infection and the PG level (Miki, 2011; Sipponen & Graham, 2007). However, the applicability of an ELISA kit for H. pylori infection and PG detection in Bhutan has not been examined.
In our previous study, we found that the overall prevalence of H. pylori infection in Bhutan was 73.4 % when subjects were considered to be H. pylori-positive by at least one of the following tests: rapid urease test (CLO test), culture, histology, immunohistochemistry and H. pylori serology (Vilaichone et al., 2013). The serological test showed significantly higher positive rates than the CLO test, culture, histology and immunohistochemistry (P<0.001, P<0.001, P<0.01 and P<0.01, respectively) (Vilaichone et al., 2013). Therefore, we examined the application of an ELISA kit (manufactured by Eiken Company in Japan) based on a Japanese H. pylori strain for the detection of H. pylori infection in Bhutan. The sensitivity and specificity of the kit for the Japanese population have been reported to be 95.2–100 % and 76.2–80.0 %, respectively (Fujioka & Tokieda, 2000; Matsuo et al., 2001). H. pylori antibody titres varied greatly depending on the test kit used (Burucoa et al., 2013; Miki, 2011). In our separate study, 334 Bhutanese samples were examined by H. pylori culture, the rapid urease test and histology. We found that the sensitivity and specificity of the ELISA kit for the Bhutanese population were 94.8 % and 70.7 %, respectively, comparable with the results obtained in Japan. Furthermore, in this study we measured anti-CagA antibody titres using the CagA ELISA kit described above. Among the 10 samples positive for the serum H. pylori antibody but negative by the rapid urease test, culture and histology, eight samples were positive for anti-CagA antibody. This suggests that most of the data on H. pylori serology were reliable. However, for future studies it might be preferable to develop a domestic ELISA kit using H. pylori strains obtained in Bhutan.
In the study of the Japanese population, Group A (73.1 %) was found to be the most dominant, followed by Group B (16.3 %), Group C (9.3 %) and Group D (1.0 %) (Miki, 2011). In the current study of the Bhutanese population, the rates of Group B (53.8 %) and Group C (17.3 %) were higher than those obtained in Japan (Miki, 2011). The proportion of Group C was approximately 20 % in those aged ≤29 and 30–39 years in Bhutan, which was higher than in Japan (≤5 %) (Yamaoka & Nakajima, 2009). Furthermore, the prevalence of PG-positive subjects in Bhutan (19.4 %) was also higher than in Chinese, Malaysian and Indian populations in Singapore (6.8 %, 6.3 % and 14.2 %, respectively) (Ang et al., 2005). This suggests that the high incidence of GC can be attributed to the high-risk gastric mucosal status in Bhutan. Furthermore, PG-positive rates in Bhutan were higher even in the younger population. It is possible that the advanced atrophy in young people contributes to the high incidence of GC in this country. In addition, gastritis staging according to the OLGA system showed that the proportion of stages I and II gastritis was high when Bhutan was compared with the USA, where 74.7 % (328/439) of the population was stage 0 (Rugge et al., 2007). Interestingly, the prevalence of H. pylori infection was high in India and Bangladesh, which are located near Bhutan, despite the low incidence of GC (Miwa et al., 2002). These differences can be attributed to differences in ethnic genetic background (i.e. the Bhutanese are mainly of East Asian origin, whereas those from India are mainly of Indo-European origin), including variations in gastric acid secretion and genetic polymorphisms in proinflammatory cytokines (Ang et al., 2005; Miwa et al., 2002). In addition, it is possible that the virulence of H. pylori strains is different in Bhutan and India. It has been reported that strains from India, which possess mainly the Western-type CagA (Mukhopadhyay et al., 2000), are less virulent than the East Asian-type CagA strains (Yamaoka, 2010); H. pylori strains in Bhutan might possess the East Asian-type CagA. A study to investigate virulence factors of H. pylori strains in Bhutan is now in progress. The genetic diversity of infective H. pylori strains, as well as environmental factors such as personal hygiene and dietary habits, are also thought to contribute to the geographical differences in the prevalence of H. pylori.
In the three areas of Bhutan investigated in the current study, the highest prevalence of H. pylori infection was found in Punakha, followed by Wangdue and Thimphu. According to the National Bureau in Bhutan, Thimphu is the most developed city of the three (National Statistics Bureau in Bhutan, http://www.nsb.gov.bt). For example, the main source of drinking water in Thimphu is indoor tap water (48.6 %). On the other hand, it is a less frequent source in Punakha (17.2 %) and Wangdue (12.6 %), where the main source of drinking water is outdoor water pipes. Likewise, the main type of toilet facility in Thimphu is an independent flush toilet in the house (39.6 %). These are less common in Punakha (13.4 %) and Wangdue (8.9 %), where the pit latrine is a major type of toilet. Household size has also been reported to be a risk factor for a high H. pylori infection rate (Azevedo et al., 2009; Goh et al., 2011). In Bhutan, the national average household size is 4.6 persons; in Thimphu and in Punakha it is 4.6 persons, and in Wangdue, 4.5 persons. Low family income is also associated with high H. pylori infection rates (Grad et al., 2012). Although we could not obtain information on family incomes for each area, 58.9 % of subjects in Thimphu are reported to be employed in industry or service, whereas 61.1% in Punakha and 49.1% in Wangdue work in the agriculture sector (National Statistics Bureau in Bhutan, http://www.nsb.gov.bt). Altogether, sanitary conditions but not household size correlate with H. pylori infection rates in Bhutan. These findings support the notion that environmental factors, including poor living conditions, are associated with higher H. pylori infection rates (Azevedo et al., 2009; Goh et al., 2011).
Although the prevalence of H. pylori infection in Bhutan decreased with age, we need to be cautious about the decreasing infection rate. The extent of advanced atrophy and intestinal metaplasia is no longer ideal for the growth of H. pylori (Asaka et al., 1994; Craanen et al., 1992). With the establishment of H. pylori infection, the serological test for infection becomes positive (Group B; H. pylori-positive/PG-negative), and as the infection spreads, the PG test also turns positive (Group C; H. pylori-positive/PG-positive). Intestinal metaplasia develops and spreads in the presence of atrophic gastritis, leading to a reduction of H. pylori load in the stomach, which results in negative H. pylori serology (Group D; H. pylori-negative/PG-positive) (Ohata et al., 2004). Thus, Group D comprises subjects with metaplastic gastritis. Therefore, negative H. pylori serology does not rule out the possibility of previous exposure to the infection, especially for the elderly. In fact, Group D was found in patients older than 60 years. Nevertheless, Group A was found in 33.3 % of this age category. As described above, these patients were also negative for serum CagA antibodies, suggesting that the majority of the Group A population are true negative for H. pylori infection. The use of antibiotics in Bhutan might also affect the infection rate. Antibiotics are frequently used to treat any infectious disease in Bhutan (Sethi et al., 2013). Therefore, H. pylori could be eradicated during antibiotic therapy directed towards other infectious diseases. Longitudinal prospective studies are necessary to further clarify H. pylori infection rates in Bhutan.
Our study had several limitations. Based on GC screening conducted in Japan, we used PG I ≤70 ng ml−1 and PG I/II ratio ≤3.0 as cut-off values (Miki, 2011). Variable cut-off values for PG I and the PG I/II ratio have been applied in previous studies (Brenner et al., 2007), but a meta-analysis showed that a PG I level ≤70 ng ml−1 and PG I/II ratio ≤3 had a sensitivity of 57 % and specificity of 80 % in screening for atrophic gastritis to detect GC (Miki, 2006). However, the serum PG level can be affected by ethnic background. In fact, the prevalence of low PG levels was highest in the Indian compared with the Chinese and Malay populations, even after adjustment for sex and H. pylori prevalence (Ang et al., 2005). This showed that the serum PG criterion cannot be used for GC screening in the Indian population (Fock et al., 2008). Other factors, such as age, sex, height, body weight, body surface area, smoking and drinking habits, might be related to PG I and PG II levels (Kim & Jung, 2010). Therefore, different cut-off values used in different studies might affect the sensitivity and specificity of the results (Brenner et al., 2007; Leung et al., 2008). In the Chinese population, the cut-off values for PG I and the PG I/II ratio used for the effective detection of atrophic gastritis were 82.3 ng ml−1 and 6.05, respectively (Cao et al., 2007). In this study, the PG I/II ratio significantly inversely correlated with atrophy score, especially in the corpus. Multiple logistic regression analysis also showed that the PG status was significantly associated with the presence of atrophy in the corpus. We found that the PG test had high specificity for the detection of atrophy (94.1 % and 87.5 % in the antrum and corpus, respectively); however, the sensitivity of this test was low (20.5 % and 30.7 % in the antrum and corpus, respectively). Nevertheless, PG screening deserves further evaluation as a non-invasive test, and future studies are needed to define the optimal PG cut-off values for GC screening in Bhutan. In addition, although the infection rates were found to be associated with socioeconomic status in each area, more detailed information on each individual is necessary, because an intra-familial infection route has also been suggested (Goh et al., 2011). Unfortunately, we could not obtain information on familial relationships, which could have helped elucidate H. pylori infection routes in Bhutan.
In conclusion, we found that the high incidence of GC in Bhutan can be attributed to a high prevalence of H. pylori infection and advanced gastric mucosal atrophy. Serum PG levels were associated with mucosal atrophy in Bhutan, similar to in other countries. In Bhutan, the incidence of GC is quite high when compared with that in neighbouring countries. Therefore, this country could be a good model for a pilot programme for the early detection of and preventive interventions in GC.
Acknowledgements
We thank Y. Kudo, K. Ito and S. Chaithongrat for their technical assistance. Seiji Shiota received a grant from Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (23790798). Varocha Mahachai and Ratha-korn Vilaichone received a grant from the National Research University Project of Thailand Office of Higher Education Commission. Yoshio Yamaoka received grants from the National Institutes of Health (DK62813) and Grants-in-Aid for Scientific Research from MEXT of Japan (22390085, 22659087, 24406015 and 24659200). The authors declare no competing interests.
Abbreviations:
- ASR
age-standardized incidence rate
- CagA
cytotoxin-associated gene A
- GC
gastric cancer
- OLGA
Operative Link for Gastritis Assessment
- PG
pepsinogen
References
- Ang T. L., Fock K. M., Dhamodaran S., Teo E. K., Tan J. (2005). Racial differences in Helicobacter pylori, serum pepsinogen and gastric cancer incidence in an urban Asian population. J Gastroenterol Hepatol 20, 1603–1609 10.1111/j.1440-1746.2005.03898.x [DOI] [PubMed] [Google Scholar]
- Asaka M., Kimura T., Kato M., Kudo M., Miki K., Ogoshi K., Kato T., Tatsuta M., Graham D. Y. (1994). Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer 73, 2691–2694 [DOI] [PubMed] [Google Scholar]
- Azevedo N. F., Huntington J., Goodman K. J. (2009). The epidemiology of Helicobacter pylori and public health implications. Helicobacter 14 (Suppl 1), 1–7 10.1111/j.1523-5378.2009.00703.x [DOI] [PubMed] [Google Scholar]
- Bornschein J., Selgrad M., Wex T., Kuester D., Malfertheiner P. (2012). Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterol 12, 10 10.1186/1471-230X-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H., Rothenbacher D., Weck M. N. (2007). Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer 121, 2782–2786 10.1002/ijc.22992 [DOI] [PubMed] [Google Scholar]
- Burucoa C., Delchier J. C., Courillon-Mallet A., de Korwin J. D., Mégraud F., Zerbib F., Raymond J., Fauchère J. L. (2013). Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter 18, 169–179 10.1111/hel.12030 [DOI] [PubMed] [Google Scholar]
- Cao Q., Ran Z. H., Xiao S. D. (2007). Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis 8, 15–22 10.1111/j.1443-9573.2007.00271.x [DOI] [PubMed] [Google Scholar]
- Correa P. (1992). Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52, 6735–6740 [PubMed] [Google Scholar]
- Craanen M. E., Dekker W., Blok P., Ferwerda J., Tytgat G. N. (1992). Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut 33, 16–20 10.1136/gut.33.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. (1996). Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20, 1161–1181 10.1097/00000478-199610000-00001 [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127, 2893–2917 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- Fock K. M., Talley N., Moayyedi P., Hunt R., Azuma T., Sugano K., Xiao S. D., Lam S. K., Goh K. L. & other authors (2008). Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol 23, 351–365 10.1111/j.1440-1746.2008.05314.x [DOI] [PubMed] [Google Scholar]
- Fujioka T., Tokieda M. (2000). [Validity of serum anti-Helicobacter pylori antibody using enzyme immunoassay for the diagnosis in eradication of Helicobacter pylori]. Jpn J Med Pharm Sci 43, 573–579 (in Japanese). [Google Scholar]
- Goh K. L., Chan W. K., Shiota S., Yamaoka Y. (2011). Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 16 (Suppl 1), 1–9 10.1111/j.1523-5378.2011.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad Y. H., Lipsitch M., Aiello A. E. (2012). Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 175, 54–59 10.1093/aje/kwr288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group (1994). Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61, 1–241 [PMC free article] [PubMed] [Google Scholar]
- Kim N., Jung H. C. (2010). The role of serum pepsinogen in the detection of gastric cancer. Gut Liver 4, 307–319 10.5009/gnl.2010.4.3.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. K., Wu M. S., Kakugawa Y., Kim J. J., Yeoh K. G., Goh K. L., Wu K. C., Wu D. C., Sollano J. & other authors (2008). Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 9, 279–287 10.1016/S1470-2045(08)70072-X [DOI] [PubMed] [Google Scholar]
- Malaty H. M. (2007). Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol 21, 205–214 10.1016/j.bpg.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Matsuo K., Hamajima N., Suzuki T., Nakamura T., Matsuura A., Tominaga S. (2001). Better ROC Curves for a Regionally Developed Helicobacter Pylori Antibody Test. Asian Pac J Cancer Prev 2, 155–156 [PubMed] [Google Scholar]
- Miki K. (2006). Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 9, 245–253 10.1007/s10120-006-0397-0 [DOI] [PubMed] [Google Scholar]
- Miki K. (2011). Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - “ABC method”. Proc Jpn Acad, Ser B, Phys Biol Sci 87, 405–414 10.2183/pjab.87.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Go M. F., Sato N. (2002). H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol 97, 1106–1112 10.1111/j.1572-0241.2002.05663.x [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A. K., Kersulyte D., Jeong J. Y., Datta S., Ito Y., Chowdhury A., Chowdhury S., Santra A., Bhattacharya S. K. & other authors (2000). Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol 182, 3219–3227 10.1128/JB.182.11.3219-3227.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata H., Kitauchi S., Yoshimura N., Mugitani K., Iwane M., Nakamura H., Yoshikawa A., Yanaoka K., Arii K. & other authors (2004). Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 109, 138–143 10.1002/ijc.11680 [DOI] [PubMed] [Google Scholar]
- Rugge M., Genta R. M., OLGA Group (2005). Staging gastritis: an international proposal. Gastroenterology 129, 1807–1808 10.1053/j.gastro.2005.09.056 [DOI] [PubMed] [Google Scholar]
- Rugge M., Meggio A., Pennelli G., Piscioli F., Giacomelli L., De Pretis G., Graham D. Y. (2007). Gastritis staging in clinical practice: the OLGA staging system. Gut 56, 631–636 10.1136/gut.2006.106666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Golparian D., Bala M., Dorji D., Ibrahim M., Jabeen K., Unemo M. (2013). Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from India, Pakistan and Bhutan in 2007-2011. BMC Infect Dis 13, 35 10.1186/1471-2334-13-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota S., Matsunari O., Watada M., Yamaoka Y. (2010). Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol 5, 1885–1893 10.2217/fmb.10.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen P., Graham D. Y. (2007). Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol 42, 2–10 10.1080/00365520600863720 [DOI] [PubMed] [Google Scholar]
- Suerbaum S., Michetti P. (2002). Helicobacter pylori infection. N Engl J Med 347, 1175–1186 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- Vilaichone R. K., Mahachai V., Shiota S., Uchida T., Ratanachu-ek T., Tshering L., Tung N. L., Fujioka T., Moriyama M., Yamaoka Y. (2013). Extremely high prevalence of Helicobacter pylori infection in Bhutan. World J Gastroenterol 19, 2806–2810 10.3748/wjg.v19.i18.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y. (2010). Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol 7, 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka M., Nakajima S. (2009). Prevalence of subjects at a high or very high risk of gastric cancer in Japan. Gut Liver 3, 95–100 10.5009/gnl.2009.3.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]


