Abstract
Converging evidence from cellular, electrophysiological, anatomic, and behavioral studies suggests that the remodeling of synapse structure and function is a critical component of cognition. This modulation of neuroplasticity can be achieved through the actions of numerous extracellular signals. Moreover, it is thought that it is the integration of different extracellular signals regulation of neuroplasticity that greatly influences cognitive function. One group of signals that exerts powerful effects on multiple neurologic processes is estrogens. Classically, estrogens have been described to exert their effects over a period of hours to days. However, there is now increasing evidence that estrogens can rapidly influence multiple behaviors, including those that require forebrain neural circuitry. Moreover, these effects are found in both sexes. Critically, it is now emerging that the modulation of cognition by rapid estrogenic signaling is achieved by activation of specific signaling cascades and regulation of synapse structure and function, cumulating in the rewiring of neural circuits. The importance of understanding the rapid effects of estrogens on forebrain function and circuitry is further emphasized as investigations continue to consider the potential of estrogenic-based therapies for neuropathologies. This review focuses on how estrogens can rapidly influence cognition and the emerging mechanisms that underlie these effects. We discuss the potential sources and the biosynthesis of estrogens within the brain and the consequences of rapid estrogenic-signaling on the remodeling of neural circuits. Furthermore, we argue that estrogens act via distinct signaling pathways to modulate synapse structure and function in a manner that may vary with cell type, developmental stage, and sex. Finally, we present a model in which the coordination of rapid estrogenic-signaling and activity-dependent stimuli can result in long-lasting changes in neural circuits, contributing to cognition, with potential relevance for the development of novel estrogenic-based therapies for neurodevelopmental or neurodegenerative disorders.
I. Introduction
It was first proposed by Ramón y Cajal (1911) that individual neurons form the basic building blocks of the nervous system. This led to the understanding that neurons do not act in isolation but act as a population of physically interconnected cells in a network or neural circuit. Activity in neural circuits is essential for normal brain processes including cognition and behavior. Understanding the principles of information processing by neural circuits will guide us in delineating how the brain transduces environmental cues into physiologic responses, cognition, and complex behaviors. One way to understand how neural circuits react to such stimuli is to study how individual neurons respond to various extracellular signals and to uncover the underlying molecular mechanisms that allow these events to occur.
Steroid hormones, including estrogens, have long been known to influence nervous system development and function (Bueno and Pfaff, 1976; Toran-Allerand, 1976; Losel and Wehling, 2003). Estrogens are among the most studied steroid hormones and have consistently been shown to affect a broad range of physiologic functions, including reproductive, developmental, cardiovascular, and neuronal function (McEwen and Alves, 1999; Nilsson et al., 2001; Lee and Pfaff, 2008; Brinton, 2009; Levin, 2011). Over recent years, there has been a growing appreciation of the complex actions of estrogens within the brain. In addition to their actions in the hypothalamus (Kelly et al., 2005), it has become clear that estrogens can exert effects in multiple regions of the brain, including the cerebral cortex and hippocampus (McEwen and Alves, 1999; Brinton, 2009; Srivastava et al., 2011). The actions of estrogens in these areas are not limited to those observed in females but have also been consistently reported to occur in males as well, albeit in a sexually dimorphic manner in certain cases (Gillies and McArthur, 2010). The emerging notion that estrogens can act in multiple areas of the brain has been accompanied by clinical and basic scientific studies implicating estrogens in regulating cognitive processing and memory in both animal models and humans (Luine, 2008; Sherwin and Henry, 2008; Brinton, 2009; Henderson, 2009). The early findings that estrogens can modulate neuronal physiology and morphology (Bueno and Pfaff, 1976; Kelly et al., 1976; Toran-Allerand, 1976; Gould et al., 1990) have led to an increased focus on how this group of steroids regulates neuroplasticity in neural circuits and thus contributes to cognitive function. The effects of estrogens on cognitive function are of significant interest because of evidence that estrogens may delay the onset or ameliorate the severity of a number of psychiatric and neurodegenerative disorders, such as schizophrenia, anxiety, depression, and Alzheimer’s disease (Cahill, 2006; Kulkarni et al., 2008; Hughes et al., 2009; Gillies and McArthur, 2010; Srivastava and Penzes, 2011). Therefore, elucidating the molecular and cellular mechanisms that underlie estrogenic effects on neuroplasticity is essential not only for understanding their role in normal brain function but also their contribution to neuropathologies and the potential role of estrogens as treatment of such disorders. As with most steroids, estrogens’ actions were thought to occur mainly via the regulation of gene transcription, which often takes hours to days to manifest. Despite the fact that some of the earliest reports of estrogenic actions in the brain were of a rapid nature (Bueno and Pfaff, 1976; Kelly et al., 1976; Toran-Allerand, 1976), much of the work performed to date has focused on the long-term actions of estrogens in the nervous system. However, there are an increasing number of studies that investigate the consequence of rapid estrogenic-signaling on neuronal function and have further linked these effects with the regulation of behavior and cognition (McEwen and Alves, 1999; Kretz et al., 2004; Woolley, 2007; Luine, 2008; Brinton, 2009; Srivastava et al., 2011). In addition, investigations into the cellular and molecular underpinnings of estrogenic signaling are starting to reveal some of the critical mechanisms involved in the modulation of neuroplasticity and thus cognition.
In this review, we will discuss recent insights into rapid modulation of neuroplasticity by brain estrogens within the mammalian forebrain, focusing on its relevance for the rapid modulation of cognition. In particular, we will highlight relevant behavioral studies that indicate a role for estrogens in rapidly modulating cognitive behaviors, mediated by areas located in the forebrain (e.g., cortex and hippocampus); 2) describe the mechanisms that control the bioavailability of active estrogens within discrete regions of the brain, in particular focusing on the ability to synthesize estradiol in nervous tissue; 3) examine the cellular consequence of rapid estrogenic-signaling on plasticity of excitatory neurons, specifically focusing on synapse structure and function; and 4) explore the cellular mechanisms and pathways that potentially underlie estrogen-induced neuroplasticity in excitatory neurons.
By use of this body of literature, we will attempt to establish a model by which estrogenic modulation of neuroplasticity may be used in a physiologic context. We further argue that one way in which estrogens can modulate cognitive function is through (micro) rewiring (Chklovskii et al., 2004; DeBello, 2008) of neural circuitry by centrally (brain) synthesized estrogens. Although this may be only one of a number of mechanisms employed by estrogens to influence cognitive function, we hope this review will aid in broadening overall comprehension of the rapid actions of estrogens in neuronal tissue.
II. Definitions and Concepts
To facilitate our exploration of the mechanisms by which estrogens can regulate neuroplasticity, it is instructive to briefly highlight some basic terminology and concepts. Foremost, we use the term “estrogens” to refer to a class of steroid compounds, of which 17β-estradiol (also known as estradiol and often abbreviated to E2) is considered to be the most biologically active form (Blaustein, 2008). Throughout this review we will interchange between these terms. We use the term cognition, or cognitive function, to refer to processes such as attention, learning, and memory that require frontal brain areas including the cortex and hippocampus. Owing to the burgeoning interest in understanding the effect of rapid estrogenic signaling on neuroplasticity, it is not possible to cover all of the many interesting studies exploring rapid estrogenic signaling within the mammalian forebrain. Therefore, where possible, we direct the reader to other reviews covering more specific topics.
A. Neuroplasticity in Neural Circuits
During the initial formation of neural circuits, neuronal connections are highly "plastic," they can undergo changes in morphology and number in response to numerous stimuli. However, once a neural circuit has been formed, the connections, or synapses, between neurons retain a degree of plasticity, permitting morphologic alterations in response to a number of environmental and extracellular stimuli throughout adulthood. These stimuli include activity-dependent, neuromodulatory, and neurosteroidal signals (Alvarez and Sabatini, 2007; Bhatt et al., 2009; Holtmaat and Svoboda, 2009), and it is thought that the resultant synaptic structural plasticity is essential for normal cognitive function (Chklovskii et al., 2004; DeBello, 2008; Bhatt et al., 2009; Holtmaat and Svoboda, 2009). The majority of the excitatory synapses in the mammalian forebrain occur on specialized structures known as dendritic spines (Fig. 1). These micron-scale, actin-rich structures garnish the dendritic arbor and typically consist of a spine neck and a spine head. It is the changes in morphology and/or number of these excitatory connections that are thought to be a major driving factor in normal brain function (Fig. 1). In addition to physical modifications, alterations in the amount of information flow between neurons through the fine tuning of postsynaptic glutamate receptors is another essential component of functional circuit refinement (Malenka and Bear, 2004; Shepherd and Huganir, 2007; Kessels and Malinow, 2009). This coordination of structural and functional plasticity, which can be referred to as neuroplasticity, can influence physiologic, cognitive, and behavioral processes.
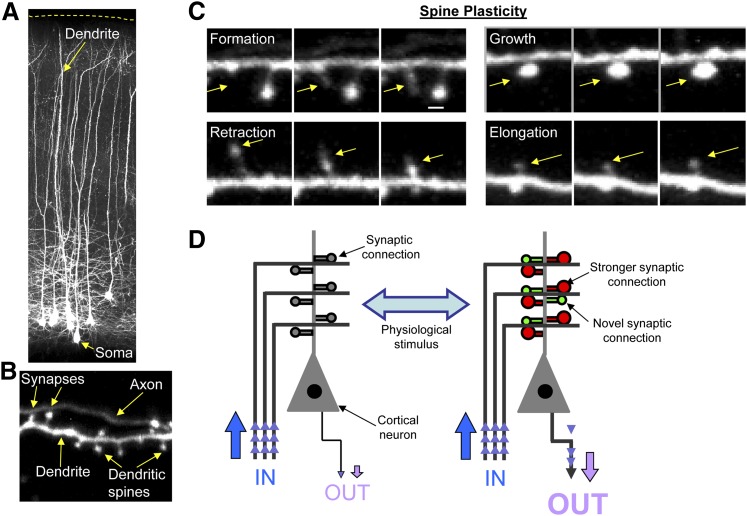
Fig. 1.
Examining neural circuits by two-photon imaging of transgenic mice expressing GFP. (A) Two-photon image of cortical pyramidal neurons in coronal sections of GFP M-line mice; a subset of layer 5 cells express GFP. The main (apical) dendrite of these cells is branched and projects to layer 1; dendritic spines are located along the dendrite. (B) High magnification image of dendrite, dendritic spines, and axon imaged by intravital two-photon microscopy. Dendritic spines protrude from dendrites, allowing neurons to make synaptic connections. Note that the axon is thinner than dendrites and does not have spines. Image demonstrates dendritic spines synapsing with an axon. (C) Examples of dendritic spine plasticity, imaged in vivo: novel spines can form (formation), whereas existing spines can change shape and size (retract, grow, or become longer); spines can also be eliminated. Dendritic spines change morphology in response to numerous extracellular stimuli; this can be a consequence of synaptic activity or neuromodulatory stimuli. Intravital two-photon images were acquired with the aid and expertise of Dr. Jack Waters, Northwestern University. (D) Schematic of cortical circuitry rewiring. The strengthening or weakening of existing synaptic connections and the addition or elimination of synaptic connections allows for the bidirectional rewiring of cortical circuits.
B. Rapid Steroid Signaling in the Nervous System?
Classically, steroid function has been described to occur via the regulation of gene transcription, a process that typically takes hours to days to manifest (Beato, 1989). However, it has been known for many years that steroids can also elicit cellular actions that occur as fast as seconds to minutes, but generally within 1 hour (Losel and Wehling, 2003). The rapid actions of steroids have been described as “nongenomic” and are characterized by the following features: rapid effects taking only seconds to minutes to manifest; actions insensitive to gene and protein synthesis inhibitors; actions initiated by steroid analogs unable to cross the plasma membrane (Losel and Wehling, 2003). Interestingly, these rapid actions of steroids are likely to be evolutionarily conserved mechanisms, as they have been described for both vertebrate and invertebrate organisms (Srivastava et al., 2005; Wehling and Losel, 2006; Evans et al., 2009; Sakamoto et al., 2012; Thomas, 2012). In the mammalian nervous system, steroids have been reported to have effects ranging from the modulation of neurotransmitter systems to the induction of signal pathways as well as effects on synaptic physiology (Paul and Purdy, 1992; McEwen and Alves, 1999; Woolley, 2007; Brinton, 2009; Lokuge et al., 2010; Melcangi et al., 2011). As will be discussed in greater detail in section V, it is becoming clear that the rapid intracellular signaling actions initiated by steroids can subsequently result in the regulation of nuclear events (Vasudevan and Pfaff, 2008), which can occur within 1 hour.
It also is important to differentiate between the sources of steroids and their action within the nervous system. It was considered for some time that the only sources of steroids that could act within the brain were limited to those produced by steroidogenic tissue (e.g., sex organs, adrenal cortex) outside of the central nervous system (CNS). However, observation made by Baulieu and colleagues in the 1980s changed our views regarding the location of steroid synthesis within the brain (Baulieu and Robel, 1990). As such, steroids acting on nervous tissue can be classified into two groups. Firstly, steroids synthesized outside the nervous system can cross the blood-brain barrier because of their lipophilic nature. Once within the brain, these steroids directly exert actions through interacting with steroid receptors within the brain; such steroids have been coined “neuroactive steroids” (Paul and Purdy, 1992; Rupprecht and Holsboer, 1999). On the other hand, the observations that steroids could accumulate in the brain even in the absence of either gonads or the adrenal cortex and that steroids levels within the brain did not always match those within the periphery, led to the proposal that steroids could be synthesized de novo within the brain (Baulieu and Robel, 1990; Do Rego et al., 2009). Following the discovery that the enzymes required for the synthesis of steroids could be localized to a number of neuronal cell types (Do Rego et al., 2009; Pelletier, 2010), steroids synthesized locally in the brain have been termed “neurosteroids” (Baulieu and Robel, 1990; Paul and Purdy, 1992). In this review, we will refer to estrogens produced outside of the nervous system as “peripherally synthesized” and those within the CNS as “centrally” or “brain-synthesized.”
III. Rapid Estrogenic Regulation of Behavior
Estrogenic regulation of behaviors, including cognitive function, has been the focus of a number of extensive reviews (Galea et al., 2008; Walf and Frye, 2008; Brinton, 2009; Frick, 2009; Henderson, 2009; Saldanha et al., 2011; Choleris et al., 2012). As such, we only wish to highlight studies that provide clues regarding the ability of rapid estrogenic signaling to modulate cognition. Specifically, we will review studies in which the time-specific administration of estrogens can modulate cognition or studies in which behavioral paradigms assess cognitive function within 1 hour. We will focus on studies from avian and rodent model systems as they have provided a large amount of information in this area.
A. Studies from Zebra Finch
Studies in the Zebra Finch songbird have demonstrated a critical role for brain-synthesized estrogens in the rapid processing of sensory information. These birds learn complex vocalizations, or songs, which require the encoding of behaviorally relevant auditory signals and subsequent reproduction of these vocalizations (London and Clayton, 2008; Mooney, 2009). The processing of these signals occurs predominately within the caudomedial nidopallium (NCM) nucleus of the Zebra Finch forebrain, which is the songbird homolog of the mammalian auditory association cortex (London and Clayton, 2008; Mooney, 2009). The NCM is rich in components required for the rapid production and detection of estrogenic signals (Saldanha et al., 2000; Remage-Healey et al., 2008; Tremere et al., 2009), offering an excellent model to investigate how brain-derived estrogens can modulate sensory encoding and processing. Rapid (within minutes) 17β-estradiol production in the NCM has been detected in response to social interactions (Remage-Healey et al., 2008). This increase in 17β-estradiol levels increases the gain of auditory-driven responses via a nongenomic mechanism and is likely also to involve inhibition of presynaptic GABAergic transmission (Tremere et al., 2009). In addition, it has been suggested that the main role for increased local production of 17β-estradiol within the NCM is to increase auditory coding efficiency (Remage-Healey et al., 2010; Tremere and Pinaud, 2011). Accordingly, acute blockade of 17β-estradiol synthesis within the NCM disrupts songbird song preference (Remage-Healey et al., 2010). Together these studies have pointed to an essential role of brain-synthesized 17β-estradiol in the rapid modulation of sensory encoding and socially relevant auditory discrimination, specifically in cortical circuitry found in the NCM of Zebra Finch songbirds.
B. Studies Using Rodent Models
Rodent models have been extensively used to investigate the role of estrogens in the control of cognitive function, including learning and memory, and are reflected by the number of publications in this area. Ovariectomy has been widely used to examine the contribution of circulating hormones to various behaviors. This approach preserves normal development and effectively removes the circulating estrogens and progesterone in adult animals. However, it is noteworthy that some androgens are produced by the adrenal cortex and thus may still be present after ovariectomy. In addition, the time between ovariectomy surgery and behavioral testing varies between studies, and thus the amount of time without these sex hormones can be significant. Moreover, the effect of ovariectomy on brain-synthesized estrogens, or the contribution of centrally produced estrogens on cognition, is less clear. These important limitations should be kept in mind when interpreting the extensive behavioral literature on ovariectomized (OVX) female rodents.
Studies in rats have yielded ample evidence that estrogens exert powerful actions on cognitive processes. OXV female rats display impaired performance in working memory and spatial navigation such as demonstrated by delayed match-to-sample tasks, T-maze alternation tasks, and various radial maze tasks (Daniel et al., 1997; Bimonte and Denenberg, 1999; Gibbs, 2007; Gibbs and Johnson, 2008). In many of these studies, these effects were reversed by an acute treatment with 17β-estradiol or other potent estrogens, including synthetic estrogen receptor modulators (SERMs) (Daniel et al., 1997; Bimonte and Denenberg, 1999; Gibbs, 2007; Gibbs and Johnson, 2008; Sherwin and Henry, 2008). Similar effects have also been observed in female OVX rats in object recognition tasks, which rely on both cortical and hippocampal processing (Ennaceur et al., 1997; Barker and Warburton, 2011). In female OVX rats, administration of 17α-estradiol or 17β-estradiol, either 30 minutes prior or immediately post-training, was able to enhance memory acquisition and consolidation (Luine et al., 2003). Interestingly, this enhancement was not observed when either estradiol isomer was administered 2 hours post-training, suggesting that there is a specific time frame in which these estrogens were able to enhance memory acquisition and consolidation (Luine et al., 2003). In a separate study, it was shown that treatment of OVX rats with the SERMs propyl pyrazole triol [PPT; selective agonist for estrogen receptor (ER) α] or diarylpropionitrile (DPN; selective agonist for ERβ) was able to enhance memory performance in object recognition tasks when administered immediately but not 60 minutes post-training (Walf et al., 2006). This indicates that both estrogen receptors can contribute to rapid estrogenic modulation of memory and have a specific time frame in which they may be effective.
It is critical to note that in the studies described above use behavioral paradigms in which, after an initial training phase, memory is tested at least 4 hours later. Nevertheless, the administration of estrogens prior to behavioral training may indicate a role of estrogens in enhancing the acquisition/formation of memory, whereas post-training administrations may be more indicative of a role for estrogen in memory consolidation (Luine et al., 2003; Luine, 2008; Walf et al., 2006). These data have been suggested to demonstrate a specific time frame, or “critical time,” in which 17β-estradiol or SERMs are effective in enhancing memory (Luine, 2008; Inagaki et al., 2010). The existence of a specific time frame for estradiol-mediated enhancements of cognition suggests that the mechanism by which estrogens enhance cognition is rapid and transient. Therefore, initiation of these estrogen-dependent mechanisms must be timed, or coordinated, with the onset of learning and memory (this potential mechanism will be explored in greater detail in section IX). A possible caveat to this interpretation lies in the fact that the administered hormones may not have been fully metabolized and are still present within the animal several hours later when testing occurs. Therefore, it is possible that the estrogens are influencing memory retrieval, rather than formation or consolidation. However, the presence of enzymes involved in the metabolism of estrogens (see section IV) in multiple brain regions diminishes viability of this alternative hypothesis.
Multiple studies have also used mouse models to investigate rapid estrogenic influences on behaviors (Frick, 2009; Choleris et al., 2012). In female OVX mice, administration of 17β-estradiol immediately after training enhances memory consolidation, as determined in an object recognition behavioral paradigm (Fernandez et al., 2008; Fan et al., 2010). In addition, 17β-estradiol rapidly (within 1 hour) increased the phosphorylation, and thus activation, of extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K) (Fernandez et al., 2008; Fan et al., 2010). These data would seemingly suggest that rapid activation of membrane/cytosolic signaling pathways are required for estradiol-mediated memory enhancements. However, memory was tested (up to) 48 hours after training and 17β-estradiol administration, providing sufficient time for gene transcription to occur. Critically, the administration of inhibitors that blocked ERK or PI3K signaling pathways, simultaneously with 17β-estradiol, abolished estradiol-dependent enhancement of memory (Fernandez et al., 2008; Fan et al., 2010). This strengthens the notion that the initiation of rapid membrane/cytosolic signaling pathways is required for estradiol enhancement of memory consolidation. However, it does not occlude the possibility that some form of cross-talk or coordinated signaling is occurring between membrane/cytosolic and nuclear compartments (see section V for a more in-depth discussion). Recently, Phan and colleagues (2011, 2012) have used “rapid learning” paradigms in which the time from the initial injection of drug to the end of the test is 45 minutes. These experiments showed that treatment of OVX female mice with specific concentrations of 17β-estradiol or PPT 15 minutes prior to training were able to increase social and nonsocial learning (Phan et al., 2011, 2012). Conversely, DPN only had a slight effect on object placement but not object recognition. The lack of effect of DPN on object recognition differs from that seen in rat (Walf et al., 2006); however, whether this is because of species difference, treatment timing (pre- vs. post-training administration), or difference in paradigms is not known. Nevertheless, these studies clearly demonstrate that estrogens are capable of modulating cognitive function in a time frame that is consistent with a nongenomic mode of action.
Studies of knockout mice lacking either of the two classic estrogen receptors (ERα, ERβ) have added support for a critical role of estrogens in rapidly modulating cognitive ability. Indeed, OVX wild-type, but not ERβ knockout (KO), mice administered with 17β-estradiol or DPN immediately after training displayed improved performance in both object recognition and placement tasks (Walf et al., 2008a). Comparisons of the ability of 17β-estradiol to influence ERα and ERβ KO mice on a Y-maze task revealed that ERα KO, but not ERβ KO, mice showed an improvement after treatment with 17β-estradiol (Liu et al., 2008), indicating a critical role for ERβ in mediating rapid estrogenic regulation of cognitive function. Interestingly, double ERα/β KO mice are still responsive to acute 17β-estradiol administration (Kudwa and Rissman, 2003), indicating that other estrogen receptors may also contribute to the rapid regulation of estrogenic actions in vivo.
A number of studies have also examined whether estrogens are able to influence cognition in male rodents. Castration of male rats impairs working memory tasks in an estrogen-sensitive manner (Kritzer et al., 2007; Luine, 2007; Aubele et al., 2008; Gibbs and Johnson, 2008), whereas male estrogen receptor knockout mice display impaired social recognition memory (Sanchez-Andrade and Kendrick, 2011). Additionally, administration of 17β-estradiol in aged male and female mice was able to improve performance in inhibitory and water maze learning tasks (Frye et al., 2005). Perhaps some of the most compelling behavioral data for a role of estrogens in influencing male cognition come from mice lacking the aromatase cytochrome P450 enzyme. Aromatase, encoded by the cyp19 gene, is the final enzyme and rate-limiting step in the biosynthesis of estrogens and is highly expressed in multiple regions of the brain (see section IV for an expanded discussion). Several strains of aromatase KO mice have been produced (Fisher et al., 1998; Honda et al., 1998; Toda et al., 2001), resulting in mice that are estrogen deficient and hyperandrogenic. Male aromatase KO mice display impairments in spatial reference memory (Martin et al., 2003), whereas severe deficits in social memory were seen in both gonadally intact and castrated male aromatase KO mice (Pierman et al., 2008). Interestingly, when gonadally intact or castrated male aromatase null mice were treated with estradiol benzoate, in association with dihydrotestosterone propionate, a recovery in social recognition was observed (Pierman et al., 2008). Collectively, these studies indicate that 17β-estradiol contributes to cognition in male rodents. Further work will be necessary to describe mechanisms of estrogen production and action in the male brain and to contrast these mechanisms with their female analogs.
The use of KO animal models should overcome many of the potential confounding issues arising from a pharmacological approach. However, current ER- and aromatase-KO mouse models are, for the most part, conventional knockouts, in which the gene of interest is lacking throughout the body. The use of alternative strategies, such as conditional gene knockout methods, would allow researchers to manipulate the expression of estrogen-related genes in a region-specific, cell type-specific, and even temporally controlled manner. For example, this would provide a more suitable system in which to interrogate the role(s) of classic and nonclassic ERs expressed in the forebrain in fast modulation of cognition. Using conditional gene knockout mice to manipulate aromatase expression would address the relative contribution of centrally/brain-synthesized estrogens in learning and memory. Region- and cell-specific ER knockout mice were used previously to understand estrogen’s role in reproduction and neuroprotection (Dupont et al., 2000; Spence et al., 2011), demonstrating the feasibility of such animal models. Another approach that has recently been employed to examine the role of ERα in sexual and aggressive behavior has been to knock down protein expression using virally encoded si- or sh-RNA interference (RNAi) constructs in a brain-specific manner (Ribeiro et al., 2012; Sano et al., 2013). Such approaches, when combined with cell-specific promoters, would potentially enable examination of ER function in both a site- and cell-specific manner. An alternative to resource-intensive transgenic mouse generation and viral-based RNAi vectors is to electroporate plasmid DNA into fetuses in utero (Taniguchi et al., 2012). This technique allows for spatially restricted gene manipulation in a temporally controlled manner without the use of episomal viral vectors (Srivastava et al., 2012c; Taniguchi et al., 2012).
C. Dose and Routes of Administration
A number of studies have also investigated what dose of estrogens is most effective at enhancing performance on various behavioral tasks (Luine et al., 2003; Inagaki et al., 2010; Phan et al., 2011). This has revealed an inverted U-shaped dose response curve as opposed to a more traditional sigmoid log-dose response curve for the enhancing actions of estrogens on cognition. For example, in OVX female rats, a single post-training subcutaneous injection of either 5 µg/kg 17β-estradiol or 1–2 µg/kg 17α-estradiol, but not lower or higher concentrations, was effective in enhancing object recognition (Inagaki et al., 2010). In female OVX mice, a single pretraining subcutaneous injection of 50 or 75 µg (per 30 g mouse) PPT was sufficient to enhance object recognition, whereas single injections of lower or higher concentrations were not (Phan et al., 2011). Interestingly, an inverse U-shaped dose-response curve has also been reported in estrogenic-mediated enhancement of spatial memory in OVX female mice (Gresack and Frick, 2006). This suggests that an inverse U-shaped pattern of estrogenic effects on memory may be applicable to a number of memory tasks. This inverted U-shaped pattern of estrogen’s effect on cognition may reflect the optimal level of receptor activation. Another interpretation of these data are that different doses can induce agonist-specific coupling, or “ligand-selective receptor conformation,” differentially coupling receptors to distinct signaling pathways or specific receptor states (e.g., dimerization, inactivation/desensitization, internalization) (Evans et al., 1995; Christopoulos and Kenakin, 2002; Srivastava et al., 2005). A similar phenomenon has been described for a Drosophila G-protein-coupled receptor activated by ecdysteroids (insect sex hormones) (Srivastava et al., 2005; Evans et al., 2009). The vast majority of studies examining the rapid effects of estrogens on cognition in rodents have used single systemic injections of 17β-estradiol. Often the concentration of estradiol within specific regions of the brain has not been measured. Thus, it is not entirely clear what is the actual concentration of estradiol acting at receptors in the brain. In addition, although a range of 17β-estradiol doses has been used in different studies, the majority are reported to fall within physiologic levels of plasma estradiol levels; however, higher doses of 17β-estradiol are thought to produce supraphysiological levels of plasma estradiol (MacLusky et al., 2005; Scharfman et al., 2007; Phan et al., 2012;). On the other hand, it has been argued that such concentrations are representative of local estradiol levels within the brain and that such concentrations are required to initiate rapid molecular and cellular responses (see section IV; Cornil et al., 2006; Hojo et al., 2009 for greater discussion). But it is not clear what effect these supraphysiological levels could have, if any, outside the central nervous system. Indeed, it is difficult to rule out effects of estrogens on peripheral systems that may contribute (directly or indirectly) to the observed effects on cognition. To circumvent these limitations, a number of studies have infused 17β-estradiol directly into the brain. The local administration of estradiol benzoate into the frontal cortex of OVX female rats 40 minutes prior to testing significantly improved performance compared with control mice in a win-shift version of the radial arm maze to test spatial working memory (Sinopoli et al., 2006). Similarly, infusion of 17β-estradiol directly into the dorsal third ventricle or dorsal hippocampus immediately post-training was also sufficient to enhance object recognition in middle-aged female OVX mice (Fernandez et al., 2008; Fan et al., 2010). Recently it has been shown that acute inhibition of local synthesis of estrogens or the acute blockage of estrogen receptors by directly infusing drugs into the rostral anterior cingulate cortex (rACC), but not the prefrontal cortex, was sufficient to block formalin-induced conditional place aversion in male, female, and OVX female rats (Xiao et al., 2013). Importantly, this indicates a critical role for centrally synthesized 17β-estradiol, specifically within the rACC, in aversive learning. It is also noteworthy that this study suggests that the acute effects of centrally synthesized estrogens, at least on pain-related aversion cognitive tests, do use overlapping mechanisms in male and female rats (Xiao et al., 2013).
The aforementioned behavioral investigations clearly show that estrogens infused directly into cortex or hippocampus are able to rapidly modulate cognition. This site-specific distinction is important because there are functional interactions between specific areas of the cortex (e.g., the prefrontal cortex) and the hippocampus required for complex behaviors (Snyder et al., 2010). Despite arguments regarding the existence of a discrete prefrontal cortex in rodents (Uylings et al., 2003), it is nevertheless clear that interactions between cortical and hippocampal areas are required for complex behaviors (Ennaceur et al., 1997; Barker and Warburton, 2011). Although the consequences of rapid estrogenic signaling on hippocampally based behaviors have been well investigated, our understanding of the influence of estrogens on cortically based behaviors is not as well developed. Therefore, to fully appreciate the extent of the modulatory actions estrogens exert in the forebrain, further investigation is needed.
Collectively, the extant literature demonstrates that estrogens are capable of rapidly influencing cognition in both male and female animals. It has also been demonstrated that infusion of an aromatase inhibitor, leading to a rapid decrease (within 30 minutes) in local estradiol levels, immediately before training attenuates behavioral responses (Xiao et al., 2013). Critically, inhibition of aromatase blocked the acquisition of song learning as assessed within 30 minutes (Remage-Healey et al., 2010). These data suggest that local estradiol production in specific brain regions can modulate cognition and, moreover, that locally produced estradiol is required for certain rapid behavioral responses. However, the contribution of peripherally versus centrally synthesized estrogens to the rapid modulation of behavior has yet to be fully explored. It is clear that a combination of approaches ranging from pharmacology to gene manipulation will be required to fully understand the contribution of estrogens to cognitive performance in a region-, cell-, and age-specific manner.
IV. Control of Estrogen Bioavailability in the Brain
A major question can be posed from the animal studies above: what is the origin of the estrogens that underlie these rapid effects? Currently there is a paucity of studies that have directly addressed this aspect of estrogen signaling experimentally, but several in-depth reviews have been written addressing this issue (Balthazart and Ball, 2006; Cornil et al., 2006; Warner and Gustafsson, 2006; Pfaff and Ribeiro, 2010). Here we only wish to highlight some specific aspects that are relevant for the estrogens' fast control over cognition in the forebrain. Specifically we consider the potential source(s) of estrogens and also the mechanisms that control the bioavailability of 17β-estradiol and its precursors, within discrete regions of the brain.
A. Peripherally Versus Centrally Synthesized Estrogens
It has been suggested that for estrogens to rapidly influence behavior, its bioavailability must be rapidly controlled (Cornil et al., 2005; Balthazart and Ball, 2006). It has been argued that the primary source of estrogens underlying these rapid actions are estrogens synthesized de novo in the brain (Cornil et al., 2006; Azcoitia et al., 2011; Saldanha et al., 2011; Srivastava and Penzes, 2011). This claim is strengthened by observations that even after removal of sex organs (gonadectomy) there are still significant levels of estrogens in both male and female brains (Yague et al., 2006; Ishii et al., 2007; Hojo et al., 2009; Boon et al., 2010; Konkle and McCarthy, 2011; Saldanha et al., 2011). However, this situation is complicated by the fact that the bioavailability of estrogens can also be influenced by the presence of peripherally or centrally produced androgen precursors [e.g., testosterone, dehydroepiandrosterone (DHEA) and androstenedione]. Indeed, the adrenal cortex is also a source of DHEA in males and females. As this tissue is not removed during gonadectomy, it can represent a potential source for the production of estrogens. Collectively, it could be suggested that there are three potential sources for the estrogens that act within the forebrain:
Source 1: Circulating estrogens produced outside the CNS;
Source 2: Estrogens produced through the conversion of circulating androgen precursors locally within nervous tissue; and
Source 3: Local estrogen synthesis directly from cholesterol sources.
Considering the rapid onset and the transient nature of the rapid modulation of cognition by estrogens, it is unlikely that fluctuations in circulating plasma levels of estrogens (Source 1) are dynamic enough to explain rapid responses in male and female brains. Rises in the level of estrogens occur over a period of hours during proestrus. Moreover, changes in circulating levels of estrogens occur on a much slower scale in males (Cornil et al., 2006; Pfaff and Ribeiro, 2010). It has been reported that plasma concentrations of estrogens are not high enough to trigger these rapid responses (Balthazart and Ball, 2006; Hojo et al., 2009). Although the physiologic relevance of the reported concentrations required to initiate rapid actions have been questioned (Warner and Gustafsson, 2006), recent reports have described picomolar to nanomolar concentrations of estrogens within the specific brain regions, compared with lower picomolar concentrations in the plasma (Ishii et al., 2007; Hojo et al., 2009; Konkle and McCarthy, 2011). This suggests that a nanomolar concentration of estradiol is required to initiate rapid molecular and cellular responses, and moreover, it is not a concentration reached by the circulating hormone. It is also important to note that changes in circulating estradiol levels would affect all estrogen-sensitive regions of the brain and would not allow for region- or cell-specific actions of estrogens.
Studies by Naftolin et al. (1971a,b) first demonstrated that nervous tissue was capable of synthesizing estradiol from androgen precursors. The central synthesis of estrogens from androgens would allow synthesis of estrogens within a time frame consistent with rapid actions and at a high enough concentration to initiate rapid responses. Moreover, it would allow estrogens to act in a region-, cell-, or even synapse-specific level (Saldanha et al., 2011; Srivastava et al., 2011). The conversion of circulating androgens (Source 2) into estrogens locally within the brain is controlled by the enzyme aromatase. As will be discussed in greater detail in the following sections, aromatase is distributed throughout the male and female brain, and its enzymatic activity can be rapidly modulated, resulting in the production of estrogens in a matter of minutes (Balthazart and Ball, 2006; Cornil et al., 2006; Azcoitia et al., 2011; Saldanha et al., 2011). Therefore, the conversion of androgens into estrogens can occur within a time frame consistent with rapid estrogenic responses in male and female brains (Rune and Frotscher, 2005; Ishii et al., 2007; Hojo et al., 2009; Azcoitia et al., 2011; Konkle and McCarthy, 2011). It must be noted that these mechanisms are dependent on the bioavailability of the androgen precursors. As such, controlling levels of the precursor would, therefore, impact local estrogen levels within the brain. This may be achieved via a number of possibilities that include variations in circulating androgen. However, whether this pathway is sufficient to account for the production of estrogens even in the absence of steroidogenic tissues outside of the brain has yet to be experimentally determined.
Evidence for the presence of multiple enzymes allowing the biosynthesis of estrogens from brain-derived cholesterol (Do Rego et al., 2009; Pelletier, 2010; Pfrieger and Ungerer, 2011) offers another mechanism for controlling the bioavailability of estrogens within the brain (Source 3) and is covered in detail in the next section. This mechanism would provide a source of estradiol independent of nonneuronal sources, but it would seem that coordinating the effect of estradiol in large or multiple areas would be difficult to achieve because of the requirement for rapid and reliable androgen precursor synthesis.
In the context of forebrain function, current experimental evidence does not allow us to identify with certainty the source of estrogens that underlies modulation of cognition. In reality, it is likely that a complex interaction between peripherally and centrally synthesized estrogens contributes to the rapid modulation of behavior by estrogens.
B. Steroidogenic Enzymes in the Central Nervous Systems
The enzyme StAR (steroidogenic acute regulatory protein) is a transport protein that regulates the transfer of cholesterol within the mitochondria membrane, which is thought to be the rate-limiting step in general steroidogenesis (Stocco, 2001). In addition to its expression in the ovary and adrenal glands, StAR is expressed widely throughout the brain (Furukawa et al., 1998; Lavaque et al., 2006), including cortical and hippocampal pyramidal neurons and astrocytes (Wehrenberg et al., 2001; Lavaque et al., 2006). Once in mitochondria membrane, the cytochrome P450 cholesterol side-chain cleavage enzyme converts cholesterol into pregnenolone, a precursor for a number of steroids, including DHEA, androstenedione, and estradiol. P450 side-chain cleavage is abundant in human cortex and hippocampus (Pelletier, 2010), and it has been shown to be coexpressed in pyramidal neurons that also express StAR in rodents (Wehrenberg et al., 2001).
The presence of the enzyme cytochrome P450 17α-hydroxylase has also been described in neurons and astrocytes (Zwain and Yen, 1999). This enzyme metabolizes pregnenolone into DHEA, which can then be metabolized into androstenedione and finally into estradiol (Zwain and Yen, 1999). A number of other enzymes including 3β-hydroxysteroid dehydrogenase) and 17β-hydroxysteroid dehydrogenase have also been identified within the brain (Wehrenberg et al., 2001; Pelletier, 2010), collectively providing a direct biochemical pathway for the biosynthesis of steroid precursors of estrogens from cholesterol or from circulating precursors. It is also important to note that many of these estrogenic precursors have actions of their own within the CNS (Rupprecht and Holsboer, 1999; Belelli and Lambert, 2005), complicating signal transduction. For discrete, centrally produced estrogens to be the primary source that modulates cognition, it would be predicted that the mechanism(s) underlying the biosynthesis of estradiol would need to be highly regulated and efficient.
Aromatase has been identified in the hypothalamus, hippocampus, visual cortex, and temporal cortex in avian, mammalian, and human brain (Rune and Frotscher, 2005; Yague et al., 2006; Boon et al., 2010; Azcoitia et al., 2011; Saldanha et al., 2011). Although the expression of aromatase has been found in glial cells, it is highly expressed in pyramidal neurons (Kretz et al., 2004; Yague et al., 2006, 2008). Enzymes, including 2- and 4-hydroxylase and catechol-O-methyltransferase, which are involved in the metabolism of estrogens into inactive (or less active) water-soluble metabolites, have also been detected within the brain (Zhu and Conney, 1998). Additionally, sulfotransferase and sulfatase enzymes have also been localized to nervous tissue (Mensah-Nyagan et al., 2000; Kríz et al., 2008). These enzymes facilitate the sulfation or the hydrolysis of steroid sulfates into their unconjugated forms (Hobkirk, 1985). Sulfated estrogens are unable to bind to ERs and thus are inactive, providing another mechanism for their inactivation. In androgen biosynthesis, these enzymes play a critical role in the conversion of DHEA to its sulfated form (DHEAS). DHEAS is a precursor for androstenedione that can be converted into estradiol (Kríz et al., 2008); the conversion of DHEA into DHEAS provides another mechanism to control the bioavailability of estrogen precursors within nervous tissue. These enzymes demonstrate that there are mechanisms in place within the brain that can metabolize estrogens into less active metabolites. Together the presence of these enzymes demonstrates that specific mechanisms required for the rapid synthesis and metabolism of estrogens in the brain exist.
C. Control of Aromatase Function
Aromatase enzyme activity has been described in several brain regions and cell types in vertebrate brains ranging from fish to humans (Naftolin et al., 1971a,b; Callard et al., 1978; MacLusky et al., 1986). Changes in aromatase activity can occur in a matter of minutes. For example, the enzyme activity of aromatase is significantly reduced after copulatory behavior (Cornil et al., 2005; Saldanha et al., 2011). Moreover, pharmacological investigations have demonstrated that acute activation of glutamate receptors or potassium-induced depolarization can rapidly (within minutes) decrease aromatase activity in the quail brain (Balthazart et al., 2001). This is mirrored by findings in Zebra Finch, where retrodialysis of glutamate reduces local 17β-estradiol concentrations within a similar time frame (Remage-Healey et al., 2008). Recently, evidence was presented that acute fluctuations in brain-synthesized 17β-estradiol levels, mediated by aromatase activity in the cortex of Zebra Finch, are controlled by specific depolarization-sensitive, calcium-dependent events (Remage-Healey et al., 2011).
More recent work has now gone on to show that aromatase activity can be regulated by phosphorylation of the aromatase protein in a Ca2+-dependent manner and by protein kinase A (PKA) and protein kinase C (PKC) (Balthazart et al., 2006; Charlier et al., 2011). Moreover, mutagenesis studies of predicted phosphorylation sites on human aromatase have revealed that a predicted PKA phosphorylation site is required for basal aromatase activity. Indeed, mutation of the serine 118 residue to alanine (S118A) was sufficient to reduce basal aromatase activity (Charlier et al., 2011). How this mutation may affect neural circuits or behavior is not known. Nevertheless, unmasking the mechanisms that rapidly control aromatase activity, and therefore local production of estrogens, will provide critical insight into the role of estrogens in the brain.
D. Synaptic Localization of Aromatase
Extensive evidence suggests that aromatase is a synaptic protein. In the hypothalamus, electron microscopy imaging has demonstrated a synaptic localization for aromatase in avian, mammalian, and human tissue (Naftolin et al., 1971a,b; Callard et al., 1978; MacLusky et al., 1986; Naftolin et al., 1996; Yague et al., 2006, 2008; Srivastava et al., 2010; Remage-Healey et al., 2011). Biochemical studies using subcellular preparations of brain tissue have detected high levels of aromatase activity in isolated presynaptic tissue preparations (Mak et al., 1985; Schlinger and Callard, 1989; Peterson et al., 2005; Remage-Healey et al., 2011), indicating a potential role of estrogens at synapses. In mature cultured cortical neurons derived from embryonic rats, we found aromatase immunoreactivity to be present at synapses (Srivastava et al., 2010). Aromatase colocalizes with the postsynaptic marker postsynaptic density protein 95 (PSD-95) and the presynaptic marker bassoon in cortical neurons. In addition, aromatase was detected in tau5-positive axonal processes, indicating that a portion of aromatase is present at presynaptic terminals (Srivastava et al., 2010). Consistent with this anatomic localization for aromatase, it has been shown that rapid (30 minutes) changes in 17β-estradiol levels, under the control of an excitatory, voltage-gated Ca2+ channel-dependent mechanism, were seen at presynaptic terminals in the NCM of songbirds (Remage-Healey et al., 2011). This study presents strong evidence that local production of estrogens within the brain is regulated by electrochemical signals, supporting a hypothesis that estrogens may be considered a neuromodulator (see Saldanha et al., 2011 for a recent in-depth review on this topic). Collectively, the presence and regulation of aromatase at presynaptic terminals places the machinery required for the de novo production of estrogens at an ideal location for this neurosteroid to act on postsynaptic structures.
Interestingly, a number of studies have also demonstrated that aromatase is located in postsynaptic structures (Naftolin et al., 1996; Kretz et al., 2004; Prange-Kiel et al., 2006). These data suggest that estrogens may be produced on either side of the synapse. Such a pattern of localization conveys great flexibility in estrogenic signaling; anterograde, retrograde, and paracrine signaling by brain-synthesized estrogens will need experimental verification but may have substantial implications for brain circuit development and function. Despite these outstanding questions, it is clear that complementary mechanisms are in place within the brain for the tight temporal and spatial regulation of the synthesis and metabolism of estrogens.
V. Coupling of Estrogen Receptors to Second Messenger Systems
There is a considerable amount of evidence indicating that the rapid actions of 17β-estradiol in the nervous system involve activation of multiple kinase pathways, including the mitogen-activated protein kinase (MAPK)/ERK pathway, the phospholipase C (PLC) pathway, PKC, PI3K/Akt (also referred to as protein kinase B), and PKA pathways (Srivastava et al., 2011; Scott et al., 2012). Despite the canonical concept of ERs as transcription factors, it is clear now that the ERα, ERβ, and the G-protein-coupled receptor (GPCR), G protein-coupled estrogen receptor 1 (also known as GPR30) can mediate rapid estrogenic signaling (Brinton, 2009; Prossnitz and Barton, 2011; Srivastava et al., 2011), providing a mechanism for coupling rapid estrogenic signaling with intracellular signaling cascades. Importantly, rapid signaling events may also be mediated by yet uncharacterized cell surface signaling molecules, such as ERX and the STX-sensitive Gq-membrane estrogen receptor (Toran-Allerand, 2004; Micevych and Kelly, 2012) (Fig. 2). It is also possible that the different rapid effects of 17β-estradiol are mediated by different combinations of the above receptor types in different neuronal cell types (Raz et al., 2008; Spary et al., 2009; Scott et al., 2012; Akama et al., 2013; Srivastava and Evans, 2013). There is also increasing evidence that a subpopulation of ERs are found at extranuclear sites and specifically at synapses, a subcellular localization consistent with the ability of these receptors to couple to second messenger signaling pathways. Others have suggested that rapid actions of estrogens are mediated by splice variants of ERs (Toran-Allerand, 2004; Zhao et al., 2005; Chung et al., 2007; Ishunina and Swaab, 2008; Ishii et al., 2011; Kobayashi et al., 2011; Wu et al., 2012).
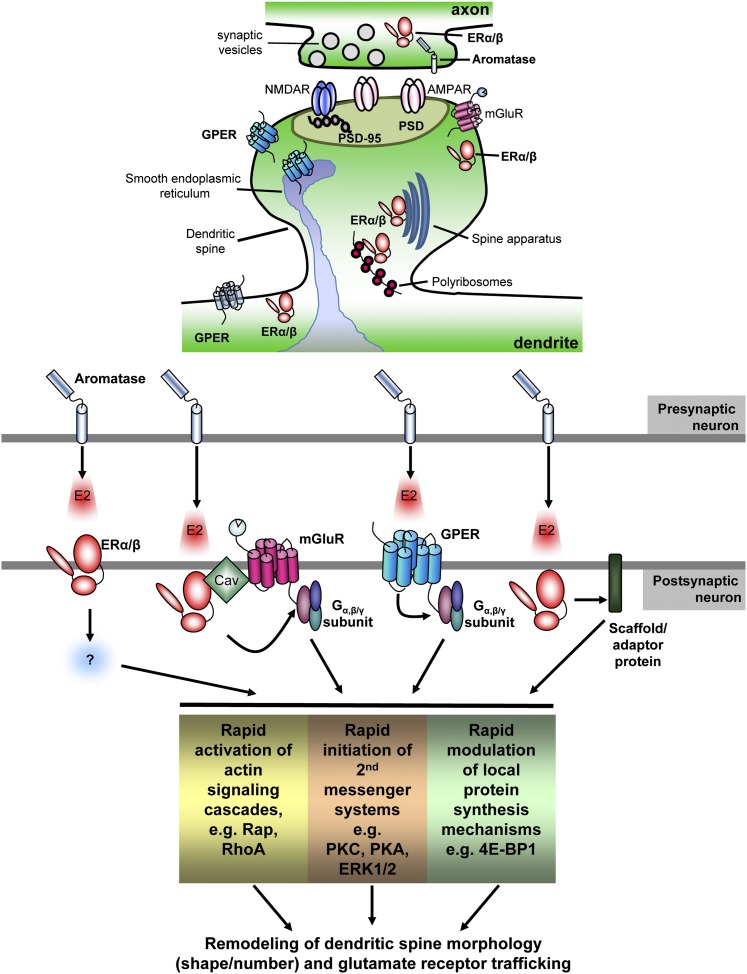
Fig. 2.
General schematic of the localization of ERs and signaling cascade engaged during rapid estrogenic signaling. All 3 forms of ERs (ERα, ERβ, and GPER) have been localized to pre- and postsynaptic structures where they are thought to associate with lipid-rich structures and spine organelle, including the plasma membrane spine apparatus and endoplasmic reticulum. Emerging evidence suggests that rapidly synthesized estrogens within the brain, mediated by synaptically located aromatase, is the source of rapid estrogenic signaling in the brain. Synthesis and “release” of estrogens onto postsynaptic cells results in the activation of ERs and the rapid transactivation of other membrane receptors or (direct) association with signaling molecules. The functional coupling of ERs via these mechanisms thus allows activation of second messenger systems and multiple intracellular cascades that ultimately lead to the regulation of the cytoskeleton, trafficking of proteins, and even the rapid synthesis of proteins, resulting in the remodeling of synapse structure and function.
A. Subcellular Localization of Estrogen Receptors ERα, ERβ, and GPER1
The presence of receptors specific for estrogens has been well documented with ERα, ERβ and GPER1 expression being detected in several regions of the brain (Brinton, 2009; Hughes et al., 2009; Gillies and McArthur, 2010; Prossnitz and Barton, 2011). A detailed review of ERα and ERβ structure and function has been published elsewhere (Nilsson et al., 2001). ERα has been observed in the extracellular regions of neurons in the cortex and hippocampus of mouse, rat, rhesus monkey and humans, albeit with a higher expression in the latter region (Milner et al., 2001; Adams et al., 2002; Kritzer, 2002; Mitra et al., 2003; Milner et al., 2005; Gonzalez et al., 2007; Wang et al., 2010). Electron microscopy has located ERα immunoreactivity in dendritic spines, where it associates with spine apparati and/or polyribosomes in rat and rhesus monkey forebrain (Milner et al., 2001; Wang et al., 2010) (Fig. 2). In presynaptic structures, ERα-labeled unmyelinated axons and axon terminals containing synaptic vesicles form asymmetric (excitatory) and symmetric (inhibitory) synapses. A recent study supports a presynaptic localization of ERα in female hippocampus, where it localizes with both glutamate and GABA containing synaptic vesicles (Tabatadze et al., 2013). ERα has also been shown to be expressed in astrocytes that are often found near the spines of pyramidal cells (Kritzer, 2002; Milner et al., 2005) and microglia (Sierra et al., 2008).
It is thought that ERβ is expressed more highly compared with ERα in the cortex and hippocampus in mouse, rat, and humans (Mitra et al., 2003; Gonzalez et al., 2007; Handa et al., 2012) and is also expressed in the cerebellum and hypothalamic nuclei (Mitra et al., 2003). In a similar manner to ERα, ERβ has been shown to be expressed in both nuclear and extranuclear compartments (Kritzer, 2002; Gonzalez et al., 2007). ERβ immunoreactivity has also been reported on or near the plasma membrane of somata and dendritic shafts and spines in pyramidal neurons (Kritzer, 2002; Milner et al., 2005; Gonzalez et al., 2007; Mitterling et al., 2010). Within spines, ERβ was found to reside at the base of spines as well as within spines (Milner et al., 2005; Mitterling et al., 2010). Furthermore, ERβ has been localized in axons and axon terminals and both in the cytoplasm and on endomembranes near mitochondria (Yang et al., 2004; Milner et al., 2005). ERβ immunoreactivity is present primarily in pyramidal cells but also is found in interneurons and a few glial cells (Kritzer, 2002; Milner et al., 2005; Fan et al., 2006). In addition, neurons generated by adult neurogenesis in the dentate gyrus of the hippocampus also express ERβ (Herrick et al., 2006).
Some controversy surrounds whether estrogens are the true ligand for GPER1 (Langer et al., 2010); nevertheless, there are numerous reports that have indicated that this receptor is highly responsive to acute estradiol treatment (Prossnitz and Maggiolini, 2009; Maggiolini and Picard, 2010; Nadal et al., 2011). Investigations into the localization of this receptor within the brain have shown expression in several areas, including the cortex, hippocampus, and hypothalamus (Brailoiu et al., 2007; Hazell et al., 2009; Hammond et al., 2011). In pyramidal neurons, GPER1 has been shown to be expressed at the plasma membrane and in the cytoplasm, as well as along the dendritic processes (Hazell et al., 2009; Hammond et al., 2011). In hippocampal neurons, GPER1 has been shown to localize to synaptic and extrasynaptic regions within dendritic spines (Akama et al., 2013). Furthermore, GPER1 was shown to interact with PSD-95, suggesting that the receptor may couple to singling pathways within dendritic spines through its interaction with this scaffold protein (Akama et al., 2013; Srivastava and Evans, 2013).
A caveat to these studies is the dubious specificity of antibodies raised against ERs. For example, evidence has been presented that the commercially available antisera for ERβ recognize seemingly specific bands in knockout and null mouse tissue (Snyder et al., 2010). This has been further supported by evidence that ERβ-specific antibodies demonstrate cross-reactivity for ERα (Wu et al., 2012). Such data clearly demonstrate the requirement for highly specific antibodies that specifically recognize ERs and/or their splice variants, as has been recently reported (Wu et al., 2012). As such, caution should be taken when attempting to interpret the subcellular localization of ERs. These technical points, when considered with the methodological approaches used, may help to account for the disparities in localization and/or expression profile of the various ERs reported in the literature.
B. Surface Expression of ERs
A number of groups have attempted to determine whether extranuclear ERs are inserted into the plasma membrane or whether they simply associate with the plasma membrane (Fig. 2). Several mechanisms have been proposed for both possibilities. On the one hand, work using bovine serum albumin-conjugated 17β-estradiol has demonstrated that binding of this compound to extracellular sites was capable of increasing intracellular Ca2+ levels (Wu et al., 2011). It is noteworthy that one potential issue of conjugated forms of 17β-estradiol (either to bovine serum albumin or horseradish peroxidase) is the presence of unconjugated 17β-estradiol, which could cloud the potential membrane actions of the steroid (Stevis et al., 1999). The possibility of surface-expressing receptors has also been suggested by various biochemical studies. Using surface biotinylation assays, Dominguez and Micevych (2010) demonstrated the surface expression of ERα and potential splice variants in mixed sex hypothalamic cultures, supporting evidence from reports using conjugated 17β-estradiol that ERs are expressed on the surface.
ERs have also been shown to associate with lipid-rich microdomains, such as caveolae, through the palmitoylation of ERα and ERβ at a conserved palmitoylation motif in the ligand binding domain of these receptors (Pedram et al., 2007). Palmitoylation is known to increase the association of ERs with caveolae rafts, which provide a local environment rich in signaling proteins to which these receptors could couple. However, the mechanisms that underlie the palmitoylation of ERs in neurons have yet to be fully determined. It has also been demonstrated that methylation of ERα at Arg260 by the protein arginine N-methyltransferase 1 enzyme promotes cytoplasmic localization and its interaction in a protein complex with PI3K and Src signaling proteins (Le Romancer et al., 2008). This mechanism has been shown to couple ERα with the insulin-like growth factor and PI3K in adult rat brain (Mendez et al., 2003). As both palmitoylation and methylation are reversible modifications, it is possible that these two mechanisms fit better with the hypothesis that rapid membrane actions by 17β-estradiol are achieved by ERs that dynamically shuttle between the membrane and the nucleus. In this scenario, ERs destined for the nucleus are capable of first initiating local signaling events before translocating to the nucleus and participating in the regulation of gene transcription (Beyer et al., 2003). Another potential mechanism that has been proposed is the identification of splice variants of both ERα and ERβ in neuronal tissue with extranuclear expression (Price et al., 2001; Ishii et al., 2011). Splice variants for both receptors can generate receptors lacking specific motifs, such as the nuclear localizing signal, or even the N or C termini (Price et al., 2001; Chung et al., 2007; Ishunina and Swaab, 2008; Ishii et al., 2011; Kobayashi et al., 2011). Therefore, it is plausible to suggest that these splice variants could function solely as a membrane ER, capable of coupling to second messenger signaling pathways. As of yet, the functional implications of ER splice variants in the CNS remain to be determined.
As GPER1 is a seven-transmembrane receptor, it would be expected to be expressed at the plasma membrane. However, this has been the source of some controversy. Several studies have suggested that GPER1 is indeed incorporated into plasma membrane (Akama et al., 2013; Srivastava and Evans, 2013), where it colocalizes with concanavalin A, a marker of the membrane (Filardo and Thomas, 2012), thus placing it in an ideal location to couple with Gα and β/γ subunits to initiate intracellular signaling. On the other hand, GPER1 has been shown to colocalize with markers of the endoplasmic reticulum (Prossnitz and Maggiolini, 2009). Although the majority of GPCRs are expressed in the plasma membrane, it is becoming accepted that some GPCRs may be functionally expressed at intracellular sites (Gobeil et al., 2006). This is particularly true of GPCRs with lipophilic ligands. It is also interesting to point out that there is some evidence that G-protein βγ subunits can be targeted to the endoplasmic reticulum, where they subsequently associate with G-protein α subunits, providing the requisite machinery for GPER1 to initiate signaling. Thus it can be speculated that GPER1 exists at both subcellular locations and translocates from the cell surface to the endoplasmic reticulum or vice versa. Consistent with such a mechanism, it was recently shown that GPER1 localizes to the plasma membrane and traffics to intracellular sites via cytokeratin intermediate filaments (Sanden et al., 2011). However, whether such a mechanism is used in neurons is not known. However, it is noteworthy that given the fact that GPER1’s ligand estrogen is membrane permeable, either intracellular or plasma membrane localization of the receptor would not rule out its function as an estrogen receptor.
C. Scaffold Protein Mediators of ER Signaling
The extranuclear localization of ERs, whether at the plasma membrane or at cytoplasmic sites, begs the question of how these receptors can couple to classic second messenger signaling cascades. It has been shown that GPER1 directly couples with Gi or Gq/11 small G-proteins (Prossnitz and Maggiolini, 2009) and potentially couples to signaling proteins via its interaction with PSD-95 (Akama et al., 2013); therefore, it can directly regulate intracellular signaling. Conversely, the classic ERs seemingly require specialized mechanisms to couple with signaling cascades (Raz et al., 2008). Although investigation of these mechanisms are currently lacking in neuronal cell types, studies from breast cancer cells and endothelial cells have identified a number of ER-interacting proteins that can scaffold ERs to signaling proteins (Fig. 2).
The proline-, glutamic acid-, and leucine-rich protein (PELP)1, or modulator of nongenomic actions of estrogen receptor (MNAR), was first identified as a novel binding partner of ERα, with high expression in human brain, testes, and mammary glands (Vadlamudi et al., 2001; Khan et al., 2005;). PELP1/MNAR contains a conserved LXXLL motif that has been shown to interact with the AF-2 domain of steroid receptors and an SRC homology 3 domain (SH3 domain), which serves as a binding site for SH3 domain proteins (Vadlamudi et al., 2001; Khan et al., 2005). Although PELP1/MNAR has been shown to be required for rapid, nongenomic signaling in breast cancer cells (Boonyaratanakornkit, 2011), the role of this protein in regulating either ERα or ERβ signaling in neurons has yet to be established. Another protein called striatin is a 110-kDa protein that contains a putative caveolin-binding motif, a Ca2+-calmodulin binding site, and has been localized to synapses of neurons in the striatum and cortex (Castets et al., 1996; Gaillard et al., 2006). In nonneuronal cells, striatin has been shown to interact with ERα at residues 183–253, which mediates it ability to complex with Gαi G-proteins and activate rapid MAPK- and Atk-dependent signaling (Lu et al., 2004). Currently it is not clear whether other ERs interact with striatin, and furthermore, it is not known whether this occurs in neurons and what impact this scaffold protein has on rapid estrogenic signaling in the brain.
D. ER Interactions with Metabotropic Glutamate Receptors
Another mechanism by which ERs may initiate rapid signaling pathways is via the functional coupling of ERs to other GPCRs. Indeed, several studies have shown that membrane-localized ERα and ERβ are capable of activating multiple metabotropic glutamate receptors independent of glutamate, leading to downstream second messenger signaling (Boulware et al., 2005; Grove-Strawser et al., 2010) (Fig. 2). In female-derived hippocampal neurons, 17β-estradiol stimulation of membrane ERα was found to trigger mGlu1a signaling. This led to the activation of Gq-mediated stimulation of phospholipase C, PKC, and inositol trisphosphate signaling and eventual MAPK-dependent cAMP response element-binding (CREB) phosphorylation. In the same population of neurons, membrane-localized ERα and ERβ could also trigger activation of mGlu2, leading to Gi/o-coupled decreases in cAMP and subsequent attenuation of L-type calcium channel-dependent CREB phosphorylation. Interestingly, caveolin-1 expression is essential for the functional coupling and compartmentalization of ERα with mGlu1a. In contrast, isolation of ERα and ERβ with mGlu2 is achieved via expression of caveolin-3. In the CA1 region of the hippocampus, 17β-estradiol has been shown to suppress inhibitory synaptic transmission via an ERα/mGlu1-dependent mobilization of the retrograde endocannabinoid anandamide (Huang and Woolley, 2012). Importantly, this effect is seen only in female rats, demonstrating a sex-specific mechanism. Nevertheless, it provides a sex-specific mechanism for the similar coupling of rapid estrogenic-signaling with signaling pathways.
E. Cooperation of Rapid Nongenomic and Genomic Signaling
As described above, a range of signaling pathways is activated within 1 hour of exposure to estradiol (Losel and Wehling, 2003; Raz et al., 2008; Lokuge et al., 2010; Srivastava et al., 2011). However, it is also emerging that there is considerable cross-talk, or a convergence, between rapidly activated signaling cascades and transcriptional machinery (Vasudevan and Pfaff, 2007, 2008; McDevitt et al., 2008; Ordonez-Moran and Munoz, 2009) (Fig. 2). These suggestions partly come from the ability of estrogens to activate Akt and ERK-pathways, which are known to regulate transcriptional machinery. Although several studies have reported that estrogens can engage such mechanisms in neuroblastoma cells (Vasudevan and Pfaff, 2007), there is currently relatively little evidence that this can occurs in neurons. It is also important to note that the regulation of transcriptional machinery by these signaling pathways occur in addition to their effects on cellular processes within the cytosol (Thomas and Huganir, 2004; Cohen and Greenberg, 2008). Indeed, although regulation of transcriptional machinery may occur within the time frame of rapid responses (within 1 hour), it is not clear whether the resulting gene products can influence cellular events within this time. Nevertheless, it is likely that extranuclear and nuclear signaling initiated by rapid estrogenic-signaling act either in cooperation to enhance or in parallel to increase signaling diversity within neurons.
Epigenetic modifications of histones, the core proteins required for the packaging of tightly coiled chromatin, are essential transcriptional regulatory mechanisms (Riccio, 2010). The phosphorylation or acetylation of histones is associated with the initiation of gene transcription, whereas the methylation of DNA is generally associated with the repression of gene transcription (Berger, 2007). Importantly, in neuronal cells, extracellular signals can impact epigenetic mechanisms through Ca2+ signals, the translocation of ERK or other soluble cytosolic proteins to the nucleus (Cohen and Greenberg, 2008; Jordan and Kreutz, 2009; Day and Sweatt, 2011; Maze et al., 2013). Indeed, epigenetic mechanisms are now thought to play a critical role in the formation and consolidation of memory and other cognitive functions (Cohen and Greenberg, 2008; Jordan and Kreutz, 2009; Day and Sweatt, 2010, 2011; Maze et al., 2013). In a series of investigations, Frick and colleagues demonstrated that estradiol-mediated enhancement of memory consolidation occurred through the cross-talk between the rapid activation of cytosolic signaling and regulation of epigenetic mechanisms (Frick et al., 2011; Zhao et al., 2010, 2012). They showed that the infusion of estradiol into the dorsal hippocampus resulted in acetylation of the H3 histone protein as well as DNA methylation within 30 minutes. Moreover, infusions of pharmacological inhibitors of H3 histone acetylation or DNA methylation immediately after training blocked estradiol’s ability to enhance memory consolidation (Zhao et al., 2010, 2012). These studies, although still at a relatively early stage, provide compelling evidence that signals generated in the cytosol by 17β-estradiol can rapidly lead to epigenetic alterations, which is required for the modulation of memory consolidation. However, it is not clear what the relationship is between cytosolic and epigenetic signaling. For example, is the consequence of this cross-talk to produce proteins that can reinforce the cellular actions initiated by rapid estrogenic signaling? As future studies in this emerging area are performed, details such as the identity of epigenetically modified gene loci, the identity of affected cells, the temporal dynamics of these modifications, and the role that they play in relation to rapid estrogenic cytosolic signaling will be revealed.
In addition to the control of epigenetic mechanisms, there is now evidence emerging that rapid estrogenic signaling may also affect local protein synthesis. Ribosomes, translation factors, and mRNA are present not only in the neuronal soma but also in dendrites and dendritic spines (Steward and Schuman, 2001). Numerous reports suggest that that local protein synthesis in the vicinity of the synapse can support long-lasting synaptic plasticity without engaging transcriptional processes in the neuronal soma. Local protein synthesis can occur in a matter of minutes if the target mRNA is present at the site of translation (Steward and Schuman, 2001; Klann et al., 2004), which would indicate that such a mechanism can occur within a rapid time frame. Estrogens have been shown to regulate protein synthesis, mainly through a translation-dependent mechanism. However, 17β-estradiol stimulates the rapid activation of specific signal transduction pathways, such as the activation of Akt, a key signal transduction intermediate that initiates protein translation by alleviating the downstream translational repression of eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) (Akama and McEwen, 2003) (Fig. 2). Specifically, estrogen rapidly (within 1 hour) increases the phosphorylation of Akt as well as the phosphorylation of eukaryotic initiation factor 4E-binding protein 1, which suggests a mechanism leading to protein translation of dendrite-localized mRNA transcripts in the hippocampus in vivo. Mirroring this, it has been shown that 17β-estradiol can activate the mammalian target of rapamycin signaling pathway, via ERK and Akt kinases, leading to an increase in the phosphorylation of eukaryotic initiation factor 4E-binding protein 1 in the dorsal hippocampus of female mice within 5 minutes, indicating that local protein synthesis is occurring (Fortress et al., 2013). Moreover, inhibition of ERK, Atk, or mammalian target of rapamycin was sufficient to block estradiol-induced object memory consolidation, potentially linking a role of rapid estrogenic-regulation of local proteins synthesis (Fortress et al., 2013). Importantly, it should be noted that the current evidence indicates that estrogens can rapidly regulate signaling pathways that can subsequently convergence on local protein synthesis mechanisms; regulation of this machinery is likely to occur in addition to other cellular responses. Indeed it remains to be seen what protein(s) are synthesized in response to the regulation of local protein synthesis. Moreover, whether these proteins are required for the initial cellular effects that occur within a rapid time frame or whether they function as part of a mechanism that reinforces these initial cellular events has yet to be determined. Nevertheless, the potential that estrogens can rapidly induce local protein synthesis offers a novel mechanism by which long-term influences on synaptic plasticity and neural circuitry may be achieved (Fig. 2).
VI. Estrogenic Modulation of Neural Circuits
The rapid modulatory effects of estrogens on cognition suggest that modifications of specific neural circuitry are occurring. Evidence has been presented that estrogen can regulate neurogenesis and even the remodeling of gross neuronal morphology that may contribute to the modulation of cognition (Galea et al., 2008; Brinton, 2009). However, these mechanisms alone cannot account for the rapid time frame in which estrogens influence cognition. Another prominent mechanism by which estrogens could modulate cognition is through the rapid fine tuning of synaptic structure and function. The resultant change in neuronal connectivity is likely to be a fundamental mechanism of rapid estrogenic modulation of cognition.
A. Structural Remodeling of Neural Circuits
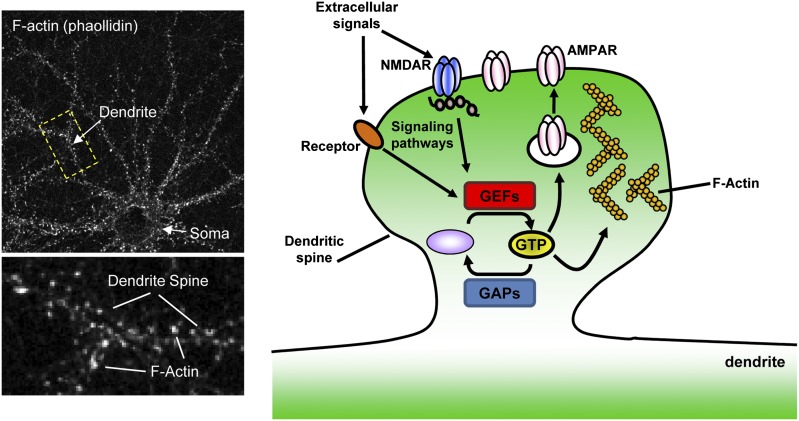
Glutamatergic synapses (excitatory synapses), the focus of substantial research attention, comprise the majority of connections between pyramidal neurons in the forebrain and predominantly occur on dendritic spines (Fig. 1). These synapses are highly plastic and play essential roles in learning, memory, and cognition (Bhatt et al., 2009; Holtmaat and Svoboda, 2009). It is within the spine head of these specialized structures that the postsynaptic density (PSD) is found, a region rich in postsynaptic proteins including the glutamate receptors N-methyl-d-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Fig. 2). Dendritic spines exhibit both transient and enduring lifetimes, persisting from minutes to years in vivo (Bhatt et al., 2009; Holtmaat and Svoboda, 2009). A myriad of dendritic spine morphologies are observed in the brain, and the notion that spine structure is highly correlated with important synaptic properties, and thus cognition, has become a recurrent theme over the last decade (Kasai et al., 2010). For example, large dendritic spines are likely to feature large PSDs and make strong connections, whereas small dendritic spines are indicative of weak connections and may be highly plastic. Importantly, dendritic spines are not static structures and exhibit a wide spectrum of structural reorganizations, ranging from formation and elimination to more subtle changes in size and shape (Fig. 1) (Bonhoeffer and Yuste, 2002). One key sequela of this structural dynamism is the ability to sample the surrounding neuropil for incident axons (Konur and Yuste, 2004), a phenomenon that we examine closely in section IX. Critically, dendritic spines undergo structural reorganization in response to a number of extracellular signals, ranging from formation and elimination to more subtle changes in size and shape (Fig. 1) (Xie et al., 2007; Penzes et al., 2008; Bhatt et al., 2009; Jones et al., 2009; Woolfrey et al., 2009; Srivastava and Penzes, 2011). Indeed, the complementary mechanisms of spinogenesis (Tada and Sheng, 2006) and spine pruning (Segal, 2005) are essential components of circuit fine tuning (Fig. 1D). Overall, agents that modulate dendritic spine linear density and morphology are critical determinants of glutamatergic circuit function.
B. Remodeling of Dendritic Spines by Estrogens
Classic studies by Woolley, Gould, and McEwan were the first to demonstrate that dendritic spine density in the CA1 both fluctuated over the estrus cycle and that OVX-induced loss of dendritic spines could be rescued by chronic treatment with 17β-estradiol (Gould et al., 1990; Woolley et al., 1990). Dendritic spines on layer II/III and layer V pyramidal neurons in the sensorimotor cortex, as well as on neurons in the medial nucleus of the amygdala, also vary over the course of the estrus cycle (Rasia-Filho et al., 2004; Chen et al., 2009). Furthermore, in young and aged OVX rhesus monkeys, long-term replacement with 17β-estradiol increased spine number on cortical neurons in the dorsolateral prefrontal cortex (Hao et al., 2007; Dumitriu et al., 2010). Overall, these reports demonstrate the effects of continuous/cyclic estrogen treatment on controlling spine number.
A number of studies have attempted to investigate the effects of centrally synthesized estrogens on dendritic spines. In cultured hippocampal neurons and hippocampal slices, inhibition of aromatase activity by letrozole (reversible aromatase inhibitor) produces a decrease in 17β-estradiol levels within the cultured medium and a concurrent loss of dendritic spines (Kretz et al., 2004). Importantly, this loss of dendritic spines was rescued by exogenous application of 17β-estradiol (Zhou et al., 2007). We have reported similar effects in cultured rat cortical neurons; treatment of cultures with androstatrienedione (irreversible aromatase inhibitor) also reduced dendritic spine density in cortical pyramidal neurons (Srivastava et al., 2008). Interestingly, letrozole treatment of cycling or OVX mice was also sufficient to reduce dendritic spine density in hippocampal neurons (Zhou et al., 2010). Together, these studies suggest that estrogens synthesized locally within discrete brain regions can regulate dendritic spine density independently of the circulating hormone. However, there is currently a lack of studies that investigate the ability of centrally synthesized estrogens to regulate dendritic spine function in vivo.
C. Rapid Regulation of Dendritic Spines by 17β-Estradiol in Cortical and Hippocampal Neurons
During early postnatal development, dendritic protrusions first appear as long, thin, highly motile structures known as filopodia, which can initiate synaptic contacts with nearby axons (Ziv and Smith, 1996). This initial contact between pre- and postsynaptic sides is a key step in synaptogenesis. Indeed, the structural similarities between these dendritic protrusions and dendritic spines suggest that filopodia are precursors of dendritic spines. In young, cultured cortical neurons, treatment with 10 nM 17β-estradiol resulted in a rapid increase in the number of filopodia. This effect was maximal after 20 minutes of treatment but was transient because the number of filopodia returned to pretreatment levels by 60 minutes (Sanchez et al., 2009). The ability of 17β-estradiol to rapidly remodel dendritic filopodia in young, developing neurons indicates a role for estrogens in modulating synaptogenesis during early development of cortical circuitry.
Following the formation of synapses, dendritic spines predominate and are significantly more stable than filopodial protrusions. However, spines still display the ability to respond to a number of stimuli and change shape, size, and number (Bhatt et al., 2009; Holtmaat and Svoboda, 2009; Srivastava, 2012). Using cultured rat cortical neurons, grown for at least 21 days in vitro to allow development of mature dendritic spines with distinct head structure and that contain PSD-95 (Xie et al., 2007; Srivastava et al., 2008, 2010; Jones et al., 2009; Woolfrey et al., 2009), we investigated the ability of estrogens to rapidly modulate spine morphology and density. Treatment with 17β-estradiol (10 nM) increased the number of dendritic spines within 30 minutes. Interestingly, spine density returned to pretreatment levels by 60 minutes (Srivastava et al., 2008). Time-lapse imaging further demonstrated that 17β-estradiol-induced spines were selectively eliminated. Importantly, 17β-estradiol-induced spines were juxtaposed to presynaptic terminals, suggesting that they were making synaptic connections (Srivastava et al., 2008). Close examination of dendritic spine morphology revealed that 17β-estradiol-induced spines had a thin morphology. A similar result was also reported after 17β-estradiol treatment of cortical neurons in the rACC (Xiao et al., 2013). This indicates the formation of highly dynamic synapses that can readily be either potentiated and stabilized or eliminated (Xie et al., 2005; Penzes et al., 2011b). A number of groups have proposed that the presence of 17β-estradiol, in sufficient enough concentration to initiate rapid responses, at synapses is subject to tight temporal regulation and, furthermore, that the initial cellular actions would be independent of transcription and translation (Balthazart and Ball, 2006; Saldanha et al., 2011; Srivastava and Penzes, 2011). Thus, it could be reasoned that estradiol must be able to modulate dendritic spines even when present only for a short amount of time. Concordant with this prediction, exposure of cortical neurons to 17β-estradiol for 5 minutes was able to transiently increase spine density over 60 minutes. Furthermore, 17β-estradiol-dependent increases in dendritic spine density was unaffected by the protein synthesis inhibitor cycloheximide D. This strongly suggests that the immediate effects of 17β-estradiol on dendritic spines are independent of translational regulation (Srivastava et al., 2008).
In hippocampal slices made from 12-week-old male rats, Kawato and colleagues observed a rapid increase in dendritic spine density after 30 minutes of 1 nM 17β-estradiol treatment, but was maximal after 2 hours. Morphologic analysis also revealed an increase in the number of thin dendritic spines and filopodia-like protrusions (Murakami et al., 2006; Mukai et al., 2007). These results parallel a recent study of 17β-estradiol remodeling of dendritic spines in CA1 cells of hippocampal slice cultures prepared from both male and female mice. Time-lapse imaging of these slices revealed that 17β-estradiol increased the rate of novel dendritic spine formation and subsequent synapse formation. Intriguingly, 17β-estradiol-induced dendritic spines were preferentially eliminated upon removal of the steroid without affecting the rate at which pre-existing dendritic spines were eliminated (Mendez et al., 2011). Taken together, these studies demonstrate that estradiol can rapidly, and often transiently, increase the rate of formation and elimination of novel dendritic spines in forebrain neurons without affecting pre-established networks (Mendez et al., 2011). We will explore the potential implications of this transient modulation of synaptic connectivity and its relevance for the remodeling of neural circuits and cognitive function in section IX.
D. The Role of Specific ERs in Spine Formation
Recently, we showed that treatment of cultured rat cortical neurons with WAY-200070 [7-bromo-2-(4-hydroxyphenyl)-1,3-benzoxazol-5-ol], an ERβ-selective agonist (Liu et al., 2008), resulted in a rapid (within 30 minutes) increase in the number of thin spines (Srivastava et al., 2010). Moreover, we found that the majority of dendritic spines following WAY-200070 treatment overlapped with the presynaptic marker bassoon and that the majority of spines were positive for PSD-95, suggesting the formation of functional spines that make connections with presynaptic partners (Srivastava et al., 2010). We did not observe an increase in PSD-95 proteins levels; however, we did observe a reduction of PSD-95 puncta within the cytosol/dendritic shaft of cells concurrent with an increase of puncta within spines. Thus, it appeared that a redistribution of PSD-95 from cytosolic regions into ERβ-induced nascent spines underlies this process rather than an overall increase in PSD-95 protein expression (Srivastava et al., 2010).
In male rat hippocampal slices, treatment with PPT (ERα selective agonist) but not DPN (ERβ selective agonist) increased dendritic spine density in CA1 pyramidal neurons within 2 hours (Murakami et al., 2006; Mukai et al., 2007). Moreover, this increase in spine number was dependent on NMDA receptors, inasmuch as blockade of this receptor abolished PPT-induced spine increase (Murakami et al., 2006; Mukai et al., 2007). This pharmacological profile differs to that seen in cultured rat cortical neurons. However it should be noted that spine density was only assessed 2 hours after treatment, and thus it is possible that activation of ERβ has an effect at an earlier time point. Conversely, 17β-estradiol and PPT, but not DPN, treatment resulted in a decrease in the number of CA3 thorny synapses (complex postsynaptic structures consisting of multiple heads) located in the stratum lucidum after 2 hours of treatment (Tsurugizawa et al., 2005). However, when individual spines on CA3 pyramidal neurons were examined, none of the tested compounds had an effect on dendritic spine density (Tsurugizawa et al., 2005). It is not clear why these estrogenic compounds have opposing effects on distinct pyramidal neurons within the hippocampus, but these studies clearly indicate cell-specific effects; it is likely that differences in the local environments of signaling proteins in each cell type underlie the disparity in these rapid estrogenic responses.
More recently, the effect of ER selective agonists on rapid hippocampal-based learning tasks and concurrent changes in spine density on CA1 neurons was investigated within the same animals (Phan et al., 2011). These experiments revealed that PPT-induced rapid enhancement of social recognition, object recognition, and placement is mirrored by an increase in dendritic spine density. On the other hand, treatment with DPN had a complex effect on behavior, with an impairment of social recognition and a modest improvement in object placement, concomitant with a reduction in spine density in CA1 neurons (Phan et al., 2011). Inasmuch as behavioral testing occurred 40 minutes after drug treatment, these findings provide strong evidence that, concurrent with rapid ER-dependent modulation of learning, there are changes in dendritic spine density. Moreover, these data support the hypothesis that estrogenic-induced alterations in dendritic spine density may be a cellular correlate of rapid estrogenic modulation of cognitive function.
E. Role of Inhibitory Neurons and Astrocytes in 17β-Estradiol-Mediated Spinogenesis
The studies discussed above focus on the effects of estrogens on excitatory neurons. The contribution of estrogenic regulation of inhibitory neurons and astrocytes in the regulation of dendritic spines has been explored in a number of studies (Murphy et al., 1998; Amateau and McCarthy, 2002; Zhou et al., 2007; Azcoitia et al., 2010; Wojtowicz and Mozrzymas, 2010) but not always in a time frame consistent with that of rapid actions. Previous studies suggested that the ability of estradiol to increase spine density is through a reduction of inhibitory and an increase in excitatory drive in hippocampal neurons (Murphy et al., 1998; Wojtowicz and Mozrzymas, 2010). Treatment of culture hippocampal neurons with 17β-estradiol for between 24 and 48 hours reduced the expression of glutamate decarboxylase-positive neurons, and γ-aminobutyric acid (GABA) receptor mediated miniature inhibitory postsynaptic currents (Murphy et al., 1998). Moreover, blocking GABAA/B receptor function with mercaptopropionic acid resulted in an increase in spine density and a concurrent decrease in glutamate decarboxylase expression. The authors concluded that the long-term actions of estradiol on dendritic spines involve the inhibition of GABAergic function (Murphy et al., 1998). Few studies have investigated the possible contribution of GABAergic signaling to rapid estrogenic effects on spines. In cultured rat cortical neurons we inhibited GABA receptor function using picrotoxin. However, no effect on spine density was observed within 30 minutes. It should be noted that picrotoxin is selective for GABAA receptors, but the contribution of GABAB receptors on rapid changes in spine density was not tested. Nevertheless, this suggests that, at least in cortical neurons, GABAA receptors do not contribute to estradiol’s ability to rapidly modulate dendritic spines (Srivastava et al., 2008).
It is increasingly becoming apparent that astrocytes and neurons are involved in a tripartite partnership at synapses, where astrocytes take part in active interactions with neurons (Perea et al., 2009). These cells respond to synaptic transmission and help to regulate and process synaptic information under basal and active conditions (Perea and Araque, 2007; Panatier et al., 2011). Interestingly, estradiol can initiate rapid cellular responses in astrocytes regulating their function (Azcoitia et al., 2010; Micevych et al., 2010), and these cells are capable of synthesizing estradiol (Yague et al., 2006; Azcoitia et al., 2011). However, it is not known what role, if any, astrocytes play in mediating the rapid responses of estrogens, be it as a source of estradiol or by directly contributing to the remodeling of spine morphology/number. Therefore, it would be worthwhile in future studies to further interrogate glia-neuron interactions in the context of rapid estradiol signaling (see Azcoitia et al., 2011 for further discussion).
Collectively, these studies provide a potential cellular mechanism by which rapid estrogenic signaling can lead to the fine tuning of synaptic connectivity in developing and established neuronal circuitry. It is noteworthy that there are a number of discrepancies in the literature regarding the contribution, or lack thereof, of ERs to the remodeling of dendritic spines. Some of these discrepancies can be attributed to the use of cells from different developmental time points, concentrations of agonists or antagonists used, region specific tissue (i.e., cortex as opposed to hippocampus), or even treatment timing. It is, however, somewhat surprising that loss of function studies, such as using tissue from ER knockout mice, or RNAi approaches to silence specific receptor subtypes have not been used to elucidate the relative contribution of specific ERs to the regulation of dendritic spines. Future studies using conditional ER or aromatase knockout animals to isolate the actions of estrogens in a brain region and/or cell-specific manner, in combination with in vivo imaging approaches to examine dendritic spines (Bhatt et al., 2009; Srivastava et al., 2012b), will provide unprecedented insight into the contribution of centrally synthesized estrogens on the rapid modulation of synapse structure.
VII. Regulation of Synaptic Function by Estrogens
The effects of acute 17β-estradiol treatment on the intrinsic excitability of neurons have been widely reported (Kelly et al., 1976; Nabekura et al., 1986; Wong and Moss, 1991; Mermelstein et al., 1996). In addition, 17β-estradiol has been shown to rapidly modulate synaptic transmission and plasticity (Woolley, 2007). Two prominent cellular mechanisms thought to underlie rapid, activity-dependent synaptic tuning are long-term potentiation (LTP) and long-term depression (LTD), both of which have received extensive research attention (Kessels and Malinow, 2009). Both LTP and LTD are thought to be critical mechanisms in the encoding and storage of information (Cooke and Bliss, 2006; Kerchner and Nicoll, 2008). It is widely accepted that the bidirectional trafficking of NMDA and AMPA receptors is a key mechanism in controlling synaptic transmission and plasticity, and thus a critical mechanism in the refinement of neuronal circuitry. Here we review some recent studies that provide an insight into the rapid regulation of synaptic function by estrogens.
A. Estrogenic Modulation of Long-term Potentiation and Long-term Depression
In hippocampal slices taken from male rats, 17β-estradiol increases the magnitude of LTP induced by high-frequency stimulation (Foy et al., 1999). In a similar manner, 17β-estradiol has also been shown to enhance LTP at CA3-CA1 synapses in response to theta burst stimulation in male rat hippocampal slices (Kramar et al., 2009). This enhancement of theta burst-inducted LTP was dependent on ERβ, but not ERα, and required actin polymerization (Kramar et al., 2009). Dependence on actin polymerization is consistent with the remodeling of dendritic spines but could also reflect the role of actin remodeling in the trafficking of AMPA receptors (Gu et al., 2010). NMDA receptors have also been implicated in estrogenic facilitation of LTP at CA3-CA1 synapses in OXV mice (Smith and McMahon, 2006); however, whether this is a sex-specific mechanism is not clear. Moreover, in intact cycling females, the magnitude of LTP has also been reported to vary: induction of LTP is greater during proestrus (high estrogen levels) compared with diestrus (low estrogen levels) (Bi et al., 2001), consistent with previous reports of the fluctuations of dendritic spine density and ER expression levels over the estrus cycle in the hippocampus (Woolley and McEwen, 1992; Mitterling et al., 2010; Waters et al., 2011).
In the male rat hippocampus, 17β-estradiol has been reported to enhance LTD in CA1, CA3, and dentate gyrus with estrogenic facilitation of LTD occurring via an ERα-, but not ERβ-dependent pathway (Mukai et al., 2007). Although this demonstrates that estrogens can modulate multiple forms of synaptic plasticity, it is also interesting to note the reported importance of ERβ for LTP and ERα for LTD (Mukai et al., 2007; Kramar et al., 2009). ER receptor expression diversity among cells could thus favor potentiation versus depression in response to estrogen. Recently it was also shown that 17β-estradiol can facilitate the induction of LTP in layer 2/3 neurons of the rACC in both male and female rats (Xiao et al., 2013), demonstrating that estrogens can affect synaptic plasticity in brain regions outside the hippocampus in males and females. However, inasmuch as the majority of studies investigating the effects of estrogens on synaptic plasticity have focused primarily at CA3-CA1 synapses, further research is required to fully understand the modulatory effects of these compounds on synaptic transmission and plasticity throughout the forebrain.
B. Rapid Estrogenic Modulation of Glutamate Receptor Trafficking in Cortical Neurons
To fully understand the rapid modulation of LTP and LTD by estrogens, it is necessary to determine their effects on NMDA and AMPA receptors. Indeed, synaptic strengthening by LTP is achieved through at least two complementary mechanisms: lateral diffusion of extrasynaptic surface AMPA receptors into the postsynaptic density (Ehlers et al., 2007; Opazo et al., 2012) and insertion of endosomal-resident AMPA receptors into specialized perisynaptic exocytic zones (Kennedy et al., 2010). However, the effects of rapid estrogenic-signaling on the trafficking of NMDA and AMPA receptors have been far less studied. Multiple studies have indicated that structural and functional changes in synapses often go hand in hand (Matsuzaki et al., 2001; Kasai et al., 2010). For example, enlargement of dendritic spine size in response to activity-dependent stimulation is accompanied with an increase in synaptic GluA1-containing AMPA receptors and thus an increase in AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) (Xie et al., 2007). Conversely, shrinkage of dendritic spine size is mirrored by a loss of surface GluA2-containing AMPA receptors and reduced AMPA receptor-mediated mEPSCs (Woolfrey et al., 2009). However, this is not always the case (Segal, 2010). Synapses containing NMDA receptors, but lacking AMPA receptors (silent synapses), are known to be rapidly potentiated during plasticity and are considered a major mechanism for the remodeling of neuronal circuits (Isaac et al., 1995; Kerchner and Nicoll, 2008; Ashby and Isaac, 2011). In cultured rat cortical neurons, we found that acute treatment with 17β-estradiol induced the removal of the GluA1-containing AMPA receptors from synapses at 30 minutes, while inducing insertion of the GluN1-containing NMDA receptors to synapses. Remarkably, by 60 minutes of treatment, GluA1 and GluN1 synaptic content had returned to control levels (Srivastava et al., 2008). Time-lapse imaging of GFP-tagged GluA1 demonstrated that GluA1 was being internalized from pre-existing dendritic spines and returning into the same spine without entering nascent spines (Srivastava et al., 2008). Electrophysiological recordings of AMPA receptor-mediated mEPSCs demonstrated that 17β-estradiol induced a transient reduction in AMPA receptor-mediated mEPSC frequency, but not amplitude, indicating a change in the number of active synapses (Srivastava et al., 2008). Together, these data indicate that 17β-estradiol transiently increases the number of synapses containing NMDA receptors but lacking AMPA receptors, consistent with the formation of silent synapses. Interestingly, in cortical slices of the rACC, application of 17β-estradiol induced a robust increase in the ratio of NMDA/AMPA EPSCs, indicating the formation of silent synapses (Xiao et al., 2013). Critically, the effect of 17β-estradiol on the ratio of NMDA/AMPA EPSC corroborates the trafficking of GluN1-containing NMDA receptors and GluA1-containing AMPA receptors reported in cultured cortical neurons (Srivastava et al., 2008; Xiao et al., 2013).
C. Modulation of Glutamate Receptor Trafficking in Hippocampal Neurons
A limited number of studies have also assessed the effects of estrogen on glutamate receptor trafficking in hippocampal neurons. Estrogenic modulation of synaptic transmission, as determined through the measurement of extracellular postsynaptic potentials in the CA1, evoked by the stimulation Schaffer collateral pathway, was shown to be dependent on calpain (Zadran et al., 2009). This work in acute hippocampal slices also revealed a distinct mode of action of 17β-estradiol on AMPA receptors. Treatment of 30 minutes with 17β-estradiol resulted in an increase in levels of membrane GluA1-, but not GluA2/3-containing AMPA receptors (Zadran et al., 2009). This increase in membrane GluA1 was mediated via a MAPK- and calpain-dependent pathway (Zadran et al., 2009). It is likely that the differences seen in GluA1 trafficking in hippocampal and cortical neurons is due to the different signaling mechanisms activated by 17β-estradiol in these different cell types.
Collectively, these data indicate that estrogenic enhancements of LTP, LTD, and synaptic transmission are mediated by specific ERs and require activation of mechanisms underlying the trafficking of glutamate receptors to the membrane and rearrangement of the actin cytoskeleton. Such findings indicate that concurrent alterations of synaptic structure and glutamate receptor trafficking are required for estrogen-induced modulation of synaptic transmission and plasticity. However, more in-depth studies of how specific ERs regulate both the synaptic expression of NMDA and AMPA receptors and their subunits are required to fully understand how estrogens can rapidly modulate synaptic function.
VIII. Molecular Mechanisms Underlying the Remodeling of Dendritic Spines
Dendritic spines are proteinaceous structures and are estimated to contain over 1000 different proteins (Emes et al., 2008), including scaffold proteins, receptors, signaling proteins, and cytoskeletal proteins (Fig. 3). A major component of these postsynaptic structures is the actin cytoskeleton, which is a key regulator of spine morphology (Fig. 3) (Fischer et al., 1998; Hotulainen et al., 2009); tight control of the actin cytoskeleton is crucial to proper synaptic function (Yoshihara et al., 2009; Hotulainen and Hoogenraad, 2010; Penzes and Cahill, 2012). But what are the putative molecular mechanisms that connect extracellular signals, such as estrogens, to the remodeling of dendritic spines? Multiple signaling pathways, many involving the superfamily of small GTPase proteins, impinge on the actin cytoskeleton, linking extracellular signals with spine remodeling (Penzes et al., 2008; Penzes and Cahill, 2012). Accordingly, 17β-estradiol has been shown to regulate both small GTPase signaling and actin dynamics in non-neuronal cells (Sanchez et al., 2010a), suggestive that estrogenic signaling-mediated spine remodeling may employ such mechanisms.
Fig. 3.
Dendritic spines and the cytoskeleton. Immunofluorescence staining with phalloidin, a marker of endogenous F-actin in cortical neurons, reveals enrichment of actin in dendrites and dendritic spines. Schematic drawing of how extracellular signals can act via specific receptors and act via small GTPases to regulate actin dynamics and/or receptor trafficking. The dynamic actin cytoskeleton confers much of the structure of the dendritic spines, and alterations in synaptic expression of glutamate receptors (e.g., AMPA receptors) are thought to play a major role in modulating synaptic function.
A. Key Determinants of Dendritic Spine Morphology
The morphologic malleability of dendrite spines is a result of a dynamic actin cytoskeleton (Fischer et al., 1998; Hotulainen et al., 2009). Spines are rich repositories of filamentous and monomeric actin (Fig. 3) and achieve both stability and dynamism through a turnover process known as treadmilling, where monomers are simultaneously added to the barbed end (at the spine periphery) and removed from the pointed end of the filament (near the spine’s core) (Star et al., 2002; Frost et al., 2010). Members of the Ras superfamily of small GTPases are molecular switches that regulate diverse cellular functions (Takai et al., 2001). Small GTPases exist in binary “on” and “off” states when bound to GTP and GDP, respectively. Perhaps best studied among these family members are Rac1 and RhoA, which have potent and opposite effects on the structure of dendritic spines (Tashiro and Yuste, 2004). Each GTPase can be regulated by a variety of different guanine nucleotide exchange factors (GEFs), which facilitate the binding of GTP by the GTPase, and GTPase activating proteins (GAPs), which catalyze the hydrolysis of GTP to GDP. GEFs and GAPs allow for both signaling diversity and spatial specificity. Indeed, by responding to extracellular signals including neuromodulators and neuronal activity, GEFs can achieve bidirectional control over spine morphology and synaptic strength by regulating their target GTPases (Xie et al., 2007; Penzes et al., 2008, 2011b; Jones et al., 2009; Woolfrey et al., 2009).
Downstream of small GTPases are a series of effector proteins that convey signaling to direct regulators of the actin cytoskeleton. This includes the p21-activated kinases (PAKs) (Manser et al., 1994): when active (phosphorylated) PAK1 can phosphorylate LIM-kinase (Lin11, Isl-1, and Mec-3 domain kinase) that in turn inhibits cofilin activity (Edwards et al., 1999). Members of the Wiskott-Aldrich syndrome protein (WASP) family bind both monomeric and filamentous actin (Egile et al., 1999) and are relieved from autoinhibition by Rho-GTPases (Kim et al., 2000). N-WASP, a brain enriched WASP, appears to be critical for spine and excitatory synapse formation (Wegner et al., 2008). Small GTPases also exert control over the similar WASP-family verprolin-homologous protein (WAVE) family. These proteins play a role in spine maintenance (Soderling et al., 2007) and formation (Kim et al., 2006); deficient WAVE1 expression is accompanied by spatial memory deficits in mice (Soderling et al., 2003).
The Arp2/3 complex is a well-studied actin nucleator and facilitator (Goley and Welch, 2006). The Arp2/3 complex is downstream of Rho family GTPases, WASP and WAVE proteins (Takenawa and Suetsugu, 2007), and is likely instrumental in dendritic spine remodeling during spine growth (Hotulainen et al., 2009). As mentioned above, cofilin is another critical determinant of actin skeletal dynamics and competes with the Arp2/3 complex by severing and debranching actin filaments (Chan et al., 2009). Although prolonged cofilin activation promotes a reduction in spine size (Shi et al., 2009), it appears that a transient burst of cofilin activity is required for spine growth during chemically induced LTP (Gu et al., 2010). A recent review of small GTPase control of the actin cytoskeleton covers these pathways in greater detail (Penzes and Cahill, 2012). Overall, a stereotyped spine-morphogenic signaling cascade begins with an extracellular signal that is conveyed to GEFs or GAPs that control small GTPase activity, which in turn influences actin-binding proteins through small GTPase effectors.
B. Convergent Actin Regulating Pathways Underlie Rapid Estrogenic-Mediated Dendritic Spine Remodeling in Cortical Neurons
Investigations into the molecular mechanisms of estrogenic modulation of dendritic spines in cortical neurons have revealed the involvement of multiple pathways that converge onto the actin cytoskeleton. In young developing cortical neurons, 17β-estradiol-mediated filopodia formation occurs via the activation of a c-SRC/Rac1/Cdk5/WAVE1/Arp2/3 pathway and a RhoA/ROCK-2/moesin cascade (Sanchez et al., 2009). Although phosphorylation of WAVE1 was only achieved by 17β-estradiol and PPT, but not DPN, suggesting an ERα-dependent pathway, the direct effects of ERα activation on filopodial formation was not tested. Interestingly, silencing of moesin by siRNAs only attenuated 17β-estradiol-induction of filopodia, suggesting only a partial role for a moesin-dependent pathway in this cellular event. Nevertheless, these data directly link 17β-estradiol-signaling with the rearrangement of the actin cytoskeleton via parallel pathways involving WAVE1/Arp2/3 and moesin in developing cortical neurons.
In more developed cortical neurons, the morphology of 17β-estradiol-induced nascent spines is suggestive of Rap pathway activation, because this small GTPase results in the formation of highly dynamic, thin dendritic spines when active (Xie et al., 2005; Woolfrey et al., 2009; Penzes et al., 2011b). Accordingly we observed a rapid time-dependent increase in active Rap (GTP bound) levels after treatment with 17β-estradiol in vitro. In situ inhibition of Rap signaling, through the overexpression of RapGAP or dominant-negative Rap (RapN17), blocked 17β-estradiol-induced spinogenesis. In contrast, pharmacological inhibition of the closely related small GTPase, Ras, did not block 17β-estradiol-induced nascent spine formation (Srivastava et al., 2008). When the activity of Rac was examined, a modest decrease was observed after 60 minutes of 17β-estradiol treatment. It is possible that this small inhibition of Rac activity is sufficient to drive 17β-estradiol-induced spine numbers back to a level similar to control, because previous studies have shown that Rac inhibition reduces spine numbers (Tashiro and Yuste, 2004). Further investigation of the downstream targets of Rap signaling revealed that ERK1/2 were required for 17β-estradiol-induced spinogenesis, consistent with a role for this kinase in the remodeling of dendritic spines and acquisition of learning and memory (Thomas and Huganir, 2004). The PDZ containing protein AF-6 (also known as afadin) is a direct target of Rap and is required for Rap-dependent spine plasticity (Xie et al., 2005; Srivastava et al., 2012a). After treatment of cultured cortical neurons with 17β-estradiol, AF-6 clustered to synapses, paralleling 17β-estradiol’s effects on dendritic spine density; interfering with AF-6 function through the overexpression of a mutant AF-6 with an inactive PDZ domain prevented estradiol-dependent spinogenesis. Taken together, these data suggested that 17β-estradiol signaling via a Rap/ERK/AF-6-dependent pathway is required for increased dendritic spine number in mature cortical neurons (Srivastava et al., 2008).
Examination of the signaling pathways activated after ERβ stimulation revealed an increase in the phosphorylation (activation) of PAK and ERK1/2 kinases in dendritic spines as well as in the dendritic shaft, consistent with cytoskeleton rearrangements and spine formation (Srivastava et al., 2010). Although ERs have been localized to dendritic spines and dendritic shaft (Mitra et al., 2003; Milner et al., 2005), it is not clear how activation of these receptors can lead to the formation of nascent dendritic spines. Recent work investigating the spatiotemporal pattern of small GTPase activity after the activation of single dendritic spines, has demonstrated that small GTPase signals spread from the activated spine into the surrounding dendritic shaft (Yasuda and Murakoshi, 2011; Murakoshi and Yasuda, 2012). Although the significance of this signal spreading is not fully understood, this is a speculative mechanism by which the trafficking of cytosolic proteins and/or the initiation of nascent dendritic spines can be achieved (Yasuda and Murakoshi, 2011; Murakoshi and Yasuda, 2012). Therefore, it is interesting to consider that phosphorylation of PAK and ERK1/2 in spines, as well as in the dendritic shaft, may be part of a mechanism mobilizing cytosolic PSD-95 and the formation of novel synapses. However, further investigation is required to validate this model.
C. Molecular Mechanisms of Dendritic Spine Remodeling in Hippocampal Neurons
Investigation into the molecular mechanisms that underlie estrogenic-dependent spinogenesis in hippocampal neurons has revealed several potentially overlapping mechanisms. In the hippocampal CA1 region, it has been reported that rapid induction of spinogenesis on basal and apical dendrites by 17β-estradiol is dependent on NMDA receptor function (Murakami et al., 2006; Mukai et al., 2007). Conversely, 17β-estradiol-mediated loss of dendritic thorns on CA3 neurons does not require NMDA receptors (Tsurugizawa et al., 2005). Blocking of AMPA receptors by CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) seemed to differentially affect rapid estrogenic signaling according to cell type. Rapid loss of dendritic thorns by 17β-estradiol in CA3 neurons could be blocked by CNQX; however, in CA1 neurons, estrogenic signaling-induced spinogenesis was only blocked by CNQX on basal dendrites but not apical dendrites (Tsurugizawa et al., 2005; Murakami et al., 2006; Mukai et al., 2007). This may suggest that cell type is important for determining both rapid estrogenic effects and the underlying molecular mechanisms and that there are compartment-specific (i.e., apical versus basal dendrites) 17β-estradiol signaling mechanisms. Such a scenario is not unprecedented because differential mechanisms have been described for the development and maintenance of apical and basal architecture (Romand et al., 2011; Srivastava et al., 2012c). If distinct regions of the dendritic tree contain specific molecular mechanisms that control dendritic arborization, it is plausible that a similar subcompartmental specialization may exist for the regulation of synapse structure.
A number of studies have also demonstrated that estrogenic signaling can activate LIM-kinase (p-LIMK) and subsequently phosphorylate cofilin (p-cofilin), resulting in the inhibition of cofilin activity (Yildirim et al., 2008; Kramar et al., 2009; Yuen et al., 2011). In the dorsal hippocampus of mice, p-LIMK levels were increased during the high-estradiol phase of proestrus (Spencer et al., 2008). Work performed in acute rat hippocampal slices treated with 17β-estradiol rapidly resulted in increased polymerization of actin through a RhoA/ROCK/cofilin-dependent pathway (Kramar et al., 2009). Although these data strongly support this pathway in the control of actin rearrangement, the requirement of this pathway in directly regulating dendritic spine morphology was not tested. Nevertheless, as activation of a p-LIMK/p-cofilin cascade can control the polymerization of actin (potentially via a RhoA/ROCK-dependent pathway), it provides another mechanism by which estrogens may control the remodeling of dendritic spines in hippocampal neurons.
D. Divergent and Parallel Pathways in Estrogenic Control of Spine Morphology
As discussed in this and in section V, there are important differences in the morphologic plasticity pathways activated in young, developing, cortical neurons and those employed in more mature neurons. One potential explanation of this signaling divergence could be attributed to differential developmental expression of ERs (Gonzalez et al., 2007) and signaling proteins, including regulators of small GTPase pathways (Penzes et al., 2008). Moreover, the mechanisms underlying estrogen-dependent spinogenesis seemingly differ according to brain region. This could be attributed to region-specific expression of ERs (Mitra et al., 2003) and signaling proteins that regulate the remodeling of dendritic spines (Penzes et al., 2008). Thus, separate regions of the brain may well employ distinct molecular mechanisms to transduce rapid estrogenic signaling into morphologic changes (Fig. 2). Ultimately, these discrete mechanisms may be important for how estrogens modulate neuroplasticity in each area.
It is also important to note that, to a great extent, the immediate changes in spine morphology seen in response to extracellular signals are transcription/translation independent, consistent with small GTPase-dependent regulation of the actin cytoskeleton. This is also congruent with our reports that the rapid induction of spine formation by 17β-estradiol was independent of protein synthesis but dependent on small GTPase activity (Srivastava et al., 2008). However, activation of ERK-dependent pathways may point to the initiation of transcriptional mechanisms (see also section V). An increase in cAMP-response element-binding (CREB) protein phosphorylation has also been reported in hippocampal neurons after acute 17β-estradiol treatment (O'Neill et al., 2008) and is required for long-term (24–48 hours) estrogenic-increases in dendritic spines (Murphy and Segal, 1997). As such, it should be recognized that parallel signaling cascade may be activated that, although not required for the immediate actions of 17β-estradiol on spines, can contribute to other aspects of neural circuit remodeling.
IX. Estrogens and “Two-Step Wiring Plasticity”
As discussed in sections VI and VII, the rapid effects of estrogens on synapse structure and function are often transient. Time-lapse imaging demonstrates that rapid estrogenic signaling has little to no effect on the structure of existing synapses but almost exclusively results in the formation of new connections (Srivastava et al., 2008; Mendez et al., 2011). So how do these transient cellular effects relate to rapid estrogenic modulation of cognition? One hypothesis is that estrogens are capable of enhancing plasticity or rewiring of neural circuits and thus facilitate information storage. Two methods of cortical circuit rewiring in the adult brain have been proposed. In the first instance, changes in synaptic strength, and thus the capacity for information flow between existing synaptic connections, is believed to be a critical mechanism underlying information processing and storage in the cortex. An alternative proposal postulates that information storage can be accomplished by increasing or decreasing the number of functional synapses between two cells (Chklovskii et al., 2004; Le Be and Markram, 2006; DeBello, 2008), thereby augmenting the storage capacity of cortical networks. Theoretical work, backed by evidence from in vivo and in vitro studies (Bourne and Harris, 2008; Kasai et al., 2010; Segal, 2010), has posited that cortical networks feature many points where dendrites and axons are sufficiently close for additional synapses to be formed (Chklovskii et al., 2004). Together, these two mechanisms constitute “wiring plasticity,” whereby altering synaptic efficacy and changing the connectivity between cells are the underlying mechanisms of neuronal circuit rewiring and critical to the acquisition of behaviors and general cognitive function (DeBello, 2008) (Fig. 1D). It is important to note that this “wiring” model is not limited to the remodeling of synapses but accounts for the potential that both axons and dendrites can undergo structural alterations in the mature brain, albeit under specialized circumstances (Chklovskii et al., 2004; Holtmaat and Svoboda, 2009). The term “micro-rewiring” specifically focuses on the remodeling of synapses in reference to linking circuit remodeling with behavior (DeBello, 2008). However, we do not discount the potential for estrogenic regulation of axonal and/or dendritic gross structure. As such, we do not distinguish between the terms “wiring plasticity” and “micro-rewiring” but accept that our current description may be better described by the latter terminology.
Recent experimental evidence has linked the rewiring of neural circuits with behavior. For example, in vivo imaging of cortical motor neurons has demonstrated increased dendritic spine density concurrent with the acquisition of a learned motor behavior (Yang et al., 2009). Moreover, in Zebra Finches, the acquisition of singing behavior is accompanied by enlargement and stabilization of dendritic spines in forebrain neurons (Roberts et al., 2010). These in vivo imaging studies elegantly demonstrate that cortical circuit remodeling accompanies behavioral modification and implicate circuit rewiring as a powerful mechanism for information storage in the cortex. To date, the molecular and cellular mechanisms underlying such circuit plasticity remain mysterious. Here we propose that cortical circuit rewiring is a compelling candidate mechanism for the rapid modulation of cognition by estrogens.
A. Estradiol-Induced “Two-Step Wiring Plasticity”
Recognizing the ability of 17β-estradiol to rapidly induce spine formation and silence a subset of synapses in cortical neurons led us to several questions: what is the physiologic role of such a transient increase in silent synaptic connections, what relevance does this increase have for the rewiring of cortical circuits, and how do these effects relate to estradiol’s ability to rapidly modulate cognition? As discussed in section III, behavioral studies indicate that there is a specific time frame in which rapid estrogenic signaling is effective in enhancing memory acquisition/consolidation. Thus, it could be further suggested that the cellular mechanism underlying estrogen's enhancement of cognition must also be transient. This idea would be consistent with the transient effect of 17β-estradiol on synapse structure and function (Srivastava et al., 2008). In vivo work has demonstrated that cortical neurons can make transient synapses. This is achieved when nascent spines sample presynaptic contacts and form connections; however, these spines are thought to lack AMPA receptors and are therefore unlikely to be functional (Holtmaat et al., 2005). Moreover, these novel connections are not permanent and retract unless a subsequent Hebbian-like or activity-dependent stimulus is provided that stabilizes or holds the new connections (Holtmaat et al., 2005; Fu and Zuo, 2011). Furthermore, activity-like stimuli are also thought to potentiate these connections via the insertion of AMPA receptors, making them functional (Chklovskii et al., 2004; Holtmaat et al., 2005). Therefore, it has been proposed that information can be stored through a “sample and hold” or “two-step” model (Holtmaat et al., 2005). Because even adult cortex is sparsely connected, this model represents a mechanism that features enormous capacity for information storage (Fig. 1) (Chklovskii et al., 2004; Le Be and Markram, 2006; DeBello, 2008; Srivastava, 2012).
On the basis of these observations, it could be hypothesized that 17β-estradiol’s ability to transiently induce nascent silent synaptic connections may serve to “prime” neurons to respond to activity-dependent stimuli with greater efficacy, thus participating in a “two-step” model of (micro-)wiring plasticity. Estrogenic signaling-induced nascent, immature spines, would sample the presynaptic environment forming new synapses containing NMDA but lack AMPA receptors. If a second stimulus is not applied, these novel connections retract, allowing the cell to return to its “resting state” (Fig. 4). However, if a second, activity-dependent stimulus is applied, these newly formed synapses may become stabilized and potentiated, leading to an increase in connectivity between cortical neurons and enhanced network storage capacity.
Fig. 4.
Estrogen-induced “two-step wiring plasticity” in cortical neurons. This form of “wiring” plasticity can be divided into three distinct phases. Phase 1: treatment with 17 β-estradiol induces the formation of novel spines, which form connections with pre-synaptic partners within 30 minutes. Concurrently GluA1-containing AMPA receptors are removed from existing spines, and GluN1-containing NMDA receptors are trafficked into nascent spines. Overall, this results in an increased number of connections between cells and a reduction in AMPA receptor transmission. Phase 2A: effect on dendritic spines and glutamate receptors is transient: 60 minutes after treatment, estradiol-induced spines are preferentially eliminated and GluA1-containing AMPA receptors and GluN1-containing NMDA receptors return to control levels. Therefore, the number of connections returns to control levels, and AMPA receptor-transmission returns to normal. Or, Phase 2B: addition of a second synaptic activity-like stimulus results in the stabilization of estradiol-induced spines and a trafficking of GluA1-containing AMPA receptors back into existing and nascent synapses as observed immediately after the completion of treatment or 24 hours post-treatment. This combined treatment may lead to long-lasting (24 hour) increase in connectivity and increase synaptic communication.
Treatment with 17β-estradiol followed by an activity-dependent stimulus, achieved through activation of NMDA receptors by a chemical LTP paradigm (Srivastava et al., 2008; Xie et al., 2007), resulted in a stabilization of 17β-estradiol-induced spines that persisted for up to 24 hours. Importantly, these spines overlapped with the presynaptic marker bassoon, demonstrating that the combined treatment of 17β-estradiol and an activity-dependent stimulus had induced a long-term increase in connectivity in neurons. Furthermore, investigations of the effect of these two treatments on synapse function revealed that there was an increase in the surface expression of GluA1 at synapses that was paralleled by an increase in overall AMPA receptor-mediated transmission (Fig. 4). Previous reports demonstrated that Ca2+/calmodulin-dependent protein kinase II (CaMKII) is required for NMDA receptor-dependent changes in spine morphology and GluA1 insertion into synapses (Xie et al., 2007). Indeed, the combined treatment of 17β-estradiol and activity-dependent stimuli resulted in an increase in active levels of CaMKII (Srivastava et al., 2008). However, whether this kinase directly plays a role in mediating the stabilization of increased connectivity and transmission by these two extracellular signals is currently not known. In summary, these data demonstrate that the combined treatment of 17β-estradiol and activity-dependent stimulus was able to induce both a long-lasting increase in synaptic connectivity and enhanced synaptic communication between neurons (Fig. 4).
B. Physiologic Relevance of “Two-Step Wiring Plasticity”
Our cellular and molecular studies into rapid action of 17β-estradiol on cortical neurons have led us to propose the following model: 17β-estradiol can rapidly “prime” neurons to respond to subsequent synaptic activity-like stimuli with greater efficacy (Srivastava et al., 2008). This is achieved by 17β-estradiol modulation of spine structure and synapse function in neural circuits. To understand the complex mechanisms underlying this form of wiring plasticity termed “two-step wiring plasticity” (TSWP), we have divided it into three conceptual phases (see Fig. 4):
Phase 1: 17β-estradiol transiently increases the number of dendritic spines and generates silent synapses by removing GluA1-containing AMPA receptors from existing spines and inserting GluN1-containing-NMDA receptors into nascent synapses. Increased physical connectivity and generation of silent synapses places the neurons in a “primed” state, ready to respond to subsequent stimuli with greater efficacy.
Phase 2a: Without a second stimulus, 17β-estradiol-induced novel spines are preferentially eliminated, and GluA1-containing AMPA receptors and GluN1-containing NMDA receptors return to pre-existing spines. This mechanism allows the cell to return to a “resting state.”
Phase 2b: Addition of a subsequent activity-dependent stimulus leads to persistence of 17β-estradiol-induced spines and the trafficking of GluA1-containing AMPA receptors into both pre-existing and novel spines. This results in long-term (at least 24 hours) increase in synaptic connectivity and transmission.
On the basis of our current data, it is possible that the priming of dendritic spines in cortical neurons may serve to augment the acquisition or consolidation of certain behaviors. Evidence that 17β-estradiol initiated rapid signaling responses can improve performance on behavioral tasks when administered in a time-specific manner (Luine, 2008; Walf and Frye, 2008; Frick, 2009) supports a role for TSWP in the acquisition/consolidation of learned behaviors. This is further supported by studies demonstrating that estrogens can enhance performance in rapid learning paradigms, concurrent with increases in dendritic spine density (Phan et al., 2012). Furthermore, Xiao and colleagues (2013) demonstrated that 17β-estradiol-dependent acquisition of learned aversion behavior occurs concurrently with the facilitation of NMDA receptor-mediated synaptic transmission and increased dendritic spine density in the rACC. Collectively these data indicate that estrogens may act as a neuromodulator, with the capability of rapidly influencing cognition through the fine tuning of neural circuitry (Saldanha et al., 2011; Srivastava et al., 2011), and it is certainly compelling to consider that a mechanism similar to TSWP may contribute to the modulation of cognitive function.
C. “Two-Step Wiring Plasticity” Molecular Underpinnings—A Convergence of Pathways
Elucidation of the molecular pathways activated after acute 17β-estradiol treatment in cortical neurons has offered insight into the critical signals required for this form of microrewiring to occur. Activation of signaling pathways that are consistent with spinogenesis and subsequently with spine stabilization is required. Moreover, mechanisms required for the trafficking of synaptic proteins, including NMDA and AMPA receptors and PSD-95, to nascent and existing synapses would also be needed. Our current data suggest that activation of a Rap/AF-6/ERK1/2 pathway is crucial for the 17β-estradiol-mediated increase in spine density seen in Phase 1. During Phase 2a, it is possible that a decrease in Rac activity may be required to induce the retraction of 17β-estradiol-induced spines. Conversely, during Phase 2b, activation of Rac via an NMDA receptor/CaMKII pathway as previously shown (Xie et al., 2007) may be required for the stabilization of nascent spines and the potentiation of silent synapses (Fig. 4). Although our understanding of the molecular mechanisms of TSWP are currently in their infancy, it should be noted that this form of (micro-)rewiring may not be limited just to the pairing of estrogens and activity-dependent stimuli. It could involve the convergence of other signals such as estrogens and brain-derived neurotrophic factor (BDNF) (Srivastava et al., 2013) or other neuromodulatory signals with activity-dependent stimuli.
Because the aforementioned studies were performed using an in vitro system (Srivastava et al., 2008, 2010; Srivastava, 2012), it will be important to confirm the effects of TSWP within more intact systems and eventually in vivo. However, the use of in vitro systems offers an excellent platform for dissecting the potential molecular mechanisms underlying this form of plasticity. One of the more compelling aspects to this model lies in the flexibility it offers for information storage. It has been speculated that because changes in synaptic strength are restricted by the number of receptors/ion channels that can be trafficked to the PSD of dendritic spines, there is also a theoretical limit for changes in information flow (Chklovskii et al., 2004). On the other hand, changes in the number of connections is only limited by the number of potential synapses, offering a greater flexibility in the capacity of information storage; according to geometric analysis of cortical neurons, nearly all neighboring neurons have the capacity to become connected (Chklovskii et al., 2004; Stepanyants and Chklovskii, 2005; DeBello, 2008). Therefore, by inducing both changes in synaptic strength of existing connections and increasing the overall number of connections, TSWP is a model that offers an enormous capability for the storage of information in a physiologic context. Disruption of such a mechanism could greatly impact brain function. On the other hand, gaining insight into the mechanistic underpinnings of TSWP could provide powerful therapies for a variety of brain pathologies (Srivastava and Penzes, 2011).
D. Neural Circuits and Pathology
Deficits in cognitive function, notably in working, spatial, and reference memory, as well as social interactions, are core features of a great number of neurologic disorders (DSM-IV, 2000). Inasmuch as there is increasing evidence for an intimate link between dendritic spines and cognition it may not be surprising that multiple neuropathologies are strongly associated with disruptions of neural circuits (van Spronsen and Hoogenraad, 2010; Penzes et al., 2011a). It is currently posited that dendritic spine dysmorphogenesis can lead to defective or excessive synapse function and connectivity resulting in disruptions in neural circuitry (see Tau and Peterson, 2010; van Spronsen and Hoogenraad, 2010; Penzes et al., 2011a for recent reviews on this topic). Dysregulation of the complex mechanisms that control dendritic spine structure and function may contribute to these synaptic irregularities and contribute to the cognitive deficits seen in many of these disorders (Gray and Roth, 2007; Insel, 2010). Understanding the cellular mechanisms by which dendritic spine morphogenesis occurs will not only expand our knowledge of normal brain function, but that of abnormal brain function as well. Harnessing structural plasticity may offer a powerful future therapeutic avenue for treating neuropathologies (Gray and Roth, 2007; Insel, 2010).
E. Estrogenic Regulation of Neural Circuitry and Disease
The potential role(s) of estrogens in psychiatric and neurodegenerative diseases, as well as their potential beneficial actions as a therapeutic has been extensively reviewed elsewhere (Hughes et al., 2009; Kulkarni, 2009; Gillies and McArthur, 2010; Sanchez et al., 2010b; Nilsson et al., 2011; Srivastava and Penzes, 2011; Torrey and Davis, 2012; Brinton, 2013). Owing to the neuroprotective and neurotrophic effects of 17β-estradiol it is not surprising that its use as an adjunct treatment in a number of disorders has been investigated. Results for a limited number of clinical studies have indicated a potential beneficial role of estrogens in disorders such as schizophrenia (Cyr et al., 2000; Kulkarni et al., 2008; Hughes et al., 2009; Kulkarni, 2009; Sanchez et al., 2010b). However, it must be noted that long-term treatments of women aged 65 years with conjugated equine estradiol and medroxyprogesterone showed no beneficial effect of this treatment in protection against cognitive decline. Conversely, these trials suggested a potential increase in cognitive decline and increased risk for a number of risk factors for cardiovascular problems, stroke, and cancer (Rossouw et al., 2002; Espeland et al., 2004; Shumaker et al., 2004). Although there is controversy regarding the results of these findings (Craig et al., 2005), it is clear that caution must be taken when examining the potential beneficial effects of estrogens in psychiatric and neurodegenerative disorders. An alternative approach would be to mimic estrogenic-mediated positive effects by modulating specific ERs (Zhao et al., 2005; Hughes et al., 2009) and/or regulating 17β-estradiol intracellular molecular targets. Such strategies could exploit the beneficial effects of estrogens without the harmful side effects.
Although many studies have focused on the neuroprotective effects of estrogens, there is growing evidence that the beneficial effects of 17β-estradiol in psychiatric and neurodegenerative diseases are mediated, in part, through the modulation of neural circuitry. The antidepressive effect of 17β-estradiol in a learned helplessness model of depression occurs concurrently with an increase in spinogenesis in CA1 neurons (Hajszan et al., 2010). As loss of dendritic spines is thought to contribute to depressive symptoms (Nestler et al., 2002) could the antidepressive effects of 17β-estradiol be driven in part by an increase in the number of synaptic connections? Furthermore, selective activation of ERβ has antidepressive-like effects in a number of cognitive tests (Walf et al., 2008b); this is in addition to ERβ-mediated modulation of synapse structure and function (Liu et al., 2008; Srivastava et al., 2010). It is also interesting to note that the actions of antidepressants are speculated to occur by increasing the plasticity of neurons, allowing them to respond to subsequent experience-dependent plasticity with greater efficacy (Castren and Hen, 2013), a mechanism that bears similarity to TSWP. Recently it was shown that rapid estradiol signaling, acting via a mechanism identical to TSWP, was sufficient to rescue spine loss induced by soluble beta amyloid (Aβ) oligomers cultured hippocampal neurons (Logan et al., 2011). Despite the potential beneficial effects of 17β-estradiol in neurodegenerative and psychiatric disorders through the modulation of synaptic structure and function, it will remain important to continually assess any 17β-estradiol-induced harmful side effects. As such, a greater understanding of the molecular and cellular mechanisms underlying 17β-estradiol’s effects on both neuroprotection and neuronal circuitry will likely identify new restitutive targets in the development of certain CNS disorders.
X. Summary and Future Directions
This review of the how rapid estrogenic signaling can modulate neuroplasticity and thus influence cognition and highlights the complexities of uncovering the underlying cellular and molecular mechanisms that govern these effects. Converging lines of research indicate that rapid estrogenic signaling can influence behavior (Galea et al., 2008; Walf and Frye, 2008; Frick, 2009; Choleris et al., 2012). The application of estrogens either 30 minutes before or immediately after an initial training phase can enhance cognitive performance even when tested several hours or even days later. Estrogen treatment activates multiple signaling pathways within 1 hour, and blocking many of these pathways abolishes estrogen's ability to enhance cognition. This suggests that the signaling pathways regulated by rapid estrogen signaling can modulate cognition. Moreover, recent studies using rapid learning paradigms provide strong evidence that estrogens can influence cognition within 1 hour (Choleris et al., 2012). These studies also demonstrate that estrogens act on both cortical and hippocampal systems to affect multiple behaviors; however, the underlying neural circuitry for many of these behaviors are not well understood. Functional interactions between specific areas of the cortex (e.g., the prefrontal cortex) and the hippocampus are required for complex behaviors (Euston et al., 2012). Although the consequences of rapid estrogenic signaling on hippocampally based behaviors have been well investigated, in comparison, our understanding of the influence of estrogens on cortically based behaviors are not as well developed. Therefore, to fully appreciate the extent of the modulatory actions of estrogens on cognition it is critical to consider the effects it has on both areas.
Another issue that we attempted to highlight is the complex question of the source, or sources, of estrogens that result in these rapid responses. A popular hypothesis is that centrally produced estrogens underlie the rapid actions seen within the brain (Cornil et al., 2006; Garcia-Segura, 2008; Saldanha et al., 2011). There is much evidence that supports this hypothesis, which has led to the idea that centrally synthesized estrogens are not only neurosteroids but may also be neuromodulators. This hypothesis, also referred to as “synaptocrine” signaling, was reviewed in depth recently (Balthazart and Ball, 2006; Garcia-Segura, 2008; Saldanha et al., 2011). Although it may be argued that estrogens synthesized in the periphery cannot achieve sufficient concentration to initiate rapid cellular responses, it is clear that controlling the bioavailability of androgens in the circulating system would impact estradiol synthesis within the brain. Thus, it is clear that the interplay between peripheral and central sources of estrogens is not straightforward. A critical question still remaining is what are the physiologic conditions and circumstances in which sufficient concentrations of estrogens are produced to initiate rapid responses and how these mechanisms are controlled in specific brain regions? To fully understand the interaction between these sources of estrogens it is necessary to develop strategies to manipulate either or both sources.
At the molecular level we have begun to elucidate the mechanisms that contribute to rapid estrogenic responses. However, much of our understanding of how estrogen receptors couple to signaling pathways currently relies heavily on investigations in non-neuronal cells. As such, more detailed studies in neurons are required to validate these observations and to further dissect the molecular responses of rapid estrogenic signaling within the CNS. Estrogens have been shown to activate multiple signaling pathways dependent on cell type and even between brain regions. One possible explanation of how this signaling diversity arises comes from recent evidence that ERs can directly or indirectly interact with other receptors (Boulware et al., 2005; Vivacqua et al., 2009; Akama et al., 2013; Srivatsava and Evans, 2013). Formation of such receptor complexes would be dependent on the expression of specific receptors within a cell that could result in complex pharmacological profiles and enable coupling to multiple signaling cascades (see Srivatsava and Evans, 2013 for further discussion). Nevertheless, we are now beginning to understand how the signaling pathways activated in response to rapid estrogenic signaling can result in changes in neuroplasticity and cognition. Interestingly, emerging evidence suggests that the intracellular pathways rapidly activated by estrogens can also regulate transcriptional and translational machinery (Vasudevan and Pfaff, 2007; Ordonez-Moran and Munoz, 2009; Srivastava et al., 2011). Moreover, behavioral studies indicate that this mechanism is important for rapid estrogenic modulation of cognition (Zhao et al., 2010; Fortress et al., 2013). In the future it will be important to clarify what role this cross-talk takes and whether the gene and/or protein products of this regulation are required for the initial cellular actions or if they reinforce the cellular effects of rapid estrogenic signaling, enabling long-term changes in neural circuitry and cognition.
Estrogens have consistently been shown to rapidly regulate synapse structure and function (Woolley, 2007; Srivastava et al., 2011). The underlying signaling mechanisms can differ across developmental time points and according to brain region. In response to rapid estrogen signaling, neurons within the CA1 of the hippocampus display distinct cellular responses, pharmacological profiles, and activation of signaling pathways compared with neurons in other hippocampal regions or the cortex. Furthermore, it is noteworthy that in many cases the cellular and molecular responses elicited by rapid estrogenic signaling are transient, leading to questions of how this can result in the modulation of cognition. We hypothesize that pairing rapid estrogen signaling with activity-dependent stimuli, as part of a “two-step” model of circuit rewiring, results in long-lasting changes in neuronal connectivity. This model of circuit remodeling displays similar temporal characteristics to the reported rapid actions of estrogens on cognition, and we suggest that TSWP represents one of the cellular mechanisms that underlies estrogenic modulation of cognition. It is also interesting to consider that TSWP could be used to describe the interaction of estradiol and other stimuli (e.g., BDNF) (Srivastava et al., 2013) or a general model of circuit remodeling that can be applied to other neuromodulators is currently unknown.
In summary, although many questions remained to be resolved, there is substantial evidence that the rapid regulation of neuroplasticity by estrogens occurs concurrently with the modulation of cognition. However, the source of estrogens responsible for this rapid signaling in vivo is not clear. The mechanisms underlying the modulation of cognition by estrogens are due to the rapid activation of signaling pathways and changes in neural circuitry driven by alterations in synapses structure and function. Interestingly, it is emerging that there is cross-talk between rapid estrogenic cytosolic signaling and transcriptional/translational machinery, and such signaling mechanisms could cooperate to produce long-term changes in neuroplasticity. In addition, we described a cellular model that integrates in vitro and in vivo observations of neural remodeling to explain how rapid estrogen signaling can lead to long-term changes in cognition. Collectively these investigations argue that the rapid modulation of neuroplasticity by estrogens play an important role in regulating cognition but may also be important for our understanding of how estrogens could be beneficial in neuropathologies.
Acknowledgments
The authors thank Harrison Lowder for help in editing this manuscript.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- CREB
cAMP -esponse element binding
- DHEA
dehydroepiandrosterone
- DHEAS
sulfated form of DHEA
- DPN
diarylpropionitrile
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- GABA
γ-aminobutyric acid
- GAP
GTPase-activating proteins
- GEF
guanine nucleotide exchange factors
- GPCR
G-protein-coupled receptor
- KO
knockout
- LTD
long-term depression
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- mEPSC
miniature excitatory postsynaptic currents
- MNAR
modulator of nongenomic actions of estrogen receptor
- NCM
caudomedial nidopallium
- NMDA
N-methyl-d-aspartic acid
- OVX
ovariectomized
- PAK
p21-activated kinases
- PELP
proline-, glutamic acid-, and leucine-rich protein
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- p-LIMK
LIM-kinase
- PPT
propyl pyrazole triol
- PSD
postsynaptic density protein
- rACC
rostral anterior cingulate cortex
- RNAi
RNA interface
- SERM
synthetic estrogen receptor modulators
- StAR
steroidogenic acute regulatory protein
- TSWP
two-step wiring plasticity
- WASP
Wiskott-Aldrich syndrome protein
- WAVE
WASP-family verprolin-homologous protein
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Srivastava, Woolfrey, Penzes.
Footnotes
This work was supported by grants from the Brain & Behavior Research Foundation [formerly National Alliance for Research on Schizophrenia and Depression (NARSAD)] Young Investigators Award [Grant 18087]; a Royal Society International Exchange Grant [Grant IE111183]; a postdoctoral American Heart Association fellowship [Grant 0825719G] (to D.P.S.); a predoctoral AHA fellowship (to K.M.W.); and NARSAD, National Alliance for Autism Research, the Alzheimer’s Association, Brain Research Foundation, and National Institutes of Health National Institute of Mental Health [Grants R01MH071316 and R01MH097216] (to P.P.).
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. (2002) Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci 22:3608–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. (2003) Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci 23:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Thompson LI, Milner TA, McEwen BS. (2013) Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem 288:6438–6450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. (2007) Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 30:79–97 [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. (2002) A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci 22:8586–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Isaac JT. (2011) Maturation of a recurrent excitatory neocortical circuit by experience-dependent unsilencing of newly formed dendritic spines. Neuron 70:510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. (2008) Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav 54:244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. (2010) Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci 32:1995–2002 [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM. (2011) Estradiol synthesis within the human brain. Neuroscience 191:139–147 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. (2001) Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol 13:63–73 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. (2006) Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology 147:359–366 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. (2006) Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29:241–249 [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. (2011) When is the hippocampus involved in recognition memory? J Neurosci 31:10721–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. (1990) Neurosteroids: a new brain function? J Steroid Biochem Mol Biol 37:395–403 [DOI] [PubMed] [Google Scholar]
- Beato M. (1989) Gene regulation by steroid hormones. Cell 56:335–344 [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. (2005) Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6:565–575 [DOI] [PubMed] [Google Scholar]
- Berger SL. (2007) The complex language of chromatin regulation during transcription. Nature 447:407–412 [DOI] [PubMed] [Google Scholar]
- Beyer C, Pawlak J, Karolczak M. (2003) Membrane receptors for oestrogen in the brain. J Neurochem 87:545–550 [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. (2009) Dendritic spine dynamics. Annu Rev Physiol 71:261–282 [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. (2001) Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA 98:13391–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. (1999) Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24:161–173 [DOI] [PubMed] [Google Scholar]
- Blaustein JD. (2008) An estrogen by any other name. Endocrinology 149:2697–2698 [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. (2002) Spine motility. Phenomenology, mechanisms, and function. Neuron 35:1019–1027 [DOI] [PubMed] [Google Scholar]
- Boon WC, Chow JD, Simpson ER. (2010) The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res 181:209–232 [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V. (2011) Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids 76:877–884 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. (2005) Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. (2008) Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci 31:47–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. (2007) Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321 [DOI] [PubMed] [Google Scholar]
- Brinton RD. (2009) Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 30:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. (2013) Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol 9:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno J, Pfaff DW. (1976) Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res 101:67–78 [DOI] [PubMed] [Google Scholar]
- Cahill L. (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484 [DOI] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ. (1978) Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology 103:2283–2290 [DOI] [PubMed] [Google Scholar]
- Castets F, Bartoli M, Barnier JV, Baillat G, Salin P, Moqrich A, Bourgeois JP, Denizot F, Rougon G, Calothy G, et al. (1996) A novel calmodulin-binding protein, belonging to the WD-repeat family, is localized in dendrites of a subset of CNS neurons. J Cell Biol 134:1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Hen R. (2013) Neuronal plasticity and antidepressant actions. Trends Neurosci 36:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Beltzner CC, Pollard TD. (2009) Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr Biol 19:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Harada N, Balthazart J, Cornil CA. (2011) Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology 152:4199–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Yan YT, Wang TJ, Chen LJ, Wang YJ, Tseng GF. (2009) Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex 19:2719–2727 [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. (2004) Cortical rewiring and information storage. Nature 431:782–788 [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. (2012) Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol 33:140–159 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374 [DOI] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. (2007) Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol 505:249–267 [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. (2008) Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol 24:183–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. (2006) Plasticity in the human central nervous system. Brain 129:1659–1673 [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. (2006) Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res 1126:2–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. (2005) Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology 146:3809–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG. (2005) The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 4:190–194 [DOI] [PubMed] [Google Scholar]
- Cyr M, Calon F, Morissette M, Grandbois M, Di Paolo T, Callier S. (2000) Drugs with estrogen-like potency and brain activity: potential therapeutic application for the CNS. Curr Pharm Des 6:1287–1312 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. (1997) Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav 32:217–225 [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. (2010) DNA methylation and memory formation. Nat Neurosci 13:1319–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. (2011) Epigenetic mechanisms in cognition. Neuron 70:813–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBello WM. (2008) Micro-rewiring as a substrate for learning. Trends Neurosci 31:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. (2009) Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol 30:259–301 [DOI] [PubMed] [Google Scholar]
- Dominguez R, Micevych P. (2010) Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci 30:12589–12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-IV (2000) Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Society, Washington, DC. [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. (2010) Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci 1204:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. (2000) Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1:253–259 [DOI] [PubMed] [Google Scholar]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF. (1999) Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol 146:1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. (2007) Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron 54:447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes RD, Pocklington AJ, Anderson CN, Bayes A, Collins MO, Vickers CA, Croning MD, Malik BR, Choudhary JS, Armstrong JD, et al. (2008) Evolutionary expansion and anatomical specialization of synapse proteome complexity. Nat Neurosci 11:799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. (1997) Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113:509–519 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, et al. Women’s Health Initiative Memory Study (2004) Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD, Robb S, Cheek TR, Reale V, Hannan FL, Swales LS, Hall LM, Midgley JM. (1995) Agonist-specific coupling of G-protein-coupled receptors to second-messenger systems. Prog Brain Res 106:259–268 [DOI] [PubMed] [Google Scholar]
- Evans PD, Srivastava DP, Reale V. (2009) Rapid, non-genomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. Ecdysone: Structures and Functions, p. 425–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. (2010) Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci 30:4390–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Warner M, Gustafsson JA. (2006) Estrogen receptor beta expression in the embryonic brain regulates development of calretinin-immunoreactive GABAergic interneurons. Proc Natl Acad Sci USA 103:19338–19343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. (2008) Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28:8660–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. (2012) Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153:2953–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. (1998) Rapid actin-based plasticity in dendritic spines. Neuron 20:847–854 [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. (1998) Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. (2013) Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem 20:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. (1999) 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol 81:925–929 [DOI] [PubMed] [Google Scholar]
- Frick KM. (2009) Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav 55:2–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Zhao Z, Fan L. (2011) The epigenetics of estrogen: epigenetic regulation of hormone-induced memory enhancement. Epigenetics 6:675– 680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. (2010) Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67:86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. (2005) Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res 1036:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Zuo Y. (2011) Experience-dependent structural plasticity in the cortex. Trends Neurosci 34:177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. (1998) Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. J Neurochem 71:2231–2238 [DOI] [PubMed] [Google Scholar]
- Gaillard S, Bailly Y, Benoist M, Rakitina T, Kessler JP, Fronzaroli-Molinières L, Dargent B, Castets F. (2006) Targeting of proteins of the striatin family to dendritic spines: role of the coiled-coil domain. Traffic 7:74–84 [DOI] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. (2008) Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Expl Psych 62:247–260 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM. (2008) Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol 20:705–712 [DOI] [PubMed] [Google Scholar]
- Gibbs RB. (2007) Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav 52:352–359 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. (2008) Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149:3176–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. (2010) Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62:155–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. (2006) G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol 84:287–297 [DOI] [PubMed] [Google Scholar]
- Goley ED, Welch MD. (2006) The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 7:713–726 [DOI] [PubMed] [Google Scholar]
- González M, Cabrera-Socorro A, Pérez-García CG, Fraser JD, López FJ, Alonso R, Meyer G. (2007) Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol 503:790–802 [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. (1990) Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10:1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. (2007) Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull 33:1100–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. (2006) Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 84:112–119 [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. (2010) Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, et al. (2010) ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci 13:1208–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky NJ, Leranth C, Duman RS. (2010) Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biol Psychiatry 67:168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. (2011) GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Ogawa S, Wang JM, Herbison AE. (2012) Roles for oestrogen receptor β in adult brain function. J Neuroendocrinol 24:160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. (2007) Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA 104:11465–11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. (2009) Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202:223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW. (2009) Estrogens, episodic memory, and Alzheimer’s disease: a critical update. Semin Reprod Med 27:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. (2006) Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res 1121:46–58 [DOI] [PubMed] [Google Scholar]
- Hobkirk R. (1985) Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol 63:1127–1144 [DOI] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, et al. (2009) Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology 150:5106–5112 [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10:647–658 [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. (2005) Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45:279–291 [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. (1998) Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun 252:445–449 [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. (2010) Actin in dendritic spines: connecting dynamics to function. J Cell Biol 189:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Llano O, Smirnov S, Tanhuanpää K, Faix J, Rivera C, Lappalainen P. (2009) Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol 185:323–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. (2012) Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74:801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ZA, Liu F, Marquis K, Muniz L, Pangalos MN, Ring RH, Whiteside GT, Brandon NJ. (2009) Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol 2:215–236 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. (2010) Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav 58:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. (2010) Rethinking schizophrenia. Nature 468:187–193 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. (1995) Evidence for silent synapses: implications for the expression of LTP. Neuron 15:427–434 [DOI] [PubMed] [Google Scholar]
- Ishii H, Shoda Y, Yomogida K, Hamada T, Sakuma Y. (2011) Identification of C-terminally and N-terminally truncated estrogen receptor α variants in the mouse. J Steroid Biochem Mol Biol 124:38–46 [DOI] [PubMed] [Google Scholar]
- Ishii H, Tsurugizawa T, Ogiue-Ikeda M, Asashima M, Mukai H, Murakami G, Hojo Y, Kimoto T, Kawato S. (2007) Local production of sex hormones and their modulation of hippocampal synaptic plasticity. Neuroscientist 13:323–334 [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. (2008) Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol 24:93–98 [DOI] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. (2009) Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA 106:19575–19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Kreutz MR. (2009) Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci 32:392–401 [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. (2010) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33:121–129 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. (1976) Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res 114:152–157 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Rønnekleiv OK. (2005) Estrogen signaling in the hypothalamus. Vitam Horm 71:123–145 [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. (2010) Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 141:524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9:813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61:340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Hadman M, Wakade C, De Sevilla LM, Dhandapani KM, Mahesh VB, Vadlamudi RK, Brann DW. (2005) Cloning, expression, and localization of MNAR/PELP1 in rodent brain: colocalization in estrogen receptor-alpha- but not in gonadotropin-releasing hormone-positive neurons. Endocrinology 146:5215–5227 [DOI] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. (2000) Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404:151–158 [DOI] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S, et al. (2006) Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442:814–817 [DOI] [PubMed] [Google Scholar]
- Klann E, Antion MD, Banko JL, Hou L. (2004) Synaptic plasticity and translation initiation. Learn Mem 11:365–372 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ishii H, Sakuma Y. (2011) Identification of novel splicing events and post-transcriptional regulation of human estrogen receptor α F isoforms. Mol Cell Endocrinol 333:55–61 [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. (2011) Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152:223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Yuste R. (2004) Imaging the motility of dendritic protrusions and axon terminals: roles in axon sampling and synaptic competition. Mol Cell Neurosci 27:427–440 [DOI] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. (2009) Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci 29:12982–12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, et al. (2004) Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci 24:5913–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF. (2002) Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male and female rats. Cereb Cortex 12:116–128 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. (2007) Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav 51:183–194 [DOI] [PubMed] [Google Scholar]
- Kríz L, Bicíková M, Hampl R. (2008) Roles of steroid sulfatase in brain and other tissues. Physiol Res 57:657–668 [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. (2003) Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol 15:978–983 [DOI] [PubMed] [Google Scholar]
- Kulkarni J. (2009) Oestrogen—a new treatment approach for schizophrenia? Med J Aust 190(4, Suppl):S37–S38 [DOI] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, Burger H. (2008) Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry 65:955–960 [DOI] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. (2010) A critical review of fundamental controversies in the field of GPR30 research. Steroids 75:603–610 [DOI] [PubMed] [Google Scholar]
- Lavaque E, Sierra A, Azcoitia I, Garcia-Segura LM. (2006) Steroidogenic acute regulatory protein in the brain. Neuroscience 138:741–747 [DOI] [PubMed] [Google Scholar]
- Le Bé JV, Markram H. (2006) Spontaneous and evoked synaptic rewiring in the neonatal neocortex. Proc Natl Acad Sci USA 103:13214–13219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Romancer M, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L. (2008) Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol Cell 31:212–221 [DOI] [PubMed] [Google Scholar]
- Lee AW, Pfaff DW. (2008) Hormone effects on specific and global brain functions. J Physiol Sci 58:213–220 [DOI] [PubMed] [Google Scholar]
- Levin ER. (2011) Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol 25:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, et al. (2008) Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11:334–343 [DOI] [PubMed] [Google Scholar]
- Logan SM, Sarkar SN, Zhang Z, Simpkins JW. (2011) Estrogen-induced signaling attenuates soluble Aβ peptide-mediated dysfunction of pathways in synaptic plasticity. Brain Res 1383:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. (2010) The rapid effects of estrogen: a mini-review. Behav Pharmacol 21:465–472 [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. (2008) Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci 11:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösel R, Wehling M. (2003) Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4:46–56 [DOI] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. (2004) Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci USA 101:17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. (2007) The prefrontal cortex, gonadal hormones and memory. Horm Behav 51:181–182 [DOI] [PubMed] [Google Scholar]
- Luine VN. (2008) Sex steroids and cognitive function. J Neuroendocrinol 20:866–872 [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. (2003) Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144:2836–2844 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. (2005) The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146:287–293 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Goldman-Rakic PS. (1986) Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci USA 83:513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Picard D. (2010) The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol 204:105–114 [DOI] [PubMed] [Google Scholar]
- Mak P, Zeen R, and Callard GV(1985) Subcellular distribution and reaction kinetics of aromatase in goldfish brain, in Program of the 67th Annual Meeting of The Endocrine Society; Baltimore, MD:265. [Google Scholar]
- Malenka RC, Bear MF. (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21 [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40–46 [DOI] [PubMed] [Google Scholar]
- Martin S, Jones M, Simpson E, van den Buuse M. (2003) Impaired spatial reference memory in aromatase-deficient (ArKO) mice. Neuroreport 14:1979–1982 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. (2001) Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Noh KM, Allis CD. (2013) Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacology 38:3–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. (2008) New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol 290:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307 [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Panzica G, Garcia-Segura LM. (2011) Neuroactive steroids: focus on human brain. Neuroscience 191:1–5 [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. (2003) Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res 112:170–176 [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM, Muller D. (2011) Estradiol promotes spine growth and synapse formation without affecting pre-established networks. Hippocampus 21:1263–1267 [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Beaujean D, Do-Rego JL, Mathieu M, Vallarino M, Luu-The V, Pelletier G, Vaudry H. (2000) In vivo evidence for the production of sulfated steroids in the frog brain. Comp Biochem Physiol B Biochem Mol Biol 126:213–219 [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. (1996) Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Bondar G, Kuo J. (2010) Estrogen actions on neuroendocrine glia. Neuroendocrinology 91:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Kelly MJ. (2012) Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology 96:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. (2005) Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol 491:81–95 [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. (2001) Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol 429:355–371 [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, et al. (2003) Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. (2010) Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol 518:2729–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. (2009) Neurobiology of song learning. Curr Opin Neurobiol 19:654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, et al. (2007) Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem 100:950–967 [DOI] [PubMed] [Google Scholar]
- Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, et al. (2006) Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun 351:553–558 [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R. (2012) Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci 35:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. (1998) Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 18:2550–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. (1997) Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA 94:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. (1986) Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science 233:226–228 [DOI] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Soriano S, Ripoll C, Fuentes E, Quesada I, Ropero AB. (2011) Role of estrogen receptors alpha, beta and GPER1/GPR30 in pancreatic beta-cells. Front Biosci (Landmark Ed) 16:251–260 [DOI] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. (1996) Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63:149–155 [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. (1971a) Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol 51:795–796 [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. (1971b) Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab 33:368–370 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. (2002) Neurobiology of depression. Neuron 34:13–25 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Koehler KF, Gustafsson JA. (2011) Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov 10:778–792 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. (2001) Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- O’Neill EE, Blewett AR, Loria PM, Greene GL. (2008) Modulation of alphaCaMKII signaling by rapid ERalpha action. Brain Res 1222:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Sainlos M, Choquet D. (2012) Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol 22:453–460 [DOI] [PubMed] [Google Scholar]
- Ordóñez-Morán P, Muñoz A. (2009) Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle 8:1675–1680 [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, Robitaille R. (2011) Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146:785–798 [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. (1992) Neuroactive steroids. FASEB J 6:2311–2322 [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. (2007) A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282:22278–22288 [DOI] [PubMed] [Google Scholar]
- Pelletier G. (2010) Steroidogenic enzymes in the brain: morphological aspects. Prog Brain Res 181:193–207 [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME. (2012) Deconstructing signal transduction pathways that regulate the actin cytoskeleton in dendritic spines. Cytoskeleton (Hoboken) 7:426–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, Srivastava DP. (2008) Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol 18:405–413 [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. (2011a) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Woolfrey KM, Srivastava DP. (2011b) Epac2-mediated dendritic spine remodeling: implications for disease. Mol Cell Neurosci 46:368–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317:1083–1086 [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431 [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. (2005) Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci 272:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Ribeiro AC. (2010) Theoretical consequences of fluctuating versus constant liganding of oestrogen receptor-alpha in neurones. J Neuroendocrinol 22:486–491 [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Ungerer N. (2011) Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res 50:357–371 [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. (2012) Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37:2299–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. (2011) Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology 152:1492–1502 [DOI] [PubMed] [Google Scholar]
- Pierman S, Sica M, Allieri F, Viglietti-Panzica C, Panzica GC, Bakker J. (2008) Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm Behav 54:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carrétero J, Rune GM. (2006) Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus 16:464–471 [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. (2001) A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology 142:2039–2049 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. (2011) The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7:715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Maggiolini M. (2009) Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol 308:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. (1911) Histology of the Nervous System of Man and Vertebrates, Oxford University Press, New York [Google Scholar]
- Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. (2004) Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience 126:839–847 [DOI] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. (2008) Rapid estrogen signaling in the brain. Neurosignals 16:140–153 [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. (2010) Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA 107:3852–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. (2011) Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci 31:10034–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. (2008) Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, Pfaff DW. (2012) siRNA silencing of estrogen receptor-α expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc Natl Acad Sci USA 109:16324–16329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A. (2010) Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci 13:1330–1337 [DOI] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. (2010) Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 463:948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand S, Wang Y, Toledo-Rodriguez M, Markram H. (2011) Morphological development of thick-tufted layer v pyramidal cells in the rat somatosensory cortex. Front Neuroanat 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. (2005) Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience 136:833–842 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. (1999) Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci 22:410–416 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Takahashi H, Matsuda K, Nishi M, Takanami K, Ogoshi M, Sakamoto T, Kawata M. (2012) Rapid signaling of steroid hormones in the vertebrate nervous system. Front Biosci (Landmark Ed) 17:996–1019 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. (2011) Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32:532–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. (2000) Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol 423:619–630 [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrade G, Kendrick KM. (2011) Roles of α- and β-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Horm Behav 59:114–122 [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Flamini MI, Baldacci C, Goglia L, Genazzani AR, Simoncini T. (2010a) Estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol Endocrinol 24:2114–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AM, Flamini MI, Fu XD, Mannella P, Giretti MS, Goglia L, Genazzani AR, Simoncini T. (2009) Rapid signaling of estrogen to WAVE1 and moesin controls neuronal spine formation via the actin cytoskeleton. Mol Endocrinol 23:1193–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MG, Bourque M, Morissette M, Di Paolo T. (2010b) Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:e43–e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandén C, Broselid S, Cornmark L, Andersson K, Daszkiewicz-Nilsson J, Mårtensson UE, Olde B, Leeb-Lundberg LM. (2011) G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol Pharmacol 79:400–410 [DOI] [PubMed] [Google Scholar]
- Sano K, Tsuda MC, Musatov S, Sakamoto T, Ogawa S. (2013) Differential effects of site-specific knockdown of estrogen receptor α in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. Eur J Neurosci 37:1308–1319 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. (2007) Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci 26:2595–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. (1989) Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology 49:434–441 [DOI] [PubMed] [Google Scholar]
- Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. (2012) Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol 33:85–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. (2005) Dendritic spines and long-term plasticity. Nat Rev Neurosci 6:277–284 [DOI] [PubMed] [Google Scholar]
- Segal M. (2010) Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci 31:2178–2184 [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. (2007) The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23:613–643 [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. (2008) Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 29:88–113 [DOI] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. (2009) Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci 29:8129–8142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, et al. Women’s Health Initiative Memory Study (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. (2008) Steroid hormone receptor expression and function in microglia. Glia 56:659–674 [DOI] [PubMed] [Google Scholar]
- Sinopoli KJ, Floresco SB, Galea LA. (2006) Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem 86:293–304 [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. (2006) Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci 26:8517–8522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Smejkalova T, Forlano PM, Woolley CS. (2010) Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods 188:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD. (2007) A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci 27:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. (2003) Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA 100:1723–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spary EJ, Maqbool A, Batten TF. (2009) Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat 38:185–196 [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, et al. (2011) Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci USA 108:8867–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. (2008) Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience 155:1106–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP. (2012) Two-step wiring plasticity—a mechanism for estrogen-induced rewiring of cortical circuits. J Steroid Biochem Mol Biol 131:17–23 [DOI] [PubMed] [Google Scholar]
- Srivatsava DP and Evans PD (2013) GPER 1 trials and tribulations of a Membrane Oestrogen Receptor. J Neuroendocrinol 10.1111/jne.12071 [published ahead of print]. [DOI] [PubMed]
- Srivastava DP, Copits BA, Xie Z, Huda R, Jones KA, Mukherji S, Cahill ME, VanLeeuwen JE, Woolfrey KM, Rafalovich I, et al. (2012a) Afadin is required for maintenance of dendritic structure and excitatory tone. J Biol Chem 287:35964–35974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Jones KA, Woolfrey KM, Burgdorf J, Russell TA, Kalmbach A, Lee H, Yang C, Bradberry MM, Wokosin D, et al. (2012b) Social, communication, and cortical structural impairments in Epac2-deficient mice. J Neurosci 32:11864–11878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Penzes P. (2011) Rapid estradiol modulation of neuronal connectivity and its implications for disease. Front Endocrinol (Lausanne) 2:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F. (2011) Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci 31:16056–16063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Evans PD. (2013) Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience 239:17–33 [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Anderson CT, Smith KR, Russell TA, Lee H, Yasvoina MV, Wokosin DL, Ozdinler PH, et al. (2012d) An autism-associated variant of Epac2 reveals a role for Ras/Epac2 signaling in controlling basal dendrite maintenance in mice. PLoS Biol 10:e1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. (2008) Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA 105:14650–14655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. (2010) Estrogen receptor ß activity modulates synaptic signaling and structure. J Neurosci 30:13454–13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. (2005) Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci 25:6145–6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. (2002) Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci 5:239–246 [DOI] [PubMed] [Google Scholar]
- Stepanyants A, Chklovskii DB. (2005) Neurogeometry and potential synaptic connectivity. Trends Neurosci 28:387–394 [DOI] [PubMed] [Google Scholar]
- Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. (1999) Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology 140:5455–5458 [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. (2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24:299–325 [DOI] [PubMed] [Google Scholar]
- Stocco DM. (2001) Tracking the role of a star in the sky of the new millennium. Mol Endocrinol 15:1245–1254 [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Smejkalova T, Woolley CS. (2013) Distribution and posttranslational modification of synaptic ERα in the adult female rat hippocampus. Endocrinology 154:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. (2006) Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 16:95–101 [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. (2001) Small GTP-binding proteins. Physiol Rev 81:153–208 [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8:37–48 [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Young-Pearse T, Sawa A, Kamiya A. (2012) In utero electroporation as a tool for genetic manipulation in vivo to study psychiatric disorders: from genes to circuits and behaviors. Neuroscientist 18:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Yuste R. (2004) Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci 26:429–440 [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. (2010) Normal development of brain circuits. Neuropsychopharmacology 35:147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5:173–183 [DOI] [PubMed] [Google Scholar]
- Thomas P. (2012) Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen Comp Endocrinol 175:367–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. (2001) A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19). J Endocrinol 168:217–220 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. (1976) Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res 106:407–412 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. (2004) Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology 145:1069–1074 [DOI] [PubMed] [Google Scholar]
- Torrey EF, Davis JM. (2012) Adjunct treatments for schizophrenia and bipolar disorder: what to try when you are out of ideas. Clin Schizophr Relat Psychoses 5:208–216 [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. (2009) Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci 29:5949–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. (2011) Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci 31:3271–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugizawa T, Mukai H, Tanabe N, Murakami G, Hojo Y, Kominami S, Mitsuhashi K, Komatsuzaki Y, Morrison JH, Janssen WG, et al. (2005) Estrogen induces rapid decrease in dendritic thorns of CA3 pyramidal neurons in adult male rat hippocampus. Biochem Biophys Res Commun 337:1345–1352 [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. (2003) Do rats have a prefrontal cortex? Behav Brain Res 146:3–17 [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, Kumar R. (2001) Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem 276:38272–38279 [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. (2007) Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev 28:1–19 [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. (2008) Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29:238–257 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, Fuqua SA, Andò S, Maggiolini M. (2009) G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells. Mol Endocrinol 23:1815–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. (2008) Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids 73:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. (2008a) Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem 89:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. (2008b) Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci 122:974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. (2006) Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem 86:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. (2010) Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci 30:12770–12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Gustafsson JA. (2006) Nongenomic effects of estrogen: why all the uncertainty? Steroids 71:91–95 [DOI] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. (2011) Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res 1379:86–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. (2008) N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem 283:15912–15920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Lösel R. (2006) Non-genomic steroid hormone effects: membrane or intracellular receptors? J Steroid Biochem Mol Biol 102:180–183 [DOI] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. (2001) Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem 76:1879–1886 [DOI] [PubMed] [Google Scholar]
- Wójtowicz T, Mozrzymas JW. (2010) Estradiol and GABAergic transmission in the hippocampus. Vitam Horm 82:279–300 [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. (1991) Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res 543:148–152 [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, et al. (2009) Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci 12:1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. (2007) Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. (1990) Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10:4035–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12:2549–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Chen S, Brinton RD. (2011) Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res 1379:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Subramaniam M, Negron V, Cicek M, Reynolds C, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. (2012) Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem 113:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Yang Y, Zhang Y, Zhang XM, Zhao ZQ, Zhang YQ. (2013) Estrogen in the anterior cingulate cortex contributes to pain-related aversion. Cereb Cortex 23:2190–2203 [DOI] [PubMed] [Google Scholar]
- Xie Z, Huganir RL, Penzes P. (2005) Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron 48:605–618 [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56:640–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Muñoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. (2006) Aromatase expression in the human temporal cortex. Neuroscience 138:389–401 [DOI] [PubMed] [Google Scholar]
- Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. (2008) Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res 1209:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, et al. (2004) Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA 101:4130–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Murakoshi H. (2011) The mechanisms underlying the spatial spreading of signaling activity. Curr Opin Neurobiol 21:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, Akama KT, McEwen BS, Milner TA, Morrison JH. (2008) Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience 152:360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D. (2009) Dendritic spine formation and stabilization. Curr Opin Neurobiol 19:146–153 [DOI] [PubMed] [Google Scholar]
- Yuen GS, McEwen BS, Akama KT. (2011) LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res 1379:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. (2009) 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci USA 106:21936–21941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, O’Neill K, Diaz Brinton R. (2005) Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Brain Res Rev 49:472–493 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. (2012) Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci 32:2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. (2010) Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA 107:5605–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. (2010) Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 151:1153–1160 [DOI] [PubMed] [Google Scholar]
- Zhou L, Lehan N, Wehrenberg U, Disteldorf E, von Lossow R, Mares U, Jarry H, Rune GM. (2007) Neuroprotection by estradiol: a role of aromatase against spine synapse loss after blockade of GABA(A) receptors. Exp Neurol 203:72–81 [DOI] [PubMed] [Google Scholar]
- Zhu BT, Conney AH. (1998) Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 19:1–27 [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. (1996) Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17:91–102 [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. (1999) Dehydroepiandrosterone: biosynthesis and metabolism in the brain. Endocrinology 140:880–887 [DOI] [PubMed] [Google Scholar]