Abstract
This study explored whether physical health problems are related to psychotic symptoms independently of a mental disorder diagnosis. A total of 224,254 subjects recruited for the World Health Organization World Health Survey were subdivided into those with both a lifetime diagnosis of psychosis and at least one psychotic symptom in the 12 months prior to the evaluation, those with at least one psychotic symptom in the past 12 months but no lifetime diagnosis of psychosis, and those without psychotic symptoms in the past 12 months and without a lifetime diagnosis of psychosis. The three groups were compared for the presence of medical conditions, health problems, and access to health care. Medical conditions and health problems (angina, asthma, arthritis, tuberculosis, vision or hearing problems, mouth/teeth problems, alcohol consumption, smoking, and accidents), medication consumption, and hospital admissions (but not regular health care visits) were more frequent in individuals with psychotic symptoms but no psychosis diagnosis, compared to those with no symptoms and no diagnosis. The number of medical conditions increased with the number of psychotic symptoms. Given the sample analyzed, this trend seems to be independent from the socio-economic development of the country or the specific health care system.
Keywords: Psychotic symptoms, physical health, medical conditions, access to health care, multinational study
Psychotic disorders have been associated with a mortality rate double that of the general population (1,2) and a shortening of life expectancy by up to 20 years (3). Physical comorbidities are major contributors to morbidity and mortality in people with schizophrenia and other psychotic disorders (1,2,4-8). The impact of cardiovascular and metabolic illnesses has been consistently reported (9-12), and evidence is beginning to accumulate regarding the role of infectious diseases, respiratory illness, and the abuse of different substances, amongst others (1,7,12-16). Lifestyle factors (such as sedentarism, inadequate diet and smoking), treatment with antipsychotics, and unequal access to health care have been suggested as contributors to poorer physical health among people with psychosis (6,17-19). Immune mechanisms and inflammation may also play a role, mediating not only the brain expression of the disorder, but also the concurrent systemic problems (20).
Approximately 3% of the general population have a psychotic disorder (21). However, the prevalence of psychotic symptoms in population-based studies is significantly higher, ranging from 0.7 to 45.8% for the presence of at least one psychotic symptom in a recent world-wide cross-national study (22). The negative impact of psychotic symptoms on functioning seems not to be restricted to individuals with diagnosable psychotic disorders (23,24). Evidence regarding whether medical conditions and other indices of physical health are related to the presence of psychotic symptoms independently of an established mental disorder diagnosis is still preliminary (25). Moreover, nearly all of the available information regarding the larger than expected comorbidity of physical illnesses with schizophrenia and other psychoses comes from studies conducted in industrialized countries, mostly single-country studies (7,26).
In this paper, we present data from the World Health Organization (WHO) World Health Survey (WHS), an international study including countries with different levels of socio-economic development (27). We explored the differential load of physical illnesses and the access to treatment for those illnesses in subjects presenting psychotic symptoms (with or without a psychosis diagnosis) and subjects without psychotic symptoms. We hypothesized that the presence of psychotic symptoms (independent of a psychosis diagnosis) would be related to medical pathology, regardless of each country's socio-economic level.
Methods
Sample
We included individuals from the 52 countries covered by the WHS: 18 from the African region, 13 from the European region, 7 from the Americas region, 5 from the Asian region, 5 from the South-East Asian region, and 4 from the Eastern Mediterranean region. Fifteen countries were classified in the high or upper-middle economic levels, according to the World Bank, and 37 in the lower-middle or low levels. All samples were drawn from a current national frame using a multi-stage cluster design enabling each household and individual respondent to be assigned a known non-zero probability of selection. The sampling guidelines and the summary descriptions of the sampling procedures are available from the WHS website (http://www.who.int/healthinfo/survey/en/index.html).
Informed consent was obtained from all respondents, and the study was cleared by ethical review committees at each site. The individual global response rate was 98.5%, with the final sample comprising 224,254 subjects. All interviews were conducted by specifically trained interviewers. A standard procedure for training and quality control was implemented at all sites and supervised periodically, as per the specified guidelines.
Measures
All respondents were interviewed using the standardized WHS instrument from the WHO. The interview collected data on health status, socio-demographic characteristics, consumption of alcohol and tobacco, lifestyle, household economic status (based on a list of permanent income indicators), and information about functioning, health status and quality of life. Lifetime diagnosis and treatment of psychosis and presence of psychotic symptoms during the last 12 months were assessed. Lifetime diagnosis and symptoms during the last 12 months of asthma, arthritis and angina pectoris were also recorded. Alcohol consumption was coded using two groups and one dummy variable, with lifetime abstainers and occasional drinkers (those who consumed a total of 15 or less units in the previous week, but no more than 4 units on one occasion) being the reference category (87.4%), and occasional heavy drinkers (those who consumed a total of 15 or more units in the previous week, but no more than 4 units on one occasion) and heavy drinkers (those who consumed 5 or more units on at least one occasion) comprising the other category. Smoking was dichotomized as not currently smoking any type of tobacco versus currently smoking tobacco daily (23.8%).
Assessment of psychotic symptoms
Individual questions based on the WHS version of the Composite International Diagnostic Interview (CIDI 3.0) (28) were included to assess the presence of psychotic symptoms, including delusional mood, delusions of reference and persecution, delusions of control, and hallucinations, over the past 12 months. The response format for all the above questions was dichotomous (yes/no). The psychosis module of the CIDI has shown high concordance with clinician ratings (29).
Assessment of specific medical conditions
The diagnosis of angina was based on an algorithm derived from the Rose questionnaire (30). Asthma and arthritis were established according to dichotomous questions (yes/no) about lifetime diagnosis of those illnesses (31). Responders were regarded as having diabetes mellitus if they responded “yes” to the question “Have you ever being diagnosed with diabetes (high blood sugar)?”. The diagnosis of tuberculosis was established on the basis of questions about a cough lasting more than three weeks and including blood in cough or phlegm. Lifetime treatment and medication use over the previous 2 weeks were also assessed for all conditions.
The number of comorbid non-psychiatric illnesses, including angina pectoris, asthma, diabetes, arthritis and tuberculosis, was assessed. Results about the association between diabetes and psychotic symptoms in this sample were already reported (32); therefore diabetes was only considered to estimate the global amount of comorbid non-psychiatric illnesses. Information was also included about the self-reported presence (yes/no) of vision problems (and of cataracts during the previous 5 years in people 60 years and over), hearing problems, teeth problems, and road traffic or other injuries in the 12 months before the interview.
Assessment of access to health care
Information about health system use and responsiveness was also collected. Occurrence and length of overnight stays in health centers during the last 5 years, as well as treatment at home or as an outpatient, were included in the analyses, not considering stays potentially attributable to psychiatric reasons. Satisfaction with health systems in the country (from 1, very satisfied, to 5, very unsatisfied), self-reported health dissatisfaction (from 1, very satisfied to 5, very unsatisfied), and perceived lack of health (from 1, very good, to 5, very bad) were also assessed.
Medications being taken at the time of the interview were reported. A total scale of number of medicines being consumed was calculated (range: 0-6), excluding medication for psychiatric problems.
Statistical analysis
We first performed a series of binomial logistic regression analyses comparing subjects with a lifetime diagnosis of psychosis and psychotic symptoms in the last 12 months (N=1,306) and subjects with at least one psychotic symptom in the last 12 months but no psychotic diagnosis (N=27,648) vs. individuals without psychotic symptoms and no lifetime diagnosis of psychosis (N=195,300). In all of these analyses we statistically controlled for age, gender, World Bank category of the country, and country of the individual (including 51 dummy variables as covariates referred to the country of the sample), the last two in order to control for potential differences in the functioning of health services. Independent variables included in those series of analyses were specific medical conditions and access to health care.
Then, to test the global impact of psychotic symptoms and diagnosis on health, we compared the total number of non-psychiatric illnesses (including angina pectoris, arthritis, asthma, diabetes and tuberculosis) and the consumption of medicines prescribed by a medical professional between individuals with lifetime psychotic diagnosis and psychotic symptoms in the last 12 months and individuals with at least one psychotic symptom and no diagnosis vs. individuals without psychotic symptoms and no diagnosis of psychosis.
Comparisons were performed through t-tests for unrelated samples, adjusting the probability level to control for family-wise type I error (Bonferroni's correction). To assess the strength of these associations, effect sizes (Hedge's g) were calculated for associations with continuous variables. Hedge's g with large samples provides values that are very similar to Cohen's d (33), for which the following arbitrary rules of thumb are often used: effect sizes below .20 are regarded as not relevant, between .20 and .50 as low, between .50 and .80 as moderate, and over .80 as high. Also, with the aim of indirectly testing both overall health and health-service responsiveness, the same comparisons were carried out using the total number of medicines currently being taken (excluding those for psychiatric problems) as the dependent variable. Additionally, we analyzed the differences in the number of illnesses and of medicines currently being taken across the continuum of psychotic symptoms (number of symptoms, range: 0-4) through one-way ANOVAs, with the number of symptoms reported as the independent variable and post-hoc comparisons (Scheffé) between specific groups. Patients with a previous diagnosis of psychosis and no psychotic symptoms in the past 12 months were not included in any analysis.
All analyses were carried out with the statistical package STATA, version 11.0 (Stata Corp, 2010). Significance was set at α=0.05.
RESULTS
In binary logistic regression analyses, all somatic variables considered had statistically significant ORs (p<0.001). Thus, compared with subjects without psychotic symptoms and no psychotic diagnoses, those with at least one psychotic symptom in the last 12 months and no psychotic diagnosis had a higher probability of also reporting angina pectoris, asthma, arthritis, tuberculosis, vision or hearing problems, cataracts (in people with 60 years or over), mouth or teeth problems during the previous year, and high alcohol consumption during the previous week, of being smokers, and of having had more road accidents or other injuries in the previous year (Table 1). The comparison of subjects with psychotic symptoms and a diagnosis of psychosis vs. those with no symptoms and no diagnosis produced very similar results, although suggesting stronger associations. In fact, comparing the two columns in Table 1, almost all 95% CIs did not overlap (the only exceptions were those for cataracts, alcohol consumption and smoking), with a higher range in the case of subjects with a diagnosis of psychosis.
Table 1.
Physical diseases and health problems in subjects with psychotic symptoms and no psychotic diagnosis and in those with psychotic symptoms plus lifetime psychotic diagnosis vs. subjects without psychotic symptoms or diagnosis
| Psychotic symptoms without psychosis diagnosis OR (95% CI) | Psychotic symptoms plus psychosis diagnosis OR (95% CI) | |
|---|---|---|
| Angina pectoris | 2.50 (2.38/2.62) | 3.98 (3.38/4.68) |
| Asthma | 1.81 (1.72/1.91) | 3.71 (3.16/4.75) |
| Arthritis | 1.80 (1.73/1.86) | 2.85 (2.50/3.25) |
| Tuberculosis | 2.87 (2.66/3.11) | 4.72 (3.73/5.97) |
| Vision problems | 1.67 (1.59/1.75) | 2.16 (1.80/2.58) |
| Cataracts (people over 60) | 1.39 (1.24/1.57) | 2.15 (1.28/3.61) |
| Hearing problems | 1.56 (1.46/1.67) | 2.27 (1.80/2.85) |
| Alcohol consumption (occasionally heavy/heavy) | 1.27 (1.24/1.30) | 1.14 (1.11/1.17) |
| Smoking (% currently yes) | 1.18 (1.15/1.21) | 1.30 (1.14/1.48) |
| Mouth or teeth problems (last year) | 1.63 (1.58/1.67) | 2.06 (1.83/2.32) |
| Road traffic or other injuries (1 year) | 2.34 (2.23/2.44) | 3.21 (2.72/3.79) |
All results are significant compared to individuals without diagnosis of psychosis nor psychotic symptoms in the prior 12 months
Values in bold indicate non-overlapping 95% CIs between the two columns
As shown in Table 2, the presence of psychotic symptoms was related to an increased probability of health dissatisfaction, worse self-rated health, higher dissatisfaction with health care in the country, and higher self-reported consumption in the two previous weeks of medicines for most conditions included in the study, except for HIV. There was also a statistically significant positive effect for at least one overnight stay in hospital during the previous five years (excluding psychiatric reasons), and for the length of the stay. Comparing people with psychotic symptoms and a lifetime diagnosis of psychosis vs. those without diagnosis and symptoms, there was also an increased probability of most variables in the former, except for consumption of medicines for HIV or asthma, as well as for the length of the last overnight stay in a hospital for non-psychiatric reasons.
Table 2.
Health system care indicators in subjects with psychotic symptoms and no psychotic diagnosis and in those with psychotic symptoms plus lifetime psychotic diagnosis vs. subjects without psychotic symptoms or diagnosis
| Psychotic symptoms without psychosis diagnosis OR (95% CI) | Psychotic symptoms plus psychosis diagnosis OR (95% CI) | |
|---|---|---|
| Overnight stays (excluding psychiatric) | 1.33 (1.28/1.38)a | 1.91 (1.65/2.22) |
| Length of stay (excluding psychiatric) | ||
| 3-5 days | 1.15 (1.07/1.25)a | 1.34 (0.99/2.22) |
| 6-14 days | 1.22 (1.11/1.34)a | 1.41 (1.00/1.99)a |
| More than 15 days | 1.37 (1.22/1.54)a | 1.45 (0.94/2.22) |
| Health care attention, excluding overnight and psychiatric (12 months) | 1.79 (0.69/4.62) | 0.67 (0.09/4.97) |
| Health dissatisfaction | 1.28 (1.26/1.29)a | 1.51 (1.44/1.60)a |
| Self-rated lack of health | 1.42 (1.41/1.44)a | 1.77 (1.69/1.86)a |
| Dissatisfaction with health care in country | 1.12 (1.11/1.13)a | 1.11 (1.05/1.16)a |
| Prescribed medicines used last two weeks | ||
| Arthritis | 1.63 (1.49/1.79)a | 1.94 (1.36/2.76)a |
| Angina pectoris | 1.30 (1.15/1.47)a | 2.43 (1.65/3.59)a |
| Asthma | 1.20 (1.03/1.39)a | 1.44 (0.80/2.57) |
| Tuberculosis | 1.86 (1.29/2.69)a | 3.75 (1.37/10.29)a |
| HIV/AIDS | 1.10 (0.66/1.83) | 2.95 (0.87/10.03) |
| Other (non-psychiatric) | 1.41 (1.35/1.48)a | 1.64 (1.36/1.99)a |
| Total medicines (0–6) | 1.31 (1.26/1.35)a | 1.54 (1.36/1.75)a |
Significant results using as comparison group individuals without diagnosis of psychosis nor psychotic symptoms in the prior 12 months
Values in bold indicate non-overlapping 95% CIs between the two columns
Comparing the two columns in Table 2, there was an overlap in ORs, except for hospital stay in the previous 5 years, consumption of medicines for angina, health dissatisfaction, self-reported lack of health, and total number of medicines currently being taken, for which the presence of diagnosis plus psychotic symptoms in the last 12 months was associated with a higher probability.
The mean number of somatic illnesses was significantly higher (t=34.0; p<0.001; g=1.05, 95% CI: 0.99-1.11) in subjects with a psychotic diagnosis and psychotic symptoms (0.79±0.97) than in those with no psychotic symptoms or diagnosis (0.24±0.52). Similarly, it was significantly higher (t= 57.8; p<0.001; g=0.40, 95% CI: 0.39-0.41) in individuals with at least one psychotic symptom but no psychotic diagnosis (0.46±0.72) than in those without diagnosis or symptoms. Among subjects with psychotic symptoms, the association with somatic illnesses was stronger in subjects with a lifetime diagnosis of psychosis than in those without, as shown by the not overlapping CIs in the effect sizes.
As shown in Figure 1, the number of somatic illnesses increased with the number of reported psychotic symptoms (F=969.3; p<0.001). Post-hoc comparisons (Scheffé) indicated that people reporting four psychotic symptoms had significantly more somatic illnesses (0.68±0.88) than the other groups (p<0.001 in all comparisons); people with three symptoms (0.52±0.78) had more illnesses (p<0.001) than people with two (0.45±0.71), one (0.40±0.66) or zero (0.24±0.52) symptoms; people with two symptoms had more illnesses (p<0.001) than those with one or no symptoms; and people reporting one symptom had more illnesses than those without symptoms and no lifetime diagnosis of psychosis (p<0.001).
Figure 1.
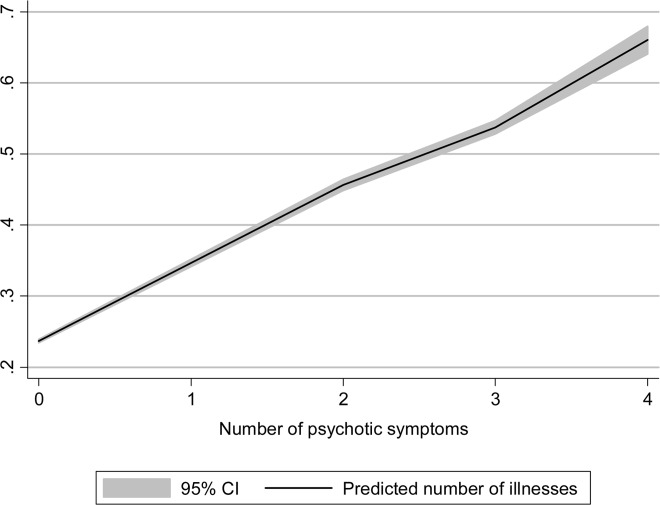
Linear prediction of number of illnesses according to the number of psychotic symptoms, with 95% confidence bands. Illnesses included: angina pectoris, arthritis, asthma, diabetes, and tuberculosis (range 0-5).
Individuals with psychotic symptoms in the past 12 months and a lifetime diagnosis of psychosis had consumed more medicines prescribed by a medical professional (excluding psychiatric medications) than persons without psychotic symptoms or diagnosis (0.19±0.48 vs. 0.14±0.41, t=4.1; p<0.001). Likewise, people with at least one psychotic symptom took more medicines than those without symptoms or diagnosis (0.16±0.43 vs. 0.14±0.41, t=7.8, p<0.001). When comparing the amount of medicines consumed by subjects with different numbers of reported psychotic symptoms, there was a clear overall omnibus difference (F=18.1, p<0.001). However, post-hoc comparisons (Scheffé) indicated that differences were only in the direction of a higher consumption for those with one (0.17±0.44; p=0.012), or two (0.17±0.44; p=0.021) as compared to persons with four psychotic symptoms (0.14±0.40), and with one, two or three symptoms (0.16±0.41; p<0.001 in all comparisons) as compared to those without psychotic symptoms (0.14±0.41).
DISCUSSION
The presence of even isolated psychotic symptoms may confer risk for medical comorbidities. Our results indicate that individuals with psychotic symptoms but no psychotic diagnosis, compared to those without psychotic diagnosis or symptoms, present more lifetime medical conditions and health problems, more (and longer) non-psychiatric overnight stays in hospital (with no differences in other health care indicators), and higher consumption of non-psychiatric medicines prescribed by a professional. Most results are replicated, and in most comparisons the effects are higher, when subjects with a diagnosis of psychosis plus psychotic symptoms over the last 12 months are included in the analyses.
Although our results are in line with previous work suggesting that psychotic illnesses are related with worse physical health (4,5,7,8,34) and higher rates of various medical conditions such as angina pectoris or cardiovascular problems (10-12,35), asthma or pulmonary problems (13,35-37) and tuberculosis (38,39), they suggest that this relationship is not dependent on the presence of a psychotic disorder, but that the critical factor is having experienced at least one psychotic symptom. The same applies to the association of psychotic symptoms with other health-related factors, such as presence of accidents, including automobile accidents (40), mouth or teeth problems (7,13,41,42), smoking (4,10-12,18), alcohol abuse (4,5,43) or hearing and vision problems (13,44,45). Although a lower frequency of rheumatoid arthritis has been previously reported for patients with schizophrenia (7,46), this negative association was not replicated in a population-based analysis (47), which also found that the incidence of arthritis in parents of schizophrenia patients was higher than in parents of controls. Unlike most previous studies on psychosis, the term arthritis in this study was not restricted to rheumatoid arthritis. A positive relationship between schizophrenia and rheumatic diseases has also been found previously (47,48).
The use of health care services was higher in persons with psychotic symptoms, particularly in cases with more severe problems needing inpatient attention. Regular visits to health services, however, were not more common among subjects with psychotic symptoms. A possible explanation for this finding could be that patients with psychotic symptoms have worse access to the usual filter systems, given their pathology-driven difficulties in engaging in routine medical care and interpreting illness-related signs (49,50). To be identified by the health system, somatic illnesses in individuals with psychosis may need to be more severe, or to have progressed enough to require treatment in hospital settings (51). Self-rated lack of health and dissatisfaction with health, higher in people with vs. without psychotic symptoms in our sample, also point in that direction.
In all analyses, country of origin, gender, age, and socio-economic status were statistically controlled for. Therefore, it can be assumed that the results of the present study are globally independent of the country, the socio-economic status or the development level of health systems in each country.
The higher frequency of medical conditions among people with psychoses in this international sample replicates previous results from single countries, but the finding that similar medical complications are present among subjects with isolated psychotic symptoms regardless of their country of origin, has not been, to our knowledge, previously reported, and points to a greater disadvantage of these individuals, even if they are not to develop a full psychotic illness or if they are in the earlier stages of psychotic processes. Our results may be explained by the existence of a physiopathological link based on genetic, inflammatory, immunological and/or metabolic mechanisms, underlying the relationship between psychotic symptoms and physical diseases (20,52-54). The higher frequency of smoking and excessive alcohol consumption, found in our study among subjects with psychotic symptoms, could also mediate the association between those symptoms and physical diseases. Promoting general physical health and improving screening methods for comorbid medical conditions (55-58) seems to be relevant in persons with psychotic symptoms across countries, even if they do not meet criteria for a psychotic disorder.
The cross-sectional nature of this study does not make it possible to address the direction of the causal link between medical conditions and psychotic symptoms. Likewise, the lack of data on potential determinants of severity or disability associated with psychotic symptoms, such as the number and frequency of episodes, episode length, age at onset, and episode severity, limits the generalization of results to the whole continuum of persons with psychotic symptoms. In addition, the range of psychotic experiences was limited, and not assessed by a clinical interview. Longitudinal studies with more experienced interviewers are needed in order to analyze the natural history of these symptoms in the general population (59).
The strengths of the study include the large sample size and the worldwide scope, including all regions of the world with all levels of development. Most of the research in the domain of psychotic experiences has been conducted in Western countries, and little is known about regions in which multiple economic, cultural or social factors or differences in the health systems can markedly affect the distribution of psychotic symptoms. The present study was performed with nationally representative samples of non-institutionalized persons, avoiding potential problems associated with clinical samples, such as Berkson's bias (60). Notably, country effects, including the categorization of the country's economic status (in addition to individual age and gender), were statistically controlled for.
In conclusion, the present study shows that the presence of at least one psychotic symptom, independent of a psychotic disorder diagnosis, is related to more comorbid medical problems, risky lifestyle behaviours, and an increased use of health services for chronic medical conditions, involving overnight stays in hospitals. Given the sample analyzed, this trend seems to hold worldwide, regardless of the socio-economic development of the country or the specific health care system. Patients with psychotic disorders and even with psychotic symptoms not fulfilling diagnostic criteria for a psychotic disorder should be screened for additional medical problems, and general practitioners should be trained in the identification of patients with these problems, in order to optimize the functioning of health systems and avoid the problems and additional costs associated with comorbid conditions.
Acknowledgments
This work was supported by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, CIBERSAM, Madrid Regional Government (S2010/BMD-2422 AGES), European Union Structural Funds, Fundación Alicia Koplowitz, Fundación Mutua Madrileña, ERA-NET NEURON (Network of European Funding for Neuroscience Research) and the World Health Organization.
References
- 1.Brown S, Kim M, Mitchell C, et al. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196:116–21. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 3.Tiihonen J, Lonnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–7. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 4.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25:83–8. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]
- 5.von Hausswolff-Juhlin Y, Bjartveit M, Lindstrom E, et al. Schizophrenia and physical health problems. Acta Psychiatr Scand. 2009;119(Suppl. 438):15–21. doi: 10.1111/j.1600-0447.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- 6.De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leucht S, Burkard T, Henderson J, et al. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116:317–33. doi: 10.1111/j.1600-0447.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69:514–9. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- 9.Arango C, Bobes J, Kirkpatrick B, et al. Psychopathology, coronary heart disease and metabolic syndrome in schizophrenia spectrum patients with deficit versus non-deficit schizophrenia: findings from the CLAMORS study. Eur Neuropsychopharmacol. 2011;21:867–75. doi: 10.1016/j.euroneuro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 10.De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–24. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Kilbourne AM, Morden NE, Austin K, et al. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry. 2009;31:555–63. doi: 10.1016/j.genhosppsych.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Truyers C, Buntinx F, De Lepeleire J, et al. Incident somatic comorbidity after psychosis: results from a retrospective cohort study based on Flemish general practice data. BMC Fam Pract. 2011;12:132. doi: 10.1186/1471-2296-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkup J, Akincigil A, Hoover DR, et al. Use of Medicaid data to explore community characteristics associated with HIV prevalence among beneficiaries with schizophrenia. Public Health Rep. 2011;126(Suppl. 3):89–101. doi: 10.1177/00333549111260S314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saiz-Ruiz J, Saiz-Gonzalez MD, Alegria AA, et al. Impact of the Spanish Consensus on Physical Health of Patients with Schizophrenia. Rev Psiquiatr Salud Ment. 2010;3:119–27. doi: 10.1016/j.rpsm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Chen YH, Lin HC. Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr Bull. 2011;37:1088–94. doi: 10.1093/schbul/sbq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arango C, Bobes J, Aranda P, et al. A comparison of schizophrenia outpatients treated with antipsychotics with and without metabolic syndrome: findings from the CLAMORS study. Schizophr Res. 2008;104:1–12. doi: 10.1016/j.schres.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Bobes J, Arango C, Garcia-Garcia M, et al. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. 2010;119:101–9. doi: 10.1016/j.schres.2010.02.1030. [DOI] [PubMed] [Google Scholar]
- 19.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–7. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick B. Schizophrenia as a systemic disease. Schizophr Bull. 2009;35:381–2. doi: 10.1093/schbul/sbn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perala J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Nuevo R, Chatterji S, Verdes E, et al. The continuum of psychotic symptoms in the general population: a cross-national study. Schizophr Bull. 2012;38:475–85. doi: 10.1093/schbul/sbq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossler W, Riecher-Rossler A, Angst J, et al. Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res. 2007;92:1–14. doi: 10.1016/j.schres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Addington J, Penn D, Woods SW, et al. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99:119–24. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha S, Scott J, Varghese D, et al. The association between physical health and delusional-like experiences: a general population study. PLoS One. 2011;6:e18566. doi: 10.1371/journal.pone.0018566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCloughen A, Foster K, Huws-Thomas M, et al. Physical health and wellbeing of emerging and young adults with mental illness: an integrative review of international literature. Int J Ment Health Nurs. 2012;21:274–88. doi: 10.1111/j.1447-0349.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 27.Ustun TB, Chatterji S, Mechbal A, et al. Household sample surveys in developing and transition countries. New York: Department for Economic and Social Affairs; 2005. Quality assurance in surveys: standards, guidelines and procedures; pp. 199–230. [Google Scholar]
- 28.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper L, Peters L, Andrews G. Validity of the Composite International Diagnostic Interview (CIDI) psychosis module in a psychiatric setting. J Psychiatr Res. 1998;32:361–8. doi: 10.1016/s0022-3956(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 30.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 31.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 32.Nuevo R, Chatterji S, Fraguas D, et al. Increased risk of diabetes mellitus among persons with psychotic symptoms: results from the WHO World Health Survey. J Clin Psychiatry. 2011;72:1592–9. doi: 10.4088/JCP.10m06801. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- 34.Oud MJ, Meyboom-de Jong B. Somatic diseases in patients with schizophrenia in general practice: their prevalence and health care. BMC Fam Pract. 2009;10:32. doi: 10.1186/1471-2296-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filik R, Sipos A, Kehoe PG, et al. The cardiovascular and respiratory health of people with schizophrenia. Acta Psychiatr Scand. 2006;113:298–305. doi: 10.1111/j.1600-0447.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Lee HC, Lin HC. Prevalence and risk of atopic disorders among schizophrenia patients: a nationwide population based study. Schizophr Res. 2009;108:191–6. doi: 10.1016/j.schres.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen MS, Benros ME, Agerbo E, et al. Schizophrenia in patients with atopic disorders with particular emphasis on asthma: a Danish population-based study. Schizophr Res. 2012;138:58–62. doi: 10.1016/j.schres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Ohta Y, Nakane Y, Mine M, et al. The epidemiological study of physical morbidity in schizophrenics -- 2. Association between schizophrenia and incidence of tuberculosis. Jpn J Psychiatry Neurol. 1988;42:41–7. doi: 10.1111/j.1440-1819.1988.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 39.Volkov VP. Respiratory diseases as a cause of death in schizophrenia. Probl Tuberk Bolezn Legk. 2009;6:24–7. [PubMed] [Google Scholar]
- 40.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 41.Janardhanan T, Cohen CI, Kim S, et al. Dental care and associated factors among older adults with schizophrenia. J Am Dent Assoc. 2011;142:57–65. doi: 10.14219/jada.archive.2011.0029. [DOI] [PubMed] [Google Scholar]
- 42.Arnaiz A, Zumarraga M, Diez-Altuna, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188:24–8. doi: 10.1016/j.psychres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Wisdom JP, Manuel JI, Drake RE. Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiatr Serv. 2011;62:1007–12. doi: 10.1176/appi.ps.62.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viertio S, Laitinen A, Perala J, et al. Visual impairment in persons with psychotic disorder. Soc Psychiatry Psychiatr Epidemiol. 2007;42:902–8. doi: 10.1007/s00127-007-0252-6. [DOI] [PubMed] [Google Scholar]
- 45.Prager S, Jeste DV. Sensory impairment in late-life schizophrenia. Schizophr Bull. 1993;19:755–72. doi: 10.1093/schbul/19.4.755. [DOI] [PubMed] [Google Scholar]
- 46.de la Fontaine L, Schwarz MJ, Riedel M, et al. Investigating disease susceptibility and the negative correlation of schizophrenia and rheumatoid arthritis focusing on MIF and CD14 gene polymorphisms. Psychiatry Res. 2006;144:39–47. doi: 10.1016/j.psychres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Eaton WW, Byrne M, Ewald H, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–8. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- 48.Sundquist K, Li X, Hemminki K, et al. Subsequent risk of hospitalization for neuropsychiatric disorders in patients with rheumatic diseases: a nationwide study from Sweden. Arch Gen Psychiatry. 2008;65:501–7. doi: 10.1001/archpsyc.65.5.501. [DOI] [PubMed] [Google Scholar]
- 49.Roberts L, Roalfe A, Wilson S, et al. Physical health care of patients with schizophrenia in primary care: a comparative study. Fam Pract. 2007;24:34–40. doi: 10.1093/fampra/cml054. [DOI] [PubMed] [Google Scholar]
- 50.Kilbourne AM, McCarthy JF, Welsh D, et al. Recognition of co-occurring medical conditions among patients with serious mental illness. J Nerv Ment Dis. 2006;194:598–602. doi: 10.1097/01.nmd.0000230637.21821.ec. [DOI] [PubMed] [Google Scholar]
- 51.Munk-Jorgensen P, Mors O, Mortensen PB, et al. The schizophrenic patient in the somatic hospital. Acta Psychiatr Scand. 2000;102(Suppl. 407):96–9. doi: 10.1034/j.1600-0447.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Egea E, Bernardo M, Heaphy CM, et al. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009;35:437–42. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson DC, Ettinger ER. Schizophrenia and diabetes. Int Rev Neurobiol. 2002;51:481–501. doi: 10.1016/s0074-7742(02)51014-x. [DOI] [PubMed] [Google Scholar]
- 54.Ferentinos P, Dikeos D. Genetic correlates of medical comorbidity associated with schizophrenia and treatment with antipsychotics. Curr Opin Psychiatry. 2012;25:381–90. doi: 10.1097/YCO.0b013e3283568537. [DOI] [PubMed] [Google Scholar]
- 55.De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10:138–51. doi: 10.1002/j.2051-5545.2011.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(Suppl. 2):S41–5. doi: 10.1016/S0924-9338(10)71706-5. [DOI] [PubMed] [Google Scholar]
- 57.Tosh G, Clifton A, Bachner M. General physical health advice for people with serious mental illness. Cochrane Database Syst Rev. 2011:CD008567. doi: 10.1002/14651858.CD008567.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Bobes J, Alegria AA, Saiz-Gonzalez MD, et al. Change in psychiatrists' attitudes towards the physical health care of patients with schizophrenia coinciding with the dissemination of the consensus on physical health in patients with schizophrenia. Eur Psychiatry. 2011;26:305–12. doi: 10.1016/j.eurpsy.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Stanghellini G, Langer AI, Ambrosini A, et al. Quality of hallucinatory experiences: differences between a clinical and a non-clinical sample. World Psychiatry. 2012;11:110–3. doi: 10.1016/j.wpsyc.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946;2:47–53. [PubMed] [Google Scholar]


