Abstract
Elevated levels of clusterin (CLU), a stress-induced and secreted cytoprotective chaperone, are associated with advanced tumor stage, metastasis, treatment resistance, and adverse outcome in several cancers. Custirsen, a second-generation antisense oligonucleotide, inhibits CLU production in tumor cells and reduces serum CLU levels. A Phase 2 study evaluated custirsen in combination with second-line chemotherapy in men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed while on or within 6 months of first-line docetaxel-based chemotherapy. Exploratory analyses evaluated serum CLU levels during custirsen treatment and correlative clinical effects on prostate-specific antigen (PSA) response, overall survival, and any relationship between serum CLU and PSA. Men with mCRPC were treated with mitoxantrone/prednisone/custirsen (MPC, n = 22) or docetaxel retreatment/prednisone/custirsen (DPC plus DPC-Assigned, n = 45) in an open-label, multicenter study. Subject-specific profiles of PSA and serum CLU levels during treatment were characterized using statistical modeling to compute subject-specific summary measures; these measures were analyzed for relationship to survival using proportional hazard regression. Estimated individual serum CLU response profiles were scored as below or at/above the median level for the population through 100 days postrandomization. Median survival was longer for subjects scoring below the median serum CLU level compared with subjects at/above the median level, respectively (MPC: 15.1 months vs. 6.2 months; DPC-Pooled: 17.0 months vs. 12.1 months). Lowered serum CLU levels during custirsen treatment when in combination with either chemotherapy regimen were predictive of longer survival in mCRPC. These results support further evaluation of serum CLU as a therapeutic biomarker.
Aside from PSA, there are currently no other prognostic or predictive biomarkers that can be used to guide treatment response in metastatic castration resistant prostate cancer (mCRPC). In a Phase 2 study, men with mCRPC were treated with prednisone and custirsen plus either mitoxantrone or docetaxel retreatment. Statistical modeling was used to compute subject-specific summary measures of PSA and serum clusterin levels at baseline and at Day 100 of treatment, followed by a regression analysis to evaluate relationship to overall survival. In this analysis, reduced serum clusterin levels during treatment were predictive of longer survival. These results currently support further evaluation of serum clusterin as a therapeutic biomarker in three ongoing Phase 3 clinical trials.
Keywords: Antisense oligonucleotide, chemotherapy, clusterin, custirsen, mCRPC
Introduction
Clusterin (CLU) functions to protect cells from many varied therapeutic stressors that induce apoptosis, including androgen or estrogen withdrawal, radiation, and cytotoxic chemotherapy 1–4. CLU expression is regulated by HSF1 (also YB-1, EGR-1) and functions like small heat shock proteins (Hsps) to chaperone and stabilize conformations of proteins at times of cell stress 5. As a functional homologue of small Hsps, CLU has chaperone activity with a potent ability to inhibit stress-induced protein aggregation 6–7. CLU interacts with stressed cell-surface proteins (e.g., receptors) to inhibit pro-apoptotic signal transduction. CLU inhibits endoplasmic reticular (ER) stress by retro-translocating from the ER to the cytosol to inhibit aggregation of intracellular proteins and prevent apoptosis 8.
CLU also suppresses p53-activating stress signals and stabilizes the cytosolic Ku70-Bax protein complex to inhibit Bax activation 9. CLU specifically interacts with conformationally-altered Bax to inhibit apoptosis in response to chemotherapeutic drugs 10. In addition, CLU increases Akt phosphorylation levels and cell survival rates 11. CLU induces epithelial-mesenchymal transformation by increasing Smad2/3 stability and enhancing TGF-β-mediated Smad transcriptional activity 12. CLU also promotes prostate cancer cell survival by increasing NF-κB nuclear transactivation, acting as a ubiquitin-binding protein that enhances COMMD1 and I-kB proteasomal degradation via interaction with E3 ligase family members 13.
In preclinical models, CLU confers treatment resistance 14,15, while CLU inhibition potentiates activity of anti-cancer therapies 3,17. CLU expression has been correlated with higher serum prostate-specific antigen (PSA), higher clinical stage, metastatic disease, and shorter recurrence free and overall survival in prostate, bladder, and non-small cell lung (NSCL) cancers 1–23. An overall schema illustrating the role of CLU in cancer cell survival is shown in Figure 1.
Figure 1.
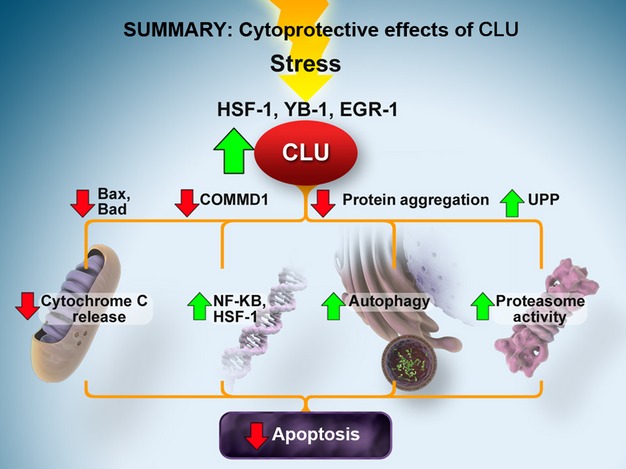
Role of CLU in cancer cell survival.
Custirsen (OGX-011, TV-1011) is a second-generation antisense oligonucleotide (ASO) with affinity for CLU mRNA. Administration of custirsen has been shown to reduce CLU production within tumor cells and to lower serum CLU levels 22–26. Reduced CLU has been associated with enhanced treatment response in murine and human in vitro and in vivo models 18–31. In previous prostate cancer clinical studies evaluating the effects of custirsen treatment, tumor cell CLU levels were reduced by >94% and serum CLU levels were reduced by ~30% 22,25.
In a previously reported randomized Phase 2 study of subjects with metastatic castration-resistant prostate cancer (mCPRC), administration of custirsen with first-line docetaxel significantly reduced serum CLU levels within the first treatment cycle compared with docetaxel only 26. A survival benefit was also observed with custirsen plus docetaxel (median survival 23.8 months; 95% CI, 16.2 months-not reached) compared with docetaxel only (median survival 16.9 months; 95% CI, 12.8–25.8 months).
The initial objective of this study was to evaluate the feasibility of adding custirsen to either mitoxantrone/prednisone (MPC) or docetaxel/prednisone retreatment (DPC) as second-line chemotherapy in subjects with mCRPC who had disease progression within 6 months of completing first-line docetaxel-based chemotherapy. Results for the randomized study (42 subjects treated with custirsen and chemotherapy) have been previously published 22. Twenty-five additional subjects were treated with DPC under a protocol amendment, resulting in 67 total subjects randomized or assigned to custirsen treatment who received custirsen and chemotherapy. The objective of the statistical analyses reported herein was to evaluate the role of serum CLU at baseline and during treatment on overall survival. PSA assessments were also of interest in order to evaluate any relationship between changes in serum CLU and PSA during treatment and as a reference for assessing the relationship between serum CLU and survival.
Material and Methods
Study design
This was an open-label, randomized study of custirsen combined with second-line chemotherapy (docetaxel retreatment or mitoxantrone) in subjects with mCRPC. Based on a review of safety and efficacy data, the study was amended upon completion of randomization to allow additional enrollment into the docetaxel retreatment arm. The study was conducted at 10 Canadian sites. Study design and methods have been previously described 22. The study and amendment were reviewed and approved by all sites and Research Ethics Boards, and all subjects provided written informed consent.
Study treatment involved three loading doses of custirsen (OncoGenex Technologies, Inc., Vancouver, BC, Canada), 640 mg, administered on separate days as a 2-hour IV infusion during a 9-day period, followed by weekly administration on Day 1, 8, and 15 of each 21-day cycle. Prophylactic premedications included ibuprofen or acetaminophen. Subjects received prednisone (5 mg) orally twice daily for the treatment period unless intolerant. Chemotherapy consisted of either docetaxel (75 mg/m2 IV, 60 min) or mitoxantrone (12 mg/m2 IV, 30 min) administered on Day 1 of 21-day cycles. Up to nine cycles were permitted.
Serum CLU was assessed in samples collected at baseline, Day 1 of each cycle, and end of study treatment. Samples were analyzed utilizing solid-phase ELISA in microplate format (BioVendor Clusterin ELISA kit; Laboratorni medicina a.s., Czech Republic) designed for quantitative measurement of human CLU. Mayo Clinical Trial Services (Rochester, MN) validated and performed all serum CLU assays. PSA levels were assessed at clinical sites at baseline, Day 1 of each cycle, end of treatment visit, and during off-treatment follow-up. Overall survival (OS) was defined as the time from start of study treatment to death; OS was censored at the date of last follow-up for subjects still alive.
Statistical analyses
Analyses focused on evaluation of chemotherapy regimen (mitoxantrone vs. docetaxel), serial PSA and serum CLU assessments, and survival. PSA and CLU were defined as biomarkers. Each subject's serial biomarker measurements through 1 year were fit to a quadratic model, and the estimated model in its functional form was used as a source of subject-specific summary measures or features 32. This method of feature extraction blunts the influence of subject-specific measurement variability and also enables use of data from subjects with missing baseline assessments. A Day 100 interval was chosen for estimating subjects' biomarker features as this represents approximately the time to complete four cycles of chemotherapy. The Day 100 interval puts the focus on early biomarker changes in response to study intervention and reduces interference from events preventing further biomarker assessments. PSA was analyzed as the base 10 logarithm of PSA, which is typically used in statistical models that evaluate PSA because the distribution of PSA has an extended right tail 33. For both PSA and CLU, subjects were excluded from analysis only if they had fewer than four assessments or if assessments ended before 21 days following initiation of study treatment; these exclusions were required by the statistical modeling used and are considered too few in number to induce significant bias.
For each biomarker and subject, baseline and follow-up values were computed from the estimated model for that subject. The biomarker baseline value was computed from the function at time zero. The Day 100 follow-up biomarker result from treatment, henceforth simply called the result for each biomarker, was the maximum or minimum value from the function at time zero through Day 100. If the quadratic function had a critical value (first derivative equal zero) in the interval 0 to 100, then the result was computed from the function at the time of this critical value; otherwise, the estimated follow-up level was computed from the model at Day 100. These computed baseline and result levels from each subject and biomarker were used to compute additional subject measures, such as change from baseline. In some cases, features were dichotomized for analytic purposes using a rounded value near the median.
Extracted features were analyzed for relationships to outcomes using graphics, descriptive statistics, Kaplan–Meier estimation, and proportional hazard regression. In this exploratory study, P values are used as indicators of model term inclusion and do not have meaning for assessing probabilistic hypothesis testing. A step-down hierarchical procedure was used in order to eliminate noncontributory terms in the models being explored, with criterion for inclusion of a model term being P < 0.1.
Results
Subjects
Forty-five subjects were randomized and 42 received treatment with custirsen in combination with chemotherapy, designated as MPC and DPC. Based on a review of safety and efficacy data in the randomized study, the original protocol was amended to enroll an additional 25 subjects (designated DPC-Assigned) who received the same intervention as the DPC group. When the DPC and DPC-Assigned groups are pooled, the resulting group is designated DPC-Pooled. The criteria for exclusion of individual subjects induced by using the quadratic modeling methodology resulted in the loss of 4/67 subjects, leaving 63 analyzable subjects.
Extraction of profile features
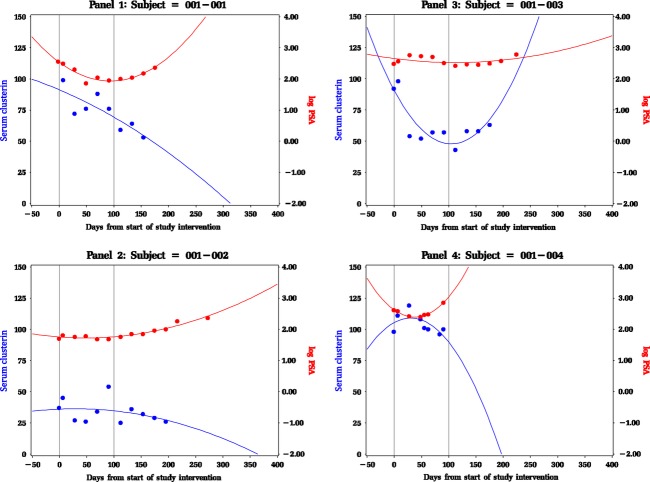
Figure 2 shows measured serial CLU assessments and log PSA values by time for the first 4 of 63 subjects; these subjects illustrate the variety observed in the individual subject profiles and the quadratic model that fits to these subjects. Three subjects did not have measured baseline serum CLU assessments; the quadratic model was used to estimate a time = 0 value for these subjects. The median time to last serum CLU assessment within the first year for the 63 analyzable subjects was 156 days (range, 44–327 days; interquartile range [IQR], 94–197 days). The median time to last PSA assessment within the first year was 176 days (range, 44–365 days; IQR, 112–252 days).
Figure 2.
Serial serum CLU and log PSA values by time. Shown is a sample from the graphs created to assess the general shape of the individual longitudinal outcomes. These graphs also show the fit of the quadratic model to each individual's data.
Figures 3 and 4 show CLU and log PSA features for each subject's quadratic model, with lines connecting subject-specific biomarker baseline and Day 100 results. Median serum CLU baseline and Day 100 results were 64.7 μg/mL and 46.9 μg/mL, respectively; cutpoints of 64 and 47 are close to values in previous studies 22–23. Group-specific median log PSA at baseline and Day 100 were 2.08 ng/mL and 2.06 ng/mL, respectively, leading to cutpoints of 2. Cutpoints were subsequently used to partition subjects at baseline and Day 100 into low and high groups.
Figure 3.
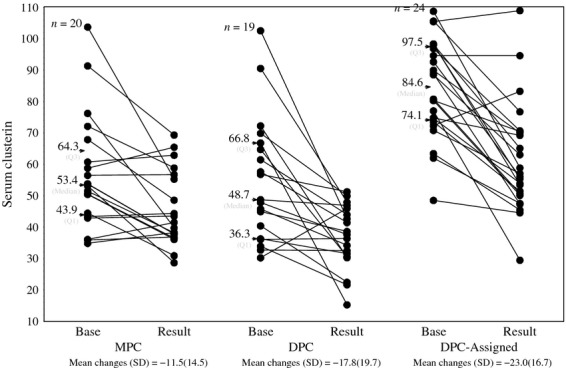
Serum CLU features and mean change by treatment group. For each group the extracted features of the 100-day CLU profile are plotted. These graphs show the general trend in response to the implementation of intervention.
Figure 4.
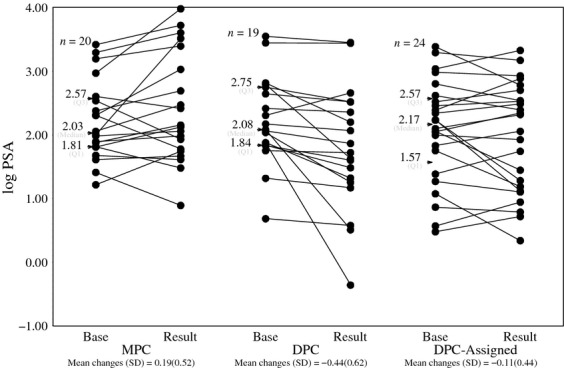
Log PSA features and mean change by treatment group. For each group the extracted features of the 100-day log(PSA) profile are plotted. These graphs show the general trend in response to the implementation of intervention.
Estimated mean change values for CLU and log PSA are also shown in Figures 3 and 4. Estimates of mean changes from baseline to Day 100 did not exhibit a notable difference between groups. For subsequent analyses, DPC and DPC-Assigned groups were pooled.
Subject characteristics and disposition
Baseline demographics at study entry did not differ materially between the three groups except for baseline CLU in the DPC-Assigned group, which was notably higher (Table 1).
Baseline and Day 100 descriptive statistics for 63 subjects analyzed
| Item | MPC | DPC | DPC-Assigned |
|---|---|---|---|
| Age | |||
| N | 20 | 19 | 24 |
| Mean | 63.8 | 67.1 | 64.6 |
| Median | 61 | 67 | 62.5 |
| IQR | 56.5–72.5 | 62–76 | 59.0–71.5 |
| Range | 49.0–81.0 | 48–80 | 53.0–80.0 |
| Baseline PSA level | |||
| N | 20 | 19 | 24 |
| Mean | 478.5 | 515.0 | 394.6 |
| Median | 105.5 | 119.2 | 120.5 |
| IQR | 62.0–371.9 | 67.3–469.0 | 38.4–384.2 |
| Range | 16.8–2630.2 | 4.8–3570.8 | 3.1–2405.2 |
| Baseline log PSA | |||
| N | 20 | 19 | 24 |
| Mean | 2.22 | 2.22 | 2.07 |
| Median | 2.03 | 2.08 | 2.17 |
| IQR | 1.81–2.57 | 1.84–2.75 | 1.57–2.57 |
| Range | 1.21–3.42 | 0.68–3.55 | 0.48–3.39 |
| Estimated log PSA result | |||
| N | 20 | 19 | 24 |
| Mean | 2.42 | 1.79 | 1.96 |
| Median | 2.14 | 1.72 | 2.19 |
| IQR | 1.76–3.21 | 1.25–2.52 | 1.15–2.62 |
| Range | 0.89–3.98 | −0.36 to 3.45 | 0.34–3.33 |
| Baseline CLU | |||
| N | 20 | 19 | 24 |
| Mean | 56.9 | 54.7 | 84.4 |
| Median | 53.4 | 48.7 | 84.6 |
| IQR | 43.9–64.3 | 36.3–66.8 | 74.1–97.5 |
| Range | 34.8–103.7 | 10.2–102.6 | 48.5–108.8 |
| Estimated CLU result | |||
| N | 20 | 19 | 24 |
| Mean | 45.4 | 38.9 | 61.4 |
| Median | 40.6 | 37.6 | 56.6 |
| IQR | 37.3–56.0 | 30.5–45.9 | 50.4–70.2 |
| Range | 28.6–69.3 | 15.2–51.3 | 29.4–109.0 |
The median number of treatment cycles was 6 in the MPC group and 7 in the DPC-Pooled group. Twenty-three of 67 subjects (34%) completed all 9 cycles. The percentage of subjects discontinuing treatment was similar for the MPC (64%) and DPC-Pooled (67%) groups. The main reason subjects discontinued treatment was progressive disease (42%). Additional reasons for discontinuation were adverse event (9%), withdrawal of consent (8%), physician decision (3%), and symptomatic disease progression (3%). No deaths occurred prior to Day 100.
Effect of custirsen on serum CLU levels and PSA response
Changes in serum CLU results from baseline to Day 100 are shown by group in Figure 3. Mean changes are −11.5, −17.8, and −23.0 μg/mL for MPC, DPC, and DPC-Assigned groups, respectively; there is little evidence of between-group differences in these changes (P = 0.0944, one-way ANOVA). Across all three groups, 51/63 (81.0%) subjects had decreases; the overall mean change was −17.8 μg/mL and was significantly different from zero (P < 0.001) despite large standard deviations. Thus, the data as analyzed indicate that initiation of study intervention resulted in CLU decreases in most subjects.
Results in log PSA from baseline to Day 100 are shown by group in Figure 4. There is no evidence of substantial change in any group (mean changes +0.19, −0.44, and −0.11 ng/mL for MPC, DPC, and DPC-Assigned groups, respectively, with large standard deviations). Across all three groups, the mean change was −0.11 and was not significantly different from zero (P = 0.127).
There was no evidence of correlation between change in log PSA result and change in serum CLU result (data not shown). The Pearson correlation coefficient between change in log PSA and change in CLU was −0.065 (P = 0.61).
Overall survival
Subjects were followed for a minimum of 36 months after the first dose of custirsen. Of 63 subjects analyzed in the statistical models, 57 had died.
Kaplan–Meier estimates of survival are shown in Figure 5 for the MPC and DPC-Pooled groups and Day 100 CLU results (low vs. high, defined with cutpoint of 47 μg/mL). In both chemotherapy groups, median survival was longer among subjects with a low Day 100 CLU result compared with subjects with a high CLU result. In the MPC group, median survival was 15.1 months for subjects with a low CLU result versus 6.2 months for subjects with a high CLU result. Similarly, in the DPC-Pooled group, median survival was 17.0 months for subjects with a low CLU result, versus 12.1 months for subjects with a high CLU result.
Figure 5.
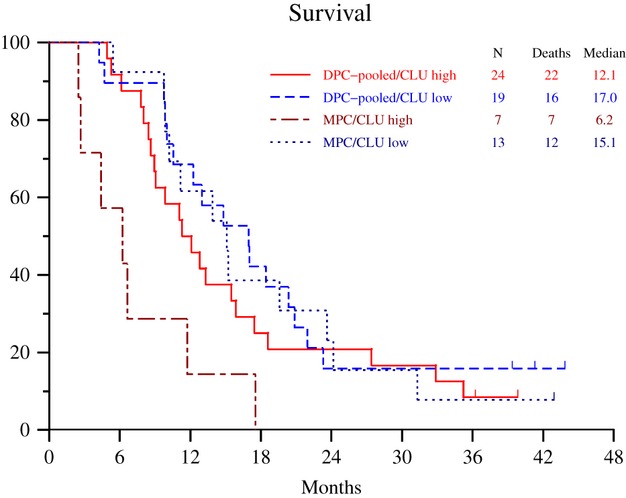
Kaplan–Meier estimates of survival by treatment group. The proportional hazard regression modeling (Table 2) suggested that Kaplan–Meier estimates from the four subgroups shown are a good representation of the data. In particular note that the difference between high versus low CLU response applies regardless of the type of chemotherapy.
Proportional hazard regression modeling for survival was performed based on the following explanatory indicator variables: chemotherapy, baseline low PSA, baseline low CLU, decrease in PSA result, and low Day 100 CLU result. The starting model included all five explanatory variables and all possible interactions to provide a complete description of the data.
Baseline CLU level did not contribute to survival outcome in this model either as an explanatory variable or in any interaction. There was also no contribution or interaction between the log PSA and the low Day 100 CLU result variables in the model. Estimates from the model are shown in Table 2. Because the best fit model has no baseline CLU variable and there are no interactions between log PSA and the low Day 100 CLU result variables, the relationship between the low CLU result and survival can be illustrated without taking PSA into account at either baseline or posttherapy.
Proportional hazard regression modeling for survival
| Variable1,2 | df | Parameter estimate | Standard error | Chi-square | Pr > Chi sq | |
|---|---|---|---|---|---|---|
| Chemotherapy | 1 | −3.13848 | 0.70529 | 19.8019 | <.0001 | |
| (0 = mitoxantrone vs. 1 = docetaxel) | ||||||
| Low baseline CLU | Not contributory | |||||
| (0 = CLU > 64 vs. 1 = CLU ≤ 64) | ||||||
| Low baseline log PSA | 1 | −1.70813 | 0.66505 | 6.5967 | 0.0102 | |
| (0 = log PSA > 2 vs. 1 = log PSA ≤ 2) | ||||||
| Low CLU result | 1 | −2.47114 | 0.70122 | 12.4190 | 0.0004 | |
| (0 = CLU > 47 vs. 1 = CLU ≤ 47) | ||||||
| PSA favorable change | 1 | −3.87441 | 0.91230 | 18.0360 | <.0001 | |
| (0 = no decrease vs. 1 = decrease) | ||||||
| Interaction between chemotherapy and low baseline log PSA | 1 | 3.02546 | 0.96097 | 9.9121 | 0.0016 | |
| Interaction between chemotherapy and PSA favorable change | 1 | 2.87465 | 1.00921 | 8.1136 | 0.0044 | |
| Interaction between chemotherapy and low CLU result | 1 | 2.54501 | 0.79178 | 10.3318 | 0.0013 | |
| Interaction between low baseline PSA and PSA favorable change | 1 | 3.87054 | 1.16147 | 11.1051 | 0.0009 | |
| Two-way interactions including baseline CLU | All not contributory | |||||
| Two-way interactions: Low baseline log PSA by low baseline CLU and low log PSA result by low baseline CLU | Neither contributory | |||||
| Three-way interaction: chemotherapy by low baseline PSA by PSA favorable change | 1 | −5.83264 | 1.44222 | 16.3556 | <.0001 | |
| Three-way interactions including any CLU variable | All not contributory | |||||
| Four-way interactions | All not contributory | |||||
| Five-way interaction | Not contributory | |||||
Biomarker variables are based on feature extraction from the individual patient quadratic models.
The starting model included the five explanatory variables (i.e., chemotherapy regimen, baseline CLU, baseline log PSA, low CLU result, PSA favorable change) plus all possible interactions. The model used a step-down hierarchical procedure with the criterion for conclusion being a two-sided P < 0.1. Shown is the final model from the selection procedure.
The model does include the two-way interaction between chemotherapy and the low posttherapy CLU result variable. Chemotherapy-specific hazard ratio estimates (95% CI) are 0.272 (0.105–0.706) for MPC and 0.742 (0.389–1.416) for DPC-Pooled. These chemotherapy-specific hazard ratio estimates are less than unity, which suggests that the low CLU result variable is prognostic of improved survival specific to chemotherapy regimen. This prognostic relationship of chemotherapy-specific low Day 100 CLU results variable can be seen in the Kaplan–Meier estimates of survival shown in Figure 5.
Discussion
The fact that survival was longer among subjects with a low serum CLU result during treatment compared with subjects with a high CLU result is consistent with preclinical data linking CLU expression to inhibition of treatment-induced cell death and with retrospective analyses of factors predictive for survival. Increased CLU levels have been reported in several cancers, including prostate, ovarian, and bladder and have been associated with poor prognostic features 20–35. In prostate cancer, Miyake et al. 36 showed that both serum CLU level and density (serum CLU level/prostate volume) were significantly higher in patients with prostate cancer compared with patients with benign prostatic hyperplasia. In addition, higher serum CLU levels in patients with prostate cancer were significantly associated with major prognostic factors such as high pretreatment serum PSA levels, advanced clinical stage, metastatic disease, and high percent of positive biopsy cores. Their findings showed that biochemical recurrence-free survival was significantly shorter in patients who had elevated CLU density at the time of radical prostatectomy compared with similar patients with normal CLU density.
Yang et al. 34 showed that CLU appeared to be both a biomarker associated with ovarian cancer and a prognostic factor associated with shorter survival. Overexpression of CLU in ovarian cancer tissue samples was found more often in advanced stage disease (P = 0.0001) compared with earlier disease. In addition, average survival time in patients with CLU overexpression was significantly shorter than in those with normal CLU expression (P = 0.033).
In patients with transitional cell carcinoma (TCC) of the bladder, Hazzaa et al. 21 observed that serum CLU levels were significantly higher in patients with TCC (invasive and superficial) compared with nontumor controls. The sensitivity and specificity of serum CLU as a tumor marker for TCC of the bladder were 80% and 91%, respectively. Furthermore, they showed that mean CLU mRNA expression in invasive disease tissue was significantly higher than in superficial disease or normal tissue 21. In addition, CLU expression levels correlated significantly with tumor grade and multiplicity but not with tumor size. They speculated that increased CLU expression was involved in TCC tumorigenesis and progression, especially since the recurrence-free survival time of patients with CLU overexpression was significantly shorter than that of patients with CLU underexpression (9.8 months vs. 35.2 months, respectively).
Another small retrospective study in patients with nonmuscle-invasive bladder cancer found a statistically significant association between bladder tissue CLU expression, measured by IHC, and progression to muscle-invasive disease following initial transurethral resection of the bladder tumor (TURBT) 37. The study authors suggest that tissue CLU in TURBT specimens may help identify patients who might benefit from more aggressive treatment earlier in disease.
Treatment with custirsen has been previously reported to reduce CLU levels and to be associated with longer survival outcomes in mCRPC and non-small cell lung cancer (NSCLC) 22–26. In a randomized, Phase 2 study in 82 patients with mCRPC evaluating docetaxel/prednisone as first-line chemotherapy with and without custirsen treatment, serum CLU levels were evaluated during the first cycle in all patients 26. Custirsen treatment significantly reduced serum CLU levels by a mean decrease of 26% by the end of cycle 1, compared to a slight increase of 0.9% in patients treated with only docetaxel/prednisone. Results from this study showed that, although there was no difference in tumor response rate, median survival for patients treated with custirsen plus docetaxel/prednisone was 23.8 months compared to 16.9 months for patients treated with docetaxel/prednisone only. In a single-arm, Phase 2 study of 81 patients with NSCLC evaluating custirsen with a first-line gemcitabine plus platinum regimen, 95% of patients had reductions in serum CLU from baseline by cycle 3. Mean reduction from baseline was 25 μg/mL (P < 0.0001; paired t-test for baseline vs. minimum CLU during treatment); median minimum serum CLU level was 38 μg/mL 23. Patients who had serum CLU levels ≤38 μg/mL during study treatment had a median survival of 27.1 months compared to 16.1 months for patients with serum CLU levels >38 μg/mL (P = 0.02).
Previously published results for the 42 randomized patients on this study showed that custirsen treatment significantly decreased serum CLU levels, with possible correlations between low serum CLU levels post treatment and prolonged survival 22. The additional analyses described herein were performed on all 63 analyzable subjects and characterized changes in each subject's Day 100 serum CLU and log PSA result following custirsen treatment, with assessment of the relationship of these changes to survival outcome. Low serum CLU results during treatment appeared to be highly predictive of improved survival outcome. In contrast, no relationship between PSA results and survival or between PSA results and serum CLU results was evident in this dataset. In other trial analyses, using different methods, a correlation between PSA decline and survival has been shown 38.
Our analysis was limited by not accounting for other baseline prognostic factors; thus, there may be confounding factors (e.g., overall disease burden, performance status) leading to bias. Although our analysis showed that baseline serum CLU levels were not prognostic for survival, the study design may have confounded these results; reduction in serum CLU levels due to custirsen treatment being administered to all subjects could have interfered with detection of a true baseline prognostic effect. In addition, one could further speculate that treatment stress in a control arm could result in increased CLU levels above baseline 26, and that increased CLU levels due to chemotherapy stress could be associated with treatment resistance or worse outcome. Ongoing randomized Phase 3 studies evaluating chemotherapy with and without custirsen will provide data on the prognostic value of baseline serum CLU as well as other possible confounding baseline factors.
The statistical modeling of the type of data analyzed is unavoidably difficult because multiple other outcomes are being related to the survival outcome. This is in contrast to models where only baseline attributes are used to predict survival. The potential biases inherent in relating multiple outcomes should be appreciated 39. As is typical of general statistical modeling efforts, many choices must be defined in the process of creating a model and some of these choices are driven by the data. The objective is to create a model that describes the data well enough to allow relationships between variables to be reliably assessed. In the words of George E. P. Box 40, “Essentially all models are wrong, but some are useful.” The main choices made for the modeling described herein included: minimum number of four serial biomarker assessments and assessments had to go beyond 20 days; quadratic shape of patient longitudinal profiles (ascertained to be adequate by viewing each patient profile); and 100 day cutoff for feature extraction (based on completion of four chemotherapy cycles, maximizing inclusion while defining a landmark for avoiding bias of early deaths given no deaths occurred before Day 100). These choices only disqualified four of the 67 subjects.
In summary, increased CLU levels have been previously correlated with various clinical and pathological parameters in a variety of tumors both as a possible prognostic biomarker and as a therapeutic biomarker during custirsen treatment. The ability to monitor serum CLU levels during custirsen treatment, whether combined with chemotherapy or with other cancer therapies (e.g., hormone or radiation), might allow individual assessments for potential survival benefit. The potential for serum CLU levels to serve as a therapeutic biomarker for custirsen treatment is being evaluated in ongoing Phase 3 studies enrolling over 2700 patients (clinical trial registration numbers: NCT01188187, NCT01578655, and NCT01630733). Prospective analyses from these Phase 3 trials will assess the validity of monitoring serum CLU level as a therapeutic biomarker.
Acknowledgments
Medical writing support was provided by Karen Wisont, an employee of OncoGenex Pharmaceuticals, Inc.
Conflict of Interest
None declared.
References
- July LV, Akbari M, Zellweger T, Jones EC, Goldenberg SL, Gleave ME. Clusterin expression is significantly enhanced in prostate cancer cells following androgen withdrawal therapy. Prostate. 2002;50:179–188. doi: 10.1002/pros.10047. [DOI] [PubMed] [Google Scholar]
- Flanagan L, Whyte L, Chatterjee N, Tenniswood M. Effects of clusterin over-expression on metastatic progression and therapy in breast cancer. BMC Cancer. 2010;10:107. doi: 10.1186/1471-2407-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger T, Chi K, Miyake H, Adomat H, Kiyama S, Skov K, et al. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin. Cancer Res. 2002;8:3276–3284. [PubMed] [Google Scholar]
- Zellweger T, Miyake H, July LV, Akbari M, Kiyama S, Gleave ME. Chemosensitization of human renal cell cancer using antisense oligonucleotides targeting the antiapoptotic gene clusterin. Neoplasia. 2001;3:360–367. doi: 10.1038/sj.neo.7900174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loison F, Debure L, Nizard P, Michel P, le Goff D, le Drean Y. Up-regulation of the clusterin gene after proteotoxic stress: implication of HSF1-HSF2 heterocomplexes. Biochem. J. 2006;395:223–231. doi: 10.1042/BJ20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J. Biol. Chem. 1999;274:6875–6881. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, et al. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21:2312–2322. doi: 10.1096/fj.06-7986com. [DOI] [PubMed] [Google Scholar]
- Nizard P, Tetley S, Watrin Y, Le Drean T, Wilson P, Le Goff MR, et al. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic. 2007;8:554–565. doi: 10.1111/j.1600-0854.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Lourda M, Antonelou MH, Kletsas D, Gorgoulis VG, Papassideri IS, et al. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin. Cancer Res. 2009;15:48–59. doi: 10.1158/1078-0432.CCR-08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat. Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- Ammar H, Closset JL. Clusterin activates survival through the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2008;283:12851–12861. doi: 10.1074/jbc.M800403200. [DOI] [PubMed] [Google Scholar]
- Lee KB, Jeon JH, Choi I, Kwon OY, Yu K, You KH. Clusterin, a novel modulator of TGF-beta signaling, is involved in Smad2/3 stability. Biochem. Biophys. Res. Commun. 2008;366:905–909. doi: 10.1016/j.bbrc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Zoubeidi A, Ettinger S, Beraldi E. Clusterin facilitates COMMD1 and I-kB degradation to enhance NF-kB activity in prostate cancer cells. Mol. Cancer Res. 2010;8:119–130. doi: 10.1158/1541-7786.MCR-09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake H, Nelson C, Rennie PS, Gleave ME. Acquisition of chemoresistant phenotype by overexpression of the antiapoptotic gene testosterone-repressed prostate message-2 in prostate cancer xenograft models. Cancer Res. 2000;60:2547–2554. [PubMed] [Google Scholar]
- Miyake H, Nelson C, Rennie PS, Gleave ME. Testosterone-repressed prostate message-2 is an antiapoptotic gene involved in progression to androgen independence in prostate cancer. Cancer Res. 2000;60:170–176. [PubMed] [Google Scholar]
- Miyake H, Hara S, Zellweger T, Kamidono S, Gleave ME, Hara I. Acquisition of resistance to Fas-mediated apoptosis by overexpression of clusterin in human renal-cell carcinoma cells. Mol. Urol. 2001;5:105–111. doi: 10.1089/10915360152559585. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Kamidono S, Gleave ME. Synergistic chemsensitization and inhibition of tumor growth and metastasis by the antisense oligodeoxynucleotide targeting clusterin gene in a human bladder cancer model. Clin. Cancer Res. 2001;7:4245–4252. [PubMed] [Google Scholar]
- Sowery RD, Hadaschik BA, So AI, Zoubeidi A, Fazli L, Hurtado-Coll A, et al. Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int. 2008;102:389–397. doi: 10.1111/j.1464-410X.2008.07618.x. [DOI] [PubMed] [Google Scholar]
- Miayake H, Tolcher A, Gleave ME. Chemosensitization and delayed androgen-independent recurrence of prostate cancer with the use of antisense Bcl-2 oligodeoxynucleotides. J. Natl. Cancer Inst. 2000;92:34–41. doi: 10.1093/jnci/92.1.34. [DOI] [PubMed] [Google Scholar]
- Miyake H, Muramaki M, Furukawa J, Kurahashi T, Fujisawa M. Serum level of clusterin and its density in men with prostate cancer as novel biomarkers reflecting disease extension. Urology. 2010;75:454–459. doi: 10.1016/j.urology.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Hazzaa SM, Elashry OM, Afifi IK. Clusterin as a diagnostic and prognostic marker for transitional cell carcinoma of the bladder. Pathol. Oncol. Res. 2010;16:101–109. doi: 10.1007/s12253-009-9196-3. [DOI] [PubMed] [Google Scholar]
- Saad F, Hotte S, North S, Eigl B, Chi K, Czaykowski P, et al. Randomized phase II trial of custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG Trial P-06c. Clin. Cancer Res. 2011;17:5765–5773. doi: 10.1158/1078-0432.CCR-11-0859. [DOI] [PubMed] [Google Scholar]
- Laskin JJ, Nicholas G, Lee C, Gitlitz B, Vincent M, Cormier Y, et al. Phase I/II trial of custirsen (OGX-011), an inhibitor of clusterin, in combination with a gemcitabine and platinum regimen in patients with previously untreated advanced non-small cell lung cancer. J. Thorac. Oncol. 2012;7:579–586. doi: 10.1097/JTO.0b013e31823f459c. [DOI] [PubMed] [Google Scholar]
- Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, Powers J, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2′-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J. Natl. Cancer Inst. 2005;97:1287–1296. doi: 10.1093/jnci/dji252. [DOI] [PubMed] [Google Scholar]
- Chi KN, Siu LL, Hirte H, Hotte SJ, Knox J, Kollmansberger C, et al. A phase I study of OGX-011, a 2′-methoxyethyl phosphorothioate antisense to clusterin, in combination with docetaxel in patients with advanced cancer. Clin. Cancer Res. 2008;14:833–839. doi: 10.1158/1078-0432.CCR-07-1310. [DOI] [PubMed] [Google Scholar]
- Chi KN, Hotte SJ, Yu EY, Tu D, Eigl BJ, Tannock I, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010;28:4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- Zellweger T, Miyake H, Cooper S, Chi K, Conklin BS, Monia BP, et al. Antitumor activity of antisense clusterin oligonucleotides is improved in vitro and in vivo by incorporation of 2′-O-(2-methoxy)ethyl chemistry. J. Pharmacol. Exp. Ther. 2001;298:934–940. [PubMed] [Google Scholar]
- Muramaki M, So A, Hayashi N, Sowery R, Miyake H, Fujisawa M, et al. Chemosensitization of gemcitabine-resistant human bladder cancer cell line both in vitro and in vivo using antisense oligonucleotide targeting the anti-apoptotic gene, clusterin. BJU Int. 2009;103:384–390. doi: 10.1111/j.1464-410X.2008.08098.x. [DOI] [PubMed] [Google Scholar]
- July LV, Beraldi E, So A, Fazli L, Evans K, English JC, et al. Nucleotide-based therapies targeting clusterin chemosensitize human lung adenocarcinoma cells both in vitro and in vivo. Mol. Cancer Ther. 2004;3:223–232. [PubMed] [Google Scholar]
- Hoeller C, Pratscher B, Thallinger C, Winter D, Fink D, Kovacic B, et al. Clusterin regulates drug-resistance in melanoma cells. J. Invest. Dermatol. 2005;124:1300–1307. doi: 10.1111/j.0022-202X.2005.23720.x. [DOI] [PubMed] [Google Scholar]
- So A, Sinnemann S, Huntsman D, Fazli L, Gleave M. Knockdown of the cytoprotective chaperone, clusterin, chemosensitizes human breast cancer cells both in vitro and in vivo. Mol. Cancer Ther. 2005;4:1837–1849. doi: 10.1158/1535-7163.MCT-05-0178. [DOI] [PubMed] [Google Scholar]
- Senn S, Stevens L, Chaturvedi N. Repeated measures in clinical trials: simple strategies for analysis using summary measures. Statist. Med. 2000;19:861–877. doi: 10.1002/(sici)1097-0258(20000330)19:6<861::aid-sim407>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J. Clin. Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- Yang GF, Li XM, Xie D. Overexpression of clusterin in ovarian cancer is correlated with impaired survival. Int. J. Gynecol. Cancer. 2009;19:1342–1346. doi: 10.1111/IGC.0b013e3181a83ed9. [DOI] [PubMed] [Google Scholar]
- Wei L, Xue T, Wang J, Chen B, Lei Y, Huang Y, et al. Roles of clusterin in progression, chemoresistance and metastasis of human ovarian cancer. Int. J. Cancer. 2009;125:791–806. doi: 10.1002/ijc.24316. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara S, Arakawa S, Kamidono S, Hara I. Over expression of clusterin is an independent prognostic factor for nonpapillary renal cell carcinoma. J. Urol. 2002;167:703–706. doi: 10.1016/S0022-5347(01)69130-4. [DOI] [PubMed] [Google Scholar]
- Ekici S, Eroğlu A, Doğan Ekici AI, Türkeri L. Clusterin immunoreactivity as a predictive factor for progression of non-muscle-invasive bladder carcinoma. Urol. Int. 2011;86:31–35. doi: 10.1159/000321692. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jr, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99–16. J. Natl. Cancer Inst. 2006;98:516–521. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J. Clin. Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- Box GEP, Draper NR. Empirical model building and response surfaces. New York, NY: John Wiley & Sons; 1987. [Google Scholar]