Abstract
Success of cancer vaccination is strongly hampered by immune suppression in the tumor microenvironment (TME). Interleukin (IL)-6 is particularly and highly produced by triple-negative breast cancer (TNBC) cells, and has been considered as an important contributor to immune suppression in the TME. Therefore, we hypothesized that IL-6 reduction may improve efficacy of vaccination against TNBC cancer through improved T-cell responses. To prove this hypothesis, we investigated the effect of curcumin, an inhibitor of IL-6 production, on vaccination of a highly attenuated Listeria monocytogenes (Listeriaat), encoding tumor-associated antigens (TAA) Mage-b in a TNBC model 4T1. Two therapeutic vaccination strategies with Listeriaat-Mage-b and curcumin were tested. The first immunization strategy involved all Listeriaat-Mage-b vaccinations and curcumin after tumor development. As curcumin has been consumed all over the world, the second immunization strategy involved curcumin before and all therapeutic vaccinations with Listeriaat-Mage-b after tumor development. Here, we demonstrate that curcumin significantly improves therapeutic efficacy of Listeriaat-Mage-b with both immunization strategies particularly against metastases in a TNBC model (4T1). The combination therapy was slightly but significantly more effective against the metastases when curcumin was administered before compared to after tumor development. With curcumin before tumor development in the combination therapy, the production of IL-6 was significantly decreased and IL-12 increased by myeloid-derived suppressor cells (MDSC), in correlation with improved CD4 and CD8 T-cell responses in blood. Our study suggests that curcumin improves the efficacy of Listeriaat-Mage-b vaccine against metastases in TNBC model 4T1 through reversal of tumor-induced immune suppression.
This study is focused on improving cancer vaccination by reducing immune suppression. Here we demonstrate that curcumin improves vaccine efficacy of Listeria-Mage-b by converting myeloid-derived suppressor cells into an immune stimulating phenotype, that is, through reducing IL-6 and increasing IL-12 production, in correlation with improved T cell responses and a dramatic reduction in the number of metastases. The novel results of this study may be a platform for improvement of other cancer vaccines by curcumin.
Keywords: Cancer vaccines, curcumin, metastases, T cells, triple-negative breast cancer
Introduction
Triple-negative breast cancer (TNBC), defined as tumors lacking estrogen receptor (ER), progesterone receptor (PR), and HER2/neu accounts for about 20% of all breast cancers, and is particularly increased in black women 1. Women with TNBC represent high-grade tumors that are large and commonly associated with regional node metastases, and recur at distant sites, especially within the first 5 years of diagnosis 2. The absence of any specific targeted therapy for TNBC or basal subtype limits the therapeutic options to cytotoxic therapy 3–4, indicating the need for new therapeutic approaches. Immunotherapy may be our best and most benign option for preventing or curing TNBC. However, immune suppression in the tumor microenvironment (TME) remains as a potential limitation to immunotherapy. Myeloid-derived suppressor cells (MDSC) are one of the most important players in mediating TME-associated immune suppression, with tumor-associated macrophages (TAM), Tregs, and M2 macrophages also playing a role 5–8. Interleukin (IL)-6 is one of such immune suppressive cytokines that is frequently and highly produced by metastatic breast cancers in humans and mice, and particularly by TNBC 9,10. TNBC are enriched for stem-like breast cancer cells (CD44+/CD24−/low), which are typically aggressive and highly resistant to current therapies 12–15. These stem-like breast cancer cells produce high levels of IL-6, and have the capacity to metastasize 16. Moreover, IL-6 is capable of converting dormant breast cancer cells into an actively growing tumor.
IL-6 is a potent regulator of dendritic cell (DC) differentiation in vivo, and is able to turn on the expression of signal transducer and activator of transcription (STAT)3 in DC 17. However, high levels of STAT3 can prevent DC from maturation and subsequent presentation of antigens 18. This in turn may lead to T-cell unresponsiveness. In a previous study, we found high levels of IL-6 produced by breast cancer cells and by immune cells in their TME in an aggressive TNBC mouse model 4T1 19. This IL-6 strongly reduced T-cell responses to Mage-b, but elimination of IL-6 using anti-IL-6 antibodies restored T-cell responses to Mage-b in vitro 20.
Agents that are able to inhibit IL-6 are of great value for immunotherapies against TNBC and other IL-6-producing cancers. One such agent could be curcumin. Curcumin (diferuloylmethane), a polyphenol derived from the plant Curcumina longa, commonly called turmeric, has a broad anticancer effect through downregulating transcription factor NFkB thereby affecting downstream genes such as c-myc, Bcl-2, COX-2, NOS, Cyclin D1, TNFα, and MMP9 21. Curcumin is also known for reducing immune suppressive cytokines such as IL-6 through the NFkB pathway 22. It has been shown that curcumin improves therapeutic efficacy of doxorubicin or of B16-R lysate against B16-R melanoma in mice, and that curcumin prevents tumor-induced T cell apoptosis in mice 23–24. In a previous study, we developed a Listeriaat-based vaccine expressing tumor-associated antigen (TAA) Mage-b 20. Mage-b is homologous to Mage-a 25, and its human homologue MAGE-A is expressed in 26% of the TNBC 26. Vaccination with Listeriaat-Mage-b showed to be highly effective against metastatic breast cancer in a TNBC model 4T1 in a preventive setting 20. However, Listeriaat-Mage-b was less effective in a therapeutic setting because of immune suppression in the TME. Here, we demonstrate that curcumin improved therapeutic efficacy of Listeriaat-Mage-b by reducing the production of IL-6 and increasing the production of IL-12, in correlation with improved T-cell responses in blood of the TNBC 4T1 model. Most important, we found a dramatic effect of the combination therapy on the metastases without having side effects. The results of this study may provide new opportunities to improve efficacy of other types of vaccines and/or against other IL-6-producing cancers.
Material and Methods
Mice
Normal female BALB/c (3-month-old) mice were obtained from Charles River and maintained in the animal husbandry facility Albert Einstein College of Medicine according to the Association and Accreditation of Laboratory Animal Care (AACAC) guidelines. All mice were kept under Bsl-2 condition as required for Listeria vaccinations.
Cells and cell culture
The TNBC 4T1 cell line, derived from a spontaneous mammary carcinoma in a BALB/c mouse 27, was cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mmol/L mixed nonessential amino acids, 2 mmol/L l-glutamine, insulin (0.5 USP units/mL), penicillin (100 units/mL), and streptomycin (100 μg/mL).
Listeriaat-based vaccine
In this study, a highly attenuated Listeria monocytogenes (Listeriaat) has been used for the delivery of TAA Mage-b in vivo, as described previously 20. The Listeriaat plasmid pGG-34, expresses the positive regulatory factor A (prfA) and one of the virulence genes Listeriolysin O (LLO) 28. The coding region for the C-terminal part of the LLO (cytolytic domain that binds cholesterol in the membranes) protein in the plasmid has been deleted, but Listeriaat is still able to escape the vacuole 29. Mutations have been introduced into the prfA gene and in the LLO, which further reduced the pathogenicity of the Listeriaat 28. The background strain XFL-7 lacks the prFA gene and retains the plasmid in vitro and in vivo 29. Listeriaat-Mage-b, expressing nucleotide fragment 311–660 of mouse Mage-b, was developed earlier in our laboratory 20.
Curcumin
As indicated in the text below, a dose of curcumin (95% curcuminoid) (Alfa Aesar, Ward Hill, MA) of 0.8 or 2 g/kg (20 or 50 mg/mouse) in olive oil was administered orally. Piperine (black pepper) of 20 mg/kg (0.48 mg/mouse) was added to the olive oil in all studies with curcumin. Piperine improves the bioavailability with 2000%, and has been successfully used in humans and animals 30. Piperine is a known inhibitor of hepatic and intestinal glucuronidation, a process that breaks down curcumin in vivo 31–32.
Immunization and tumor challenge
In this study, two different immunization protocols were tested. The first immunization protocol consisted of three therapeutic immunizations with Listeriaat-Mage-b and curcumin. Briefly, mice received 0.5 × 105 4T1 tumor cells in the mammary fat pad on day 0, then 0.5 × 107 CFU of Listeriaat-Mage-b, or Listeriaat or saline intraperitoneally (ip) on days 2, 9, and 16, and finally curcumin orally (50 mg curcumin + 0.48 mg black pepper in olive oil/mouse) on days 4, 5, 6, 11, 12, and 13 (Immunization protocol A). All mice were euthanized on day 17 and analyzed for the number of metastases and tumor growth. All untreated 4T1 mice developed a primary tumor in the mammary fat pad that extended to the chest cavity lining and metastasized predominantly to the mesenteric lymph nodes (MLN), and less frequently to the diaphragm, portal liver, spleen, and kidneys within 14 days (metastases were visible as nodules and counted by eye) as described previously 20.
The second immunization protocol consisted of three therapeutic immunizations with Listeriaat-Mage-b, but curcumin was administered before tumor development. Briefly, mice received curcumin orally (50 mg curcumin + 0.48 mg black pepper in olive oil/mouse) on days 0, 1, and 2, then 0.5 × 105 4T1 tumor cells in the mammary fat pad on day 5, and finally three therapeutic immunizations (ip) with 1 × 104 CFU Listeriaat-Mage-b, Listeriaat, or saline on days 8, 11, and 14 (Immunization protocol B). All mice were euthanized on day 16 and analyzed for metastases and tumor growth as described above.
Flow cytometry analysis
Cells were isolated from spleen and blood as described previously 33. Briefly, red blood or spleen cells were lysed according to standard protocols, and the remaining leukocyte population was used for analysis. Single cell suspensions were also obtained from primary tumors using GentleMacs combined with a mild treatment of the cells using Collagenase, Dispase, and DNAse I, according to the manufacturer's instructions (Miltenyi Biotec Inc, Auburn, CA).
Cells were first incubated with an Fc blocker (anti-CD16), and subsequently with the antibodies for the identification of different cell types. For MDSC, anti-CD11b and -Gr1 antibodies were used. CD11b+Gr1low represents monocytic MDSC (mMDSC), and CD11b+Gr1high granulocytic MDSC (gMDSC). Anti-CD8 antibodies were used to identify CD8 T cells and anti-CD4 to identify CD4 T cells. Anti-CD45 antibodies were used to identify the leukocyte population in the primary tumors. To detect the production of intracellular lymphokines the cytofix/cytoperm kit from Pharmingen (San Diego, CA) according to the manufacturer's instructions, and antibodies to IL-6, IL-12, and IFNγ were used. Appropriate isotype controls were used for each sample. Depending on the sample size, 10,000–500,000 cells were acquired by scanning using a Fluorescence Activated Cell sorter (flow cytometry) (BD-FACS-Calibur, Franklin Lakes, NJ), and analyzed using Flojo software as described previously 33. Cell debris and dead cells were excluded from the analysis based on scatter signals and use of Fixable Blue or Green Live/Dead Cell Stain Kit (Invitrogen, Grand Island, NY). In blood and spleens, MDSC were analyzed in the total live gated leukocyte population, and T cells in the total live gated lymphocyte population. In the tumor cell suspension, MDSC and T cells were analyzed in the total live gated CD45+ (leukocyte) population. All antibodies were purchased from BD Biosciences (San Diego, CA) Pharmingen.
Cell proliferation, mitotic index, and apoptosis
Cell proliferation
4T1 cells (2000 cells in 0.1 mL) were cultured with different doses of curcumin in dimethyl sulfoxide (DMSO) for 72 h, then cell viability was analyzed by 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) method using a microtiter plate reader at a wave length of 570 nm.
Mitotic index
Sections of 1 mm thick of primary tumors of mice treated with Listeriaat-Mage-b and curcumin or with saline were stained with hematoxylin and eosin (H and E) and subsequently analyzed for the number of cells in mitosis by light microscopy.
Apoptosis
Early and late apoptosis was analyzed by Annexin-V and TUNEL assay, respectively. For the Annexin-V assay, 4T1 tumor cells were cultured with or without 100 μmol/L of curcumin for 24 h, and subsequently incubated with Annexin-V antibodies (BD Biosciences), for the detection of apoptosis. For the TUNEL Assay, the ApoTag® In Situ Apoptosis detection (Millipore, Billerica, MA) was used. Briefly, slides were deparaffinized through graded alcohols to PBS. TUNEL staining was performed using ApopTag® In Situ Apoptosis Detection Kit (Millipore). Briefly, samples were Proteinase K digested (20 μg/mL) for 15 min at room temperature. Endogenous peroxidases were blocked using 3% H2O2 for 5 min at RT. Samples were washed and placed in Equilibration Buffer for 10 sec followed by TdT enzyme incubation in reaction buffer for 1 h at 37°C. Samples were incubated in the Anti-Digoxigenin, washed and developed using DAB (3, 3′-diaminobenzidine). Slides were briefly counterstained in hematoxylin and mounted using Permount (Fisher Scientific, Pittsburgh, PA). From each tissue, two sections were analyzed, and from each section the number of apoptotic cells in 10 fields were counted by light microscopy. The TUNEL assay and Mitotic Index analyses were performed in the Laboratory of Dr. Rani Sellers, Director of Histology and Comparative Pathology Core Facility, Albert Einstein College of Medicine.
Pathological examination
All pathological analyses were performed by Dr. Rani Sellers, Director of Histology and Comparative Pathology Core Facility, Albert Einstein College of Medicine. Briefly, normal tissues such as kidneys, heart, lungs, liver, and spleen were fixed in 10% formaldehyde for 48 h and then kept in 70% ethanol until use. Sections of 1 mm thick were stained with H and E, and analyzed for pathological damage by light microscopy.
Results
Curcumin administered after tumor development significantly improved therapeutic effect of Listeriaat-Mage-b in the 4T1 model
Here, we tested whether curcumin could improve the efficacy of Listeriaat-Mage-b vaccination in the model 4T1. Listeriaat-Mage-b and curcumin were alternately administered after tumor development (Immunization protocol A). As shown in Figure 1A, the number of metastases in the mice that received Listeriaat-Mage-b and curcumin was significantly lower compared to all control groups. Also the tumor weight in the mice that received Listeriaat-Mage-b and curcumin was significantly lower than in the mice that received Listeriaat or curcumin alone, but not compared to the mice that received Listeriaat-Mage-b alone (Fig. 1B). Curcumin alone had no significant effect on the tumor weight or metastases compared to the saline group.
Figure 1.
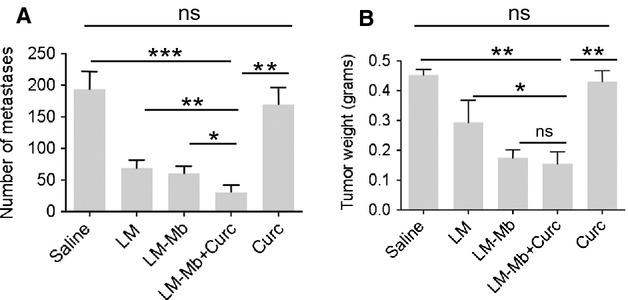
Significant reduction in the number of metastases by therapeutic immunizations with Listeriaat-Mage-b and curcumin in 4T1 tumor-bearing mice. BALB/c mice were immunized with Listeriaat-Mage-b and treated with curcumin after tumor development (Immunization protocol A), and analyzed for the frequency of metastases (A) and tumor weight (B). This experiment was performed two times with five mice per group. Average of two experiments. Mann–Whitney P < 0.05 is significant. *P < 0.05, ** P < 0.01, *** P < 0.001, ****P < 0.0001; ns, not significant. All groups were compared to LM-Mb+Curc. In addition, curcumin alone was compared to the saline group.
Curcumin administered before tumor development also significantly improved therapeutic effect of Listeriaat-Mage-b in the 4T1 model
As curcumin is frequently used in food all over the world, we tested whether curcumin could improve therapeutic vaccine efficacy of Listeriaat-Mage-b when consumed before tumor development (Immunization protocol B). Here, we used a low dose of Listeriaat-Mage-b (104 CFU) at a high frequency (every 3 days; four times totally) in order to obtain a continuous delivery of Listeriaat-Mage-b in vivo without having side effects. Using this immunization protocol, the number of metastases in the mice that received Listeriaat-Mage-b and curcumin was significantly decreased compared to all control groups (Fig. 2A). Also the tumor weight in the mice that received Listeriaat-Mage-b and curcumin was significantly lower compared to all control groups (Fig. 2B). Curcumin alone had also a significant effect on the metastases and primary tumors compared to the saline group (Fig. 2B). The growth kinetics of the primary tumors was analyzed as well in mice that received Listeriaat-Mage-b and curcumin, and confirmed the results shown in Figure 2B; that is, on day 14 the tumor size in mice that received Listeriaat-Mage-b and curcumin was significantly lower compared to all other control groups (Fig. S1).
Figure 2.
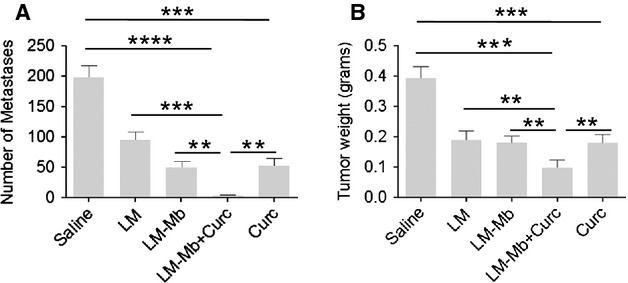
Significant reduction in the number of metastases by preventive administration of curcumin followed by therapeutic immunization with Listeriaat-Mage-b in 4T1 tumor-bearing mice. BALB/c mice were treated with curcumin before tumor development and immunized with Listeriaat-Mage-b after tumor development (Immunization protocol B), and analyzed for the frequency of metastases (A) and tumor weight (B). This experiment was performed three times with five mice per group. Average of three experiments. Mann–Whitney P < 0.05 is significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant. All groups were compared to LM-Mb+Curc. In addition, curcumin alone was compared to the saline group.
The combination therapy with curcumin before and Listeriaat-Mage-b after tumor development was slightly but significantly more effective against the metastases than curcumin and Listeriaat-Mage-b both after tumor development (Fig. 3A and B); that is, the number of metastases in the combination therapy with curcumin before tumor development was 4 ± 1, and after tumor development 31 ± 12 (Mann–Whitney P = 0.0017).
Figure 3.
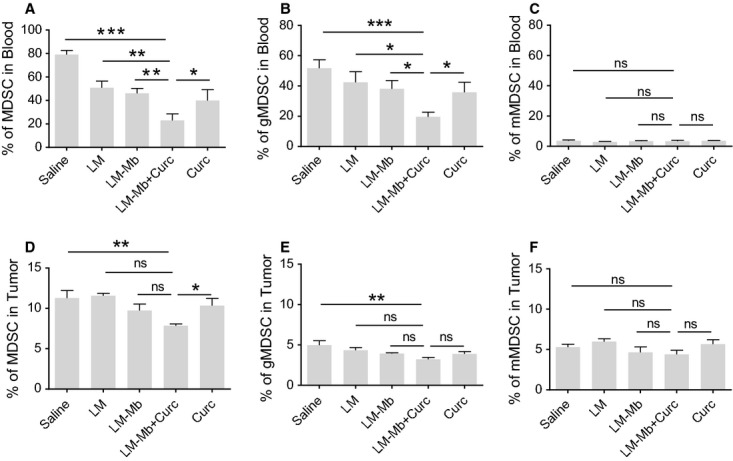
The effect of Listeriaat-Mage-b and curcumin on MDSC in 4T1 tumor-bearing mice. BALB/c mice were treated with curcumin before tumor development and immunized with Listeriaat-Mage-b after tumor development (Immunization protocol B), and analyzed for MDSC (CD11b+Gr1+) (A), gMDSC (CD11b+Gr1high) (B), and mMDSC (CD11b+Gr1low) (C) in blood and for MDSC (D), gMDSC (E), and mMDSC (F) in primary tumors using flow cytometry. All groups were compared to Lm-Mb+Curc. Flow cytometry profiles of MDSC of each group (saline, Listeriaat, Listeriaat-Mage-b, Listeriaat-Mage-b and curcumin, curcumin) are presented in Figure S3. This experiment was performed three times with five mice per group. Average of three experiments. Mann–Whitney P < 0.05 is significant. *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001; ns, not significant.
The effects of Listeriaat-Mage-b and curcumin on MDSC in vivo
As MDSC strongly contributes to immune suppression in the TME, we analyzed the effect of the combination therapy on MDSC in blood and primary tumors of mice immunized according to immunization protocol B. In total blood, the percentage of MDSC was extremely high (~80%) (Fig. 3A). This percentage was strongly reduced to ~20% by the combination of Listeriaat-Mage-b and curcumin compared to the saline group, but was also significantly lower compared to all other control groups (Fig. 3A). More detailed analysis showed that gMDSC was predominantly responsible for the strong decrease in percentage of MDSC (Fig. 3B and C). In the primary tumors, the percentage of MDSC was much lower than in blood (~12%), and the effect of Listeriaat-Mage-b and curcumin on MDSC was much less robust than in blood. The combination therapy slightly but significantly reduced the percentage of MDSC and gMDSC (but not of mMDSC) compared to the saline or curcumin groups only (Fig. 3D–F).
Curcumin reduced the production of IL-6 in primary tumors and in MDSC
Here, we analyzed the effect of curcumin on the production of IL-6 in total tumor cell lysates, in MDSC of primary tumors and blood, and in serum of the 4T1 model. In the tumor cell lysates, we found that the curcumin significantly reduced IL-6 levels compared to the saline group (Fig. 4A). In the primary tumor, the IL-6 production in mMDSC was significantly reduced by curcumin compared to Listeriaat-Mage-b (Fig. 4C), but IL-6 was not produced by gMDSC (Fig. 4B). In blood, the IL-6 production in gMDSC (Fig. 4D) and mMDSC (Fig. 4E) was significantly reduced by curcumin compared to the Listeriaat-Mage-b group. In serum, IL-6 was undetectable and therefore not shown.
Figure 4.
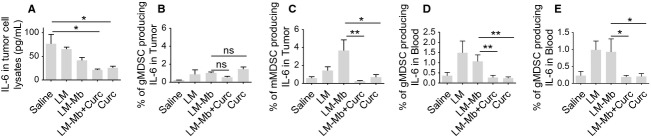
Effects of Listeriaat-Mage-b and curcumin on IL-6 in 4T1 tumor-bearing mice. Curcumin treatment before tumor development followed by immunizations with Listeriaat-Mage-b after tumor development (Immunization protocol B), significantly reduced IL-6 levels in primary tumors as shown here by ELISA (A), and the intracellular production of IL-6 by mMDSC in primary tumors (B, C) and by gMDSC and mMDSC in blood (D, E) as shown here by flow cytometry. In A, the curcumin-containing groups were compared to the saline group, while in B, C and E, D, the curcumin-containing groups were compared to Lm-Mb. These experiments were repeated three times with five mice per group, and the results were averaged. Mann–Whitney P < 0.05 is significant *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant.
Also Listeriaat-Mage-b reduced IL-6 levels in the primary tumors (tumor cell lysates) (Fig. 4A), but not in MDSC in blood and primary tumors (Fig. 4B–E). Moreover, Listeriaat-Mage-b significantly increased the production of IL-6 in sub populations of the MDSC (with an exception of gMDSC in tumors), probably to protect themselves from immune clearance, but as mentioned above curcumin strongly reduced the IL-6 production in both types of MDSC in blood and primary tumor (Fig. 4C–E).
Curcumin administered before and Listeriaat-Mage-b after tumor development improved the IL-12 production by MDSC and T-cell responses to Mage-b
Here, we analyzed the IL-12 production in subpopulations of gMDSC and mMDSC in blood of mice that received the combination of curcumin before and Listeriaat-Mage-b after tumor development. A significant increase was found in the percentage of IL-12-producing gMDSC and mMDSC in the combination group compared to all other groups (Fig. 5A and B), but not in the primary tumor (data not shown). These results raised the question whether the lower number of MDSC (Fig. 3), the decreased IL-6 levels (Fig. 4) and increased IL-12 production (Fig. 5A and B) induced by Listeriaat-Mage-b and curcumin, could improve T-cell responses in vivo. For this purpose, we analyzed the production of IFNγ by CD4 and CD8 T cells in blood and primary tumors in vaccinated and control mice by flow cytometry. IFNγ is a marker for T-cell activation. The cells were analyzed in all groups without restimulation in order to determine whether the T cells were activated in vivo by the combination therapy compared to the control groups. It appeared that the combination of Listeriaat-Mage-b and curcumin significantly improved the percentage of CD4 and CD8 T cells producing intracellular IFNγ compared to all control groups in blood (Fig. 5C and D), but not in tumors (data not shown). We also analyzed T-cells responses in the spleen upon restimulation with Mage-b in vitro. As shown in Figure 5E, Listeriaat-Mage-b and curcumin strongly improved the number of CD8 T cells to Mage-b, secreting extracellular IFNγ.
Figure 5.
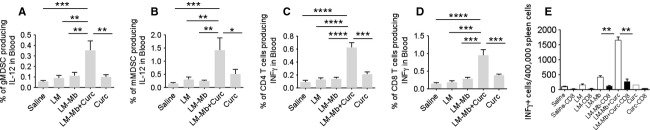
The combination of Listeriaat-Mage-b and curcumin increased IL-12 production by MDSC and improved T-cell responses in 4T1 tumor-bearing mice. Curcumin treatment before tumor development followed by immunizations with Listeriaat-Mage-b after tumor development (Immunization protocol B) significantly increased the percentage of gMDSC (A) and mMDSC (B) producing intracellular IL-12 in blood of 4T1 tumor-bearing mice. This correlated with a significant increase in the percentage of CD4 (C) and CD8 T (D) cells producing intracellular IFNγ (activation marker for T cells) in blood of 4T1-tumor-bearing mice as shown here by flow cytometry. CD8 T-cell responses (extracellular production of IFNγ) were also analyzed in the spleen in vitro upon restimulation with Mage-b by ELISPOT, and a significant higher number of CD8 T cells was found in the spleen that received Listeriaat-Mage-b and curcumin compared to all other groups (E). These experiments were repeated three times with five mice per group, and the results were averaged. Mann–Whitney P < 0.05 is significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Curcumin inhibited proliferation of tumor cells and killed tumor cells through apoptosis
Several reports describe that curcumin inhibits proliferation and kills tumor cells through apoptosis, including breast tumor cells 34,35. We found that curcumin inhibited the growth of 4T1 tumor cells in vitro (Fig. 6A), and mitosis of the tumor cells in vivo (Fig. 6B). In addition, we found that curcumin killed tumor cells through apoptosis in vitro as shown by Annexin-V (early apoptosis) (Fig. 6C), and in the primary tumors in vivo as shown by the TUNEL assay (late apoptosis) (Fig. 6D). A representative example of apoptotic cells by the TUNEL assay is shown in Figure 6E.
Figure 6.
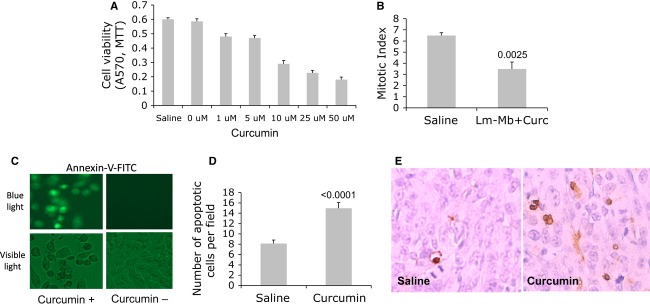
Curcumin inhibited proliferation and killed 4T1 tumor cells through apoptosis. 4T1 tumor cells were cultured with different doses of curcumin for 72 h, and cell viability was analyzed by MTT (A). We also analyzed the Mitotic Index in tumors of mice that received curcumin or saline (B). 4T1 tumor cells were cultured with 100 μmol/L of curcumin in vitro for 24 h, and subsequently incubated with anti-Annexin-V antibodies for the detection of early apoptosis (C). Primary tumors of mice that received curcumin or saline (according Immunization protocol B) were analyzed for the detection of late apoptosis in vivo by the TUNEL assay (D). Apoptotic cells in the primary tumor by the TUNEL assay and light microscopy are shown in (E). Representative of two experiments in A, C, D. Average of two experiments in B and D. n = 5 mice per group. Unpaired t test P < 0.05 is significant. Magnification light microscopy in C and E is 400×. In A, curcumin was dissolved in DMSO and then diluted to the final concentrations of 1–50 μmol/L. The 0 μmol/L represents DMSO without curcumin.
Listeriaat-Mage-b is nonpathogenic and curcumin is nontoxic
In a previous study we have shown that Listeriaat-Mage-b is nonpathogenic 37, while curcumin, consumed through food all over the world, is nontoxic 32. However, the combination of Listeriaat-Mage-b and curcumin has never been tested. Here, we demonstrate by pathological examination of various normal tissues (as kidney, heart, lungs, liver, and spleen) in tumor-bearing mice that the combination of Listeriaat-Mage-b and curcumin is nonpathogenic and nontoxic, but primarily activated the innate immune system. Most obvious was the increased extramedullary myeloid hematopoiesis in the spleen and liver of mice that received Listeriaat-Mage-b and curcumin compared to the saline group. An example of extramedullary myeloid hematopoiesis in the liver is shown in Figure S2. An overview of pathological analysis of normal tissues of tumor-bearing mice that received Listeriaat-Mage-b and curcumin is shown in Table S1.
Discussion
Patients with TNBC have the poorest prognosis. One of the main problems of current therapies against TNBC is their inability to target metastases and their high toxicity. They do not respond to therapies that target ER, PR, and HER2/neu because their tumors lack the expression of these receptors/molecules, and other types of therapies such as tyrosine kinase inhibitor sunitinib, targeting vascular endothelial growth factor (VEGF), or therapies targeting c-kit or Flt2, or bevacizumab, a human antibody to VGEF 38–42, are under investigation but with moderate success. In the study presented here, we developed two nontoxic vaccination strategies in a preclinical TNBC mouse model 4T1. We demonstrated that three therapeutic vaccinations with a highly attenuated nonpathogenic Listeriaat-based vaccine, expressing TAA Mage-b, and nontoxic curcumin significantly reduced the number of metastases compared to Listeriaat-Mage-b or curcumin alone. Curcumin alone had no significant effect on the primary tumors or metastases. Others described that curcumin killed tumor cells in vitro 43–47. However, tumor cells may react differently to curcumin in vitro than in vivo because in vitro bioavailability and the immune system do not play a role and higher concentrations can be obtained in vitro compared to the in vivo situation. Also, the time point of administering curcumin, the concentration of curcumin, and the type of cancer may determine the antitumor effect of curcumin. For instance, others reported that in the Lewis Lung model, curcumin was not effective against metastases and that the time point of administration of curcumin was important 48.
We also tested three administrations of curcumin before tumor development followed by three immunizations with Listeriaat-Mage-b after tumor development. This immunization protocol was slightly but significantly more effective against the metastases compared to Listeriaat-Mage-b and curcumin both after tumor development. Most interestingly, curcumin alone significantly reduced the number of metastases and tumor growth, in contrast to administering curcumin after tumor development. These results suggest that consuming curcumin before cancer develops may provide an advantage over consuming curcumin after cancer develops in the battle against metastatic breast cancer.
Curcumin is known for reducing the production of IL-6 49–50. Here, we demonstrate that curcumin significantly reduced the production of IL-6 in vivo in the primary tumors (tumor cell lysates), and in MDSC of blood and primary tumors. Also Listeriaat-Mage-b reduced the production of IL-6 significantly in the tumor primary tumors, but IL-6 production was even more reduced by the combination of Listeriaat-Mage-b and curcumin.
MDSC are important regulators of the immune system in the TME 5–6, and therefore became one of our most important targets in this study. As mentioned above, curcumin reduced the production of IL-6 significantly in MDSC in blood and primary tumors. To our surprise, the combination of Listeriaat-Mage-b and curcumin significantly increased the production of IL-12 in gMDSC and mMDSC in blood (but not in tumors). It has been reported that IL-12 activates naïve and mature CD4 and CD8 T cells 51–52, which may have happened in this study as well. An interesting observation was that the combination of Listeriaat-Mage-b and curcumin significantly reduced the number of MDSC (predominantly gMDSC) in blood of the TNBC model 4T1. Various factors may have played a role here. It is possible that MDCS infected with Listeriaat-Mage-b became a target for Listeriaat- and Mage-b-specific T-cell and perhaps NK-cell responses because the combination therapy improved these immune responses to Listeriaat and Mage-b by reducing IL-6, and increasing IL-12 production. As Listeriaat 37 and curcumin kill 4T1 tumor cells directly (this study), it is also possible that the combination therapy prevented the tumor cells from growing in the early phase of treatment, and consequently prevented migration of the MDSC to the TME. We found that curcumin alone decreased the percentage of MDSC in blood (although this effect was much stronger when Listeriaat-Mage-b was combined with curcumin). Reduction in the percentage of MDSC by curcumin was also found by others in a xenograft model of colon cancer 53. They concluded that reduction in IL-6 production by curcumin reduced the mobilization of MDSC to the primary tumors. Others found that activated T cells might express Fas ligand and induce apoptosis of Fas+ MDSC 54. In conclusion, various pathways may lead to the reduction in MDSC and more analysis is required.
The decrease in IL-6 and increase in IL-12 production, the improved CD4 and CD8 T-cell responses in blood and spleen, and the dramatic reduction in the number of metastases by the combination therapy strongly suggest that T-cell responses contributed to the effect on the metastases. However, this strong reduction by the combination therapy is not only an effect of Mage-b-specific T-cell responses. As shown previously, Listeriaat exhibits several pathways to kill tumor cells; that is, Listeriaat infects tumor cells in vivo and in vitro, and kills tumor cells directly through high levels of reactive oxygen species (ROS) 37. Moreover, we have shown that Listeriaat-activated CD8 T cells eliminated Listeriaat-infected tumor cells in vivo 37. In addition, we have shown that curcumin kills 4T1 tumor cells through apoptosis (this study). Therefore, it is most likely that the synergistic effects of the multiple pathways of Listeriaat-Mage-b and curcumin as described above, are responsible for the overall strong therapeutic effect on the metastases in this TNBC model 4T1.
The therapeutic effect of the combination therapy was strong but less pronounced on the primary tumors compared to the metastases. It is possible that the production of IL-6 was not sufficiently reduced in the primary tumors (IL-6 was reduced in the tumor cell lysates by ~65% by Listeriaat-Mage-b and curcumin treatment), and other inhibitory cytokines likes TGFβ, which is highly produced by 4T1 tumor cells 55, may play here a role as well. However, most primary tumors can be removed by surgery, radiation, or chemotherapy, while metastases are unresectable and usually chemoresistant despite aggressive and toxic follow-up 56.
The highly attenuated Listeriaat of this study is nonpathogenic, and are naturally cleared by the immune system within 3–5 days 37, which is different from wild type Listeriaat that multiplies in hepatocytes in the liver or epithelial cells of the gastrointestinal tract 57–58. Moreover, the side effects of the combination therapy of Listeriaat-Mage-b and curcumin in the 4T1 model were minimal; that is, primarily induction of inflammatory responses in the liver and spleen and no significant findings were observed in other normal tissues such as heart, lungs, and kidneys. Therefore, Listeriaat-Mage-b and curcumin may be of value as a nontoxic adjuvant therapy, to prevent the development of metastases in TNBC patients that produce IL-6 and express MAGE. This study may be a platform for improvement of other cancer vaccines by curcumin and against other IL-6-producing cancers.
Acknowledgments
This work was supported by NCI grant 1R21CA129470-01A1, NIA/NCI grant 1RO1 AG023096-01, and Reliable Cancer Therapies (RCT). We like to thank Lydie Tesfa of the FACS Facility Core of Einstein and Lisa Scandiuzzi of Xingxing Zang's laboratory for their excellent assistance with the flow cytometry.
Conflict of Interest
Bharat Aggarwal has filed several patents with respect to curcumin. US classification: 514/679; International classification: A61K3112; issued 6 April 1999.
ELISPOT
Restimulation of spleen cells of vaccinated or control mice was performed as described previously [20]. Briefly, 2×105 spleen cells were transfected with pcDNA3.1-Mage-b plasmid DNA and pCMV-GM-CSF plasmid DNA) (1 μg of each plasmid DNA), using the Nucleofector kit of AMAXA (Gaithersburg, MD). Two days later, the frequency of IFNγ-producing cells was determined by ELISPOT for both restimulation assays according to standard protocols (Pharmingen, San Diego, CA), using an ELISPOT reader (CTL Immunospot S4 analyzer, Cellular Technology Ltd, Cleveland, OH). Spleen cells were depleted for CD8 T cells using magnetic bead depletion techniques according to the manufacturer's instructions (Miltenyi Biotec Inc, Auburn, CA). FACS analysis demonstrated that ≥90% of all CD8 T cells were depleted.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S3. The effect of Listeriaat-Mage-b and curcumin on MDSC in 4T1 tumor-bearing mice (Flow cytometry profile).
References
- Stead LA, Lash TL, Sobieraj JE, Chi DD, Westrup JL, Charlot M, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent R, Traudeau M, Pritchard KI, Hanna WH, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185(HER2/neu) monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J. Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J. Clin. Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Cuervo C, Harris KW, Kallman L, Vaananen HK, Selander KS. Tumor necrosis factor-alpha induces interleukin-6 production via extracellular-regulated kinase 1 activation in breast cancer cells. Breast Cancer Res. Treat. 2003;80:71–78. doi: 10.1023/a:1024443303436. [DOI] [PubMed] [Google Scholar]
- Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadle H, Gerald WL, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9:R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XS, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, et al. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11:367–376. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- Idowu MO, Kmieciak M, Dunur C, Burton RS, Grimes MM, Powers CN, et al. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum. Pathol. 2012;43:364–373. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Liu S, Wicha MS. Targeting breast cancer stem cells. J. Clin. Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta LLC, Almendo V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J. Clin. Invest. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa SI, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- Xie J, Qian J, Wang S, Epstein ME, Freeman J, III, Yi Q. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J. Immunol. 2003;171:4792–4800. doi: 10.4049/jimmunol.171.9.4792. [DOI] [PubMed] [Google Scholar]
- Gravekamp C, Leal B, Denny A, Bahar R, Lampkin S, Kim SH, et al. In vivo responses to vaccination with Mage-b, GM-CSF and thioglycollate in a highly metastatic mouse breast tumor model, 4T1. Cancer Immunol. Immunother. 2008;57:1067–1077. doi: 10.1007/s00262-007-0438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Castro F, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br. J. Cancer. 2008;99:741–749. doi: 10.1038/sj.bjc.6604526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- Odot J, Albert P, Carlier A, Tarpin M, Madoulet C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int. J. Cancer. 2004;111:381–387. doi: 10.1002/ijc.20160. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Mandal D, Saha B, Sen GS, Das T, Sa G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J. Biol. Chem. 2007;282:15954–15964. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- de Backer O, Verheyden AM, Martin B, Godelaine D, Brasseur E, de Plean R, et al. Structure, chromosomal location, and expression pattern of three mouse genes homologous to the human MAGE genes. Genomics. 1995;28:74–83. doi: 10.1006/geno.1995.1108. [DOI] [PubMed] [Google Scholar]
- Curigliano G, Viale G, Ghioni M, Jungbluth AA, Bagnardi V, Spagnoli GC, et al. Cancer-testis antigen expression in triple-negative breast cancer. Ann. Oncol. 2011;22:98–103. doi: 10.1093/annonc/mdq325. [DOI] [PubMed] [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary-tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- Gunn G, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PSSR. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine – evidence that piperine is a potent inhibitor of drug-metabolism. J. Pharmacol. Exp. Ther. 1985;232:258–262. [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, et al. Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br. J. Cancer. 2009;101:1329–1337. doi: 10.1038/sj.bjc.6605329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets. 2005;5:117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H. Targeted therapy in breast cancer: current status and future directions. Jpn. J. Clin. Oncol. 2010;40:711–716. doi: 10.1093/jjco/hyq037. [DOI] [PubMed] [Google Scholar]
- Bernard-Marty C, Lebrun F, Awada A, Piccart MJ. Monoclonal antibody-based targeted therapy in breast cancer: current status and future directions. Drugs. 2006;66:1577–1591. doi: 10.2165/00003495-200666120-00004. [DOI] [PubMed] [Google Scholar]
- Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Lieberman DS, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J. Clin. Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- Lin NU, Minetta LA, Younger J, Come SE, Ewend M, Gordon GJ, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios CH, Liu MC, Lee SH, Vanlemmens L, Ferrero JM, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res. Treat. 2010;121:121–131. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Kang HJ, Moon A. Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Arch. Pharm. Res. 2001;24:349–354. doi: 10.1007/BF02975105. [DOI] [PubMed] [Google Scholar]
- Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Nguyen M. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer. 2002;98:234–240. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Rodriguez S, Ramachandran R, Nair PKR, Fonseca H, Khatib Z, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25:3293–3302. [PubMed] [Google Scholar]
- Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Hohneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29:779–789. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- Zong H, Wang F, Fan QX, Wang LX. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol. Biol. Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- Yan L. Dietary supplementation with curcumin enhances metastatic growth of Lewis lung carcinoma in mice. Int. J. Cancer. 2013;132:269–275. doi: 10.1002/ijc.27683. [DOI] [PubMed] [Google Scholar]
- Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int. J. Cancer. 2006;119:1268–1275. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Wolf SF, Sieburth D, Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells. 1994;12:154–168. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev. Res. 2012;5:205–215. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–5390. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Subramaniam V, Abdalla S, Jothy S, Prud'homme GJ. Tranilast inhibits the growth and metastasis of mammary carcinoma. Anticancer Drugs. 2009;20:334–345. doi: 10.1097/CAD.0b013e328327994e. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Racz P, Tenner K, Mero E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab. Invest. 1972;26:694–700. [PubMed] [Google Scholar]
- Rosen H, Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J. Exp. Med. 1987;166:1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S3. The effect of Listeriaat-Mage-b and curcumin on MDSC in 4T1 tumor-bearing mice (Flow cytometry profile).


