Significance
One of the oldest debates in cognitive neuroscience concerns the degree of functional specialization present in the human brain. Prior work has discovered several highly specialized components dedicated to particular mental functions, like face recognition or motion perception. However, our cognitive versatility suggests the additional existence of more general-purpose machinery. Building on prior neuroimaging evidence, along with neurophysiological evidence from non-human primates, we searched for such domain-general brain regions in humans. Seven diverse demanding cognitive tasks produced overlapping activation at the individual-subject level in a number of frontal and parietal brain regions. Thus, human cognition arises from hardware that includes not only specialized components, but also very general-purpose ones that plausibly enable us to solve novel problems.
Keywords: Multiple-demand system, cognitive control
Abstract
Unlike brain regions that respond selectively to specific kinds of information content, a number of frontal and parietal regions are thought to be domain- and process-general: that is, active during a wide variety of demanding cognitive tasks. However, most previous evidence for this functional generality in humans comes from methods that overestimate activation overlap across tasks. Here we present functional MRI evidence from single-subject analyses for broad functional generality of a specific set of brain regions: the same sets of voxels are engaged across tasks ranging from arithmetic to storing information in working memory, to inhibiting irrelevant information. These regions have a specific topography, often lying directly adjacent to domain-specific regions. Thus, in addition to domain-specific brain regions tailored to solve particular problems of longstanding importance to our species, the human brain also contains a set of functionally general regions that plausibly endow us with the cognitive flexibility necessary to solve novel problems.
A striking feature of the human brain is that it contains cortical regions specialized for particular mental tasks, from perceiving visual motion, to recognizing faces, understanding language, and thinking about others’ thoughts (e.g., refs. 1 and 2). However, an equally striking feature of human cognition is our ability to solve novel problems on the fly for which we cannot have ready-made, specialized brain machinery. We innovate on recipes when a key ingredient is missing, we think through the possible causes—and possible solutions—when our car breaks down on the highway, and we invent white lies on the spot in awkward social situations. How are we so cognitively versatile and innovative, and what brain regions endow us with the ability to solve new problems that neither our evolutionary history nor our individual experience has specifically prepared us for?
Based on previous neuroimaging data, a plausible neural substrate for cognitive flexibility is provided by a specific set of frontal and parietal brain regions the activity of which does not appear to be closely tied to specific cognitive demands. Instead, activity in these regions increases for a wide range of complex behaviors (e.g., refs. 3 and 4). Comprising this network are regions on the dorsolateral surface of the frontal lobes (along the inferior frontal sulcus/middle frontal gyrus), parts of the insular cortex, regions along the precentral gyrus, presupplementary and supplementary motor area (preSMA, SMA), parts of the anterior/mid cingulate, and regions in and around the intraparietal sulcus. We will refer to these regions as the multiple-demand or MD system, following Duncan (4). Across both human and nonhuman primate studies, these regions are commonly linked to cognitive or executive control processes likely involved in many different kinds of behavior (e.g., refs. 5 and 6), including focused attention, goal maintenance, strategy selection, performance monitoring, and other activities. In line with the apparent functional generality observed with functional MRI (fMRI), single-cell recording studies have shown that many neurons in the frontal and parietal lobes exhibit substantial flexibility, adapting their response properties to code the specific information required in current behavior (e.g., refs. 7–12).
However, although the neurophysiological evidence from nonhuman primates is tantalizing, it is not obvious that similar functional generality would be present in humans. Indeed, previous work has established that larger brains are associated with greater functional differentiation of brain regions (e.g., refs. 13–15; see ref. 16 for a recent discussion). At present, most evidence in humans comes from studies that rely on traditional group analyses (e.g., refs. 17–19) or metaanalyses of activation peaks pooled across studies (e.g., refs. 20–25). These methods have been shown to overestimate activation overlap across tasks because of intersubject variability in neuroanatomy (e.g., refs. 26–28). In particular, a group analysis can reveal overlapping activations for two tasks even when these tasks activate distinct, nonoverlapping brain regions in each subject. The functional generality that emerges in the group analysis would be spurious in this case, obscuring the true underlying architecture. Thus, most of the available evidence that has been offered for the functional generality of the MD system in the human brain is in principle consistent with the opposite hypothesis, that this network is actually composed of a set of distinct but nearby regions, each engaged during different tasks.
The concern is not just an esoteric theoretical possibility. We recently showed that the widely claimed overlap between language and executive processes in the frontal lobes (e.g., ref. 29) is not found in analyses of individual subjects (30). Might prior findings of overlap in activation across multiple demands, also primarily based on group analyses and comparisons across studies, also disappear in analyses of individual subjects? Very few prior studies have tested this question. One study found such overlap for three attentional tasks in parietal regions (31), and another found overlap across four tasks in frontal regions (32). Here we build on these initial results to test for the existence of functionally general brain regions more stringently and comprehensively.
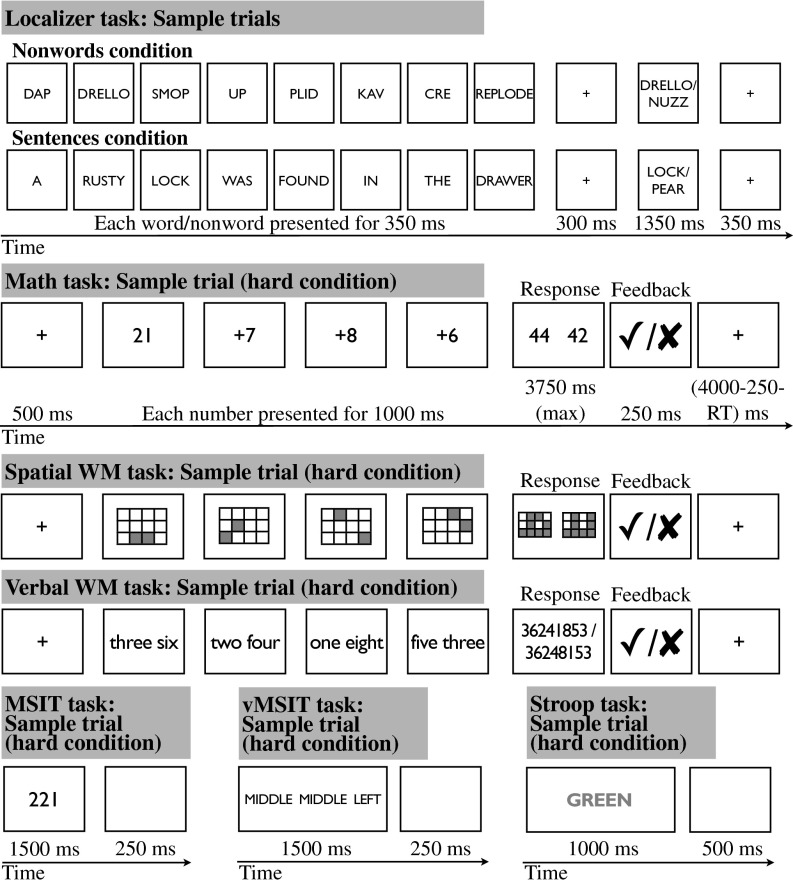
To this end, we test the engagement of the MD regions in seven diverse tasks (Fig. 1). Each task has a difficult condition and an easier control condition. To test for activation overlap among these tasks, we first used one contrast as a “localizer” to identify regions in each subject individually that are sensitive to cognitive demand, and then we measured the responses of these functional regions of interest (fROIs) to the other tasks.
Fig. 1.
A schematic illustration of the seven tasks. (Note: The timing for the spatial WM and verbal WM tasks is identical to the timing of the Math task.) (Adapted from ref. 2.)
The tasks were chosen to vary along several dimensions. First, the tasks differed in the kinds of representations they involved: four tasks [the localizer task, verbal working memory (WM), verbal multisource interference task (vMSIT), and Stroop] used verbal representations, two tasks (math and MSIT) used Arabic numerals, and the spatial WM task used spatial locations. Second, the tasks differed in the kinds of cognitive processes they taxed most strongly: the localizer task required participants to read sequences of nonwords or sentences and to respond to a memory probe at the end of each; WM tasks required keeping sets/sequences of elements in memory for a brief period; the math task required manipulating representations and storing/updating intermediate results; and the two MSIT tasks and Stroop required inhibiting a prepotent response and selecting a task-relevant response. Finally, six tasks required a manual response (a button press), and one task (Stroop) required a vocal response.
Note that these tasks were not designed to be directly comparable to one another, even though they have all been shown to produce behavioral difficulty effects with the hard condition, leading to lower accuracies and longer reaction times (2, 27). As a result, differences among tasks in the magnitude of the overall response or in the size of the hard > easy effect may be hard to interpret. In the present study, we focus on whether a given brain region does or does not show a significantly greater response during the hard compared with the easy condition of each task. Furthermore, these tasks were not designed to test any particular hypotheses about specific functions of any of the MD brain regions (although in the Discussion we briefly relate these data to some existing proposals). What is important about this set of tasks is their diversity in terms of the representations they involve and the mental processes they engage, which provides the critical test of whether the MD regions get engaged by a variety of cognitive tasks.
Results
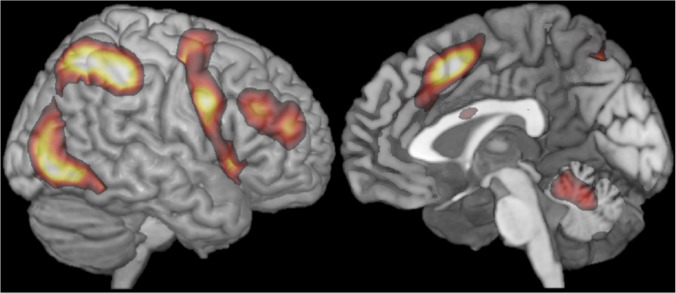
Consistent with previous findings, traditional group analyses revealed several regions in the frontal and parietal cortices that are robustly activated by the hard > easy contrast across tasks (SI Text, Figs. S1 and S2, and Table S1). In Fig. 2 we show the aggregate representation of these activation maps, highlighting their most stable features. In particular, we see activation in the premotor regions of the precentral gyrus, going as far inferiorly as the posterior parts of the inferior frontal gyrus and the nearby anterior insula/frontal operculum, along the middle frontal gyrus, and in and around the intraparietal sulcus. On the dorsomedial frontal surface, we see activation in the SMA and preSMA, and in some of the tasks we additionally observe activation in anterior/mid cingulate cortex (ACC). Furthermore, consistent with previous findings, we see activation in the inferior posterior temporal and adjacent occipital regions, presumably because of the attentional enhancement of visual representations in the more difficult conditions. Finally, we observe activity in the cerebellum, consistent with work implicating some regions of the cerebellum in high-level cognitive functions (e.g., refs. 33–35).
Fig. 2.
A group-level representation of the MD activity based on average activity in left and right hemispheres. To create this representation, we (i) reflected left hemisphere data to the right, (ii) averaged the resulting 14 (7 contrasts × 2 hemispheres) t-maps, and (iii) thresholded the map at t = 1.5. The map (including a parcellated version) can be downloaded at http://imaging.mrc-cbu.cam.ac.uk/imaging/MDsystem.
These results are broadly consistent with prior work, but like the prior work leave open the important possibility that activations for different tasks do not in fact overlap at the critical level of individual subjects. In the present paper we test this hypothesis, focusing on the frontal and parietal regions. To do so, we first defined fROIs in each subject by intersecting that subject’s localizer activation (thresholded at P < 0.001, uncorrected) with a set of key anatomical ROIs previously implicated (e.g., ref. 20) in domain-general functions (see SI Text and Fig. S3 for a demonstration that similar results obtain when different tasks are used as localizers). The fROI definition procedure adopted here is similar to the group-constrained subject-specific approach recently introduced by Fedorenko et al. (27; see also ref. 36), except that anatomical parcels are used instead of group-level functional parcels to constrain the selection of subject-specific voxels. [A complementary analysis using the group-constrained subject-specific method on the whole brain (27, 36) found the same pattern of results, with no new frontal/parietal functionally general regions.] For all regions, fROIs could be defined in at least 70% of the subjects (and for 11 of 18 regions fROIs could be defined in >80% of the subjects; see Table S2 for details).
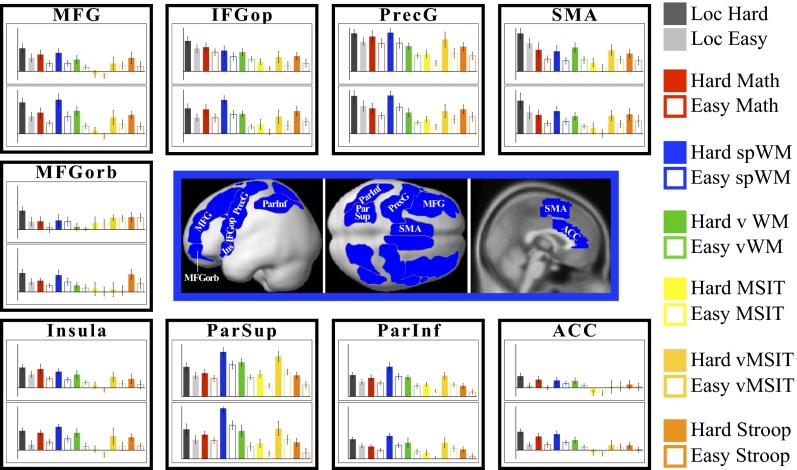
Fig. 3 shows the response profiles of the individually defined fROIs across hard and easy conditions for each of the seven tasks (see Table S3 for the statistics). The responses to the localizer task conditions are estimated using independent data (a left-out run not used for fROI definition). Most of the regions show reliable hard > easy effects for all (or six of seven) tasks. [Furthermore, at least half of the voxels in these fROIs on average show individually significant hard > easy effects within individual subjects for the other six tasks (Fig. S4)]. These regions include parts of the precentral gyrus bilaterally, the opercular part of the inferior frontal gyrus (IFG) bilaterally, the right middle frontal gyrus, the SMA bilaterally, the inferior and superior parietal cortex bilaterally, and the insula bilaterally. Three of the regions—the orbital parts of the middle frontal gyrus in the left hemisphere and, to some extent, in the right hemisphere, and the left ACC—show weaker results, with significant hard > easy effects for fewer tasks.
Fig. 3.
Average responses across subjects (expressed in percent BOLD signal change relative to the fixation baseline) of individually defined fROIs to the hard and easy condition of each of the seven tasks. In the center we show the anatomical parcels used to constrain the selection of voxels in individual subjects in blue. Each individual subject’s fROI constituted only a small portion of the anatomical parcel (see SI Text and Table S2 for details). For each region, the Upper bar graph represents the profile of the left hemisphere region, and the Lower bar graph, the right hemisphere region. The values on the y axis range from −0.04 to 2 for all fROIs except for the ParSup and ParInf fROIs where they range from 0 to 2.8. Error bars represent SEMs. IFGop, opercular part of the inferior frontal gyrus; MFG, middle frontal gyrus; MFGorb, orbital part of the middle frontal gyrus; ParInf, the inferior parietal cortex; ParSup, the superior parietal cortex; PrecG, precentral gyrus; SMA, supplementary motor area.
Is this pattern of increased response to greater difficulty across tasks restricted to these regions, or does the brain as a whole show the same pattern? As discussed next, functionally general responses demonstrated above appear to be spatially restricted to the MD system. First, (2; see also 37–38) showed that brain regions sensitive to linguistic stimuli—responding more strongly to meaningful and structured stimuli, like sentences, than to various degraded versions of those stimuli (e.g., lists of unconnected words or nonwords)—respond weakly or not at all during the same demanding cognitive tasks examined here. These regions instead appear to require a particular type of stimulus (language) to drive them, much as many high-level visual regions require specific visual information (e.g., ref. 1). Language-responsive regions thus show a very different profile of response from the MD regions identified here, despite often lying immediately adjacent to the MD cortex (30; see Fig. S5 for sample activation maps showing both contrasts).
Second, to test the idea that any large anatomical region—like the ones used in the current analysis—contains a substantial number of voxels that respond more to difficult conditions than to easier conditions across tasks, we performed an analysis using four control regions: bilateral superior and middle temporal pole regions, which have been implicated in a wide range of mental processes (e.g., ref. 39) but are not considered to be part of the MD system. The analysis procedures were identical to those used above, and the results are shown in Fig. S6. Although in each region a little less than half of the subjects have a few localizer-responsive voxels (seven voxels per region per subject on average), (i) the nonwords > sentences effect does not replicate in a left-out run (ts < 1.5), and (ii) these voxels do not show the hard > easy effect for any of the other six tasks (ts < 1.5). These results indicate that it is not the case that any large anatomical region contains voxels with the properties of the MD system. (Note that these results are not simply a result of signal dropout in the temporal poles. For example, the language-responsive portions of the same regions—that is, regions defined by the sentences > nonwords contrast—show highly robust and replicable responses in the left-out run: ts > 3.8, Ps < 0.0005.)
Finally, in addition to the language-responsive regions and regions in the temporal poles discussed above, a set of brain regions that have become known as the “default mode network” (e.g., ref. 40) show a response profile that is essentially the opposite of that exhibited by the regions of the MD system. These regions—which include parts of the medial temporal lobe, parts of the medial prefrontal cortex, the posterior cingulate cortex, and the precuneus—deactivate in response to demanding cognitive tasks.
In summary, the activations reported here and in previous studies—greater responses to more cognitively demanding conditions—albeit extensive, are spatially restricted to a specific set of brain regions: the MD system.
Discussion
Using the most stringent method currently available for human fMRI (activation overlap within individual subjects), we found that brain regions throughout the MD system respond across a wide range of demanding cognitive tasks. These results extend previous conclusions about the MD system in three key respects. First, almost all prior claims of functional generality were based on group analyses (e.g., refs. 17–19) or metaanalyses of activation peaks pooled across studies (e.g., refs. 20–25), where apparent activation overlap could have resulted spuriously from data in which each subject individually shows functional segregation (e.g., ref. 28). Extending previous reports focusing on selected parts of the MD system (30–32), the present data show that activation overlap is not an artifact of group averaging, but is present within individual subjects. Second, by testing a large number of tasks varying in both the content and operations invoked, we demonstrate broader functional generality of the MD response than previously shown. Finally, the present study characterizes the spatial topography of the MD system (Fig. 2) and its regional specificity (Figs. S5 and S6), in which abutting regions often show strikingly different profiles.
Taken together, these results provide both the strongest evidence to date for the existence of the MD system in humans, and a richer anatomical and functional characterization of that system. More generally, the present data show that the strikingly domain-specific brain regions that have been characterized over the last 15 y (e.g., ref. 1) are complemented by an extensive system of brain regions exhibiting the diametrically opposite property of broad functional generality.
In the present paper we have focused on the similarity in the functional profiles among the regions of the MD system. However, we assume that some functional differentiation exists among these regions, and it remains an important goal for future research to identify such dissociations. We briefly mention a number of recent proposals here. Some proposals have attempted to link particular MD regions to specific cognitive functions. For example, Aron et al. (41) have argued for the specific role of the right IFG in inhibitory control (see ref. 42 for a similar proposal with respect to the left IFG). Others (43–45) have argued for a distinction between the ACC, critical in detecting conflict, and the lateral prefrontal cortex, responding to the ACC signal with strengthened cognitive control (cf. 46–47). Still others (48; see 49 and 50 for a related proposal) have hypothesized that an anterior–posterior gradient exists on the lateral surface of the frontal lobes, with the more anterior regions supporting more hierarchically complex operations. A few studies have reported differences between frontal and parietal regions (e.g., refs. 51–55), including differences in the timing of neural activity (e.g., refs. 56 and 57), or between the left and right hemisphere MD regions (e.g., refs. 58–61). Many investigations using a variety of methodologies have argued for the existence of subnetworks within the broader MD system, with the number and functional interpretation of these subnetworks varying across proposals (e.g., refs. 6, 62–70). For example, Dosenbach et al. (62) have proposed that the MD system includes two subsystems: one subserving transient control and the other subserving more sustained control processes. Based on resting-state correlation analyses, Power et al. (66) identified three subnetworks in the cortices previously linked to cognitive control: the dorsal attention network, the fronto-parietal task control network, and the cingulo-opercular task control network (see ref. 71, for example, for a functional dissociation between the first two networks). In evaluating these proposals further in future studies and in developing new ones, it is important to remember that all parts of the extended MD system can be driven by increased task difficulty of many forms, including simple tasks with little obvious element of hierarchical control (e.g., refs. 72–75). In our data, the main exception was the rather weak MD pattern observed for the most anterior part of the lateral prefrontal cortex, and for the left ACC. Regardless of what the true functional organization of the MD system turns out to be—and in line with single unit evidence for flexible coding of task-relevant information across multiple MD regions (e.g., refs. 7–12)—we suspect that control representations and processes generally involve close interaction and information exchange among multiple MD regions. At the level of resolution of fMRI, the result is broadly similar activity across multiple task domains.
Parts of the MD system resemble regions that have been linked to oculomotor control (e.g., refs. 76 and 77). Indeed, in some of our tasks (e.g., spatial WM), the hard condition plausibly required more eye movements than the easy condition during at least some stages of the task. Importantly, though, the materials were presented foveally in the main localizer task and the Stroop task, so it is unlikely that all MD activity is a result of differential eye movements. Furthermore, even if MD regions contribute to eye movement control, this contribution may in part reflect domain-general attentional processes, consistent with reports that cortical regions implicated in oculomotor control are apparently engaged more broadly in other goal-directed behaviors, like reaches (e.g., refs 78–80, but see also ref. 81).
Our results have several implications for future studies. The ability to properly define the MD brain regions (using functional localization in individual subjects) is important for making progress in understanding the properties and functional organization of this system. First, as discussed above, both frontal and parietal MD brain regions are located near brain regions that have different, sometimes opposing, functional profiles (SI Text and Fig. S5). This situation, especially in combination with anatomical variability of those regions across subjects, makes the use of common stereotaxic space problematic for characterizing the MD or nearby regions. Identifying ROIs functionally in individual subjects enables a more precise characterization of their roles in cognition. (Of course, individual-subject analyses may not always be feasible/practical; in these cases, group-level ROIs shown in Fig. 2 may provide a useful alternative.) Second, and relatedly, the use of MD fROIs makes possible stronger tests of possible dissociations among MD regions than have been conducted in the past, by testing for significant ROI by functional contrast interactions (e.g., refs. 82 and 83). Such evidence is stronger than simply showing that a given contrast reaches significance in some regions but not others, a pattern of results that may reflect a difference in significance but not a significant difference (see refs. 84–85 for discussion). Finally, to interpret findings from single domains (e.g., number processing, syntactic processing, imagery, episodic memory retrieval), care must be taken when the activations lie near the MD regions. This caveat is especially important when contrasting a more versus a less difficult condition, and when it is critical to know whether the resulting activations originate within the functionally general MD regions. One solution would be to include a demanding task that has nothing to do with the manipulation of interest and yet robustly activates MD regions, so that activations for the target task can be dissociated from generic difficulty-based MD activations, if the two are indeed distinct. [The particular localizer contrast used in the present study may not be the best one for future investigations of the MD system. Reassuringly, similar functional profiles are observed across different localizer contrasts (SI Text and Fig. S3). The optimal long-term solution might be to use a combination of two or more MD tasks for localization purposes, to identify the most robust and stable MD voxels.]
Of course, even at the single-subject level, the resolution of fMRI does not come close to that of single-cell recordings in nonhuman primates. Nevertheless, complementing our results, several studies have used multivoxel pattern analyses (86) to show that MD regions code many specific properties of attended stimuli, responses and tasks in the fine-grained patterns of spatial activity (e.g., refs. 32, 87–93). Such results dovetail with analogous findings of widespread, adaptive coding of task-relevant information in single neurons of frontal and parietal cortex (8, 12).
Ever since Gall, Flourens, and Broca, neuroscientists have debated whether the human brain is made up of highly specialized components, each conducting a very specific mental process, or whether instead each brain region is broadly engaged in a wide range of cognitive tasks. Careful individual-subject–based analyses of fMRI data over the last 15 y have revealed a high degree of functional specificity for at least a few regions of the human brain (1). Here we use similar individual-subject–based analyses of fMRI data to provide strong evidence for the functional generality of a set of regions in the frontal and parietal lobes that are broadly engaged in a wide range of tasks, from mental arithmetic, to holding information in working memory, to filtering and suppressing task-irrelevant information. The evidence presented here for the broad functional generality of these regions comes together with prior evidence for the extreme domain-specificity of other brain regions to paint a rich and fascinating picture of the functional architecture of the human brain: Human cognition is accomplished by the joint efforts of both highly specialized and very general-purpose cognitive and neural mechanisms.
Materials and Methods
Participants.
Forty right-handed participants (28 females) from the Massachusetts Institute of Technology (MIT) and the surrounding community were paid for their participation. All subjects were native speakers of English between the ages of 18 and 50, had normal or corrected-to-normal vision, and were naive as to the purposes of the study. All participants gave informed consent in accordance with the requirements of Internal Review Board at MIT.
Design.
The tasks included reading sequences of nonwords and sentences and performing a memory-probe task after each (the localizer task), and one or more of the following: an arithmetic task, a spatial and a verbal WM task, two versions of the MSIT (94), and the classic Stroop task (Fig. 1). Each task used a blocked design and included a harder and an easier condition. We used the nonwords > sentences contrast as the localizer contrast because it was present in each of the 40 participants (but see SI Text and Fig. S3 for a demonstration that the results are similar when other contrasts are used as localizers).
Procedure.
Each participant was run on the localizer manipulation, and between 13 and 16 participants performed each of the other tasks. In the localizer task, participants (n = 40) read sequences of pronounceable nonwords and sentences, presented one nonword/word at a time. A memory probe appeared after each stimulus and participants decided whether the probe appeared in the preceding stimulus. This task is more difficult in the nonwords—compared with the sentences—condition. [Because of script/experimenter errors, behavioral data for the localizer task in the current dataset were recorded for 31 of the 40 participants: accuracies: nonwords 80.3%, sentences 84.5% (P < 0.005); reaction times: nonwords 778.4 ms, sentences 668.9 ms (P < 0.0001).] For the timing information for this and other tasks, see ref. 2. In the math task participants (n = 13) saw a number (range 11–30) and added three addends to it (of size 2–4 or 6–8 in the easy and hard condition, respectively). After each trial, participants had to choose the correct sum in a two-alternative forced-choice question. In the spatial WM task, participants (n = 16) saw a 3 × 4 grid and kept track of four or eight locations in the easy and hard conditions, respectively. After each trial, participants had to choose the grid with the correct locations in a two-alternative forced-choice question. In the Verbal WM task, participants (n = 13) kept track of four or eight digit-names in the easy and hard condition, respectively. Digits were presented as words (e.g., “three”) to prevent chunking. After each trial, participants had to choose the correct digit sequence in a two-alternative forced-choice question. In the MSIT (94), participants (n = 15) saw triplets of digits (possible digits included 0, 1, 2, and 3) and pressed a button (1, 2, or 3) corresponding to the identity of the nonrepeated digit. In the easy condition, the position of the nonrepeated digit corresponded to the position of the response button, and the other digits were not possible responses (e.g., 100); in the hard condition, the position of the nonrepeated digit did not correspond to the position of the button, and the other digits were possible responses (e.g., 212). The vMSIT, n = 14, was identical to the MSIT task, except that digits (0, 1, 2, and 3) were replaced with words (NONE, LEFT, MIDDLE, and RIGHT). In the Stroop task, participants (n = 14) saw a word and overtly named the color of the word’s font. In the easy condition, the words were noncolor adjectives (HUGE, CLOSE, GUILTY) matched to the color adjectives in length and lexical frequency; in the hard condition, the words were color adjectives (BLUE, GREEN, YELLOW) and on half of the trials in each block the font color did not match the color that the word indicated.
fMRI Data Acquisition.
Structural and functional data were collected on the whole-body 3 Tesla Siemens Trio scanner with a 32-channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted structural images were collected in 176 sagittal slices with 1-mm isotropic voxels (TR = 2,530 ms, TE = 3.48 ms). Functional, blood-oxygenation level-dependent (BOLD), data were acquired using an echo-planar imaging sequence (with a 90° flip angle and using GRAPPA with an acceleration factor of 2), with the following acquisition parameters: 31 4 mm thick near-axial slices acquired in the interleaved order (with 10% distance factor), 2.1 mm × 2.1 mm in-plane resolution, field of view in the phase encoding (A>>P) direction 200 mm and matrix size 96 mm × 96 mm, TR = 2000 ms, and TE = 30 ms. The first 10 s of each run were excluded to allow for steady-state magnetization.
Statistical Analyses.
MRI data were analyzed using SPM5 (www.fil.ion.ucl.ac.uk/spm) and custom Matlab scripts (available from http://web.mit.edu/evelina9/www/funcloc.html and www.nitrc.org/projects/spm_ss). Each subject’s data were motion-corrected and then normalized in a common brain space (the Montreal Neurological Institute template) and resampled into 2-mm isotropic voxels. Data were then smoothed using a 4-mm Gaussian filter and high-pass–filtered (at 200 s). For the individual-subject fROI analyses, we used the wfu_pickatlas tool (95) to create anatomical ROI masks for brain regions within which MD activity has been previously reported (Fig. 3). (For the ACC region, we edited the mask manually to restrict the region to the dorsal part of the anterior cingulate.) We then intersected each anatomical ROI with each subject’s activation map for the localizer contrast (thresholded at P < 0.001, uncorrected), to define each subject’s fROIs. (No spatial contiguity constraints were imposed on these fROIs: any voxel that passed the specified threshold and fell within the boundaries of the anatomical parcel was included in the fROI definition. However, visual examination of the resulting fROIs revealed that for most subjects and regions a fROI took the form of a set of contiguous voxels with occasional small noncontiguous sets nearby.) These regions were defined in each hemisphere separately, for a total of 18 fROIs. To estimate the responses of these fROIs to various conditions, we averaged the responses across the voxels in each individual fROI and then averaged these values across subjects for each region. To estimate the responses to the localizer conditions, we used all but the first run to define the fROIs and the first run to estimate the responses, so that the data used to estimate the effects were always independent of the data used for ROI definition (e.g., refs. 96 and 97).
Supplementary Material
Acknowledgments
We thank Michael Behr and Eyal Dechter for help with the experimental scripts and with running the participants; Alfonso Nieto-Castañon for help with analyses; Daniel Mitchell for help with creating the anterior cingulate cortex parcel; Christina Triantafyllou, Steve Shannon, and Sheeba Arnold for technical support; members of the N.K. laboratory, Saxe laboratory, and Gibson laboratory for helpful discussions; Alex Kell and Jason Fischer for comments on earlier drafts of the manuscript; and the Athinoula A. Martinos Imaging Center at McGovern Institute for Brain Research, Massachusetts Institute of Technology. This research was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Award K99HD-057522 (to E.F.); a grant from the Ellison Medical Foundation (to N.K.); National Science Foundation Grant 0904625 (to N.K. and P. Golland); and by the Medical Research Council (United Kingdom) Intramural Program MC_A060_5PQ10.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315235110/-/DCSupplemental.
References
- 1.Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA. 2010;107(25):11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc Natl Acad Sci USA. 2011;108(39):16428–16433. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 4.Duncan J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2(11):820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 8.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 9.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291(5502):312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 10.Cromer JA, Roy JE, Miller EK. Representation of multiple, independent categories in the primate prefrontal cortex. Neuron. 2010;66(5):796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy JE, Riesenhuber M, Poggio T, Miller EK. Prefrontal cortex activity during flexible categorization. J Neurosci. 2010;30(25):8519–8528. doi: 10.1523/JNEUROSCI.4837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443(7107):85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- 13.Kaas JH. The evolution of complex sensory systems in mammals. J Exp Biol. 1989;146:165–176. doi: 10.1242/jeb.146.1.165. [DOI] [PubMed] [Google Scholar]
- 14.Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain Mind. 2000;1(1):7–23. [Google Scholar]
- 15.Striedter GF. Principles of Brain Evolution. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 16.Barrett HC. A hierarchical model of the evolution of human brain specializations. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10733–10740. doi: 10.1073/pnas.1201898109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nystrom LE, et al. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11(5 Pt 1):424–446. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- 18.Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci USA. 2006;103(26):10023–10028. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chein JM, Moore AB, Conway ARA. Domain-general mechanisms of complex working memory span. Neuroimage. 2011;54(1):550–559. doi: 10.1016/j.neuroimage.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 20.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 21.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 22.Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25(1):22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56(4):2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 24.Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends Cogn Sci. 2012;16(6):338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niendam TA, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxe R, Brett M, Kanwisher N. Divide and conquer: A defense of functional localizers. Neuroimage. 2006;30(4) doi: 10.1016/j.neuroimage.2005.12.062. 1088–1096, discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 27.Fedorenko E, Hsieh P-J, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N. New method for fMRI investigations of language: Defining ROIs functionally in individual subjects. J Neurophysiol. 2010;104(2):1177–1194. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage. 2012;63(3):1646–1669. doi: 10.1016/j.neuroimage.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumstein S, Amso D. Neural plasticity and dynamic functional organization: Insights from functional neuroimaging. Perspect Psychol Sci. 2013;8(1):44–48. doi: 10.1177/1745691612469021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedorenko E, Duncan J, Kanwisher N. Language-selective and domain-general regions lie side by side within Broca’s area. Curr Biol. 2012;22(21):2059–2062. doi: 10.1016/j.cub.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23(4):747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- 32.Stiers P, Mennes M, Sunaert S. Distributed task coding throughout the multiple demand network of the human frontal-insular cortex. Neuroimage. 2010;52(1):252–262. doi: 10.1016/j.neuroimage.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 33.Schmahmann J, editor. The Cerebellum and Cognition. New York: Academic; 1997. [Google Scholar]
- 34.Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6(3):184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 35.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Julian JB, Fedorenko E, Webster J, Kanwisher N. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. Neuroimage. 2012;60(4):2357–2364. doi: 10.1016/j.neuroimage.2012.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Monti MM, Parsons LM, Osherson DN. The boundaries of language and thought in deductive inference. Proc Natl Acad Sci USA. 2009;106(30):12554–12559. doi: 10.1073/pnas.0902422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monti MM, Parsons LM, Osherson DN. Thought beyond language: Neural dissociation of algebra and natural language. Psychol Sci. 2012;23(8):914–922. doi: 10.1177/0956797612437427. [DOI] [PubMed] [Google Scholar]
- 39.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 40.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 41.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci. 2005;5(3):263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- 43.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 44.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 46.Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007;17(7):1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinband J, et al. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57(2):303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 49.Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 50.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10(9):659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postle BR, et al. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci. 2006;18(10):1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- 52.Montojo CA, Courtney SM. Differential neural activation for updating rule versus stimulus information in working memory. Neuron. 2008;59(1):173–182. doi: 10.1016/j.neuron.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: A parametric functional magnetic resonance imaging study. J Neurosci. 2010;30(10):3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci. 2013;16(1):98–104. doi: 10.1038/nn.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philipp AM, Weidner R, Koch I, Fink GR. Differential roles of inferior frontal and inferior parietal cortex in task switching: Evidence from stimulus-categorization switching and response-modality switching. Hum Brain Mapp. 2013;34(8):1910–1920. doi: 10.1002/hbm.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bisiacchi PS, Cona G, Schiff S, Basso D. Modulation of a fronto-parietal network in event-based prospective memory: An rTMS study. Neuropsychologia. 2011;49(8):2225–2232. doi: 10.1016/j.neuropsychologia.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Shomstein S, Kravitz DJ, Behrmann M. Attentional control: Temporal relationships within the fronto-parietal network. Neuropsychologia. 2012;50(6):1202–1210. doi: 10.1016/j.neuropsychologia.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Esposito M, et al. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 59.Thiebaut de Schotten M, et al. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 60.Asanowicz D, Marzecová A, Jaśkowski P, Wolski P. Hemispheric asymmetry in the efficiency of attentional networks. Brain Cogn. 2012;79(2):117–128. doi: 10.1016/j.bandc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchida A, Fellows LK. Are core component processes of executive function dissociable within the frontal lobes? Evidence from humans with focal prefrontal damage. Cortex. 2013;49(7):1790–1800. doi: 10.1016/j.cortex.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Dosenbach NUF, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13(4):507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nomura EM, et al. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci USA. 2010;107(26):12017–12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hampshire A, Highfield RR, Parkin BL, Owen AM. Fractionating human intelligence. Neuron. 2012;76(6):1225–1237. doi: 10.1016/j.neuron.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 68.Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: A meta-analysis. Hum Brain Mapp. 2012;33(1):130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23(2):223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. J Cogn Neurosci. 2003;15(8):1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- 73.Tombu MN, et al. A unified attentional bottleneck in the human brain. Proc Natl Acad Sci USA. 2011;108(33):13426–13431. doi: 10.1073/pnas.1103583108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crittenden B, Duncan J. Cereb Cort. 2012. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. 10.1093/cercor/bhs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reynolds JR, O’Reilly RC, Cohen JD, Braver TS. The function and organization of lateral prefrontal cortex: A test of competing hypotheses. PLoS ONE. 2012;7(2):e30284. doi: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99(1):133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hutchison RM, et al. Functional connectivity of the frontal eye fields in humans and macaque monkeys investigated with resting-state fMRI. J Neurophysiol. 2012;107(9):2463–2474. doi: 10.1152/jn.00891.2011. [DOI] [PubMed] [Google Scholar]
- 78.Levy I, Schluppeck D, Heeger DJ, Glimcher PW. Specificity of human cortical areas for reaches and saccades. J Neurosci. 2007;27(17):4687–4696. doi: 10.1523/JNEUROSCI.0459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beurze SM, de Lange FP, Toni I, Medendorp WP. Spatial and effector processing in the human parietofrontal network for reaches and saccades. J Neurophysiol. 2009;101(6):3053–3062. doi: 10.1152/jn.91194.2008. [DOI] [PubMed] [Google Scholar]
- 80.Heed T, Beurze SM, Toni I, Röder B, Medendorp WP. Functional rather than effector-specific organization of human posterior parietal cortex. J Neurosci. 2011;31(8):3066–3076. doi: 10.1523/JNEUROSCI.4370-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci. 2010;30(39):13053–13065. doi: 10.1523/JNEUROSCI.1644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirose S, et al. Sub-centimeter scale functional organization in human inferior frontal gyrus. Neuroimage. 2009;47(2):442–450. doi: 10.1016/j.neuroimage.2009.04.094. [DOI] [PubMed] [Google Scholar]
- 83.Derrfuss J, Vogt VL, Fiebach CJ, von Cramon DY, Tittgemeyer M. Functional organization of the left inferior precentral sulcus: Dissociating the inferior frontal eye field and the inferior frontal junction. Neuroimage. 2012;59(4):3829–3837. doi: 10.1016/j.neuroimage.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 84.Duncan J, Owen A. Dissociative methods in the study of frontal lobe function. In: Monsell S, Driver J, editors. Attention and Performance XVIII, 567-76. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 85.Nieuwenhuis S, Forstmann BU, Wagenmakers E-J. Erroneous analyses of interactions in neuroscience: A problem of significance. Nat Neurosci. 2011;14(9):1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- 86.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 87.Li S, Ostwald D, Giese M, Kourtzi Z. Flexible coding for categorical decisions in the human brain. J Neurosci. 2007;27(45):12321–12330. doi: 10.1523/JNEUROSCI.3795-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esterman M, Chiu Y-C, Tamber-Rosenau BJ, Yantis S. Decoding cognitive control in human parietal cortex. Proc Natl Acad Sci USA. 2009;106(42):17974–17979. doi: 10.1073/pnas.0903593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cole MW, Etzel JA, Zacks JM, Schneider W, Braver TS. Rapid transfer of abstract rules to novel contexts in human lateral prefrontal cortex. Front Hum Neurosci. 2011;5:142. doi: 10.3389/fnhum.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reverberi C, Görgen K, Haynes J-D. Compositionality of rule representations in human prefrontal cortex. Cereb Cortex. 2012;22(6):1237–1246. doi: 10.1093/cercor/bhr200. [DOI] [PubMed] [Google Scholar]
- 91.Woolgar A, Hampshire A, Thompson R, Duncan J. Adaptive coding of task-relevant information in human frontoparietal cortex. J Neurosci. 2011;31(41):14592–14599. doi: 10.1523/JNEUROSCI.2616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stokes MG, et al. Dynamic coding for cognitive control in prefrontal cortex. Neuron. 2013;78(2):364–375. doi: 10.1016/j.neuron.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tark K-J, Curtis CE. Deciding where to look based on visual, auditory, and semantic information. Brain Res. 2013;1525:26–38. doi: 10.1016/j.brainres.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bush G, Shin LM. The Multi-Source Interference Task: An fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1(1):308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- 95.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 96.Vul E, Kanwisher N. Begging the question: The non-independence error in fMRI data analysis. In: Hanson SJ, Bunzl M, editors. Foundational Issues in Human Brain Mapping. Cambridge, MA: MIT Press; 2010. pp. 71–91. [Google Scholar]
- 97.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.